Summary:
Recent studies have provided evidence that lymphovenous bypass—microsurgical re-routing of divided lymphatics to an adjacent vein—performed at the time of lymph node dissection decreases the rate of lymphedema development. Immediate lymphatic reconstruction in this setting is technically demanding, and there is a paucity of literature describing the details of the surgical procedure. In this report, we review the literature supporting immediate lymphatic reconstruction and provide technical details to demystify the operation for surgeons who wish to provide this option to their patients.
Introduction and Literature Review
Secondary lymphedema is a common complication of lymph node dissection (LND) and is a major source of morbidity in patients who are treated for solid malignancies. It is estimated that 16%–49% of patients who undergo LND develop this disease.1–5 Despite compliance with lifelong compression garments and manual lymphatic drainage, patients may still show disease progression.
Traditionally, surgical treatment of lymphedema—lymphovenous bypass, lymph node transplantation, or liposuction—has been reserved for patients who have already developed the disease. Recent reports, however, have suggested that lymphatic surgery may also be effective in preventing lymphedema if the lymphatic system is repaired at the time of LND.6–17 Boccardo et al first described Lymphatic Microsurgical Preventative Healing Approach (LYMPHA)—referred in this article as immediate lymphatic reconstruction (ILR)—in an initial study of 19 patients.7,18,19 In a follow-up study, they reported a 4% rate of lymphedema after ALND for treatment of breast cancer over a 4-year follow-up period in 74 patients.8 Feldman et al reported a 12.5% lymphedema rate after ALND at a mean follow-up of 6-months, compared with their historical rate of 30.6%.6 Singhal and colleagues described a modification to the original technique using fluorescein isothiocyanate (FITC) to differentiate lymphatics draining the arm versus those draining the breast when the breast surgeon has used blue dye for sentinel node mapping.10 Early data utilizing this technique demonstrated a lymphedema rate of 12.5% versus a control group rate of 40%.16 With increased experience and more robust data utilizing this same technique, Johnson et al report a rate of 3.1% rate of lymphedema with 11.4 months of follow-up.17 The cost-effectiveness of ILR (after ALND ± radiation) was examined and found to be the more cost-effect treatment option (compared with ALND ± radiation without ILR) for node-positive breast cancer.1
While these results are promising, studies with longer term follow-up and a larger number of patients are needed to establish the results. The authors are currently working to provide long-term data from ongoing prospective cohort studies (RS, DS). Additionally, a randomized control trial is underway comparing ALND without ILR and ALND with ILR in patients undergoing breast cancer treatment (MC, BM, JD). A total of 174 patients will be recruited to the trial and will be followed for 2 years postoperatively.
Immediate lymphatic reconstruction has not only been used for breast cancer, but also for melanoma and gynecological cancers. Morotti et al described ILR for 12 lower extremities following inguinal lymph node dissection and reported only mild lymphedema in 1 extremity (8.3%).13 Takeishi et al reported a decreased incidence in lymphedema in 7 patients undergoing intrapelvic ILR after hystero-oophorectomy and intrapelvic LND.15 Cakmakoglu et al successfully performed ILR in 22 patients with ALND or inguinal lymph node dissection for treatment of melanoma.12 These promising reports, coupled with the high risk of developing the permanent and disabling consequences of lymphedema, have resulted in an increased demand for ILR (Table 1).
Table 1.
Literature Review
| Authors | Year Published | Type of Study | Number of Patients | Nodal Surgery/Indication | Extremity | Average FU (mo) | Lymphedema Rate |
|---|---|---|---|---|---|---|---|
| Takeishi et al | 2006 | Prospective cohort | 7 (14 extremities) | PLND for uterine carcinoma | LE | 14 | 7.14% |
| Boccardo et al | 2009 | Prospective cohort | 19 | ALND for breast cancer | UE | 12 | 0% |
| Boccardo et al | 2011 | Randomized controlled trial | 49 | ALND for breast cancer | UE | 18 | 4.34% versus 30.43% |
| Morotti et al | 2013 | Prospective cohort (with historical control) | 8 (12 extremities) | ILND for vulvar carcinoma | LE | 6.2 | 8.33% versus 25% in historical control |
| Boccardo et al | 2014 | Prospective cohort | 71 | ALND for breast cancer | UE | 48 | 4.05% |
| Feldman et al | 2015 | Prospective cohort (with historical control) | 27 | ALND for breast cancer | UE | 6 | 12.5% versus 30.6% in historical control |
| Spiguel et al | 2017 | Retrospective review of prospective cohort | 13 | ALND for breast cancer | UE | NA | NA |
| Hahamoff et al | 2019 | Retrospective review of prospective cohort | 87 | ALND for breast cancer | UE | 15 (ALND + ILR), 20 (ALND alone) | 12.5 % in ALND + ILR (n = 8) versus 40% in ALND alone (n = 10) |
| Johnson et al | 2020 | Prospective cohort | 32 | ALND for breast cancer | UE | 11.4 | 3.10% |
| Cakmakoglu et al | 2020 | Prospective cohort | 22 | ALND (n = 10) ILND (n = 12) for melanoma | UE and LE | 6 (14 patients), 12 (4 patients) | 4.50% |
ALND, axillary lymph node dissection; FU, follow-up; ILND, inguinal lymph node dissection; LE, lower extremity; PLND, pelvic lymph node dissection; UE, upper extremity.
Although lymphovenous bypass (LVB) for ILR is simple in concept, it can be a challenge to apply in the operating room. For example, if a standard en bloc lymphadenectomy is performed, finding a suitable vein of adequate length and appropriate caliber can be difficult, if not impossible. There is a learning curve for the ablative surgeon—namely identifying and preserving appropriate recipient veins during the LND. For the reconstructive surgeon, intimate knowledge of the venous and lymphatic anatomy, reliable identification of target lymphatic channels, and proper vein selection are fundamentally important but not well described in the literature. Consequently, the learning curve for the reconstructive surgeon can be steep and frustrating as well.
The purpose of this study was to provide surgeons with an in-depth description of the technical details of ILR. We will discuss all aspects of ILR, including preoperative planning, lymphatic channel and vein selection, back-up venous options, anastomotic technique, and postoperative care. Techniques and technology will undoubtedly evolve; the goal is to provide a solid foundation to make ILR more approachable.
Preparation
ILR is currently an option for patients undergoing axillary or groin LND. Patients undergoing LND, and especially those having adjuvant radiation, are at a high risk for developing lymphedema and, therefore, the most appropriate patients for this operation. To our knowledge, ILR has not been for patients who are treated with neck dissection. Specialized microsurgical instruments are required for successful ILR (Fig. 1). These include an operating microscope, microsurgical instruments, and agents for visualizing lymphatic channels. The patient’s entire extremity, including the hand or foot, is prepared circumferentially in the field for maximum exposure.
Fig. 1.
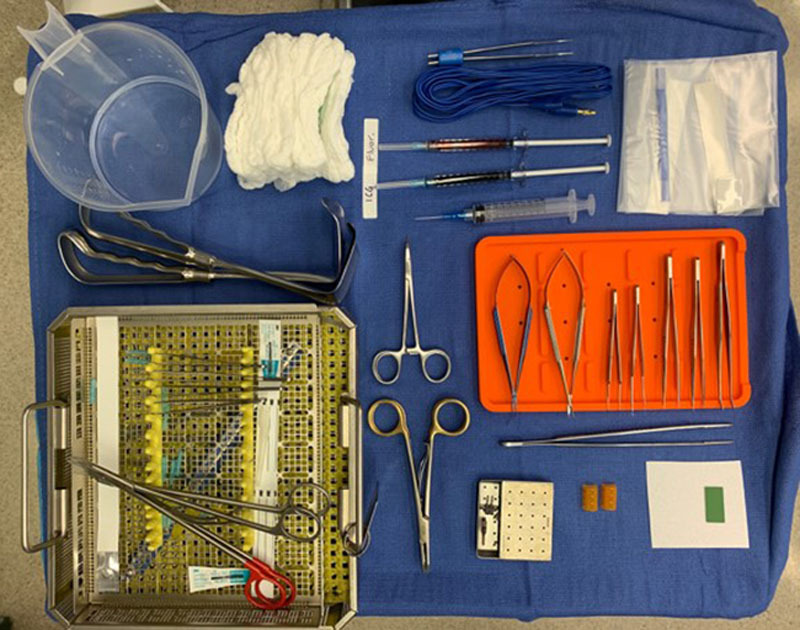
ILR instruments and equipment setup.
Communication with the ablative surgeon is paramount for incision placement and preservation of a suitable vein for bypass. Two of the most common pitfalls we initially encountered were a low axillary incision limiting visualization of the field and inadequate vein length for bypass. ALND and subsequent ILR can be accomplished either through the mastectomy incision or separate axillary incision. Low axillary incisions can be problematic for visualization; so we recommend incising high in the axilla to provide a direct view of the severed lymphatic channels. It is customary for the oncologic surgeon to clip veins at their origin, making ILR all but impossible. Scrubbing in with the ablative surgeon during the lymphadenectomy is essential; a learning curve of about 5 cases is typically adequate. With appropriate communication and practice, the surgical oncologists can also preserve a suitable length of the vein (6–7 cm) to facilitate microsurgical anastomosis without tension.
Identification of Lymphatic Channels
There are a number of agents available that are commonly used to identify lymphatic channels: indocyanine green (ICG), lymphazurin (isosulfan blue), and fluorescein isothiocyanate (FITC).7,10 ICG is mixed to a concentration of 2.5 mg/ml.20 Blue dye is generally not diluted. FITC solution is formulated by mixing 2 ml AK-FLOUR 10% (Akorn Inc, Lake Forest, Ill.) and 8 ml normal saline for a 2% concentration. All dyes are injected subdermally with a 30-gauge needle, using a 1 ml syringe. If performed before LND, the dye has time to fill the lymphatic channels before their transection. However, coordination with the oncologic surgeons can sometimes be difficult and injection of dye following LND may be more feasible.
We first perform ICG lymphangiography of the entire limb to become familiar with the patient’s lymphatic anatomy and establish a baseline. This may reveal pre-existing abnormalities sometimes observed following chemotherapy, and will identify alternative pathways draining into the supraclavicular lymph nodes.21 The authors reliably visualize the entire lymphatic tree of the upper limb with 0.1 ml of ICG injected into the first webspace, third webspace, and volar wrist (Fig. 2). (See Video 1 [online], which displays the subdermal injection of ICG into the third webspace, 0.1 cm3.)
Fig. 2.
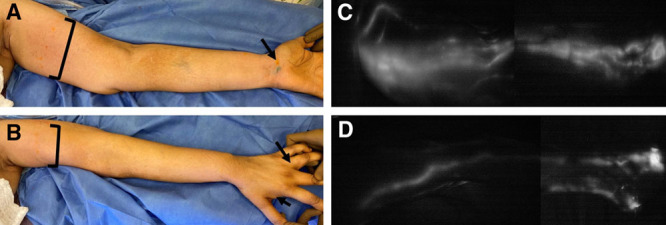
Upper extremity injection sites and ICG lymphangiogram with SPY-PHI. A, B, ICG is injected at 0.1 ml into the dermis of the first and third webspace, and the volar wrist. FITC is injected at 0.1 ml into the dermis of the first and third webspace, and the volar wrist. Additional FITC injections are done into the dermis across the medial upper arm (4–5 points with 0.1 ml each) and deeper, just above muscle fascia (1 point with 0.3 ml). C, D, ICG lymphangiogram showing the standard volar, radial, and ulnar lymphatic bundles merging at the upper inner arm.
Video 1. Video 1 from “Immediate Lymphatic Reconstruction: Technical Points and Literature Review”.
An additional injection 4 cm proximal to the elbow crease over the cephalic vein may provide extra coverage of the Mascagni-Sappey pathway into the supraclavicular nodes.22 Four injection sites are used for the lower limb: the first and third webspace and the medial and lateral ankle. Once injected, ICG is rapidly bound by albumin, thus limiting its uptake to the lymphatic system, where it can be visualized with a near infra-red camera. Although ICG reliably images the superficial lymphatic system, the deep system is not seen because sensitivity rapidly diminishes at depths > 1–2 cm below the skin.10,23
Following baseline ICG lymphangiography, the microscope is brought in to identify divided lymphatic channels in the field. Although ICG alone can be used, many surgeons find the additional use of FITC or blue dye helpful in delineating the lymphatic channels. These are injected at a similar volume (0.1 ml) into the dermis of the hand/wrist or foot/ankle.10 Additional dye injections are made more proximally across the medial upper arm using 4–5 injections each with 0.1 ml of agent and an additional deep injection just above muscle fascia of 0.3 ml to identify deep lymphatics in the field (Fig. 2). FITC excites in the visible spectrum; therefore, this dye can be visualized through the microscope binoculars with special filters such as the YELLOW 560 package on a Pentero 900D Microscope (Carl Zeiss Inc, Germany) or a multi-contrast yellow fluorescence filter on the Mitaka MM51 microscope (Mitaka Kohki Co., Ltd, Japan) (Fig. 3). In contrast, blue dye can be directly visualized without any special equipment. The benefit of using FITC is that it decreases the risk of severe allergic reaction noted in 1%–3% patients who have a sensitivity to blue dye.10,24 Additionally, if blue dye was also used for sentinel lymph node identification, it can be difficult to differentiate the target lymphatic channels draining the limb from those draining the breast. The downside of using FTIC is that specialized equipment, such as a Mitaka microscope with filter, is necessary to visualize the dye. It is preferable for the oncologic surgeon to clip the lymphatic channels, which creates a solid seal and leads to dilation of the vessels and accumulation of dye in the lumen (Fig. 4). In contrast, cauterizing lymphatic channels often results in an incomplete seal, dye spillage, and a smaller, decompressed lymphatic channel. Massage or elevation of the arm can help facilitate dye movement to the axilla.
Fig. 3.
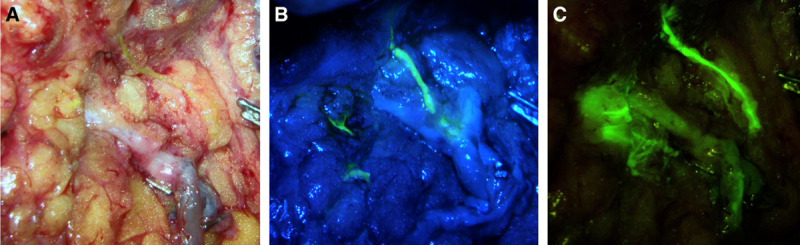
Visualization of lymphatic channels with FITC using the Mitaka microscope. A, With no filter, the lymphatic channel is slightly visible under white light. B, With a low-contrast fluorescence filter, there is visibility of non-fluorescent tissue and some fluorescence within the lymphatic channel. C, With a high-contrast fluorescence filter, there is poor visibility of non-fluorescent tissue, but excellent fluorescence seen within the lymphatic channel.
Fig. 4.
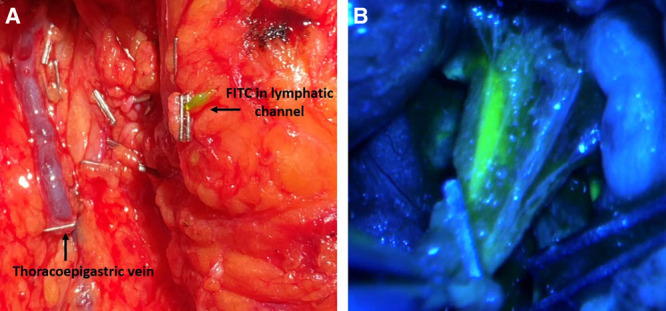
Lymphatic channel with FITC injected after ALND. A, Without the microscope, FITC is seen accumulating in the lymphatic channel up to the clip, dilating the lymphatic channel, on the right. Thoracoepigastric vein with clip on the left. B, Using the Mitaka microscope with a low-contrast fluorescence filter, there is visibility of surrounding tissue and fluorescence within the lymphatic channel.
As a way to examine different lymphosomes—superficial lymphatic territories that drain to specific lymph nodes—another method utilizes both blue dye and fluorescein.11,25 Blue dye can be injected to the lateral upper arm to capture lymphatic channels draining the lateral lymphosome. FITC is injected to the hand/wrist and medial upper arm to capture lymphatic channels draining the medial lymphosome. During ILR, the surgeon can then delineate which lymphosome the transected lymphatic channels are draining.
The selection of which lymphatic channels to bypass—if more than one are located—is not well defined. The number of lymphatic channels bypassed depends on the location of the channels and the number of veins/vein branches available for anastomosis. Larger channels that have a high output should be targeted first. These are generally found in proximity to the axillary vein. If all lymphatic channels appear equal in caliber and drainage, the anatomy and reach of the vein will dictate which channels are chosen for bypass.
Vein Choice Upper Extremity
Multiple vein options exist for ILR in the axilla. We prefer, as a first choice, the thoracoepigastric vein (sometimes referred to as the accessory vein) (Fig. 5). This vein is located at a superficial to medium depth (just below the clavipectoral fascia) arising perpendicular from the axillary vein, coursing into the level 1 axillary lymph nodes, anterior and parallel to the thoracodorsal vessels. The intercostal brachial cutaneous nerve is frequently found at approximately the depth of the thoracoepigastric vein, which runs perpendicular to it. This vein usually runs within the lymph node package and, as a result, should be dissected from the lymph nodes before completing the dissection, thereby preserving a suitable length of the vein (6–7 cm) necessary to reach the transected axillary lymphatic channels. The preserved vein length also increases the likelihood that a valve will be present, thus decreasing the potential for venous backflow into the transected lymphatic channels.
Fig. 5.
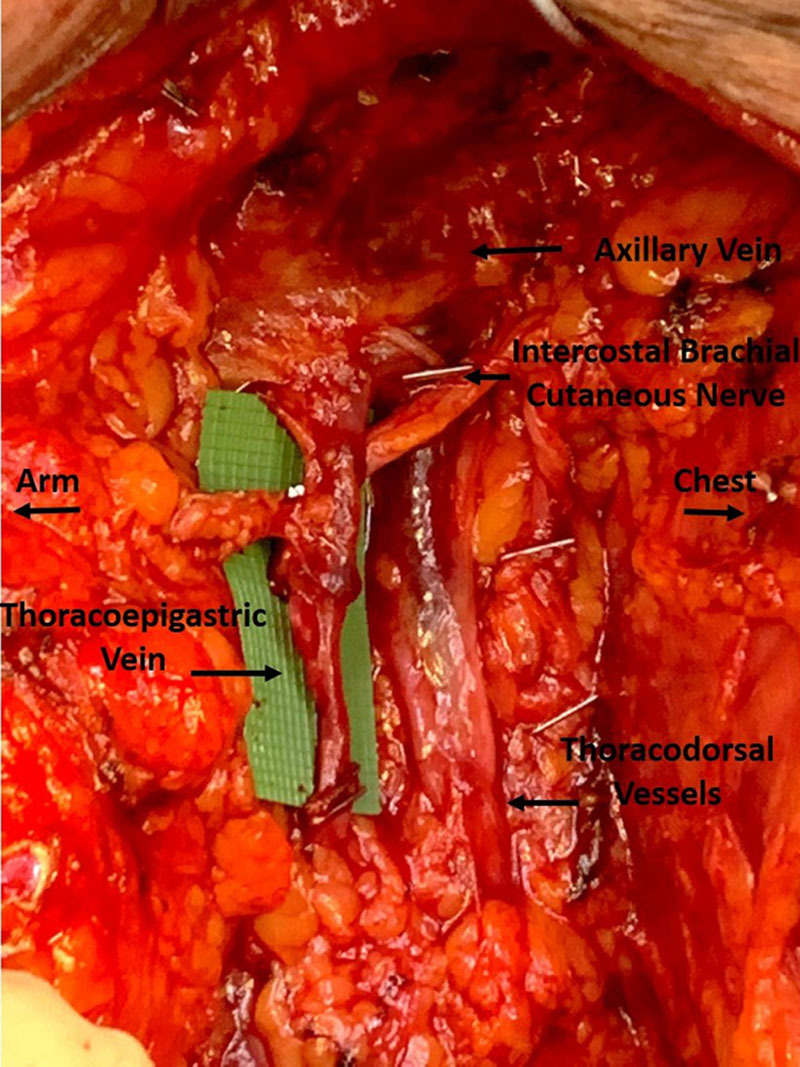
Thoracoepigastric (sometimes referred to as accessory) vein in axilla. The vein was dissected to a length of 6 cm, and there is a valve present at the midpoint. The intercostal brachial nerve is crossing posteriorly, and the thoracodorsal vessels are seen posterior to the thoracoepigastric vein.
In some cases, the breast surgeon will need to remove the thoracoepigastric vein if they believe there is tumor involvement/encasement or otherwise cannot remove the ALND specimen without its sacrifice. In these cases, secondary vein options exist: the lateral thoracic vein, the medial pectoral vein, circumflex scapular vein, thoracodorsal vein, or any unnamed vein generally found more lateral to the thoracodorsal vessels (Fig. 6). The lateral thoracic vein courses superficially on the lateral chest wall, medial and parallel to the long thoracic nerve, and has an associated artery.26 Some references will note the thoracoepigastric vein is actually a distal runoff of the lateral thoracic vein. However, according to other references, and in our experience, the thoracoepigastric vein and lateral thoracic vein are separate entities. The medial pectoral vein is found on the undersurface of the pectoralis major muscle, running with the medial pectoral nerve. Both the lateral thoracic vein and medial pectoral vein need to be dissected to a longer length (7–10 cm) than that of the thoracoepigastric vein to be able to reach the lymphatics of the upper inner am. The circumflex scapular vein runs lateral and posterior to the thoracodorsal vein. These veins can either come together as the subscapular vein before joining the axillary vein or can enter the axillary vein separately.27 Unnamed veins can also commonly be found lateral to and around the same depth of the thoracodorsal vessels. In some cases, short vein grafts harvested from the axilla can be anastomosed to other suitable (but short) veins to provide adequate length to reach the axilla. This is simple to do with an anastamotic coupler. We avoid using the thoracodorsal vein owing to its size with a high risk of backbleeding and to preserve this option for future breast reconstruction or salvage. The small branches of the thoracodorsal vein can still be used for ILR.
Fig. 6.
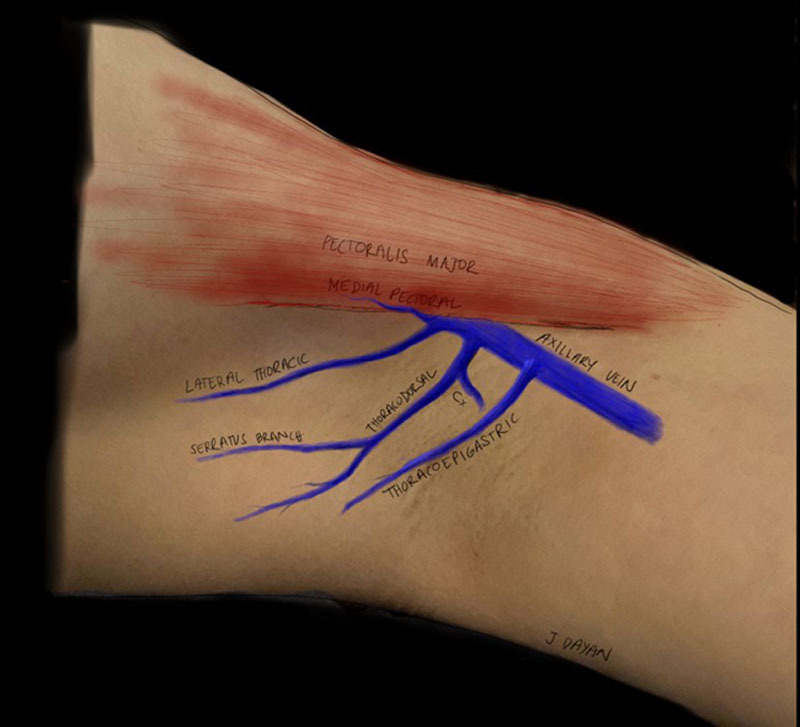
Alternative vein choices in the axilla. Illustrated are the medial pectoral vein running on the undersurface of the pectoralis major muscle, the lateral thoracic vein running on the lateral chest wall, the thoracodorsal vein and circumflex scapular vein (Cx) that are deeper in the axilla, and the thoracoepigastric vein (sometimes referred to as accessory) running more superficial (just below the clavipectoral fascia) to the thoracodorsal vessels.
Vein Choice Lower Extremity
Multiple vein choices also exist for ILR of the groin. In our experience, there is no first choice analogous to the thoracoepigastric vein to be used for ILR of the groin. The vein choice primarily depends on the location of the transected lymphatic channels (Fig. 7). The external pudendal vein is best positioned for medial lymphatic channels and can branch directly from the femoral vein or from the greater saphenous vein.26 The superficial epigastric vein or the superficial circumflex iliac vein are best for lateral lymphatic channels and can enter the femoral vein independently or in a common trunk.26,28,29 Any of the veins can be dissected to a length that, when transected and rotated, can be used for the opposite area, if needed.
Fig. 7.
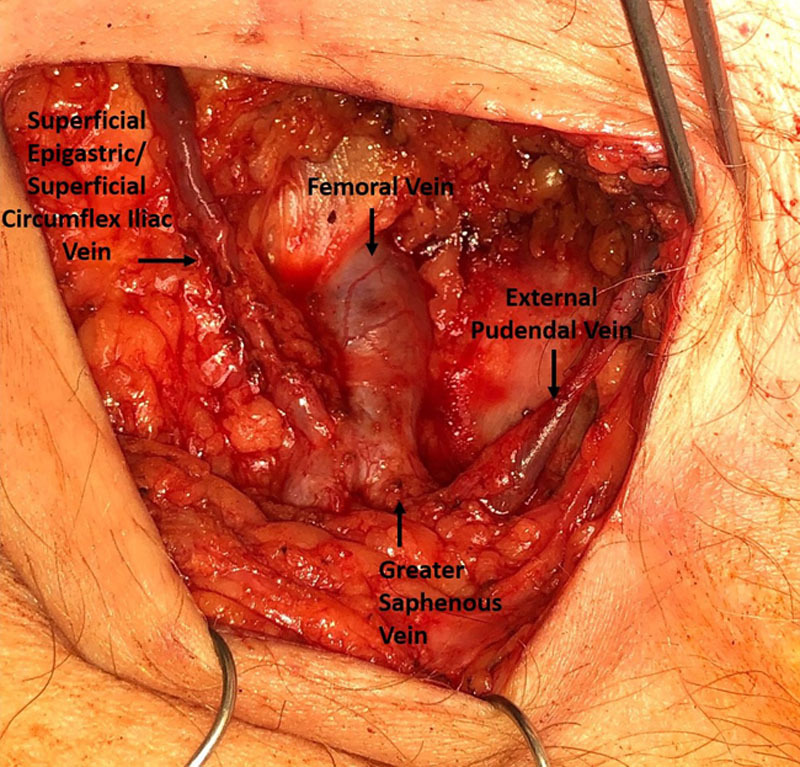
Vein choices in the groin. The superficial epigastric vein and superficial circumflex iliac vein course laterally from the femoral vein. The external pudendal vein courses medially.
Anastomotic Technique
Most descriptions of ILR include intussusception of all identified lymphatic channels into the end of the chosen vein. However, there can be a large size mismatch between the vein and the lymphatic channel(s), especially in cases where anastomosis is of only 1 or 2 lymphatics. This leads to a potential for leakage. Additionally, when multiple lymphatic channels are identified, they can be located far from one another, making intussusception into 1 vein difficult and can cause kinking at the site where the lymphatic channels enter into the vein. Also, intussusception of multiple lymphatic channels into one vein can lead to twisting and occlusion of the lymphatics. One possible approach to this problem is to use terminal or side branches of the vein to enable multiple lymphovenous anastomoses (Fig. 8). Using branches decreases the potential for significant backflow from the vein because valves are commonly found at branch points. In addition, the main vein can have many branches along its length offering multiple choices for anastomoses, both for the best vessel diameter match and for optimal geometry. Anastomoses of lymphatic channels to vein branches can be 1:1 or 2:1 ratio, ensuring lymphatics are not tangled with one another. Use of branches can also lead to a better orientation, allowing bypass of multiple lymphatic channels that may be far away from each other. Finally, using a smaller branch provides a better size match for anastomosis that may prevent leakage.
Fig. 8.
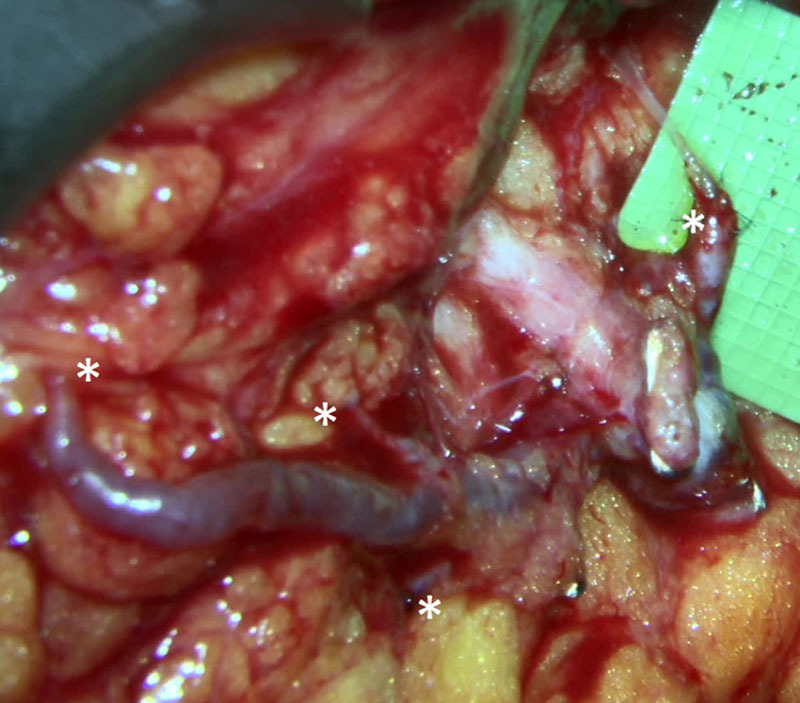
Thoracoepigastric vein with 4 branches and 4 separate anastomosis (*) of lymphatic channels at different locations and different depths in the axilla visualized through the Mitaka microscope (image is focused to deepest anastomosis; therefore, several proximal anastomosis appear blurred).
For each anastomosis, the authors prefer the intussusception technique, although end to end can also be performed for larger lymphatic channels. Standard microsurgical instruments are used. To begin the anastomosis, the end the lymphatic channel is trimmed and perilymphatic tissue is removed from the portion to be intussuscepted. A temporary U-stitch using 9-0 or 10-0 nylon is performed through the vein, through the advential layer (or perilymphatic tissue) of the lymphatic channel, and back through the vein, to pull the lymphatic into the vein. In an alternate approach, the vein is first sutured to the caudal perilymphatic tissue before placement of the U-stitch.30 An angled jeweler forceps is helpful to coax the lymphatic into position within the vein. (See Video 2 [online], which displays anastomosis using the intussusception technique. The perilymphatic tissue is trimmed from the lymphatic channel at the transected end. A U-stitch is placed from the vein to the perilymphatic tissue (or adventia) and back through the vein. Additional sutures are placed from the vein edge to the lymphatic adventia.)
Video 2. Video 2 from “Immediate Lymphatic Reconstruction: Technical Points and Literature Review”.
Additional interrupted sutures are placed from the adventia of the lymphatic channel to the cut edge of the vein. The initial U-stitch should be removed if there is uncertainty that that lymphatic suture was only through adventia. The suture is unnecessary after the lymphatic has been secured inside the vein, and removal minimizes foreign material inside the vessel lumen. If a large size mismatch occurs between the lymphatic(s) and the vein, closing a portion of the end of the vein with sutures will help prevent leakage.
ILR takes between 30 and 60 minutes to complete, in most cases. With experience, the breast surgeon can dissect a suitable vein during the ALND. If a vein is prepared during ALND, the time needed for the plastic surgeon to perform ILR is shortened. Once ILR is complete, a strip test can be done to assess patency of the anastomosis (Fig. 9). Additionally, ICG and/or flourescein can be seen crossing the anastomosis, with dye filling in the vein.
Fig. 9.
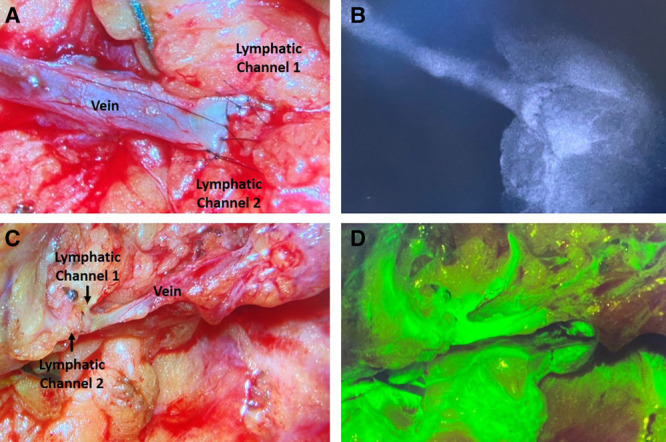
Verification of anastomotic patency using ICG and FITC. A, B, Two lymphatic channels anastomosed to the end of a vein with sutures closing the vein to itself centrally to prevent leakage. ICG seen flowing through the anastomosis, into the vein. C, D, Two lymphatic channels anastomosed to end of a vein branch. FITC seen flowing through the anastomosis, into the vein.
Postoperative Care
Postoperative management following ILR is the same as the recommendations for LND with 2 notable exceptions. First, when placing a surgical drain, care must be taken to ensure the drain will not disrupt the anastomosis. Placement of the drain is more challenging in the groin than in the axilla. Fast-absorbing gut sutures can be used to keep the drain away from the anastomosis. Second, for upper extremity ILR, patients are asked to refrain from raising their arm higher than 90 degrees for 2 weeks. After 2 weeks, patients resume full range of motion.
In the long term, although lymphoscintigraphy has been suggested to show patency of ILR at 1–4 years after operation, the authors feel that lymphoscintigraphy better shows the overall lymphatic drainage (not the specific ILR anastomosis) and therefore currently do not perform this routinely.8 ICG lymphangiography is generally done in office at 1 year postoperative and every year after this to evaluate for signs of lymphedema in the extremity. Extremity volume measurements—by manual circumference or perometry—are conducted every 6 months postoperative and compared with baseline preoperative values.
CONCLUSIONS
ILR is a highly technical operation with a steep learning curve. This article may provide surgeons with a more detailed description than what has been previously published. We hope this information assists and encourages surgeons to offer ILR to patients as part of their cancer care.
Footnotes
Published online 17 February 2021.
Disclosure: Joseph H. Dayan is a paid consultant for the Stryker Corporation. The other authors have no conflicts to disclose.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Johnson AR, Asban A, Granoff MD, et al. Is immediate lymphatic reconstruction cost-effective? Ann Surg. 2019. (E-pub ahead of print.) [DOI] [PubMed] [Google Scholar]
- 2.Warren AG, Brorson H, Borud LJ, et al. Lymphedema: A comprehensive review. Ann Plast Surg. 2007;59:464–472. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol. 2008;26:5213–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 5.Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377. [DOI] [PubMed] [Google Scholar]
- 6.Feldman S, Bansil H, Ascherman J, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol. 2015;22:3296–3301. [DOI] [PubMed] [Google Scholar]
- 7.Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol. 2009;16:703–708. [DOI] [PubMed] [Google Scholar]
- 8.Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: Over 4 years follow-up. Microsurgery. 2014;34:421–424. [DOI] [PubMed] [Google Scholar]
- 9.Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol. 2011;18:2500–2505. [DOI] [PubMed] [Google Scholar]
- 10.Spiguel L, Shaw C, Katz A, et al. Fluorescein isothiocyanate: A novel application for lymphatic surgery. Ann Plast Surg. 2017;78(6S suppl 5):S296–S298. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AR, Bravo MG, James TA, et al. The all but forgotten mascagni-sappey pathway: Learning from immediate lymphatic reconstruction. J Reconstr Microsurg. 2020;36:28–31. [DOI] [PubMed] [Google Scholar]
- 12.Cakmakoglu C, Kwiecien GJ, Schwarz GS, et al. Lymphaticovenous bypass for immediate lymphatic reconstruction in locoregional advanced melanoma patients. J Reconstr Microsurg. 2020;36:247–252. [DOI] [PubMed] [Google Scholar]
- 13.Morotti M, Menada MV, Boccardo F, et al. Lymphedema microsurgical preventive healing approach for primary prevention of lower limb lymphedema after inguinofemoral lymphadenectomy for vulvar cancer. Int J Gynecol Cancer. 2013;23:769–774. [DOI] [PubMed] [Google Scholar]
- 14.Boccardo F, De Cian F, Campisi CC, et al. Surgical prevention and treatment of lymphedema after lymph node dissection in patients with cutaneous melanoma. Lymphology. 2013;46:20–26. [PubMed] [Google Scholar]
- 15.Takeishi M, Kojima M, Mori K, et al. Primary intrapelvic lymphaticovenular anastomosis following lymph node dissection. Ann Plast Surg. 2006;57:300–304. [DOI] [PubMed] [Google Scholar]
- 16.Hahamoff M, Gupta N, Munoz D, et al. A lymphedema surveillance program for breast cancer patients reveals the promise of surgical prevention. J Surg Res. 2019;244:604–611. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AR, Fleishman A, Granoff MD, et al. Evaluating the impact of immediate lymphatic reconstruction for the surgical prevention of lymphedema. Plast Reconstr Surg. 2021. (in press). [DOI] [PubMed] [Google Scholar]
- 18.Johnson AR, Singhal D. Immediate lymphatic reconstruction. J Surg Oncol. 2018;118:750–757. [DOI] [PubMed] [Google Scholar]
- 19.Coriddi M, Dayan J, Mehrara B. Nomenclature in lymphedema surgery. Plast Reconstr Surg. 2020;146:385e–386e. [DOI] [PubMed] [Google Scholar]
- 20.Wiser I MB, Coriddi M, Kenworthy E, et al. Preoperative assessment of upper extremity secondary lymphedema. Cancers. 2020;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suami H, Scaglioni MF. Anatomy of the lymphatic system and the lymphosome concept with reference to lymphedema. Semin Plast Surg. 2018;32:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson AR, Bravo MG, James TA, et al. The all but forgotten mascagni-sappey pathway: Learning from immediate lymphatic reconstruction. J Reconstr Microsurg. 2020;36:28–31. [DOI] [PubMed] [Google Scholar]
- 23.Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg. 2008;36:230– 23–6. [DOI] [PubMed] [Google Scholar]
- 24.Liang M, Carson WE, III. Biphasic anaphylactic reaction to blue dye during sentinel lymph node biopsy. World J Surg Oncol. 2008;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suami H. Lymphosome concept: Anatomical study of the lymphatic system. J Surg Oncol. 2017;115:13–17. [DOI] [PubMed] [Google Scholar]
- 26.Netter F. Atlas of Human Anatomy. 4th ed. Philadelphia, Pa.: Saunders Elsevier; 2006. [Google Scholar]
- 27.Lhauaire M, Hivelin M, Derder M, et al. Anatomical variations of the subscapular pedicle and its terminal branches: An anatomical study and a reappraisal in the light of current surgical approaches. Surg Radiol Anat. 2019;41:385–392. [DOI] [PubMed] [Google Scholar]
- 28.Kita Y FY, Arikawa M, Kagaya Y, et al. Anatomy of the arterial and venous systems of the superficial inferior epigastric artery flap: A retrospective study based on computed tomographic angiography. J Plast Reconstr Aesthet Surg. 2019;28;S1748–6815(19)30516-9. [DOI] [PubMed] [Google Scholar]
- 29.Zenn M JG. Reconstructive Surgery: Anatomy, Technique, and Clinical Applications. St. Louis, Missouri: Quality Medical Publishing; 2012. [Google Scholar]
- 30.Johnson AR, Bravo MG, Pardo JM, et al. Immediate lymphatic reconstruction. In: Hanasono MM, Mardini S, Morani SL, et al., eds. Reconstructive Surgery: A Problem-Based Approach. New York, N.Y.: Thieme; 2021. [Google Scholar]


