Abstract
Objectives:
To characterize tracheal cartilage morphology in mouse models of Fgfr2-related craniosynostosis syndromes. To establish relationships between specific Fgfr2 mutations and tracheal cartilaginous sleeve (TCS) phenotypes in these mouse models.
Methods:
Postnatal day-0 knock-in mouse lines with disease specific genetic variations in the Fgfr2 gene (Fgfr2C342Y/C342Y, Fgfr2C342Y/+, Fgfr2+/Y394C, Fgfr2+/S252W and Fgfr2+/P253R) as well as line-specific controls were utilized. Tracheal cartilage morphology as measured by gross analyses, microcomputed-tomography, and histopathology were compared using Chi-squared and single-factor analysis of variance statistical tests.
Results:
A greater proportion of rings per trachea were abnormal in Fgfr2C342Y/+ tracheas (63%) than Fgfr2+/S252W (17%), Fgfr2+/P253R (17%), Fgfr2+/Y394C (12%) and controls (10%) (p <.001 for each vs Fgfr2C342Y/+). TCS segments were found only in Fgfr2C342Y/C342Y (100%) and Fgfr2C342Y/+ (72%) tracheas. Cricoid and 1st tracheal ring fusion was noted in all Fgfr2C342Y/C342Y and 94% of Fgfr2C342Y/+ samples. The Fgfr2C342Y/C342Y and Fgfr2C342Y/+ groups were found to have greater areas and volumes of cartilage than other lines on gross analysis and microcomputed-tomography. Histologic analyses confirmed TCS among the Fgfr2C342Y/C342Y and Fgfr2C342Y/+ groups, without appreciable differences in cartilage morphology, cell size or density; no histologic differences were observed among other Fgfr2 lines compared to controls.
Conclusion:
This study found TCS phenotypes only in the Fgfr2C342Y mouse lines. These lines also had increased tracheal cartilage compared to other mutant lines and controls. These data support further study of the Fgfr2 mouse lines and the investigation of other Fgfr2 variants to better understand their role in tracheal development and TCS formation.
Keywords: FGFR2, tracheal cartilaginous sleeve, tracheal anomalies, craniosynostosis, Crouzon syndrome, Apert syndrome, Beare-Stevenson syndrome
Introduction:
Tracheal cartilaginous sleeve (TCS) is a life-threatening airway malformation often found in patients with syndromic craniosynostosis.1 The condition results from the vertical fusion of tracheal rings – forming a solid C-shaped “sleeve” of cartilage along the anterior and lateral trachea.2 Abnormal airway architecture in those with TCS is thought to alter airway dynamics and airway clearance, and may not allow adequate airway growth to support the respiratory requirements of a developing child.3 A 90% mortality rate has been reported by 2 years of age without tracheotomy.4
With the exception of rare reports, TCS is associated only with craniosynostosis syndromes caused by mutations in fibroblast growth factor receptor 2 (FGFR2), such as Apert, Beare-Stevenson, Crouzon, and Pfeiffer syndromes.3,5 The FGFRs are a family of four receptor tyrosine kinases, each composed of a split intracellular-kinase domain, single transmembrane domain, and ligand-binding extracellular domain. The extracellular domain contains three immunoglobulin (Ig)-like domains, with ligand binding occurring at the second and third Ig-like domains. The receptors dimerize when complexed with fibroblast growth factors (FGFs) or other receptor-specific signaling molecules. This dimerization leads to the activation of downstream signaling cascades involved in a wide array of cellular responses including regulation of cell proliferation, differentiation and migration.6–8 Gain-of-function mutations upregulate these processes and are associated with the aforementioned craniosynostoses syndromes.7
Protein variants FGFR2C342Y (p.Cys342Tyr) and FGFR2Y375C (p.Tyr375Cys) are associated with Crouzon and Beare-Stevenson syndromes, respectively, and result in ligand-independent FGFR2 activation.8–12 Meanwhile, variants FGFR2S252W (p.Ser252Trp) and FGFR2P253R (p.Pro253Arg) account for 98% of mutations seen in human Apert syndrome. Both occur at the linker-region between Ig-like loops II and III and enhance receptor binding affinity, resulting in ligand-dependent activation of FGFR2.7,12,13
While TCS has been described in patients with several different FGFR2 mutations, a clear genotype-phenotype relationship has not been established. Such a relationship is of clinical importance as TCS is notoriously difficult to diagnose and early treatment is critical.3 In this study, we systematically characterized tracheal cartilage morphology using knock-in mouse models of ligand-independent (Crouzon/Fgfr2C342Y, Beare-Stevenson/Fgfr2Y394C) and ligand-dependent (Apert/Fgfr2S252W and Fgfr2P253R) activation to establish genotype-phenotype correlations.
We hypothesized that genotype-phenotype correlation would be demonstrated and that mice with ligand-independent activating Fgfr2 mutations would demonstrate TCS that are more severe and occur at greater frequency than those with ligand-dependent Fgfr2 mutations.
Materials and Methods:
Animal models and preparation:
Generation of mouse models are described elsewhere.14–17 Fgfr2C342Y/(C342Y/+) mice were maintained on a CD1 outbred background whereas Fgfr2+/Y394C, Fgfr2+/S252W and Fgfr2+/P253R mice were maintained on C57BL/6J inbred backgrounds. Line-specific controls were utilized for analyses (Fgfr2+/+). Mouse lines were maintained at the Icahn School of Medicine for ongoing experiments. Previous studies have demonstrated high neonatal lethality rates for mice with Fgfr2C342Y/C342Y, Fgfr2+/S252W and Fgfr2+/P253R genotypes.15–17 Thus, postnatal day-0 (P0) mice were euthanized per local Institutional Animal Care and Use Committee (IACUC) protocols. Mouse thoraces were harvested and fixed in 4% paraformaldehyde. Tracheas were dissected and removed en bloc with thyroid cartilages and mainstem bronchi. Genotype was determined using line specific PCR assays.14–17 Animal protocols were approved by the Icahn School of Medicine Institutional Animal Care and Use Committee.
Tracheal morphology evaluation:
Tracheas were fixed in 95% ethanol. Alcian blue staining was carried out according to standard protocols.18
Alcian blue-stained tracheas were examined under stereomicroscopy (Nikon Instruments Inc., Melville, NY) and rings individually classified according to a modified version of the classification system described by Permakumar et al.19 “Normal” ring types were defined as those present on at least 20% of control specimens. “Abnormal” ring types were present on fewer than 20% of control tracheas and those classified as “Other”. Two sleeve-type ring segments were defined: “Complete cartilaginous sleeve” and “Partially sleeved rings.” A tracheal segment was defined as 1/3rd tracheal length – superior, middle, inferior. Analysis was performed for 86 specimens (n = 5 Fgfr2C342Y/C342Y/+; 32 Fgfr2C342Y/+; 5 Fgfr2+/Y394C; 6 Fgfr2+/S252W; 5 Fgfr2+/P253R; 33 Fgfr2+/+). The ring type classification system is detailed in Figure 1.
Figure 1:
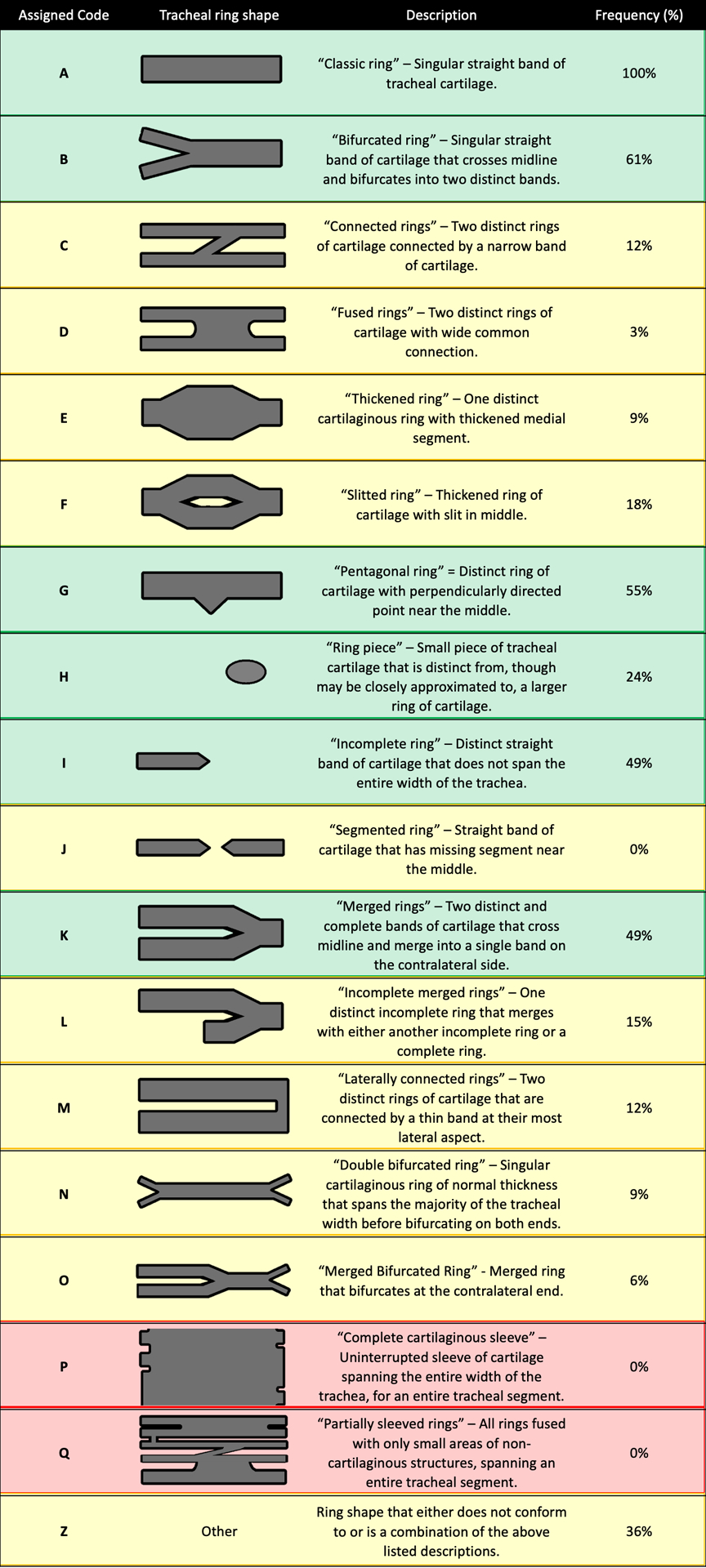
Tracheal ring types, their assigned codes, descriptions and frequencies at which they were observed in control specimens. “Normal” tracheal ring types (frequency in controls ≥ 20%) are highlighted in green whereas “Abnormal” and “Sleeve-Type” are highlighted in yellow and red, respectively.
Note: Sleeve-type segments are classified both as “Abnormal” and “Sleeve-Type” for analyses.
The presence or absence of fusion of the cricoid and 1st tracheal rings were noted.
Cartilage dimensions assessment:
Images of 80 alcian blue whole-mount stained tracheas (n = 5 Fgfr2C342Y/C342Y/+; 30 Fgfr2C342Y/+; 5 Fgfr2+/Y394C; 5 Fgfr2+/S252W; 5 Fgfr2+/P253R; 30 Fgfr2+/+) were obtained in an anterior-to-posterior orientation. Using these images, photographic area containing stained cartilage was manually highlighted and extracted using Adobe Photoshop CC 2017.0.0 (Adobe Inc., San Jose, CA) and imported into ImageJ software 1.52a (National Institutes of Health) where cartilage area was calculated.20 Only the cartilage present between the superior border of the cricoid cartilage and the carina was included. Thus, the amount of cartilage visible on the 2-dimensional images, was quantified as cartilage area.
Methods for additional cartilage dimensions are provided in Supplemental Methods.
Microcomputed-tomography assessment:
After serial alcohol dehydration tracheas were stained in 1% phosphotungstic acid for 7 days and then washed with tap water.21 Microcomputed-tomography (μCT) was performed using a SkyScan-1076 microtomography system (Bruker Corporation, Billerica, MA). μCT settings are provided in Supplemental Methods. Images were reconstructed with NRecon v1.7.0.4. software (Bruker Corporation, Billerica, MA) for segmentation and data acquisition with 3D Slicer v4.11.0 (www.slicer.org).22
Volumetric models of tracheal cartilage and airway lumens were obtained by manual segmentation. Cartilage and airway volumes between the superior border of the cricoid cartilage and the inferior-most point of the common tracheal airway were calculated using the Segment Statistics module. Cross-sectional areas along the entire airway lumen were computed and the mean and minimum cross-sectional areas, as well as the cross-sectional area at the cricoid, were recorded. Tracheal cartilage volumes were obtained for 35 samples (n = 4 Fgfr2C342Y/C342Y/+; 5 Fgfr2C342Y/+; 3 Fgfr2+/Y394C; 3 Fgfr2+/S252W; 4 Fgfr2+/P253R; 16 Fgfr2+/+) and airway volumes for 33 (n = 4 Fgfr2C342Y/C342Y/+; 4 Fgfr2C342Y/+; 2 Fgfr2+/Y394C; 3 Fgfr2+/S252W; 4 Fgfr2+/P253R; 16 Fgfr2+/+).
Histopathologic evaluation:
Fixed tracheas were paraffin-embedded, sectioned at 5 microns and stained with hematoxylin and eosin and Movat pentachrome. Images were captured with a digital camera mounted on a Nikon Eclipse 80i microscope and analyzed using NIS-Elements Advanced Research Software v4.13 (Nikon Instruments Inc., Melville, NY). The number of chondrocytes per area of hyaline cartilage was assessed on 5 random images taken at 40x for 2 samples per group. The number of chondrocytes is expressed as a fraction of the total cartilage area. All sections were reviewed by a board-certified pathologist with expertise in airway histopathology (GHD).
Statistical analysis:
Statistical analysis was performed using the Real Statistics Resource Pack Software v6.8.1.23 Chi-squared statistic with post hoc Fisher Exact Test was utilized to compare groups for categorical variables. For continuous variables, two-tailed T-Test assuming equal variances was used for comparisons involving two groups, and single-factor analysis of variance (ANOVA) with post hoc Tukey HSD was used for comparisons with greater than two groups. An alpha level of 0.05 was used for all statistical procedures. Frequencies are reported for categorical variables whereas means ± standard deviations are reported for continuous variables.
Line-specific control groups were pooled for analyses between Fgfr2 variant groups.
Results:
Tracheal morphology evaluation:
Representative whole-mount images are provided in Figure 2A–F. The mean proportions of abnormal rings per trachea for the Fgfr2C342Y/C342Y (100±0%) and Fgfr2C342Y/+ (63±27%) groups were significantly greater than all other groups, which were similar to controls (10±8%) (p < .001 Fgfr2C342Y/C342Y and Fgfr2C342Y/+ vs each other group) (F(5, 80) = 43.49, p < .001) (Table 1, Figure 2G).
Figure 2:
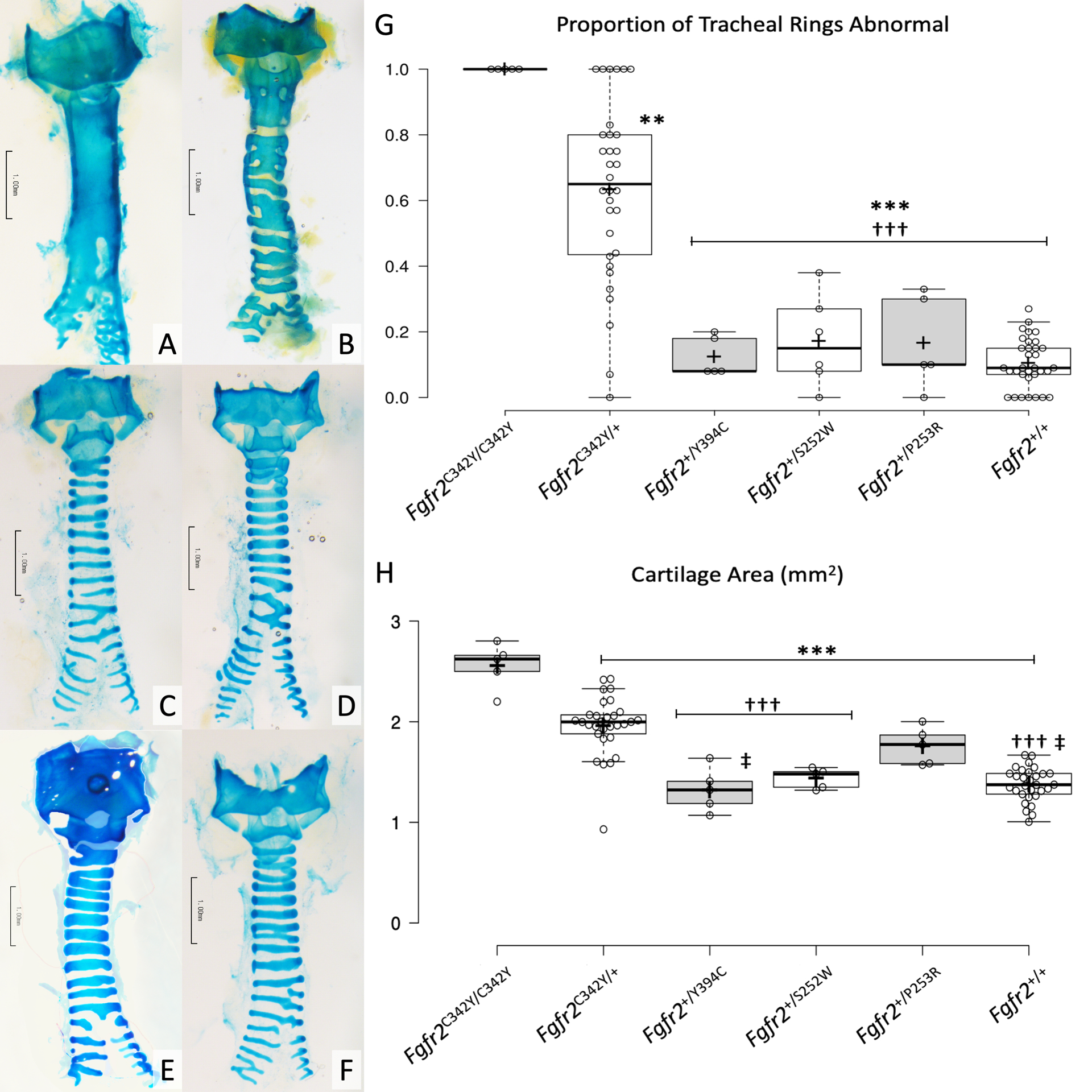
Panels A-F represent alcian blue stained whole-mount specimens, A) Fgfr2C342Y/C342Y, B) Fgfr2C342Y/+, C) Fgfr2+/Y394C, D) Fgfr2+/S252W, E) Fgfr2+/P253R and F) Fgfr2+/+. Scale bars = 1.0 mm.
Panels G and H demonstrate box plots representing the proportion of tracheal rings that were abnormal and cartilage area, respectively. Center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; plus signs represent sample means; data points are plotted as open circles.
** p < .01 vs Fgfr2C342Y/C342Y
*** p < .001 vs Fgfr2C342Y/C342Y
††† p < .001 vs Fgfr2C342Y/+
‡ p < .05 vs Fgfr2+/P253R
Table 1:
Proportion of abnormal rings per trachea on tracheal morphology evaluation data comparisons between Fgfr2 gene variants and controls (pooled). n – number of samples per group. SD – standard deviation.
| Group | n | mean (± SD) | p-value |
|---|---|---|---|
| Abnormal Rings per Trachea (mean proportion) | <.001 | ||
| Fgfr2C342Y/C342Y | 5 | 100% (±0%) | |
| Fgfr2C342Y/+ | 32 | 63% (±27%) | |
| Fgfr2+/Y394C | 5 | 12% (±6%) | |
| Fgfr2+/S252W | 6 | 17% (±14%) | |
| Fgfr2+/P253R | 5 | 17% (±14%) | |
| Fgfr2+/+ | 33 | 10% (±8%) | |
Sleeve-type segments were present only in Fgfr2C342Y/C342Y (100%) and Fgfr2C342Y/+ (72%) tracheas (X2 (5, N = 86) = 56.54, p < .001). The mean proportion of sleeve-type segments per trachea was greater in the Fgfr2C342Y/C342Y group (100±0%) than all other groups (p < .001 for Fgfr2C342Y/C342Y vs each group) (F(5, 80) = 35.36, p < .001). Greater mean proportion of sleeve-type segments per trachea was also noted among the Fgfr2C342Y/+ group (23±27%) as compared to the Fgfr2+/S252W (0±0%, p = 0.04) and Fgfr2+/+ (0±0%, p < .001) groups. It was also observed that sleeve-type segments tended to be present on the superior trachea, whereas the inferior trachea tended to have more normal appearing rings. No sleeve-type segments were present in any other group (Figure 2A–F).
Cricoid and 1st tracheal ring fusion was noted in all Fgfr2C342Y/C342Y and 94% of Fgfr2C342Y/+ tracheas. These frequencies were significantly greater than in all other groups, in which no cricoid and 1st tracheal ring fusion was found in any specimen (p < .001 for Fgfr2C342Y/C342Y and Fgfr2C342Y/+ vs each other group) (X2 (5, N = 86) = 78.23, p < .001).
Cartilage dimensions assessment:
Significant between group differences in whole-mount mean cartilage area among mutant groups were found (F(5, 74) = 38.03, p < .001). Cartilage area values for each group are provided in Table 2. The mean cartilage area for the Fgfr2C342Y/C342Y group was significantly greater than all other groups (p < .001 for all comparisons). The Fgfr2C342Y/+ group was the greatest among the heterozygous groups, significantly greater than the Fgfr2+/Y394C, Fgfr2+/S252W and Fgfr2+/+ groups (p < .001 for each comparison). The mean cartilage area for the Fgfr2+/P253R group was noted to be significantly greater than the Fgfr2+/Y394C (p = .04) and Fgfr2+/+ (p = .01) groups (Figure 2H).
Table 2:
Whole-mount cartilage area data comparisons between Fgfr2 gene variants and controls (pooled). n – number of samples per group. SD – standard deviation.
| Group | n | mean (± SD) | p-value |
|---|---|---|---|
| Cartilage Area (mm2) | <.001 | ||
| Fgfr2C342Y/C342Y | 5 | 2.56 (±0.23) | |
| Fgfr2C342Y/+ | 30 | 1.96 (±0.29) | |
| Fgfr2+/Y394C | 5 | 1.32 (±0.22) | |
| Fgfr2+/S252W | 5 | 1.44 (±0.10) | |
| Fgfr2+/P253R | 5 | 1.76 (±0.18) | |
| Fgfr2+/+ | 30 | 1.38 (±0.17) | |
Microcomputed-tomography assessment:
Representative μCT images are provided in Figure 3A–F. Among Fgfr2 variants, a significant between group difference was identified (F(5, 29) = 27.10, p < .001). Cartilage volume values for each group are provided in Table 3. Mean cartilage volume was greatest among Fgfr2C342Y/C342Y tracheas, followed by the Fgfr2C342Y/+ group. The Fgfr2+/+ group demonstrated the lowest mean cartilage volume, followed by the Fgfr2+/S252W group. Mean cartilage volume for Fgfr2C342Y/C342Y tracheas was significantly greater than all other groups (p < .001 for all comparisons) and mean volume among Fgfr2C342Y/+ tracheas was significantly greater than Fgfr2+/+ tracheas (p < .001). There was a trend for greater mean cartilage volume in the Fgfr2C342Y/+ group as compared to Fgfr2+/Y394C (p = 0.1) and Fgfr2+/S252W (p = .05) tracheas but significance was not reached (Figure 3G).
Figure 3:
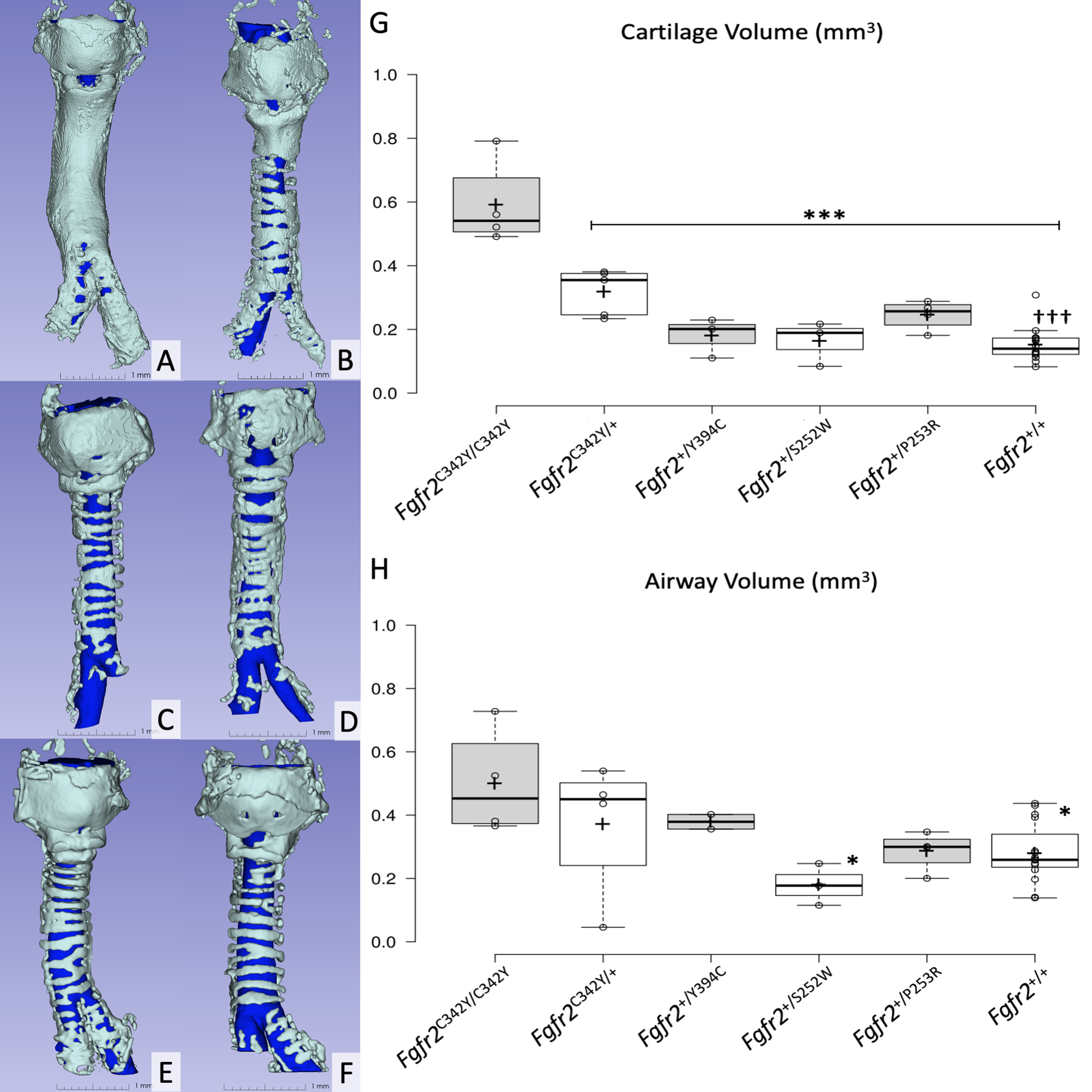
Panels A-F represent 3D-reconstructions of microcomputed-tomography specimens, A) Fgfr2C342Y/C342Y, B) Fgfr2C342Y/+, C) Fgfr2+/Y394C, D) Fgfr2+/S252W, E) Fgfr2+/P253R and F) Fgfr2+/+. Scale bars = 1.0 mm.
Panels G and H demonstrate box and whisker plots representing cartilage volume and airway volume, respectively. Center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; crosses represent sample means; data points are plotted as open circles.
* p < .05 vs Fgfr2C342Y/C342Y
*** p < .001 vs Fgfr2C342Y/C342Y
††† p < .001 vs Fgfr2C342Y/+
Table 3:
Microcomputed-tomography data comparisons between Fgfr2 gene variants and controls (pooled). n – number of samples per group. SD – standard deviation.
| Group | n | mean (± SD) | p-value |
|---|---|---|---|
| Cartilage Volume (mm3) | <.001 | ||
| Fgfr2C342Y/C342Y | 4 | 0.59 (±0.14) | |
| Fgfr2C342Y/+ | 5 | 0.32 (±0.07) | |
| Fgfr2+/Y394C | 3 | 0.18 (±0.06) | |
| Fgfr2+/S252W | 3 | 0.16 (±0.07) | |
| Fgfr2+/P253R | 4 | 0.25 (±0.05) | |
| Fgfr2+/+ | 16 | 0.15 (±0.05) | |
| Airway Volume (mm3) | .02 | ||
| Fgfr2C342Y/C342Y | 4 | 0.50 (±0.17) | |
| Fgfr2C342Y/+ | 4 | 0.37 (±0.22) | |
| Fgfr2+/Y394C | 2 | 0.38 (±0.03) | |
| Fgfr2+/S252W | 3 | 0.18 (±0.07) | |
| Fgfr2+/P253R | 4 | 0.29 (±0.06) | |
| Fgfr2+/+ | 16 | 0.28 (±0.09) | |
A significant between group difference in mean airway volumes was detected (F(5, 27) = 3.34, p = .02). Airway volume values for each group are provided in Table 3. Mean airway volume was greatest among Fgfr2C342Y/C342Y tracheas and least in the Fgfr2+/S252W group. Airway volume was similar among Fgfr2C342Y/+ and Fgfr2+/Y394C tracheas, whereas the Fgfr2+/P253R group demonstrated volumes similar to the Fgfr2+/+ group. The Fgfr2C342Y/C342Y group demonstrated significantly greater mean airway volumes than both the Fgfr2+/S252W and Fgfr2+/+ groups (p = .02 and p = .03, respectively). No other between group differences for airway volume reached statistical significance (Figure 3H).
Histopathologic evaluation:
Representative histopathologic images are provided in Figure 4A–D. Microscopic examination of coronal and transverse tracheal sections confirmed the presence of TCS in the Fgfr2C342Y/C342Y and Fgfr2C342Y/+ groups (Figure 4B,D). All other groups appeared similar to the representative images in Figure 4A,C. No other consistent structural abnormalities were seen in hyaline cartilage rings, trachealis muscles or intercartilaginous spaces. There were no distinguishable abnormalities in chondrocyte cytologic appearance, cell size, or presence of binucleated forms among groups, compared to their line-specific controls. Occasional mitotic figures within chondrocytes were seen in all cases (Figure 4C, D). Morphometric assessment of cell density per cartilage area demonstrated no significant between group differences (F(5, 12) = 1.30, p = .3) (Figure 4E).
Figure 4:
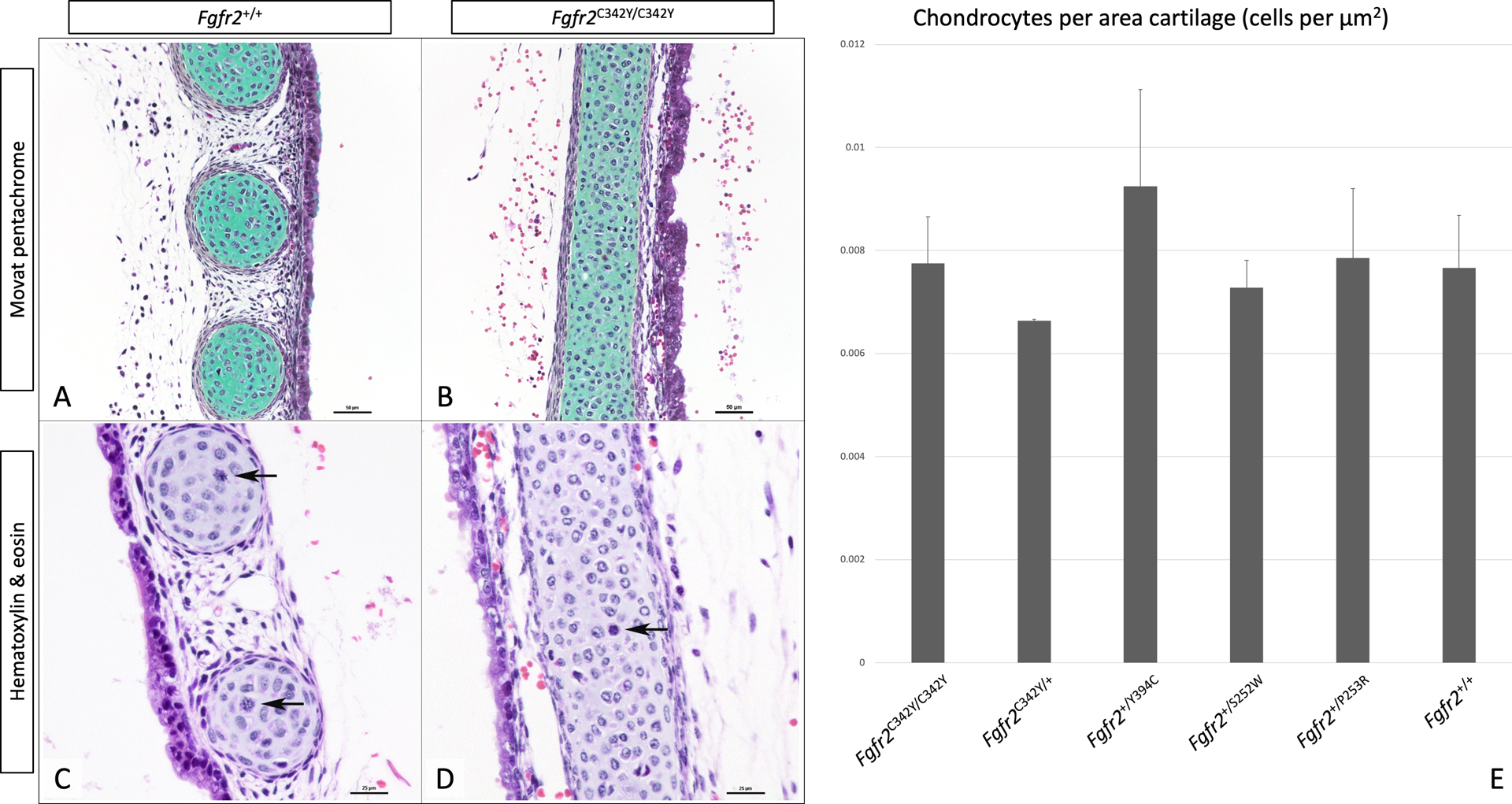
Representative images of Movat pentachrome stain (panels A, B) and hematoxylin & eosin stain (panels C, D) of Fgfr2+/+ (panels A, C), and Fgfr2C342Y/C342Y (panels B, D) highlight cartilage (stained green) and mitotic figures (arrows). Panel E shows a bar graph representing the number of chondrocytes per area of hyaline cartilage as assessed on 5 random images taken at 40x for 2 samples per group. Error bars represent standard deviations. Scale bars = 50 μm for panels A, B, and 25 μm for panels C, D.
Additional data:
Data for comparisons with individual line-specific control groups are provided in Supplemental Tables S1–S4. Additional Fgfr2 variant comparison data from evaluations of tracheal morphology, tracheal cartilage dimensions, microcomputed-tomography and histology is presented in Supplemental Table S5.
Discussion:
Given the significant clinical impact of TCS phenotypes, we evaluated tracheal morphology using knock-in mouse models of Fgfr2-associated craniosynostosis syndromes. The analysis included mouse models harboring either ligand-independent (Fgfr2C342Y, Fgfr2Y394C) or ligand-dependent (Fgfr2S252W, Fgfr2P253R) activating mutations.14–17 We hypothesized that a genotype-phenotype correlation for TCS malformations would be demonstrated among the tested mouse lines and found that tracheal abnormalities were more frequent and severe among Fgfr2C342Y lines. We also hypothesized that ligand-independent activating Fgfr2 mutations would be associated with TCS malformations at greater incidence and severity than ligand-dependent mutations. While this was seen in Fgfr2C342Y tracheas, it was not observed in the other ligand-independent group (Fgfr2Y394C).
The Fgfr2C342Y/C342Y group was most severely affected, with all tracheas demonstrating exclusively sleeve-type segments. Further, these tracheas exhibited greater amounts of cartilage and tracheal airway volumes. The presence of larger tracheal airways is interesting since tracheal stenosis has been thought possibly associated with TCS.24,25 It is theorized that the tube-like cartilage in TCS may fail to appropriately increase in size with patient growth, leading to a relatively stenotic airway.3 Since our study utilized a single experimental time point, we could not assess the impact of airway growth on stenosis. It is also possible that this observation was an artifact of fixation, with reduced dehydration-related contraction possibly associated with greater amounts of cartilage (as in TCS). Airways did appear smaller in the Fgfr2+/S252W group but were otherwise similar among heterozygous mutants and controls. Since tracheal cartilage abnormalities were generally not found in the Fgfr2+/S252W group, this difference in airway size is likely due to smaller body size, as opposed to morphologically abnormal tracheas. While body size was not assessed in our study, other research has shown P0 mice with the Fgfr2S252W mutation to be smaller than controls.16 Previous studies of P0 Fgfr2+/Y394C and Fgfr2+/P253R mice showed no difference in body size when compared to controls.14,17
Of the heterozygous groups, the Fgfr2C342Y/+ tracheas were most severely affected. Nearly all samples in this group were noted to have fusion of the cricoid and 1st tracheal ring – a finding not observed in any other heterozygous or control sample. Fgfr2C342Y/+ tracheas were also the only heterozygous specimens exhibiting sleeve-type segments, present in 75% of Fgfr2C342Y/+ tracheas. This prevalence is substantially greater than that reported in children with the mutation.3 Frequent use of bronchoscopy as a diagnostic modality in human studies may result in under-diagnosis of TCS, as the subtle and non-specific endoscopic findings make diagnosing TCS extremely difficult.2,3 Noorily et al., observed TCS in humans that frequently did not involve the entire trachea.26 Likewise, we describe a large number of tracheas with sleeved segments that do not extend the entire tracheal length. Bronchoscopy in patients with morphologically similar tracheas would be unlikely to yield a TCS diagnosis since visualization of defined tracheal ring structures could lower the surgeon’s index of suspicion.
Non-sleeve-type rings were also abnormal at high frequencies in Fgfr2C342Y/+ tracheas. In this group, the average proportion of non-sleeve-type abnormal rings per trachea was 40%, whereas other heterozygous groups were similar to controls (10%). The TCS entity has been described as a broad spectrum of cartilaginous abnormalities; samples without overt TCS, but larger burdens of morphologically abnormal rings may represent the more limited disease extent on this spectrum.26
To further examine the spectrum of tracheal cartilage abnormalities and compare genotypes, we quantified the amount of tracheal cartilage by calculating the area of stained cartilage on anterior-posterior photographs and by measuring cartilage volume on μCT scans. Of the heterozygous groups, Fgfr2C342Y/+ had the greatest amounts of cartilage followed by the Fgfr2+/P253R group; the Fgfr2+/Y394C and Fgfr2+/S252W groups had measurements similar to controls. Greater amounts of cartilage in Fgfr2+/P253R mice (ligand-dependent activation) than in Fgfr2+/Y394C mice (ligand-independent activation) was contrary to our hypothesis – that ligand-independent mutations would be associated with TCS at greater incidence and severity than ligand-dependent mutations. Despite activating the same receptor type, different mutations in Fgfr2 may cause differential activation of downstream signaling cascades in different tissues.14 Thus, the Fgfr2P253R mutation may result in signaling upregulation that, to a greater degree, affects pathways involved in tracheal cartilage formation. Interestingly, whereas the Apert-associated variant FGFR2S252W is more commonly associated with TCS in humans, the Fgfr2P253R mutation was associated with greater amounts of tracheal cartilage in our study.3,27 While this finding may also be explained by differential signaling cascade stimulation, another possible explanation would be greater overall Fgfr2 activation in the Fgfr2P253R variant. Both the Fgfr2P253R and Fgfr2S252W mutations affect the linker region between the Ig-like II and III domains, and both are associated with reduced ligand dissociation.9 However, whereas the increased affinity in the Fgfr2S252W variant is ligand-specific, the Fgfr2P253R mutation results in an indiscriminate increased affinity for FGFs.7 Indiscriminate Fgfr2 activation in the Fgfr2P253R group may lead to greater signaling pathway upregulation with subsequently increased abnormal tracheal cartilage development. Genotype-phenotype correlations between Fgfr2 mutations and cartilage phenotypes also may vary with the location or structure of the cartilage. In a study of midface phenotypes of Fgfr2 C342Y/+, Fgfr2+/S252W and Fgfr2+/P253R mutants, increased thickness of nasal cartilages were seen in Fgfr2+/S252W and Fgfr2+/P253R variants.28 A comparison of Meckel’s cartilage between these three genotypes again found significantly increased cartilage thickness only in Fgfr2+/S252W and Fgfr2+/P253R mutants.29
Genetic and phenotypic variability exists within craniosynostosis syndromes.13,30 As our understanding of genotype-phenotype relationships improves, genetic screening of these patients may become increasingly useful for prognostication, genetic counseling and treatment decision making.13 Our study demonstrates varying incidences and severities of tracheal cartilage malformations among four genotypes associated with Fgfr2 craniosynostosis syndromes and TCS phenotypes. This strengthens the notion that genotype information in craniosynostosis may help guide airway evaluation and intervention decisions.
Several limitations are worth noting. There were substantially fewer Fgfr2+/Y394C, Fgfr2+/S252W and Fgfr2+/P253R specimens than Fgfr2C342Y/+. It is possible that more severely affected tracheas would have been present in other groups, had greater numbers of specimens been available. The mouse model of Beare-Stevenson syndrome (Fgfr2+/Y394C) was characterized by Wang et al., and while several abnormalities were noted, tracheal morphology was not described.14 Though TCS has been seen in the human FGFR2Y375C variant, our results suggest that they are not present or are present at low frequency in the mouse model.3,31 Among Apert syndrome-associated FGFR2 mutations, Wang et al. observed TCS in 8% of Fgfr2+/S252W mice.16 The malformation has not been described in Fgfr2+/P253R mice.17 Additional variability may have been introduced by using mice with different genetic backgrounds. We attempted to control for this by using line-specific control groups, but variability could not be controlled in comparisons between the Fgfr2 variants with differing genetic backgrounds (i.e. Fgfr2C342Y – CD1 vs other Fgfr2 variant groups – C57Bl/6J). While at least 10 other FGFR2 mutations have been associated with TCS in humans,3 our study utilized models of four classic TCS-associated mutations available to us at the time of this study. A homozygous model was only available for the Fgfr2C342Y variant.
Conclusion:
TCS is a common life-threatening airway malformation found in patients with syndromic craniosynostosis. This study highlights the variability of TCS formation seen in murine models of Fgfr2-associated craniosynostosis syndromes. The Fgfr2C342Y variant appears to be associated with tracheal cartilage abnormalities occurring more frequently and with greater severity than other tested variants. The presence of higher risk genotypes for tracheal cartilage malformations supports further study of these lines and the investigation of other Fgfr2 variants to better understand their role in tracheal development and TCS formation. This study lays a foundation for future researchers in understanding the relationships between specific FGFR2 mutations and TCS phenotypes.
Supplementary Material
Table S1: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for Fgfr2C342Y groups and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S2: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for the Fgfr2Y394C group and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S3: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for the Fgfr2S252W group and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S4: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for the Fgfr2P253R group and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S5: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data between Fgfr2 gene variants and pooled controls. n – number of samples per group. SD – standard deviation.
Acknowledgments:
We thank Jonas Gustafson BA, Michael Bindschadler PhD, Murat Maga PhD, Neeraja Konuthula MD for their help on this project. Dr. Austin Lam was supported by T32DC000018 from the National Institute on Deafness and Other Communication Disorders during his work on this study. Dr. Ethylin Wang Jabs, Dr. Greg Holmes, and Joshua Rivera were supported in part by grants NIH/NIDCR R01 DE022988 and NIH/NICHD P01 HD078233.
Footnotes
Conflicts of Interest Statement: None of the authors have any conflicts of interests to disclose.
References:
- 1.Alli A, Gupta S, Elloy MD, Wyatt M. Laryngotracheal anomalies in children with syndromic craniosynostosis undergoing tracheostomy. J Craniofac Surg. 2013;24(4):1423–1427. doi: 10.1097/SCS.0b013e3182953b43 [DOI] [PubMed] [Google Scholar]
- 2.Inglis AF, Kokesh J, Siebert J, Richardson MA. Vertically Fused Tracheal Cartilage: An Underrecognized Anomaly. Arch Otolaryngol Head Neck Surg. 1992;118(4):436–438. doi: 10.1001/archotol.1992.01880040102017 [DOI] [PubMed] [Google Scholar]
- 3.Wenger TL, Dahl J, Bhoj EJ, et al. Tracheal cartilaginous sleeves in children with syndromic craniosynostosis. Genet Med. 2017;19(1):62–68. doi: 10.1038/gim.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lertsburapa K, Schroeder JW, Sullivan C. Tracheal cartilaginous sleeve in patients with craniosynostosis syndromes: A meta-analysis. J Pediatr Surg. 2010;45(7):1438–1444. doi: 10.1016/j.jpedsurg.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 5.Stater BJ, Oomen KPQ, Modi VK. Tracheal cartilaginous sleeve association with syndromic midface hypoplasia. JAMA Otolaryngol - Head Neck Surg. 2015;141(1):73–77. doi: 10.1001/jamaoto.2014.2790 [DOI] [PubMed] [Google Scholar]
- 6.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2 SPEC. ISS.):139–149. doi: 10.1016/j.cytogfr.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Robin NH, Falk MJ, Haldeman-Englert CR. FGFR-Related Craniosynostosis Syndromes. 1998 Oct 20 [Updated 2011 Jun 7]. In: Adam MP, Ardinger HH, Pagon RA, et al. , editors. GeneReviews® [Internet]. Seattle WA: ): University of Washington, Seattle; 1993–2019.; 1993. [Google Scholar]
- 8.Snyder-Warwick AK, Perlyn CA, Pan J, Yu K, Zhang L, Ornitz DM. Analysis of a gain-of-function FGFR2 Crouzon mutation provides evidence of loss of function activity in the etiology of cleft palate. Proc Natl Acad Sci U S A. 2010;107(6):2515–2520. doi: 10.1073/pnas.0913985107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkie AOM. Craniosynostosis: Genes and mechanisms. Hum Mol Genet. 1997;6(10 REV. ISS.):1647–1656. doi: 10.1093/hmg/6.10.1647 [DOI] [PubMed] [Google Scholar]
- 10.Webster MK, Donoghue DJ. FGFR activation in skeletal disorders: Too much of a good thing. Trends Genet. 1997;13(5):178–182. doi: 10.1016/S0168-9525(97)01131-1 [DOI] [PubMed] [Google Scholar]
- 11.Przylepa KA, Paznekas W, Zhang M, et al. Fibroblast growth factor receptor 2 mutations in Beare–Stevenson cutis gyrata syndrome. Nat Genet. 1996;13(4):492–494. doi: 10.1038/ng0896-492 [DOI] [PubMed] [Google Scholar]
- 12.Ibrahimi OA, Zhang F, Eliseenkova AV, Itoh N, Linhardt RJ, Mohammadi M. Biochemical analysis of pathogenic ligand-dependent FGFR2 mutations suggests distinct pathophysiological mechanisms for craniofacial and limb abnormalities. Hum Mol Genet. 2004;13(19):2313–2324. doi: 10.1093/hmg/ddh235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham ML, Seto ML, Ratisoontorn C, Heike CL, Hing AV. Syndromic craniosynostosis: From history to hydrogen bonds. Orthod Craniofac Res. 2007;10(2):67–81. doi: 10.1111/j.1601-6343.2007.00389.x [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhou X, Oberoi K, et al. p38 inhibition ameliorates skin and skull abnormalities in Fgfr2 Beare-Stevenson mice. J Clin Invest. 2012;122(6):2153–2164. doi: 10.1172/JCI62644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eswarakumar VP, Horowitz MC, Locklin R, Morriss-Kay GM, Lonai P. A gain-of-function mutation of Fgfr2c demonstrates the roles of this receptor variant in osteogenesis. Proc Natl Acad Sci U S A. 2004;101(34):12555–12560. doi: 10.1073/pnas.0405031101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Xiao R, Yang F, et al. Abnormalities in cartilage and bone development in the Apert syndrome FGFR2+/S252W mouse. Development. 2005;132(15):3537–3548. doi: 10.1242/dev.01914 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Sun M, Uhlhorn VL, et al. Activation of p38 MAPK pathway in the skull abnormalities of Apert syndrome Fgfr2+P253R mice. BMC Dev Biol. 2010;10:22. doi: 10.1186/1471-213X-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigueur D, Lyons KM. Whole-Mount Skeletal Staining. In: Hilton MJ, ed. Skeletal Development and Repair: Methods and Protocols. Vol 1130. Methods in Molecular Biology Humana Press; 2014:113–121. doi: 10.1007/978-1-62703-989-5_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Premakumar Y, Griffin MF, Szarko M. Morphometric characterisation of human tracheas: Focus on cartilaginous ring variation. BMC Res Notes. 2018;11(1). doi: 10.1186/s13104-018-3123-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasband WS. ImageJ, Version 1.52a. U. S. National Institutes of Health https://imagej.nih.gov/ij/, 1997–2018
- 21.Lesciotto KM, Perrine SMM, Kawasaki M, et al. Phosphotungstic acid enhanced microCT: optimized protocols for embryonic and early postnatal mice. Dev Dyn Off Publ Am Assoc Anat. 2020;249(4):573–585. doi: 10.1002/dvdy.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaiontz C Real Statistics Resource Pack software. Published online 2015.
- 24.Hockstein NG, McDonald-McGinn D, Zackai E, Bartlett S, Huff DS, Jacobs IN. Tracheal anomalies in Pfeiffer syndrome. Arch Otolaryngol - Head Neck Surg. 2004;130(11):1298–1302. doi: 10.1001/archotol.130.11.1298 [DOI] [PubMed] [Google Scholar]
- 25.Chen JC, Holinger LD. Congenital tracheal anomalies: Pathology study using serial macrosections and review of the literature. Fetal Pediatr Pathol. 1994;14(3):513–537. doi: 10.3109/15513819409024281 [DOI] [PubMed] [Google Scholar]
- 26.Noorily MR, Farmer DL, Belenky WM, Philippart AI. Congenital tracheal anomalies in the craniosynostosis syndromes. J Pediatr Surg. 1999;34(6):1036–1039. doi: 10.1016/S0022-3468(99)90787-X [DOI] [PubMed] [Google Scholar]
- 27.Zenner K, Bonilla-Velez J, Johnson K, Bly RA. Slide tracheoplasty to repair stenotic tracheal cartilaginous sleeve with advanced surgical planning. Otolaryngol Neck Surg. Published online April 14, 2020:0194599820915469. doi: 10.1177/0194599820915469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes G, O’Rourke C, Motch Perrine SM, et al. Midface and upper airway dysgenesis in FGFR2-related craniosynostosis involves multiple tissue-specific and cell cycle effects. Dev Camb Engl. 2018;145(19). doi: 10.1242/dev.166488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motch Perrine SM, Wu M, Stephens NB, et al. Mandibular dysmorphology due to abnormal embryonic osteogenesis in FGFR2-related craniosynostosis mice. Dis Model Mech. 2019;12(5). doi: 10.1242/dmm.038513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullikcn JB, Steinberger D, Kunze S, Müller U. Molecular diagnosis of bilateral coronal synostosis. Plast Reconstr Surg. 1999;104(6):1603–1615. doi: 10.1097/00006534-199911000-00001 [DOI] [PubMed] [Google Scholar]
- 31.Seki E, Enomoto K, Tanoue K, Tanaka M, Kurosawa K. Tracheal cartilaginous sleeve in patients with Beare-Stevenson syndrome. Congenit Anom. 2020;60(3):97–99. doi: 10.1111/cga.12352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for Fgfr2C342Y groups and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S2: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for the Fgfr2Y394C group and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S3: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for the Fgfr2S252W group and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S4: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data for the Fgfr2P253R group and their line-specific controls. n – number of samples per group. SD – standard deviation.
Table S5: Whole-mount morphology data, alcian blue staining measures, microcomputed-tomography and histology data between Fgfr2 gene variants and pooled controls. n – number of samples per group. SD – standard deviation.


