Abstract
Objectives
To investigate whether urban–rural location and socioeconomic factors (income, education and employment) are associated with body mass index (BMI) and waist–hip ratio (W/H-ratio), and to further explore if the associations between urban–rural location and BMI or W/H-ratio could be mediated through variations in socioeconomic factors.
Design
Cross-sectional, WHO STEPS survey of non-communicable disease risk factors.
Setting
Urban and rural areas of Myanmar.
Participants
A total of 8390 men and women aged 25 to 64 years included during the study period from September to December 2014. Institutionalised people (Buddhist monks and nuns, hospitalised patients) and temporary residents were excluded.
Results
The prevalence of overweight and obesity was higher in the urban areas and increased with increasing socioeconomic status (SES) score. Mean BMI was higher among urban residents (ß=2.49 kg/m2; 95% CI 2.28 to 2.70; p<0.001), individuals living above poverty line, that is, ≥US$1.9/day (ß=0.74 kg/m2; 95% CI 0.43 to 1.05; p<0.001), and those with high education attainment (ß=1.48 kg/m2; 95% CI 1.13 to 1.82; p<0.001) when adjusting for potential confounders. Similarly, greater W/H-ratio was observed in participants living in an urban area, among those with earnings above poverty line, and among unemployed individuals. The association between urban–rural location and BMI was found to be partially mediated by a composite SES score (9%), income (17%), education (16%) and employment (16%), while the association between urban–rural location and W/H-ratio was found to be partially mediated by income (12%), education (6%) and employment (6%).
Conclusion
Residents living in urban locations had higher BMI and greater W/H-ratio, partially explained by differences in socioeconomic indicators, indicating that socioeconomic factors should be emphasised in the management of overweight and obesity in the Myanmar population. Furthermore, new national or subnational STEPS surveys should be conducted in Myanmar to observe the disparity in trends of the urban–rural differential.
Keywords: public health, epidemiology, preventive medicine
Strengths and limitations of this study.
The study is novel in reporting associations between income, education, employment with body mass index or waist–hip ratio in Myanmar.
The study analyses a large nationally representative sample including both urban and rural populations.
The internationally recommended WHO STEPS protocol was followed.
The findings may be generalised to Myanmar’s non-institutionalised population only.
Due to cross-sectional nature of the study, causality cannot be inferred.
Introduction
Overweight and obesity pose a major economic burden to society, and are important determinants for non-communicable diseases (NCDs), including cardiovascular diseases (CVDs), diabetes, musculoskeletal disorders and certain types of cancers.1 An even greater risk seems to be associated with excess abdominal obesity.2 3 According to the WHO, 39% of adults worldwide were overweight and 13% were obese in 2016.1 In the South-East Asia Region (SEAR), the estimated proportions of overweight and obese were 21.5% and 4.6%, respectively.4 5
Urbanization, a complex socioeconomic process that gradually transforms the society from rural into urban settlements, including migrations of people from rural to urban areas, is frequently cited as the most important factor contributing to increasing overweight and obesity, explained by increased access to unhealthy foods and a less physically active lifestyle in urban areas.6–9 Moreover, among urban residents the socioeconomic status (SES) is likely to be higher, which in turn is associated with higher body mass index (BMI) in most low- and middle-income countries (LMICs).10 11 However, a recent publication by the NCD Risk Factor Collaboration comprising evidence from 2009 population-based studies on trends in mean BMI (from 1985 to 2017), showed that increasing BMI in rural areas has been the main contributor to the global rise in mean BMI over the last 33 years, while the contribution from rural to urban migration was small.12
In Myanmar, two surveys were carried out in 2004 and 2014 in the most populated and developed part of Myanmar, the Yangon region.13 14 Findings from these studies indicate increasing trends in overweight and obesity. The overall prevalence of combined overweight and obesity in urban areas of Yangon increased from 39.8% in 2004 to 40.9% in 2014, whereas in rural areas, there was an increase in overweight and obesity prevalence from 23.0% to 31.2%.14–16 In 2009, a nationwide survey in Myanmar found an overall prevalence of overweight and obesity of 18.7% and 6.8%, respectively.17 The most recent nationwide study (2015–2016), the Myanmar Demographic and Health Survey,18 included 12 160 women in reproductive age, and reported a high prevalence of overweight (28.1%) and obesity (13.1%), and a significantly higher proportion of overweight and obesity with urban residency, higher economic status and having secondary education.
Myanmar has been lagging behind neighbouring countries in terms of sociodemographic development, which could partly be due to socio-political difficulties during more than 50 years of military rule, which was gradually replaced with a democratic development in 2011.19 In order to contribute to a better understanding of socioeconomic determinants of overweight and obesity in Myanmar, we analysed a nationally representative sample of 25–64 years old men and women from 2014,20 with the following objectives: (1) to investigate associations of urban–rural location with BMI and waist–hip ratio (W/H-ratio); (2) to explore the association of selected socioeconomic characteristics (income, education and employment status) with BMI and W/H-ratio; and (3) to assess whether the potential associations between urban–rural location and BMI or W/H-ratio could be explained by (ie, mediated through) variations in socioeconomic characteristics (income, education, and employment status).
Methods
Study design, sampling and participants
A national cross-sectional survey of NCD risk factors in Myanmar (WHO STEPS survey) was conducted between September and December 2014 in 52 different townships in Myanmar.20 A detailed methodological description of the sampling and data collection has been published previously,20 and is summarised below.
The STEPS survey used a multistage cluster sampling method for the selection of townships, wards and villages, households, and eligible participants at each of the selected households. The first stage of the sampling method consisted of townships, which formed the primary sampling snits (PSUs). Overall, 52 PSUs were selected out of the total of 330 townships, using probability proportionate to size of population in each PSU. In the second stage, six secondary sampling units (SSUs), that is, wards (from urban townships) and villages (from rural townships) were selected from each chosen PSU giving a total of 312 SSUs for the whole country. The list of households with unique identification number developed from a recent listing of households was used as the sampling frame for the third stage. From each selected SSU, 30 households were chosen using a systematic random sampling method. In this sampling method, the elements to be included in the sample are selected based on a systematic rule, using a fixed sampling interval obtained by dividing population size by required sample size.21 In the fourth stage, recruitment of one eligible participant aged between 25 and 64 years was done from the selected household. The Kish sampling method was used to rank the eligible participants in each household in order of decreasing age, starting with men then women, and randomly selected using the automated programme for Kish selection in the handheld PDA (personal digital assistant).20
The study population comprises 25–64 years old men and women residing in both urban and rural areas. The following exclusion criteria were used: individuals with a mental or physical illness deemed too ill to participate, institutionalised people (Buddhist monks and nuns, armed forces, hospitalised patients, prisoners) and temporary residents (living in a locality for less than 6 months).
Altogether, a total of 8757 men and women aged 25–64 years, residing in both urban and rural areas, participated in the survey. The response rates were 94% for the questionnaire, 91% for physical measurements and 90% for biochemical measurements. The final sample for the current study included 8390 adults who participated in both STEP 1 and STEP 2, excluding 87 women who were currently pregnant, and 280 individuals with missing BMI.
Data collection and measurements
The STEPS Instrument covers the following three different levels of ‘STEPS’ of risk factor assessment. STEP (1) questionnaire survey; STEP (2) physical measurement; STEP (3) biochemical measurements. In the present study, we included variables from STEP 1 and STEP 2.
Eighteen trained teams (containing six members in each) collected the data. The English WHO STEPS Instrument (core and expanded) questionnaire V.3.0 was translated into the Myanmar version for the survey. A 5-day training was conducted at University of Medicine (2), Yangon. The data collection teams conducted a pilot survey of all steps of data collection in the wards of North Okkalapa Township, Yangon, on the fifth day of the training. Data for STEP 1 and STEP 2 were collected at the survey participant’s household during the first visit. Face-to-face interviews were conducted to collect information on sociodemographic factors and behavioural risk factors.
A Seca 217 portable stadiometer was used to measure body height without footwear and any hat or hair ties. Findings were recorded in centimetre (cm) to the nearest 0.1 cm. Body weight was measured with a pre-calibrated portable Seca Digital Floor Scale with High Capacity (Model 813) to the nearest 0.1 kilogram (kg). During weighing, the participants were requested to wear light clothing without footwear.20 Waist and hip circumference measurement were done in a private area using a Seca 201 measuring tape. The waist circumference was measured in centimetres over light clothing at the midpoint between the last palpable rib and the top of the iliac crest. The hip circumference measurement was taken by placing the tape horizontally at maximum circumference over the buttocks. Measures were taken to the nearest 0.1 cm.20
Variables
BMI and W/H-ratio
BMI is the most widely used measure of general overweight and obesity22 23 whereas W/H-ratio measures abdominal or central obesity and is a better predictor of CVD risk.24 25 Therefore, we have included both measures in our study.
BMI was calculated as weight in kilograms divided by the height in metres squared. Cut-off points for BMI were defined based on WHO recommendations: BMI of 25.0–29.9 kg/m2 was considered overweight whereas having a BMI of 30 kg/m2 or higher was considered obese.26 For comparison, BMI was also classified according to Asian specific cut-off points: BMI of 23.0–27.4 kg/m2 (overweight) and BMI of ≥27.5 kg/m2 (obesity).27 W/H-ratio was defined as the ratio of the circumference of the waist to that of the hip. Central obesity was defined as a W/H-ratio above 0.90 for men and above 0.85 for women.28
Urban–rural location
According to the ward or village tract administration law 2012, a ward is defined as an urban unit and a village is defined as a rural unit.29 Hence, the same definition was used to define urban and rural areas in the current study.
Sociodemographic factors
Age was defined as completed years of age. Education level was defined by both total number of years spent in school and by highest educational level obtained. It was categorised into seven categories: no formal schooling, less than primary school, primary school completed, secondary school completed, high school completed, college/university completed and postgraduate degree. In multivariable analyses, education level was collapsed into three groups: low level, medium level and high level education. Low level was defined as education below primary school completion; Medium level: completion of primary and secondary school; High level: completion of high school, university or postgraduate education.
Occupation was defined according to the main work status over the past 12 months and categorised as government employee, non-government employee, self-employed, non-paid, student, homemaker, retired, unemployed (able to work) and unemployed (unable to work). In multivariable analyses, employment status was collapsed into two groups: employed and unemployed. Employed group included people who were government, non-government employee, self-employed and homemakers. Non-paid, student, retired and unemployed (both able and unable to work) were categorised in the unemployed group.
Daily personal income was calculated from the entire household income divided by the total number of household members excluding the household members under 18 years of age. Income was converted from Myanmar Kyats into US dollars (US$). Exchange rate of US$1 was 970 Myanmar Kyats as of 1 September 2014.30 Cut-off values for poverty line was used as defined by World Bank: US$1.9/day.31
Statistical methods
Statistical analysis was performed in the Statistical Package for Social Sciences (SPSS) V.26.0 (Armonk, New York: IBM Corp). The characteristics of the study participants were presented in the form of frequency (N) and percentages (%) for categorical variables, and mean with SD for continuous variables. Differences in categorical variables were tested using the χ2 test or Fisher’s exact test, whereas differences in the mean for continuous variables were tested using two-tailed t-tests. Linear regression was used to estimate the association between the urban–rural location and socioeconomic factors variables (income, education and employment status) with continuous outcomes (BMI and W/H-ratio), obtaining betas (β) with 95% CIs. Potential multicollinearity between variables was assessed with variance inflation factors (VIF). A VIF value greater than 10 was considered an indication of multicollinearity; however, no significant multicollinearity was observed. We tested for heteroscedasticity by using robust estimator and there were only minor changes in the estimates, which indicates there was no problem of heteroscedasticity. For all statistical analysis, the two-tailed significance level was set to 0.05.
Based on previous literature and construction of directed acyclic graphs (DAGs),32 we identified potential confounders and mediators (see online supplemental figure 1). For objective 1, for associations of urban–rural location with BMI and W/H-ratio, age and gender were identified as confounders (see online supplemental figure 1A), and they were therefore included in multivariable models to obtain the total effect of urban–rural location on BMI and W/H-ratio.
bmjopen-2020-042561supp002.pdf (99.5KB, pdf)
For the second objective, for the association of socioeconomic characteristics (income, education and employment status) with BMI and W/H-ratio, we constructed three different DAGs (see online supplemental figure 1B–D) for confounder adjustments.
To study the statistical effect of the SES variables (income, education and employment status) together, the variables were assigned SES values (0/1) and a composite SES score was calculated. For this, education level was collapsed into two groups: high education (defined as high school completion and above) and low education (defined as education below high school completion). Participants with earnings above poverty line, high education attainment (binary) and employment were assigned SES value=1 and the lower category was assigned SES value=0. Total SES score for each participant was obtained by summing up values and total SES score was further categorised into three SES groups: low (total SES score=0), medium (total SES score=1 and 2) and high (total SES score=3) (see online supplemental table 1). We assessed the association between SES groups and BMI or W/H-ratio with adjustment for confounders (age, gender and urban–rural location).
bmjopen-2020-042561supp001.pdf (27.7KB, pdf)
The third objective exploited whether the potential association between urban–rural location and BMI or W/H-ratio was mediated through socioeconomic characteristics. We included the potential mediators income, education, employment and the composite SES score variable one by one in order to obtain the direct effect of urban–rural location on BMI and W/H-ratio, and the proportion mediated through each of the socioeconomic factors.
Patient and public involvement
No patients or public were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study.
Results
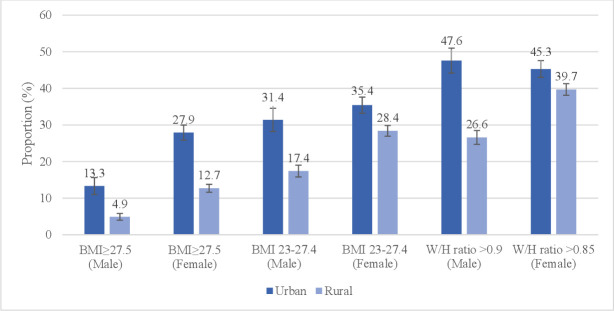
The mean age of the study participants was 44.9 years, with rural participants being slightly younger than urban participants (table 1). Nearly three-quarters of the participants (68.6%), were from rural areas (not shown in tables). The mean length of education was 7.7 years in urban areas and 4.8 years in rural areas. In urban areas, the majority had primary education only (31.9%), 6.6% had no formal schooling and 42.7% were self-employed (table 1). In the rural areas, 39.6% had primary education only, 18.8% had no formal schooling and 68.6% were self-employed. The proportion living on <US$1.9/day was 4.9% in urban areas and 17.5% in rural areas. The prevalence of overweight (WHO standard cut-off: BMI 25–29.9) and obesity (WHO standard cut-off: BMI ≥30) was 20.4% and 6.5%, respectively (not shown in tables). The prevalence of overweight (Asian cut-off: BMI 23–27.4 kg/m²) was higher in women in urban areas (35.4%) in comparison to women in rural areas (28.4%) (figure 1). Similarly, the prevalence of obesity (Asian cut-off: BMI ≥27.5 kg/m²) was higher in urban women (27.9%), compared with rural women (12.7%) (figure 1).
Table 1.
Characteristics of 25–64 years old residents in Myanmar, by gender and urban–rural location
| Variables | Total (n=8390) N (%) |
Urban | Rural | ||||
| Male (n=830) N (%) |
Female (n=1798) N (%) |
Total (n=2628) N (%) |
Male (n=2117) N (%) |
Female (n=3645) N (%) |
Total (n=5762) N (%) |
||
| Age (mean years±SD) | 44.9±10.7 | 47.0±10.8 | 46.0±10.3 | 46.4±10.4 | 44.1±10.9 | 44.4±10.7 | 44.2±10.8 |
| Age group (years) | |||||||
| 25–34 | 1689 (20.1) | 139 (16.7) | 272 (15.1) | 411 (15.6) | 488 (23.1) | 790 (21.7) | 1278 (22.2) |
| 35–44 | 2315 (27.6) | 178 (21.4) | 511 (28.4) | 689 (26.2) | 577 (27.3) | 1049 (28.8) | 1626 (28.2) |
| 45–54 | 2412 (28.7) | 269 (32.4) | 558 (31.0) | 827 (31.5) | 580 (27.4) | 1005 (27.6) | 1585 (27.5) |
| 55–64 | 1974 (23.5) | 244 (29.4) | 457 (25.4) | 701 (26.7) | 472 (22.3) | 801 (22.0) | 1273 (22.1) |
| Education (mean years±SD) | 5.7±4.1 | 8.4±3.9 | 7.5±4.2 | 7.7±4.1 | 5.3±3.4 | 4.5±3.8 | 4.8±3.7 |
| Education level | |||||||
| No formal school | 1256 (15.0) | 51 (6.1) | 122 (6.8) | 173 (6.6) | 370 (17.5) | 713 (19.6) | 1083 (18.8) |
| Less than primary school | 1912 (22.8) | 66 (8.0) | 293 (16.3) | 359 (13.7) | 434 (20.5) | 1119 (30.7) | 1553 (27.0) |
| Primary school completed | 3121 (37.2) | 265 (31.9) | 574 (31.9) | 839 (31.9) | 933 (44.1) | 1349 (37.0) | 2282 (39.6) |
| Secondary school completed | 1044 (12.4) | 214 (25.8) | 349 (19.4) | 563 (21.4) | 239 (11.3) | 242 (6.6) | 481 (8.3) |
| High school completed | 524 (6.2) | 117 (14.1) | 203 (11.3) | 320 (12.2) | 86 (4.1) | 118 (3.2) | 204 (3.5) |
| College/university completed | 499 (5.9) | 110 (13.3) | 241 (13.4) | 351 (13.4) | 53 (2.5) | 95 (2.6) | 148 (2.6) |
| Postgraduate degree | 34 (0.4) | 7 (0.8) | 16 (0.9) | 23 (0.9) | 2 (0.1) | 9 (0.2) | 11 (0.2) |
| Employment status | |||||||
| Government employee | 359 (4.3) | 81 (9.8) | 120 (6.7) | 201 (7.6) | 59 (2.8) | 99 (2.7) | 158 (2.7) |
| Non-government employee | 560 (6.7) | 88 (10.6) | 78 (4.3) | 166 (6.3) | 182 (8.6) | 212 (5.8) | 394 (6.8) |
| Self-employed | 5074 (60.5) | 495 (59.6) | 628 (34.9) | 1123 (42.7) | 1667 (78.7) | 2284 (62.7) | 3951 (68.6) |
| Non-paid | 210 (2.5) | 36 (4.3) | 28 (1.6) | 64 (2.4) | 59 (2.8) | 87 (2.4) | 146 (2.5) |
| Student | 8 (0.1) | 3 (0.4) | 0 (0.0) | 3 (0.1) | 2 (0.1) | 3 (0.1) | 5 (0.1) |
| Homemaker | 1559 (18.6) | 6 (0.7) | 811 (45.1) | 817 (31.1) | 7 (0.3) | 735 (20.2) | 742 (12.9) |
| Retired | 174 (2.1) | 61 (7.3) | 38 (2.1) | 99 (3.8) | 44 (2.1) | 31 (0.9) | 75 (1.3) |
| Unemployed (able to work) | 298 (3.6) | 47 (5.7) | 70 (3.9) | 117 (4.5) | 59 (2.8) | 122 (3.3) | 181 (3.1) |
| Unemployed (unable to work) | 144 (1.7) | 13 (1.6) | 24 (1.3) | 37 (1.4) | 38 (1.8) | 69 (1.9) | 107 (1.9) |
| Refused to answer | 4 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.0) | 0 (0.0) | 3 (0.1) | 3 (0.1) |
| Daily income US$ per day (n=7408) | |||||||
| <1.9 | 992 (13.4) | 38 (5.0) | 79 (4.8) | 117 (4.9) | 316 (17.0) | 559 (17.8) | 875 (17.5) |
| ≥1.9 | 6416 (86.6) | 727 (95.0) | 1559 (95.2) | 2286 (95.1) | 1540 (83.0) | 2590 (82.2) | 4130 (82.5) |
US$, US dollar.
Figure 1.
Proportion of participants with high body mass index (BMI) (above Asian overweight and obesity cut-off) and waist–hip ratio (W/H-ratio), with 95% CI, of 25–64 years old Myanmar residents by urban–rural location and gender.
Objective 1: association between urban–rural location and BMI or W/H-ratio
The mean BMI was higher among urban than rural residents by 2.49 kg/m2 (β=2.49 kg/m2; 95% CI 2.28 to 2.70; p<0.001) when adjusting for age and gender (table 2). Similarly, W/H-ratio was 0.015 greater in participants living in an urban area (β=0.015; 95% CI 0.011 to 0.020; p<0.001) compared with rural, when adjusting for age and gender (table 3).
Table 2.
Level of associations between urban–rural location and socioeconomic factors with BMI (kg/m2) among 25–64 years old Myanmar residents
| Variables | Category | Mean BMI±SD | Crude estimates | Adjusted estimates |
| β (95% CI) | β (95% CI) | |||
| Location | Rural | 21.9±4.1 | Ref. | Ref. |
| Urban | 24.5±5.5 | 2.62 (2.40 to 2.83)† | 2.49 (2.28 to 2.70)† ¶ | |
| Income‡ | <US$1.9/day | 21.5±4.1 | Ref | Ref. |
| ≥US$1.9/day | 22.9±4.8 | 1.44 (1.12 to 1.76)† | 0.74 (0.43 to 1.05)† ** | |
| Education | Low | 21.9±4.2 | Ref. | Ref. |
| Medium | 22.9±4.8 | 0.98 (0.76 to 1.21)† | 0.88 (0.66 to 1.10)† †† | |
| High | 24.2±5.7 | 2.28 (1.95 to 2.61)† | 1.48 (1.13 to 1.82)† †† | |
| Employment§ | Employed | 22.7±4.7 | Ref. | Ref. |
| Unemployed | 22.7±5.1 | 0.04 (−0.30 to 0.38) | −0.06 (−0.39 to 0.26)‡‡ | |
| SES | Low | 21.4±4.0 | Ref. | Ref. |
| Medium | 22.9±4.8 | 1.42 (1.09 to 1.76)† | 0.81 (0.49 to 1.14)† †† | |
| High | 24.1±4.8 | 2.65 (1.54 to 3.77)† | 1.28 (0.21 to 2.36)* †† |
*P<0.05.
†P<0.001.
‡982 out of 8390 participants with missing value for income excluded in crude and adjusted estimates.
§4 participants with missing employment status excluded in crude and adjusted estimates.
¶Adjusted for age and gender.
**Adjusted for age, gender, urban–rural location, education and employment.
††Adjusted for age, gender and urban–rural location.
‡‡Adjusted for age, gender, urban–rural location and education.
BMI, body mass index; Ref, reference category; SES, socioeconomic status; US$, US dollar.
Table 3.
Level of associations between urban–rural location and socioeconomic factors with W/H-ratio among 25–64 years old Myanmar residents
| Variables | Category | Mean W/H-ratio±SD | Crude estimates | Adjusted estimates |
| β (95% CI) | β (95% CI) | |||
| Location | Rural | 0.84±0.09 | Ref. | Ref. |
| Urban | 0.86±0.11 | 0.016 (0.012 to 0.021)‡ | 0.015 (0.011 to 0.020)‡ ** | |
| Income§ | <US$1.9/day | 0.84±0.07 | Ref. | Ref. |
| ≥US$1.9/day | 0.85±0.09 | 0.010 (0.004 to 0.016)* | 0.007 (0.001 to 0.013)* †† | |
| Education | Low | 0.84±0.08 | Ref. | Ref. |
| Medium | 0.85±0.10 | 0.005 (0.00 to 0.009)* | 0.002 (−0.003 to 0.006)‡‡ | |
| High | 0.85±0.11 | 0.006 (−0.001 to 0.013) | 0.002 (−0.006 to 0.009)‡‡ | |
| Employment¶ | Employed | 0.85±0.09 | Ref. | Ref. |
| Unemployed | 0.86±0.10 | 0.018 (0.011 to 0.025)‡ | 0.006 (−0.001 to 0.014)§§ | |
| SES | Low | 0.84±0.07 | Ref. | Ref. |
| Medium | 0.85±0.09 | 0.012 (0.005 to 0.018)† | 0.008 (0.001 to 0.014)‡‡ * | |
| High | 0.88±0.19 | 0.044 (0.022 to 0.066)‡ | 0.019 (−0.002 to 0.040)‡‡ |
Seven participants with missing W/H-ratio excluded in crude and adjusted estimates
*P<0.05.
†P<0.01.
‡P<0.001.
§982 out of 8390 participants with missing value for income excluded in crude and adjusted estimates.
¶4 participants with missing employment status excluded in all models.
**Adjusted for age and gender.
††Adjusted for age, gender, urban–rural location, education and employment.
‡‡Adjusted for age, gender and urban–rural location.
§§Adjusted for age, gender, urban–rural location and education.
Ref, reference category; SES, socioeconomic status; US$, US dollar; W/H-ratio, waist–hip ratio.
Objective 2: association between socioeconomic factors (income, education and employment status) and BMI or W/H-ratio
The socioeconomic factors income and education, but not employment were associated with BMI (table 2). Mean BMI was 0.74 kg/m2 higher among individuals living above compared with those living below poverty line (β=0.74 kg/m2; 95% CI 0.43 to 1.05; p<0.001), when adjusting for age, gender, urban–rural location, education and employment. BMI was 0.88 kg/m2 higher among individuals with medium education and 1.48 kg/m2 higher among individuals with high education (medium education vs low: β=0.88 kg/m2; 95% CI 0.66 to 1.10; p<0.001 and high education vs low: β=1.48 kg/m2; 95% CI 1.13 to 1.82; p<0.001). Moreover, BMI was higher in medium and high SES groups (medium SES vs low: β=0.81 kg/m2; 95% CI 0.49 to 1.14; p<0.001 and high SES vs low: β=1.28 kg/m2; 95% CI 0.21 to 2.36; p<0.05) (table 2).
There was an association between the socioeconomic indicators income and employment but not education, with W/H-ratio (table 3). Among those with earnings ≥US$1.9/day, the W/H-ratio was 0.007 greater (β=0.007; 95% CI 0.001 to 0.013; p<0.05) than those earning <US$1.9/day. Unemployed participants had greater W/H-ratio than employed participants in the crude estimates, but the association was attenuated in the adjusted estimates (table 3). In addition, medium and high SES groups had greater W/H-ratios than the low SES group in the crude estimates (table 3).
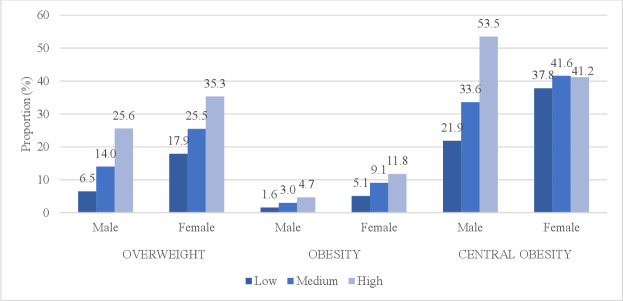
When combining income, education and employment status into a composite SES score, the prevalence of overweight (BMI 25–29.9) and obesity (BMI ≥30) increased with increasing SES score for both genders (figure 2). Similarly, the prevalence of central obesity (W/H-ratio>0.9 for men and W/H-ratio>0.85 for women) increased with increasing SES score in men, whereas in women the prevalence was almost similar in medium and high SES group (figure 2).
Figure 2.
Prevalence of overweight (BMI 25–29.9), obesity (BMI ≥30) and central obesity (W/H-ratio>0.9 for men and W/H-ratio>0.85 for women) across three levels of SES (calculated composite SES score) among 25–64 years old Myanmar residents, by gender. BMI, body mass index; SES, socioeconomic status; W/H-ratio, waist–hip ratio.
Objective 3: association between urban–rural location and BMI or W/H-ratio mediated by socioeconomic factors (income, education, and employment status)
Table 4 shows the adjusted total and direct effects of urban–rural location on BMI and W/H-ratio. There was change in the estimates for BMI after adjusting for income (table 4), which gave an indirect effect of urban–rural location through income: 2.50–2.08=0.42 kg/m2, and a mediated proportion of 17% (0.42/2.50=0.17). Similarly, adjusting for education gave an indirect effect of urban–rural location through education of 0.40 kg/m2, and a mediated proportion of 16%. When adjusting for employment, corresponding figures were 0.40 kg/m2 and mediated proportion of 16%. Adjustment for the composite SES score gave an indirect effect of urban–rural location through composite SES score of 0.22 kg/m2, and a mediated proportion of 9%. There was change in the estimates for W/H-ratio after adjusting for income, which gave an indirect effect of urban–rural location through income of 0.002, and a mediated proportion of 12%. Similarly, adjusting for education gave an indirect effect of urban–rural location through education of 0.001, and a mediated proportion of 6%, and when adjusting for employment: 0.001 (mediated proportion of 6%) (table 4). Furthermore, adjusting for composite SES score gave an indirect effect of urban–rural location through composite SES score of 0.002, and a mediated proportion of 12%.
Table 4.
Total versus direct effect of urban–rural location on BMI and W/H-ratio, among 25–64 years old Myanmar residents
| Location | BMI | W/H-ratio†† | ||||||||
| Total effect† | Direct effect through composite SES score‡ |
Direct effect through income§ | Direct effect through education¶ | Direct effect through employment** | Total effect† | Direct effect through composite SES score‡ |
Direct effect through income§ | Direct effect through education¶ | Direct effect through employment** | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Rural | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Urban | 2.50* | 2.28 (2.05 to 2.51)* | 2.08 (1.85 to 2.32)* | 2.10 (1.86 to 2.33)* | 2.10 (1.86 to 2.34)* | 0.017 (0.012 to 0.021)* | 0.015 (0.010 to 0.019)* | 0.015 (0.010 to 0.020)* | 0.016 (0.011 to 0.021)* | 0.016 (0.011 to 0.021)* |
982 out of 8390 participants with missing value for income and 4 participants with missing employment status excluded in all models for comparison. Exclusion of missing values gives slightly different total estimates from table 1.
*P<0.001.
†Adjusted for age and gender (confounders).
‡Adjusted for age, gender and composite SES score.
§Adjusted for age, gender, education, employment and income.
¶Adjusted for age, gender and education.
**Adjusted for age, gender, education and employment.
††7 participants with missing W/H-ratio excluded
BMI, body mass index; Ref, reference category; SES, socioeconomic status; W/H-ratio, waist-hip ratio.
Discussion
We found the prevalence of overweight and obesity, including central obesity to be higher in urban areas in Myanmar compared with rural areas. There was a consistent positive adjusted association between SES and BMI, while the association between SES and W/H-ratio was less consistent. Out of the socioeconomic factors, education was found to have the strongest association with BMI (general overweight and obesity), whereas income had the strongest association with W/H-ratio (central obesity). The association between urban–rural location and BMI was found to be partially mediated by the SES indicators with income, education and employment status contributing almost equally. In the association between urban–rural location and W/H-ratio, the highest proportion was mediated by income.
A previous study from Myanmar reported an 28% overall prevalence of overweight and 13% prevalence of obesity, which was higher than in the current study.18 The higher prevalence could be due to the inclusion of adult women only and the use of Asian-specific BMI cut-offs. However, our findings of a higher BMI and greater W/H-ratio in urban compared with rural areas corroborates previous studies conducted in Myanmar.14–16 Further, it is also consistent with findings of studies carried out in other countries of the SEAR,33–37 and of a global study conducted in 2010, which reported that the overall prevalence of overweight and obesity was higher in urban areas compared with their rural counterpart.38 In contrast to our findings, a recent study composed of data from 2009 population-based studies showed that BMI is rising at the same proportion or faster in rural areas compared with urban in LMICs except women in sub-Saharan Africa.12 Similar study reported that mean BMI was generally higher in rural compared with urban men in South Asia while BMI increased at a similar rate in rural and urban men in East and Southeast Asia. The study also reported that changes in rural areas are driving the increase in mean BMI globally.12 The authors suggested that improved road infrastructure and transportation has led to an increased access to high calorie foods, mechanised farming equipment, in addition to shifts from manual labour to more sedentary work,12 that is, an urbanisation of the rural areas.
There is a paucity of studies investigating the association of all three socioeconomic factors (income, education and employment status) with BMI and W/H-ratio in both male and female populations in Myanmar, hence the current study is the first to report these novel findings. In our study, higher income and higher education was associated with increased BMI, which is in accordance with a systematic review of studies investigating the association between SES and obesity in LMICs.6 Additionally, a wide scale and much larger study focusing on the association between socioeconomic factors and weight status across 53 countries in 2010 found that the prevalence of obesity was highest in the richest quintile of the participants.38 Another study from 70 low-income, middle-income and high-income countries found a strong, positive association between individual income and obesity.39 Furthermore, our result correspond with evidence from a systematic review of studies40 and a study involving non-pregnant women from 37 developing countries,41 which observed positive associations between education and obesity in low-income countries. Several studies from Bangladesh, Nepal, India and Sri Lanka also support this.34–37 42 Based on the Myanmar Demographic and Health Survey 2015–2016, 28% of Myanmar’s population is living in urban areas.43 However, Myanmar is still considered to be in the early phase of the demographic transition. Much of the current development is happening in the cities,44 which indicates that many of the rural areas in Myanmar are not yet influenced by the ongoing urbanisation of the country. The economic growth in Myanmar, has reduced the proportion of people living below the poverty line (a reduction in poverty from 48% to 25% between 2005 and 2017).45 Because of the continuing economic development of the country, there may be an increase in sedentary lifestyle, higher income and more availability of processed food in urban areas, culminating to an increased burden of overweight and obesity as diet and physical activity are its major risk factors.46 As rice is the main staple food of Myanmar, people generally consume high amounts of carbohydrates, which in turn is associated with high BMI.47 48 In urban Myanmar residents, high intakes of fat and protein have been reported.49 Moreover, the consumption of fast food and high caloric soft drinks and alcohol is higher in urban inhabitants compared with rural dwellers.48 Additionally, in a study conducted in the Yangon region of Myanmar, the prevalence of physical inactivity was low, and no difference was observed between urban and rural residents.14 However, most of the physical activity was linked to work, and high energy expenditure in the workplace was higher among rural than among urban residents.14 There may also be cultural determinants of BMI, as a larger body size often symbolises high status and good health in Myanmar, which means that people with a high SES may even prefer a larger body size.48 In LMICs, in general, high SES individuals have been found to have a higher energy intake, reflecting a greater access to both inexpensive energy dense foods and expensive higher-quality food items.50 Rural populations in high-income countries have excess BMI compared with urban populations.12 51–54 As compared with urban populations in high-income countries, rural populations often have lower income and education, limited access to healthy and fresh food choices and they have less sports facilities and recreational activities, possibly explaining the higher rural BMI.55 56 In high-income countries, the obesity risk is often higher for individuals in low SES groups compared with high SES groups,57–60 as those in the high SES groups are more likely to consume healthy foods, such as whole grains, lean meats, fish, low-fat dairy products and fruit and vegetables.61 62 They also more often have several physical activity opportunities and more knowledge about healthy choices.63
We found that the association between urban–rural location and BMI was only partially mediated by socioeconomic factors such as income, education and employment status. Our finding is in line with a study conducted in women in reproductive age in 38 LMICs, reporting that much, but not all of the urban–rural differences in BMI is driven by the socioeconomic composition (measured by household wealth).10 This indicates that other important factors could explain the urban–rural BMI difference in Myanmar, including differences in non-leisure physical activity opportunities, less energy intensive occupation in urban areas, differences in neighbourhood environment, better transportation facilities and better access to high ultra-processed and packaged food in urban areas.64–66 Future prospective studies may be able to provide information that can explain this association. Road infrastructure and transportation facility is not well developed in the rural part of Myanmar.67 As many as 40% of villages are without road access and additional 30% villages have access only part of the season, requiring the rural population to travel long distances to access markets and basic services leading to high energy expenditure.67
Strengths and limitations
The findings of this study add to the current research related to large population analyses of overweight and obesity. One of the major strengths of this study is the analyses of a large nationally representative sample of 8390 participants that constitutes both urban and rural populations in Myanmar. In addition, the internationally recommended WHO STEPS protocol was diligently followed, and the outcome measures were assessed using standardised procedures. The response rate was high, at 91%. Moreover, we used W/H-ratio as the measure of central obesity in addition to BMI. There are, however, some limitations. The study was cross-sectional which means that causality cannot be determined, for example, the temporal relationships between socioeconomic factors and obesity cannot be inferred; evidence suggests the association is likely to be bidirectional.68 69 Furthermore, 982 participants refused to provide information on their income were excluded. These have a higher likelihood of belonging to lower-income groups as most of them were unemployed, which may have given an underestimation of socioeconomic differences in BMI. At the analysis stage, 280 participants were excluded from the study due to missing BMI values. As association measures are robust, it is unlikely that this exclusion has substantially contributed to selection bias.70 Institutionalised people like monks, nuns and soldiers were excluded from the sampling frame as their lifestyle may differ from most of the general population, which means that the findings can only be generalised to Myanmar’s non-institutionalised population.
Conclusion
The current study examines the relation between urban–rural location and socioeconomic factors with general and central overweight and obesity in adult residents in Myanmar. Taken together, we found an independent and positive association between urban–rural location and BMI, partially, but not fully explained by socioeconomic factors. Mean BMI was higher among urban dwellers, individuals living above poverty line and in those with higher attained education level.
Knowledge about the significant roles played by location of living (urban and rural) and socioeconomic factors in relation to overweight and obesity can contribute to the development of well-targeted policies. Currently, mainly behavioural factors have been emphasised in prevention strategies for the management of overweight and obesity in Myanmar. Our findings imply that there should also be a focus on socioeconomic factors in order to reduce the burden of overweight and obesity. Moreover, updated national or subnational STEPS surveys should be conducted to continue monitoring the trends and urban–rural differences in overweight and obesity.
Supplementary Material
Acknowledgments
The authors acknowledge Professor Tint Swe Latt, the principle investigator, for the 2014 STEPS study. The authors are grateful to the WHO, the University of Oslo and the Norwegian Agency for Development Cooperation (Norad) for the project 'Health and Sustainable Development in Myanmar–Competence Building in Public Health and Medical Research and Education (MY-NORTH)' Project: MMY-13/0049, NORHED, UiO:211782. We would like to thank all the participants, medical doctors from the Myanmar Medical Association, township medical officers, basic health staffs and local administrative authorities from all the surveyed areas.
Footnotes
Contributors: RT contributed to the design of the article, statistical analysis, interpreted the data and drafted the manuscript. EB, CD and WPA contributed to the conception and design of the study and the article, interpretation of the data and the content of the article. All authors contributed revising the manuscript for important intellectual content and approved the final version to be published.
Funding: The research is funded by the Norwegian Agency for Development Cooperation (Norad), NORHED Project MMY-13/0049, UiO:211782: Health and Sustainable development in Myanmar - Competence Building in Public Health and Medical Research and Education (MY-NORTH).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Informed consent was obtained from the study participants; all information was handled with strict confidentiality. The participants were informed about the purpose and procedures of the study. Ethical approvals were obtained from the Norwegian Regional Committees for Medical and Health Research Ethics (REK) (Reference no. REK 2016/379) and the Ethical Review Committee of Department of Medical Research (Myanmar).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.World Health Organization . Obesity and overweight WHO, 2020. Available: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- 2.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: association for weight management and obesity prevention; NAASO, the obesity Society; the American Society for nutrition; and the American diabetes association. Obesity 2007;15:1061–7. 10.1038/oby.2007.632 [DOI] [PubMed] [Google Scholar]
- 3.Amato MC, Guarnotta V, Giordano C. Body composition assessment for the definition of cardiometabolic risk. J Endocrinol Invest 2013;36:537–43. 10.3275/8943 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Global health observatory data Repository (south-east Asia region): prevalence of overweight among adults, BMI ≥ 25, crude estimates by WHO region 2016, 2017. Available: http://apps.who.int/gho/data/view.main-searo.BMI25CREGv?lang=en
- 5.World Health Organization . Global health observatory data Repository (south-east Asia region): prevalence of obesity among adults, BMI ≥ 30, crude estimates by WHO region 2016, 2017. Available: http://apps.who.int/gho/data/view.main-searo.BMI30CREGv?lang=en
- 6.Dinsa GD, Goryakin Y, Fumagalli E, et al. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev 2012;13:1067–79. 10.1111/j.1467-789X.2012.01017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ND, Patel SA, Narayan KMV. Obesity in low- and middle-income countries: burden, drivers, and emerging challenges. Annu Rev Public Health 2017;38:145–64. 10.1146/annurev-publhealth-031816-044604 [DOI] [PubMed] [Google Scholar]
- 8.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21. 10.1111/j.1753-4887.2011.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Nations . World urbanization prospects: the 2018 revision. New York: United Nations, Department of Economic and Social Affairs, Population Division; 2019. https://population.un.org/wup/Publications/Files/WUP2018-Report.pdf. 978-92-1-148319-2. [Google Scholar]
- 10.Neuman M, Kawachi I, Gortmaker S, et al. Urban-rural differences in BMI in low- and middle-income countries: the role of socioeconomic status. Am J Clin Nutr 2013;97:428–36. 10.3945/ajcn.112.045997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaren L. Socioeconomic status and obesity. Epidemiol Rev 2007;29:29–48. 10.1093/epirev/mxm001 [DOI] [PubMed] [Google Scholar]
- 12.NCD Risk Factor Collaboration (NCD-RisC) . Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature 2019;569:260–4. 10.1038/s41586-019-1171-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Myanmar (Yangon division) steps survey 2004 fact sheet 2004, 2019. Available: https://www.who.int/ncds/surveillance/steps/Myanmar_2004_FactSheet.pdf
- 14.Htet AS, Bjertness MB, Sherpa LY, et al. Urban-Rural differences in the prevalence of non-communicable diseases risk factors among 25-74 years old citizens in Yangon region, Myanmar: a cross sectional study. BMC Public Health 2016;16:1225. 10.1186/s12889-016-3882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . WHO STEPwise approach to NCD surveillance 2004, Myanmar Disagregation of urban annd rural data (urban), 2019. Available: https://www.who.int/ncds/surveillance/steps/MyanmarSTEPSReport2004URBAN.pdf
- 16.World Health Organization . WHO STEPwise approach to NCD surveillance 2004, Myanmar Disagregation of urban annd rural data (rural), 2019. Available: https://www.who.int/ncds/surveillance/steps/MyanmarSTEPSReport2004RURAL.pdf
- 17.World Health Organization . Noncommunicable disease risk factor survey Myanmar 2009, 2011. Available: https://www.who.int/ncds/surveillance/steps/2009_STEPS_Survey_Myanmar.pdf
- 18.Hong SA, Peltzer K, Lwin KT, et al. The prevalence of underweight, overweight and obesity and their related socio-demographic and lifestyle factors among adult women in Myanmar, 2015-16. PLoS One 2018;13:e0194454. 10.1371/journal.pone.0194454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokke K, Vakulchuk R, Øverland I. Myanmar: a political economy analysis. Oslo, Norway: Norwegian Institute of International Affairs; 2018. https://nupi.brage.unit.no/nupi-xmlui/bitstream/handle/11250/2483349/NUPI_rapport_Myanmar_Stokke_Vakulchuk_%25C3%2598verland.pdf?sequence=1&isAllowed=y [Google Scholar]
- 20.World Health Organization . Report on national survey of diabetes mellitus and risk factors for non-communicable diseases in Myanmar (2014), 2018. Available: www.who.int/ncds/surveillance/steps/Myanmar_2014_STEPS_Report.pdf
- 21.Elfil M, Negida A. Sampling methods in clinical research; an educational review. Emerg 2017;5:e52. [PMC free article] [PubMed] [Google Scholar]
- 22.Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–6. 10.7326/0003-4819-122-7-199504010-00001 [DOI] [PubMed] [Google Scholar]
- 23.Pajak A, Kuulasmaa K, Tuomilehto J. Geographical variation in the major risk factors of coronary heart disease in men and women aged 35-64 years: the WHO MONICA Project/prepared by Andrzej Pajak. [et al.]. World health statistics quarterly 1988;41:115–40. [PubMed] [Google Scholar]
- 24.Bigaard J, Frederiksen K, Tjønneland A, et al. Waist circumference and body composition in relation to all-cause mortality in middle-aged men and women. Int J Obes 2005;29:778–84. 10.1038/sj.ijo.0802976 [DOI] [PubMed] [Google Scholar]
- 25.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004;79:379–84. 10.1093/ajcn/79.3.379 [DOI] [PubMed] [Google Scholar]
- 26.Lim JU, Lee JH, Kim JS, et al. Comparison of World Health organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis 2017;12:2465–75. 10.2147/COPD.S141295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization . Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. Geneva: World Health Organization; 2011. https://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf?sequence=1&isAllowed=y. 9789241501491. [Google Scholar]
- 29.The Republic of Union of Myanmar MoHA . The ward or village tract administration law 2012, 2019. Available: http://www.myanmar-law-library.org/law-library/laws-and-regulations/laws/myanmar-laws-1988-until-now/union-solidarity-and-development-party-laws-2012-2016/myanmar-laws-2012/pyidaungsu-hluttaw-law-no-1-2012-ward-or-village-administration-law-burmese.html
- 30.Central Bank of Myanmar . Reference exchange rate, 2019. Available: https://forex.cbm.gov.mm/index.php/fxrate
- 31.Ferreira FH, Chen S, Dabalen A, et al. A global count of the extreme poor in 2012: data issues, methodology and initial results 2015. http://documents1.worldbank.org/curated/en/360021468187787070/pdf/A-global-count-of-the-extreme-poor-in-2012-data-issues-methodology-and-initial-results.pdf
- 32.Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol 2016;45:1887–94. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 33.Angkurawaranon C, Jiraporncharoen W, Chenthanakij B, et al. Urban environments and obesity in Southeast Asia: a systematic review, meta-analysis and meta-regression. PLoS One 2014;9:e113547. 10.1371/journal.pone.0113547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas T, Garnett SP, Pervin S, et al. The prevalence of underweight, overweight and obesity in Bangladeshi adults: data from a national survey. PLoS One 2017;12:e0177395. 10.1371/journal.pone.0177395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui ST, Kandala N-B, Stranges S. Urbanisation and geographic variation of overweight and obesity in India: a cross-sectional analysis of the Indian demographic health survey 2005-2006. Int J Public Health 2015;60:717–26. 10.1007/s00038-015-0720-9 [DOI] [PubMed] [Google Scholar]
- 36.Biswas T, Townsend N, Islam MS, et al. Association between socioeconomic status and prevalence of non-communicable diseases risk factors and comorbidities in Bangladesh: findings from a nationwide cross-sectional survey. BMJ Open 2019;9:e025538. 10.1136/bmjopen-2018-025538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Silva AP, De Silva SHP, Haniffa R, et al. A cross sectional survey on social, cultural and economic determinants of obesity in a low middle income setting. Int J Equity Health 2015;14:6. 10.1186/s12939-015-0140-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore S, Hall JN, Harper S. Global and national socioeconomic disparities in obesity, overweight, and underweight status. J Obes 2010;2010. 10.1155/2010/514674. [Epub ahead of print: 01 04 2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masood M, Reidpath DD. Effect of national wealth on BMI: an analysis of 206,266 individuals in 70 low-, middle- and high-income countries. PLoS One 2017;12:e0178928. 10.1371/journal.pone.0178928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen AK, Rai M, Rehkopf DH, et al. Educational attainment and obesity: a systematic review. Obes Rev 2013;14:989–1005. 10.1111/obr.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteiro CA, Conde WL, Lu B, et al. Obesity and inequities in health in the developing world. Int J Obes Relat Metab Disord 2004;28:1181–6. 10.1038/sj.ijo.0802716 [DOI] [PubMed] [Google Scholar]
- 42.Al Kibria GM. Prevalence and factors affecting underweight, overweight and obesity using Asian and world Health organization cutoffs among adults in Nepal: analysis of the demographic and health survey 2016. Obes Res Clin Pract 2019;13:129–36. 10.1016/j.orcp.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 43.Mo H. Sports - MoHS/Myanmar, ICF. Myanmar demographic and health survey 2015-16. Nay Pyi Taw, Myanmar: MoHS and ICF, 2017. [Google Scholar]
- 44.The World Bank . Myanmar’s urbanization: creating opportunities for All 2019, 2019. Available: https://www.worldbank.org/en/country/myanmar/publication/myanmars-urbanization-creating-opportunities-for-all
- 45.The World Bank in Myanmar . Overview 2020, 2020. Available: https://www.worldbank.org/en/country/myanmar/overview
- 46.International Food Policy Research Institute . Global food policy report 2017. Washington, DC: International Food Policy Research Institute (IFPRI), 2017. [Google Scholar]
- 47.Unwin D, Unwin J. Low carbohydrate diet to achieve weight loss and improve HbA 1c in type 2 diabetes and pre-diabetes: experience from one general practice. Practical Diabetes 2014;31:76–9. 10.1002/pdi.1835 [DOI] [Google Scholar]
- 48.Aye T, Aung M, Oo E. Diabetes mellitus in Myanmar: socio-cultural challenges and strength. Journal of Social Health and Diabetes 2018;2:9–13. [Google Scholar]
- 49.Hlaing HH, Liabsuetrakul T. Dietary intake, food pattern, and abnormal blood glucose status of middle-aged adults: a cross-sectional community-based study in Myanmar. Food Nutr Res 2016;60:28898. 10.3402/fnr.v60.28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayén A-L, Marques-Vidal P, Paccaud F, et al. Socioeconomic determinants of dietary patterns in low- and middle-income countries: a systematic review. Am J Clin Nutr 2014;100:1520–31. 10.3945/ajcn.114.089029 [DOI] [PubMed] [Google Scholar]
- 51.Fogelholm M, Valve R, Absetz P, et al. Rural-Urban differences in health and health behaviour: a baseline description of a community health-promotion programme for the elderly. Scand J Public Health 2006;34:632–40. 10.1080/14034940600616039 [DOI] [PubMed] [Google Scholar]
- 52.Jackson JE, Doescher MP, Jerant AF, et al. A national study of obesity prevalence and trends by type of rural County. J Rural Health 2005;21:140–8. 10.1111/j.1748-0361.2005.tb00074.x [DOI] [PubMed] [Google Scholar]
- 53.Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005-2008). J Rural Health 2012;28:392–7. 10.1111/j.1748-0361.2012.00411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marques A, Peralta M, Naia A, et al. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur J Public Health 2018;28:295–300. 10.1093/eurpub/ckx143 [DOI] [PubMed] [Google Scholar]
- 55.Seguin R, Connor L, Nelson M, et al. Understanding barriers and facilitators to healthy eating and active living in rural communities. J Nutr Metab 2014;2014:1–8. 10.1155/2014/146502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liese AD, Weis KE, Pluto D, et al. Food store types, availability, and cost of foods in a rural environment. J Am Diet Assoc 2007;107:1916–23. 10.1016/j.jada.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 57.Pigeyre M, Rousseaux J, Trouiller P, et al. How obesity relates to socio-economic status: identification of eating behavior mediators. Int J Obes 2016;40:1794–801. 10.1038/ijo.2016.109 [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007;29:6–28. 10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- 59.Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychol Bull 1989;105:260–75. 10.1037/0033-2909.105.2.260 [DOI] [PubMed] [Google Scholar]
- 60.Ameye H, Swinnen J, Obesity SJ. Obesity, income and gender: the changing global relationship. Global Food Security 2019;23:267–81. 10.1016/j.gfs.2019.09.003 [DOI] [Google Scholar]
- 61.Giskes K, Avendano M, Brug J, et al. A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes Rev 2010;11:413–29. 10.1111/j.1467-789X.2009.00658.x [DOI] [PubMed] [Google Scholar]
- 62.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr 2008;87:1107–17. 10.1093/ajcn/87.5.1107 [DOI] [PubMed] [Google Scholar]
- 63.Miech R, Pampel F, Kim J, et al. The enduring association between education and mortality: the role of widening and narrowing disparities. Am Sociol Rev 2011;76:913–34. 10.1177/0003122411411276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Technology PBM. Transport, globalization and the nutrition transition food policy. Food Policy 2006;31:554–69. [Google Scholar]
- 65.Solomons NW, Gross R. Urban nutrition in developing countries. Nutr Rev 1995;53:90–5. 10.1111/j.1753-4887.1995.tb01526.x [DOI] [PubMed] [Google Scholar]
- 66.Mohammed SH, Habtewold TD, Birhanu MM, et al. Neighbourhood socioeconomic status and overweight/obesity: a systematic review and meta-analysis of epidemiological studies. BMJ Open 2019;9:e028238. 10.1136/bmjopen-2018-028238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asian Development Bank . Myanmar transport sector policy note: rural roads and access, 2016. Available: https://www.adb.org/sites/default/files/publication/189079/mya-rural-roads.pdf
- 68.Kim TJ, von dem Knesebeck O. Income and obesity: what is the direction of the relationship? A systematic review and meta-analysis. BMJ Open 2018;8:e019862. 10.1136/bmjopen-2017-019862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim TJ, Roesler NM, von dem Knesebeck O. Causation or selection - examining the relation between education and overweight/obesity in prospective observational studies: a meta-analysis. Obes Rev 2017;18:660–72. 10.1111/obr.12537 [DOI] [PubMed] [Google Scholar]
- 70.Søgaard AJ, Selmer R, Bjertness E, et al. The Oslo health study: the impact of self-selection in a large, population-based survey. Int J Equity Health 2004;3:3. 10.1186/1475-9276-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042561supp002.pdf (99.5KB, pdf)
bmjopen-2020-042561supp001.pdf (27.7KB, pdf)