Abstract
Primary hyperoxaluria type 1 (PH1) is a genetic disorder characterized by overproduction of oxalate and eventual kidney failure. Kidney failure is usually irreversible in PH1. However, in PH1 patients homozygous for the G170R mutation, pyridoxine is an enzyme co-factor and decreases urine oxalate excretion (UOx) by reducing hepatic oxalate production. We report recovery from dialysis in three PH1 patients homozygous for the G170R mutation in response to pharmacologic dose pyridoxine treatment. Median age at initiation or resumption of pyridoxine treatment was 37 years (range 20–53), and pyridoxine dose was 8.8 mg/kg/d (range 6.8–14.0 mg/kg/d). Duration of hemodialysis prior to renal recovery was 10 months (range 5–19). POx improved after recovery of renal function. At a median of 3 months (range 2–46) following discontinuation of hemodialysis, estimated glomerular filtration rate (eGFR) was 34 ml/min/1.73 m2 (range 23–52), plasma oxalate concentration (POx) was 8.8 μmol/L (4.6–11.3), and UOx was 0.93 mmol/24 hours (0.47–1.03). Kidney function was maintained during a median of 3.2 yrs (range 1.3–3.8) of follow-up. These observations suggest that kidney failure may be reversible in a subset of PH1 patients homozygous for the G170R mutation treated with pharmacologic dose pyridoxine.
INTRODUCTION
The primary hyperoxalurias constitute a group of rare autosomal recessive disorders characterized by aberrant metabolism of glyoxylate into oxalate. PH1 is the most common and severe form and accounts for 80% of cases.1 PH1 is caused by mutations in the AGXT gene which encodes the liver-specific peroxisomal enzyme alanine/glyoxylate aminotransferase (AGT). AGT normally converts glyoxylate into glycine within liver peroxisomes. When the enzyme is defective or deficient, glyoxylate is aberrantly converted into oxalate which is eliminated in the urine.2 Excessive UOx can lead to nephrolithiasis, nephrocalcinosis, and progressive chronic kidney disease (CKD).3,4
Once PH1 patients initiate maintenance dialysis, their kidney failure is generally considered irreversible and combined liver and kidney transplantation is the preferred treatment. However, a subset of PH1 patients homozygous for the G170R mutation has a more treatable phenotype in which AGT remains functional but mislocated. Pyridoxine (vitamin B6) is a chemical chaperone for AGT, increasing its activity through peroxisomal targeting.5–7 Thus treating patients with pharmacologic doses of pyridoxine (5–8 mg/kg/day) significantly reduces UOx in heterozygous patients and may even normalize UOx in homozygous patients.8,9 Approximately 9% of PH1 patients are homozygous for the G170R mutation.10 The value of reducing hepatic oxalate production with pyridoxine in patients with advanced CKD is unknown. We here report three G170R homozygous patients with kidney failure who experienced renal recovery allowing dialysis discontinuation after pharmacologic dose pyridoxine treatment. These observations suggest that reducing hepatic oxalate production even in PH1 patients with advanced CKD may be beneficial.
CASE REPORTS
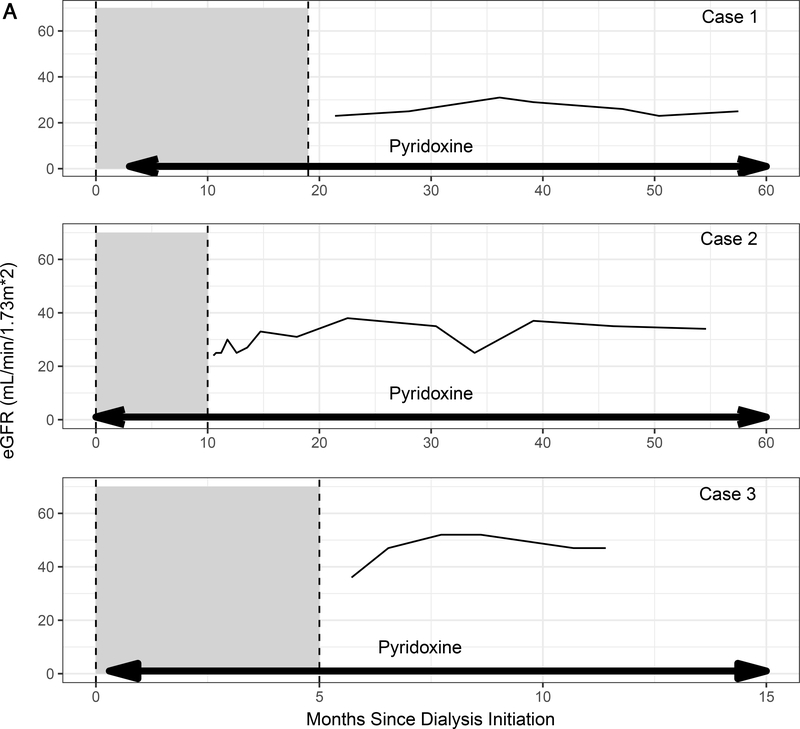
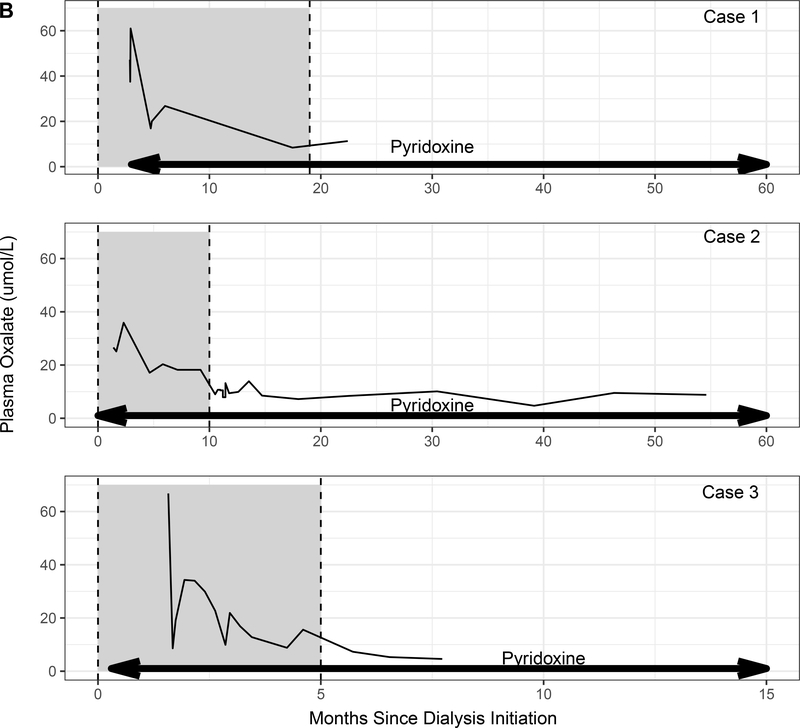
We reviewed patients enrolled in the Rare Kidney Stone Consortium (RKSC) PH Registry, an observational registry involving consented patients under approval of the Mayo Clinic IRB. Among the 41 G170R homozygous PH1 patients in the registry, 23 progressed to kidney failure, of whom 12 were treated with pyridoxine. Pyridoxine was started in 2 patients after more than one year of dialysis and in 8 patients within 3 months of dialysis initiation (including the 3 patients in this series). Subtherapeutic doses of pyridoxine were started prior to ESKD in the remaining 2 patients but did not prevent progression to kidney failure. The 3 patients reported in our series were the only patients to recover sufficient kidney function to stop dialysis. Baseline characteristics of these 3 patients are shown in the Table, and the relationship among pyridoxine treatment, eGFR, POx, and time on dialysis is depicted in Figure 1.
Table 1.
Baseline clinical and laboratory characteristics of G170R homozygous PH1 patients followed at Mayo Clinic who recovered renal function after treatment with pharmacologic dose pyridoxine
| Patient | Age at PH1 symptom1 onset (years) | Age at dialysis initiation (years) | Timing of pyridoxine initiation or resumption | Initial POx3 (μmol/L) | UOx2 before pyridoxine initiation (mmol/24 hrs) |
|---|---|---|---|---|---|
| 1 | 25 | 37 | 3 months after dialysis initiation | 47.2 on dialysis, prior to pyridoxine initiation | 0.95 |
| 2 | 2.5 | 20 | At time of dialysis initiation | 26.6 on dialysis, 6 weeks after pyridoxine initiation | 2.34 |
| 3 | 33 | 53 | Within 1 week after dialysis initiation | 67.9 on dialysis, 6 weeks after pyridoxine resumption | 1.874 |
Symptoms = nephrolithiasis or development of chronic kidney disease
normal Uox ≤ 0.46 mmol/24 hrs
normal Pox < 1.8 mcmol/L, level obtained immediately prior to dialysis session
2 months after pyridoxine started.
Figure 1.
(A) Estimated glomerular filtration rate (eGFR) and (B) plasma oxalate levels in relation to pyridoxine initiation (arrows). Time on dialysis is shown as the shaded area between dotted lines.
Case 1:
A woman developed nephrolithiasis at age 25. At age 37, she presented to an outside institution with a creatinine of 14 mg/dL. Kidney biopsy showed acute tubular injury, calcium oxalate crystals within the tubules and interstitium, and interstitial fibrosis involving 50% of the sample. UOx was 1.32 mmol/24 hrs. She was started on thrice weekly hemodialysis and referred to our institution three months later for transplant evaluation. On presentation to our institution, her pre-dialysis POx was 47.2 μmol/L (normal < 1.8 μmol/L) and and UOx was 0.95 mmol/24 hr (normal <0.46 mmol/24 hr). AGXT genetic testing revealed homozygosity for the G170R mutation. Three months after initiation of hemodialysis, she was started on pyridoxine therapy (14 mg/k/d).
Approximately 20 months after initiation of hemodialysis (17 months after initiation of pyridoxine) hemodialysis was discontinued. Three months after discontinuation of hemodialysis, the patient had a creatinine of 2.5 mg/dL (eGFR 23 ml/min/1.73 m2). POx was 11.3 μmol/L, and Uox was 1.03 mmol/24 hr. After 4.6 years on pyridoxine (3.2 years after dialysis discontinuation), her creatinine remained 2.3 mg/dl.
Case 2:
A 2.5-year-old girl presented to an outside institution with bilateral nephrolithiasis. A diagnosis of PH was presumed due to an elevated UOx. She was started on pyridoxine (2.5 mg/kg/d) but self-discontinued the medication at age 16. At the age of 20, she presented with a creatinine of 5.2 mg/dL. CT imaging showed bilateral nephrolithiasis without obstruction. Kidney biopsy showed acute tubular injury, oxalate crystals within the tubules, and interstitial fibrosis involving 75% of the interstitium. POx was not measured, and UOx was 2.34 mmol/24 hrs/1.73m2. She was started on thrice weekly hemodialysis, and pyridoxine was resumed.
One month after starting dialysis, she was referred to our institution for transplant evaluation. AGXT genetic testing revealed G170R homozygosity. POx obtained 6 weeks after starting hemodialysis was 26.6 μmol/L. Her pyridoxine dose was increased to 6.8 mg/kg/day. Five months after starting pyridoxine, dialysis was reduced to twice weekly and eventually discontinued 11 months after starting pyridoxine. Her creatinine remained stable at 1.8 mg/dl (eGFR 34 ml/min/1.73 m2) 3.8 years after dialysis discontinuation, with POx was 8.8 μmol/L, and UOx was 0.93 mmol/day. Following an obstructive stone episode complicated by acute kidney injury, she experienced a decline in kidney function and resumed hemodialysis 6.5 years after it was originally discontinued.
Case 3:
A 33-year-old woman developed renal failure of unclear etiology and was initiated on hemodialysis. After 3 months of hemodialysis, she received a living donor kidney transplant (KT) elsewhere which failed within 5 years due to oxalate nephropathy. Five months after KT failure, she presented to our institution on peritoneal dialysis. AGXT genetic testing revealed homozygosity for the G170R mutation, and she was started on pyridoxine (7.8 mg/kg/d). Ten months later, she received a second living donor KT elsewhere. Post-transplant, she was continued on pyridoxine. Thirteen years after her second KT, she had an iothalamate clearance of 59 ml/min/1.73 m2, POx of 4.2 μmol/L, and a UOx of 0.51 mmol/24 hr. She subsequently developed septic shock and acute kidney injury in the setting of a perforated cecal ulcer attributed to lymphoma. She was initiated on continuous hemodialysis and transitioned to intermittent hemodialysis every 4–5 days. Pyridoxine was temporarily held due to her inability to tolerate oral intake but resumed approximately one week after dialysis initiation. Seven weeks after dialysis initiation, she was transferred to our facility. On presentation, POx was 66.8 μmol/L, and UOx was 1.87 mmol/24 hr. Hemodialysis was increased to 6 days per week. A renal allograft biopsy showed acute tubular injury with associated calcium oxalate crystals and interstitial fibrosis involving 40% of the sample. Subsequently, dialysis was tapered to twice weekly. Due to recovery of kidney function, dialysis was able to be stopped 6 months after it started. Two months after dialysis discontinuation, the patient had stable renal allograft function with an eGFR of 52 ml/min/1.73m2, POx of 4.6 μmol/L, and UOx of 0.47 mmol/1.73 m2/24 hr. Pyridoxine was continued at 10 mg/kg/d. After five months off dialysis, eGFR was 47 ml/min/1.73m2 and urine oxalate 0.46 mmol/24 hrs. Renal allograft function was maintained until her death due more than a year after discontinuing dialysis.
DISCUSSION
This case series demonstrates that kidney failure secondary to oxalate nephropathy in PH1 can be reversible following reduction in hepatic oxalate production. We report three patients homozygous for the G170R mutation with severe oxalate nephropathy who experienced renal recovery allowing dialysis discontinuation after treatment with pyridoxine 8.8 mg/kg/d (range 6.8–14.0). Median duration of dialysis prior to renal recovery was 10 months (range 5–19). Renal recovery was sustained for a median of 3.2 years (range 1.3–6.5). Our findings challenge the conventional wisdom that kidney failure in PH1 is always irreversible. Rather, even advanced CKD can be reversed if hepatic production of oxalate can be reduced. We speculate that the prompt initiation of pyridoxine within months after kidney failure, before kidney damage becomes irreversible, accounts for the improved outcome in these patients. Overall, 3/8 patients (38%) started on pharmacologic dose pyridoxine within 3 months of dialysis initiation experienced renal recovery, a much higher rate of renal recovery than that seen in the general population (1.0–2.4%).11,12 Pyridoxine is a co-factor for AGT and other enzymes involved in amino acid metabolism. Specifically, pyridoxine is involved in neurotransmitter synthesis, homocysteine regulation, and gluconeogenesis.13 The G170R mutation results in functional but mistargeted AGT, with the potential for enhanced enzyme activity when sufficient pyridoxine is present. By contrast, most other mutations result in nonfunctional or deficient enzymes.5–7,14 Thus patients with G170R mutations are more likely to experience renal recovery when treated with pyridoxine. Another method of reducing hepatic oxalate production in PH1 patients regardless of genotype is siRNA therapy.15 In PH1, use of siRNA technology targeting the hepatic enzyme GO has been proposed. GO is responsible for converting glycolate to glyoxylate, the immediate precursor to oxalate. Thus inhibiting GO reduces the primary substrate necessary for oxalate formation and knocking out GO in AGT-deficient mice results in significantly lower UOx.16 A clinical trial of siRNA inhibition of GO has shown promising reductions in UOx in PH1 patients with preserved kidney function.17,18 Another potential target of siRNA technology in PH is the hepatic isoform of LDH (LDHa) which catalyzes the final step from glyoxylate to oxalate. siRNA therapy targeting LDH in mice has been shown to reduce oxalate production, and clinical trials of LDH inhibition using siRNA technology are currently underway.19,20
Our series suggests that reducing hepatic oxalate production may be of benefit even in PH1 patients with advanced CKD. Thus new or emerging treatments may allow for recovery of kidney function in a subset, even after kidney failure ensues.
Acknowledgements
We want to thank the staff of the Mayo Clinic Hyperoxaluria Center, referring physician Dr. Christoph Eggert, and the many physicians and patients who have contributed to the RKSC PH Registry.
Patient Protections The authors declare that the patients reported here provided consent for use of their medical records for research and that this work was performed under approval of the Mayo Clinic Institutional Review Board.
Support Funding for this work was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); the RKSC (U54DK083908), a member of the Rare Diseases Clinical Research Network of the Office of Rare Diseases Research; the National Center for Advancing Translational Sciences of the National Institutes of Health; and the Oxalosis and Hyperoxaluria Foundation (OHF). Dr. Lorenz is supported by the NIDDK (Award Number DK 123313). The funders had no role in defining the content of this manuscript.
Financial DisclosureDr. Lieske reports receiving grants from Allena, Alnylam, Dicerna, OxThera, Retrophin, and Siemens; receiving honoraria from Alnylam, American Board of Internal Medicine (ABIM), Dicerna, Orfan, OxThera, and Retrophin; serving as a consultant for ABIM, Allena, Alnylam, Dicerna, Orfan, OxThera, Retrophin, and Siemens; and serving as a scientific advisor or member of ABIM, Kidney International, and the OHF; all outside of the submitted work. Dr. Milliner reports receiving grants from Allena, Alnylam, Dicerna, and OxThera; receiving honoraria from Alnylam; serving as a consult for Allena, Alnylam, Dicerna, and OxThera; serving on an advisory committee for Alnylam, a monitoring committee for a clinical trial conducted by Dicerna, and on a data safety monitoring board for a clinical trial by OxThera, all outside of the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hopp K, Cogal AG, Bergstralh EJ, et al. Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. J Am Soc Nephrol. 2015;26(10):2559–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369(7):649–658. [DOI] [PubMed] [Google Scholar]
- 3.Milliner DS, Wilson DM, Smith LH. Phenotypic expression of primary hyperoxaluria: comparative features of types I and II. Kidney Int. 2001;59(1):31–36. [DOI] [PubMed] [Google Scholar]
- 4.Hoppe B, Beck BB, Milliner DS. The primary hyperoxalurias. Kidney Int. 2009;75(12):1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fargue S, Knight J, Holmes RP, Rumsby G, Danpure CJ. Effects of alanine:glyoxylate aminotransferase variants and pyridoxine sensitivity on oxalate metabolism in a cell-based cytotoxicity assay. Biochim Biophys Acta. 2016;1862(6):1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fargue S, Rumsby G, Danpure CJ. Multiple mechanisms of action of pyridoxine in primary hyperoxaluria type 1. Biochim Biophys Acta. 2013;1832(10):1776–1783. [DOI] [PubMed] [Google Scholar]
- 7.Miyata N, Steffen J, Johnson ME, Fargue S, Danpure CJ, Koehler CM. Pharmacologic rescue of an enzyme-trafficking defect in primary hyperoxaluria 1. Proc Natl Acad Sci U S A. 2014;111(40):14406–14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer-Kuhn H, Kohbrok S, Volland R, et al. Vitamin B6 in Primary Hyperoxaluria I: First Prospective Trial after 40 Years of Practice. Clin J Am Soc Nephrol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenz EC, Michet CJ, Milliner DS, Lieske JC. Update on oxalate crystal disease. Curr Rheumatol Rep. 2013;15(7):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harambat J, Fargue S, Acquaviva C, et al. Genotype-phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int. 2010;77(5):443–449. [DOI] [PubMed] [Google Scholar]
- 11.Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23(6):606–613. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald JA, McDonald SP, Hawley CM, et al. Recovery of renal function in end-stage renal failure--comparison between peritoneal dialysis and haemodialysis. Nephrol Dial Transplant. 2009;24(9):2825–2831. [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health Vitamin B6. Accessed June 22, 2020. https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/
- 14.Lorenz EC, Lieske JC, Seide BM, et al. Sustained pyridoxine response in primary hyperoxaluria type 1 recipients of kidney alone transplant. Am J Transplant. 2014;14(6):1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milliner DS. siRNA Therapeutics for Primary Hyperoxaluria: A Beginning. Mol Ther. 2016;24(4):666–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Higueras C, Luis-Lima S, Salido E. Glycolate Oxidase Is a Safe and Efficient Target for Substrate Reduction Therapy in a Mouse Model of Primary Hyperoxaluria Type I. Mol Ther. 2016;24(4):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frishberg Y, Deschenes G, Cochat P, Magen D, Groothoff J, Hulton SA, et al. A safety and efficacy study of lumasiran, an investigational RNA interference (RNAi) therapeutic, in adult and pediatric patients with primary hyperoxaluria type 1. Eur Urol Suppl 2019;18(1):e388–e389. [Google Scholar]
- 18.McGregor T, Hulton S, Frishberg Y, Cochat P, Groothoff J, Magen D, et al. Interim Results from the Ongoing Phase 2 Open-Label Extension Study of Lumasiran, an Investigational RNA Interference (RNAi) Therapeutic, in Patients with Primary Hyperoxaluria Type 1 (PH1) [Kidney Week abstract 414]. J Am Soc Nephrol. 2019;30:870. [Google Scholar]
- 19.Wood KD, Holmes RP, Erbe D, Liebow A, Fargue S, Knight J. Reduction in urinary oxalate excretion in mouse models of Primary Hyperoxaluria by RNA interference inhibition of liver lactate dehydrogenase activity. Biochim Biophys Acta Mol Basis Dis. 2019;1865(9):2203–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai C, Pursell N, Gierut J, et al. Specific Inhibition of Hepatic Lactate Dehydrogenase Reduces Oxalate Production in Mouse Models of Primary Hyperoxaluria. Mol Ther. 2018;26(8):1983–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]