Abstract
A previously healthy 53-year-old man was hospitalised for 12 days due to COVID-19 with shortness of breath. A few days after discharge from hospital, the patient developed fever and severe pain in several joints in the lower extremities. The pain was so severe that the patient was unable to stand on his feet. Synovial fluid from the right-side knee contained a high number of polynuclear cells and a few mononuclear cells. Microscopy, culture and PCR tests for bacterial infection were all negative. Furthermore, the patient tested negative for rheumatoid factor, anti-cyclic citrullinated peptide and human leukocyte antigen (HLA)-B27. Thus, the condition was compatible with reactive arthritis. The condition improved markedly after a few days’ treatment with non-steroid anti-inflammatory drugs and prednisolone.
Keywords: COVID-19, immunology, rheumatology
Background
The rapid global spread of SARS-CoV-2 constitutes a major human threat. COVID-19, the disease caused by SARS-CoV-2, usually manifests as pneumonia with hypoxia. In severe cases, the virus activates the immune system leading to systemic inflammation and immune dysregulation. Complications include kidney failure, myocardial dysfunction, thrombotic events and gastrointestinal symptoms.1 Due to the novelty of the disease, the picture of post-COVID-19 sequelae is still unclear. We here report a case of reactive arthritis after COVID-19.
Case presentation
A 53-year-old man developed fatigue, shortness of breath and fever up to 40°C 2 days after attending a seminar at work. He took a throat swab test for SARS-CoV-2 infection, and the test result was positive. Due to persistent fever and increasing pulmonary symptoms, he was admitted at Randers Regional Hospital. At admission, there was hypoxia <90% despite oxygen supply, and the patient was transferred to the intensive care unit at Aarhus University Hospital. For the next 12 days, he gradually improved without the need for mechanical ventilation. At discharge, the patient was stable and had no fever, and C reactive protein (CRP) levels were normal.
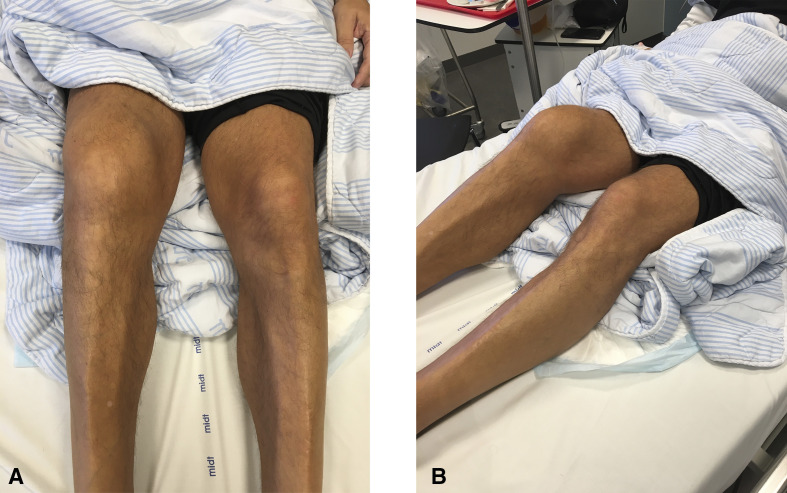
Four days later, the patient presented again at Randers Regional Hospital with severe pain in several joints in the lower extremities. The pain begun shortly after he was discharged and affected the right knee, both ankles and the lateral side of the left foot. This prevented the patient from walking, and he was barely able to stand on his feet. Correspondingly, at examination, the right knee and the ankles were found swollen, warm, tender and slightly reddish (figure 1A, B). Since the debut of respiratory distress at the first admission related to COVID-19, he had consistently felt some shortness of breath and tiredness, and these symptoms still prevailed. There were no other symptomatic complaints or objective findings. Body temperature was 37.7℃ measured rectally. Blood pressure was 144/92 mm Hg, heart rate 92 beats per minute, respiratory rate 18/minute and peripheral saturation 93%–95%.
Figure 1.
(A, B) The right knee was markedly swollen.
The patient was slightly overweight (body mass index=26.5 kg/m2) but had no previous or concomitant illnesses, and he did not take any medications prior to admission. He informed that his mother had general osteoarthritis and gout. There were no predispositions to autoimmune diseases.
Investigations
At the first admission, a throat swab was positive for SARS-CoV-2 RNA by PCR. At the second admission, the throat swab was negative, but a tracheal secretion test turned out positive. Thus, the patient was kept in isolation during both hospitalisations.
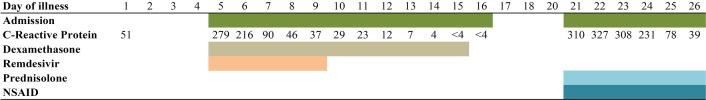
During both admissions, the patient had elevated acute-phase reactants. CRP peaked at 279 mg/L and 327 mg/L at the first and the second admission, respectively (figure 2). Total leucocyte count was normal during first admission although the number of lymphocytes was decreased to 0.66×109 cells/L (normal range: 1.30–3.50×109 cells/L). However, lymphocytes normalised after 5 days of hospitalisation. During the second admission, the total leucocyte count was elevated to 13.6×109 cells/L (normal range: 3.50–10×109 cells/L) with elevated neutrophils.
Figure 2.
Days of illness and corresponding admissions, C reactive protein and treatment given. NSAID, non-steroid anti-inflammatory drug.
At the second admission, X-ray examination of all major joints was made. There was a profound accumulation of fluid in the right knee and the right-side talocrural joint. There were no signs of arthritis.
Synovial fluid was aspirated from the right knee. Unfortunately, the sample for cell count was misplaced. By light microscopy, a large proportion of polynuclear cells and a minor part of mononuclear cells were detected. There were no crystals when viewed in polarised microscope. Both synovial fluid and blood cultures were negative. SARS-CoV-2 PCR was not performed on joint fluid.
To investigate the presence of other rheumatological diseases, we measured rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies—both were within normal range. Also, the patient was HLA-B27 negative. Other negative analyses were HIV testing, antinuclear antibodies, antimyeloperoxidase antibodies, antiproteinase 3 antibodies and antibodies against the glomerular basal membrane.
Differential diagnosis
At the second admission, the patient presented with inflamed joints shortly after a viral infection. As other viruses are well known to cause reactive arthritis, we immediately suspected this diagnosis from the anamnesis. There are no validated diagnostic criteria or definitive laboratory tests for reactive arthritis. The diagnosis is based on a clinical assessment following the exclusion of other differential diagnoses.2
Swollen inflamed joints are also seen in septic arthritis, but this condition most often presents as monarthritis.3 Before giving antibiotic therapy, we aspirated synovial joint fluid. We intended to perform a synovial fluid leucocyte count because this may give a hint to the cause of the inflammation. Unfortunately, as mentioned above, the vial sent for analysis was lost. Synovial fluid and blood cultures were negative, and we thus ruled out septic arthritis.
Rheumatoid arthritis (RA) may also cause sterile inflamed joints; nevertheless, several aspects made this diagnosis less likely. Our patient had not previously experienced swollen joints, and we found it unlikely that such an autoimmune disease would be revealed shortly after treatment with dexamethasone (see the Treatment section). The serological tests for RF and anti-CCP were both negative. The patient had involvement of one knee and both ankles. In RA, the typical sites of joint involvement at disease onset are the smaller joints in the hands and feet with a symmetrical pattern, although the condition may present differently. Finally, a large proportion of patients with RA have family members who are also affected; in this case, there was no history of autoimmune diseases in the family. Altogether, we did not find that the patient had RA.
Treatment
Initially, the patient presented with classic COVID-19 symptoms requiring oxygen therapy. Blood saturation was satisfactory at 95% by high-flow oxygen (up to 60 L/min and 88% oxygen), and mechanical ventilation was avoided. At first day of admission, we initiated antiviral therapy with 200 mg intravenous remdesivir followed by 100 mg daily for a total of 5 days.4 The patient also received 6 mg intravenous dexamethasone for 10 days5 in line with Danish and most international guidelines at this particular time of the pandemic.
Broad-spectrum antibiotic treatment (piperacillin/tazobactam) was immediately administered intravenously at the second admission, and this treatment was continued until synovial fluid culture came out negative. Treatment for reactive arthritis was also initiated at the first day of admission. This treatment consisted of the non-steroid anti-inflammatory drug (NSAID) ibuprofen 400 mg orally three times a day and prednisolone 25 mg orally once daily. As presented in figure 2, the anti-inflammatory drugs had a significant effect on the CRP levels, and the patient improved clinically. After 5 days, the patient had recovered and was able to walk again, and he was discharged from hospital. Prednisolone was given for 6 days, and the patient still takes ibuprofen at the time of writing this report.
Outcome and follow-up
By January 2021, 4 months after COVID-19 symptom debut, the patient has completely recovered from both COVID-19 and reactive arthritis. He has returned to work and reports that he is feeling well.
Discussion
Reactive arthritis is a well-known complication after an infection. Often, it is related to infections in other body compartments with a mucosal entry such as the urogenital (Chlamydia) and digestive (Campylobacter, Salmonella and Shigella) systems. Individuals with the HLA-B27 allele or with a family history of spondyloarthritis apparently have an increased risk of developing reactive arthritis.2
Since the emergence of COVID-19, only a few cases of post-COVID-19 reactive arthritis have been published.6–9 There seems to be some common traits in these case reports. Joint inflammation and elevated CRP appeared approximately 1 week after COVID-19 symptoms had dissolved. Age span in the other cases was 47–73 years, and all cases involved were men. All cases were affected in the lower extremities including the knees,6 9 ankle7 and metatarsophalangeal and interphalangeal joints.8 However, one case also had involvement of the wrist and shoulder.
Recommended first-choice treatment for reactive arthritis caused by other pathogens is NSAIDs and glucocorticoids. If these agents are insufficient, disease-modifying antirheumatic drugs may be attempted. In our case and two of the previously reported cases,7 8 NSAID and glucocorticoid therapy was successful. One patient received etoricoxib and intra-articular triamcinolone,9 and the remaining two cases spontaneously recovered without therapy.6
Learning points.
Reactive arthritis may occur after COVID-19.
Clinical and laboratory presentation of reactive arthritis triggered by COVID-19 resembles reactive arthritis due to other pathogens.
Non-steroid anti-inflammatory drugs and prednisolone have successfully been used for treatment.
Footnotes
Contributors: BLH, MLFH and MS were involved in planning, conducting, reporting, conception and design, analysis and interpretation of data. BLH drafted the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 2.Wendling D, Prati C, Chouk M, et al. Reactive arthritis: treatment challenges and future perspectives. Curr Rheumatol Rep 2020;22:29. 10.1007/s11926-020-00904-9 [DOI] [PubMed] [Google Scholar]
- 3.Mathews CJ, Weston VC, Jones A, et al. Bacterial septic arthritis in adults. The Lancet 2010;375:846–55. 10.1016/S0140-6736(09)61595-6 [DOI] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813–26. 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 2020. 10.1056/NEJMoa2021436. [Epub ahead of print: 17 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokogawa N, Minematsu N, Katano H, et al. Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis 2020:annrheumdis-2020-218281. 10.1136/annrheumdis-2020-218281 [DOI] [PubMed] [Google Scholar]
- 7.Ono K, Kishimoto M, Shimasaki T, et al. Reactive arthritis after COVID-19 infection. RMD Open 2020;6:e001350. 10.1136/rmdopen-2020-001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saricaoglu EM, Hasanoglu I, Guner R. The first reactive arthritis case associated with COVID‐19. J Med Virol 2020:jmv.26296. 10.1002/jmv.26296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew IY, Mak TM, Cui L, et al. A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol 2020;26:233. 10.1097/RHU.0000000000001560 [DOI] [PMC free article] [PubMed] [Google Scholar]