Abstract
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in clinical practice and induces cardiac dysfunction and stroke. The development of AF requires a trigger and also an electroanatomic substrate capable of both initiating and perpetuating AF. In the past decade, ectopic beats originating from the pulmonary veins (PV) have been identified as a source of paroxysmal AF. Thus, strategies that target the PV, including the PV antrum, are the cornerstone of most AF ablation procedures. Recently, alternative technologies to radiofrequency catheter ablation for paroxysmal AF such as balloon ablation modalities have been developed. The purpose of this review is to discuss cryoballoon ablation for paroxysmal AF.
Key Words: Atrial fibrillation, Catheter ablation, Cryoballoon
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in clinical practice, inducing cardiac dysfunction and stroke.1,2 AF depends on the interaction between triggers and anatomical substrates,3,4 and the isolation of the pulmonary veins (PV) and elimination of non-PV triggers form the basis of an AF ablation strategy that is highly effective in most patients with paroxysmal AF.5–7 Therefore, point-by-point ablation of the circumference of the PV using an irrigated-tip radiofrequency (RF) catheter is the cornerstone of therapy for symptomatic paroxysmal AF refractory to anti-arrhythmic drugs. Recently, advanced balloon ablation technology using a variety of energy modalities, such as cryoballoon (CB; Arctic-Front Advance; Medtronic, Minneapolis, MN, USA), hot balloon (Toray Industries, Tokyo, Japan), and laser balloon (HeartLight; CardioFocus, Marlborough, MA, USA), which are placed in the PV ostia to isolate the PV circumferentially, has become available for the treatment of paroxysmal AF. Multiple studies for each balloon ablation technology have proven that each technology is as effective as RF catheter ablation (RFCA) in treating paroxysmal AF.8–10 The balloon ablation modalities, however, have various challenges that need to be overcome, such as the need for additional touch-up ablation; the durability of balloon-based PV isolation; anatomical difficulties; and complications including PV stenosis, thromboembolic complications, cardiac perforation with pericardial tamponade, esophageal fistula, and phrenic nerve palsy (PNP).11 Further development of the technology and methodology of balloon-based ablation is therefore needed, in order to reduce these problems.
In this article, we review the current clinical experience of CB ablation in the treatment of paroxysmal AF.
Catheter Ablation of Paroxysmal AF
According to the expert consensus statement, catheter ablation is recommended for symptomatic paroxysmal AF refractory to anti-arrhythmic drugs (class I/level of evidence A), or it may even be considered as first-line therapy, after weighing the risks of the ablation procedure (class IIa/B).12 PV isolation is the cornerstone of catheter ablation strategies for AF, especially the paroxysmal condition. Numerous studies have shown that the elimination of PV triggers could lead to better long-term clinical outcomes for paroxysmal AF patients.12 Human and animal studies and computer simulation models have also indicated that the wave front arising from the rotor area with high dominant frequency (DF) accompanying peripheral heterogenous activation through an inhomogeneous atrial substrate gives rise to fibrillatory conduction.13–16 The highest incidence of wavebreaks and the beat-to-beat variability in the direction and velocity of wave front propagation have also been shown to be associated with highly fractionated atrial signals located at the boundaries of the high DF regions.13–16 Several clinical studies reported that complex fractionated atrial electrograms were observed at adjacent areas with high DF in patients with paroxysmal and non-paroxysmal AF.17,18 Lin et al reported that the highest DF was located in the arrhythmogenic PV or its ostium in the PV-AF patients, and that those patients also had a significant frequency gradient from the PV ostium to the left atrium (LA).19 Similarly, a recent study demonstrated a close relationship between the arrhythmogenic PV and the critical atrial substrate near the PV manifesting abnormal fibrillatory electrograms.20 Furthermore, regions with the most fractionated electrogram were mostly located in the periphery of the high DF regions. Most of the critical atrial substrates identified on automated electrogram analysis were located <15 mm from the PV ostium,20 indicating the possible need to extend the area of circumferential PV isolation even in patients with paroxysmal AF. RFCA is a well-established procedure for paroxysmal AF that achieves PV isolation via consecutive, transmural point-by-point RF energy applications. In contrast, CB-based PV isolation has emerged as an effective therapy to treat paroxysmal AF and has become a substitute for RFCA due to the advantage of easier catheter manipulation and shorter procedure time compared with RFCA. The efficacy and safety of CB ablation therapy have also been shown to be similar to those of RFCA in patients with paroxysmal AF.21–24 For paroxysmal AF patients without advanced structural remodeling of the PV and LA, the 28-mm CB could be sufficient to ablate a large area including the PV antrum, similar to the area obtained in wider circumferential ipsilateral PV isolation using RFCA.25,26
Evolution of CB Technology
Cryotherapy has a long history of use as a treatment for frostbite and tumor destruction. The ridged, hand-held cryothermal ablation probes have been used for surgical arrhythmia treatment since the 1970 s.27–29 As described previously, the mechanism of cryoablation is based on the Joule-Thomson effect (the change in temperature of an expanding gas) to achieve tissue temperatures between −30℃ and −90℃.30 Cryoablation is realized through the delivery of pressurized cryorefrigerant (currently liquefied nitrous oxide) to the distal aspect of the inner balloon via an ultrafine injection tube. Nitrous oxide has a boiling temperature of −88.47℃, providing adequate cooling power and safety margins for use in cardiac tissue ablation.31 As the refrigerant cools, ice forms at the tissue contact site, causing the balloon to adhere to the tissue, stabilizing it in place for the duration of freezing. Progressive cooling to below −40℃ results in the formation of intracellular ice crystals. Cell and tissue damage, however, occur both during the freezing process and afterward, in sequential stages (freeze, thaw, hemorrhage and reactive inflammation, and replacement fibrosis).31,32 The extent of scarring yielding electrically silent tissues depends on the acute damage phase (the freeze/thaw cycle), and involves several variables. As compared with RFCA lesions, cryoablation lesions result in preservation of tissue architecture with less damage to large vascular structures or to the endocardium. Histologically, cryothermy results in the creation of well-demarcated homogeneous lesions that are less arrhythmogenic and thrombogenic than the ragged, indistinct lesions associated with RFCA.33
The use of cryoablation for PV isolation may offer certain advantages. The tissue-catheter adhesion during cryoablation can result in improved catheter stability for creating lesions. The cryoablation is associated with reduced pain and discomfort given that the afferent pain fibers are frozen as opposed to stimulated thermally.34 Cryoablation also has a lower risk of thrombus formation and, consequently, of systemic thromboembolic stroke, because it is associated with decreased activation of platelets and of the coagulation cascade as compared with RFCA lesions.35 Cryoablation leaves the connective tissue matrix intact and decreases the risk of steam pops.34 Furthermore, the lack of circulation, around the center of the cryoablation lesion results in uniform tissue necrosis.34 Thus, the cryoablation lesions result in preservation of tissue architecture with less damage to large vascular structures or to the endocardium as compared with RFCA lesions.
The introduction of second-generation CB (CB2) has improved balloon based-PV isolation. Compared with first-generation equipment, the CB2 ablation devices have a better cooling capacity and seem to reduce the procedure duration.36–38 Real-time PV potential monitoring during CB application is feasible with an over-the-wire mapping catheter (Achieve), and monitoring of the time from the beginning of cryofreezing until PV isolation is now possible. CB2 has a high procedural success rate and convincing clinical outcome data for paroxysmal AF. The FIRE AND ICE trial proved the non-inferiority of CB2- to RF-based PV isolation with regard to efficacy and safety for treatment of PAF.24 In consequence, the latest AF ablation guidelines state that PV isolation should be performed using either RF or CB catheters.12 In general, CB ablation protocols predominantly use a fixed freeze cycle duration of 180–240 s (Table), often followed by a bonus freeze cycle. Recently, modified freezing protocols aimed at shorter and fewer CB applications have demonstrated similar clinical outcomes.39,40 After identifying the time to PV isolation (TTI) as an essential indicator of durable PV isolation, the latest ablation strategies have been implemented the individual TTI.41 The weakness of CB2, however, is that TTI could be recorded only in 55–85% of cases, even at experienced centers. The longer distal tip of the CB2 limits proper visualization of PV potentials because it prevents proximal positioning of the Achieve catheter close to the CB’s surface (Figure 1). The third-generation CB (CB3) incorporates a 40% shorter tip, facilitating a more proximal Achieve catheter position. As a consequence, a higher rate of real-time PV recordings was noted for the CB3 compared with CB2.42,43 In October 2018, a fourth-generation CB (CB4) was released (Figure 1), and it has been available for use in Japan since April 2019. The CB4 with a shorter distal tip is safe and effective, and allows determination of TTI in 78–85% of PV.44–46 Several randomized clinical trials have been carried out to investigate the efficacy and long-term outcomes of CB ablation (Table), which were almost identical to those of RFCA.8,47–51 Furthermore, CB-based PV isolation seems to be less operator dependent and more reproducible than RF-based PV isolation in the setting of paroxysmal AF ablation,52 although various parts of the procedure, such as the freezing protocol, were not standardized.
Table.
Clinical Trials of CB Ablation in Patients With Paroxysmal AF
| Trial | Publication year (ref. no.) |
n (CB/RF) |
AF type |
Generation of CB |
CB procedure |
Average CB application time (s) |
Procedure time LA dwell time (min) |
Mean follow-up (months) |
Primary endpoints/ outcome |
|---|---|---|---|---|---|---|---|---|---|
| FIRE and ICE | 2016 (8) | 374/376 | PAF | 1 st and 2nd | 300 s (CB1), 240 s (CB2) |
124.4 vs. 140.9 | 124.4±39.8 92.3±31.4 |
18 | 65.4% (CB) vs. 64.1% (RF) |
| CRYO-Japan PMS |
2016 (47) | 616 (CB) | PAF | 2nd | 180–240 s | LS,† 156.7±31.9 LI,† 149.3±35.2 RS,† 150.4±33.5 RI,† 154.9±34.3 |
150.2±48.9 95.9±35.4 |
6 | 88.4% |
| STOP AF PAS |
2019 (50) | 344 (CB) | PAF | 2nd | 240 s | 214.4±1.5 | N/A 80.4±31.4 |
36 | 64% |
| RADICOOL | 2018 (48) | 452 (CB) | PAF | 2nd | 180 or 240 s | N/A | N/A 63 |
12 | 87% |
| FREEZE AF | 2019 (49) | 2,329/1,860 | PAF (70.4%) |
1 st, 2nd, 3rd | N/A | N/A | N/A 94.2±37.1 |
N/A | 69.3% (CB) vs. 60.6% (RF) |
| ESC-EHRA registry |
2019 (51) | 982/3,675 | PAF (75.4%) |
2nd | N/A | 133.6 vs. 174.6 | 133.6±45.2 N/A |
12 | 70.2% (CB) vs. 68.2% (RF) |
†Using 28-mm CB. AF, atrial fibrillation; CB, cryoballoon; CB1/2, first-/second-generation CB; LA, left atrium; LI, left inferior; LS, left superior; PAF, paroxysmal atrial fibrillation; RF, radiofrequency; RI, right inferior; RS, right superior.
Figure 1.
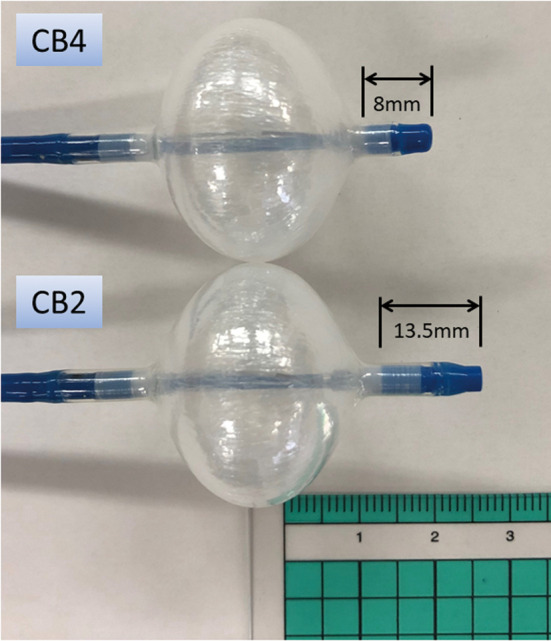
Comparison of the second-generation and fourth-generation cryoballoons (CB2 and CB4, respectively). The CB4 has a shorter tip to enhance visualization of pulmonary vein (PV) potential by enabling more proximal placement of the Achieve mapping catheter in the PV.
CB Ablation Technique
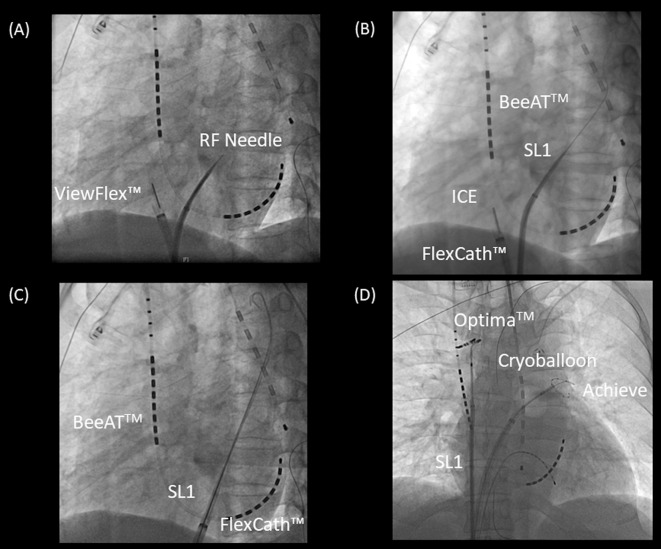
Many researchers worldwide have reported on the CB ablation technique.8,47–51 CB ablation is simple and fast, even in the hands of beginners, as compared with RFCA, despite the learning curve needed to master the skills of CB ablation.53–55 In brief, a single transseptal puncture is made using an RF needle (Baylis Medical, Montreal, QC, Canada) through an 8-Fr sheath (SL1, AF Division, Abbott/St. Jude Medical, Minneapolis, MN, USA) under intracardiac echocardiography guidance (ViewFlexTM Xtra ICE catheter, Abbott/St. Jude Medical, Figure 2A,B). Then, a 15-Fr steerable sheath (FlexCath Advance, Medtronic) is inserted into the LA as a guide for the wire of the SL1 sheath (Figure 2C). After the Flexcath sheath is inserted into the LA, the SL1 sheath is placed at the superior vena cava (SVC). A spiral circular mapping catheter (Optima; Abbott/St. Jude Medical) is placed at the SVC through the SL1 sheath to monitor the diaphragmatic compound motor action potentials (CMAP) during phrenic nerve pacing for each CB application at right PV (Figure 2D). The 4-Fr deflectable 4-pole electrode catheter is placed into the apex of the right ventricle for sudden AV block during the CB procedure. PV isolation was performed with a single balloon technique using a CB2 (the CB4 has been available since April 2019). A 28-mm CB catheter was used in all of the patients. A spiral mapping catheter (Achieve, Medtronic) was used to advance the CB and map the PV potentials. Complete sealing at the antral aspect of the PV was confirmed on injection of contrast medium. The freezing protocol has been applied based on the CRYO-Japan PMS study.47 An 11–20-mm or 15–25-mm circular mapping catheter (InquiryTM OptimaTM, Abbott/St. Jude Medical, St. Paul, MN, USA), according to PV diameter, was used to map all of the PV after CB applications to confirm electrical isolation. If electrical isolation was not achieved, additional touch-up ablation was performed with a conventional RF catheter.
Figure 2.
Representative cryoballoon (CB) procedure in a patient with paroxysmal atrial fibrillation (AF). (A) A single transseptal puncture was performed using a radiofrequency (RF) needle (Baylis Medical, Montreal, QC, Canada) through an 8-Fr sheath (SL1, AF Division, Abbott/St. Jude Medical, Minneapolis, MN, USA) under intracardiac echocardiography (ICE) guidance (ViewFlexTM Xtra ICE catheter, Abbott/St. Jude Medical). (B) After transseptal puncture under ICE guidance, the SL1 sheath is advanced into the left atrium (LA) through the inner wire and pulled back into the right atrium (RA). (C) The FlexCath sheath is advanced into the LA via the transseptal puncture as a guide for the SL1 inner wire. (D) After the Optima mapping catheter is placed at the superior vena cava (SVC) through the SL1 sheath to observe the compound motor action potentials on the right side, the CB ablation procedure is started from the left superior pulmonary vein (PV), followed by the left inferior PV, the right inferior PV, and the right superior PV.
CB Ablation: Precautions
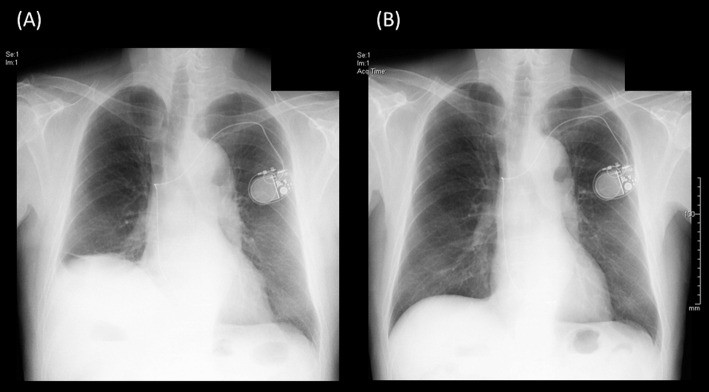
Although CB ablation has emerged as an effective, alternative treatment option to traditional point-by-point RFCA for paroxysmal AF, there are several issues with CB ablation. Knowledge of the close anatomical relationships between the PV, the right phrenic nerve, esophagus and the bronchial tree is critical to understanding why these structures are prone to collateral injury during CB ablation. This collateral injury is usually associated with a low nadir balloon temperature.56 In several multi-center clinical trials, the common major complication was PNP, including transient injury, which has been seen in 3.2–13.5%.8,36,57 In contrast, almost zero patients in the RFCA group had PNP. We also encountered 2 cases of transient PNP. Figure 3 shows the recovery process in one of them with PNP after CB ablation. Recently, the continuous monitoring of CMAP has become standard in clinical practice, and this involves immediate termination with a single or double stop technique when the CMAP significantly decreases.
Figure 3.
Acute phrenic nerve palsy after cryoballoon (CB) ablation: chest X-ray (A) 1 day after and (B) 6 months after the CB ablation procedure.
Regarding unusual complications, late onset coronary spasm after CB ablation has been reported.58 We also encountered a similar case of late-onset coronary spasm after CB procedure. Although no remarkable change was noted on 12-lead electrocardiogram (ECG) during CB ablation (Figure 4A), the ST segment in the inferior leads gradually rose approximately 30 min after the CB procedure, and a severe spasm of the right coronary artery was noted on emergency coronary angiography (Figure 4B). After intra-coronary nitroglycerin, the 12-lead surface ECG and spastic right coronary artery returned to normal (Figure 4B). The CB procedure induces acute and late-onset coronary artery spasm in susceptible patients,58 which is suggested to be modulated by CB by imbalance in the autonomic nerve system via affected ganglionated plexi close to the PV ostium. The 12-lead ECG should be continuously monitored throughout the procedure with special attention to ischemic changes.
Figure 4.
Representative case of severe spasm in the right coronary artery after cryoballoon ablation (CBA). (A) On surface electrocardiogram (ECG) the inferior leads showed ST-segment elevation, and emergency coronary angiography of the right coronary artery showed severe spasm. (B) After intracoronary injection of nitroglycerine, the right coronary artery spasm and surface ECG resolved. ISP, isoproterenol; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Furthermore, attention is needed to confirm the isolation of carina regions after CB ablation (Figure 5). Takigawa et al reported that a double-Lasso guided ipsilateral PV isolation may miss a non-isolation of the PV carina. In that study, the PV carina in 21% of patients remained non-isolated after the procedure.59 Furthermore, the existence of non-isolated PV carina even after double-Lasso guided successful PV isolation was an independent predictor of AF recurrence during a mean follow-up of 19±13 months.59 In our patient, residual PV potential was detected at the right PV carina region even after successful Achieve catheter-guided CB ablation (Figure 5C). Additional RF applications in the PV antral area using a 4-mm open-irrigated tip catheter (FlexAbilityTM, Abbott/St. Jude Medical) completely eliminated the residual conduction between the LA and right PV (Figure 5D). Even though isolation of all PV is confirmed by Achieve catheter after CB ablation, a myocardial connection between the right PV carina and LA may persist after CB ablation, which may require additional RF applications. Therefore, careful mapping around each PV including the carina region is necessary to ensure complete electrical PV antral isolation after CB ablation.
Figure 5.
(A,B) After cryoballoon (CB) ablation, there were no residual pulmonary vein (PV) potentials inside any of the PV. Moreover, exit block to the left atrium (LA) was confirmed only in the right superior PV (RSPV). (C) A roving circular catheter recorded sporadic PV potentials from the right PV carina. (D) Additional radiofrequency applications around the right PV carina completely eliminated the conduction between the LA and right PV.
Minimally Invasive and Tailored CB Ablation
There are differences in CB protocol between institutions (Table). Acute PV isolation is a well-known physiological predictor of durable isolation if real-time acute PV isolation can be monitored using the Achieve mapping catheter.41 Time to PV isolation, however, has been documented in all targeted PV in only 37% patients.60 Thus, it could be difficult to determine the end-point of cryotherapy using monitoring of time to PV isolation. The optimal dosage of cryotherapy to achieve durable PV isolation is still unclear. Creatine kinase (CK) has been reported to be significantly increased after CB ablation as compared with RF ablation.61,62 Moreover, Casella et al reported that troponin I and CK-MB were significantly increased after CB ablation as compared with after ablation with other energy sources.63 This strongly indicates that CB ablation could induce great myocardial injury. Thus, unnecessary CB applications could lead to severe collateral complications, even though the locations of residual PV-LA conduction breakthrough were usually only limited points.64 Furthermore, lower nadir balloon temperature and longer application could lead to collateral injuries.56 Recently, several studies have investigated ways to reduce cryotherapy time.65,66 Pott et al reported that CB ablation with TTI-guided cryoenergy titration could be non-inferior to the fixed CB ablation strategy.66 Their TTI-guided CB ablation protocol was modified such that freeze duration was 120 s for TTI <30s, and 180 s for TTI 30–60 s, and the fixed CB ablation protocol included a 240-s bonus freeze following freeze cycles of 240 s.66 TTI-guided cryoenergy titration leads to reduced procedure duration and fluoroscopy time and appears to be as effective as a fixed ablation strategy. In the Pott et at study, real-time PV isolation was observed in 81.5% of PV.66 CB4 could record real-time online PV potential with a low incidence of CB dislodgment.44–46 Further research into tailored CB ablation protocols using CB4 to reduce unnecessary cryoenergy applications is required.
Conclusions
The evolution of CB ablation as a single-shot effective and safe ablation approach targeted at the PV triggers in paroxysmal AF has generated a huge surge of interest in this technique. CB ablation may be able to control this most common cardiac arrhythmia by producing a lasting sinus rhythm. Further, these technological advances could result in better procedure guidance and more durable lesions. The new CB4, with a 40% shortened tip compared with the previous version, was recently introduced in an attempt to improve the visualization of real-time recording of PV potentials during the CB procedure, and thus, can more clearly visualize TTI, which is an important predictor of successful and durable PV isolation.67,68 The technological progress that has led to this tailored minimally invasive approach for avoiding excessive cryotherapy to the atrium and collateral damage, as described in this review, will need further study to explore and elucidate the pathophysiological mechanisms of this most common arrhythmia.
Disclosures
S.H. is a consultant to Japan Life Line and Johnson & Johnson, and has received Speaker’s Honoraria from Japan Life Line, Medtronic, Abbott, Bayer, Biotronik, Boehringer-Ingelheim, Bristol-Myers, Daiichi-Sankyo Pharmaceutical Company, and Pfizer. The other authors declare no conflicts of interest.
References
- 1. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE.. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995; 98: 476–484. [DOI] [PubMed] [Google Scholar]
- 2. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al.. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997; 96: 2455–2461. [DOI] [PubMed] [Google Scholar]
- 3. Nattel S.. New ideas about atrial fibrillation 50 years on. Nature 2002; 415: 219–226. [DOI] [PubMed] [Google Scholar]
- 4. Wyse DG, Gersh BJ.. Atrial fibrillation: A perspective: Thinking inside and outside the box. Circulation 2004; 109: 3089–3095. [DOI] [PubMed] [Google Scholar]
- 5. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al.. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339: 659–666. [DOI] [PubMed] [Google Scholar]
- 6. Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al.. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999; 100: 1879–1886. [DOI] [PubMed] [Google Scholar]
- 7. Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, et al.. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003; 107: 3176–3183. [DOI] [PubMed] [Google Scholar]
- 8. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al.. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374: 2235–2245. [DOI] [PubMed] [Google Scholar]
- 9. Sohara H, Ohe T, Okumura K, Naito S, Hirao K, Shoda M, et al.. HotBalloon ablation of the pulmonary veins for paroxysmal AF: A multicenter randomized trial in Japan. J Am Coll Cardiol 2016; 68: 2747–2757. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt B, Neuzil P, Luik A, Osca Asensi J, Schrickel JW, Deneke T, et al.. Laser balloon or wide-area circumferential irrigated radiofrequency ablation for persistent atrial fibrillation: A multicenter prospective randomized study. Circ Arrhythm Electrophysiol 2017; 10: e005767. [DOI] [PubMed] [Google Scholar]
- 11. Miyazaki S, Tada H.. Complications of cryoballoon pulmonary vein isolation. Arrhythm Electrophysiol Rev 2019; 8: 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al.. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017; 14: e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J.. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation 1998; 98: 1236–1248. [DOI] [PubMed] [Google Scholar]
- 14. Berenfeld O, Mandapati R, Dixit S, Skanes AC, Chen J, Mansour M, et al.. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the Langendorff-perfused sheep heart. J Cardiovasc Electrophysiol 2000; 11: 869–879. [DOI] [PubMed] [Google Scholar]
- 15. Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J.. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation 2000; 101: 194–199. [DOI] [PubMed] [Google Scholar]
- 16. Suenari K, Hirao H, Okamoto M, Kihara Y, Chen SA.. Differences of biatrial substrate properties in patients with different types of AF. J Atr Fibrillation 2012; 5: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin YJ, Tai CT, Kao T, Chang SL, Lo LW, Tuan TC, et al.. Spatiotemporal organization of the left atrial substrate after circumferential pulmonary vein isolation of atrial fibrillation. Circ Arrhythm Electrophysiol 2009; 2: 233–241. [DOI] [PubMed] [Google Scholar]
- 18. Stiles MK, Brooks AG, Kuklik P, John B, Dimitri H, Lau DH, et al.. High-density mapping of atrial fibrillation in humans: Relationship between high-frequency activation and electrogram fractionation. J Cardiovasc Electrophysiol 2008; 19: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 19. Lin YJ, Tai CT, Kao T, Tso HW, Higa S, Tsao HM, et al.. Frequency analysis in different types of paroxysmal atrial fibrillation. J Am Coll Cardiol 2006; 47: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 20. Suenari K, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, et al.. Relationship between arrhythmogenic pulmonary veins and the surrounding atrial substrate in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2011; 22: 405–410. [DOI] [PubMed] [Google Scholar]
- 21. Vogt J, Heintze J, Gutleben KJ, Muntean B, Horstkotte D, Nolker G.. Long-term outcomes after cryoballoon pulmonary vein isolation: Results from a prospective study in 605 patients. J Am Coll Cardiol 2013; 61: 1707–1712. [DOI] [PubMed] [Google Scholar]
- 22. Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, Hormann P, et al.. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: The prospective, randomized, controlled, noninferiority FreezeAF Study. Circulation 2015; 132: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holda MK, Klimek-Piotrowska W, Holda J.. Cryoballoon or radiofrequency ablation for atrial fibrillation. N Engl J Med 2016; 375: 1100. [DOI] [PubMed] [Google Scholar]
- 24. Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M, et al.. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: Reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016; 37: 2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cronin EM, Wisnoskey BJ, Rizzo RA, Niebauer MJ, Dresing TJ, Cantillon DJ.. Real-time guidewire localization using impedance-based electroanatomic mapping: Experimental results and clinical validation during cryoballoon ablation of atrial fibrillation. Europace 2013; 15: 1669–1676. [DOI] [PubMed] [Google Scholar]
- 26. Miyazaki S, Taniguchi H, Hachiya H, Nakamura H, Takagi T, Iwasawa J, et al.. Quantitative analysis of the isolation area during the chronic phase after a 28-mm second-generation cryoballoon ablation demarcated by high-resolution electroanatomic mapping. Circ Arrhythm Electrophysiol 2016; 9: e003879. [DOI] [PubMed] [Google Scholar]
- 27. Gallagher JJ, Sealy WC, Anderson RW, Kasell J, Millar R, Campbell RW, et al.. Cryosurgical ablation of accessory atrioventricular connections: A method for correction of the pre-excitation syndrome. Circulation 1977; 55: 471–479. [DOI] [PubMed] [Google Scholar]
- 28. Harrison L, Gallagher JJ, Kasell J, Anderson RH, Mikat E, Hackel DB, et al.. Cryosurgical ablation of the A-V node-His bundle: A new method for producing A-V block. Circulation 1977; 55: 463–470. [DOI] [PubMed] [Google Scholar]
- 29. Gallagher JJ, Anderson RW, Kasell J, Rice JR, Pritchett EL, Gault HJ, et al.. Cryoablation of drug-resistant ventricular tachycardia in a patient with a variant of scleroderma. Circulation 1978; 57: 190–197. [DOI] [PubMed] [Google Scholar]
- 30. Piccini JP, Daubert JP.. Cryoablation of atrial fibrillation. J Interv Card Electrophysiol 2011; 32: 233–242. [DOI] [PubMed] [Google Scholar]
- 31. Avitall B, Kalinski A.. Cryotherapy of cardiac arrhythmia: From basic science to the bedside. Heart Rhythm 2015; 12: 2195–2203. [DOI] [PubMed] [Google Scholar]
- 32. Andrade JG, Khairy P, Dubuc M.. Catheter cryoablation: Biology and clinical uses. Circ Arrhythm Electrophysiol 2013; 6: 218–227. [DOI] [PubMed] [Google Scholar]
- 33. Hirao T, Nitta J, Adachi A, Takahashi Y, Goya M, Hirao K.. First confirmation of histologic changes in the human heart after cryoballoon ablation. HeartRhythm Case Rep 2019; 5: 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bredikis A, Wilber D.. Cryoablation of cardiac arrhythmias. Philadelphia: Saunders, 2011.
- 35. Khairy P, Chauvet P, Lehmann J, Lambert J, Macle L, Tanguay JF, et al.. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation 2003; 107: 2045–2050. [DOI] [PubMed] [Google Scholar]
- 36. Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, et al.. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: First results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol 2013; 61: 1713–1723. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt M, Dorwarth U, Andresen D, Brachmann J, Kuck KH, Kuniss M, et al.. Cryoballoon versus RF ablation in paroxysmal atrial fibrillation: Results from the German Ablation Registry. J Cardiovasc Electrophysiol 2014; 25: 1–7. [DOI] [PubMed] [Google Scholar]
- 38. Straube F, Dorwarth U, Vogt J, Kuniss M, Heinz Kuck K, Tebbenjohanns J, et al.. Differences of two cryoballoon generations: Insights from the prospective multicentre, multinational FREEZE Cohort Substudy. Europace 2014; 16: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 39. Ciconte G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, Mugnai G, et al.. Single 3-minute freeze for second-generation cryoballoon ablation: One-year follow-up after pulmonary vein isolation. Heart Rhythm 2015; 12: 673–680. [DOI] [PubMed] [Google Scholar]
- 40. Heeger CH, Wissner E, Mathew S, Hayashi K, Sohns C, Reissmann B, et al.. Short tip-big difference? First-in-man experience and procedural efficacy of pulmonary vein isolation using the third-generation cryoballoon. Clin Res Cardiol 2016; 105: 482–488. [DOI] [PubMed] [Google Scholar]
- 41. Watanabe R, Okumura Y, Nagashima K, Iso K, Takahashi K, Arai M, et al.. Influence of balloon temperature and time to pulmonary vein isolation on acute pulmonary vein reconnection and clinical outcomes after cryoballoon ablation of atrial fibrillation. J Arrhythm 2018; 34: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Su W, Aryana A, Passman R, Singh G, Hokanson R, Kowalski M, et al.. Cryoballoon Best Practices II: Practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm 2018; 15: 1348–1355. [DOI] [PubMed] [Google Scholar]
- 43. Ferrero-de-Loma-Osorio Á, García-Fernández A, Castillo-Castillo J, Izquierdo-de-Francisco M, Ibáñez-Críado A, Moreno-Arribas J, et al.. Time-to-effect-based dosing strategy for cryoballoon ablation in patients with paroxysmal atrial fibrillation: Results of the plusONE Multicenter Randomized Controlled Noninferiority Trial. Circ Arrhythm Electrophysiol 2017; 10: pii: e005318. [DOI] [PubMed] [Google Scholar]
- 44. Straube F, Dorwarth U, Pongratz J, Bruck B, Wankerl M, Hartl S, et al.. The fourth cryoballoon generation with a shorter tip to facilitate real-time pulmonary vein potential recording: Feasibility and safety results. J Cardiovasc Electrophysiol 2019; 30: 918–925. [DOI] [PubMed] [Google Scholar]
- 45. Mathew S, Rottner L, Warneke L, Maurer T, Lemes C, Hashiguchi N, et al.. Initial experience and procedural efficacy of pulmonary vein isolation using the fourth-generation cryoballoon: A step forward? Acta Cardiol, doi:10.1080/00015385.2019.1677373. [DOI] [PubMed] [Google Scholar]
- 46. Moltrasio M, Sicuso R, Fassini GM, Riva SI, Tundo F, Dello Russo A, et al.. Acute outcome after a single cryoballoon ablation: Comparison between Arctic Front Advance and Arctic Front Advance PRO. Pacing Clin Electrophysiol 2019; 42: 890–896. [DOI] [PubMed] [Google Scholar]
- 47. Okumura K, Matsumoto K, Kobayashi Y, Nogami A, Hokanson RB, Kueffer F, et al.. Safety and efficacy of cryoballoon ablation for paroxysmal atrial fibrillation in Japan: Results from the Japanese Prospective Post-Market Surveillance Study. Circ J 2016; 80: 1744–1749. [DOI] [PubMed] [Google Scholar]
- 48. Su W, Orme GJ, Hoyt R, Baker J, Compton S, Fellows C, et al.. Retrospective review of Arctic Front Advance Cryoballoon Ablation: A multicenter examination of second-generation cryoballoon (RADICOOL trial). J Interv Card Electrophysiol 2018; 51: 199–204. [DOI] [PubMed] [Google Scholar]
- 49. Hoffmann E, Straube F, Wegscheider K, Kuniss M, Andresen D, Wu LQ, et al.. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace 2019; 21: 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Knight BP, Novak PG, Sangrigoli R, Champagne J, Dubuc M, Adler SW, et al.. Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: Final results from STOP AF Post-Approval Study. JACC Clin Electrophysiol 2019; 5: 306–314. [DOI] [PubMed] [Google Scholar]
- 51. Mortsell D, Arbelo E, Dagres N, Brugada J, Laroche C, Trines SA, et al.. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: A study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace 2019; 21: 581–589. [DOI] [PubMed] [Google Scholar]
- 52. Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi F, et al.. Results from a multicentre comparison of cryoballoon vs. Europace 2017; 19: 48–57. [DOI] [PubMed] [Google Scholar]
- 53. Chen S, Schmidt B, Bordignon S, Bologna F, Nagase T, Perrotta L, et al.. Practical techniques in cryoballoon ablation: How to isolate inferior pulmonary veins. Arrhythm Electrophysiol Rev 2018; 7: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leitz P, Monnig G, Guner F, Dechering DG, Wasmer K, Reinke F, et al.. Comparing learning curves of two established “single-shot” devices for ablation of atrial fibrillation. J Interv Card Electrophysiol 2018; 53: 317–322. [DOI] [PubMed] [Google Scholar]
- 55. Tokuda M, Yamashita S, Matsuo S, Kato M, Sato H, Oseto H, et al.. Clinical significance of early recurrence of atrial fibrillation after cryoballoon vs. radiofrequency ablation: A propensity score matched analysis. PLoS One 2019; 14: e0219269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kulkarni N, Su W, Wu R.. How to prevent, detect and manage complications caused by cryoballoon ablation of atrial fibrillation. Arrhythm Electrophysiol Rev 2018; 7: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mugnai G, de Asmundis C, Ciconte G, Irfan G, Saitoh Y, Velagic V, et al.. Incidence and characteristics of complications in the setting of second-generation cryoballoon ablation: A large single-center study of 500 consecutive patients. Heart Rhythm 2015; 12: 1476–1482. [DOI] [PubMed] [Google Scholar]
- 58. Watanabe T, Hachiya H, Miyazaki S, Nakamura H, Taniguchi H, Iesaka Y.. Recurrent and late-onset coronary spasms after cryoballoon ablation procedure in a patient with atrial fibrillation. HeartRhythm Case Rep 2016; 2: 421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takigawa M, Yamada T, Yoshida Y, Ishikawa K, Aoyama Y, Yamamoto T, et al.. The incidence and clinical significance of non-isolation of the pulmonary vein carina after encircling ipsilateral pulmonary veins isolation for paroxysmal atrial fibrillation: A pitfall of the double-Lasso technique. Europace 2013; 15: 33–40. [DOI] [PubMed] [Google Scholar]
- 60. Stabile G, Tondo C, Curnis A, Lunati M, Manfrin M, Molon G, et al.. Efficacy of cryoballoon ablation in patients with paroxysmal atrial fibrillation without time to pulmonary vein isolation assessment. Int J Cardiol 2018; 272: 118–122. [DOI] [PubMed] [Google Scholar]
- 61. Kizilirmak F, Gokdeniz T, Gunes HM, Demir GG, Cakal B, Guler GB, et al.. Myocardial injury biomarkers after radiofrequency catheter and cryoballoon ablation for atrial fibrillation and their impact on recurrence. Kardiol Pol 2017; 75: 126–134. [DOI] [PubMed] [Google Scholar]
- 62. Kurose J, Kiuchi K, Fukuzawa K, Mori S, Ichibori H, Konishi H, et al.. The lesion characteristics assessed by LGE-MRI after the cryoballoon ablation and conventional radiofrequency ablation. J Arrhythm 2018; 34: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Casella M, Dello Russo A, Russo E, Al-Mohani G, Santangeli P, Riva S, et al.. Biomarkers of myocardial injury with different energy sources for atrial fibrillation catheter ablation. Cardiol J 2014; 21: 516–523. [DOI] [PubMed] [Google Scholar]
- 64. Yokoyama K, Tokuda M, Matsuo S, Isogai R, Tokutake K, Kato M, et al.. Pulmonary vein re-mapping after cryoballoon ablation for atrial fibrillation. Europace 2018; 20: 943–948. [DOI] [PubMed] [Google Scholar]
- 65. Nakano T, Suenari K, Suruga K, Takemoto H, Hashimoto Y, Tomomori S, et al.. New minimally invasive and tailor-made strategy for cryoballoon ablation in patients with paroxysmal atrial fibrillation. Eur Heart J 2019; 40: P4760; ehz745.1136. [DOI] [PubMed] [Google Scholar]
- 66. Pott A, Kraft C, Stephan T, Petscher K, Rottbauer W, Dahme T.. Time-to-isolation guided titration of freeze duration in 3rd generation short-tip cryoballoon pulmonary vein isolation: Comparable clinical outcome and shorter procedure duration. Int J Cardiol 2018; 255: 80–84. [DOI] [PubMed] [Google Scholar]
- 67. Chierchia GB, Mugnai G, Stroker E, Velagic V, Hunuk B, Moran D, et al.. Incidence of real-time recordings of pulmonary vein potentials using the third-generation short-tip cryoballoon. Europace 2016; 18: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 68. Aryana A, Kowalski M, O’Neill PG, Koo CH, Lim HW, Khan A, et al.. Catheter ablation using the third-generation cryoballoon provides an enhanced ability to assess time to pulmonary vein isolation facilitating the ablation strategy: Short- and long-term results of a multicenter study. Heart Rhythm 2016; 13: 2306–2313. [DOI] [PubMed] [Google Scholar]