Abstract
Objective
Osteoporosis is a common disease in postmenopausal women. Several studies have analysed the associations between dietary supplementation with probiotics and bone health in postmenopausal women, but the results are still controversial. We conducted this meta-analysis to assess the effects of probiotics supplement on bone mineral density (BMD) and bone turnover markers for postmenopausal women.
Design
Systematic review and meta-analysis.
Methods
We systematically searched PubMed, EMBASE and the Cochrane Library from their inception to November 2020 for randomised controlled trials (RCTs) assessing probiotic supplements and osteoporosis in postmenopausal women. Study-specific risk estimates were combined using random-effect models.
Results
Five RCTs (n=497) were included. Probiotic supplements were associated with a significantly higher BMD in the lumbar spine (standardised mean difference, SMD=0.27, 95% CI 0.09 to 0.44) than in control. There was no difference between probiotic supplements and BMD in hips (SMD=0.22, 95% CI −0.07 to 0.52). Collagen type 1 cross-linked C-telopeptide levels in the treatment groups were significantly lower than those of the placebo group (SMD=−0.34, 95% CI −0.60 to −0.09). In subgroup meta-analysis, levels of bone-specific alkaline phosphatase, osteoprotegerin, osteocalcin and tumour necrosis factor did not differ between the probiotic and placebo groups.
Conclusions
We conclude cautiously that supplementation with probiotics could increase lumbar BMD. More RCTs are recommended to validate or update these results.
Keywords: bone diseases, hip, spine, nutrition & dietetics
Strengths and limitations of this study.
This is the first meta-analysis on the effectiveness of probiotic supplements on bone status in postmenopausal women.
We included only high-quality randomised controlled trials to improve the level of evidence.
The limited number of reports prevented us from conducting subgroup analysis and made it difficult to draw firm conclusions.
Introduction
Osteoporosis is characterised by low bone mineral density (BMD) and deteriorated bone microstructure, leading to reduced bone strength and increased susceptibility to fractures.1 Osteoporosis and fracture occur commonly in postmenopausal women, who experience a natural decline in endogenous oestrogen, reducing BMD (on average 2%–5% BMD/year)2 and adverse effects on bone microarchitecture.
Currently, many medications are used in osteoporosis to decrease bone resorption or increase bone formation. Large randomised controlled trials (RCTs) showed that oestrogen therapy (such as red clover isoflavone supplementation) was effective for preventing and treating osteoporosis in postmenopausal women.3–5 However, this remains controversial because of the increased risk of cancer, including endometrial, breast and ovarian cancer.6 Nevertheless, other antiresorptive agents are not widely used because of their side effects, high prices and poor compliance on the part of patients; these include bisphosphonates, calcitonin and raloxifene. Therefore, complementary and dietary therapies are more acceptable to some patients. Also, natural treatments are increasingly requested by patients.7 It was shown that calcium and vitamin D supplements effectively improved bone microarchitecture and health8; however, supplementation with calcium and vitamin alone is not sufficient to halt menopausal bone loss.9
Therefore, alternative ways to prevent and treat osteoporosis are sought. Probiotics are popular dietary therapies that have favourable effects on the skeletal system.10 Probiotics are ‘live microorganisms that when administered in adequate amounts will confer a health benefit on the host’ defined by the Food and Agricultural Organisation/WHO,11 such as bacillus subtilis, lactobacillus and other mixed strains. They are affordable and have fewer side effects.
To our knowledge, there has been no systematic review or meta-analysis of RCTs with probiotics in the treatment arms, analysing the effect of probiotics in postmenopausal-related osteoporosis. Therefore, this systematic review and meta-analysis were performed to provide an overview of the effects of dietary probiotic supplements in postmenopausal related bone resorption in women and to inform researchers of new potential sources of bias to be addressed in future clinical trials.
Methods and analysis
Data sources and search strategies
A literature search of relevant studies was performed in PubMed, EMBASE and the Cochrane Library. A comprehensive search strategy was developed. The protocol was drafted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.12 The keywords were as follows: ‘probiotics’, ‘probiotic supplement’, ‘bone,’ ‘osteoporosis’, ‘osteopenia’, BMD’, ‘bone turnover’ and ‘postmenopausal’ (search queries available in online supplemental table 1). References of retrieved articles were also scanned to identify any additional relevant studies. Two independent reviewers (JY and GC) conducted this work. Discrepancies were resolved by consensus of the two reviewers. If required, the final disposition was determined by MC.
bmjopen-2020-041393supp001.pdf (34.5KB, pdf)
Inclusion and exclusion criteria
Inclusion criteria are as follows: (1) RCTs and prospective cohort studies; (2) consideration of postmenopausal women as patients, consideration of probiotic supplement as interventions, consideration of placebo as a comparison and consideration of the change of BMD and bone turnover markers (BTM) as outcomes; (3) BMD was measured by dual-energy X-ray absorptiometry (DXA) and BTM was measured using blood tests at baseline, and the end of trial; (4) administered probiotics for more than 6 months and (5) English language original articles indexed up to November 2020.
Exclusion criteria are as follows: (1) absence of critical data for meta-analysis and (2) low-quality articles according to Cochrane checklist.
Data extraction and quality assessment
The characteristics of the relevant articles were extracted and recorded independently by two reviewers (JY and GC) as follows: first author’s name, year, area, age (mean or range), type of probiotic supplement, dose design, course of treatment, number of cases, number of controls and bone status (as shown in table 1). The Cochrane Collaboration’s tool13 was used for assessing the risk of bias. Six domain-based evaluations (selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias) were used in the tool to assess the possible bias of RCTs. The results were displayed as low risk, unclear risk or high risk of bias.
Table 1.
Characteristics of included randomised controlled trials in the meta-analysis
| Study | Year | Area | Age | Blinding | Type of probiotic supplement | No of treatment | No of placebo | Course of treatment | BMD | BTM |
| Jansson | 2019 | Sweden | T: 59.1 P: 58.1 |
Double blind | Three Lactobacillus strains* | 126 | 123 | 12 months | lumbar spine hip | N/A |
| Takimoto | 2018 | Japan | T: 57.5 P: 57.8 |
Double blind | Bacillus subtilis C-3102 | 31 | 30 | 6 months | lumbar spine hip | CTX |
| Nilsson | 2018 | Sweden | T: 76.4 P: 76.3 |
Double blind | Lactobacillus reuteri 6475 | 32 | 36 | 12 months | Lumbar spine hip | CTX BALP TNF |
| Jafarnejad | 2017 | Iran | T: 58.9 P: 57.3 |
Double blind | Seven probiotic b acteria species† | 20 | 21 | 6 months | Lumbar spine hip | CTX BALP OPG OC TNF |
| Lambert | 2017 | Denmark | T: 60.8 P: 62.9 |
Double blind | Lactic acid bacteria and soflavones | 38 | 40 | 12 months | Lumbar spine hip | CTX OPG OC |
*Lactobacillus paracasei DSM 13434, L. plantarum DSM 15312and L. plantarum DSM 15313.
†L. casei, Bifidobacterium longum, L. acidophilus, L. rhamnosus, L. bulgaricus, Bifidobacterium breve and Streptococcus thermophilus.
BALP, bone-specific alkaline phosphatase; BMD, bone mineral density; BTM, bone turnover marker; CTX, collagen type 1 cross-linked C-telopeptide; N/A, not available; OC, osteocalcin; OPG, osteoprotegerin; TNF, tumour necrosis factor.
Statistical analysis
The mean relative change from baseline to the end of the course and SD were used to express the effect of the probiotic supplement on bone status in postmenopausal women. If the original studies did not provide the mean relative change and SD, we converted the data using a common method.14 15 The pooled effects of included studies were expressed in terms of standardised mean difference (SMD) with 95% CI. Q test and I2 index were used to evaluate heterogeneity among the included results. Meta-regression was conducted to determine whether different types of probiotic supplements would introduce sources of heterogeneity. Random-effects model and subgroup analysis were used in the face of heterogeneity. Forest plots and funnel plots were produced, and publication bias was tested using Begg’s test and the weighted Egger test.16 17 Sensitivity analysis was conducted to verify the impact of each study on the pooled results. In the sensitivity analyses, each study was omitted to recalculate the pooled estimates. All analyses were performed using STATA V.12.0 (StataCorp).
Patient and public involvement
Patient and public involvement is not applicable for this meta-analysis.
Results
Search results and characteristics of identified studies
A total of 604 articles were identified from the initial searches of PubMed and EMBASE, and 547 articles were removed because of absence of relevance. Nine articles were retained after reviewing the abstract according to the exclusion criteria. Finally, five RCTs18–22 satisfied the inclusion criteria and entered this meta-analysis after full-text review. All the five RCTs had low risk of bias (available in online supplemental table 2).
bmjopen-2020-041393supp002.pdf (38.7KB, pdf)
A detailed overview of the selection process is outlined in figure 1.
Figure 1.
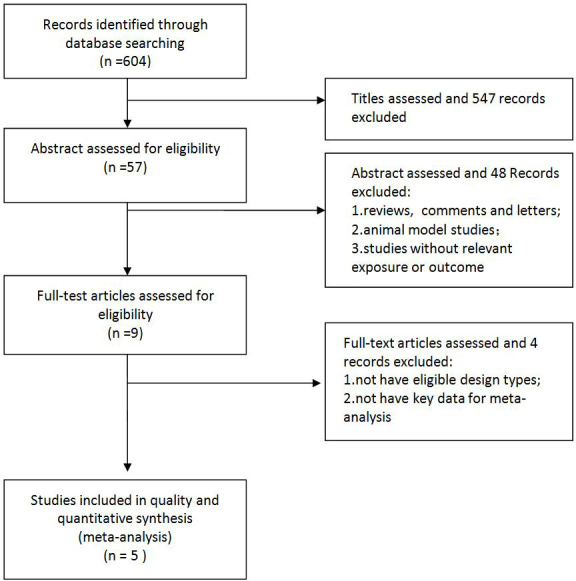
Flow diagram of the studies search process.
A total of 497 postmenopausal women completed these trials. Among the five trials, two were conducted in Asia (one in Japan,18 the other in Iran20 and the other three were in Europe (two in Sweden,19 22 the last one in Denmark).21 All trials were randomised using the double-blinded method. Each trial identified the type of probiotic supplements used and described the dosage design. Three studies considered treatment with probiotics only,18–20 while the other two studies included treatment with combined isoflavone and probiotics.21 22 All studies provided BMD data from DXA scans at the lumbar spine and total hip. Collagen type 1 cross-linked C-telopeptide (CTX), bone-specific alkaline phosphatase (BALP), osteoprotegerin (OPG), osteocalcin (OC) and tumour necrosis factor (TNF) were used as BTMs. Details of the characteristics are displayed in table 1 and online supplemental table 3.
bmjopen-2020-041393supp003.pdf (73.4KB, pdf)
Probiotic supplements and lumbar spine BMD
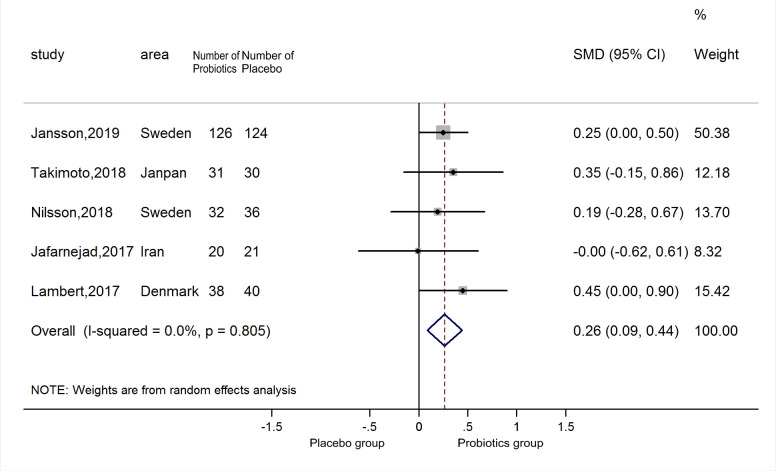
A total of five estimates were included in the meta-analysis. The meta-regression results also showed no source of heterogeneity from various types of probiotics (p=0.987). Therefore, the five estimates were incorporated into the pooled analysis. Compared with the placebo group, the lumbar spine BMD level of the supplementary group was higher (SMD=0.26, 95% CI 0.09 to 0.44), with no heterogeneity (p=0.805; I2=0.0) (figure 2). The funnel plot was symmetrical (online supplemental figure 1) and excluded publication bias (Begg’s test zc=0.73, p=0.462; Egger’s test t=−0.22, p=0.843). Sensitivity analyses indicated that the positive result was robust (online supplemental figure 2).
Figure 2.
Forest plots of meta-analysis on probiotics supplements and lumbar spine BMD. BMD, bone mineral density.
bmjopen-2020-041393supp004.pdf (25.8KB, pdf)
bmjopen-2020-041393supp005.pdf (35.6KB, pdf)
Probiotics supplements and total hip BMD
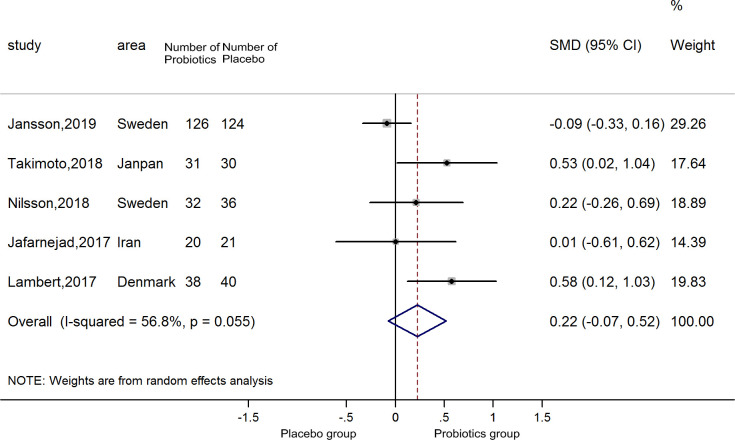
Overall, five estimates of the association between probiotics supplement and hip BMD were included in the meta-analysis. The meta-regression results revealed that various types of probiotics were not a source of heterogeneity (p=0.237). Therefore, we brought the five estimates into the pooled analysis. There was no difference between probiotic supplements and BMD in hips (SMD=0.22, 95% CI −0.07–0.52), with no heterogeneity (p=0.055; I2=56.8) (figure 3). The funnel plot is shown in online supplemental figure 3; it was symmetrical, excluding publication bias (Begg’s test zc=−0.24, p=1.00; Egger’s test t=1.59, p=0.209). Sensitivity analyses indicated that the positive result was affected by the Jansson trial (online supplemental figure 4).
Figure 3.
Forest plots of meta-analysis on probiotics supplements and hip BMD. BMD, bone mineral density.
bmjopen-2020-041393supp006.pdf (26.3KB, pdf)
bmjopen-2020-041393supp007.pdf (26.3KB, pdf)
Probiotic supplements and BTM
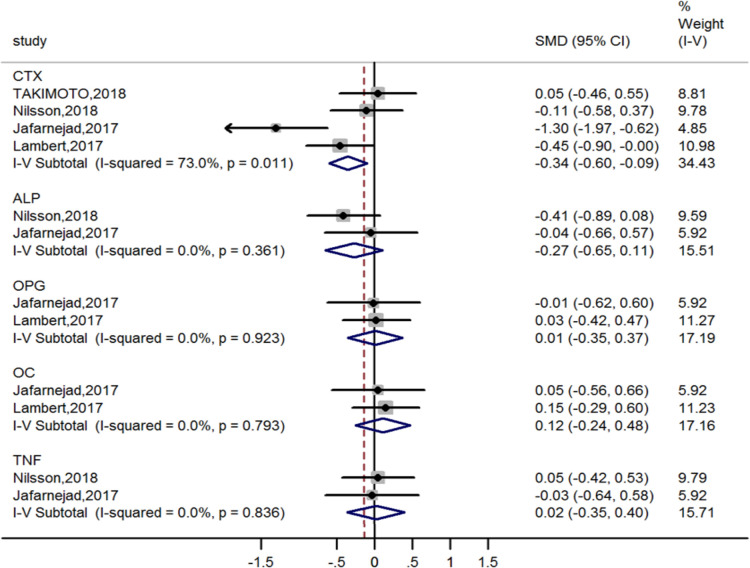
Four estimates of CTX and two estimates of BALP, OPG, OC and TNF were incorporated into the pooled analysis. The results suggested that probiotic supplements help decrease the supplementary group’s body CTX level compared with the placebo group (SMD=−0.34, 95% CI −0.60 to −0.09) with substantial heterogeneity. There was no evidence that probiotic supplements were associated with BALP, OPG, OC and TNF (figure 4).
Figure 4.
Forest plots of meta-analysis on probiotics supplements and bone turnover markers. ALP, alkaline phosphatase; CTX; cross-linked C-telopeptide; OC, osteocalcin; OPG, osteoprotegerin; SMD, standardised mean difference; TNF, tumour necrosis factor.
Discussion
Main findings
This meta-analysis included five RCTs with low risk of bias and 497 postmenopausal women. The results provides evidence that dietary probiotics supplement can slow bone resorption in postmenopausal women. Daily supplementation with probiotics for 24 weeks to 12 months significantly decreased BTM CTX (compared with placebo) in postmenopausal women. BMD loss at the lumbar spine was significantly lower in the treatment group.
Bone loss occurs throughout life following maturation and is accelerated following menopause in women.23 Postmenopausal women have an increased risk of fragility fractures. Using a naturally-occurring bacterium to significantly reduce the annual bone loss in this group of patients is a new concept that could lead to a paradigm shift in osteoporosis prevention. Previous studies in animals demonstrated that supplementation with specific bacterial strains increases bone density and protect against osteoporosis.24–26 Kim et al27 reported that the administration of Lactobacillus casei 393 significantly increased BMD in ovariectomised rats. For the first time, the present meta-analysis systemically demonstrated that this probiotic also works in humans.
The lumbar spine and hip are the most suitable organs to assess bone metabolism. The vertebrae and metaphyses of long bones, rich in trabecular bone, have a higher turnover rate than cortical bones in the axis of long bones. Therefore, medications and diseases affecting the lumbar spine and hip are identified earlier than in other skeletal segments.28 The vertebrae and hips are easily accessible for measuring BMD. Therefore, the lumbar spine and hip BMD were suitable primary outcome variables in the present studies. McCabe et al29 showed that oral administration of L. probiotics identified a 45% increase in hip and vertebral trabecular bone volume fraction in male mice. In another study, the administration of L. plantarum and L. paracasei to ovariectomised mice showed increased trabecular number compared with sham-ovariectomised control groups.30 Our meta-analysis showed, in the probiotics group, both total hip and lumbar vertebrae BMD were at significantly higher levels than those of the control.
CTX and BALP were chosen as critical BTMs. Because BMD depends on the dynamic balance of bone formation and resorption, bone turnover markers are also important parameters analysed in our meta-analysis. The measurement of CTX has been taken as a marker of bone resorption; osteoclasts produce it during bone resorption.31 Therefore, the increased levels of serum CTX indicated increased bone resorption. Subgroup included three RCT studies, suggesting that probiotic supplements’ ingestion significantly reduced the bone resorption marker CTX. Another study from Japan18 showed that the probiotics group had significantly lower uNTx (urinary type I collagen cross-linked N-telopeptide) levels than the placebo group at 12 weeks of treatment. uNTx is another fragment of type I collagen generated during resorption detected in urine; therefore, this also suggested that probiotics inhibit bone resorption by suppressing osteoclast activity. BALP is another well-known BTM, an indicator of osteoblast proliferation that is thought to be a bone formation marker.32 However, the present meta-analysis showed no significant changes in BALP. Similarly, no differences were detected in levels of biochemical markers for bone metabolic indices (OPG, OC).
The mechanism of action
The mechanisms of action of probiotics are as follows. Probiotics have many functional properties in humans. They function in the gastrointestinal system by modifying the microbiota composition, intestinal barrier function and the immune system, which feeds back systemic benefits to the host, including bone health. Moreover, probiotic function modifying physiological homeostasis of the intestinal flora can also benefit bone metabolism.33 Gastrointestinal inflammation and systemic inflammation are close to enhanced generation of potent osteoclastogenic cytokines as the leading cause of bone loss.34 35 Probiotics can restore the balance of the gut microbiota, preventing or moderating gut and systemic inflammation and allowing absorption of nutrients, especially in older adults.36
Furthermore, probiotics decrease levels of inflammatory mediators and cytokines in the gut and bone marrow.37 These changes give bone cell signals, including osteoblasts, osteoclasts and stem cells, significantly affecting bone homoeostasis. Endocrine factors (such as serotonin and incretins) secreted by the intestine also remarkably affect bone cells.33 Anti-inflammatory effects are among the underlying mechanisms by which probiotics benefit bone metabolism. There is evidence that arginine deiminase, produced by the probiotic L. brevis CD2, has an anti-inflammatory effect.38 Supplementation of probiotics may reduce the expression of proinflammatory and osteolytic cytokines, including TNF-α. These cytokines alter antiosteoclastogenic cytokine expression, leading to enhanced osteoclast formation and inhibited osteoblast activity.39 Some studies found that probiotic supplementation reduces TNFα, IL - 17(interferon 17) and RANKL (Receptor activator of nuclear factor-kappa ligand) expression levels in ovariectomised mice.40 These changes give bone cell signals, such as osteoblasts, osteoclasts and stem cells, significantly affecting bone homeostasis. More clinical trials are needed in the future to elucidate the relationship between the administration of probiotics and anti-inflammatory effects.
Limitations and strengths
Our study has some limitations. First, only five RCTs with specific population groups satisfied our inclusion criteria. The limited number of reports and specific population groups focusing on the association between the probiotic supplement and BMD and BTMs prevented us from conducting subgroup analysis and drawing conclusive summaries. Furthermore, the insufficient number of estimates inflates the impact of the results of a particular study and the conclusions may change on the publication of future studies. Second, although meta-regression was used to determine that various types of probiotic supplements did not impact the pooled results, dosage design and course of treatment could also introduce bias. Third, in Lambert et al’s study,21 probiotics plus isoflavones were used as a treatment regimen, rather than probiotics alone. This may cause some bias; however, we did not want to ignore this valuable study. Third, the units describing BMD change were inconsistent among the five reports. Nilsson et al’s study19 and Jansson et al’s study22 applied T score to describe BMD change, while the other three studies used g/cm2 instead. We could only calculate SMD rather than the weighted mean difference. Fifth, unfortunately, we did not find a relevant prospective cohort for this meta-analysis. Thus, our results of a meta-analysis should be interpreted with caution.
Our research also has some strengths. First, to our knowledge, this is the first meta-analysis describing the evidence of the association of probiotic supplements and bone status in postmenopausal women. Second, there is little heterogeneity between the included articles and the fixed-effects model used to calculate the results. Third, all included RCTs were of high quality.
Implications and future research
This systematic review and meta-analysis are useful for multidisciplinary clinicians to evaluate their practices and make a proper clinical decision. The beneficial effects of probiotic supplements may infect probiotic indication in postmenopausal women with osteoporosis. More RCT studies from different regions are needed to validate our argument and help answer research questions about probiotic supplements, dose and the optimal duration.
Conclusion
Our systematic review and meta-analysis showed that probiotic supplementations in postmenopausal women were associated with preserving lumbar spine BMD. The results should be interpreted with caution and more high-quality RCTs are needed to validate or update these results. An appropriate supplement of probiotics could be recommended to improve bone status in postmenopausal women.
Supplementary Material
Acknowledgments
The authors thank the researchers of the included articles for information relevant to the analysis. We appreciate the help from Robert P Lindeman for revising the language in this manuscript.
Footnotes
JY and GC contributed equally.
Contributors: MC and JY conceived and designed the meta-analysis; GC, SY and CL searched the literature; JY, GC and SY analysed the data; JY contributed analysis tools; JY and GC wrote the paper; JY and MC revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplemental information. No additional data available.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. The Lancet 2002;359:1929–36. 10.1016/S0140-6736(02)08761-5 [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008;93:861–8. 10.1210/jc.2007-1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Liu X. [Effects on blood fat and bone density of postmenopausal women fed by soy protein with isoflavone]. Zhonghua Yi Xue Za Zhi 2014;94:215–7. [PubMed] [Google Scholar]
- 4.Clifton-Bligh PB, Nery M-L, Clifton-Bligh RJ, et al. Red clover isoflavones enriched with formononetin lower serum LDL cholesterol-a randomized, double-blind, placebo-controlled study. Eur J Clin Nutr 2015;69:134–42. 10.1038/ejcn.2014.207 [DOI] [PubMed] [Google Scholar]
- 5.Thorup AC, Lambert MN, Kahr HS, et al. Intake of novel red clover supplementation for 12 weeks improves bone status in healthy menopausal women. Evid Based Complement Alternat Med 2015;2015:1–11. 10.1155/2015/689138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer 2017;84:60–8. 10.1016/j.ejca.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 7.De Franciscis P, Colacurci N, Riemma G, et al. A nutraceutical approach to menopausal complaints. Medicina 2019;55:544. 10.3390/medicina55090544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonen S, Vanderschueren D, Haentjens P, et al. Calcium and vitamin D in the prevention and treatment of osteoporosis - a clinical update. J Intern Med 2006;259:539–52. 10.1111/j.1365-2796.2006.01655.x [DOI] [PubMed] [Google Scholar]
- 9.Gonnelli S, Cepollaro C, Pondrelli C, et al. Bone turnover and the response to alendronate treatment in postmenopausal osteoporosis. Calcif Tissue Int 1999;65:359–64. 10.1007/s002239900713 [DOI] [PubMed] [Google Scholar]
- 10.Zaiss MM, Jones RM, Schett G, et al. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest 2019;129:3018–28. 10.1172/JCI128521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food And Agriculture Organization Organization . Guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO Working Group. Ontario, Canada: Food and Agriculture Organization of the United Nations and World Health Organization, 2002. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green S, Editors Higgins JPT. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration, 2011. [Google Scholar]
- 14.Pagano MGK. Principles of biostatistics. Second Edition. Massachusetts: Duxbury, 2000. [Google Scholar]
- 15.Kaiser L. Adjusting for baseline: change or percentage change? Stat Med 1989;8:1183–90. 10.1002/sim.4780081002 [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a RANK correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998;316:61–6. 10.1136/bmj.316.7124.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takimoto T, Hatanaka M, Hoshino T, et al. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci Microbiota Food Health 2018;37:87–96. 10.12938/bmfh.18-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson AG, Sundh D, Bäckhed F, et al. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med 2018;284:307–17. 10.1111/joim.12805 [DOI] [PubMed] [Google Scholar]
- 20.Jafarnejad S, Djafarian K, Fazeli MR, et al. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr 2017;36:497–506. 10.1080/07315724.2017.1318724 [DOI] [PubMed] [Google Scholar]
- 21.Lambert MNT, Thybo CB, Lykkeboe S, et al. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr 2017;106:ajcn153353–20. 10.3945/ajcn.117.153353 [DOI] [PubMed] [Google Scholar]
- 22.Jansson P-A, Curiac D, Lazou Ahrén I, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol 2019;1:e154–62. 10.1016/S2665-9913(19)30068-2 [DOI] [PubMed] [Google Scholar]
- 23.Bliuc D, Nguyen ND, Alarkawi D, et al. Accelerated bone loss and increased post-fracture mortality in elderly women and men. Osteoporos Int 2015;26:1331–9. 10.1007/s00198-014-3014-9 [DOI] [PubMed] [Google Scholar]
- 24.Mutuş R, Kocabagli N, Alp M, et al. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci 2006;85:1621–5. 10.1093/ps/85.9.1621 [DOI] [PubMed] [Google Scholar]
- 25.Britton RA, Irwin R, Quach D, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol 2014;229:1822–30. 10.1002/jcp.24636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues FC, Castro ASB, Rodrigues VC, et al. Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food 2012;15:664–70. 10.1089/jmf.2011.0296 [DOI] [PubMed] [Google Scholar]
- 27.Kim JG, Lee E, Kim SH, et al. Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. Int Dairy J 2009;19:690–5. 10.1016/j.idairyj.2009.06.009 [DOI] [Google Scholar]
- 28.Seeman E. Chapter 1 - modeling and remodeling: the cellular machinery responsible for the gain and loss of bone’s material and structural strength. Cambridge, Massachusetts: Academic Press, 2008: 1–28. [Google Scholar]
- 29.McCabe LR, Irwin R, Schaefer L, et al. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol 2013;228:1793–8. 10.1002/jcp.24340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parvaneh K, Ebrahimi M, Sabran MR, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate SPARC and BMP-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015;2015:1–10. 10.1155/2015/897639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szulc P, Delmas PD. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int 2008;19:1683–704. 10.1007/s00198-008-0660-9 [DOI] [PubMed] [Google Scholar]
- 32.Scholz-Ahrens KE, Adolphi B, Rochat F, et al. Effects of probiotics, prebiotics, and synbiotics on mineral metabolism in ovariectomized rats — impact of bacterial mass, intestinal absorptive area and reduction of bone turn-over. NFS Journal 2016;3:41–50. 10.1016/j.nfs.2016.03.001 [DOI] [Google Scholar]
- 33.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–9. 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 34.Ali T, Lam D, Bronze MS, et al. Osteoporosis in inflammatory bowel disease. Am J Med 2009;122:599–604. 10.1016/j.amjmed.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciucci T, Ibáñez L, Boucoiran A, et al. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 2015;64:1072–81. 10.1136/gutjnl-2014-306947 [DOI] [PubMed] [Google Scholar]
- 36.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 2013;6:39–51. 10.1177/1756283X12459294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin YP, Thibodeaux CH, Peña JA, et al. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis 2008;14:1068–83. 10.1002/ibd.20448 [DOI] [PubMed] [Google Scholar]
- 38.Maekawa T, Hajishengallis G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J Periodontal Res 2014;49:785–91. 10.1111/jre.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin R, Lee T, Young VB, et al. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis 2013;19:1586–97. 10.1097/MIB.0b013e318289e17b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J-Y, Chassaing B, Tyagi AM, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 2016;126:2049–63. 10.1172/JCI86062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041393supp001.pdf (34.5KB, pdf)
bmjopen-2020-041393supp002.pdf (38.7KB, pdf)
bmjopen-2020-041393supp003.pdf (73.4KB, pdf)
bmjopen-2020-041393supp004.pdf (25.8KB, pdf)
bmjopen-2020-041393supp005.pdf (35.6KB, pdf)
bmjopen-2020-041393supp006.pdf (26.3KB, pdf)
bmjopen-2020-041393supp007.pdf (26.3KB, pdf)