Background:
Cognitive impairment is frequently reported by silicone breast implant (SBI) patients. The aim of our study is to investigate whether subjective cognitive failure indeed is more frequent in a cohort of SBI patients compared with healthy controls (HCs). Furthermore, the severity of this cognitive failure and a possible relation to other symptoms as well as the duration of SBI exposure was examined. In addition, we assessed the effect of ruptures and reinterventions on cognitive failure severity.
Methods:
A cohort study was performed, including 376 women and consisting of 3 different groups of patients; 143 SBI patients (group 1), 94 age- and sex-matched HC patients (group 2), and 139 women with SBI and health issues who registered themselves at a Dutch foundation for women with illness due to SBI (group 3). All patients filled in the Cognitive Failure Questionnaire (CFQ). The American College of Rheumatology Fibromyalgia Diagnostic Criteria (2010) were used to score other symptoms.
Results:
Completed CFQ data from 222 patients were available for analysis: n = 79 for group 1, n = 62 for group 2, and n = 81 for group 3. SBI patients from group 3 had a significantly higher prevalence of subjective cognitive dysfunction (CFQ score ≥ 43) compared with SBI patients from group 1 and HC (60.5% versus 13.9% and 12.9%; P = 0.000). Linear regression showed a statistically significant relation between subjective cognitive functioning scores and other symptoms (P = 0.000). Implant duration as well as rupture rate and reinterventions were not found to significantly influence CFQ scores.
Conclusion:
An increased risk of cognitive failure in consecutive SBI patients when compared with HCs could not be found.
INTRODUCTION
Silicone breast implants (SBIs) may be associated with symptoms such as fatigue, arthralgias, myalgias, pyrexia, and sicca symptoms.1 In addition, patients may also complain that they develop cognitive impairment, characterized by memory loss, word finding problems, and concentration problems. Up until now, no studies have been performed to assess cognitive complaints in SBI patients compared with healthy controls (HCs).
In 1994, Shoaib and Patten2,3 first described a neurological disease in patients with SBIs with multiple sclerosis-like symptoms and/or atypical motor neuron disease. They reported 100 women with SBIs from which 84 patients underwent a magnetic resonance imaging of the brain.4 Nineteen of these 84 women showed multiple white matter lesions and 13 women showed multiple small ischemic lesions.
In 2011, Shoenfeld and Agmon-Levin5 proposed that above described (atypical) neurological symptoms may be due to adjuvant activity of SBIs. Later on, we and others described large cohorts of patients with SBI that were diagnosed as suffering from “Autoimmune/Inflammatory Syndrome Induced by Adjuvants” (ASIA-syndrome).6–8 Next to cognitive impairment, these patients also frequently report fatigue, arthralgias, myalgias, dry eyes, dry mouth, pyrexia, stroke, and/or multiple sclerosis-like symptoms.1 The prevalence of ASIA, due to silicone incompatibility or so called breast implant illness, and also the pathogenesis, however, at present are unknown.9 In the last decade, over 5000 Dutch women with SBIs made their complaints public online on the World Wide Web and registered themselves at a Dutch foundation for women with illness due to breast implants.10
The aim of this cohort study is to investigate the prevalence of cognitive failure in a cohort of SBI patients compared with control patients, as well as to evaluate the severity of this cognitive failure. Furthermore, we evaluated the existence of a possible relation between cognitive failure severity and the presence of other symptoms that are frequently reported in SBI patients as well as the duration of SBIs exposure. At last, we assessed the effect of patients reported ruptures and reinterventions on cognitive failure severity.
PATIENTS AND METHODS
Patients and Controls
The study included 376 women consisting of 3 different groups of patients. Group 1 consisted of 143 SBI patients that had been operated between 1997 and 2004 in the Maastricht University Medical Center, Maastricht, the St. Anna Hospital, Geldrop or the Maxima Medical Center, Eindhoven, The Netherlands. Patients were detected by archived surgery reports of the participating hospitals. The time span for the period of breast implantation from 1997 to 2004 was chosen based on our previous study, in which we reported the occurrence of onset of clinical symptoms after a median period of 4 years after time of breast implantation and a diagnosis of ASIA after a median period of 13 years after breast implantation.7 Group 2 consisted of 94 age- and sex-matched HC friends of the patients of group 1, which were selected according to the following exclusion criteria: (history of) SBIs and/or (history of) breast cancer. Group 3 consisted of 139 women with SBI and health issues who registered themselves at a Dutch foundation for women with illness due to breast implants (in Dutch: Steunpunt voor Vrouwen met Siliconen implantaties), matched for the period of breast implantation to patients of group 1. All women who were registered with known address details and had been operated between 1997 and 2004 were contacted and invited. All subjects were invited by post to complete a questionnaire after signing the informed consent form. The questionnaire consisted of a general questionnaire, the Dutch version of the 2010 American College of Rheumatology Fibromyalgia Diagnostic Criteria, and the Cognitive Failure Questionnaire (CFQ). Paper questionnaires were distributed by the clinical researcher (M.J.L.C.). They were coded with a unique number in advance to anonymize obtained data. Written informed consent for participation in this study was obtained from all subjects. The study was approved by the local Medical Ethics board of the Maastricht University Medical Center, The Netherlands.
Questionnaires
The prevalence of self-reported cognitive failure was assessed by filling in the CFQ, completed by all patients between July 2016 and July 2017. The CFQ, developed by Broadbent et al,11 is a self-report questionnaire consisting of 25 items assessing impairment in attention, perception, memory, and motor functioning in everyday life. In addition to these 25 items, all participants indicated on a 5-point Likert scale if they had experienced an increase in cognitive failures in the past 5 years (termed “increase,” ranging from “no increase” score 1, to “very strong increase” score 5), and to what extent these cognitive impairments affected their daily life, ranging from “no hindrance at all” (score 1) to “very much [a] hindrance” (score 5), how worried they were about these cognitive failures, ranging from “not worried at all” (score 1) to “very much worried” (score 5) and finally how annoying they found their cognitive failure, ranging from “not annoying at all” (score 1) to “very much annoying” (score 5). The total CFQ score was calculated by summing up all items. A high CFQ score is defined as a score ≥43, based on reference data from healthy volunteers (n = 1357) who participated in the Maastricht Aging Study.12 Cognitive failure severity is defined as the mean of the total CFQ scores, measured for all the participants in the group.
The somatic symptoms from the SS Scale score of the American College of Rheumatology Fibromyalgia Diagnostic Criteria (2010) were used to score other symptoms (range 0–6).13 These symptoms included: arthralgia, myalgia, fatigue/unrefreshed sleep, cognitive difficulties (eg, memory loss, concentration problems), sicca (eg, dry eyes and/or dry mouth), and pyrexia. A clinical score was calculated by summing up the total amount of symptoms (range 0–6).
Rupture rates were identified by the patient’s questionnaires respectively self-reporting. Duration of silicone exposure is counted from the year of implantation to year of explantation (mean in years ± SD).
Statistical Analysis
Continuous variables were reported as mean and SD and percentages were reported for categorical variables. Differences in mean CFQ scores between 2 groups were compared using Student’s t test for independent samples or the Mann–Whitney U test and differences in mean scores between more than two groups were compared using analysis of variance. Comparisons among groups were performed by the Chi-square or the Fisher’s exact tests for categorical variables. Pearson’s correlation and (unadjusted and adjusted) linear regression coefficients were used to estimate the crude and adjusted associations between the clinical score of other symptoms, age and duration of silicone exposure of breast implant(s) in relation to total CFQ scores. Statistical analyses were performed using SPSS (version 22.0). All statistical tests were 2-tailed, and a significance level of P ≤0.05 was used as indicated statistical significance.
RESULTS
Of the 376 patients, 231 (61.4%) returned the questionnaires. Because of incomplete data, 9 patients had to be excluded. In total from 222 patients, CFQ data were completed and available for analysis: n = 79 (55%) for group 1, n = 62 (66%) for group 2 and n = 81 (58%) for group 3. The demographic characteristics of the included SBI patients from the consecutive cohort (group 1) and from the cohort with previous reported health issues (group 3) are summarized in Table 1. The mean age of the HC patients was 43.01 (±14.29) years.
Table 1.
Demographic Characteristics of Included SBI Patients with (Group 3) and without (Group 1) Previously Reported Health Issues
| Variable | SBI Patients Group 1 (n = 79) | SBI Patients Group 3 (n = 81) | P (2-sided) |
|---|---|---|---|
| Age, mean in years ± SD | 57.77 ± 10.18 | 53.89 ± 9.36 | 0.015* |
| Reason of breast implantation, n (%) | |||
| Cosmetic | 52 (65.8) | 62 (76.6) | 0.134 |
| Reconstruction after breast cancer | 25 (31.6) | 15 (18.5) | |
| BRCA mutation | 12 (15.2) | 4 (4.9) | |
| Other | 2 (2.6) | 4 (4.9) | |
| Reintervention, n (%) | |||
| Yes | 53 (67.1) | 69 (85.2) | 0.007* |
| No | 26 (32.9) | 12 (14.8) | |
| Rupture rate of breast implant(s), n (%) | |||
| Yes | 21 (26.6) | 24 (29.6) | 0.668 |
| No | 58 (73.4) | 57 (70.4) | |
| Duration of silicone exposure, mean in years ± SD | 16.2 ± 10.2 | 15.4 ± 7.2 | 0.004* |
| Clinical score,† mean ± SD | 2.4 ± 1.7 | 4.1 ± 1.4 | 0.008* |
*Statistical significant results (P < 0.05).
†Clinical score: amount of other symptoms (range 0–6).
In group 3 (ie, SBI patients with previous reported health issues), a significantly higher prevalence of subjective cognitive dysfunction (CFQ score ≥ 43) was observed compared to SBI patients from group 1 (consecutive patients) and HCs (60.5% versus 13.9% and 12.9%; P = 0.000) (Fig. 1). No difference in the prevalence of subjective cognitive dysfunction could be found for SBI patients from group 1 compared with HCs (13.9% versus 12.9%; P = 1.000).
Fig. 1.
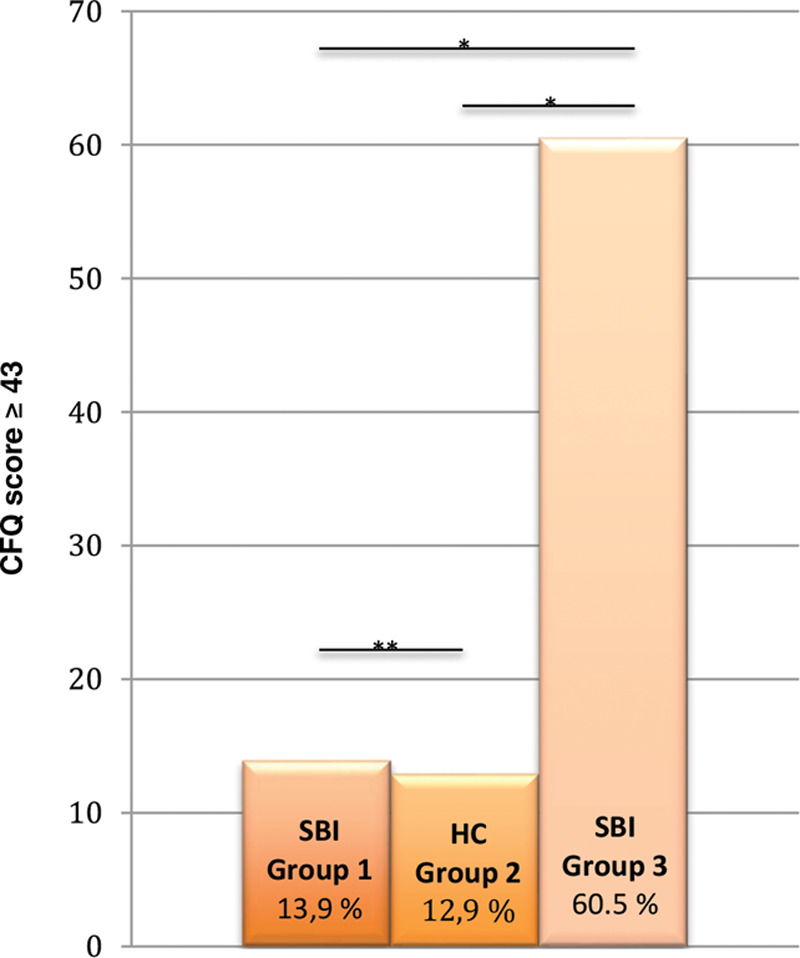
Prevalence of cognitive failure. *Statistical significant results (P < 0.05). **NS.
SBI patients from group 1 had CFQ scores comparable to CFQ scores of HC, but significantly lower than SBI patients from group 3 (P = 0.000) (Fig. 2). SBI patients from group 3 experienced significantly higher increases in cognitive failures in the past 5 years and were more impaired, annoyed, and worried about these cognitive failures compared with HCs and SBI without previous reported health issues (all P = 0.000).
Fig. 2.
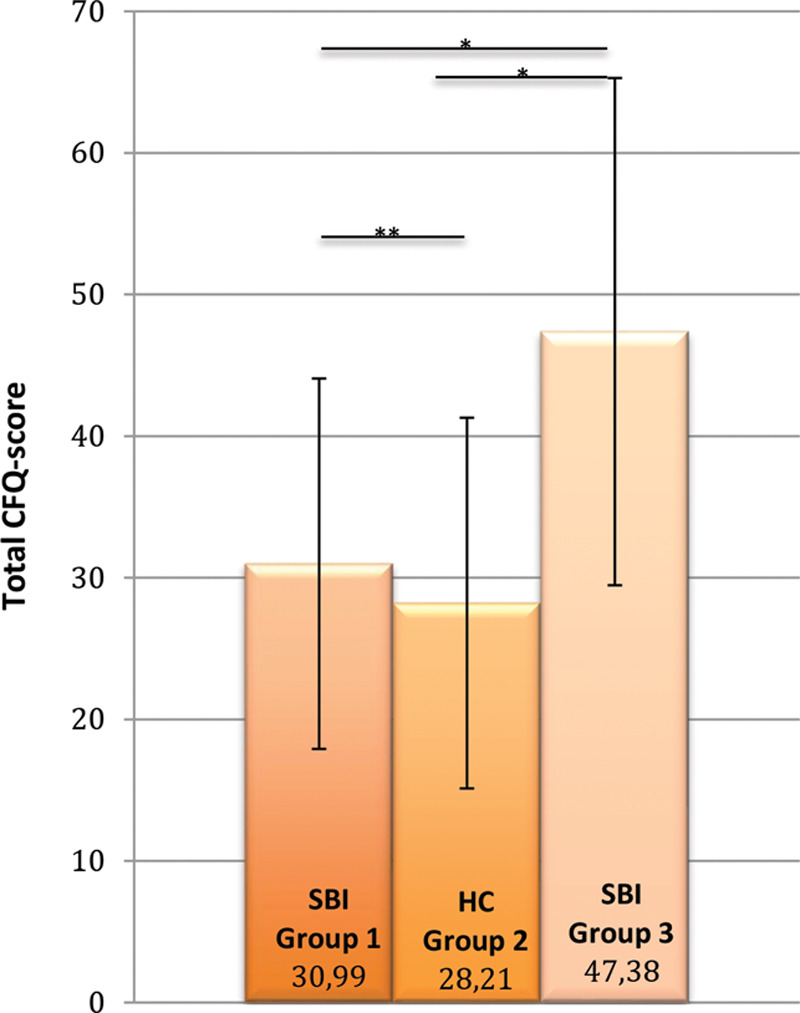
Severity of cognitive failure. *Statistical significant results (P < 0.05). **NS.
Total CFQ scores are positively correlated with the clinical score of other symptoms in SBI patients from either group 1 and/or group 3 (P = 0.000) (Fig. 3). Also after adjustment for age, a correlation between total CFQ scores and the total clinical score remained present (P = 0.004) (Table 2). Total CFQ scores are not correlated with duration of silicone exposure itself in SBI patients from either group 1 or group 3 (P = 0.596).
Fig. 3.
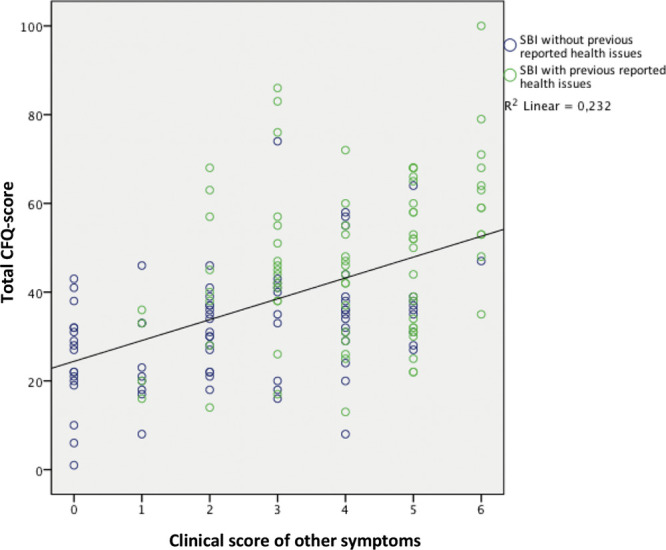
Relationship between total CFQ-scores and other clinical symptoms in SBI patients. Linear regression: the y axis represents the value of the CFQ-scores, values range from 1 to 100: a high CFQ-score was defined as a score ≥43. The x axis represents the clinical score, which was calculated by summing up the amount of other symptoms (range 0–6). The blue circles in the scatterplot represent the SBI patients without previous reported health issues (group 1), and the green circles represent the SBI patients with previous reported health issues (group 3). The black line is the linear regression with a regression coefficient of 4.698 ± 0.68 and an R2 of 0.232 ± 15.32 (P = 0.000).
Table 2.
Pearson’s Correlation and Crude and Adjusted Linear Regression Coefficients of Clinical Scores and Age in Relation to Total CFQ Scores
| Independent | Dependent | Crude | ||||
|---|---|---|---|---|---|---|
| R | R2 | B | CI | P | ||
| Clinical score* | Total Cognitive Failure Score | 0.481 | 0.232 | 4.698 | 3.354–6.043 | 0.000 |
| Age | Total Cognitive Failure Score | −0.248 | 0.062 | −0.422 | −0.685 to −0.158 | 0.002 |
| Independent | Dependent | Adjusted | ||||
| Clinical score† | Total Cognitive Failure Score | 0.527 | 0.277 | 4.381 | 3.092–5.670 | 0.004 |
B, regression coefficient; CI, confidence interval.
*Clinical score: amount of other symptoms (range 0–6).
†Adjusted for age.
Self-reported ruptures of SBIs do not influence CFQ scores of SBI patients from group 1 (P = 0.211) as well as CFQ scores of SBI patients from group 3 (P = 0.991) (Table 3). Moreover, CFQ scores were not found to be influenced by reintervention of the SBIs in SBI patients from group 1 (P = 0.850) as well as in SBI patients from group 3 (P = 0.504) (Table 4).
Table 3.
Mean Total CFQ Scores in SBI Patients with (Group 3) and without (Group 1) Ruptures of Their SBIs
| SBI Patients Group 1 (n = 79) | SBI Patients Group 3(n = 81) | |||||
|---|---|---|---|---|---|---|
| Rupture of SBI | + (n = 21) | − (n = 58) | P(2-sided) | + (n = 24) | − (n = 57) | P(2-sided) |
| Total CFQ scores, mean ± SD | 34.57 ± 16.76 | 30.50 ± 10.89 | 0.211 | 47.42 ± 17.86 | 47.37 ± 18.10 | 0.991 |
Table 4.
Mean Total CFQ Scores in SBI Patients with (Group 3) and without (Group 1) Reintervention of Their SBIs
| SBI Patients Group 1 (n = 79) | SBI Patients Group 3 (n = 81) | |||||
|---|---|---|---|---|---|---|
| Reintervention of SBI | + (n = 53) | − (n = 26) | P(2-sided) | + (n = 69) | − (n = 12) | P(2-sided) |
| Total CFQ scores, mean ± SD | 31.77 ± 13.46 | 31.19 ± 11.28 | 0.850 | 47.94 ± 18.33 | 44.17 ± 15.59 | 0.504 |
DISCUSSION
This cohort study was aimed at exploring the prevalence and severity of subjective cognitive dysfunction in SBI patients compared with controls. Our data showed that consecutive SBI patients from group 1 have the same amount (CFQ score ≥ 43) and severity of cognitive difficulties (mean of total CFQ score) as measured with the CFQ compared to sex- and age-matched HCs (13.9% versus 12.9% and 30.99 versus 28.21; P > 0.05). Furthermore, these SBI patients have less frequent and less severe cognitive failure than SBI patients who registered themselves at a Dutch foundation for women with illness due to breast implants (60.5% and 47.38%; P = 0.000). Our study raises awareness that SBI patients who made their complaints public are a selected group of patients with multiple symptoms including higher cognitive failure scores. The higher frequency and severity of cognitive failure in these latter silicone breast patients suggests that in these patients similar psychological mechanisms are operative as in conditions characterized by so-called central sensitizations such as fibromyalgia and/or chronic fatigue syndrome. A major difference with chronic fatigue syndrome and fibromyalgia, however, is that under these conditions reversibility has only infrequently been described, whereas cognitive difficulties and other symptoms may improve and/or disappear after explantation of the breast implants.1,8,14
Silicone can migrate outside the outer shell after SBI rupture, but also migration with an intact shell, the so-called gel bleed have been described.1 Also, the duration of silicone breast implantation time is positively correlated to the rupture rate or leakage of the SBIs.15–17 In previous studies, it is postulated that implant rupture and aging can be an important factor for an inflammatory response or triggering of the immune system as reaction to migration of silicone particles throughout the body.18 In our study, however, no relation of cognitive failure severity with self-reported rupture rate and/or duration of silicone implant exposure was found. Therefore, the results of this study imply that rupture of SBIs and leakage of silicone exposure are not responsible for the cognitive difficulties that SBI patients experience.
The results of our study clearly show that cognitive failure occurred more frequently in the group of SBI patients with other self-reported symptoms. We admit that a statistical difference between group 1 and group 3 could be previously expected because of the selection bias of group 3. Due to this selection bias, we cannot conclude that there is an increased risk to develop cognitive failure after SBI in general. In fact, in the group of SBI patients without other self-reported symptoms, which represents the general SBI population the most, an increased risk to develop cognitive failure could not be found at all in comparison to HCs. We can conclude that the selected SBI patients, with previously self-reported symptoms, cannot represent the overall general SBI patients. In SBI patients with other self-reported symptoms, chronic reactions of the immune system, however, may have resulted in the symptoms of cognitive failure. Otherwise, social media may have an influence suggesting that the concerns that these patients are sharing with each other may have contribute to the development of symptoms of cognitive failure.19
This is the first study that measures subjective cognitive failure in consecutive SBIs patients compared with controls. A limitation of this cohort study is that a subjective screening instrument is used for the assessment of cognitive dysfunction. Subjective cognitive functioning is, however, not equal to objective cognitive functioning.20 Broadbent et al11 described “cognitive failure” as a cognitive error that occurs during the performance of a task that a person normally would execute successfully. Beliefs about cognitive changes are strongly influenced by self-efficacy beliefs, personality, vitality, and coping styles.21 Zuckerman et al.22 reviewed the literature about the link between breast implants and self-esteem, quality of life and the risk of suicide suggesting that breast implants are related to risks to mental health. Without studying cognitive function before implants, it is, however, not possible to make firm conclusions. Depression and fatigue are both important issues regarding cognition.
CONCLUSIONS
Subjective cognitive failure is a substantial problem in SBI patients that registered themselves with a patient organization. In the current study, we could not find an increased risk of cognitive failure in consecutive SBI patients, without previously self-reported symptoms, when compared with HCs. Implant duration, rupture rate, and reinterventions were not found to be related to CFQ scores in all of the patients that we studied. Prospective cognitive function studies should be performed, in which cognitive function will be measured before SBI and after long-term follow-up before definitive conclusions can be drawn.
Footnotes
Published online 17 February 2021.
Presented (partially) at the 7th EURAPS Research Council Meeting on May 16, 2018 in Madrid, Spain.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Cohen Tervaert JW, Colaris MJ, van der Hulst RR. Silicone breast implants and autoimmune rheumatic diseases: myth or reality. Curr Opin Rheumatol. 2017;29:348–. [DOI] [PubMed] [Google Scholar]
- 2.Shoaib BO, Patten BM. An atypical motor neuron disease syndrome in patients with breast implants. Can J Neurol Sci. 1994;1:64–69. [Google Scholar]
- 3.Shoaib BO, Patten BM. An MS-like syndrome in women with silicone breast implants or silicone fluid injections in breast. Neurology. 1994;44:37–44. [Google Scholar]
- 4.Shoaib BO, Patten BM, Calkins DS. Adjuvant breast disease: an evaluation of 100 symptomatic women with breast implants or silicone fluid injections. Keio J Med. 1994;43:79–87. [DOI] [PubMed] [Google Scholar]
- 5.Shoenfeld Y, Agmon-Levin N. “ASIA” - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36:4–8. [DOI] [PubMed] [Google Scholar]
- 6.Cohen Tervaert JW, Kappel RM. Silicone implant incompatibility syndrome (SIIS): a frequent cause of ASIA (Shoenfeld’s syndrome). Immunol Res. 2013;56:293–298. [DOI] [PubMed] [Google Scholar]
- 7.Colaris MJL, de Boer M, van der Hulst RR, et al. Two hundreds cases of ASIA syndrome following silicone implants: a comparative study of 30 years and a review of current literature. Immunol Res. 2017;65:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maijers MC, de Blok CJ, Niessen FB, et al. Women with silicone breast implants and unexplained systemic symptoms: a descriptive cohort study. Neth J Med. 2013;71:534–540. [PubMed] [Google Scholar]
- 9.Healing Breast Implant Illness. Breast implant safety. 2020. Available at: http://healingbreastimplantillness.com/breast-implant-safety. Accessed April 18, 2017.
- 10.Meldpunt Klachten Siliconen. Dutch foundation for women with illness due to breast implants. Available at: https://www.meldpuntklachtensiliconen.com. Accessed February 06, 2020.
- 11.Broadbent DE, Cooper PF, FitzGerald P, et al. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21:1–16. [DOI] [PubMed] [Google Scholar]
- 12.Ponds R, van Boxtel M, Jolles J. De “cognitive failure questionnaire” als maat voor subjectief functioneren. Tijdschrift voor neuropsychologie. 2006;1:37–42. [Google Scholar]
- 13.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600–610. [DOI] [PubMed] [Google Scholar]
- 14.de Boer M, Colaris M, van der Hulst RRWJ, et al. Is explantation of silicone breast implants useful in patients with complaints? Immunol Res. 2017;65:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters W, Keystone E, Smith D. Factors affecting the rupture of silicone-gel breast implants. Ann Plast Surg. 1994;32:449–451. [DOI] [PubMed] [Google Scholar]
- 16.Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003;138:801–806. [DOI] [PubMed] [Google Scholar]
- 17.Seigle-Murandi F, Lefebvre F, Bruant-Rodier C, et al. Incidence of breast implant rupture in a 12-year retrospective cohort: evidence of quality discrepancy depending on the range. J Plast Reconstr Aesthet Surg. 2017;70:42–46. [DOI] [PubMed] [Google Scholar]
- 18.Nesher G, Soriano A, Shlomai G, et al. Severe ASIA syndrome associated with lymph node, thoracic, and pulmonary silicone infiltration following breast implant rupture: experience with four cases. Lupus. 2015;24:463–468. [DOI] [PubMed] [Google Scholar]
- 19.Tang SYQ, Israel JS, Afifi AM. Breast implant illness: Symptoms, patient concerns, and the power of social media. Plast Reconstr Surg. 2017;140:765e–766e. [DOI] [PubMed] [Google Scholar]
- 20.Walitt B, Čeko M, Khatiwada M, et al. Characterizing “fibrofog”: subjective appraisal, objective performance, and task-related brain activity during a working memory task. Neuroimage Clin. 2016;11:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponds RW, Commissaris KJ, Jolles J. Prevalence and covariates of subjective forgetfulness in a normal population in The Netherlands. Int J Aging Hum Dev. 1997;45:207–221. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerman DM, Kennedy CE, Terplan M. Breast implants, self-esteem, quality of life, and the risk of suicide. Womens Health Issues. 2016;26:361–365. [DOI] [PubMed] [Google Scholar]


