Abstract
Infection with the liver fluke, Opisthorchis felineus, caused by the consumption of infected raw or undercooked cyprinid fish is common in humans and carnivores in the middle Ob River basin (Tomsk region, Russia) and can lead to diseases in humans. The goal of this study was the status of fish infection with O. felineus metacercariae in order to assess the role of fish in the infection of the human population in the middle Ob River basin.
Of the 14 Cyprinidae species recorded in the middle Ob River basin, we examined 6 cyprinid species for prevalence and intensity of infection with O. felineus metacercariae. Five of these species (Leuciscus idus, Leuciscus leuciscus, Rutilus rutilus, Abramis brama, and Carassius gibelio) are of commercial value, while the common bleak is an object of amateur fishing. In addition, we conducted a survey of the fish consumption habits as part of a community-based cross-sectional study in the rural Shegarsky district, Tomsk region, Russian Federation.
O. felineus metacercariae were observed in muscles of all examined species except for the Prussian carp. The ide is the main infection source in the Ob River (prevalence of infection, 100%, and intensity, 50.5 metacercariae per fish) and the common dace in the Tom River (91.1% and 12.7 metacercariae). Although the two alien species (bream and common bleak) are susceptible to infection with O. felineus metacercariae, the prevalence of infection in these fish and in the roach did not exceed 3%. The prevalence and intensity of infection in fish increased with age and size. The infection characteristics of fish in different water flows were different. The cyprinid species account on average for 69.8% of the commercial fish harvested in the Tomsk region. According to epidemiological survey, among 600 participants, 87.5% (n = 525) of respondents consumed river fish, with the most popular fish being cyprinids including Prussian carp, dace, ide and bream, followed by pike and perch.
Thus, the epizootological state of the water flows in the middle Ob River basin is adverse with respect to opisthorchiasis, as indicated by high infection rates of liver fluke metacercariae in ide and common dace, which are abundant species of high commercial value. An extremely high prevalence of infection suggests a strong transmission cycle with a high rate of infection from infected humans and/or animal reservoir hosts to snails and fish. In addition to treating humans, there should be a strong focus on identifying these potential reservoir hosts to reduce subsequent infection in humans. Furthermore, since the intensity of infection in humans is determined by the presence of fish species such as ide and dace in the diet, they should be included in a dietary change campaign by eliminating the consumption of raw fish.
Keywords: Opisthorchiasis, Commercial stock, Cyprinid fish species, Epidemiology, Epizootology
Highlights
-
•
Commercial cyprinid species play the key role in maintaining opisthorchiasis foci.
-
•
The ide and common dace are the main source of human and animal infection.
-
•
Measures to control Opisthorchis felineus should target these two species of fish.
-
•
The invasion of fish increases with their age and size.
1. Introduction
Trematodes (flukes) of the family Opisthorchidae cause liver and bile duct diseases in humans and carnivores. Persistent liver fluke infection correlates with malignant neoplasms of the hepatobiliary system (Aksorn et al., 1981; Fedorova et al., 2017, Fedorova et al., 2016; Lim, 2011; Thunyaharn et al., 2013).
Three liver fluke species are of the highest epidemiological importance: namely, Opisthorchis viverrini (Poirier, 1886) Stiles & Hassal, 1896, causing opisthorchiasis in Southeast Asia (Cambodia, Lao People's Democratic Republic, Thailand, and Vietnam); Clonorchis sinensis Looss, 1907, pertinent to China, Republic of Korea and northern Vietnam; and O. felineus Rivolta, 1884, responsible for the health problems in Europe, Russia, Kazakhstan, and Ukraine (Haswell-Elkins and Levri, 2003; World Health Organization, 1995).
Up to 600–750 million people worldwide are at risk of infection with liver flukes (Marcos et al., 2008; Mordvinov et al., 2012; Ogorodova et al., 2015; Petney et al., 2013), including 601 million at risk of C. sinensis infections (570 million in China and Taiwan); 67.3 million people are exposed to O. viverrini infection; and 12.5 million people are exposed to O. felineus infection (Keiser and Utzinger, 2009, Keiser and Utzinger, 2005).
In Russia, opisthorchiasis still remains among the most relevant and socially significant challenges, accounting for 78.5% of all recorded helminthiases in this country (Federal Service on Surveillance in the Sphere of Consumer Rights Protection and Human Welfare, 2018, 2018). Russia encompasses two-thirds of the world distribution range of O. felineus (Resolution no. 179 of the Federal Service for Supervision of Consumer Rights Protection and Human Well-being of December 12, 2016). According to the official statistical data, up to 26,000 human cases are recorded annually. The real number of cases may be approximately 15-fold higher (Letter no. 1/12315-2018-27 of the Federal Service for Supervision of Consumer Rights Protection and Human Well-being of September 24, 2018). The Tomsk region (middle Ob River basin) is among the hyperendemic areas of Russia where a prevalence of O. felineus infection is 60.2% (Fedorova et al., 2017, Fedorova et al., 2018, Fedorova et al., 2020).
O. felineus has a complex life cycle that includes a freshwater snail of the genus Bithynia as the first intermediate host and river fish of the family Cyprinidae, which is the second intermediate host (Petney et al., 2018, Petney et al., 2013). The definitive hosts are mammalian (cats, dogs, foxes and various fish-eating mammals, including humans) who are infected by raw (or undercooked – for human) fish containing metacercariae of O. felineus. After ingestion, the metacercariae ascend into the biliary ducts, where they develop into adults and start releasing eggs. Eggs together with faeces of definitive hosts fall into the surface waters of rivers or lakes where they are ingested by snails. A wide range of various fish-eating mammals are responsible for circulating of O. felines (Petney et al., 2018, Petney et al., 2013; Yurlova et al., 2017).
The fish in the Ob-Irtysh basin were examined for infestation with the liver fluke metacercariae with varying intensity. A large volume of data on fish infestation in the Novosibirsk, Tomsk and Tyumen regions was accumulated in the 1990s–2000s (Fattakhov, 2002, Fattakhov, 1996, Fattakhov, 1990; Fedorov et al., 2001; Gafina, 1996; Krivenko and Filatov, 1990; Sous’ and Rostovtsev, 2006; Bocharova, 2007). The major carriers of the liver fluke metacercariae in Western Siberia are cyprinid fish, primarily ide, common dace, and roach (Bocharova, 2007; Karpenko et al., 2008; Pel'gunov, 2012; Romanov et al., 2017).
A high prevalence of metacercariae contaminated cyprinid fish is one of the most important factors influencing the opisthorchiasis morbidity rate in humans. However, not only biological and ecological factors drive opisthorchiasis transmission. Some cultural preferences, including fishing and traditional consumption of undercooked river fish, also contribute to the high prevalence of opisthorciasis in the region. Previous study has shown the importance of river fish to the local population in terms of nutrition, food culture and community life (Zvonareva et al., 2018). Therefore, opisthorchiasis remains a significant problem in the Tomsk region despite prevention efforts and it is important to assess the prevalence of metacercariae-infected fish among commonly used and commercially demanded species of cyprinid fish.
The goal of this study was the status of fish infection with O. felineus metacercariae in order to assess the role of fish in the infection of the human population in the middle Ob River basin. A better understanding of the species of fish primarily responsible for transmission can help to improve food quality and safety and provide data to assist the development of an effective strategy for the targeted prevention and control of O. felineus infection in Western Siberia.
2. Materials and methods
2.1. Study area
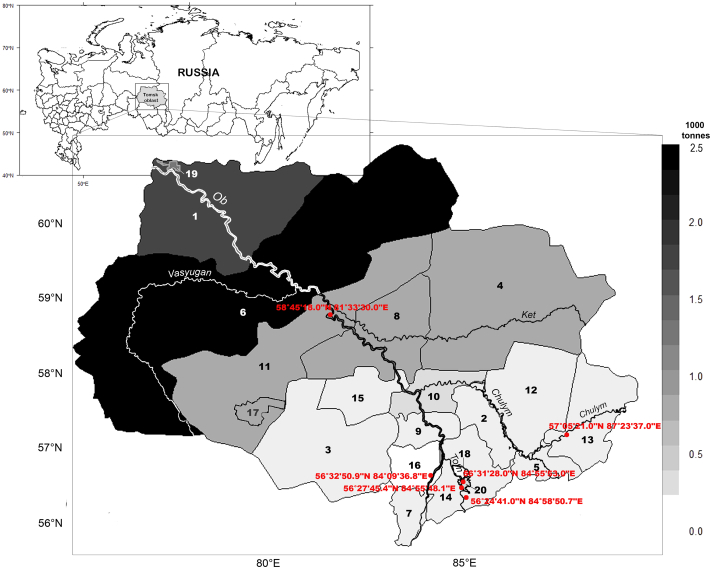
The study was conducted in the Tomsk region situated in the southeast of Russia (Western Siberian Plain), which covers an area of 314,391 km2 of the middle Ob River basin that stretches over 600 km from north to south and 780 km from west to east (Fig. 1). The region contains 18,000 rivers, rivulets, and other streams with a total length of approximately 97,000 km. The length of the Ob River part within the Tomsk region is 1170 km (Savichev, 2010). Tomsk city is the capital of the Tomsk region.
Fig. 1.
Commercial fish stocks in the Tomsk region (Resource and Ecological Atlas of the Tomsk Region, 2004): 1, Aleksandrovsky district; 2, Asinovsky district; 3, Bakcharsky district; 4, Verkhneketsky district; 5. Zyryansky district; 6, Kargasoksky district; 7, Kozhevnikovsky district; 8, Kolpashevsky district; 9, Krivosheinsky district; 10, Molchanovsky district; 11, Parabelsky district; 12, Pervomaysky district; 13, Teguldetsky district; 14, Tomsky district; 15, Chainsky district; and 16, Shegarsky district, and the cities of 17, Kedroviy; 18, Seversk; 19, Strezhevoy; and 20, Tomsk. Red points indicate sampling locations. The map is based on GDM data (www.gadm.org), version 3.4, April 2018, and visualization using R (3.6.1) (http://CRAN.R-project.org/package=sp). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)`
The study was conducted in two stages, including epidemiological stage and further fish harvesting and contamination assessment.
2.2. Survey of the fish consumption habits
We conducted the survey of fish consumption habits as part of a community-based cross-sectional study in the rural Shegarsky district, Tomsk region, Russian Federation (Fedorova et al., 2020). Shegarsky district displays high fish densities due to two main rivers: the Ob (East) and the Shegarka (West) rivers and a rich network of small rivers and lakes. Inhabitants of 37 rural settlements in the district lead a typical rural/farmer lifestyle. Fishing is an important part of the community life and river fish is a staple food (Zvonareva et al., 2018).
The survey was approved by the Ethics Committee of Siberian State Medical University (# 4815 of 27 June 2016). All household members (aged seven years and older) present on the survey day were enrolled in the study. Written informed consent was obtained from adult and adolescent (14 years and older) study participants. Parents or legal guardians of children under 14 years signed the informed consent forms. The study was conducted in the period from 1 July 2016 to 30 April 2017. Using the cluster approach, nine of the 37 villages were selected, namely Melnikovo, Kargala, Voznesenka, Batkat, Pobeda, Monostyrka, Malobragino, Novoilyinka, and Voronovka. For each village, a list of randomly selected households proportional to village population size was obtained. Within survey the clinical interviewing was performed. The questionnaire was developed based on the insights gained from in-depth interviews conducted during an earlier qualitative study (Zvonareva et al., 2018). After a pilot test with 15 volunteers, the questionnaire was revised to improve clarity. The questionnaire includes questions about fishing and river fish consumption. The survey was conducted by trained interviewers. More details of the study can be found in (Fedorova et al., 2020).
2.3. Fish harvesting and contamination assessment
The harvestable stock of cyprinid fish in the Tomsk region was assessed in 2004–2018 (according to the official data kindly provided by the Upper Ob River regional office of the Federal Agency for Fishery).
The fish infection rate was assayed in 2016–2018 using the individuals harvested in the Basandaika River (second-order tributary; 56°24′41.00″N, 84°58′50.70″E), Tom River (first-order tributary; 56°27′45.4″N, 84°55′48.1″E), Chulym River (first-order tributary; 57°05′20.93″N, 87°23′36.95″E), Ob River in the Shegarsky (56°32′50,92″N, 84°09′36.83″E) and Parabelsky (58°45′18.0″N, 81°33′30.0″E) districts, and in several lakes (56°31′28″N, 84°55′53″E) of the Tomsky district (Tomsk region) (Fig. 1).
Commercial and non-commercial (by-catch) fish were selected from commercial catches. Commercial dragnet with a size of 700 × 1.85 × 11.84 m was used.
In total, 1226 individuals were assayed in 2016–2018, including 75 ides (age cohorts of 2 to 8 years); 463 daces (age cohorts of 1 to 6 years), 216 roaches (age cohorts of 1 to 5 years), 255 bleaks (age cohorts of 1 to 4 years), 145 breams (1 to 9 years), and 72 Prussian carps (2 to 7 years).
The fish were identified to a species level and gender to measure the standard length (SL, cm) and body weight (W, g). Otoliths were used to determine the age. The back of the fish's head was transected to extract otoliths. In small fish, otoliths were examined as a whole, and in large fish they were separated. Examination was performed using a stereo microscope, up to 90×, MSP-1, LOMO. One transparent and one opaque zone together were taken as an indicator of annual growth.
O. felineus metacercariae were identified using a standard patented compression method by examining the fish muscles from both body sides (Voronin et al., 2019). We performed a complete parasitological dissection of the fish and microscoped all the muscles. Pieces of the dorsal musculature of fish and scrapings from the inner side of the skin are pressed between two glasses or a compressor and examined under the MBS stereoscopic microscope at different magnifications. The detected metacercariae are separated from the surrounding tissues using dissecting needles and transferred to a glass slide in a drop of water and microscoped. If two species of opisthorchid metacercariae simultaneously parasitize in fish, Pseudamphistomum truncatum cysts are seen to be distinctly larger than O. felineus ones. When P. truncatum and Paracoenogonimus ovatus metacercariae cysts are found in fish, the latter are noticeably smaller and round in shape. If O. felineus metacercariae cysts and P. ovatus cysts of similar size are simultaneously detected in fish, the latter is round in shape and have a thick hyaline capsule. The comparison of O. felineus and P. truncatum metacercariae cysts shows that the secretory vesicle of the former is translucent with granular structure, whereas in P. truncatum it is larger and completely dark and impervious to light (Voronin et al., 2019).
This method is reliable, and it was widely used for detection and identification of muscle metacercariae of trematodes by Russian scientists in 1946–2005 (Bauer, 1987; Beer, 2005; Beer et al., 1987; Bocharova, 1971; Fedorov et al., 2001; Myasoedov, 1960; Titova, 1965, Titova, 1953). We used this method to reliably compare our results with findings of previous studies. We found other trematode larvae in the fish muscles, including Metorchis billis and Paracoenogonimus ovatus. These data were not included in the present study, but Paracoenogonimus ovatus was used for valid identification of O. felineus larvae (Voronin et al., 2019).
The prevalence of infection, PI (the percentage of infested individuals relative to the total number of examined ones), intensity of infection, II (the minimal and maximal counts of parasites in infected individuals), and their average values were determined. The II value was determined by dividing the total count of parasites by the number of infested fishes. The index of abundance (the average number of larvae per examined individual) was calculated by dividing the total number of the observed larvae by the number of examined individuals. The mean error of PI was calculated as.
where p is the prevalence of infection, % and n is the number of examined individuals.
The fish species are ordered in the Results section according to the intensity of their infection. Statistical analyses were performed using R (3.6.1), a free software environment for statistical computing and graphics. Continuous data were presented in mean and standard deviation, while nominal variables were presented in percent. Statistical data processing involved Pearson correlation coefficient (r) and nonparametric Kruskal–Wallis test (Zar, 2010). A P-value of 0.05 or less was considered significant.
3. Results
3.1. Survey of the fish consumption habits
In total, 600 participants were involved from 388 randomly selected households in nine villages with a mean age of 44.9 ± 19.7 years. Among 600 participants, 29.7% were male and 16.1% were children/adolescents aged 7 to 18 years.
Of the 600 participants, 87.5% (n = 488) consumed river fish and 94.16% (n = 565) indicated that someone from their family members ate river fish. A total of 60.06% of respondents where at least one family member ate fish listed fishermen as the main source of fish in their family, 53.1% of respondents got fish from other people and 33.98% bought river fish in a store.
There is a fisherman in 57.89% of households, and 34.3% of respondents fish themselves (including children). About half of the fishermen (47.9%) go fishing 1–2 times a month and 28.2% do this 3–4 times a month, 16.8% fish several times a week and 7.2% go fishing every day. The majority (99.5%) of fishermen eat caught fish and 26.8% of them sell or give out the caught fish. In 61.5% of households pets are fed with river fish.
According to the results of the survey, the most consumed fish are Prussian carp, pike, dace, perch, ide, and bream. Among these fish, cyprinids are most popular. The majority of these fish species belong to the cyprinid family and pose the greatest danger in terms of opisthorchiasis infection (Table 1).
Table 1.
Fish species most commonly consumed according to the surveya.
| Fish | n | % |
|---|---|---|
| Prussian carp | 337 | 64.19 |
| Pike | 334 | 63.62 |
| Dace | 149 | 28.38 |
| Perch | 123 | 23.43 |
| Ide | 98 | 18.67 |
| Bream | 81 | 15.43 |
| Roach | 75 | 14.3 |
| Zander | 74 | 14.10 |
| Burbot | 56 | 10.67 |
| Don't remember | 40 | 7.62 |
| Chinese sleeper | 34 | 6.48 |
| Tench | 7 | 1.33 |
| Ruff | 7 | 1.33 |
| Minnow | 6 | 1.14 |
| Gudgeon | 5 | 0.95 |
| Catfish | 2 | 0.38 |
Cyprinid fish are given in bold.
3.2. Fish harvesting and contamination assessment
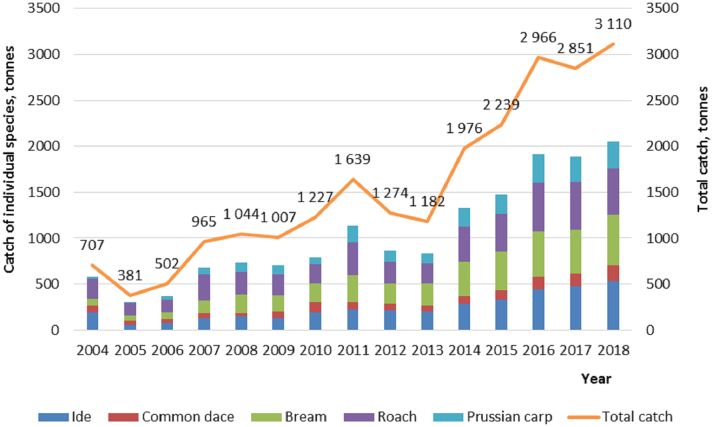
The commercial fish stock of the Tomsk region was analyzed using the data provided by the Upper Ob River regional office of the Federal Agency for Fishery in 2004–2018 to assess the role of each cyprinid species in the middle Ob River basin (Fig. 2). Three species of fish (ide, bream and roach) prevail in fish catches. Each fish species accounts for approximately 16–18% of the total catch. These three species add up to 49.1–68.0%. On the average, this amounts to over half (55.1%) of commercial harvest. The common dace is somewhat less commercially important, accounting for 3.1–11.6% of the total harvest. The commercial significance of the Prussian carp is similar to that of the common dace (2.7–11.5%), whereas the common bleak, an object of the amateur fishing, has not been taken into account in the commercial harvest statistics.
Fig. 2.
Data on the volumes of commercial fishing in the Tomsk region in 2004–2018 (according to the official data kindly provided by the Upper Ob River regional office of the Federal Agency for Fishery).
Thus, these six species of cyprinids constantly found in the middle Ob River basin are objects of commercial or amateur fishing. The share of the cyprinids accounts on the average for 69.8% of the total fish harvest in the Tomsk region.
Therefore, these six cyprinid species, which are the major harvested objects in the Tomsk region, were assayed: common bream Abramis brama Linnaeus, 1758; common bleak Alburnus alburnus Linnaeus, 1758; Prussian carp Carassius gibelio Bloch, 1782; ide Leuciscus idus Linnaeus, 1758; common dace L. leuciscus Linnaeus, 1758; and roach Rutilus rutilus Linnaeus, 1758.
3.2.1. Ide
The ide was harvested in the Ob River in two districts of the Tomsk region, Shegarsky and Parabelsky. The ide is known to migrate for long distances (over 500 km) to its spawning and feeding sites (Kulíšková et al., 2009; Petkevich, 1953; Winter and Fredrich, 2003). This allowed us to pool the two available samples.
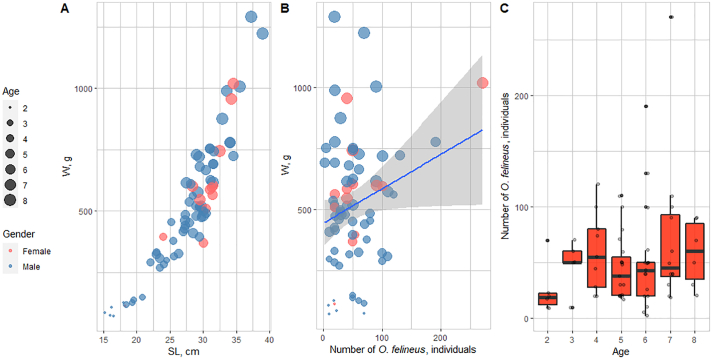
O. felineus metacercariae were counted in the muscles of ide individuals aged 2+ to 8+ with a body length (SL) of 15.2–34.5 cm (average, 27.9 cm) and body weight (W) of 72–1290 g (average, 519 g) (Fig. 3A). All ide individuals were of commercial size, i.e., they are actively harvested with fishing gear and are in demand of population.
Fig. 3.
Size, age, and infection characteristics of the ide in the middle Ob River basin (2016–2018). A: Size (SL, standard length; W, weight) and age characteristics. B: Dependence of the intensity of infection on the body weight. C: Dependence of the intensity of infection of the ide on the age.
Metacercariae were observed in all examined individuals. The prevalence of infection in the ide was 100% and the mean intensity of infection amounted to 52.6 metacercariae with a variation from 2 to 190 larvae per fish (Table 2).
Table 2.
Characteristics of the infection of cyprinid fish by O. felineus larvae (middle Ob River basin, 2016–2018).
| Species | Infection of fish |
Parasitic load |
||||||
|---|---|---|---|---|---|---|---|---|
| PI ± mPI, % | II, individuals, M ± s.e./(min–max) | Index of abundance, individuals | Examined in total | Number of infected | Uninfected/infected, tons | Total number of metacercariae, 106 individuals | Number metacercariae, 103/ton M ± s.e. | |
| Ide Leuciscus idus | 100 | 50.5 ± 5.3/(2–190) | 50.5 ± 5.28 | 75 | 75 | 0/483.1 | 65.78 | 136.16 ± 16.94 |
| Common dace L. leuciscus | 91.1 ± 1.3 | 13.7 ± 1.2/(1−302) | 12.5 ± 1.17 | 463 | 422 | 13.6/139.5 | 52.38 | 342.11 ± 55.19 |
| Common bleak Alburnus alburnus | 2.4 ± 0.9 | 1 | 0.02 ± 0.01 | 255 | 6 | – | – | 1.29 ± 0.54 |
| Bream Abramis brama | 2.1 ± 1.2 | 1.3 ± 0.3/(1–2) | 0.03 ± 0.02 | 145 | 3 | 492.6/10.6 | 0.14 | 0.29 ± 0.17 |
| Roach Rutilus rutilus | 1.8 ± 0.9 | 3.5 ± 1.3/(1–7) | 0.06 ± 0.04 | 216 | 4 | 510.9/9.4 | 0.49 | 0.93 ± 0.55 |
The dependences of the intensity of infection on the body weight, body length, and age were compared. As is shown, the intensity of infection varies considerably. We have observed the correlation between the intensity of infection and the body weight (Pearson's coefficient r = 0.23, p < 0.05) (Fig. 3B). The correlation between the intensity of infection and the body length is statistically insignificant.
Comparison of the infection characteristics with age has shown that the individuals of younger ages have a lower intensity of infection as compared with the adult fish (Fig. 3C).
The ide is among the dominant species in the harvests, with the share amounting to 13.2–26.9% of the total harvest. The average commercial ide harvest in 2004–2018 in the Tomsk region amounted to 241.9 tons (61.1 to 535.1 tons). A high rate of liver fluke infection of the ide suggests that this fish species is a dominant intermediate host that maintains the opisthorchiasis focus in the middle Ob River basin.
3.2.2. Common dace
The common dace from the Tom River in the vicinity of the city of Tomsk was most comprehensively examined for the infection with liver fluke metacercariae. This fish species forms local populations without migrating for long distances. Therefore, the harvesting sites were taken into account when examining the common dace muscles for O. felineus metacercariae.
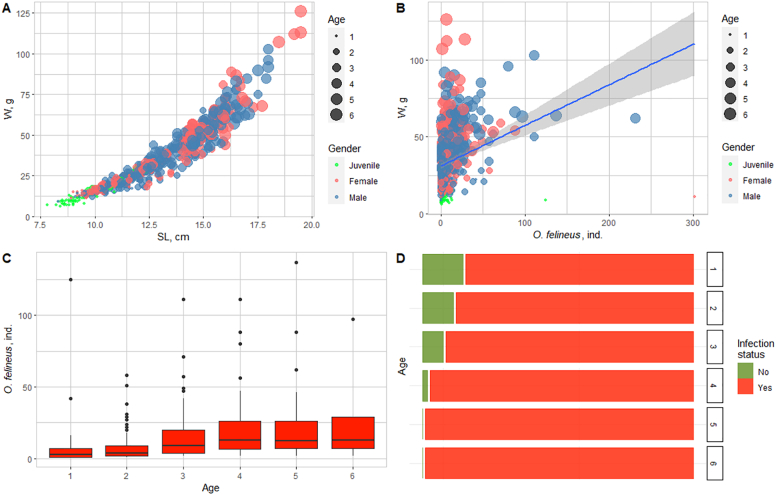
The harvested common dace individuals were aged 1+ to 6+ years (mean age, 2.6 years). The body length varied from 7.8 to 19.5 cm (mean, 12.9 cm) and weight ranged from 6.0 to 126.0 g (mean, 36.2 g) (Fig. 4A).
Fig. 4.
Common dace infection in the middle Ob River basin (2016–2018). A: Size (SL, standard length; W, weight) and age characteristics. B: Dependence of the intensity of infection on the body weight. C: Dependence of the intensity of infection on the age in the Tom River. D: Dependence of the prevalence of infection of the age in the Tom River.
The mean prevalence of infection of the common dace in the Tom River over the observation period (2016–2018) was 91.1%, and the intensity of infection considerably varied from 1 to 302 metacercariae with the mean of 13.8 metacercariae per fish (Table 2).
The dependences of the intensity of infection on the sizes of fish and age were compared. Comparison of the common dace infection versus age and gender revealed certain patterns. The correlation was observed between the intensity of infection and the age of the common dace (Pearson's coefficient r = 0.22, p < 0.05), body length (r = 0.23, p < 0.05) and body weight (r = 0.24, p < 0.05) (Fig. 4B). The intensity of infection of the common dace increased with age: from 6.6 at the age of 1+ to 23.4 at the age of 5+ and older (Fig. 4C). The individual variation of the intensity of infection in the common dace is very high regardless of age.
The prevalence of infection on the average increased with age from 85.1% at the age of 1+ to 100% starting from 5+ years and older (in males, from the age of 4+) (Fig. 4D).
Males and females do not exhibit statistically significant difference in the infection characteristics.
The commercial value of the common dace is several fold lower as compared with the ide, amounting on the average to 87.4 tons (32.7 to 145.1 tons) (Fig. 2). However, we have shown that the infection characteristics of this fish species are very high and comparable to those of the ide. Both the prevalence and intensity of infection of the common dace increase with the fish age and size, similar to the patterns observed in the ide. Therefore, the common dace is a dominant contributor to the maintenance of the opisthorchiasis focus in the middle Ob River basin but to a lesser degree, since its commercial value and infection characteristics are lower.
As for the other cyprinid fish species, both the prevalence and intensity of infection were rather low. Thus, it was impossible to find out the correlations between the infection characteristics and the structural characteristics of fish populations.
3.2.3. Common bleak
The examined common bleak individuals were harvested in different water bodies of the middle Ob River basin, namely, Ob, Tom, and Basandaika rivers. The fish abundances in these rivers were approximately comparable, which allowed us to pool the harvested individuals in one sample. The examined harvests contained the individuals aged 1+ to 4+ years (mean, 2.3 years) with a body length of 8.7–15.5 cm (mean, 11.9) and weight of 10–35 g (mean, 18.6 g) (Fig. 5).
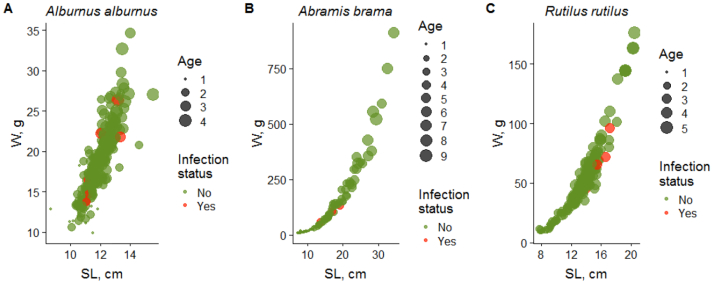
Fig. 5.
Size (SL, standard length; W, weight) and age characteristics of cyprinid fishes and the rate of their infection with O. felineus larvae in the middle Ob River basin (2016–2018).
Six fish aged 2+ years were infected; the mean prevalence of infection was 2.4% and intensity accounted for only one metacercaria per individual. (Fig. 5A).
3.2.4. Bream
The bream was harvested in the Ob River (Shegarsky district). The individuals in harvests were aged 1+ to 9+ years (mean, 2.1) with prevalence of young fish. The body length was 7.1–34.5 cm (mean, 13.5 cm) and weight was 7.0–912.0 g (mean, 92.7 g). This is an alien species for the Siberian water bodies; it was artificially introduced to the Novosibirsk Reservoir from rivers of the European Russia, rapidly acclimated, and spread over the entire Ob River basin. Currently, the bream is second in the list of commercial fish species in the middle Ob River basin. The average bream harvest was 196.51 tons, varying from 52.3 to 425.1 tons (Fig. 2).
Of the 145 bream individuals examined only 3 were infested; all of them were fish aged 3+ years (Fig. 5B).
3.2.5. Roach
The roach was sampled in different water bodies of the middle Ob River basin, namely, Ob, Chulym, and Tom rivers. The harvests contained the individuals aged 1+ to 5+ years (mean, 2.7 years) with a body length of 7.6 to 20.5 cm (mean, 12.8 cm) and weight of 8 to 176 g (mean, 46.1 g) (Fig. 5C). Most individuals were of commercial size. The roach has a considerable commercial value, following the bream and ide in the list of commercial species of the region. The average harvest of the roach was 309.04 tons (137.1 to 531.7 tons) (Fig. 2). Liver fluke larvae were found in four roach individuals; all of them were aged 3+ years. The mean prevalence of infection was 1.8% and the mean intensity was 3.5 metacercariae per fish (one–seven).
3.2.6. Prussian carp
The Prussian carp was harvested in two lakes near Tomsk (Lakes Kerepet’ and Igumenskoe). The examined individuals aged 2+ to 7+ years had a length of 10.5–25.7 cm (mean, 16.3 cm), and weight of 34–624 g (mean, 140.4 g). All individuals were of commercial size. The mean harvest of the Prussian carp was 139.94 tons (from 9.10 to 311.36 tons) (Fig. 2).
The muscles of all examined Prussian carp individuals were not infected with liver fluke larvae.
Thus, we have examined six cyprinid species (ide, bream, roach, common dace, Prussian carp, and common bleak) for the infection with O. felineus metacercariae. Four of these species are aboriginal and two are alien (bream and common bleak). All species except for the common bleak are of considerable commercial value in the middle Ob River basin. The ide, bream, and roach are the main commercial cyprinid species. Their harvesting amounts to approximately half of the total fish harvest in the Tomsk region. The Prussian carp and common dace play a smaller part.
4. Discussion
Most of the world literature on opisthorchiasis is devoted to human morbidity, and relatively few data are available on the infection of cyprinid fish. In a recent survey, 70% of cyprinid fish from Thailand and 27% from Lao Peoples Democratic Republic were found to be infected with O. viverrini (Bürli et al., 2018; Sithithaworn et al., 2012; Vonghachack et al., 2017). However, the prevalence of C. sinensis infection in fish in Vietnam was quite low and attained 1.9–5.1% (Thu et al., 2007).
In the Russian Federation, O. felines infection occurs in populations that inhabit the shores of the Ob, Irtysh, Ural, Volga, Kama, Don, Dnieper, North Dvina, and Biryusa rivers, with highest reported annual incidences in Western Siberia (Beer, 1997; Onishhenko et al., 2009; “Russian Ministry of Health”, 2004; Zavojkin et al., 1991). The largest world focus of opisthorchiasis is associated with the Ob–Irtysh basin because the environmental conditions support an active O. felineus lifecycle there. A vast floodplain of the Ob-Irtysh basin, rich in lakes and meadows, and a developed network of first- and second-order tributaries contributes to abundance of snails from the family Bithyniidae. Close coexistence and cohabitation of these snails and cyprinid fish in the same habitats allow for the full liver fluke life cycle. The cyprinid fish are the leader group in the Ob–Irtysh basin in abundance and species diversity. The ichthyofauna in the Ob River middle reaches comprises 43 species belonging to two classes, nine orders, 12 families, and 30 genera. Ten species (23% of the total number of species) appeared via introduction, either planned or random. The family Cyprinidae currently contains 9 native (ide, common dace, roach, Prussian carp, crucian carp, river minnow, lake minnow, gudgeon, and tench) and 4 introduced (carp, bream, common bleak, and sunbleak) species (Joganzen and Petkevich, 1951; Babkina et al., 2013). Of the 13 species, five are of commercial value (bream, Prussian carp, ide, dace, and roach), while the remaining species are objects of amateur fishing (Romanov et al., 2017).
The liver fluke larvae were discovered for the first time in the muscles of fish inhabiting Siberian water bodies by N.N. Plotnikov and L.K. Zerchaninov in 1932 (Plotnikov and Zerchaninov, 1932). According to their data, the prevalence of infection was 47.6% for the ide, 55.8% for the common dace, and 10% for the roach.
The studies into the fish infection with O. felineus metacercariae in the middle Ob River basin were commenced by S.D. Titova in 1936 (publications of 1946–1965) (Titova, 1965, Titova, 1953) and continued by V.S. Myasoedov (1953–1960) (Myasoedov, 1960), and T.A. Bocharova (1971–2005) (Bocharova, 2007, Bocharova, 1971). The liver fluke larvae were detected only in the ide, common dace, and roach and were not observed in the muscles of the pike, sunbleak, common bleak, bream, gudgeon, lake minnow, Prussian carp, perch, and zander (Bocharova, 2007). Our studies have expanded the host range by adding two species alien for this region – the bream and common bleak. The bream is a species of commercial value and the common bleak is an important object of amateur fishing. They also appeared to be infected with O. felineus metacercariae; however, both the prevalence and intensity of infection were very low (Table 2) (Simakova et al., 2019).
Our study of the infection of commercial fish species with O. felineus metacercariae carried out in 2016–2018 showed that Leuciscus species are most infected, with the ide heading the list. The prevalence of ide infection amounted to 100% with an intensity of 50.5 metacercariae per fish. The common dace is the second in this list with the prevalence of infection of 91.1% and intensity of 13.7 metacercariae per fish. The ide and common dace still exhibit high infection characteristics, being the main carriers of O. felineus metacercariae. The prevalence of infection of the remaining examined fish species did not exceed 3% (Table 2). The Prussian carp (Carassius gibelio) was free of the liver fluke.
The earlier studies on the prevalence of infection of the roach demonstrated a decrease in its infection from 15.0 to 8.5% (Bocharova, 2007). Our study shows tendency to further decrease in the roach infection. Currently, the prevalence of roach infection is below 2% (Table 2).
Thus, the main commercial fish species in the middle Ob River basin are the source of infection of humans and carnivores with the liver fluke.
The main source of infection in the Ob River is the ide, and in the Tom River, the common dace is the main source of infection. The infection characteristics increase with the fish age, which was also noted by earlier studies. In particular, the prevalence of infection in the common dace increased with age from 19.9% in under year hatchlings to 98.8% in the individuals aged 4+ years. This fact can be explained by the following factors:
– an increase in the area of infection with cercariae when fish grow in size and
– accumulation of metacercariae in fish muscles during fish life.
The number of metacercariae per unit of fish mass (ton) is greater in the common dace, which is 2.5 times more than in the ide. However, the total number of metacercariae in 2016–2018 in the ide was higher as compared with the common dace owing to its greater intensity of infection and large volumes of catch in the Tomsk region. The smallest number of the larvae was recorded in the bream (Table 2). Thus, the ide and common dace provide a stable maintenance of the opisthorchiasis focus.
According to the data provided by the Tomsk Center of Hygiene and Epidemiology, liver fluke eggs are regularly found in the wastewater of urban settlements. In particular, three wastewater samples (assayed in 2015–2018) near Tomsk contained liver fluke eggs. It is thus evident that the wastewater discarded into the Tom River near the city contains viable eggs of the helminths and is not always properly cleaned from them. This contributes to an increase and spread of the infection within and in the vicinity of the city, being one of the factors that determines high infection characteristics in the Tom River running through the city. As has been earlier demonstrated, the infection of the common dace in several floodplain lakes in the vicinity of densely populated settlements in the Vasyugan River lower reaches was maximal as compared with other water bodies and amounted to 100% (Bocharova, 2007).
The long-term data suggest an extremely adverse epizootological state of the water streams in the middle Ob River basin with respect to opisthorchiasis. An indicator of this adverse factor is a high infection of the ide and common dace by liver fluke metacercariae, since these species, on the one hand, are the main source of infection in all studied sites and, on the other hand, are of high commercial value. The ide migrates for long distances and thus can considerably contribute to the spread of this infection. The common dace contributes to maintenance of local opisthorchiasis foci in the settlements located on river banks.
The specific features in the geographic position and hydrological regime of the rivers running in the Tomsk region (middle Ob River basin) contribute to the wide spread of the O. felineus opisthorchiasis since they create the optimal conditions for the liver fluke intermediate hosts –Codiella snails and cyprinid fish species, which play a major role in maintenance of the focus of liver fluke infection.
5. Conclusions
The Tomsk region (middle Ob River basin) situated in the largest opisthorchiasis focus has the highest rates of human infection with this parasite. This is evidenced not only by high infection characteristics of the commercial and abundant cyprinid fish species (ide and common dace), but also by several socioeconomic factors, namely, developed fishing, consumption of fish and fish products by population ignoring cooking safety and disinfection practices, an increased number of amateur fishermen, uncontrolled export of fish and fish products from opisthorchiasis foci, and illegal fish vending. All these factors create the conditions for stable maintenance of the O. felineus opisthorchiasis.
The prevalence of infection in some fish species is extremely high. This suggests that there is a strong transmission cycle with a lot of infected humans and/or animal reservoir hosts that infect a lot of snails. This cause an urgent need for identifying these reservoir hosts.
Most of the force of infection in humans is driven by the ide and common dace, since these species are highly infected and are highly important commercially caught fish. Control measures should target these fish species and include either campaigns suggesting appropriate cooking of fish or ensuring its deep freezing. This study is part of the planned integrated interdisciplinary project on opisthorchiasis in the middle Ob River basin in the Tomsk region, which focuses not only on the fish infection rate, but also on the infection rates of the intermediate host, Bithyniidae snails, and the definitive host, domestic and wild animals.
Funding
The study was supported by the Ministry of Science and of Education the Russian Federation (project no. 0721-2020-0019), the program for elevating the competitive ability of Tomsk State University (no 8.2.22.2019). NC acknowledges funding from the Swiss National Science Foundation grant number 31003A_163057.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank E.A. Interesova, I.P. Rossomakhin and N.K. Valova (Novosibirsk Branch, Russian Federal Research Institute of Fisheries and Oceanography) for their assistance in sampling. We thank Thomas Smith for helpful discussions and suggestions for the analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fawpar.2021.e00113.
Appendix A. Supplementary data
Food preferences of the population
Conclusion of the ethical commission
Household questionnaire
Individual questionnaire
References
- Aksorn N., Roytrakul S., Kittisenachai S., Leelawat K., Chanvorachote P., Topanurak S., Bauer O.N., Musselius V.A., Strelkov Y.A. Diseases of pond fishes. Legk. Pishch. Promyshl. 1981 [in Russian] [Google Scholar]
- Babkina I.B., Petlina A.P., Shestakova A.S. Morphological and ecological features of bleak (Alburnus alburnus (L.)) of Lower Tomsk. Tomsk State Pedagog. Univ. Bull. 2013;8(136):61–69. [Google Scholar]
- Bauer O.N., editor. Key to Parasites of Freshwater Fishes of the USSR Fauna. Parasitic Metazoans. Nauka; Leningrad: 1987. [Google Scholar]
- Beer S.A. Parasitological monitoring in Russia (the basic concepts) Mediczinskaya Parazitol. i Parazit. Bolezn. 1997;1:3–8. [PubMed] [Google Scholar]
- Beer S.A. KMK; Moscow: 2005. The Biology of Opisthorchiasis Agent. [Google Scholar]
- Beer S.A., Belyakova Y.V., Sidorov E.G. Nauka; Alma-Ata: 1987. Methods for Studying the Intermediate Hosts of Opisthorchiasis Agent. [Google Scholar]
- Bocharova T.A. The Diseases and Parasites of the Fish in the Ice Sea Province (within the USSR) Tyumen; 1971. Opisthorchiasis foci in the Tomsk oblast; pp. 52–53. [Google Scholar]
- Bocharova T.A. Izd. Tomsk. Univ. Tomsk; Tomsk: 2007. Opisthorchiasis Agent and Other Muscle Parasites of the Cyprinid Fish in the Lover Tom River Basin. [Google Scholar]
- Bürli C., Harbrecht H., Odermatt P., Sayasone S., Chitnis N. Mathematical analysis of the transmission dynamics of the liver fluke, Opisthorchis viverrini. J. Theor. Biol. 2018;439:181–194. doi: 10.1016/j.jtbi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Fattakhov R.G. Nauka; Novosibirsk: 1990. The Distribution of Opisthorchiid Metacercariae in Cyprinid Fish in Western Siberia, in: Parasites and Diseases of Hydrobionts in the Ice Sea Province; pp. 128–131. [Google Scholar]
- Fattakhov R.G. The Tasks and Problems in Fish Management in Siberian Inner Water Bodies. Tomsk. 1996. The distribution of Opisthorchis felineus (Riv., 1884) and Metorchis bilis (Braun., 1790) larvae in populations of the roach Rutilus rutilus (L.) and sunbleak Leucaspius delineates (Heck) pp. 109–110. [Google Scholar]
- Fattakhov R.G. Infection of fish by opisthorchiasis agent in Russia and several adjacent countries. Mediczinskaya Parazitol. i Parazit. Bolezn. 2002;1:62. [Google Scholar]
- Federal Service on Surveillance in the Sphere of Consumer Rights Protection and Human Welfare . 2018. On the state of sanitary and epidemiological wellbeing of population in the Russian Federation in 2017: a state report, Moscow. [Google Scholar]
- Fedorov V.G., Belov G.F., Naumov V.A. Topical Problems in Infectology and Parasitology, Proc. of the First International Conference Devoted to 110th Anniversary of Prof. K.N. Vinogradov's Discovery of Liver Fluke in Humans. Tomsk. 2001. The problem of human trematodiases in Western Siberia; p. 34. [Google Scholar]
- Fedorova O.S., Kovshirina Y.V., Kovshirina A.E., Fedotova M.M., Deev I.A., Petrovskii F.I., Filimonov A.V., Dmitrieva A.I., Kudyakov L.A., Saltykova I.V., Mikhalev E.V., Odermatt P., Ogorodova L.M. Analysis of the morbidity of Opisthorchis felineus infection and malignant neoplasms of the hepatobiliary system in the Russian Federation. Bull. Sib. Med. 2016;15(5):147–158. doi: 10.20538/1682-0363-2016-5-14-158. [DOI] [Google Scholar]
- Fedorova O.S., Kovshirina Y.V., Kovshirina A.E., Fedotova M.M., Deev I.A., Petrovskiy F.I., Filimonov A.V., Dmitrieva A.I., Kudyakov L.A., Saltykova I.V., Odermatt P., Ogorodova L.M. Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: a review of medical statistics. Parasitol. Int. 2017;66(4):365–371. doi: 10.1016/j.parint.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Fedorova O.S., Fedotova M.M., Sokolova T.S., Golovach E.A., Kovshirina Y.V., Ageeva T.S., Kovshirina A.E., Kobyakova O.S., Ogorodova L.M., Odermatt P. Opisthorchis felineus infection prevalence in Western Siberia: a review of Russian literature. Acta Trop. 2018;178:196–204. doi: 10.1016/j.actatropica.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Fedorova O.S., Fedotova M.M., Zvonareva O.I., Mazeina S.V., Kovshirina Y.V., Sokolova T.S., Golovach E.A., Kovshirina A.E., Konovalova U.V., Kolomeets I.L., Gutor S.S., Petrov V.A., Hattendorf J., Ogorodova L.M., Odermatt P. Opisthorchis felineus infection, risks, and morbidity in rural Western Siberia, Russian Federation. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafina T.E. The Tasks and Problems in Fish Management in Siberian Inner Water Bodies. Tomsk. 1996. On the current state of the helminth fauna of the main commercial; fish species in the Ob Reservoir tail pond; pp. 105–106. [Google Scholar]
- Haswell-Elkins M., Levri E. Food-borne trematodes. In: Cook G., Zumla A., editors. Manson's Tropical Diseases. W.B. Saunders Ltd; London: 2003. pp. 1471–1486. [Google Scholar]
- Joganzen B.G., Petkevich A.N. Acclimatization of fish in Western Siberia. Proc. Baraba branch VNIORKH. 1951;5:3–204. [Google Scholar]
- Karpenko S.V., Chechulin A.I., Yurlova N.I., Serbina E.A., Vodyanitskaya S.N., Krivopalov A.V., Fedorov K.P. Characteristics of opisthorchiasis foci in southern West Siberia. Contemp. Probl. Ecol. 2008;1(5):517–521. doi: 10.1134/s1995425508050019. [DOI] [Google Scholar]
- Keiser J., Utzinger J. Emerging foodborne trematodiasis. Emerg. Infect. Dis. 2005;11:1507–1514. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Food-borne trematodiases. Clin. Microbiol. Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivenko V.V., Filatov V.G. Parasites and Diseases of Hydrobionts in the Ice Sea Province. Nauka, Novosibirsk. 1990. Cyprinid fish are intermediate hosts of trematodes in the Tura–Pyshma Interfluve; pp. 131–135. [Google Scholar]
- Kulíšková P., Horký P., Slavík O., Jones J.I. Factors influencing movement behaviour and home range size in ide Leuciscus idus. J. Fish Biol. 2009;74:1269–1279. doi: 10.1111/j.1095-8649.2009.02198.x. [DOI] [PubMed] [Google Scholar]
- Letter no. 1/12315–-2018-27 of the Federal Service for Supervision of Consumer Rights Protection and Human Well-being of September 24 . 2018. On implementation of the Resolution of the Chief State Sanitary Inspector of the Russian Federation of December 12, 2016 “On Prevention of Distribution of the Parasitic Diseases Transmitted via Fish and Fish Products in the Russian Federation.”. n.d. [Google Scholar]
- Lim J.H. Liver flukes: the malady neglected. Korean J. Radiol. 2011;12:269–279. doi: 10.3348/kjr.2011.12.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos L.A., Terashima A., Gotuzzo E. Update on hepatobiliary flukes: fascioliasis, opisthorchiasis and clonorchiasis. Curr. Opin. Infect. Dis. 2008;21:523–530. doi: 10.1097/QCO.0b013e32830f9818. [DOI] [PubMed] [Google Scholar]
- Mordvinov V.A., Yurlova N.I., Ogorodova L.M., Katokhin A.V. Opisthorchis felineus and Metorchis bilis are the main agents of liver fluke infection of humans in Russia. Parasitol. Int. 2012;61:25–31. doi: 10.1016/j.parint.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Myasoedov V.S. Tomsk Gos. Univ; Tomsk: 1960. The Epidemiology of Opisthorchiasis. [Google Scholar]
- Ogorodova L.M., Fedorova O.S., Sripa B., Mordvinov V.A., Katokhin A.V., Keiser J., Odermatt P., Brindley P.J., Mayboroda O.A., Velavan T.P., Freidin M.B., Sazonov A.E., Saltykova I.V., Pakharukova M.Y., Kovshirina Y.V., Kaloulis K., Krylova O.Y., Yazdanbakhsh M. Opisthorchiasis: an overlooked danger. PLoS Negl. Trop. Dis. 2015;9(4):1–11. doi: 10.1371/journal.pntd.0003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishhenko D., Darvin V., Lysak M. Acute cholаngitis in residents of opisthorchosis endemic area. Ann. Hir. Gepatologii. 2009;14:38–43. [Google Scholar]
- Pel'gunov A.N. The problems in opisthorchiasis and fish tapeworm disease in the Irtysh River lower reaches. Ross. Parazitol. Zhurnal. 2012;3:68–73. [Google Scholar]
- Petkevich A.N. On the biology of migratory fish of the Ob River middle and upper reaches. Proc. Barabinskogo Otd. VNIORKh. 1953;6(1):3–23. [Google Scholar]
- Petney T.N., Andrews R.H., Saijuntha W., Wenz-Mücke A., Sithithaworn P. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int. J. Parasitol. 2013;43(12−13):1031–1046. doi: 10.1016/j.ijpara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Petney T.N., Andrews R.H., Saijuntha W., Tesana S., Prasopdee S., Kiatsopit N., Sithithaworn P. Taxonomy, ecology and population genetics of Opisthorchis viverrini and its intermediate hosts. Adv. Parasitol. 2018;101:1–39. doi: 10.1016/bs.apar.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Plotnikov N.N., Zerchaninov N.K. Materials on the biology of Opisthorchis felineus (Rivolta, 1884) on treating opisthorchiasis. Mediczinskaya Parazitol. i Parazit. Bolezn. 1932;1:130–139. [Google Scholar]
- Resolution no. 179 of the Federal Service for Supervision of Consumer Rights Protection and Human Well-being of December 12 . 2016. On Prevention of Distribution of the Parasitic Diseases Transmitted via Fish and Fish Products in the Russian Federation. n.d. [Google Scholar]
- Resource and Ecological Atlas of the Tomsk Oblast . 2004. Pechatnaya manufaktura., Tomsk. [Google Scholar]
- Romanov V.I., Interesova E.A., Dyldin Y.V., Babkina I.B., Karmanova O.G., Vorobiev D.S. An annotated list and current state of ichthyofauna of the Middle Ob River basin. Int. J. Environ. Stud. 2017;74(5):818–830. doi: 10.1080/00207233.2017.1288547. [DOI] [Google Scholar]
- Russian Ministry of Health . N22FTS; 2004. Imported Helminth Infections in the Russian Federation, the Information Letter of Russian Ministry of Health, January 16, 2004. [Google Scholar]
- Savichev O.G. Izd. Tomsk. Politekhn. Univ; Tomsk: 2010. Aquatic Resources of the Tomsk Oblast: A Monograph. [Google Scholar]
- Simakova A.V., Babkina I.B., Khodkevich N.E., Babkin A.M., Interesova E.A. Infestation of alien cyprinid fishes with trematode Opisthorchis felineus Rivolta, 1884 in the Middle Ob River basin. Russ. J. Biol. Invasions. 2019;10(2):178–180. doi: 10.1134/S2075111719020115. [DOI] [Google Scholar]
- Sithithaworn P., Andrews R.H., Van De N., Wongsaroj T., Sinuon M., Odermatt P., Nawa Y., Liang S., Brindley P.J., Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol. Int. 2012;61(1):10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sous’ S.M., Rostovtsev A.A. Parasites of Fish in the Novosibirsk Oblast. Gosrybtsentr, Tyumen. 2006. Opisthorchiasis, metorchiasis, and fish tapeworm disease. Prevention. Part 1. [Google Scholar]
- Thu N.D., Dalsgaard A., Loan L.T.T., Murrell K.D. Survey for zoonotic liver and intestinal trematode metacercariae in cultured and wild fish in an Giang Province, Vietnam. Korean J. Parasitol. 2007;45:45–54. doi: 10.3347/kjp.2007.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunyaharn N., Promthet S., Wiangnon S., Suwanrungruang K., Kamsa-ard S. Survival of cholangiocarcinoma patients in northeastern Thailand after supportive treatment. Asian Pac. J. Cancer Prev. 2013;14:7029–7032. doi: 10.7314/apjcp.2012.14.11.7029. [DOI] [PubMed] [Google Scholar]
- Titova S.D. The fish of western Siberia as the vectors of opisthorchiasis and fish tapeworm disease and the measures for controlling these diseases. Tr. Tomsk. Univ. 1953;125:261–266. [Google Scholar]
- Titova S.D. Izd. Tomsk. Gos. Univ; Tomsk: 1965. The Fish Parasites of Western Siberia. [Google Scholar]
- Vonghachack Y., Odermatt P., Taisayyavong K., Phounsavath S., Akkhavong K., Sayasone S. Transmission of Opisthorchis viverrini, Schistosoma mekongi and soil-transmitted helminthes on the Mekong Islands, Southern Lao PDR. Infect. Dis. Poverty. 2017;6:1–15. doi: 10.1186/s40249-017-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin V.N., Kudryavtseva T.M., Kuznetsova E.V., Dudin A.S. RU Pat. 2708990: C1 A61D 99/00 (2006/01) 2019. Method of life-time differential diagnostic of metacercariae of opisthorchid flukes. [Google Scholar]
- Winter H.V., Fredrich F. Migratory behaviour of ide: a comparison between the lowland rivers Elbe, Germany, and Vecht, The Netherlands. J. Fish. Biol. 2003;63:871–880. doi: 10.1046/j.1095-8649.2003.00193.x. [DOI] [Google Scholar]
- World Health Organization . Report of a WHO study group; Geneva: 1995. Control of Foodborne Trematode Infections. [PubMed] [Google Scholar]
- Yurlova N.I., Yadrenkina E.N., Rastyazhenko N.M., Serbina E., Glupov V.V. Opisthorchiasis in Western Siberia: epidemiology and distribution in human, fish, snail, and animal populations. Parasitol. Int. 2017;66(4):355–364. doi: 10.1016/j.parint.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Zar J.H. Pearson Prentice-Hall; Upper Saddle River, NJ: 2010. Biostatistical Analysis. [Google Scholar]
- Zavojkin V., Darchenkova D., Zelya O. The structure of the nosological area of opisthorchiasis in Ob-Irtysh basin. Mediczinskaya Parazitol. i Parazit. Bolezn. 1991;6:25–28. [PubMed] [Google Scholar]
- Zvonareva O., Odermatt P., Golovach E.A., Fedotova M.M., Kovshirina Y.V., Kovshirina A.E., Kobyakova O.S., Fedorova O.S. Life by the river: neglected worm infection in Western Siberia and pitfalls of a one-size-fits-all control approach. Crit. Public Health. 2018;28:534–545. doi: 10.1080/09581596.2017.1378425. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Food preferences of the population
Conclusion of the ethical commission
Household questionnaire
Individual questionnaire