Abstract
Animal studies have established that acute alcohol increases neural inhibition and that frequent intoxication episodes elicit neuroadaptive changes in the excitatory/inhibitory neurotransmission balance. To compensate for the depressant effects of alcohol, neural hyperexcitability develops in alcohol use disorder and is manifested through withdrawal symptoms. It is unclear, however, whether neuroadaptive changes can be observed in young, emerging adults at lower levels of consumption in the absence of withdrawal symptoms. Here we used an anatomically-constrained magnetoencephalography method to assess cortical excitability in two independent sets of experiments. We measured early visual activity 1) in social drinkers during alcohol intoxication vs. placebo conditions and 2) in parallel cohorts of sober binge (BDs) and light drinkers (LDs). Acute alcohol intoxication attenuated early sensory activity in the visual cortex in social drinkers, confirming its inhibitory effects on neurotransmission. In contrast, sober BDs showed greater neural responsivity compared to a matched group of LDs. A positive correlation between alcohol consumption and neural activity in BDs is indicative of cortical hyperexcitability associated with hazardous drinking. Furthermore, neural responsivity was positively correlated with alcohol intake in social drinkers whose drinking did not reach binge levels. This study provides novel evidence of compensatory imbalance reflected in the downregulation of inhibitory and upregulation of excitatory signaling associated with BD in young, emerging adults. By contrasting acute effects and a history of BD, these results support the mechanistic model of allostasis. Direct neural measures are sensitive to synaptic currents and could serve as biomarkers of neuroadaptation.
Keywords: allostasis, alcohol, GABA, Magnetoencephalography
INTRODUCTION
Alcohol consumption is pervasive in western cultures with binge drinking (BD) being the dominant pattern of alcohol misuse during adolescence and early adulthood1. With rising prevalence rates and harmful consequences2, hazardous alcohol intake is an increasingly important public health concern. Furthermore, brain development continues at least until the age of 253 and this period overlaps with the age-range in which BD most frequently occurs1,3, rendering critical brain maturation processes vulnerable to excessive alcohol exposure.
Binge, or heavy episodic drinking, is characterized by consuming large quantities of alcohol in a short time interval interspersed with periods of abstinence. It is commonly defined as drinking enough alcohol to reach blood alcohol concentration (BAC) of 0.08%, which is approximated with an intake of 5/4 drinks for males/females within a 2-hour interval4. However, many individuals, particularly those in their early 20’s, consume alcohol at much higher levels5, which is accompanied by harmful consequences that scale with intake2. An individual’s drinking patterns and the associated deleterious effects emerge from a complex interplay of psychosocial and heritable factors whose influence varies over the lifespan6. Given BDś repeated and hazardous exposure to alcohol, it is likely that BD results in long-term neural changes that persist beyond the binge episode, but the human evidence for this is limited7. Because most individuals engaging in high-intensity drinking are young and vulnerable to neurotoxicity, elucidating the neural alterations associated with BD patterns is of paramount importance.
Animal studies provide extensive and detailed pharmacological evidence on alcohol-induced changes in neurotransmission8. As illustrated in Figure 1, alcohol acutely enhances GABA mediated inhibition9 while reducing the excitatory glutamatergic activity10, in addition to affecting other receptor targets6. It is well established that short-term acute alcohol intoxication has depressant effects overall, as reflected in sedation, anxiolysis, and a range of behavioral impairments6. Human studies confirm that acute intoxication blunts overall neural responsivity11,12 and dysregulates long-range co-oscillations13.
Figure 1.
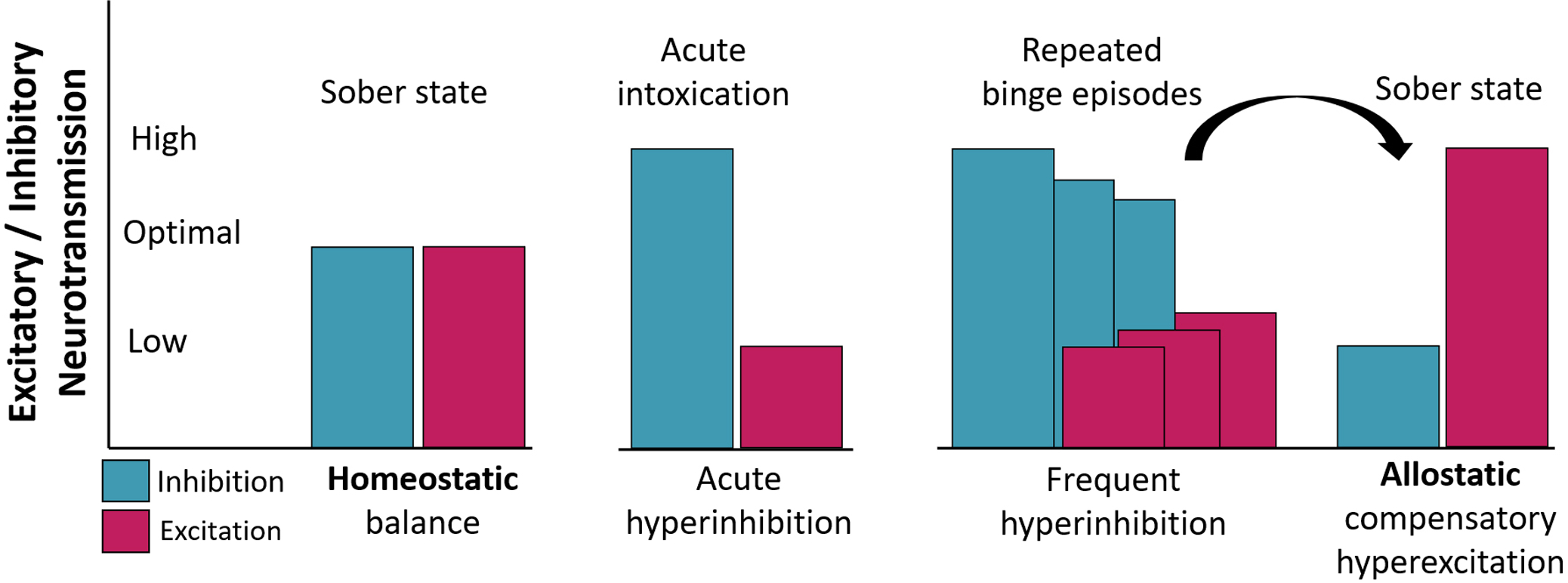
A model of neuroadaptive changes in excitatory/inhibitory (E/I) neurotransmission balance as a function of acute intoxication and frequent binge drinking. Acute intoxication enhances GABA-mediated inhibition and reduces glutamatergic excitation. Consistent with a model of allostasis20–22, repeated binge episodes result in neuroadaptive changes affecting the E/I balance. To compensate for the depressant effects of alcohol, inhibitory signaling is downregulated and the excitatory function is enhanced, maintaining allostatic balance when alcohol is present in the system. In a sober state, however, these countervailing neuroadaptive changes are reflected in neural hyperexcitability.
The allostatic model of BD (Figure 1) posits that repeated episodes of hazardous intake elicit neuroadaptive changes to compensate for the depressant effects of alcohol and restore equilibrium. These countervailing effects include down-regulation of GABAA receptors leading to decreased inhibitory neurotransmission, as well as upregulation of glutamatergic, primarily NMDA, receptor expression which increases excitation8. Overall, these neuroadaptive changes are reflected in enhanced hyperexcitability upon cessation of drinking as manifested in withdrawal symptoms including insomnia, irritability, and anxiety14. Nota bene: terms such as excitation, inhibition, hyperexcitability, or depressant effects refer to the basic neurotransmission processes and not to behavioral or systems level effects. Neuroadaptive changes also underlie the development of alcohol tolerance, physiological dependence, and the risk of relapse. They are manifested as long-lasting CNS hyperexcitability termed “subacute withdrawal syndrome”15. These counter-regulated effects have been formalized in an influential neurobiological opponent-process theory of motivation16,17. It encompasses a range of allostatic (i.e. “stability through change”) mechanisms18 that characterize the development of compulsive drinking and maintenance of alcohol use disorders (AUD) primarily through hedonic and motivational dysregulation. This conceptual framework is supported by extensive evidence from animal studies. In contrast, human research on neuroadaptation to hazardous drinking is exceedingly scarce19. Withdrawal symptoms, indirect indicators of neuroadaptation, are commonly assessed with subjective self-reports, endocrine assays, and peripheral measures20. However, currently lacking is an objective, direct measure of neural hyperexcitability that is sensitive to neuroadaptive changes in the absence of tremors, palpitations, or other withdrawal symptoms.
Electrophysiological methods, comprising magneto- and electroencephalography (MEG/EEG), reflect postsynaptic neural currents directly, making them well suited for pharmacological studies11 and granting them millisecond temporal resolution. Furthermore, these non-invasive direct measures are sensitive to differences in neural activity between binge drinkers (BDs) and light drinkers (LDs) in the absence of deficits in cognitive performance or neuropsychological impairments21–25.
To examine the balance of the excitatory/inhibitory (E/I) signaling within the allostasis framework (Figure 1), we focused on the stimulus-induced early neural responsivity of the occipital cortex and were not concerned with the cognitive processing stages downstream26. We and others have shown that acute alcohol intoxication attenuates early visual responses in social drinkers27–31, as predicted by an alcohol-induced increase in GABAergic activity and reduction of glutamatergic signaling8. Furthermore, the sensitivity of the early visual response to alcohol is consistent with proton magnetic resonance spectroscopy, (1H-MRS) evidence of high concentrations of GABA in the visual cortex32 and with the finding that stimulus-evoked activity is dominated by synaptic inhibition during wakefulness33. Therefore, following a mechanistic model of allostasis8,16,17, we hypothesized that habitual BD results in enduring neural hyperexcitability as manifested as greater visually-evoked activity in individuals engaging in BD compared to LD.
Our overall aim was to examine the basic tenets of the allostasis framework in humans. In two independent experiments we compared early sensory responsivity to visual stimuli in cohorts of young adults. To examine the effects of acute alcohol intoxication on early visual activity, we administered a moderate alcohol dose and placebo to a group of social drinkers in two counterbalanced sessions. Furthermore, to investigate the allostatic indices of neuroadaptation, we compared the early visual responsivity between matched cohorts of sober BDs and LDs. Data for each experiment were acquired with equivalent MEG devices and analyzed using the same methods. We used an anatomically-constrained MEG (aMEG) technique in which distributed source modeling of the MEG signal is informed by structural MRI and cortical reconstruction, to obtain noise-normalized source estimates of early visual responses during acute intoxication vs placebo and in BD vs LD groups. The resulting “brain movies” reflect statistical parametric maps of estimated neural activity in time domain and provide insight into the spatio-temporal characteristics of stimulus processing with exquisite temporal resolution34.
METHODS
Participants
Two independent cohorts of young, healthy, right-handed adults participated in the two arms of this study as detailed below and described in previous companion publications23,29,35. One group comprised social drinkers who took part in an acute alcohol challenge/placebo experiment in a within-subject design. The other cohort comprised demographically matched groups of BDs and LDs who were scanned when sober. Across both experiments, all participants were medication-free and reported no current health issues, no history of seizures or neuropsychiatric disorders, and no head injuries resulting in a loss of consciousness. They reported no family history of alcohol or drug abuse and had never been in treatment or arrested for drug or alcohol related offenses. All participants gave written informed consent approved by the relevant institutional review boards.
Social Drinkers: Acute Intoxication vs. Placebo
Twenty-two young adults (12 males, mean ± SD = 24.9 ± 4.5 years of age) took part in all sessions of the experiment in the Boston area (USA)29,35. They were all non-smokers who reported no alcoholism-related symptoms, describing themselves as light social drinkers. They reported drinking alcohol 2.0 ± 1.1 times per week in low-to-moderate amounts (2.8 ± 0.9) drinks per drinking day. Potential participants were excluded if they reported exceeding moderate drinking levels, defined by NIAAA as consuming up to 14 drinks for men and 7 drinks for women per week on average, or if they reported engaging in binge drinking.
Men and women did not differ in the quantity or frequency of drinking and no individuals abstaining from alcohol were enrolled. Each participant took part in both alcohol and placebo MEG sessions in a within-subject design, in addition to undergoing a structural MRI scan. The two experimental MEG sessions were administered 16 ± 13 days apart in a counterbalanced manner so that half of the participants received alcohol in the first, and placebo in the second session. Both sessions followed the same protocol with the exception of the imbibed beverage.
During the alcohol session, a beverage containing 0.6 g/kg of ethanol for men and 0.55 g/kg for women was administered as a cocktail of 20% v/v of vodka (Gray Goose, Bacardi Limited) mixed with orange juice29,35 targeting blood alcohol concentration of ~0.06%. An equivalent amount of orange juice was given as placebo. Word stimuli were presented in white letters on the black background and subtended the visual angle of 2.8 deg. The stimuli were shown on the screen for 300 ms every 2.5 sec. A fixation string (XXXX) was presented at all other times. Behavioral and neural indices of the processing induced by a lexical visual word recognition task have been reported elsewhere29,35. At the end of each session participants completed a series of questions on subjective effects of the administered beverage. They were provided transportation to their homes.
Binge vs. Light Drinkers: No beverage administered
Fifty-one first-year students of the Complutense University of Madrid (Madrid, Spain) participated in both, the MEG and MRI sessions23. They were assigned to a binge drinking (BD) group (N = 25, 13 females) or to a light drinking (LD) group (N = 26, 14 females) based on a questionnaire and a semi-structured interview inquiring about their alcohol and other drug use. Participants provided a record of their daily alcohol consumption indicating the type(s) and the quantity of the beverage(s) they consumed in the past month as well as the length of time (in hours) it took them to imbibe these beverages (Table 1). Their estimated Blood Alcohol Concentration (est-BAC) was calculated based on the information they provided for the past month, as well as their gender and weight23. We considered the estBAC to roughly represent the max BD level reached by each participant in the previous month. Participants reaching an estBAC of 0.08% or above at least once during the past month were classified as BDs. The low drinking (LD) group consisted of students who never achieved that BAC (Table 1). It is important to note that the legal drinking age in Spain is 18 years old. Participants completed the Alcohol Use Disorders Identification Test (AUDIT)36 and those scoring 20 or above were excluded for having AUD. Four participants reported smoking tobacco regularly (3 BDs and 1 LD).
Table 1.
Demographic and drinking-related variables of Light drinkers and Binge drinkers
| Light drinkers | Binge drinkers | p-values | |
|---|---|---|---|
| N (females) | 26 (14) | 25 (11) | 0.76 (Chi2) |
| Age | 18±0.8 | 18±0.4 | 0.93 |
| Estimated BAC | 0.017±0.02 | 0.17±0.072 | 0.021* |
| Months drinking | 4.55±3.71 | 20.67±10.45 | 0.013* |
| Age of 1st drink | 16.6±1.01 | 14.92±1.08 | 0.93 |
| Drunk days/past month | 0 | 4.84±2.56 | 0.00* |
| Total AUDIT score | 1.7±1.2 | 8.5±2.6 | 0.015* |
estBAC: maximal estimated Blood Alcohol Concentration reached during the past month; AUDIT: Alcohol Use Disorders Identification Test. Group mean ± SD is given for all continuous variables. Mean differences were computed with independent sample t-tests.
Participants performed an equal probability Go/NoGo task requiring target detection. Blue or green squares or circles subtended the visual angle of 3.4 deg and were presented for 100 ms every 1,400 ± 200 ms.
Data acquisition and analysis
MEG
The two experiments were carried out independently using equivalent Vectorview instruments (Elekta-Neuromag) located in magnetically and electrically shielded rooms. Both data sets were analyzed with the same processing stream in the Spatio-Temporal Brain Imaging Laboratory in San Diego. High-density, whole-head MEG signals were recorded from 204 channels comprising 102 pairs of planar gradiometers. Precise co-registration with the structural MRI images was achieved by digitizing the nasion and preauricular points, the position of magnetic coils attached to the skull, and a large array of random points with a 3Space Isotrak II system. Data analysis relied on custom-made MATLAB (Mathworks, Natick, MA) routines and publicly available packages including FieldTrip, EEGLab37, and MNE. Epochs extending −300 to 700 ms relative to stimulus onset were bandpass filtered 0.5 and 40 Hz, downsampled to 250 Hz, and baseline corrected using the prestimulus period. A combination of automatic threshold rejection and an independent component analysis method37 was used to remove eye-blinks and other artifacts. Comparable number of trials (N = 200) were averaged in both experiments.
An anatomically-constrained linear minimum-norm estimation procedure was used to obtain noise-normalized source estimates26,34. Noise covariance was calculated from the prestimulus periods across data epochs and used for inverse calculation resulting in “brain movies” or dynamic statistical parametric maps (dSPM) of the estimated noise-normalized dipole strengths. The estimated source dipole strengths are expressed as the square root of an F-statistic reflecting the likelihood that a particular patch of cortex is more active than the baseline at each timepoint. Group average dSPMs of the placebo – alcohol differences are shown in Figure 2, along with group averages of the timecourses of a region-of-interest (ROI) estimated to the medial occipital cortex (Occ). Similarly, dSPM differences showing LDs < BDs average activity are shown in Figure 3, together with average timecourses of the Occ ROI. Comparisons of the visual activity during acute intoxication vs placebo and in BDs vs LDs were carried out with the appropriate t-tests on the timecourses within 100–180 ms in the Occ ROI. It is important to note that, even though the two experiments did not use the same visual paradigm, we focused on the early activation of the retinotopic visual cortex which is sensitive to low-level visual features. Furthermore, the comparisons were carried out within each experimental setup respectively. The factor of gender was included initially, but because it exerted no significant effects, the reported results were pooled across the gender factor.
Figure 2.
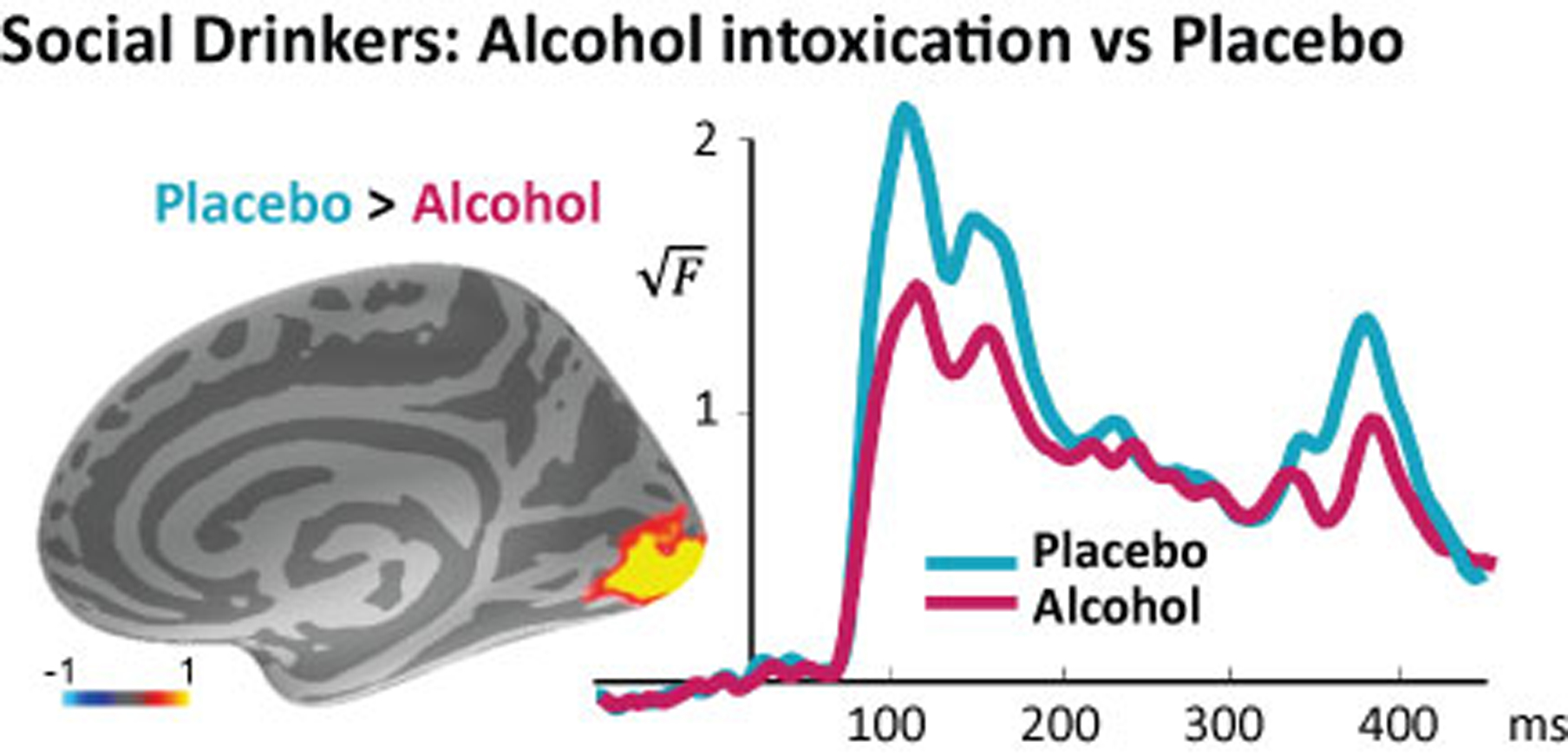
MEG source estimate of the group average differential activity between placebo vs alcohol conditions (left) in the occipital cortex (Occ). Group average time courses of the estimated noise-normalized dipole strength in the Occ (right). Acute alcohol intoxication decreased the early visual activity in the occipital cortex within the time window indicated with a bar on the x-axis.
Figure 3.
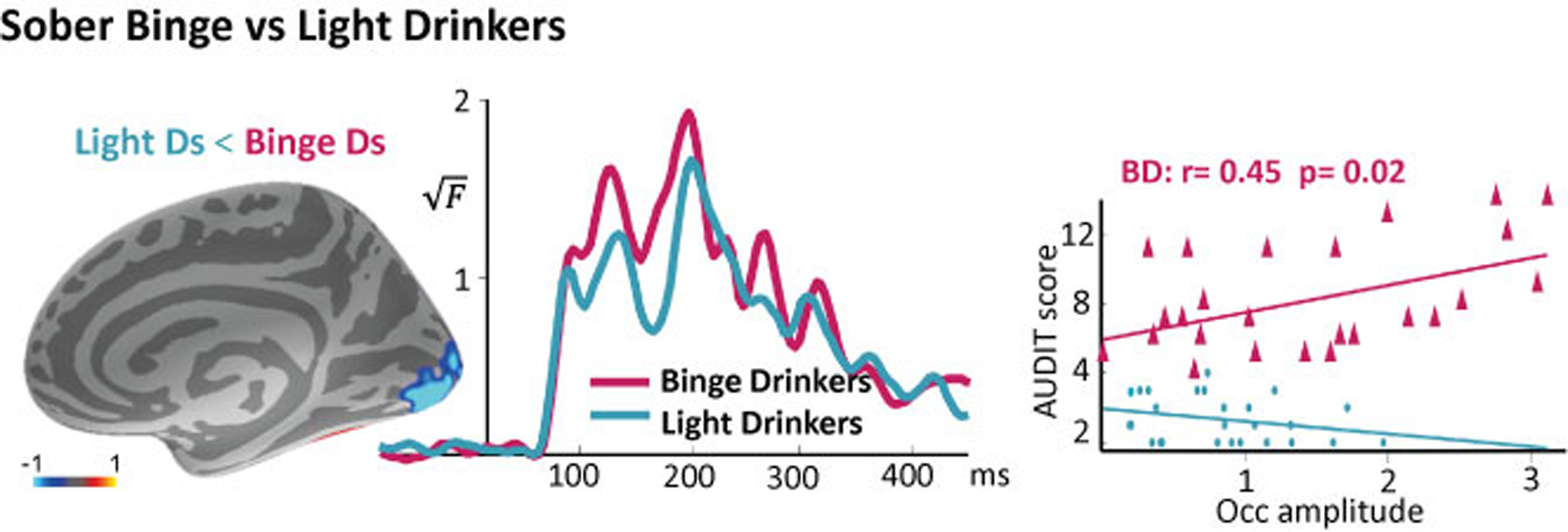
Group average difference map of activity estimates in LDs minus BDs (left). The time courses associated with the activity in the occipital cortex indicate hyperexcitability in BDs (middle). Visual responsivity in BDs correlates with hazardous levels of their alcohol intake (right) as measured with AUDIT (Alcohol Use Disorders Identification Test).
MRI
Structural MRI scans were obtained with standard T1 protocols23,35. Each participant’s cortical surface was reconstructed from the T1-weighted images with FreeSurfer and served to constrain inverse estimates, defining the solution space with approximately 5000 free-rotating dipoles spaced 7 mm apart. The inner skull surface was derived from the segmented MRI data and used as a boundary element model of the volume conductor in the forward calculations. For purposes of group averaging, the reconstructed individual surfaces were morphed into and average representation based on the sulcal-gyral pattern alignment26,34.
RESULTS
Social drinkers: Alcohol intoxication vs placebo
We compared the early visual responses during alcohol vs. non-alcohol beverage conditions in a within-subject design in social drinkers. These individuals drink occasionally at low to moderate levels, but their consumption does not reach binge levels. As expected, we found that alcohol significantly attenuated early responses in the occipital cortex (Figure 2), t(23) = 3.09, p < 0.01. The alcohol-induced attenuation of the visual response confirms that alcohol exerts inhibitory effects on neurotransmission as mediated by increasing GABA and decreasing glutamatergic function. This effect is especially visible in the visual cortex due to its relatively high concentration of GABA32. Please note that the stimulus length was 300 ms. Consequently, the sensitivity of the visual cortex to the onset and offset of the visual stimuli is reflected in the peaks at ~100ms and ~400ms respectively, visible in the time courses.
Sober Binge vs Light Drinkers
To test the mechanistic predictions of the allostasis account, we assessed whether BDs show altered early sensory responses compared to LDs, as it would be expected based on their heavier alcohol intake. As shown in Figure 3, the early responsivity of the occipital cortex was greater in BDs compared to LDs, t (51) = 2.06, p < 0.05 which is indicative of cortical hyperexcitability. Furthermore, it correlated positively with the total AUDIT score in the BD group, r = 0.45, p < 0.05, indicating that greater visual response amplitudes are associated with heavier drinking levels. Because the stimuli were presented for 100 ms, the “on” and “off” visual responses are visible as peaks at ~100 and ~200 ms respectively.
How much is too much? Hyperexcitability is associated with non-binge consumption in social drinkers
Recent evidence form very large scale studies combining data from millions of participants indicates that there are no “safe drinking” levels and that harmful health outcomes are minimized by zero alcohol intake38. Therefore, we wondered whether the hyperexcitability signature of the BD group would be mirrored in social drinkers who imbibe at moderately high levels. We found that the visual responsivity of social drinkers when sober was positively correlated with the number of drinks consumed per drinking day, r= 0.61, p= 0.01 (Figure 4). This finding suggests that hyperexcitability, which is indicative of compensatory neuroadaptation, is detectable even at non-binge levels of alcohol consumption.
Figure 4.
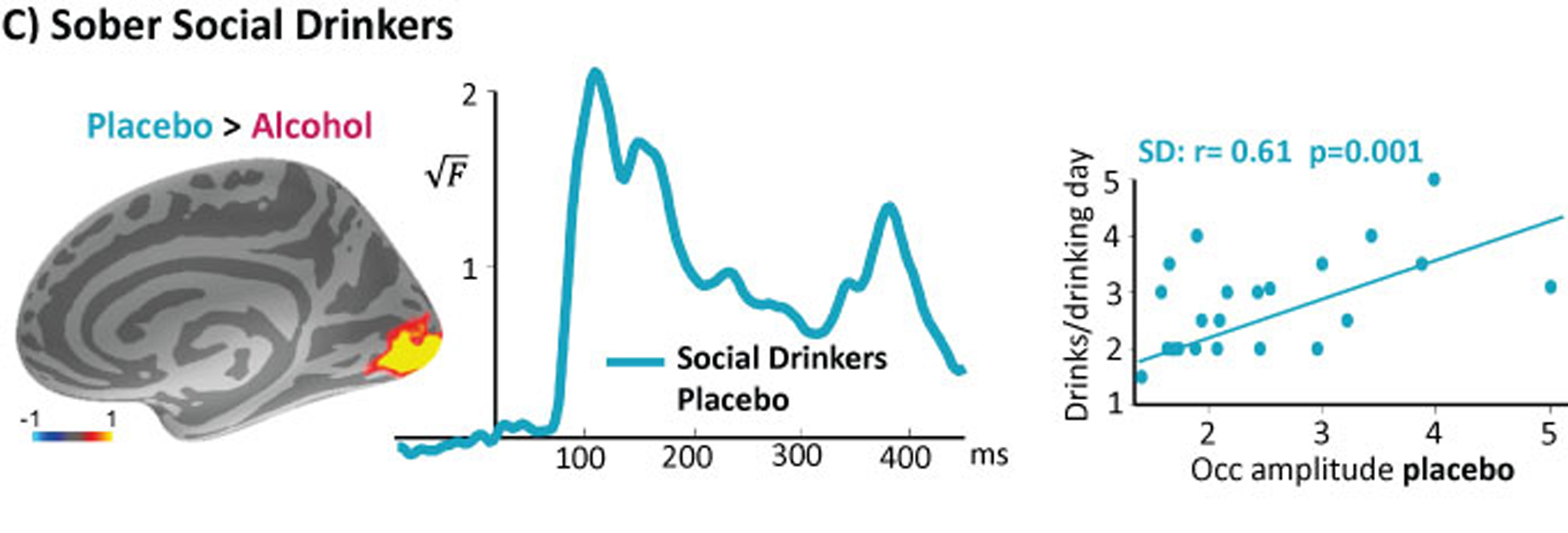
Group average early visual activity estimated to the occipital cortex in sober social drinkers is positively correlated with alcohol intake on a typical drinking day, suggesting that hyperexcitability is associated with consumption even when it does not reach binge levels.
DISCUSSION
The present study examined neuroadaptive hyperexcitability associated with BD in the context of the effects of acute intoxication on the early cortical responsivity. The results are the first to provide novel human evidence in support of a mechanistic conceptual framework of allostasis16,17,39 (Figure 1). Acute alcohol intoxication attenuated early sensory activity in the visual cortex of social drinkers (Figure 2), confirming its inhibitory effects on neurotransmission. In contrast, sober BDs showed greater early responses to visual stimuli compared to a matched group of LDs (Figure 3). The occipital activity in BDs correlated positively with their AUDIT scores, which is indicative of cortical hyperexcitability associated with hazardous drinking. This finding is additionally supported by a positive correlation between neural responsivity during the placebo condition in social drinkers and their alcohol intake (Figure 4). The results suggest that cortical hyperexcitability is associated with BD patterns but can also be detected at non-binge levels of alcohol intake, which could be leveraged to provide individually-tailored feedback on “how much is too much” and promote health and wellness.
The aMEG method employed here is directly sensitive to synaptic currents in real time, which underlies its excellent temporal resolution. Estimates of the spatial origin of the signal can be obtained by constraining inverse solutions to the cortical surface reconstructed from the structural MRI34. Estimates are especially reliable when the generating structures are circumscribed, as is the case with early sensory processing. In order to probe cortical excitability in response to visual stimulation, we focused on the occipital cortex, whose functional circuitry has been studied extensively. It has been well established that GABAergic inhibitory interneurons are essential for fine-tuning the visual cortical network which underlies the specificity of the visual cortex responses40 and that inhibition dominates stimulus-evoked visual activity in awake mice33. The human visual cortex is characterized by relatively high GABA concentration as measured by 1H-MRS32, which is related to more effective performance on visual discrimination tasks41. Acute alcohol intoxication exerts pharmacological effects on multiple brain systems6. However, its primary targets are the two major and most plentiful neurotransmitters, glutamate and its metabolic product GABA. Acute alcohol strengthens inhibitory effects by primarily facilitating the GABAA receptor function, and it also decreases excitatory transmission by blocking the NMDA as well as AMPA glutamate receptor function8–10. Acute alcohol intoxication is accompanied by well-known anxiolytic effects which are similar to those of the pharmacological sedatives such as benzodiazepines and barbiturates, owing to mutual cross-tolerance. Therefore, the attenuation of the early visual response observed during intoxication in the present study is consistent with alcohol-induced inhibitory effects42. Dampening of the early visual response is well established and has been reported in numerous MEG studies27–31,35. Similarly, decreased activity of the visual areas during intoxication has been observed in fMRI studies43.
In contrast, greater evoked visual responses were recorded in sober BDs compared to cohort-matched LDs in the present study. Moreover, the increased visual responsivity correlated with AUDIT scores in BDs which is suggestive of harmful drinking. This finding is indicative of hyperexcitability reflecting neuroadaptive changes due to excessive alcohol intake within a mechanistic framework of allostasis (Figure 1). Drinking at binge- or higher levels of intake results in downregulated GABA-mediated inhibition and upregulated excitatory function8–10,42. Indeed, neurochemistry of the visual cortex is characterized by high plasticity. A direct relationship between GABA levels and activity of the visual cortex has been demonstrated by Lunghi and colleagues44 who used a short-term (2.5 hr) monocular deprivation to manipulate GABA plasticity. The deprivation reduced GABA levels as measured with 1H-MRS and increased the amplitude of the early cortical visual evoked potentials which is consistent with the pharmacological mechanism underlying our results. Therefore, the enhanced early visual response observed in the present study is in agreement with GABA downregulation as as a result of high levels of alcohol intake. This responsivity change could potentially serve as a biomarker of hyperexcitability due to neuroadaptation to alcohol in the absence of frank withdrawal symptoms. Broadly similar findings have been reported in the auditory domain in individuals with AUD by Ahveninen and colleagues19. They observed greater peak amplitude of a positive middle-latency auditory evoked potential deflection with a latency of ~30 ms in individuals with AUD. The peak amplitude correlated negatively with the duration of abstinence indicating that neural hyperexcitability in the primary auditory cortex dissipates with time as long as one refrains from drinking.
Because alcohol acts as an indirect GABA agonist while antagonizing the excitatory glutamatergic system, the relative balance of these two neurotransmitters is of particular interest. Imaging studies have used 1H-MRS to provide in vivo information about regionally-specific chemical composition of brain tissues with an emphasis on low-molecular-weight compounds containing hydrogen. Despite technical challenges presented by spectral overlap of the relevant metabolites, this method is suitable for investigating neurochemical signatures of allostatic changes associated with excessive drinking. Lower GABA levels have been reported in the anterior cingulate cortex (ACC) in BDs which is associated with reduced cognitive performance and more deleterious alcohol use consequences45. This finding is broadly compatible with the present results and may be indicative of neuroadaptive changes at the level of neural signaling in association with BD. Similarly, it has been reported that individuals with AUD have lower levels of GABA46 and higher levels of glutamate in the ACC during an early withdrawal stage47.
The results of the current study confirm the applicability of the allostatic model to humans, as it was originally based on animal research focusing on the reward and stress circuitry16,17,39,48. It has inspired a large number of animal studies investigating the neural basis of reward and motivational mechanisms by outlining stages of addiction and their neurobiological characteristics. The theory proposes that intoxication initially induces positive reinforcement which gradually shifts to negative reinforcement as a result of chronic heavy drinking and the accompanying neuroadaptive changes. Tolerance to alcohol develops over time and is reflected in a lowered hedonic homeostatic set point which is termed allostasis. At this stage, it is hypothesized that withdrawal from alcohol produces changes in the reward signaling opposite to those experienced during acute positive reinforcement, so alcohol dependent individuals drink to stave off withdrawal. The neurochemical changes predicted by the opponent process allostasis framework may contribute to compulsive alcohol consumption and increase vulnerability to relapse and the addiction cycle39,48. This stage is termed the “dark side” of addiction17,48 and is associated with increased anxiety and depression which are known AUD comorbidities49. The neuroadaptive changes can unfold in a protracted manner, depending on each individual’s environmental and social influences, genetic predisposition, and other factors, and may be accompanied by the slow attrition of cognitive faculties50.
It is important to note that the extent of neural changes and deficits in BD may not be fully appreciated based on behavioral measures only. Many studies employ highly functioning young adult BDs (often college students) who perform well on cognitive tests and who show only subtle or no deficits on neuropsychological assessments when compared to LDs7. However, when the neural function is measured directly, group differences are readily apparent in the absence of performance deficits. Such evidence from EEG and MEG studies shows altered neural responses in BDs across different cognitive tasks indicating impact across multiple neurofunctional systems21–24. This further underscore the greater sensitivity of electrophysiological measures to neural changes associated with BD.
In sum, the present study examined the basic tenets of the mechanistic model of allostasis in the context of acute intoxication and BD16,17. To that end, we used an anatomically-constrained MEG method to measure early visual activity in the occipital cortex in social drinkers during alcohol challenge vs. placebo, and in parallel cohorts of sober BDs and LDs. The major findings are as follows: a moderate alcohol dose attenuated early visually-evoked activity by increasing inhibitory signaling and concurrently decreasing glutamatergic excitation. In contrast, sober BDs manifested greater early visual responses in comparison to light drinkers, which is consistent with compensatory neuroadaptive changes reflected in the downregulation of inhibitory and upregulation of excitatory signaling. This is further supported by the observed correlation between the visual responsivity and the level of alcohol intake in both experiments.
Given the cross-sectional nature of this type of observational cohort studies, it is not possible to make causal inferences about the factors underlying the changes of the early visual response. Although the observed differences in neural responsivity between BDs and LDs correlated with AUDIT scores indicating association with alcohol consumption, a possibility that these differences precede alcohol consumption cannot be excluded.
Taken together, these results are in agreement with mechanistic predictions of the allostasis model16–18,39,48. The magnitude of early visual response can potentially serve as a sensitive biomarker of neuroadaptation. Such neural indices could be leveraged to monitor hyperexcitability and to provide insight on “how much is too much”. In the context of recent developments in wearable biosensors, it may be possible to use similar signals for individually-tailored interventions to preempt heavy drinking and promote a healthy lifestyle.
Supplementary Material
ACKNOLEDGEMENTS:
This work was supported by the National Institutes of Health, R01-AA016624, and the National Drug Plan, SPI/2010/051, (Spain). We are grateful to Lauren Beaton and Brendan Cox for their contributions.
REFERENCES
- 1.SAMHSA. Substance Abuse and Mental Health Services Administration. Results from 2012 Natl. Survery Drug Use Heal 13–4795 (2013). [Google Scholar]
- 2.Haber JR, Harris-Olenak B, Burroughs T & Jacob T Residual effects: young adult diagnostic drinking predicts late-life health outcomes. J. Stud. Alcohol Drugs 77, 859–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobus J & Tapert SF Neurotoxic effects of alcohol in adolescence. Annu. Rev. Clin. Psychol 9, 703–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute of Alcohol and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA newsletter, 3 p. 3. (2004). [Google Scholar]
- 5.Terry-McElrath YM & Patrick ME Intoxication and binge and high-intensity drinking among US young adults in their mid-20s. Subst. Abus 37, 597–605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spanagel R Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol. Rev 89, 649–705 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Petit G, Maurage P, Kornreich C, Verbanck P & Campanella S Binge drinking in adolescents: a review of neurophysiological and neuroimaging research. Alcohol Alcohol 49, 198–206 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Roberto M & Varodayan FP Synaptic targets: Chronic alcohol actions. Neuropharmacology 122, 85–99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl). 205, 529–64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovinger DM, White G & Weight FF Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243, 1721–4 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Marinkovic K Neuropsychopharmacology: Recent MEG investigations. in Magnetoencephalography: From signals to dynamic cortical networks (ed. Supek Selma, Aine CJ) 875–900 (2014). [Google Scholar]
- 12.Kovacevic S et al. Theta oscillations are sensitive to both early and late conflict processing stages: Effects of alcohol intoxication. PLoS One 7, 1–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinkovic K, Beaton LE, Rosen BQ, Happer JP & Wagner LC Disruption of frontal lobe neural synchrony during cognitive control by alcohol intoxication. J. Vis. Exp (2019). doi: 10.3791/58839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker HC Alcohol dependence, withdrawal, and relapse. Alcohol Res. Health 31, 348–61 (2008). [PMC free article] [PubMed] [Google Scholar]
- 15.Begleiter H & Porjesz B Persistence of a ‘subacute withdrawal syndrome’ following chronic ethanol intake. Drug Alcohol Depend. 4, 353–357 (1979). [DOI] [PubMed] [Google Scholar]
- 16.Koob G & Le Moal M Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Koob GF & Le Moal M Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. B Biol. Sci 363, 3113–3123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 22, 108–24 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Ahveninen J et al. Post-withdrawal changes in middle-latency auditory evoked potentials in abstinent human alcoholics. Neurosci. Lett 268, 57–60 (1999). [DOI] [PubMed] [Google Scholar]
- 20.King AC, Hasin D, O’Connor SJ, McNamara PJ & Cao D A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol. Psychiatry 79, 489–98 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Holcomb LA, Cruz SM & Marinkovic K Altered oscillatory brain dynamics of emotional processing in young binge drinkers. Cogn. Affect. Behav. Neurosci 18, 43–57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Affan RO et al. High-intensity binge drinking is associated with alterations in spontaneous neural oscillations in young adults. Alcohol 70, 51–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correas A et al. Decreased event-related theta power and phase-synchrony in young binge drinkers during target detection: An anatomically-constrained MEG approach. J. Psychopharmacol 33, 335–346 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holcomb LA, Huang S, Cruz SM & Marinkovic K Neural oscillatory dynamics of inhibitory control in young adult binge drinkers. Biol. Psychol 146, 107732 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurage P et al. Cerebral effects of binge drinking: respective influences of global alcohol intake and consumption pattern. Clin. Neurophysiol 123, 892–901 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Marinkovic K et al. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron 38, 487–97 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell AE, Sumner P, Singh KD & Muthukumaraswamy SD Acute effects of alcohol on stimulus-induced gamma oscillations in human primary visual and motor cortices. Neuropsychopharmacology 39, 2104–2113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marinkovic K, Halgren E & Maltzman I Effects of alcohol on verbal processing: an event-related potential study. Alcohol. Clin. Exp. Res 28, 415–23 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinkovic K, Rosen BQ, Cox B & Kovacevic S Event-related theta power during lexical-semantic retrieval and decision conflict is modulated by alcohol intoxication: anatomically constrained MEG. Front. Psychol 3, 121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen BQ, Padovan N & Marinkovic K Alcohol hits you when it is hard: intoxication, task difficulty, and theta brain oscillations. Alcohol. Clin. Exp. Res 40, 743–752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beaton LE, Azma S & Marinkovic K When the brain changes its mind: Oscillatory dynamics of conflict processing and response switching in a flanker task during alcohol challange. PLoS One (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans CJ, McGonigle DJ & Edden RAE Diurnal stability of γ-aminobutyric acid concentration in visual and sensorimotor cortex. J. Magn. Reson. Imaging 31, 204–209 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Haider B, Häusser M & Carandini M Inhibition dominates sensory responses in the awake cortex. Nature 493, 97–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dale AM et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron 26, 55–67 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Marinkovic K, Rosen BQ, Cox B & Hagler DJ Spatio-temporal processing of words and nonwords: Hemispheric laterality and acute alcohol intoxication. Brain Res. 1558, 18–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders JB, Aasland OG, Babor TF, de la Fuente JR & Grant M Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 88, 791–804 (1993). [DOI] [PubMed] [Google Scholar]
- 37.Delorme A & Makeig S EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Griswold MG et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 392, 1015–1035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George O, Le Moal M & Koob GF Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiol. Behav 106, 58–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura Y, Dantzker JLM & Callaway EM Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–73 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Edden RAE, Muthukumaraswamy SD, Freeman TCA & Singh KD Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci 29, 15721–15726 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clapp P, Bhave SV & Hoffman PL How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res. Health 31, 310–39 (2008). [PMC free article] [PubMed] [Google Scholar]
- 43.Calhoun VD et al. Alcohol intoxication effects on visual perception: An fMRI study. Hum. Brain Mapp 21, 15–26 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lunghi C, Emir UE, Morrone MC & Bridge H Short-term monocular deprivation alters GABA in the adult human visual cortex. Curr. Biol 25, 1496–501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silveri MM et al. Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcohol. Clin. Exp. Res 38, 969–979 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prisciandaro JJ et al. Brain glutamate, GABA, and glutamine levels and associations with recent drinking in treatment‐naïve individuals with Alcohol Use Disorder versus light drinkers. Alcohol. Clin. Exp. Res 43, acer.13931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermann D et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol. Psychiatry 71, 1015–21 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Koob GF & Le Moal M Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat. Neurosci 8, 1442–4 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Petrakis I & Krystal J Neuroscience: implications for treatment. Alcohol Health Res. World 21, 157–60 (1997). [PMC free article] [PubMed] [Google Scholar]
- 50.Oscar-Berman M & Marinković K Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol. Rev 17, 239–57 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


