Abstract
Per- and polyfluoroalkyl substances (PFAS) are ubiquitously detected in populations worldwide and may hinder kidney function. The objective of the study was to determine longitudinal associations of plasma PFAS concentrations with estimated glomerular filtration rate (eGFR) and evaluate whether a lifestyle intervention modify the associations. We studied 875 participants initially randomized to the lifestyle or placebo arms in the Diabetes Prevention Program (DPP, 1996–2002) trial and Outcomes Study (DPPOS, 2002–2014). We ran generalized linear mixed models accounting a priori covariates to evaluate the associations between baseline PFAS concentrations and repeated measures of eGFR, separately, for six PFAS (PFOS, PFOA, PFHxS, EtFOSAA, MeFOSAA, PFNA); then used quantile-based g-computation to evaluate the effects of the six PFAS chemicals as a mixture. The cohort was 64.9% female; 73.4% 40–64 years-old; 29.4% with hypertension; 50.5% randomized to lifestyle intervention and 49.5% to placebo and had similar plasma PFAS concentrations as the general U.S. population in 1999–2000. Most participants had normal kidney function (eGFR >90 mL/min/1.73 m2) over the approximately 14 years of follow-up. We found that plasma PFAS concentrations during DPP were inversely associated with eGFR during DPPOS follow-up. Each quartile increase in baseline plasma concentration of the 6 PFAS as a mixture was associated with 2.26 mL/min/1.73 m2 lower eGFR (95% CI: −4.12, −0.39) at DPPOS Year 5, approximately 9 years since DPP randomization and PFAS measurements. The lifestyle intervention did not modify associations, but inverse associations were stronger among participants with hypertension at baseline. Among prediabetic adults, we found inverse associations between baseline plasma PFAS concentrations and measures of eGFR throughout 14 years of follow-up. The lifestyle intervention of diet, exercise and behavioral changes did not modify the associations, but persons with hypertension may have heightened susceptibility.
Keywords: per- and polyfluoroalkyl substances, kidney function, eGFR, hypertension, prediabetic adults, Diabetes Prevention Program
1. INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are a large group of nonbiodegradable synthetic chemicals ubiquitously detected in the environment worldwide. First manufactured in 1950s, PFAS are widely used in industrial and consumer products, including firefighting foams, non-stick cookware, food packaging, stain-, grease- and water-resistant products (ATSDR 2018). PFAS are ubiquitously detected in human populations and many also have long elimination half-lives in humans, e.g., 5.3 years for perfluorohexanesulfonic acid (PFHxS), 3.4 years for perfluorooctanesulfonic acid (PFOS), 2.7 years for perfluorooctanoic acid (PFOA) (Li et al. 2018). More than 98% of blood samples collected from participants in the U.S. National Health and Nutrition Survey (NHANES) contained detectable concentrations of select PFAS (Calafat et al. 2007; K. Kato et al. 2011). Exposure to PFAS has been linked with obesity, diabetes, hyperlipidemia and microvascular disease, conditions that are themselves associated with poorer kidney function (A. Cardenas et al. 2017; Cardenas et al. 2019; Kirk et al. 2018; Lin et al. 2019). Furthermore, the kidney is a primary route for PFAS elimination (Kjølholt J 2015). Once certain PFAS enter the human body, it may take up to several years to eliminate them (Olsen et al. 2007) and continued exposure leads to bioaccumulation. PFAS have been shown to cause renal hypertrophy and histopathologic changes (Cui et al. 2009) and alter renal microvascular endothelial-cell permeability through increased production of reactive oxidative species. The proximal tubules have been shown to actively secrete and reabsorb PFAS (Stanifer et al. 2018).
Systematic reviews of the PFAS literature found evidence that PFAS is an emerging environmental threat to kidney health (Ferrari et al. 2019; Stanifer et al. 2018). Currently, most epidemiological evidence from population-based studies on PFAS biomarkers and kidney diseases is cross-sectional (Dhingra et al. 2017; Kataria et al. 2015; Shankar et al. 2011; Watkins et al. 2013), and reverse causation has been a main concern, i.e. that PFAS concentration in blood increases as kidney function declines. More findings from longitudinal data are needed to address the potential gap causal link between PFAS and kidney health (Ferrari et al. 2019; Stanifer et al. 2018; Wang et al. 2019). The two available studies that used longitudinal data showed contradictory findings. Blake et al found that higher serum PFHxS, perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) were associated with lower prospective measures of eGFR but N-methyl-perfluorooctane sulfonamido acetic acid (MeFOSAA) was associated with higher eGFR. However, Dhingra et al concluded reduced eGFR was the cause rather than the result of elevated serum PFOA level (Blake et al. 2018; Dhingra et al. 2017). Both studies used community-exposed cohorts which had higher PFAS exposure than the U.S. general population.
In this study, we used a longitudinal cohort of prediabetic adults to test the relationship between commonly detected PFAS and kidney function over 14 years of follow-up. We implemented directed acyclic graphs (DAGs) to assess variables that may confound the relationship and used multiple approaches to address the issue of reverse causation in this association. Our study question was whether baseline PFAS concentrations, as individual exposure and as a mixture, were associated with repeated measures of estimated glomerular filtration rate (eGFR) over time, and whether lifestyle intervention could modify this association. We hypothesized higher baseline PFAS concentrations would be associated with lower eGFR over time. We also hypothesized that an initial lifestyle intervention of diet, exercise and behavioral changes could reduce this detrimental effect.
2. METHODS
2.1. Study Population
The Diabetes Prevention Program (DPP) was a randomized controlled trial to prevent or delay the onset of type 2 diabetes using lifestyle or pharmacological intervention, relative to medication placebo (Diabetes Prevention Program Research Group 1999, 2000). The trial recruited obese and overweight adults ≥25 years with elevated fasting glucose from 27 clinical centers across the United States between 1996 and 1999, and randomized participants into three arms: a pharmacological intervention (metformin), a medication-placebo control, or a lifestyle intervention (Diabetes Prevention Program Research Group 2002b). The lifestyle intervention arm contained a goal-based behavioral intervention to achieve 7% weight loss and maintenance of weight loss; each participant had a personal lifestyle coach or case manager who delivered the intervention and provided frequent follow-up and contacts to ensure achievement and maintenance of weight and physical activity goals (Diabetes Prevention Program Research Group 2002a). Both the lifestyle and metformin interventions demonstrated effectiveness in preventing type 2 diabetes (Knowler et al. 2002). All participants were offered a modified version of the lifestyle intervention after DPP ended (Diabetes Prevention Program Research Group 2009, 2012) and were offered follow up in Diabetes Prevention Program Outcome Study (DPPOS) which started in 2002 (Diabetes Prevention Program Research Group 2015). For this analysis, we captured the exposure (plasma PFAS) during the DPP phase (from baseline recruitment to DPP intervention) and the outcome (eGFR) from DPPOS follow-up (after the DPP intervention phase ended) to allow for the prospective temporal order between exposure and outcome (eFigure 1). Participants enrolled in the DPP intervention phase for an average (standard deviation, SD) of 3.1 (0.7) years (for this sub-cohort), and had up to 11 annual visits during DPPOS; the total follow-up time since DPP baseline randomization was 13.9 (2.6) years (eTable 1).
This prospective analysis was restricted to participants initially randomized to the lifestyle and placebo arms; 957 (46.6%) had enough blood volume remaining in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Repository for plasma PFAS measurements. We did not measure PFAS among participants in the metformin arm given the unknown interaction between metformin and PFAS and the protective effect of metformin on diabetic kidney disease. Both DPP and DPPOS study phases have little missing data (only N=1 missing all eGFR measures among those with PFAS measurements) so we used complete case analysis in this study (Diabetes Prevention Program Research 2015). After excluding participants with missing covariates and removing extreme values for eGFR, the final longitudinal analysis included 875 participants (see study flow chart in eFigure 1).
All DPP/DPPOS protocols were approved by the institutional review board (IRB) at each clinical center and Harvard Pilgrim Health Care IRB reviewed and approved the protocol for this current analysis. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
2.2. Plasma PFAS Concentrations
We retrieved plasma samples stored at the NIDDK repository (https://repository.niddk.nih.gov) for analyses at the CDC laboratory. The modified on-line solid-phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry method (Andres Cardenas et al. 2017; Kayoko Kato et al. 2011), also used to analyze NHANES PFAS samples, yielded limit of detections (LOD) of 0.1 ng/mL for all PFAS. Measurements <LOD were imputed as LOD/√2 (Hornung and Reed 1990). This analysis included 6 PFAS with detection frequency >80% [PFOS, PFOA, PFHxS, N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA), MeFOSAA, and PFNA]. Concentrations of PFOS and PFOA were calculated as the sum of their respective isomers: linear perfluorooctanesulfonic acid PFOS (n-PFOS), sum of perfluoromethylheptane sulfonic acid isomers (Sm-PFOS), and sum of perfluorodimethylhexane sulfonic acid isomers (Sm2-PFOS) for PFOS; and linear perfluorooctanoic acid (n-PFOA) and sum of perfluoromethylheptanoic and perfluorodimethylhexanoic acids (Sb-PFOA) for PFOA; we performed imputation for concentrations <LOD before summing which was consistent with the method used in the Fourth National Report on Human Exposure to Environmental Chemicals (Centers for Disease Control and Prevention 2019). We calculated the average concentrations of baseline and the DPP Year 2 measures (referred to as baseline PFAS from now on), which had been shown to adequately reflect the relative PFAS body burden considering the relatively long half-lives of many PFAS (A. Cardenas et al. 2017; Cardenas et al. 2019; Lin et al. 2020a). The year of blood collection ranged between 1996 to 1999 for baseline and 1998 to 2001 for DPP Year 2. The concentrations between the two measurements did not change significantly for all participants, which was expected considering the relatively long biological half-lives of most PFAS. The temporal pattern and geometric means of all 6 PFAS were comparable to those observed in US NHANES adults (A. Cardenas et al. 2017).
2.3. Kidney function
As previously reported, DPP/DPPOS used Roche reagents on the Hitachi 917 autoanalyzer (Boehringer Mannheim, Mannheim, Germany) to measure serum creatinine (Kim et al. 2019). We extracted annual laboratory results of serum creatinine for DPPOS years 1 to 11 annual visits for our analysis; 62% of the 875 participants had all 11 annual measures and more than 90% had more than 5 measures (eTable 2). We used the 2009 Chronic Kidney Disease Epidemiology collaboration (CKD-EPI) serum creatinine equation (Levey et al. 2006) to calculate eGFR. Due to privacy protection, we did not have participants’ actual age, so we used the following “mid-point” for each age category: 38 for <40 years; 42 for 40–44 years; 47 for 45–49years; 52 for 50–54 years; 57 for 55–59 years; and 62 for 60–64 years; 67 for 65+ years). We recognize the use of 67 for the 65+ age group may introduce bias considering accelerating declines in eGFR with age, so we also performed sensitivity analyses using 70 and 75 as the imputed age for this oldest group. To avoid healthy participant bias, we included eGFR data only for DPPOS Years 1 to 8 annual visits for the final longitudinal analysis so more than 90% of the participants had eGFR measures at all annual follow-up visits; this covered approximately 4.5 to 11.4 years since baseline (time point of PFAS measurements). We also included all available DPPOS follow-up data (up to 11th annual visits) in the sensitivity analysis (eTable 1). Although eGFR is not a perfect measure of kidney function, due to data availability, we decided to use eGFR which can also produce comparable results with previous studies. We also had measures urine albumin-to-creatinine ratios (ACRs), but only at DPP baseline. We defined elevated ACR, or microalbuminuria, as ACR ≥ 30 mg/g.
2.4. Covariates
We selected covariates a priori based on study questions and used DAGs to identify potential confounders as well as mediators that could be in the causal pathway between plasma PFAS concentration and eGFR (eFigure 2). The following information was extracted directly from the NIDDK data repository: age category, sex, race/ethnicity, education, marital status, income, smoking status, treatment arm, baseline microalbuminuria, hypertension status, menopause status, diabetes status at the end of DPP phase, and time (years since randomization) of each outcome assessment. Hypertension status was defined as self-reported hypertension diagnosis, use of anti-hypertensive medications or systolic/diastolic BP ≥140/90 mmHg. We modeled time and age (using the “mid-point” age described in the previous section) as continuous variables and the rest as categorical variables (see categorization in Table 1). We evaluated dietary habits using the Dietary Approaches to Stop Hypertension (DASH) score, which we previously found to be associated with plasma PFAS concentrations (Lin et al. 2020b). We evaluated uses of kidney medications [angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs), beta-blockers] based on self-reported prescriptions.
Table 1.
Baseline characteristics of study participants
| Characteristics | N(%), or mean ± SD |
|---|---|
| Sample size, N | 875 |
| Group assignment | |
| . Lifestyle | 442 (50.5) |
| . Placebo | 433 (49.5) |
| Sex | |
| . Male | 307 (35.1) |
| . Female | 568 (64.9) |
| Race/ethnicity | |
| . Non-Hispanic White | 502 (57.4) |
| . African American | 171 (19.5) |
| . Hispanic of any race | 163 (18.6) |
| . All others | 37 (4.5) |
| Age at DPP enrollment (years) | |
| . <40 | 95 (10.9) |
| . 40–44 | 100 (11.4) |
| . 45–49 | 196 (22.4) |
| . 50–54 | 155 (17.7) |
| . 55–59 | 130 (14.9) |
| . 60–64 | 97 (11.1) |
| . >65 | 102 (11.7) |
| Educational attainment | |
| . < High school | 41 (4.7) |
| . High school/GED | 183 (20.9) |
| . College | 427 (48.8) |
| . Graduate school | 224 (25.6) |
| Marital Status | |
| . Married/Cohabitating | 590 (67.4) |
| . Single | 107 (12.2) |
| . Divorced | 138 (15.8) |
| . Widowed | 40 (4.6) |
| Income | |
| . <$20,000 | 107 (12.2) |
| . $20,000 - <$35,000 | 159 (18.2) |
| . $35,000 - <$50,000 | 173 (19.8) |
| . $50,000 - <$75,000 | 166 (19.0) |
| . ≥ 75,000 | 196 (22.4) |
| . Refused to answer | 74 (8.5) |
| Current smoker | 52 (5.9) |
| Menopausal, % among female participants | 307 (54.0) |
| Hypertension diagnosis | 262 (29.4) |
| Use of kidney medication* | 72 (8.2) |
| DASH Diet Score (0–9 range) | 2.5 ± 1.6 |
| Microalbuminuria at baseline (ACR ≥ 30 mg/g) | 41 (4.7) |
| Developed diabetes during DPP | 483 (55.2) |
Kidney medication: ACE-inhibitor, ARBs, beta-blockers
2.5. Statistical Analyses
We used descriptive analysis to report participants’ characteristics, applied visual inspection to detect outliers, and used normality tests to assess the distributions of each variable. Plasma PFAS concentrations were right-skewed, thus we log-2 transformed them. In the main analysis we examined differences in the eGFR during DPPOS by baseline PFAS plasma concentrations. Because Sm2-PFOS concentrations were <LOD in more than 50% of the samples and the median concentration was close to the LOD (0.1 ng/mL), we also treated Sm2-PFOS as a binary variable (detected vs non-detected) in the analysis. We applied generalized linear mixed models with random intercepts and slopes and used restricted maximum likelihood for estimation to estimate the longitudinal association between baseline PFAS and annual measures of eGFR during DPPOS (approximately 3–16 years after the baseline PFAS measurement, see eFigure 1 and eTable 1 for DPP/DPPOS timeline). We tested models with different correlation structures, added quadratic and cubic terms for the follow-up time based on visual inspection of scatter plot and eGFR trajectories, and evaluated interactions between parameters. We selected the final model based on study questions and model fit using the likelihood ratio test for nested models (see detailed information in Appendix A). We reported parameter estimates of the fixed effect that estimated the differences in mean eGFR per doubling of plasma PFAS concentrations across the study period adjusted for covariates; evaluated potential effect modification of the longitudinal associations by sex, treatment arm, and baseline hypertension status, by adding a multiplicative term between plasma PFAS concentrations and each effect modifier in the model; and conducted additional stratified analyses if the multiplicative term had p<0.10. We tested reverse causation of effect by using baseline eGFR at DPP as the exposure and repeated measures of plasma PFAS concentrations (measured at baseline, DPP Year 2 annual visit and DPPOS Year 10 annual visits) as outcomes in the longitudinal models (see detailed equation of the model in Appendix A).
We performed mixture analysis using quantile-based g-computation (Keil et al. 2020) to estimate the effect of the 6 PFAS as a mixture with eGFR measured at the DPPOS Year 5 (mean 8.4 years since randomization) as the outcome, a timepoint when outcome measures were available for more than 90% of the study participants (eTable 1). Quantile g-computation (Keil et al. 2020) estimates the effect of an exposure mixture index that is a weighted average of all exposure after transforming PFAS concentrations into quartiles, and produces effect estimates that can be interpreted as mean difference in eGFR across quantile range of the plasma PFAS concentrations as a mixture. We evaluated both linear and nonlinear effects and present the mixture slope with overall model confidence bounds (estimated by 1000 bootstraps) as well as pointwise comparison of the expected difference in eGFR at each quartile of PFAS concentration with respect to the 2nd quartile using the gqcomp R package (https://cran.r-project.org/web/packages/qgcomp/vignettes/qgcomp-vignette.htmlversion1.3defaults).
As a sub-analysis, we performed cross-sectional analyses of plasma PFAS concentrations with eGFR and ACR at DPP baseline using linear regression to compare with most previous literature. We assessed the linearity using generalized additive models. For sensitivity analyses, we accounted for additional variables in the longitudinal models and mixtures analysis, including baseline eGFR level, diabetic status at the beginning of DPPOS, and elevated ACR at baseline. However, since these variables were likely mediators between the associations of plasma PFAS concentrations and kidney function (eFigure 2), we did not include them in the main analysis. We also ran sensitivity analyses using 70 and 75 as the imputed age for estimating the eGFR for the 65+ age group. We performed the statistical modeling using SAS Studio and R version 3.6.0. Reporting of the manuscript follows the guidelines and checklist for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (Von Elm et al. 2007).
3. RESULTS
3.1. Participant Characteristics and Plasma PFAS Concentrations
We included 875 DPP/DPPOS participants in this analysis: 442 (50.5%) from the lifestyle arm and 433 (49.5%) from the placebo arm. At baseline, participants were 64.9% female; 57.4% non-Hispanic White, 66.4% 40–59 years old; 74.4% college graduates; 67.4% married/cohabitating; 41.4% with annual income >$50,000; 5.9% smokers; 29.4% with hypertension.; 8.2% used kidney medication, 4.7% had microalbuminuria at baseline, and 55.2% developed diabetes at the start of DPPOS follow-up (Table 1). Average plasma PFAS concentrations were comparable to the general U.S. population concentrations during 1999–2000 (Centers for Disease Control and Prevention (CDC) 2017) and did not differ across the two treatment arms. Concentrations of all PFAS were positively correlated (eFigure 3). PFOS [median (interquartile range, IQR): 27.6 ng/mL (19.2, 38.9)] and PFOA [5.5 ng/mL (3.8, 7.4)] were the two PFAS detected at the highest concentrations (Table 2).
Table 2.
Plasma concentrations of PFAS and kidney function during study follow-up
| Exposure (PFAS) | Time of assessment | N | Median (IQR) (ng/mL) | |
| PFOS | Average of baseline and DPP Year 2 | 875 | 27.6 (19.2, 38.9) | |
| n-PFOS | 875 | 19.9 (13.6, 28.2) | ||
| Sm-PFOS | 875 | 7.5 (5.2, 10.9) | ||
| Sm2-PFOS | 875 | 0.1 (0.1, 0.3) | ||
| PFOA | 875 | 5.5 (3.8, 7.4) | ||
| n-PFOA | 875 | 4.7 (3.4, 6.2) | ||
| Sb-PFOA | 875 | 0.6 (0.4, 1.0) | ||
| PFHxS | 875 | 2.4 (1.6, 3.8) | ||
| EtFOSAA | 875 | 1.2 (0.7, 2.0) | ||
| MeFOSAA | 875 | 1.1 (0.7, 1.7) | ||
| PFNA | 875 | 0.6 (0.4, 0.9) | ||
| Outcome | Time of assessment | N | Mean (STD) (mL/min/1.73 m2) | Percent of participants with normal eGFR (> 90 mL/min/1.73 m2) |
| eGFR | DPPOS Year 1 | 811 | 111.0 (40.2) | 79.4 |
| DPPOS Year 2 | 868 | 106.7 (24.4) | 79.2 | |
| DPPOS Year 3 | 845 | 105.3 (22.7) | 77.6 | |
| DPPOS Year 4 | 826 | 100.9 (18.6) | 71.2 | |
| DPPOS Year 5 | 843 | 101.2 (19.1) | 71.4 | |
| DPPOS Year 6 | 814 | 102.1 (22.0) | 70.4 | |
| DPPOS Year 7 | 780 | 99.4 (19.4) | 67.4 | |
| DPPOS Year 8 | 788 | 98.0 (19.2) | 63.3 | |
| DPPOS Year 9 | 773 | 96.9 (18.9) | 61.7 | |
| DPPOS Year 10 | 771 | 96.7 (19.6) | 58.8 | |
| DPPOS Year 11 | 748 | 96.8 (19.7) | 56.0 | |
Most participants had normal eGFR (>90 mL/min/1.73 m2) throughout DPP/DPPOS (Table 2). At baseline, the mean (STD) eGFR was 111.05 (38.0) mL/min/1.73 m2 and 87.2% had normal eGFR (Table 2).
3.2. Longitudinal Associations of PFAS with eGFR
We used DAGs to evaluate the appreciate covariates to adjust for in assessing the longitudinal relationship between baseline PFAS and repeated measures of eGFR (eFigure 2A) and described the model evaluation and selection process in detail in Appendix A. After controlling for baseline covariates including age, sex, race/ethnicity, education, marital status, income, smoking status, menopause status, DASH diet score, hypertension status, and use of kidney medication, we observed inverse associations of PFOS and PFOA isomers with eGFR; each doubling of plasma total PFOS concentrations were associated with 1.50 mL/min/1.73m2 lower eGFR (95% CI: −2.81, −0.19); and each doubling of n-PFOA and Sb-PFOA were associated with 1.49 (95% CI: −2.97, 0.00) and 0.85 (95% CI: −1.58, −0.12) mL/min/1.73m2 lower eGFR, respectively (Table 3). The effect estimates were robust with additional adjustment for diabetic status (eTable 3). When adjusting for baseline eGFR, which was a potential mediator between plasma PFAS concentration and longitudinal decline in kidney function (see eFigure 2 for DAG), significant longitudinal associations remained for Sm2-PFOS and Sb-PFOS (eTable 3). Controlling for elevated ACR at baseline strengthened the magnitude of inverse association between some plasma PFAS and eGFR (eTable 3) which suggested potential negative confounding by microalbuminuria (see eFigure 2 for DAG). We did not find evidence of effect modification by sex or the initial lifestyle intervention (eTable 4), but participants with baseline hypertension had greater decreases in eGFR per doubling of plasma PFAS concentrations (Table 3). The test for potential difference in the slope of eGFR decline by adding an interaction term between time and PFAS yield statistically insignificant result (Appendix A). Evaluation for reverse causation showed that repeated measures of plasma PFAS concentrations did not differ significantly by baseline eGFR level (data not shown). Analysis using all eGFR data from Year 1 to Year 11 shows comparable findings with associations of the same direction and similar magnitude, however, most of the 95% confidence interval were wider and crossed the null (data not shown). Sensitivity analyses using different imputed ages in estimating the eGFR for the oldest age group (65+ years) showed comparable estimates (beta changed less than 0.1), and all the statistically significant findings remained excepted for the borderline significant n-PFOA (eTable 5).
Table 3.
Associations between PFAS plasma concentration and mean change in eGFR during DPPOS follow-up estimated using generalized mixed modelsa, overall and stratified according to baseline hypertension status.
| Log-2 PFAS analyte | Full | Stratified | ||
|---|---|---|---|---|
| (N=875) | Hypertensionb (N=262) | No hypertension (N=613) | ||
| β (95% CI) | β (95% CI) | β (95% CI) | pintc | |
| Total PFOS | −1.50 (−2.81, −0.19) | −2.19 (−4.92, 0.53) | −1.44 (−2.96, 0.06) | 0.68 |
| n-PFOS | −1.34 (−2.62, −0.06) | −1.98 (−4.62, 0.66) | −1.30 (−2.79, 0.17) | 0.72 |
| Sm-PFOS | −1.56 (−2.85, −0.28) | −2.15 (−4.89, 0.57) | −1.49 (−2.96, −0.02) | 0.72 |
| Sm2-PFOSd | −1.87 (−2.88, −0.86) | −2.66 (−4.73, −0.60) | −1.58 (−2.75, −0.41) | 0.24 |
| Total PFOA | −1.35 (−2.73, 0.02) | −3.18 (−5.85, −0.51) | −0.65 (−2.30, 0.99) | 0.16 |
| n-PFOA | −1.49 (−2.97, −0.01) | −3.16 (−5.98, −0.35) | −0.83 (−2.63, 0.96) | 0.24 |
| Sb-PFOA | −0.85 (−1.58, −0.12) | −1.66 (−3.11, −0.21) | −0.55 (−1.41, 0.29) | 0.20 |
| PFHxS | 0.21 (−0.79, 1.21) | −2.35 (−4.46, −0.25) | 1.24 (0.09, 2.39) | 0.01 |
| EtFOSAA | −0.15 (−0.97, 0.65) | 0.02 (−1.63, 1.68) | −0.31 (−1.27, 0.64) | 0.53 |
| MeFOSAA | −0.62 (−1.71, 0.45) | −1.56 (−3.82, 0.69) | −0.15 (−1.40, 1.09) | 0.33 |
| PFNA | 0.18 (−0.90, 1.27) | −1.85 (−4.20, 0.49) | 0.90 (−0.33, 2.14) | 0.06 |
Note:
The generalized mixed model include random intercept and random slope for year since enrollment, the crude model used repeated measures of eGFR from the 1st to the 8th annual visit during DPPOS follow-up as the dependent variable and used baseline PFAS concentration (average between baseline and year 2 measures during DPP, log-2 transformed), treatment arm, and year since randomization as the independent variables, the model also included a second and a third order spline term for year (year*year and year*year*year); the adjusted model adjusted for baseline covariates including age, sex, race/ethnicity, education, marital status, income, smoking status, menopause status, DASH diet score, hypertension status, and use of kidney medication. N=875. The beta coefficient is interpreted as the mean difference in eGFR (mL/min/1.73m2) during DPPOS follow-up per doubling of baseline PFAS concentration.
Hypertension was defined as self-reported hypertension diagnosis, use of anti-hypertensive medications, or systolic/diastolic BP ≥140/90 mmHg.
pint: p-value of the interactive term between PFAS and baseline hypertension status (p<0.1 suggests effect modification)
β (95% CI)= −3.75 (−5.83, −1.68)** for detected vs non-detected Sm2-PFOS.
3.3. PFAS as a mixture
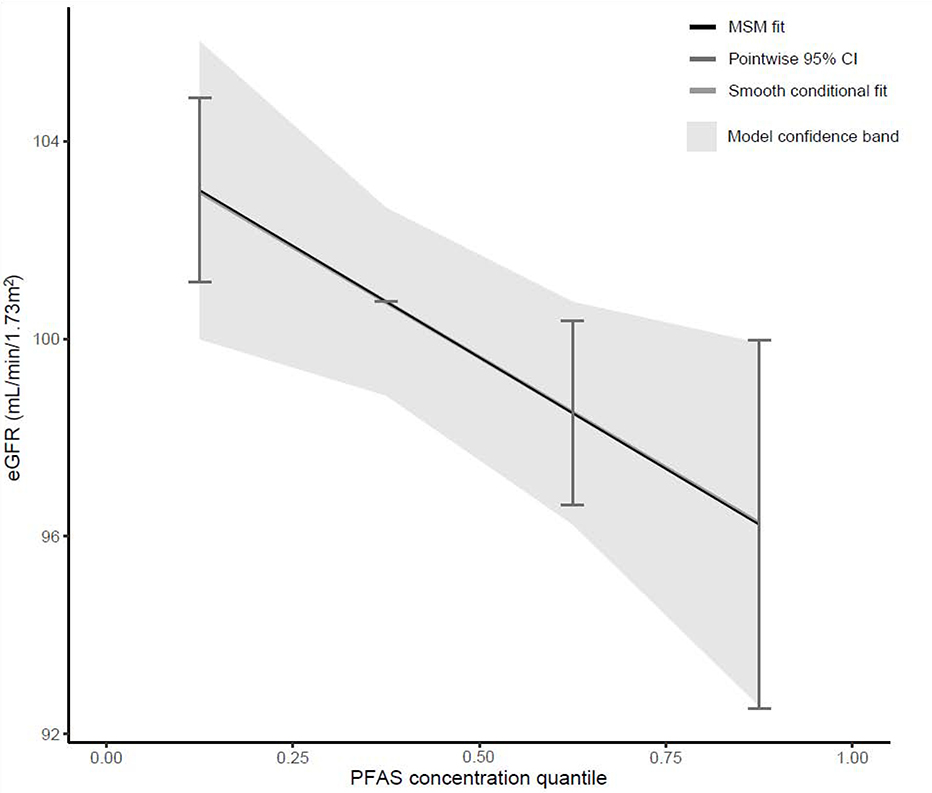
Quantile g-computation estimated a one quartile increase in PFAS mixtures to be associated with 2.26 (95% CI: −4.12, −0.39) mL/min/1.73m2 lower eGFR at year 5 in DPPOS, conditional on covariates. Sm2-PFOS and Sb-PFOA contributed to most of the weight of the mixtures (eTable 6). Estimated eGFR decreased linearly by quartile of PFAS mixture concentrations (Figure 1), and test of nonlinearity did not show significant deviation from linear effect. Controlling for baseline eGFR reduced the magnitude of the estimate but the effect remained statistically significant [Ψ: −1.81 (95% CI: −3.57. −0.04,) mL/min/1.73m2 eGFR at DPPOS Year 5 per quartile increase) (eTable 6).
Fig. 1.
Mixture effect of 6 plasma PFAS concentrations on eGFR at DPPOS Year 5.
3.4. Cross-sectional association at baseline
We evaluated cross-sectional associations of plasma PFAS concentrations with eGFR and ACR at baseline using data from 925 participants who had available data (eFigure 1); participants’ characteristics were comparable with study population of the main longitudinal analysis (eTable 7). Most PFAS were not cross-sectionally associated with eGFR at baseline, except for Sm2-PFOS [−4.66 (−8.29, −1.04) mL/min/1.73m2 per doubling or −2.90 (−4.94, −0.85) mL/min/1.73m2 comparing detectable vs non-detectable, eTable 8]. Consistent with longitudinal findings, we detected effect modification by hypertension status (eTable 8). There was no evidence of effect modification by sex or nonlinearity of effect. As a mixture, baseline plasma concentrations of these 6 PFAS were not associated with baseline eGFR in this population [ψ: −3.26 (95% CI: −1.63, 0.64) mL/min/1.73m2 eGFR per quantile increase]. Baseline ACR was inversely associated with baseline PFOA and EtFOSAA, and there was no evidence of effect modification by hypertension status (eTable 9) or baseline eGFR (data not shown).
4. DISCUSSION
In this prospective analysis incorporating approximately 14 years of kidney function data from prediabetic adults enrolled in the lifestyle and placebo arms of the DPP trial, we observed that plasma concentrations of select PFAS, individually and as a mixture, were associated with lower mean eGFR over time, or declining kidney function. These results support our initial hypothesis. However, we did not find evidence that higher baseline PFAS was associated with greater slope of eGFR decline, which could be due to the fact that most participants had normal kidney function throughout the follow-up and our data was underpowered to detect the difference in slope. Additionally there presumably was decreasing PFAS exposure after baseline (1996–1999) due to manufactures’ phasing out of PFOS and PFOA (US EPA 2016) which may have attenuated the effect. While there is evidence on the adverse effect of PFAS on kidney function (Ferrari et al. 2019; Stanifer et al. 2018; Zhao et al. 2020), most prior epidemiological studies were cross-sectional, thus limiting inferences on causality (Stanifer et al. 2018; Wang et al. 2019), and the two available studies using longitudinal data showed inconsistent results. Specifically, Black et al. observed repeated measures of higher serum PFNA, PFHxS and PFDA and lower MeFOSAA were significantly associated with lower longitudinal measures of eGFR, but found null association between PFOA and eGFR (Blake et al. 2018). Dhingra et al, on the other hand, observed significant association between PFOA and eGFR, but only when using PFOA concentration measured in the serum (cross-sectional analysis only). When they evaluated the longitudinal data with modelled PFOA serum concentrations based on external exposure, such association did not exist which led them to conclude the observed association between higher serum PFOA and reduced eGFR was a result of reverse causation (Dhingra et al. 2017). While we cannot perform a randomized intervention to examine the causal effect of PFAS exposure on kidney function, our study utilized a prospective study design and implemented multiple tests of reverse causation to establish the temporality of the relationship in order to provide a more definitive evidence of the causal influence of PFAS on kidney function. We estimated that each doubling of baseline PFOS concentrations were associated with 1.50 mL/min/1.73m2 (95% CI: −2.81, −0.19) lower eGFR during the study follow-up; and as a mixture, each quartile increase in baseline PFAS was associated with 2.26 mL/min/1.73m2 (95% CI: −4.12, −0.39) lower eGFR at the 5th annual follow-up visits of DPPOS controlling for age, sex, race/ethnicity, education, marital status, income, smoking status, baseline menopause status, baseline diet score, treatment arm,, and baseline blood pressure. When additionally controlled for baseline eGFR, the effect estimates attenuated somewhat but still stayed statistically significant. While this effect may not translate to immediate clinical manifestation on kidney health, over time and at the population level, PFAS exposure may negatively affect the kidney and compound the effects of other risk factors. Contrary to our hypothesis, we did not find evidence that the PFAS-associated decreases in eGFR were modified by an initial lifestyle intervention targeting diet, exercise and behavioral changes. However, all participants were offered a modified lifestyle intervention after the unblinding of the DPP intervention (Diabetes Prevention Program Research et al. 2009; Diabetes Prevention Program Research 2015; Goldberg et al. 2017), thus, the differential effect across the lifestyle and placebo arm might be diluted, and additional investigation is needed.
Our study observed comparable findings with some previous studies (Shankar et al. 2011; Zhao et al. 2020). Cross-sectional analyses using U.S. NHANES data (1999–2008, N=4857 adults) showed those with the highest quartile of serum PFOS and PFOA concentration (PFOS >29.5 ng/mL, PFOA: >5.9 ng/mL) had 6.7 and 5.7 ml/min/1.73 m2 lower eGFR compared to those with the 1st quartile concentrations (PFOS: <11.7 ng/mL, PFOA: <2.8 ng/mL); and the odds of CKD were 82% and 73% higher, respectively (Shankar et al. 2011). Consistently, the China C8 Study [N=1612, median (IQR) PFOS 24.2 (14.6, 37.2) ng/mL] estimated a −0.91 (−1.83, 0.00) mL/min/1.73 m2 lower eGFR per natural-log (2.7-fold) increase in serum PFOS (Wang et al. 2019); and the US C8 Health Project (Mid-Ohio Valley, N=29,641) estimated −0.98 (SE: 0.274, p=0.0003) mL/min/1.73 m2 lower eGFR comparing the 5th (>88 ng/mL) to the 1st quintile (<11.1 ng/mL) of serum PFOA, cross-sectionally (Dhingra et al. 2017)The only available longitudinal estimates were a study from 210 community residents living close to a uranium processing site in Fernald, Ohio who also had high risk of exposure of PFAS due to residential location (Blake et al. 2018). The study had repeated measures of serum PFAS and eGFR over approximately 10 years of follow-up (1999–2008). The latent models they applied were comparable to the longitudinal model we used in this analysis as both evaluated the longitudinal trend of eGFR in relation to a baseline eGFR measurement. They found −1.72 (−3.29, −0.15) mL/min/1.73 m2 lower eGFR per quartile increase (approximately 30% absolute increase) in serum PFOS adjusting for age, year of measurement, sex, education, income, BMI and marital status, which was comparable to our finding [−1.50 (−2.81, −0.19) mL/min/1.73 m2 lower eGFR per doubling of PFOS]. Compared to Blake et al, our study had a larger sample size and a narrower age range, therefore our estimates had higher precision, which was evident by the narrower 95% confidence interval. However, since our participants had fewer measures of blood PFAS and eGFR, we did not apply the repeated measures approach as done by Blake et al, thus, we cannot confirm their findings on the inverse associations of PFHxS, PFNA, PFDA and the positive association of MeFOSAA with eGFR. We should note that while this repeated measure approach applied by Black et al. can gain more power, findings from this method was also more likely to be biased by time-varying confounding so care should be taken in interpreting the results. Similar to one previous report (Wang et al. 2019), we observed stronger effects of the branched PFOS and PFOA which have higher renal clearance rates compared to linear isomers (Gao et al. 2015; Olsen et al. 2007; Russell et al. 2015; Shi et al. 2016; Worley et al. 2017; Zhang et al. 2013). The uptake of PFAS by organic anion transporters into the proximal renal tubules regulates the active secretion and reabsorption of PFAS (Weaver et al. 2010; Worley et al. 2017; Yang et al. 2010; Zhang et al. 2013). The reabsorption of PFAS is hypothesized to alter normal kidney function.
Impaired kidney function can also disrupt the balance of renal secretion and reabsorption of PFAS which in term alters blood PFAS concentrations (Olsen et al. 2007; Stanifer et al. 2018). Previous cross-sectional study from US NHANES had shown an inverted U-shaped relationship between PFAS and eGFR (Jain and Ducatman 2019a), suggesting that the level of absorption and secretion activities may be differentially affected during progressive renal decline. Thus, caution should be taken when using blood PFAS concentrations as a proxy of PFAS exposure as it may underestimate the effect of PFAS on kidney function, especially at the stage of advanced renal failure. In our study, most participants had normal kidney function, so we may have been unable to detect this inverted U-shape association. However, when we additionally adjusted for baseline microalbuminuria or elevated ACR, which often precede eGFR decline, the inverse association between blood PFAS and eGFR strengthened, confirming the potential negative confounding effect. The inverse relationship between blood PFAS concentration and ACR was previously reported in NHANES (Jain and Ducatman 2019b). The study hypothesized that elevated ACR could directly cause PFAS excretion, or that elevated ACR could also be a result of a process that also reverses the reabsorption of PFAS by the kidney, leading to higher PFAS excretion from urine and lower blood PFAS concentrations. The role of protein-binding had been noted to alter PFAS excretion (Beesoon and Martin 2015); and a recent study also showed that albumin is the major protein carrier for many long-chain PFAS including PFOS, PFOA, PFHxS, PFDA and PFNA (Forsthuber et al. 2020). Together this evidence shows that adjustment for albumin level and stages of glomerular filtration function may have important implications and further investigations are warranted.
Unreported in previous literature, we observed differential effect by hypertension status. Hypertension may accelerate renal damage and increase the susceptibility of CKD for patients with diabetes (Mennuni et al. 2014). Our previous analysis showed plasma PFAS concentrations were not associated with blood pressure (Lin et al. 2020a), thus hypertension was unlikely an intermediate variable in the causal pathway. In the hypertensive state, the kidney experiences alteration of vascular structure, change in glomerular permeability to macromolecules, and increase in glomerular, tubular and interstitial injuries (Folkow et al. 1977; Mennuni et al. 2014) which could intensify the damage of PFAS on kidney function. Hypertensive patients often experienced microalbuminuria (Palatini 2003; Rodicio et al. 1998), and the severity of microalbuminuria correlates with the severity of hypertension and responses to lowering of blood pressure levels (Parving et al. 1974). Other mechanisms that may promote renal damage include oxidative stress, endothelial dysfunction, and genetic and epigenetic factors. Additional studies are needed to better understand this differential effect.
Important strengths of our current study included the long follow-up time, prospective assessments of exposure and eGFR, multiple sensitivity analyses and tests of reverse causation to ensure the internal validity and robustness of detected associations. The current longitudinal findings fill a significant gap in the current literature (Stanifer et al. 2018). The g-computation approach provided a framework to estimate the overall effect of the multiple PFAS chemicals.
Limitations include: limited generalizability because of participants’ characteristics (all had overweight/obesity and prediabetes); potential unmeasured confounders, such as blood iron level, genetics and epigenetic variants; and findings from the 6 PFAS we examined in this study may not be generalizable to other, newer PFAS. Renal clearance of PFAS vary by carbon-chain length, side-chain functional groups and isomeric structure, and continued monitoring of the potential adverse effects of PFAS on kidney health is needed.
5. CONCLUSIONS
Among adults with prediabetes enrolled in the long-term DPP/DPPOS studies who had plasma PFAS concentrations comparable to those of the general U.S. population, higher baseline plasma concentrations were associated with lower eGFR over approximately 14 years of follow-up. An initial intensive lifestyle intervention of diet and exercise did not modify this adverse effect. However, individuals with hypertension may experience a more detrimental effect.
Supplementary Material
Highlights.
We examined the relationship between PFAS and eGFR among prediabetic adults
We found evidence of associations between plasma PFAS and adverse kidney function
Baseline plasma PFAS was inversely associated with prospective measures of eGFR
Lifestyle intervention did not modify the association
We observed stronger effect among those with baseline hypertension
Acknowledgements
The Diabetes Prevention Program (DPP) was conducted by the DPP Research Group and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the General Clinical Research Center Program, the National Institute of Child Health and Human Development (NICHD), the National Institute on Aging (NIA), the Office of Research on Women’s Health, the Office of Research on Minority Health, the Centers for Disease Control and Prevention (CDC), and the American Diabetes Association. The data [and samples] from the DPP/DPPOS included in this project were supplied by the NIDDK Central Repositories (project number 1X01DK104234). The authors acknowledge all participants in DPP and DPPOS who made this study possible. The authors thank Sharon Edelstein at the DPP/DPPOS Coordinating Center for providing support on data inquires; Jennifer Thompson in the Department of Population Medicine for providing valuable logistical support for this project; K. Kato, T. Jia, and the late X. Ye for performing the quantification of PFAS biomarkers at the Centers for Disease Control and Prevention (CDC). This work was supported by the National Institutes of Health grants R01ES024765. All persons named in the Acknowledgments section have provided the corresponding author with written permission to be named in the manuscript. The authors declare they have no actual or potential competing financial interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Funding source
Support for this research was provided by grants from the US National Institute of Environmental Health Sciences, National Institutes of Health (R01ES024765).
Abbreviations
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- DAGs
directed acyclic graphs
- DASH
Dietary Approaches to Stop Hypertension
- DPP
Diabetes Prevention Program
- DPPOS
Diabetes Prevention Program Outcomes Study
- eGFR
Estimated glomerular filtration rate
- EtFOSAA
N-ethyl-perfluorooctane sulfonamido acetic acid
- FDR
False-discovery rate
- FP
Fractional polynomials
- GAM
Generalized additive models
- IRB
institutional review board
- LOD
limit of detection
- MeFOSAA
N-methyl-perfluorooctane sulfonamido acetic acid
- NHANES
National Health and Nutrition Examination Survey
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- n-PFOA
n-perfluorooctanoic acid
- n-PFOS
n-perfluorooctane sulfonic acid
- PFAS
Per- and polyfluoroalkyl substances
- PFDA
Perfluorodecanoic acid
- PFHxS
perfluorohexane sulfonic acid
- PFNA
Perfluorononanoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonic acid
- Sb-PFOA
branched perfluorooctanoic acid isomers
- Sm2-PFOS
Perfluorodimethylhexane sulfonic acid isomers
- Sm-PFOS
Perfluoromethylheptane sulfonic acid isomers
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR. 2018. PFAS health effects. Available: https://www.atsdr.cdc.gov/pfas/health-effects.html [accessed July 27 2018].
- Beesoon S, Martin JW. 2015. Isomer-specific binding affinity of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to serum proteins. Environ Sci Technol 49:5722–5731. [DOI] [PubMed] [Google Scholar]
- Blake BE, Pinney SM, Hines EP, Fenton SE, Ferguson KK. 2018. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the fernald community cohort. Environ Pollut 242:894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. 2007. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. Population: Data from the national health and nutrition examination survey (NHANES). Environ Sci Technol 41:2237–2242. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert M-F, Calafat AM, et al. 2017. Plasma concentrations of per-and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the Diabetes Prevention Program trial. Environmental Health Perspectives 107001:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, et al. 2017. Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the diabetes prevention program trial. Environ Health Perspect 125:107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Hivert MF, Gold DR, Hauser R, Kleinman KP, Lin PD, et al. 2019. Associations of perfluoroalkyl and polyfluoroalkyl substances with incident diabetes and microvascular disease. Diabetes Care 42:1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. Fourth report on human exposure to environmental chemicals, updated tables. Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2017. Fourth national report on human exposure to environmental chemicals.
- Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. 2009. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 56:338–349. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. 2017. A study of reverse causation: Examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 125:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research G, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 2009. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research G. 2015. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 3:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 1999. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 2000. The Diabetes Prevention Program: Baseline characteristics of the randomized cohort. Diabetes care 23:1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 2002a. The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care 25:2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 2002b. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl j Med 2002:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 2009. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. The Lancet 374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 2012. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention. Diabetes care 35:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 2015. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. The lancet Diabetes & endocrinology 3:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Orlando A, Ricci Z, Ronco C. 2019. Persistent pollutants: Focus on perfluorinated compounds and kidney. Curr Opin Crit Care 25:539–549. [DOI] [PubMed] [Google Scholar]
- Folkow B, Gothberg G, Lundin S, Ricksten SE. 1977. Structural “resetting” of the renal vascular bed in spontaneously hypertensive rats (shr). Acta Physiol Scand 100:270–272. [DOI] [PubMed] [Google Scholar]
- Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschlager M, Stangl H, et al. 2020. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ Int 137:105324. [DOI] [PubMed] [Google Scholar]
- Gao Y, Fu J, Cao H, Wang Y, Zhang A, Liang Y, et al. 2015. Differential accumulation and elimination behavior of perfluoroalkyl acid isomers in occupational workers in a manufactory in China. Environ Sci Technol 49:6953–6962. [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Aroda VR, Bluemke DA, Barrett-Connor E, Budoff M, Crandall JP, et al. 2017. Effect of long-term metformin and lifestyle in the Diabetes Prevention Program and its outcome study on coronary artery calcium. Circulation 136:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene 5:46–51. [Google Scholar]
- Jain RB, Ducatman A. 2019a. Perfluoroalkyl substances follow inverted u-shaped distributions across various stages of glomerular function: Implications for future research. Environ Res 169:476–482. [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A. 2019b. Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: Serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ Res 174:143–151. [DOI] [PubMed] [Google Scholar]
- Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L. 2015. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health 14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. Journal of chromatography A 1218:2133–2137. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol 45:8037–8045. [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128:47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ricardo AC, Boyko EJ, Christophi CA, Temprosa M, Watson KE, et al. 2019. Sex hormones and measures of kidney function in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 104:1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M, Smurthwaite k, Braunig J, Trevenar S, D’Este C, Lucas R, et al. 2018. The PFAS health study: Systematic literature review. Canberra, ACT, Australia:The Australian National University. [Google Scholar]
- Kjølholt J JA, Warming M 2015. Short-chain polyfluoroalkyl substances (PFAS): A literature review of information on human health effects and environmental fate and effect aspects of short-chain pfas. Copenhagen, Denmark:Danish Ministry of the Environment. [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. 2006. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254. [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. 2019. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults-longitudinal analysis of the Diabetes Prevention Program Outcomes Study. Environ Int 129:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. 2020a. Per- and polyfluoroalkyl substances and blood pressure in pre-diabetic adults-cross-sectional and longitudinal analyses of the Diabetes Prevention Program Outcomes Study. Environ Int 137:105573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. 2020b. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: Cross-sectional results from the Diabetes Prevention Program trial. Environ Int 137:105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. 2014. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens 28:74–79. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P 2003. Microalbuminuria in hypertension. Curr Hypertens Rep 5:208–214. [DOI] [PubMed] [Google Scholar]
- Parving HH, Mogensen CE, Jensen HA, Evrin PE. 1974. Increased urinary albumin-excretion rate in benign essential hypertension. Lancet 1:1190–1192. [DOI] [PubMed] [Google Scholar]
- Rodicio JL, Campo C, Ruilope LM. 1998. Microalbuminuria in essential hypertension. Kidney Int Suppl 68:S51–54. [DOI] [PubMed] [Google Scholar]
- Russell MH, Waterland RL, Wong F. 2015. Calculation of chemical elimination half-life from blood with an ongoing exposure source: The example of perfluorooctanoic acid (PFOA). Chemosphere 129:210–216. [DOI] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. 2011. Perfluoroalkyl chemicals and chronic kidney disease in us adults. Am J Epidemiol 174:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Vestergren R, Xu L, Zhou Z, Li C, Liang Y, et al. 2016. Human exposure and elimination kinetics of chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs). Environ Sci Technol 50:2396–2404. [DOI] [PubMed] [Google Scholar]
- Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. 2018. Perfluorinated chemicals as emerging environmental threats to kidney health: A scoping review. Clin J Am Soc Nephrol 13:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. 2016. PFOA stewardship program. Available: https://www.regulations.gov/docket?D=EPA-HQ-OPPT-2006-0621 [accessed Oct 6 2020].
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. 2007. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS medicine 4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zeng XW, Bloom MS, Qian Z, Hinyard LJ, Belue R, et al. 2019. Renal function and isomers of perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS): Isomers of C8 health project in China. Chemosphere 218:1042–1049. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, et al. 2013. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 121:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. 2010. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol Sci 113:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley RR, Yang X, Fisher J. 2017. Physiologically based pharmacokinetic modeling of human exposure to perfluorooctanoic acid suggests historical non drinking-water exposures are important for predicting current serum concentrations. Toxicol Appl Pharmacol 330:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X. 2010. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1a2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci 117:294–302. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. 2013. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47:10619–10627. [DOI] [PubMed] [Google Scholar]
- Zhao J, Hinton P, Chen J, Jiang J. 2020. Causal inference for the effect of environmental chemicals on chronic kidney disease. Comput Struct Biotechnol J 18:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.