Abstract
Infective native aortic aneurysms (INAA) are aneurysms arising from infection of the aortic wall. Treatment is demanding with 5-year survival rates between 53 and 55%. The aim of our study was to evaluate the usefulness of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) in the long-term monitoring of patients with proven INAA. Fifty-three PET/CT were performed in 15 patients with INAA in this single-center retrospective cohort study and retrospective analysis of prospectively collected Vascular Graft Cohort Study (VASGRA) data. Median metabolic activity (as measured by maximum standardized uptake value, SUVmax) of the aneurysms at the initial PET/CT was high (6.8 (IQR 5.7–21.8)), and lower at the last PET/CT prior to the end of antimicrobial therapy (3.9 (IQR 2.7–6.8); n = 11) as well as in the first PET/CT after the end of the treatment (3.9 (IQR 3.0–4.4);n = 6). Compared to the course of C-reactive protein alone, PET/CT provided different (> 20% difference in trend) or altering (opposed trend) information on the course of disease in at least 14 comparisons (56%) in 11 patients (73%). The one-year and five-year freedom from all-cause lethality was 92% (95% confidence interval 57%-99%). As compared to the course of C-reactive protein, PET/CT provides different and occasionally altering information in therapy control of INAA.
Subject terms: Aneurysm, Molecular medicine
Introduction
Surgical and medical treatment of infective native aortic aneurysms (INAA) is demanding and the imminent rupture of the arterial vessel wall requires immediate surgical care1,2. Open surgical treatment includes resection of the aneurysm, extensive local debridement, and revascularization by in situ reconstruction or extra-anatomic bypass3,4. In recent years, there is increasing evidence that endovascular aortic repair (EVAR) of INAA may be an equivalent treatment option5,6, especially in cases where the anatomic location of the aneurysm precludes open surgical repair. Endovascular treatment of INAA frequently leads to secondary vascular graft infections (VGI). Treatment of secondary graft infections generally involves long-term antimicrobial therapy7,8 depending on the respective microorganisms, graft location, and involved graft material. Based on the heterogeneity of these factors, there are still many uncertainties with regard to the type and length of antimicrobial therapy7.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) can be used to assess the treatment response in VGI. Its impact on patient management has been investigated in three preliminary studies9–11, with promising results. To date, PET/CT has not been investigated in treatment response assessment of INAA. Thus, the aim of our study was to evaluate the usefulness of PET/CT in the long-term monitoring of patients with proven INAA.
Methods
Study design and definitions
Eligible participants included (a) prospectively acquired patients aged 18 years or older with proven INAA and open and/or endovascular surgery enrolled in the Vascular Graft Cohort Study (VASGRA), or (b) retrospectively acquired patients with proven INAA, who were examined at least twice with PET/CT between the years 2005 and 2018. The study was approved by the local ethics committee, namely the Kantonale Ethikkomission Zürich (protocol number 2018-01904), and we obtained written informed consent from all participants who were either prospectively enrolled or examined between the years 2016 and 2018; for subjects scanned between the years 2005 and 2015, written informed consent was waived due to retrospective inclusion by the local ethics committee, namely the Kantonale Ethikkomission Zürich (protocol number 2018-01904). All procedures were performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Diagnosis of INAA was made in an overall appraisal of clinical presentation (pain, fever, sepsis), laboratory (positive microbiological culture of aortic/aneurysmatic wall, presence of bacteria in thrombus or blood culture; elevation of inflammatory markers such as C-reactive protein and leucocytes) and imaging5. Diagnosis of secondary VGI due to placement of stentgrafts relied on the MAGIC criteria12.
In all prospectively enrolled patients, criteria for the termination of antimicrobial therapy were a combination of the absence of clinical features of infection, normal CRP, and reduced metabolic activity in PET/CT. Cure of INAA was defined as a combination of absence of clinical features, normal CRP, and reduced metabolic activity in PETCT at least three months after termination of antibiotic therapy (the latter only in the prospectively enrolled patients).
For retrospective inclusion of patients, we performed a retrospective chart review in all patients with suspected INAA, who were examined with PET/CT between the years 2005 and 2018, if the term “mycotic aneurysm”, “infective aneurysm”, or “infected aneurysm” was mentioned in the written PET/CT report, and was found to refer to an aneurysm of the thoracic, abdominal or pelvic arteries.
PET/CT examinations and patient follow-up
The study design for all consecutively and prospectively enrolled patients included consecutive PET/CT scans, at baseline, during follow-up on antimicrobial therapy, and at the end of antimicrobial treatment. If feasible, a control PET/CT three months after the end of antimicrobial therapy was performed, to document continuous cure or possible signs for recurrence of infection. For all retrospectively enrolled patients, PET/CT was performed depending on the clinical situation of the patient; the reasons for patient’s referral are given in the results section. The exact time points of follow-up examinations with regard to the baseline are demonstrated in Fig. 1. For all patients, baseline was defined as first PET/CT scan at diagnosis of INAA.
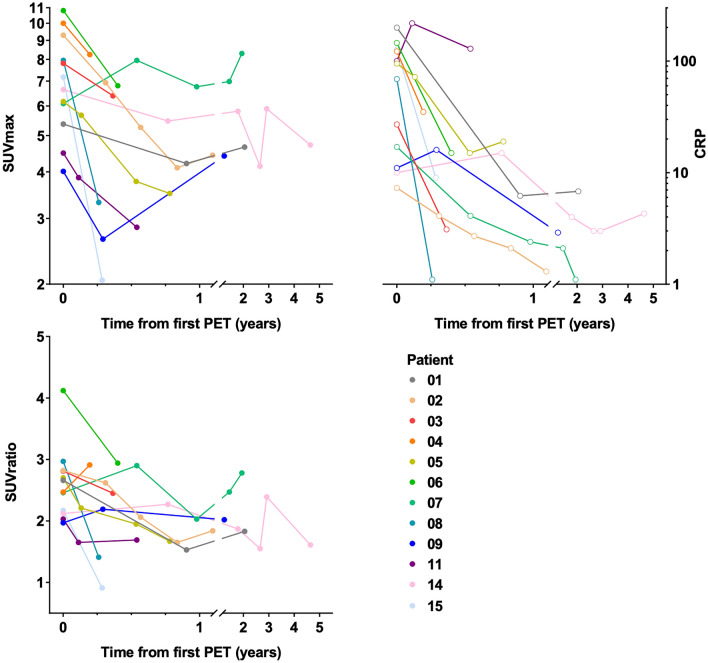
Figure 1.
Courses over time of metabolic activity in PET/CT (SUVmax and SUVratio in the two graphs on the left) and CRP (graph on the right) in patients with proven infective native aortic aneurysms. Note: PET/CT provides additional or altering information (as defined in the methods section) on the course of disease in at least 14 comparisons (56%) in 11 patients (73%), as compared to CRP alone (i.e. patients: 02, 03, 04, 05, 06, 07, 08, 09, 11, 14, 15). Abbreviations: PET: positron emission tomography; SUV: standardized uptake value; CRP: C-reactive protein.
We performed clinical follow-up of all patients by reviewing electronic patient charts. Patient data were recorded at the time of imaging, and at the last recorded clinical visit (recorded until March 2020).
Recorded data at baseline and at follow-up included patient demographics, clinical information, laboratory data (e.g. level of CRP and leucocyte count), results of microbiology, results from other diagnostic procedures, and information about treatment.
PET/CT data acquisition and image analysis
Five different types of PET/CT scanners were used within the study period between 2005 and 2018, i.e. a Discovery ST16, a Discovery VCT, two Discovery MI a Discovery 690 and a Discovery 710 (all GE Healthcare, Waukesha, WI). Body weight, height, and blood glucose level were measured prior to imaging (blood glucose levels < 12 mmol/l were accepted13). All PET/CT examinations followed basic study protocols: patients fasted for at least four hours, body-weight adjusted intravenous injection of FDG14, standardized uptake time of 60 min in supine position, non-enhanced CT scans for attenuation correction, data acquisition with arms overhead whenever possible.
All PET/CT examinations were independently analysed by two double board certified radiologists and nuclear medicine physicians, blinded to all clinical patient data. Readers determined the FDG uptake pattern in the aneurysm or graft to be focal or diffuse15. A consensus reading was performed if results differed. Furthermore, both readers quantified the FDG-uptake in the aneurysm or in the vascular graft by measuring the maximum standardized uptake value (SUVmax) in the aneurysm or graft as well as in the liver and mediastinal bloodpool (the latter as background for reference). In case SUVmax measurements were not identical among both readers concerning the measurements in the aneurysm or graft, a consensus reading was performed; in case of discordant SUVmax values for the background measurements, the mean was calculated. To compensate for differences in the sensitivity of the different PET/CT scanner generations between the years 2005 and 2018, and resulting differences in quantitative PET parameters (i.e. SUVmax), we calculated relatively scanner-independent FDG uptake ratios (i.e. SUVratio was defined as uptake in the aneurysm of graft in relation to the mediastinal blood pool). To determine differences between the course of C-reactive protein and SUVratio we calculated trends between consecutive examinations, and defined an arbitrary cut-off of > 20% between differences in trends as relevant.
Furthermore, for all baseline and follow-up PET/CT examinations, readers determined whether a INAA or a VGI was present or not, using a visual 4-point grading score. Score 1 (no signs for infected aortic aneurysm/VGI) and score 2 (most likely a non-infected aneurysm/VGI) were considered negative for infected aortic aneurysm, while score 3 (suspicion of infected aortic aneurysm/VGI) and score 4 (clear signs of infected aortic aneurysm/VGI) were considered positive for infection.
Statistical analyses
Variables were expressed as median and interquartile range (25th, 75th percentiles) or percentages. Kaplan–Meier estimates were used to describe survival at 1 and 5 years. We compared sizes and visual grading scores of INAA from initial and last CT scans using nonparametric pairwise Wilcoxon signed-rank test. Mixed-effects multilevel linear regression were applied to analyze the individual changes of SUVmax, SUVratio and CRP. Statistical analysis were performed using commercially available software (Stata/SE, Version 15.1, StataCorp, College Station, Texas).
Results
Patient population
Seven out of 21 (33%) patients with confirmed INAA were prospectively enrolled, while 14 (66%) patients were retrospectively included. Five out of 14 retrospective patients were excluded due to lack of written consent and one out of the seven prospective patients was excluded due to lack of clinical follow-up after the first PET/CT.
Thus, the final patient population consisted of 15 patients. All patients were eventually treated with open and/or endovascular repair (six prior to the first PET/CT, seven prior to the second, and two prior to the third PET/CT) (Table 1).
Table 1.
Patient demographics, data on interventional procedures, and patient outcome data of the final study population in patients with proven infective native aortic aneurysms.
| Pat | Age | Vascular surgery | Surgical technique | Location of aneurysm | Reoperation | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|
| Days after initial operation | Days after end of antimicrobial treatment | Status | Signs for recurrence | ||||||
| 01 | 62 | Y-Graft | Open | Infrarenal aorta | Second look | 1938 | 1832 | Cured | No |
| 02 | 58 | Y-EAP | Endovascular | Infrarenal aorta | n.a | 1198 | 826 | Cured | No |
| 03 | 64 | T-EVAR debranching | Endovascular | Aortic arch | n.a | 1451 | 1346 | Cured | No |
| 04 | 70 | T-EVAR debranching | Endovascular | Aortic arch | n.a | 287 | n.a | Ongoing* | n.a |
| 05 | 82 | T-EVAR | Endovascular | Descending thoracic aorta | n.a | 758 | 643 | Cured | no |
| 06 | 48 | Acute bridging EVAR bifurcation prothesis | Hybrid | Infrarenal aorta |
Resection of EVAR Extraanatomic Y-graft Open abdomen treatment |
579 | 334 | Cured | No |
| 07 | 50 |
TEVAR Renovisceral debranching |
Hybrid | Supra-/juxta-renal aorta |
Resection of parts of the duodenum due to fistula VAC-on-vessel |
2585 | n.a | Ongoing | Stable disease |
| 08 | 76 | T-EVAR | Endovascular | Descending thoracic aorta | n.a | 1289 | 1183 | Cured | No |
| 09 | 56 | Acute bridging EVAR | Hybrid | Iliac artery |
Resection of EVAR Extraanatomic Y-graft Open abdomen treatment |
2926 | 2508 | Cured | No |
| 10 | 71 |
Debranching A. mesenterica superior and T. coeliacus EVAR |
Hybrid | Descending thoracic aorta | Prognostic laparoscopy due to peri-aortic abscess | 1059 | 293 | Cured | No |
| 11 | 52 | TA-EVAR | Hybrid | Descending thoracic aorta/suprarenal | Viscerales debanching | 227 | n.a | Cured | No† |
| 12 | 40 | Y-EAP | Endovascular | Infrarenal aorta | n.a | 2798 | n.a | Cured | No‡ |
| 13 | 61 |
Renoviscerales debranching Graft implantation |
Open | Juxtarenal aorta | Open abdomen treatment | 3163 | 3109 | Cured | No |
| 14 | 61 | Graft implantation | Open | Infrarenal aorta | Open abdomen treatment | 1919 | n.a | Ongoing | Stable disease |
| 15 | 85 | EVAR | Endovascular | Infrarenal aorta | n.a | 1931 | 804 | Cured | No |
Pat: Patient number; na, not applicable.
*Patient died due to gastrointestinal bleeding, no relation to thoracic INAA.
†Patient developed 1.5 years later an infected pancreatic cyst.
‡The active intravenous drug user died 8 years later due to aortic valve endocarditis.
At the time of the initial PET/CT examination, patients had a median age of 61 years (IQR 54–85), one patient (7%) was female, six (40%) were smokers or had a history of smoking, two (13%) patients were diabetic, and six (40%) had renal insufficiency. The diagnoses of INAA were confirmed by blood culture (n = 11; 73%), culture or PCR from tissue obtained during surgical revision (n = 2; 13%) and serology (n = 2, 13%) (Table 2).
Table 2.
Data on microbiology and antimicrobial of the final study population in patients with proven infective native aortic aneurysms.
| Pat | Microbiology* | Antimicrobial treatment | ||||
|---|---|---|---|---|---|---|
| Intravenous | Oral | Strategy | Duration (days) | Complications | ||
| 01 | Escherichia coli | Piperacillin/tacobactam | Ciprofloxacin | Prolonged | 136 | None |
| 02 | Streptococcus agalactiae | Cetriaxone | Amoxicillin/clavulanic acid | Prolonged | 382 | None |
| 03 | Candida albicans | Caspofungin | Fluconazol | Prolonged | 192 | None |
| 04 | Streptococcus agalactiae | Penicillin | Clindamycin | Prolonged | 289 | Port infection |
| 05 | Streptococcus agalactiae | Ceftriaxone | Clindamycin | Prolonged | 220 | None |
| 06 | Staphylococcus aureus | Flucloxacillin |
Ciprofloxacin Rifampicin |
Prolonged | 261 | None |
| 07 |
Streptococcus gallolyticus Candida albicans Pediococcus acidilactici |
Daptomycin Ertapenem |
Fluconazole Amoxicillin |
Lifelong | Infinite | None |
| 08 | Streptococcus pneumoniae |
Ceftriaxone Gentamicin |
Moxifloxacin | Prolonged | 122 | Vestibular toxicity due to gentamicin |
| 09 | Bacteroides thetaiotaomicron/ Clostridium perfringens | Piperacillin/tacobactam |
Amoxicillin/clavulanic acid Ciprofloxacin |
Prolonged | 458 | None |
| 10 | Coxiella burnetii | na |
Doxycycline Plaquenil |
Prolonged | 783 | None |
| 11 | Streptococcus pneumoniae |
Ceftriaxone Gentamicin |
Clindamycin | Prolonged | 118 | None |
| 12 | Staphylococcus aureus | Flucloxacillin |
Ciprofloxacin Rifampicin |
Prolonged | 176 | None |
| 13 | Salmonella typhi | Ceftriaxon | Ciprofloxacin | Prolonged | 99 | None |
| 14 | Coxiella burnetii | na |
(Doxycycline) (Plaquenil) Minocycline |
Lifelong | Infinite | Allergy to doxycyclin |
| 15 | Porphyromonas gingivalis | Penicillin | Clindamycin | Prolonged | 1139 | None |
Pat: Patient number; na, not applicable.
*Microbiology resulted from blood culture (n = 11), culture or PCR from tissue obtained during surgical revision (n = 2) and serology (n = 2).
PET/CT examinations
We performed a total of 53 PET/CT scans in 15 patients (Figs. 1, 2, 3, Table 3) after intravenous injection of a median of 353 Megabecquerel of FDG (IQR 334—400). Initial imaging was performed for nine (60%) patients before and for six (40%) patients after vascular intervention, 11 (73%) received antimicrobial therapy at the time of initial imaging. The median visual 4-point grading score was 4 (11 × score 4, 3 × score 3, 1 × score 2). Eventually, all patients underwent vascular intervention with graft placement, and were treated with antibiotics (Tables 1 and 2). All aneurysms (or grafts) had increased focal FDG uptake on the initial PET/CT examination (SUVmax 6.8 (IQR 5.7–21.8); SUVmax aneurysm/graft to blood pool background ratio 2.7 (IQR 2.2–7.3)). FDG uptake in all aneurysms/grafts (100%) was higher than liver background (SUVmax 3.4 (IQR 2.7–6.1) (Figs. 1, 2, 3, Table 3).
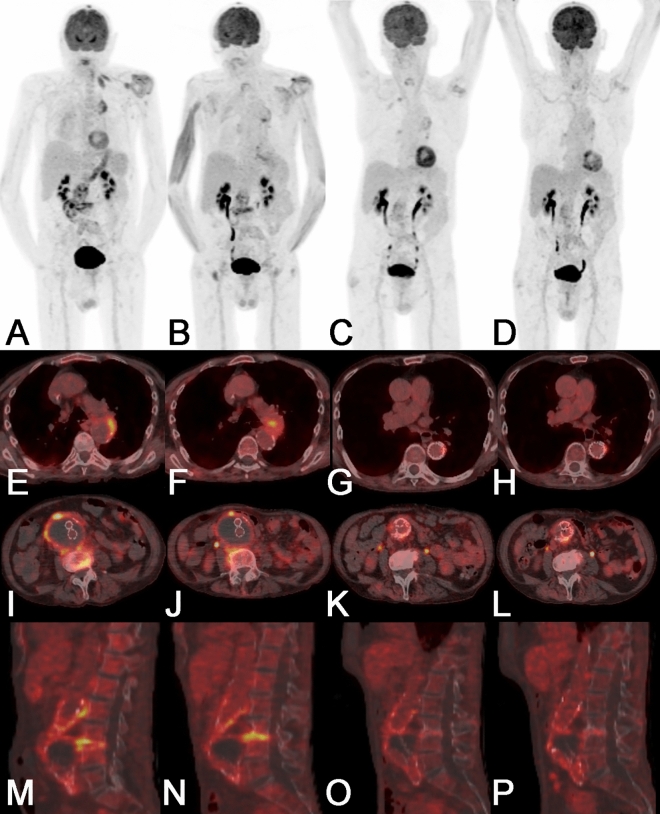
Figure 2.
PET/CT of an 82-year old male patient (patient 05 in Tables 1, 2 and 3) with a vascular graft infection and spondylodiscitis due to Streptococcus agalactiae showed a new focal FDG uptake in the wall of the thoracic aorta in September 2017. Both readers rated the thoracic finding as a infective native aneurysm despite the fact that the vessel diameter was not pathologically widened. The first PET/CT follow-up in revealed a progression of the aneurysm in size with stable increased FDG uptake; at the same time the FDG uptake of the spondylodiscitis increased while it partially decreased in the vascular graft infection; C-reactive protein and white blood cell count decreased. After subsequent thoracic endovascular repair with an Endurant II Stent Graft Systems (MEDTRONIC), two further PET/CT follow-up, before and after termination of antimicrobial therapy (223 days of therapy) showed faint residual FDG uptake in all sites of infection. At the last clinical follow-up in January 2019 the patient was in good clinical condition with no sign of infection. Note: Panels A-D show maximum intensity reconstructions of PET; Panels E-P show fused PET/CT images. Abbreviations: PET: positron emission tomography; CT: computed tomography; FDG: 18F-fluorodeoxyglucose.
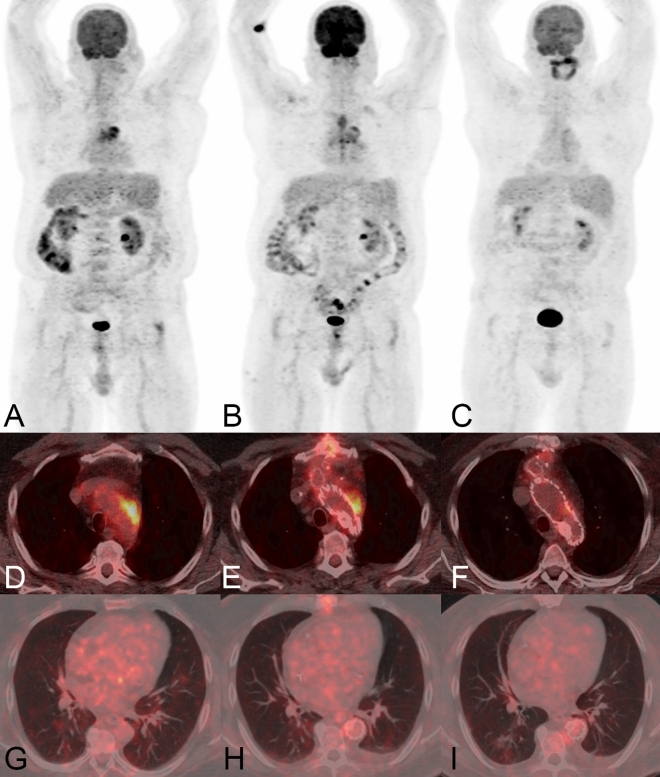
Figure 3.
A 70-year old male patient (patient 04 in Tables 1, 2 and 3) presented with chest pain and signs for infection (C-reactive protein 122 mg/L). The initial PET/CT examination showed strongly increased FDG-uptake in the wall of an aortic arch aneurysm and no other infectious foci. Both PET/CT readers suspected a infective native aneurysm; which was clinically confirmed (Streptococcus agalactiae in blood cultures). At first PET/CT follow-up (ongoing antimicrobial therapy, after thoracic endovascular repair with a Conformable GORE TAG Thoracic Endoprosthesis and debranching) a strong residual focal FDG-uptake was detected adjacent to the graft, in line with a secondary vascular graft infection. The second PET/CT follow-up (ongoing antimicrobial therapy), showed only very faintly increased FDG-uptake adjacent to the graft. However, a new pneumonia in the right lower lobe was incidentally detected. The patient died 60 days after the last PET/CT due to a gastrointestinal bleeding not related to the thoracic infective native aortic aneurysm. Note: Panels A-C show maximum intensity reconstructions of PET; Panels D-I show fused PET/CT images. Abbreviations: PET: positron emission tomography; CT: computed tomography; FDG: 18F-fluorodeoxyglucose.
Table 3.
Patient demographics, follow-up PET/CT and CT findings of the final study population in patients with proven infective native aortic aneurysms.
| Pat | PET/CT | Initial morphologic signs in CT | Morphologic signs at last CT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Size INAA in cm visual imaging score | Size INAA in cm visual imaging score | ||
| 01 |
SUVmax CRP (mg/L) |
5.4 199 |
4.4 n.a |
4.3 n.a |
4.2 6 |
4.7 7 |
*Stranding, fluid, gas | †Stranding | |
|
Days to start of AB Days to end of AB |
− 3 133 |
− 112 24 |
− 230 − 94 |
− 328 − 192 |
− 740 − 604 |
7.1 4 |
3.4 3 |
||
| 02 |
SUVmax CRP (mg/L) |
9.3 7 |
6.9 4 |
5.3 3 |
4.1 2 |
4.4 1 |
†Stranding, fluid, CE | *Lymph | |
|
Days to start of AB Days to end of AB |
− 79 303 |
− 192 190 |
− 284 98 |
− 380 2 |
− 475 − 93 |
6.7 4 |
4.3 3 |
||
| 03 |
SUVmax CRP (mg/L) |
7.8 27 |
6.4 3 |
†Stranding, CE, lymph | †None | ||||
|
Days to start of AB Days to end of AB |
− 45 147 |
− 175 17 |
2.7 4 |
Not measurable 3 |
|||||
| 04 |
SUVmax CRP (mg/L) |
10 122 |
8.2 35 |
3.9 n.a |
†Stranding, CE | *None | |||
|
Days to start of AB Days to end of AB |
6 n.a |
− 64 n.a |
− 230 n.a |
4.6 4 |
Not measurable 3 |
||||
| 05 |
SUVmax CRP (mg/L) |
6.2 95 |
5.7 72 |
3.8 15 |
3.5 19 |
†Stranding, CE, lymph | †Stranding, lymph | ||
|
Days to start of AB Days to end of AB |
− 21 199 |
− 68 152 |
− 214 6 |
− 303 − 83 |
4.4 4 |
4.4 3 |
|||
| 06 |
SUVmax CRP (mg/L) |
11 145 |
6.8 15 |
†Stranding, CE, lymph | *Stranding, fluid, CE, gas, lymph | ||||
|
Days to start of AB Days to end of AB |
− 3 258 |
− 148 113 |
6.6 4 |
4.9 3 |
|||||
| 07 |
SUVmax CRP (mg/L) |
6.1 17 |
7.9 4 |
6.8 2 |
7.0 2 |
8.3 1 |
7.9 n.a |
*Stranding, fluid, gas | *Stranding, fluid, gas |
|
Days to start of AB Days to end of AB |
− 818 n.a |
− 1011 n.a |
− 1170 n.a |
− 1337 n.a |
− 1515 n.a |
− 1702 n.a |
3.3 3 |
3.6 4 |
|
| 08 |
SUVmax CRP (mg/L) |
7.9 69 |
3.3 1 |
†Stranding, fluid, CE | *Stranding | ||||
|
Days to start of AB Days to end of AB |
− 6 116 |
− 101 21 |
5.5 4 |
3.1 3 |
|||||
| 09 |
SUVmax CRP (mg/L) |
4.0 11 |
2.6 16 |
3.9 n.a |
2.0 n.a |
4.4 3 |
*Stranding, lymph | *Stranding | |
|
Days to start of AB Days to end of AB |
− 71 387 |
− 176 282 |
− 325 133 |
− 421 37 |
− 520 − 62 |
2.7 3 |
2.7 2 |
||
| 10 |
SUVmax CRP (mg/L) |
22 31 |
5.7 n.a |
†Stranding, fluid, CE | *Stranding | ||||
|
Days to start of AB Days to end of AB |
− 11 772 |
− 721 62 |
6.6 4 |
4.0 3 |
|||||
| 11 |
SUVmax CRP (mg/L) |
4.5 100 |
3.9 218 |
2.8 129 |
†Stranding, fluid, CE | *Stranding | |||
|
Days to start of AB Days to end of AB |
40 158 |
0 118 |
− 153 na |
6.0 3 |
6.4 1 |
||||
| 12 |
SUVmax CRP (mg/L) |
6.8 146 |
2.4 n.a |
2.6 n.a |
2.0 n.a |
*Stranding, fluid | †Lymph | ||
|
Days to start of AB Days to end of AB |
− 4 n.a |
− 98 n.a |
− 464 n.a |
− 2602 n.a |
5.8 4 |
Not measurable 1 |
|||
| 13 |
SUVmax CRP (mg/L) |
1.1 23 |
2.3 n.a |
†Stranding, fluid, CE, gas | *Stranding | ||||
|
Days to start of AB Days to end of AB |
− 20 79 |
− 163 − 64 |
4.1 2 |
3.3 2 |
|||||
| 14 |
SUVmax CRP (mg/L) |
6.6 10 |
5.5 15 |
5.8 4 |
4.1 3 |
5.9 3 |
4.7 4 |
*Stranding, fluid | *Stranding, fluid |
|
Days to start of AB Days to end of AB |
24 n.a |
− 253 n.a |
− 618 n.a |
− 933 n.a |
− 1027 n.a |
− 1646 n.a |
8.3 4 |
4.2 2 |
|
| 15 |
SUVmax CRP (mg/L) |
7.2 126 |
2.1 9 |
*Stranding, fluid, lymph | Lymph | ||||
|
Days to start of AB Days to end of AB |
6 1145 |
− 96 1043 |
4.5 4 |
4.1 1 |
|||||
Pat: Patient number; AB: antimicrobial treatment; n.a.: not applicable; stranding: fat stranding; fluid: fluid collection; CE: contrast enhancement, gas: gas formation; lymph.: lymph adenopathy.
*Non-enhanced CT;† contrast-enhanced CT.
The first follow-up PET/CT (112 (IQR 95–721) days after the first PET/CT) was performed in all patients, and 13 (87%) patients received antimicrobial therapy at the time (in one patient antimicrobial therapy was started after the second PET/CT in another therapy was already terminated). The median visual 4-point grading score decreased to 3 (3 × score 4, 8 × score 3, 2 × score 2, 2 × score 1). The first follow-up showed an overall decrease in SUVmax (5.5 (IQR 3.0–8.2) and SUVmax ratio (2.2 (IQR 1.6–2.9)); SUVmax decreased in 13 and increased in two patients, SUVmax ratio decreased in 10 and increased in five patients (Figs. 1, 2, 3, Table 3).
In 11 patients, PET/CT was performed prior to the termination of the antimicrobial therapy. Compared to background activity, PET/CT showed focally increased FDG uptake in nine patients (82%) and diffuse increased FDG uptake in two patients (18%) with SUVmax 3.9 (IQR 2.7–6.8) and SUV ratio 1.7 (IQR 1.2–2.9). The FDG uptake in the aneurysm/graft was higher than blood pool background in eight (73%) patients (Fig. 2) and higher than liver background in seven (64%) patients. The median CRP was 12.0 mg/L (IQR 3–218). Of note, the median time difference between the last PET/CT prior to the termination of antimicrobial therapy and the actual end of antimicrobial therapy was a median 30 days (IQR 18–1059), and six examinations (55%) were considered to be directly linked to the decision to end treatment.
A total of eight PET/CT were performed after termination of the antimicrobial therapy treatment in six patients (SUVmax in all eight PET/CT: 4.2 (IQR 3.3–4.7); SUVmax in the first six PET/CT 3.9 (IQR 3.0–4.4). Compared to background activity, six (75%) of these examinations showed increased and focal FDG uptake (SUVmax 4.2 (IQR 3.3–4.7), SUV ratio 1.7 (IQR 1.5–4.1)), in two examinations (25%) the uptake was considered diffuse; FDG uptake was higher than blood pool background (SUVmax 2.2 (IQR 2.0–2.9)) and liver background (SUVmax 3.2 (IQR 2.7–3.6)) in all examinations (100%). The median visual 4-point grading score after termination of the antimicrobial therapy was 3 (0 × score 4, 5 × score 3, 2 × score 2, 1 × score 1).
The comparison of the last PET/CT prior to the termination of antimicrobial therapy to the first PET/CT after termination of antimicrobial therapy (feasible in six patients) showed less focal FDG-uptake (from five to four) and decreasing FDG uptake in three patients for SUVmax and in two patients for SUVratio, while the uptake increased in three patients for SUVmax and in four patients for SUVratio (SUVmax from 3.8 to 3.9 (IQR 2.5–4.4 and 3.0–4.4) and SUV ratio from 1.6 to 1.7 (IQR 0.9–1.9 and 1.5–4.1)). Notably, the two patients with a significantly rising SUV ratio (from 0.7 to 2.0 and from 0.7 to 1.2; patient 9 and 13 in Table 3) had no signs of infection at the last clinical follow-up, which was more than five and more than eight years after the last PET/CT and without antimicrobial therapy. None of these patients had clinical signs of new VGI at the last clinical follow-up.
Sizes of INAA, measured on CT images, decreased significantly by 1.45 cm on average from the initial to the last examination (p = 0.016, Table 3). In parallel, visual grading scores for INAA decreased by 1.2 on average (p < 0.01, Table 3).
Discrepancies between trends of SUVratio and CRP
With 53 PET/CT performed in 15 patients we were able to calculate 38 trends of SUVratio and 25 trends of CRP (immediate data of CRP missing for 13 PET/CT).
The decrease or increase of SUVratio was paralleled by decreasing or increasing CRP values in 16 of 25 (64%) trends, while it was opposed in nine (36%) examinations. Nineteen comparisons (76%) showed either opposed trends (as mentioned above, n = 9) and/or differences between trends of CRP and SUVratio > 20%. For these trends, PET/CT provided a conclusive explanation for the difference in five comparisons (26%) in three patients (i.e. additional foci of infection with different response to therapy: patient 05, 11, and 14 in Table 3 and Fig. 2). No apparent reason for high differences in trend could be determined for four comparisons (21%) in four patients (i.e. three opposed trends between first and second PET/CT in patient 04, 07, and 11 (Table 3) and a high 46% increase in CRP paralleled by a low 11% increase of SUVratio in patient 09 in Table 3). In five comparisons of five patients, CRP values showed very good or complete responses to therapy at the time of the first follow-up PET/CT, which were paralleled by less pronounced partial responses in SUVratio (patient 02, 03, 06, 08, and 15); further clinical follow-up could not determine which methods better described the actual clinical response to therapy (as all other clinical parameters defining cure of INAA were normal/unchanged). The final five comparisons of differences between trends of CRP and SUVratio which were > 20%, were rated to be errors as the values were very low and partly normal, i.e. between the fourth and fifth PET/CT of patient 02 (Table 3), CRP values decreased by 80% from 2.1 to 1.3, which was paralleled by a 20% increase in SUV ratio from 1.65 to 1.84.
Hence, PET/CT provided additional or altering information on the course of disease in at least 14 comparisons (56%) in 11 patients (73%), as compared to CRP alone.
Patient outcome
Patients were clinically followed for a median of 1496 days (IQR 961–3187 days) after their initial PET/CT examination and for a median of 1005 days (IQR 683–3109 days) after their last PET/CT examination. The one-year and five-year freedom from all-cause mortality was 92% (95% confidence interval [CI] 57–99%), for both. Overall mortality was 3.5% (0.9–13.8) per year. Two events occurred in 57.8 years of follow-up time; i.e. two patients (13%) died because of reasons considered unlikely to be related to INAA; one died due to gastrointestinal bleeding (location of the INAA was in the aortic arch), the other due to aortic valve endocarditis (after more than six years after successful treatment of the INAA).
The two patients (13%) on continuous antibiotic therapy, showed higher metabolic activity in the INAA (SUVmax 7.9 and 4.7, SUVratio 2.4 and 1.6) than the mean metabolic activity of those patients that were scanned prior to the termination of antimicrobial treatment (as mentioned above: SUVmax 3.9 (IQR 2.7–6.8) and SUV ratio 1.7 (IQR 1.2–2.9)). The conditions of these two patients were determined to be stable at the last clinical visit 261, and 729 days after the last performed PET/CT.
One patient (7%) showed recurrent signs of infection at the last clinical visit, which were attributed to an infected pancreatic cyst. All other patients (n = 10, 67%) did not show any signs of infection at the last clinical visit and all were without antimicrobial therapy (Table 1).
Among patients alive without antimicrobial therapy, yearly changes of SUVmax, SUVratio and CRP were -3.5 (95% CI -5.5 to -1.5, p < 0.01), -1.0 (-1.7 to -0.3, p < 0.01) and -58 (-96 to -20, p < 0.01), respectively. Among patients still on antimicrobial therapy or who died, yearly changes of SUVmax, SUVratio and CRP were -0.39 (95% CI -0.81 to 0.03, p = 0.07), -0.20 (-0.33 to -0.08, p < 0.01) and -4.6 (-14.2 to 5.1, p = 0.35), respectively.
Discussion
To the best of out knowledge, this is the first study investigating the role of PET/CT in therapy control of INAA. Our study results show: (i) PET/CT adds additional information in therapy control of INAA. (ii) Metabolic activity in the aneurysms remains slightly elevated after the end of antimicrobial therapy and therefore should not be mistaken for persistent infection.
All patients of the present study were treated with open and/or endovascular repair, and as this treatment in INAA frequently leads to secondary graft infections, we may compare the present study results to the findings of three previous publications9–11 on therapy control with PET/CT in VGI. In line with these studies9–11, we found that in addition to laboratory and clinical information, consecutive PET/CT examinations were a valuable source of information for treatment monitoring, often displaying additional and sometimes even opposed results to clinical and laboratory parameters.
Previous studies have documented a correlation between the course of CRP and SUVmax in therapy control of patients with VGI10. In the present study, only a minority of comparisons (i.e. 24%) of CRP and the metabolic activity in the aneurysm showed a similar course in therapy control of patients with INAA. The majority of cases displayed large discrepancies between trends of CRP and metabolic activity or even opposed courses. The latter, may partly be explained by additional foci of infection with different response to therapy, which was in line with results of a previous study, showing correlation between CRP and SUVmax only in a subpopulation of patients with VGI without additional infectious foci9.
However, we could identify another group of comparisons with no apparent reason for the large discrepancies between trends of CRP and metabolic activity, which may indicate, that CRP and metabolic activity in the aneurysm are two unrelated identities, which may have to be evaluated independently, when treating patients with INAA. Because of such discrepancies and the overall severity of INAA in general, we believe that in all patients with INAA, decisions for further therapy should always be based also on other parameters such as clinical aspects (general health status, fever, clinical signs of INAA and/or graft infection), and other laboratory parameters (CRP, ESR, leucocytes).
Furthermore, we documented in line with previous reports16–18, that the metabolic activity in INAA is initially high or very high, and comparable to the initial presentation of VGI in PET/CT9–11,15,19–25. Under antimicrobial therapy the metabolic activity generally decreases9–11. However, the metabolic activity remains above background level before and even after the end of medical treatment; despite the fact that we did not observe any cases of recurrent infection. High rates of residual metabolic activity were also observed in patient populations with VGI at the end of medical treatment. Husmann et al.10 observed a complete metabolic response to treatment in only 33% of patients with VGI, while we could not observe a complete response in any of our patients. The reason for this difference remains unclear, and may possibly be due to differences in the course of two similar but yet different diseases, or it may be due to bias as our study cohort was considerably smaller in number. Notably, residual metabolic activity (i.e. SUVmax values slightly above background level) in the aneurysms after the end of medical treatment should not be mistaken for persistence of infection in INAA, but may possibly represent a sterile ongoing inflammatory reaction, as none of our patients showed signs of recurrence during the follow-up of this series and an excellent patient outcome was observed.
Two patients died due to reasons not considered unrelated to INAA. The one-year and five-year freedom from all-cause mortality was 93%, and was much higher than in previously published studies, which found five-year survival rates between 53%26 and 55%7. Whether disease monitoring with PET/CT contributed to the excellent clinical outcome in the present study still remains unanswered. However, we could not find any other obvious systematic difference, which may account for the improved clinical outcome.
Limitations of the study
The present study population is heterogeneous, with varying numbers of follow-up PET/CT examinations, as well as prospectively and retrospectively included patients, which precludes standardized PET/CT intervals. Furthermore, due to the lack of comparable data in the literature, we defined an arbitrary cut-off of > 20% between differences in trends of SUVratio and CRP as relevant.
Despite these limitations, the results of the present study appear consequential and should be confirmed in further studies.
Conclusion
As compared to the course of C-reactive protein, PET/CT provides different and occasionally altering information in therapy control of INAA. Of note, metabolic activity in the aneurysms remains slightly elevated even after the end of antimicrobial therapy and should not be mistaken for persistent infection.
Acknowledgements
We are grateful to our patients for their participation in the study. We thank C. Mueller/ S. Bajrami, study nurses and Ch. Laich /C. Voegtli for administrative assistance. The members of the VASGRA Cohort Study are (in alphabetical order): A. Anagnostopoulos, B. Hasse (PI), N. Eberhard, M. Hoffmann, L. Husmann, R. Kopp, B. Ledergerber, Z. Rancic, C.A. Mestres, R. Zbinden, A. Zinkernagel.
Author contributions
B.H. and L.H. designed the study. L.H., M.H., B.L. and B.H. analysed the data. L.H. wrote the first draft, and L.H. and B.H. wrote the final version of the manuscript. The remaining investigators (A.A., N.E., M.K., K.K., I.A.B., C.A.M., Z.R.) contributed to data collection and interpretation of the data, reviewed drafts of the manuscript, and approved the final manuscript.
Funding
This study was financed within the framework of the Vascular Graft Cohort Study (VASGRA), supported by the Swiss National Science Foundation (SNF) grant 320030_184918/1. This work was also supported by the Clinical Research Priority Program of the University of Zurich for the CRPP Precision medicine for bacterial infections. M.H. is a recipient of investigator initiated study grants by GE Healthcare. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson WR, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134:e412–e460. doi: 10.1161/CIR.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 2.Sorelius K, Budtz-Lilly J, Mani K, Wanhainen A. Systematic review of the management of mycotic aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019;58:426–435. doi: 10.1016/j.ejvs.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Muller BT, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J. Vasc. Surg. 2001;33:106–113. doi: 10.1067/mva.2001.110356. [DOI] [PubMed] [Google Scholar]
- 4.Johansen K, Devin J. Mycotic aortic aneurysms: a reappraisal. Arch. Surg. 1983;118:583–588. doi: 10.1001/archsurg.1983.01390050059011. [DOI] [PubMed] [Google Scholar]
- 5.Sorelius K, et al. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation. 2016;134:1822–1832. doi: 10.1161/CIRCULATIONAHA.116.024021. [DOI] [PubMed] [Google Scholar]
- 6.Ding N, et al. CT texture analysis predicts abdominal aortic aneurysm post-endovascular aortic aneurysm repair progression. Sci. Rep. 2020;10:12268. doi: 10.1038/s41598-020-69226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorelius K, et al. Endovascular treatment of mycotic aortic aneurysms: a European multicenter study. Circulation. 2014;130:2136–2142. doi: 10.1161/CIRCULATIONAHA.114.009481. [DOI] [PubMed] [Google Scholar]
- 8.Wanhainen A, et al. Editor's choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019;57:8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Husmann L, et al. (1)(8)F-FDG PET/CT for therapy control in vascular graft infections: a first feasibility study. J. Nucl. Med. 2015;56:1024–1029. doi: 10.2967/jnumed.115.156265. [DOI] [PubMed] [Google Scholar]
- 10.Husmann L, et al. The role of FDG PET/CT in therapy control of aortic graft infection. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:1987–1997. doi: 10.1007/s00259-018-4069-1. [DOI] [PubMed] [Google Scholar]
- 11.Machelart I, et al. Graft infection after a Bentall procedure: a case series and systematic review of the literature. Diagn. Microbiol. Infect. Dis. 2017;88:158–162. doi: 10.1016/j.diagmicrobio.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Lyons OT, et al. Diagnosis of aortic graft infection: a case definition by the management of aortic graft infection collaboration (MAGIC) Eur. J. Vasc. Endovasc. Surg. 2016;52:758–763. doi: 10.1016/j.ejvs.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Eskian M, et al. Effect of blood glucose level on standardized uptake value (SUV) in (18)F- FDG PET-scan: a systematic review and meta-analysis of 20,807 individual SUV measurements. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:224–237. doi: 10.1007/s00259-018-4194-x. [DOI] [PubMed] [Google Scholar]
- 14.Morand GB, et al. Maximum standardized uptake value (SUVmax) of primary tumor predicts occult neck metastasis in oral cancer. Sci. Rep. 2018;8:11817. doi: 10.1038/s41598-018-30111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sah BR, et al. Diagnostic performance of F-FDG-PET/CT in vascular graft infections. Eur. J. Vasc. Endovasc. Surg. 2015;49(4):455–464. doi: 10.1016/j.ejvs.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Husmann L, et al. Diagnostic accuracy of PET/CT and contrast enhanced CT in patients with suspected infected aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2020;59:972–981. doi: 10.1016/j.ejvs.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Ben Shimol J, et al. The utility of PET/CT in large vessel vasculitis. Sci. Rep. 2020;10:17709. doi: 10.1038/s41598-020-73818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannsberger D, Heinola I, di Summa PG, Sorelius K. The value of 18F-FDG-PET-CT in the management of infective native aortic aneurysms. Vascular. 2021 doi: 10.1177/1708538120987971. [DOI] [PubMed] [Google Scholar]
- 19.Husmann L, Hasse B. PET-CT in vascular graft infections. Zentralbl Chir. 2017;142:502–505. doi: 10.1055/s-0043-119998. [DOI] [PubMed] [Google Scholar]
- 20.Keidar Z, Engel A, Hoffman A, Israel O, Nitecki S. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. J. Nucl. Med. 2007;48:1230–1236. doi: 10.2967/jnumed.107.040253. [DOI] [PubMed] [Google Scholar]
- 21.Keidar Z, Nitecki S. FDG-PET in prosthetic graft infections. Semin. Nucl. Med. 2013;43:396–402. doi: 10.1053/j.semnuclmed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Hasse B, et al. Vascular graft infections. Swiss. Med. Wkly. 2013;143:w13754. doi: 10.4414/smw.2013.13754. [DOI] [PubMed] [Google Scholar]
- 23.Tokuda Y, et al. Detection of thoracic aortic prosthetic graft infection with 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Eur J. Cardiothorac. Surg. 2013;43:1183–1187. doi: 10.1093/ejcts/ezs693. [DOI] [PubMed] [Google Scholar]
- 24.Fukuchi K, et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: comparison with computed tomographic findings. J. Vasc. Surg. 2005;42:919–925. doi: 10.1016/j.jvs.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Husmann L, et al. Comparing diagnostic accuracy of (18)F-FDG-PET/CT, contrast enhanced CT and combined imaging in patients with suspected vascular graft infections. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1359–1368. doi: 10.1007/s00259-018-4205-y. [DOI] [PubMed] [Google Scholar]
- 26.Luo CM, et al. Long-term outcome of endovascular treatment for mycotic aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2017;54:464–471. doi: 10.1016/j.ejvs.2017.07.004. [DOI] [PubMed] [Google Scholar]