Abstract
Purpose:
To provide guidance on how to appropriately quantitate various choriocapillaris (CC) parameters with optical coherence tomography angiography (OCTA).
Design:
Evidence-based perspective.
Methods:
Review of literature and experience of authors.
Results:
Accurate and reliable quantification of the CC using OCTA requires that the CC can be visualized and that the measurements of various CC parameters are validated. For accurate visualization, the selected CC slab must be physiologically sound, must produce images consistent with histology, and must yield qualitatively similar images when viewing repeats of the same scan or scans of different sizes. For accurate quantification, the measured inter-capillary distances (ICDs) should be consistent with known measurements using histology and adaptive optics/OCTA, the selected CC parameters must be physiologically and physically meaningful based on the resolution of the instrument and the density of the scans, the selected algorithm for CC binarization must be appropriate and generate meaningful results, and the CC measurements calculated from multiple scans of the same and different sizes should be quantitatively similar. If the Phansalkar’s local thresholding method is used, then its parameters must be optimized for the CC based on the OCTA instrument and scan patterns used. It is recommended that the window radius used in the Phansalkar method should be related to the expected average ICD in normal eyes.
Conclusions:
Quantitative analysis of the CC using commercially available OCTA instruments is complicated and researchers need to tailor their strategies based on the instrument, scan patterns, anatomy, and thresholding strategies to achieve accurate and reliable measurements.
Graphical Abstract
This Perspective provides an update on the latest optical coherence tomography angiographic strategies for imaging the choriocapillaris (CC) based on previous publications and offers guidance on how to appropriately visualize the CC and quantify various parameters based on the instrument and scan patterns used, the image processing methods chosen, and the thresholding strategies employed in order to achieve accurate and reliable measurements.
Introduction
The imaging and analysis of the choriocapillaris (CC) using optical coherence tomography angiography (OCTA) has become one of the hottest topics in ophthalmological research in recent years. Researchers have used different OCTA instruments and various analytical methods to characterize various properties of the CC in ocular diseases such as age-related macular degeneration (AMD)1, diabetic retinopathy,2 glaucoma,3 and central serous chorioretinopathy.4 Like many new techniques, the emergence of CC imaging and its quantitation has been accompanied by new challenges due to limited understanding of current technology and the inappropriate use of various algorithms. Many of the limitations and challenges associated with these strategies have been explored,5–7 but no definitive conclusions or protocols have been provided to guide researchers on how to best image the CC and perform its quantitative analysis. This report aims to summarize lessons we learned in our experience and provide evidence-based guidelines on how to appropriately image and analyze the CC flow using OCTA with a particular emphasis on swept source OCTA (SS-OCTA).
Accurate CC Visualization using OCTA
The first step in appropriately analyzing the flow within the CC is to accurately visualize the CC. The CC is a thin but dense vascular monolayer located along the inner choroid, adjacent to the Bruch’s membrane (BM). The most important step in visualizing the CC flow using OCTA is an accurate segmentation of the relevant retinal structures.
CC slab selection must be physiologically sound
OCT is an imaging modality that provides cross-sectional, depth-encoded information. Therefore, in OCT and OCTA imaging, the z-axis (depth) segmentation is the first step in visualizing any particular layer of interest. Traditionally, volumetric OCT data has been used to perform layer segmentation, as different ocular layers often have different OCT reflectivity due to their specific optical properties.8 In the case of the CC visualization, the theoretical definition of the CC slab should start from its anatomic location which is adjacent to the outer boundary of BM with the thickness of single capillary diameter.9
Ideally, the thickness of the CC slab should equal to the axial diameter of a single CC vessel, which decreases with aging, with a mean thickness of 9.8 µm in the first decade of life and 6.5 µm in the tenth decade of life.10 However, the convolutional effect of OCT resolution will artificially fatten the biological features during imaging. Therefore the thickness of the CC slab should take into account of such effects of OCT’s axial resolution (~6 µm). Thus, a CC slab of 10–20 µm thickness, located directly beneath the BM should yield the best OCTA en face images. In practice, due to the limited axial resolution of OCTA (~6 µm) and the scattering properties of these retinal layers, common OCT systems cannot individually resolve the RPE (~11 µm thick) from the BM (~2–6 µm thick in normal eyes) 10, 11 unless they are separated due to pathology. Thus, in normal eyes, the RPE/BM complex is generally presented as a wide bright band in the OCT image, and the CC slab should be defined parallel to the segmentation line of either RPE or BM. If the RPE is being segmented (upper boundary of the RPE/BM complex), the CC slab should be placed ~16 µm beneath the segmentation line, considering the thickness of RPE and BM. If the BM is being segmented (the lower boundary of the RPE/BM complex), the CC slab should be place ~4 µm below the segmentation line, considering the thickness of BM. Another approach to define the CC slab would be using averaged A-lines as previous described by multiple groups.12–14 Figure 1 shows an example of such approach, panel A and B are OCT and OCTA B-scans from previously published high resolution (~7 µm laterally, compared to ~16–20 µm in commercial OCT systems) SS-OCT data,13 and panel C shows the averaged A-line profiles through the whole volume. Two obvious peaks can be observed, one from the OCT data, one from the OCTA data, labeled with arrows. The peak from the OCT data represents the BM (positioned at 1002 µm depth) while the peak from the OCTA data represents the posterior boundary of CC (positioned at 1018 µm depth). Figure 2 shows the en face OCT and OCTA images at each depth position (single pixel). With this approach, the posterior boundary of the CC slab can be decided by identifying the CC peak from averaged OCTA A-lines and the anterior boundary of the CC slab can be decided by identifying the BM peak from the averaged OCT A-lines. Figure 1 panels D (OCTA) and E (OCT) show such a CC slab positioned from 4 µm below the BM peak to the CC posterior boundary peak.
FIGURE 1.
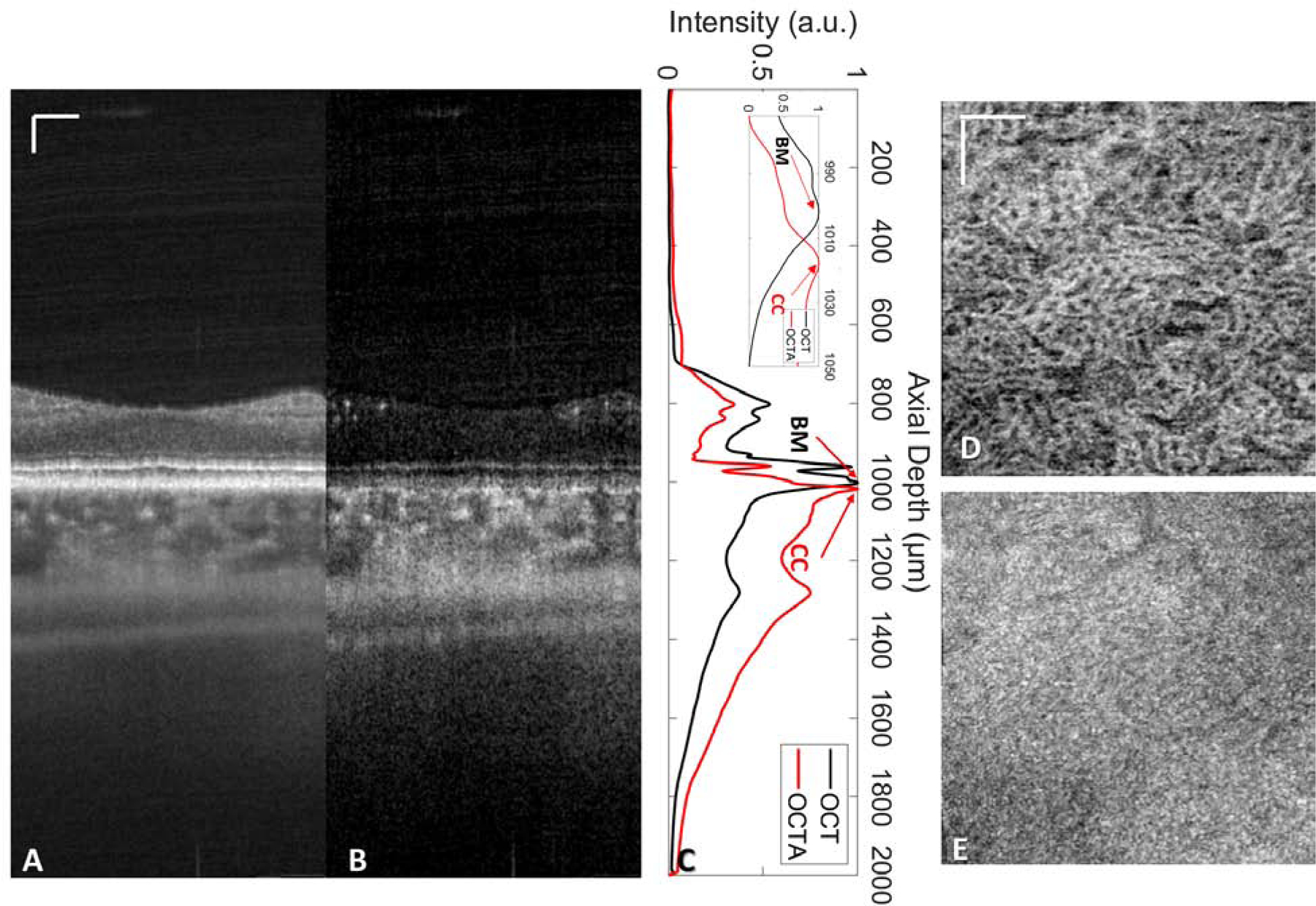
Illustration of how to define the choriocapillaris (CC) slab by averaging all A-lines in both swept-source optical coherence tomography (SS-OCT) volume and SS-OCT angiography (SS-OCTA) volume datasets using a previously published high resolution (~7 µm laterally, compared to ~16–20 µm in commercial OCT systems) SS-OCT system. 13 (A) Representative SS-OCT B-scan and (B) corresponding OCTA B-scan. (C) Averaged A-line profiles from SS-OCT (black) and SS-OCTA (red) signals, with the zoomed-in profile near the CC in the top right corner. The OCT peak at 1002 µm represents the BM, the OCTA peak at 1018 µm represents the posterior boundary of the CC. (D) En face OCTA CC image with the slab positioned at a depth of 1004~1020 µm. (E) En face OCT CC image of the same slab from D. Scale bars represent 100 µm.
FIGURE 2.
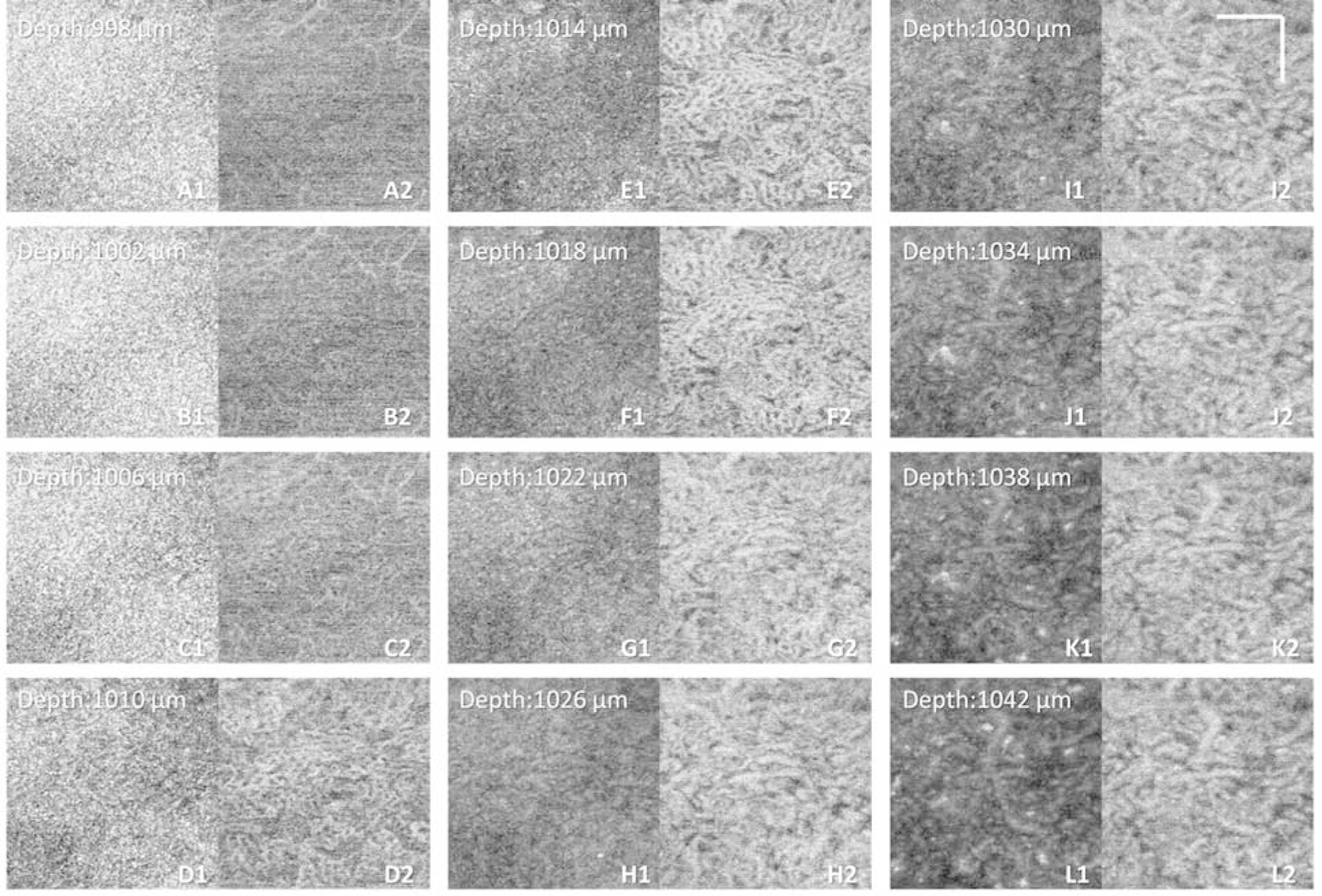
Example showing the appearances of en face swept-source optical coherence tomography (SS-OCT) and SS-OCT angiography (SS-OCTA) images at different depth position of the same dataset as Figure 1. (A1–L1) Single pixel en face OCT images at depth position of 998 µm to 1042 µm, position 1002 µm is the calculated Bruch’s membrane position, as showed in Figure 1. (A2–L2) Single pixel en face OCTA images at depth position of 998 µm to 1042 µm, position 10018 µm is the calculated choriocapillaris posterior boundary position, as showed in Figure 1. Scale bars represent 100 µm.
When generating CC slabs, researchers should always take OCT CC structure en face images into consideration, and these structural images can be used to judge if the CC slab is too deep into the choroid. For example, Figure 3 shows an example of a normal eye acquired by PLEX® Elite 9000 (Carl Zeiss Meditec., Dublin, CA) where different OCT and OCTA CC slabs were extracted using different locations offset to the BM segmentation line (manual segmentation, red dashed lines in panel A and B). Even though the OCTA CC en face flow images look similar across different slabs, the changes in the appearance of the OCT structural CC en face images are apparent, ranging from a homogeneous appearance to a variegated appearance. Researchers should be aware that if the OCT structural CC en face image appears variegated as in Figure 3 panels D and F, then your CC slab is too deep into the choroid. This could also be seen in data scanned from our lab-built high resolution SS-OCT system, where Figure 2 panel G1–L2 demonstrates clearly that the variegated OCT structural image appearances indicate that the slab is placed too deep into the choroid rather than at the real CC. It could be observed that choroidal slabs in Figure 2 panel K1 and K2 show larger diameter vasculature in the OCTA image and variegated pigments appearance in the OCT image, whereas the CC slabs in Figure 2 panel E1 and E2 show smaller diameter vasculature in the OCTA image and homogenous appearance in the OCT image.
FIGURE 3.
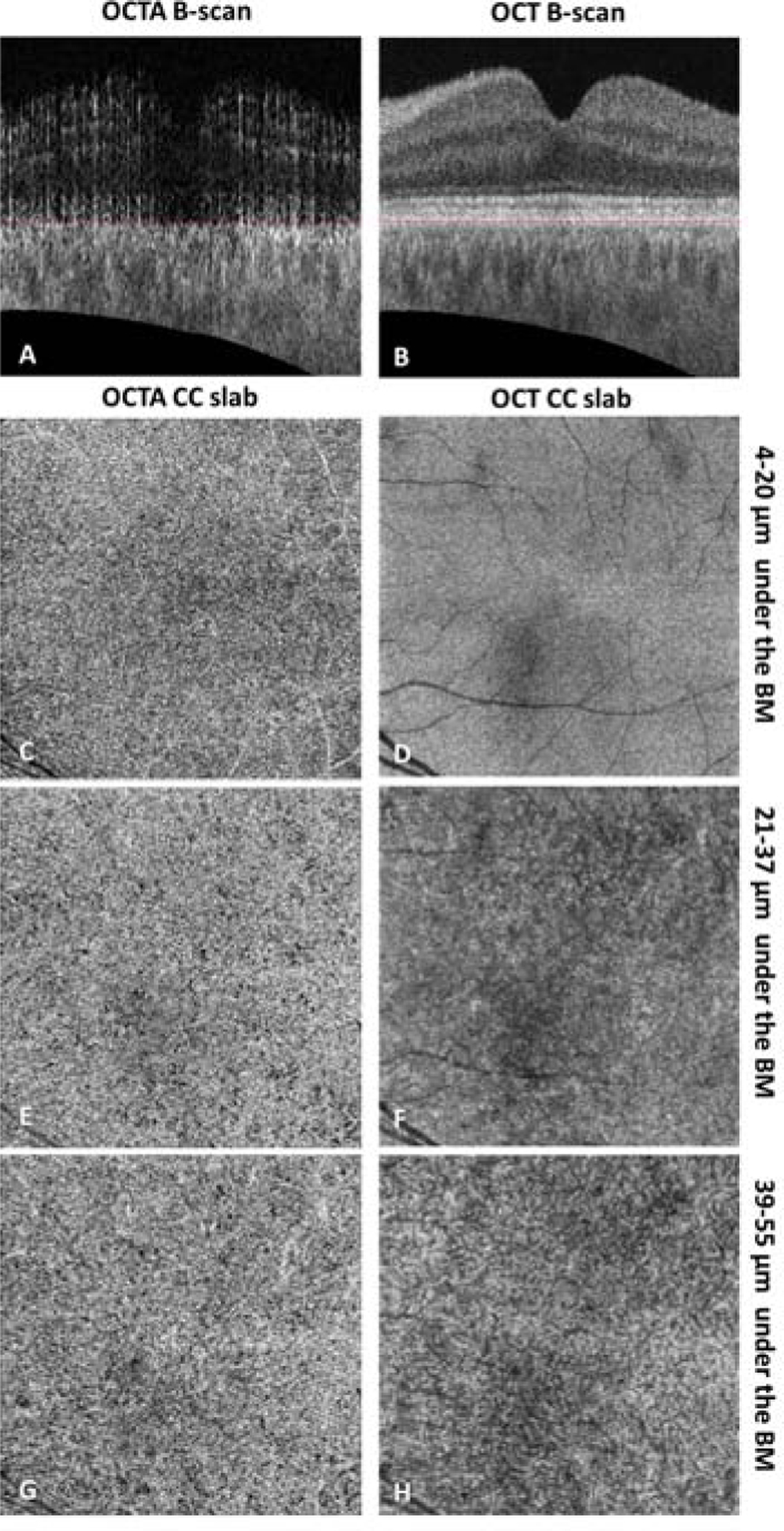
Example of PLEX® Elite 9000 swept-source optical coherence tomography angiography (SS-OCTA) choriocapillaris (CC) slab with a thickness of 15 µm located at selected positions of a 6 × 6 mm scan in a normal eye. (A–B) Cross-sectional SS-OCTA and SS-OCT B-scans showing the position of segmented Bruch’s membrane (red dashed lines). (C–D) En face SS-OCTA and SS-OCT CC images with a position of 4–20 µm under the segmented BM. (Recommended) (E–F) En face SS-OCTA and SS-OCT CC images with a position of 21–37 µm under the segmented BM. (G–H) En face SS-OCTA and SS-OCT CC images with a position of 39–55 µm under the segmented BM. OCTA CC images have been compensated for shadowing effect by using the CC structural signals, and retinal projection artifacts have been removed. Positions in micron have been rounded (1 pixel = 1.9531 µm).
Since not all research groups develop their own segmentation software to generate en face images, it is common for researchers to rely on the proprietary segmentation software on each commercial OCT instrument to generate en face OCT and OCTA images. Therefore, it is important to check and correct all segmentation lines, especially in the presence of pathology, as current automated segmentation algorithms can be often unreliable, especially along the RPE/BM layer.
Selected CC slab must show similarity in appearance compared with histology
Once anatomically correct CC slab is generated, the corresponding OCTA CC en face flow image should be visually checked for resemblance to previously published histological images. Previous studies have shown that the CC network has distinguishable morphological features at different regions.15 In the submacular region, the CC appears as a dense meshwork pattern of interconnected capillaries separated by septa while in the equatorial and peripheral regions, the CC has a lobular pattern where arterioles and venules join the segment from either the center or the periphery of the lobules. The averaged CC vessel diameter (laterally) under the macular was reported as 16–20 µm, with inter-capillary distances (ICDs) of 5–20 µm. However in the equatorial region, CC vessel diameter (laterally) was reported as 20–50 µm with ICDs of 50–200 µm.16
As previously explained,5 the CC networks under the macula cannot be fully resolved due to OCTA’s limited lateral resolution, but more widely spaced capillary networks within the equatorial regions are easier for OCTA to resolve. Registering and averaging multiple OCTA volumes is one useful approach for improving CC visualization, but averaging does not improve the optical resolution of the images so individual capillaries will still be beyond the instrument’s reach.17 Researchers should consider averaging images to confirm visually the resemblance of their OCTA CC images with histological images, particularly in the peripheral regions. While some of the capillaries within the macula will still remain unresolved, pathological flow deficits will be better defined due to a decrease in noise. Figure 4 demonstrates one such example where average OCTA CC images (previously published data17) show high degree of similarity compared with scanning electron microscope images.16 With this comparison, researcher can increase their confidence that the OCTA signals they are looking at are indeed from the CC networks.
FIGURE 4.
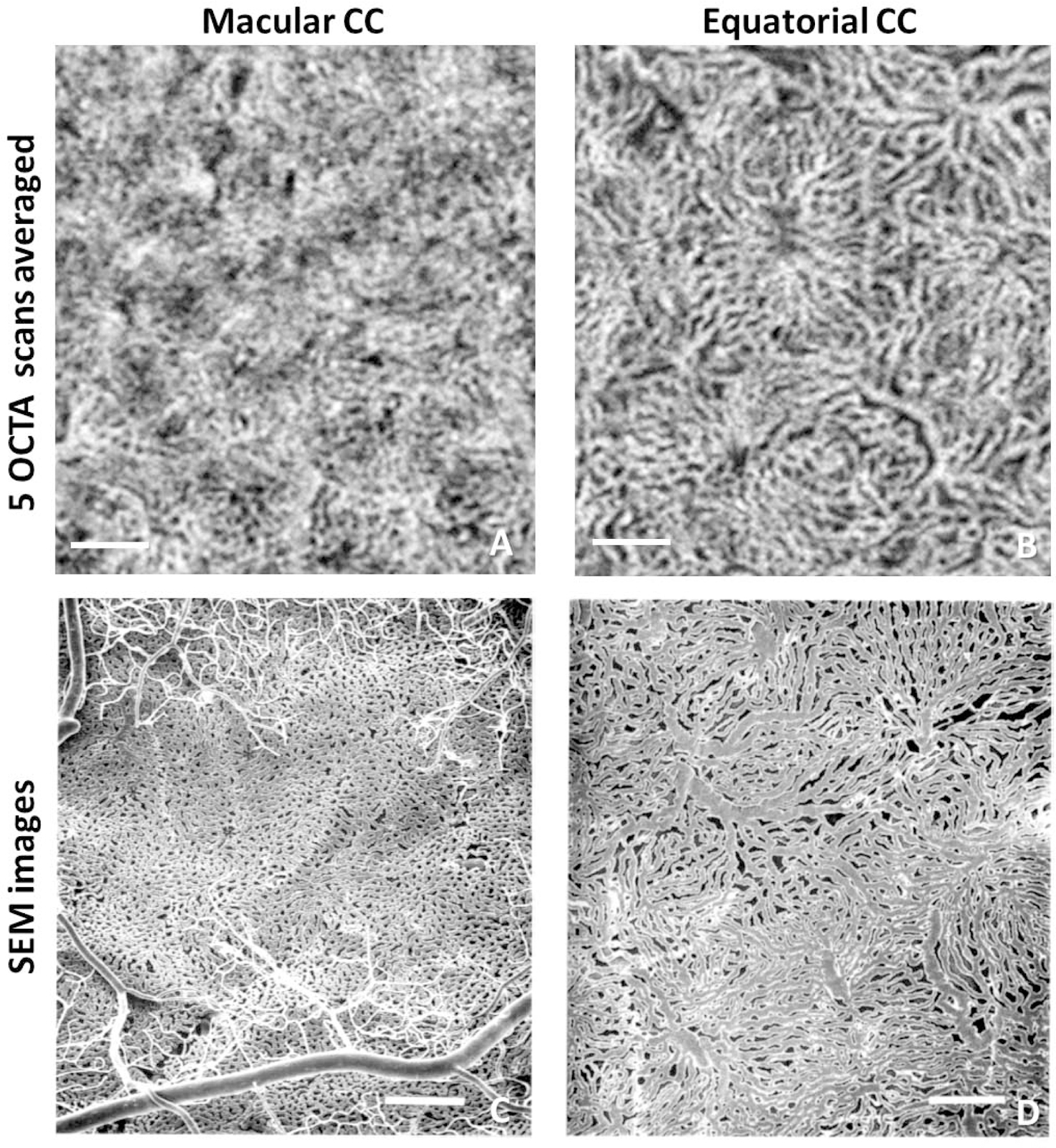
Comparison of choriocapillaris (CC) in the macular region and equatorial region using PLEX® Elite 9000 swept-source optical coherence tomography angiography (SS-OCTA) and scanning electron microscopy (SEM). (A) En face SS-OCTA CC image acquired in the macular region, 5 3×3 mm scans averaged with 300 A-scans and 300 B-scan positions. (B) En face SS-OCTA CC image acquired in the equatorial region, 5 scans averaged (same as above). (C) SEM CC image of methyl methacrylate casts under the macular region reproduced from Olver et al. with permission.16 (D) SEM CC image of corrosion casts under the equatorial region reproduced from Olver et al. with permission.16 Scale bars: 250 µm. Reprinted by permission from Springer Nature: Springer Nature, EYE, Olver J. Functional anatomy of the choroidal circulation: methyl methacrylate casting of human choroid. Eye 1990;4:(2):262–272.
Repeated scans of same and different sizes should be qualitatively similar.
In addition to comparing CC images with histological CC images, researchers should also collect data to confirm that repeated scans of the same subject, using the same scanning pattern, are qualitatively similar. Moreover, when performing repeated scans of the same subject at the same location using different scanning patterns (such as 3×3 mm scans and 6×6 mm scans), researchers should confirm that these scans are qualitatively similar. The next step in generating OCTA CC images requires that the researcher should also consider the physical limitations of OCT imaging, particularly the potential for artifacts. For example, in the cases of pathology, especially in the presence of drusen, the OCT signal can be significantly attenuated after passing through drusen. As a result, the OCTA flow signal, derived from OCT structural signal, often shows abnormally low intensity. A specially designed compensation strategy18 is helpful in such cases. While light propagation and backscattering within tissues are a complicated physical process in the presence of pathologies, the published strategy by Zhang et al.18 in which the inverted structural image derived from the CC slab is utilized to compensate the intensity of CC flow image, works well for drusen. Specific examples of this approach’s application on eyes with drusen could be found in previous publications.19, 20 This strategy also compensates for the lack of uniformity in the OCT signal across the scan area. It should be noted that this approach requires an accurate CC slab, as defined earlier in this study. For inaccurate slabs that are deeper into the choroid, especially the ones with variegated appearances in the OCT en face images, brighter signals from pigments in deeper choroid in the structural image could introduce unwanted artifacts.21 This approach have its limitations, for example signals of areas underneath migrated pigments might be beyond salvation and should be excluded instead. Different compensation strategies may be needed for other pathologies such as retinal edema or hyper-reflective materials located above the RPE/BM complex. Lastly, retinal projection artifacts22 need to be removed for optimal imaging of the CC before any quantitation is performed.
Accurate CC quantification using OCTA
Once a good visualization of the CC is obtained, the next steps should be to ensure accurate quantitation of CC parameters using OCTA.
Measured inter-capillary distances should be consistent with known measurements using histology
An important quantitative parameter that researchers should consider when dealing with OCTA CC images is the ICD. The ICD is defined as the averaged distance from the center of one capillary lumen to another. Therefore, it is substantially equivalent to one capillary width plus the width of one physiological flow void. It should be noted that in some histological studies, the ICD was defined as the distance from the edge of one capillary to another.16 Multiple groups have reported measurements of ICD values using a radially averaged power spectrum analysis with different OCTA systems.12–14, 17, 23 This measurement is performed by generating and radially averaging a two-dimensional power spectrum from OCTA CC images. The resultant peak in the averaged plot represents the most prevalent spacing, which is the averaged ICD in the CC images. Previous histological studies16 have reported that in macular regions the CC vessel diameter is about 16–20 µm while the vessel edge to edge distance is 5–20 µm. Thus, according to the definition of the ICD above, one vessel width plus one flow void width would give an ICD of about 21–40 µm. Due to OCTA’s limitations, the calculated ICDs might not match with the histological measurements exactly, but should be relatively consistent with this 21–40 µm range. This ICD measurement does not require a binarization algorithm.
Selected parameters must be physiologically and physically meaningful
As previously discussed, the limited lateral resolution of OCTA images makes it difficult to visualize the detailed macular CC vasculature network.5 Instead of developing a binarization algorithm to segment individual capillaries, most researcher have chosen to segment flow deficits (FDs), which can be larger than the typical size of physiological flow voids, within the detection sensitivity of OCTA images, and represent CC flow impairment, which is the feature of the CC that should be associated with disease. Many quantitative parameters have been introduced in various reports17, 24, 25 to describe CC FDs, but it is important for researchers to only select parameters that are physiologically and physically meaningful. Some appropriate metrics include FD density (FDD), FD number (FDN), mean FD size (MFDS) or average FD size/average FD area and ICD. Some inappropriate parameters include vessel skeleton density (VSD), vessel length density (VLD) and vessel diameter index (VDI). Generally speaking, researchers should avoid using any parameter that involves skeletonizing the binarized CC image unless they can prove that individual CC vessels have been fully resolved and segmented in their images. With the current OCTA lateral resolution, we can only reasonably attempt to measure the perfusion deficits within the CC (or FDs) but not detailed vascular morphology patterns.
Selected algorithm for CC binarization must be appropriate and generate meaningful results
To quantify the meaningful parameters mentioned above, one must binarize the OCTA CC images, which requires applying a thresholding strategy. Several thresholding algorithms have been used in the literature to segment the CC FDs. As we have discussed in a previous publication, some of those algorithms are not appropriate to the task at hand for various reasons.5 Useful methods that are available include: 1) a global thresholding method that utilizes the standard deviation (SD) values of a normal database (SD method);18 2) a thresholding method that utilizes the fuzzy C-means algorithm (FCM method);17 3) the Phansalkar local thresholding method (Phansalkar method).26 Previously, we have reported a high correlation between the SD method and the FCM method using a normative database.27 Moreover, we have also pointed out that when using the Phansalkar method, the choice of local window radius can significantly affect the appearance of binarized CC FD images and subsequent quantification.5 Many researchers26, 28–30 have used a fixed 15-pixel radius window with the Phansalkar method, even though, due to the properties of the various scan patterns, the actual pixel size ranged from 2.9 µm/pixel to 9.9 µm/pixel in these studies. The discrepancy in the physical dimensions of the window radius is particularly concerning, and this discrepancy compromises the assessment of previously published reports and makes cross validation comparisons difficult.
To explore how the choice of different window radii influences CC quantification, we have conducted a study19 where eyes scanned using a SS-OCTA instrument (PLEX® Elite 9000, Carl Zeiss Meditec, Dublin, CA) were analyzed using the Phansalkar method with window radii ranging from 1 pixel to 15 pixels (3×3 mm: 2.9 µm/pixel, 6×6 mm: 5.9 µm/pixel for images adjusted to 1024X1024 pixels). In our study, we found that larger window radii resulted in higher FDD values. With the increase of the window size, the FDN initially increased and then decreased, and the opposite trend was found for the MFDS. However, the inflection point for this change corresponded to a window radius equal to about 1–2 ICDs, which provides some justification for considering this as an optimal choice for the proper use of Phansalkar’s method. Moreover, this choice of 1–2 ICDs for the window radius yielded images that closely resembled the actual CC flow images before binarization and were substantially indistinguishable from the results obtained using the FCM approach, as demonstrated in Figure 5. After analyzing our data, we concluded the Phansalkar method can be a good approach for the CC quantification, as long as its parameters, especially the local window radius, are optimized for the OCTA CC images. Therefore, we recommend that the proper use of the Phansalkar method should include the selection of the window radius related to the expected ICD in normal eyes. Thus, for the Phansalkar method, 1–2 ICDs should be selected as the window diameter (2*radius + 1 pixels), then for a 3×3 mm scan (2.9 µm/pixel), a radius of 4–8 pixels could be chosen and for a 6×6 mm scan (5.9 µm/pixel), a radius of 2–4 pixels could be chosen. In the case of CC binarization, it is clear that global thresholding techniques and local thresholding techniques each have its own merits and limitations. Future studies may also consider employing more than one binarization techniques to ensure accurate evaluation.
FIGURE 5.
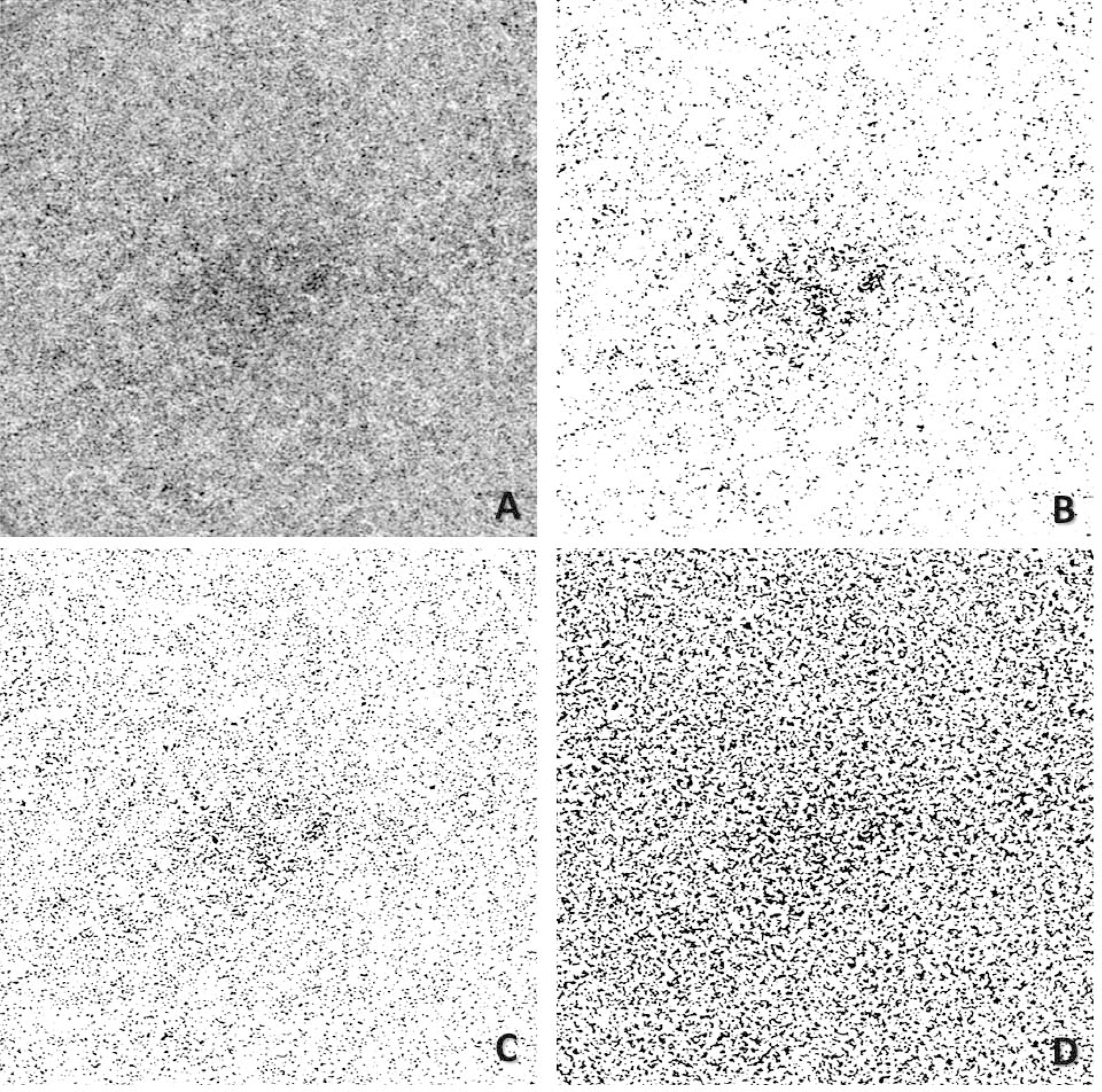
Comparison of choriocapillaris (CC) flow deficits (FDs) segmented using different algorithms with PLEX® Elite 9000 swept-source optical coherence tomography angiography (SS-OCTA). (A) 6×6 mm en face SS-OCTA CC image from a normal eye (1024×1024 pixels). (B) Segmented CC FDs binary map using the Fuzzy C-means method, black pixels represent FDs. (C) Segmented CC FDs binary map using the Phansalkar’s local thresholding method, with a window radius of 3 pixels (a diameter of 41 µm). (D) Segmented CC FDs binary map using the Phansalkar’s local thresholding method, with a window radius of 15 pixels (a diameter of 182 µm).
As we mentioned, when developing an algorithm for CC binarization, the most straightforward criterion for judging the validity of any binarization strategy is to compare the OCTA CC flow image side-by-side with the binarized CC FD image. The major FDs segmented in the binarized image should closely resemble the FDs in the original OCTA CC image. The binarized CC FD image should not appear to create false positive FDs or artificial vasculature networks. Moreover, the calculated CC FD quantitative parameters should stay consistent with histological studies. Since OCT imaging is a convolutional process with a lateral resolution of ~20 µm (imaged features will be ‘dilated’ ~20 µm larger), the CC imaged by OCTA would appear wider. Consequently, the CC perfusion density calculated from OCTA images should be expected to be considerably larger than the values calculated from histological images. Taking quantification of retinal vasculature as an example, previous histological study using confocal scanning laser microscopy have reported vessel area density (VAD, which is opposite of flow deficit density) in the ganglion cell - inner plexiform layer (GCIPL) as 22.32%±0.99% 31 whereas this value was 48.6%±1.4% from the OCTA assessment. 32 This significant difference in reported vessel density is mainly caused by OCTA’s lateral resolution. Similarly, CC density (which equals to 1.0-FDD) measured by OCTA should also result in higher values compared to the CC density measured by histological studies,10 meaning that OCTA measured FDD should be lower than the “real values”. If abnormally high FDD values are obtained using OCTA, it is possible that the selected thresholding technique has not been optimized.
Most studies have adopted a signal strength cut off as part of their data inclusion criterion, per OCTA manufactures’ recommendation. However, studies have shown positive correlation between the signal strength with quantitative vasculature metrics, even above the common cut-offs.33, 34 Therefore, it might be useful to adopt a signal normalization strategy in CC quantification. Zhang et al.22 previously normalized data with a signal strength index smaller than 9 against ones of 9, where each voxel in the OCT and OCTA volume was multiplied by a factor of 9 divided by the data’s actual signal strength index, before segmentation and en face image generation were taken place. Such strategy might not work perfectly as it is unknown how device manufactures defined their signal strength index. For future work, it might be necessary for the device manufactures to get involved in the signal normalization to obtain more accurate CC quantifications.
Multiple scans of same size and different sizes should be repeatable and correlated
Researchers should make sure that their quantitative analyses of the CC demonstrate repeatability in both normal and diseased eyes. After all, if the OCTA CC flow metrics are to be used as a biomarker for assessing disease pathology and progression, then quantitative CC metrics should be able to demonstrate good repeatability. Otherwise, it would be simply impossible to compare CC properties among patient cohorts or track disease progression. Quantitative metrics acquired from the multiple scans of the same subjects using the same scanning pattern should have good repeatability. Likewise, quantitative metrics acquired from the multiple scans of the same subjects using different scanning patterns (3×3 mm and 6×6 mm) should be highly correlated.35 Various studies have used the coefficient of variation and intraclass correlation coefficient values18 to judge the clinical usefulness of the results. When publishing new studies with new quantification approaches, it is important that researchers should investigate the reproducibility properties and compare them to those of other algorithms. Most importantly, high repeatability should not be obtained at the cost of sensitivity. For quantitative CC metrics to be used as biomarkers, researchers must demonstrate both high repeatability of measurements as well as high sensitivity in detecting pathology, and high repeatability of any given measurement is not a substitute for sensitivity.6 Just because a particular slab yields results that are repeatable doesn’t mean that the results are relevant for detecting disease. Researchers need to assess the relationship between repeatability and sensitivity.
OCTA systems typically provide multiple choices of scanning patterns. For example, in the current SS-OCTA instrument (PLEX® Elite 9000) options include 3×3 mm, 6×6 mm, 9×9 mm, 9×15 mm, and 12×12 mm scan patterns. However, not all scans are suitable for the CC quantification. Given the limited lateral resolution of the system, it is best to use images that sample the retina tissue as densely as possible. The 3×3 mm scan has a sampling (or scanning) density of 10 µm/pixel, and the 6×6 mm scan has a sampling density of 12 µm/pixel. Both scan sizes have been reported to be useful in the quantitative CC analyses. However, larger scans such as 9×9 mm, 9×15 mm and 12×12 mm scans currently employ a larger sampling density of 18 µm/pixel and 24 µm/pixel, making it almost impossible to provide reliable quantitative assessment of the CC network. The thickness measurements of the RPE, BM and CC layers also vary between the macular region to the equatorial regions.36 Therefore, one can often observe variegated appearances of CC OCT structural images from larger scans when a uniformly defined CC slab is used. This often indicates inaccurate CC visualization with larger scanning patterns.
Summary and future considerations
There are numerous issues to be considered when imaging the CC with OCTA and carrying out a quantitative analysis of such images. In our pursuit of an optimal protocol to tackle this problem, we have made some mistakes and learned many lessons. In this report we have presented a summarized checklist of how best to visualize and quantify the CC with OCTA. In each claim, we have presented specific guidelines as well as providing rationale and evidence.
In summary, we argue that, in order to achieve an accurate and reliable CC quantitative analysis using OCTA, researchers must make sure that both the visualization of the CC and the quantification of the CC are physically and physiologically justifiable. As a first step to ensure accurate visualization of the CC, the selection of the CC slab must be physiologically sound. The CC slab should be defined as a 10–20 µm thickness slab beneath the BM. Proprietary algorithms from manufactures or various research groups most often define the CC slab by segmenting either the RPE or BM. With such approaches, the difference offset from the segmentation line may depend on the specific characteristics of the segmentation and should be decided using RPE and BM thicknesses calculated from histological studies.10, 11 One could also segment a CC slab by using averaged A-lines to identify the BM and CC’s posterior boundary. Averaged OCT A-lines could be used to identify the position of the BM, and the anterior boundary of the CC should be ~2–4 µm below this BM position. Averaged OCTA A-lines could be used to identify the posterior boundary of the CC. When defining the CC slab, one should always examine the OCT structural CC en face image to avoid a variegated appearance caused by irregular structures in the deeper choroid. The generated OCTA CC images should show a similar appearance to histology. OCTA CC images under the macula should have a dense meshwork appearance, whereas in the equatorial region, the CC images should have more lobular pattern. Lastly, multiple OCTA CC images from the same and different scan sizes should be qualitatively similar.
Once a good visualization of the CC is achieved, the additional steps are necessary to ensure accurate CC quantification. If researchers choose to measure the ICD, it should be consistent with known measurement using histology (~21–40 µm).16 If researchers choose to segment and quantify FDs, the selected parameters must be physiologically and physically meaningful. Parameters like VSD, VLD and VDI should be avoided due to the inability to resolve individual CC vessels, at least in the submacular region, using current SS-OCTA systems. In the process of segmenting the CC FDs, selected algorithm for CC binarization must be appropriate and generate physically meaningful results. If the Phansalkar method is chosen, one should optimize its parameters using a window radius that is specific for the OCTA CC images. We recommend using a window radius of 1–2 ICDs. Lastly, quantitative metrics generated by the multiple scans of the same and different sizes should be repeatable and correlated.
The limited lateral resolution of OCTA is the most important reason why the quantitative analysis of the CC is so complicated. Quite simply, it is difficult to confirm accurate visualization and quantification of the CC in the macula where the imaging technology is unable to resolve the detailed microvascular network. However, this does not mean that the commercially available OCTA instruments are not useful in CC analysis. Even though OCTA cannot always resolve individual CC vessels, it is typically capable of resolving the lack of CC flow, which is why we choose to segment and quantify CC FDs instead of actual vessels. Currently, efforts have begun to develop next generation instruments that will achieve higher resolution and faster speeds for the CC imaging.13, 37 It is possible that with the technological advancements, commercial OCTA systems could achieve the ability to make CC imaging more straightforward and easier to quantitate in the future. However, for now, we strongly encourage researchers to follow the guidelines provided in this perspective so they can generate meaningful quantitative CC metrics that are similar across platforms, in order to help diagnose, follow, and predict the progression of ocular diseases.
Supplementary Material
ACKNOWLEDGMENTS:
a. Funding/Support: Research supported by grants from the National Eye Institute (R01EY024158, R01EY028753), the Salah Foundation, Carl Zeiss Meditec, an unrestricted grant from the Research to Prevent Blindness, Inc., New York, NY, and the National Eye Institute Center Core Grant (P30EY014801) to the Department of Ophthalmology, University of Miami Miller School of Medicine. The funding organization had no role in the design or conduct of this research.
b. Financial Disclosures: Dr. Gregori, Dr. Wang and Dr. Rosenfeld received research support from Carl Zeiss Meditec, Inc. Dr. Gregori and the University of Miami co-own a patent that is licensed to Carl Zeiss Meditec, Inc.
Dr. Rosenfeld also receives research support from Stealth BioTherapeutics. He is a consultant for Apellis, Biogen, Boehringer-Ingelheim, Carl Zeiss Meditec, Biogen, Chengdu Kanghong Biotech, EyePoint, Ocunexus Therapeutics, Ocudyne, and Unity Biotechnology. Philip Rosenfeld has equity interest in Apellis, Valitor, Verana Health, and Ocudyne. Dr. Wang discloses intellectual property owned by the Oregon Health and Science University and the University of Washington. Dr. Wang also receives research support from Tasso Inc, Moptim Inc, Colgate Palmolive Company and Facebook technologies LLC. He is a consultant to Insight Photonic Solutions, Kowa, and Carl Zeiss Meditec.
c. Other Acknowledgments: Figure 4 C,D are reprinted by permission from Springer Nature: Springer Nature, EYE, Olver J. Functional anatomy of the choroidal circulation: methyl methacrylate casting of human choroid. Eye 1990;4:(2):262–272.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The remaining authors have no disclosures.
REFERENCES
- 1.Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol. Vis 1999;5:(35):35. [PubMed] [Google Scholar]
- 2.Cao J, McLeod DS, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch. Ophthalmol 1998;116:(5):589–597. [DOI] [PubMed] [Google Scholar]
- 3.Spraul CW, Lang GE, Lang GK, Grossniklaus HE. Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vision Res 2002;42:(7):923–932. [DOI] [PubMed] [Google Scholar]
- 4.Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina 1994;14:(3):231–242. [DOI] [PubMed] [Google Scholar]
- 5.Chu Z, Gregori G, Rosenfeld PJ, Wang RK. Quantification of choriocapillaris with OCTA: a comparison study. Am. J. Ophthalmol 2019;208:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byon I, Nassisi M, Borrelli E, Sadda SR. Impact of Slab Selection on Quantification of Choriocapillaris Flow Deficits by Optical Coherence Tomography Angiography. Am. J. Ophthalmol 2019;208:397–405. [DOI] [PubMed] [Google Scholar]
- 7.Mehta N, Liu K, Alibhai AY, et al. Impact of Binarization Thresholding and Brightness/Contrast Adjustment Methodology on Optical Coherence Tomography Angiography Image Quantification. Am. J. Ophthalmol 2019;205:54–65. [DOI] [PubMed] [Google Scholar]
- 8.Yin X, Chao JR, Wang RK. User-guided segmentation for volumetric retinal optical coherence tomography images. J. Biomed. Opt 2014;19:(8):086020–086020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torczynski E, Tso MO. The architecture of the choriocapillaris at the posterior pole. Am. J. Ophthalmol 1976;81:(4):428–440. [DOI] [PubMed] [Google Scholar]
- 10.Ramrattan RS, van der Schaft TL, Mooy CM, De Bruijn W, Mulder P, De Jong P. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest. Ophthalmol. Vis. Sci 1994;35:(6):2857–2864. [PubMed] [Google Scholar]
- 11.Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 1996;37:(13):2724–2735. [PubMed] [Google Scholar]
- 12.Gorczynska I, Migacz J, Jonnal R, Zawadzki R, Poddar R, Werner J. Imaging of the human choroid with a 1.7 MHz A-scan rate FDML swept source OCT system. Ophthalmic Technologies XXVII: International Society for Optics and Photonics, 2017:1004510.
- 13.Zhou K, Song S, Zhang Q, Chu Z, Huang Z, Wang RK. Visualizing choriocapillaris using swept-source optical coherence tomography angiography with various probe beam sizes. Biomed. Opt. Express 2019;10:(6):2847–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurokawa K, Liu Z, Miller DT. Adaptive optics optical coherence tomography angiography for morphometric analysis of choriocapillaris. Biomed. Opt. Express 2017;8:(3):1803–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoneya S, Tso MO, Shimizu K. Patterns of the choriocapillaris. Int. ophthalmol 1983;6:(2):95–99. [DOI] [PubMed] [Google Scholar]
- 16.Olver J Functional anatomy of the choroidal circulation: methyl methacrylate casting of human choroid. Eye 1990;4:(2):262–272. [DOI] [PubMed] [Google Scholar]
- 17.Chu Z, Zhou H, Cheng Y, Zhang Q, Wang RK. Improving visualization and quantitative assessment of choriocapillaris with swept source OCTA through registration and averaging applicable to clinical systems. Sci. rep 2018;8:(1):16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Zheng F, Motulsky EH, et al. A Novel Strategy for Quantifying Choriocapillaris Flow Voids Using Swept-Source OCT Angiography. Invest. Ophthalmol. Vis. Sci 2018;59:(1):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Z, Cheng Y, Zhang Q, et al. Quantification of Choriocapillaris with Phansalkar Local Thresholding: Pitfalls to Avoid. Am. J. Ophthalmol 2020;213:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Chu Z, Wang L, et al. Validation of a Compensation Strategy Used to Detect Choriocapillaris Flow Deficits Under Drusen With Swept Source OCT Angiography. Am. J. Ophthalmol:S0002-9394 (0020) 30329–30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledesma-Gil G, Fernandez-Avellaneda P, Spaide RF. SWEPT-SOURCE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IMAGE COMPENSATION OF THE CHORIOCAPILLARIS INDUCES ARTIFACTS. RETINA;Publish Ahead of Print. [DOI] [PubMed]
- 22.Zhang Q, Zhang A, Lee CS, et al. Projection artifact removal improves visualization and quantitation of macular neovascularization imaged by optical coherence tomography angiography. Ophthalmol. retina 2017;1:(2):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Shi Y, Zhou H, et al. Accurate estimation of choriocapillaris flow deficits beyond normal intercapillary spacing with swept source OCT angiography. Quant. imag. med. surg 2018;8:(7):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacconi R, Borrelli E, Corbelli E, et al. Quantitative changes in the ageing choriocapillaris as measured by swept source optical coherence tomography angiography. Brit. J. Ophthalmol 2019;103:(9):1320–1326. [DOI] [PubMed] [Google Scholar]
- 25.Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, Sadda SR. Choriocapillaris imaging using multiple en face optical coherence tomography angiography image averaging. JAMA ophthalmol 2017;135:(11):1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaide RF. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am. J. Ophthalmol 2016;170:58–67. [DOI] [PubMed] [Google Scholar]
- 27.Chu Z, Zhang Q, Zhou H, et al. Quantifying choriocapillaris flow deficits using global and localized thresholding methods: a correlation study. Quant. imag. med. surg 2018;8:(11):1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassisi M, Baghdasaryan E, Tepelus T, Asanad S, Borrelli E, Sadda SR. Topographic distribution of choriocapillaris flow deficits in healthy eyes. PloS one 2018;13:(11):e0207638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nassisi M, Shi Y, Fan W, et al. Choriocapillaris impairment around the atrophic lesions in patients with geographic atrophy: a swept-source optical coherence tomography angiography study. Brit. J. Ophthalmol 2019;103:(7):911–917. [DOI] [PubMed] [Google Scholar]
- 30.Spaide RF. Ising model of choriocapillaris flow. Retina 2018;38:(1):79–83. [DOI] [PubMed] [Google Scholar]
- 31.Chan G, Balaratnasingam C, Paula KY, et al. Quantitative morphometry of perifoveal capillary networks in the human retina. Invest. Ophthalmol. Vis. Sci 2012;53:(9):5502–5514. [DOI] [PubMed] [Google Scholar]
- 32.Richter GM, Madi I, Chu Z, et al. Structural and Functional Associations of Macular Microcirculation in the Ganglion Cell-Inner Plexiform Layer in Glaucoma Using Optical Coherence Tomography Angiography. J. glaucoma 2018;27:(3):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brücher VC, Storp JJ, Eter N, Alnawaiseh M. Optical coherence tomography angiography-derived flow density: a review of the influencing factors. Graef Arch. Clin. Exp. Ophthalmol 2020;258:(4):701–710. [DOI] [PubMed] [Google Scholar]
- 34.Lim HB, Kim YW, Kim JM, Jo YJ, Kim JY. The importance of signal strength in quantitative assessment of retinal vessel density using optical coherence tomography angiography. Sci. rep 2018;8:(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng F, Zhang Q, Shi Y, et al. Age-dependent Changes in the Macular Choriocapillaris of Normal Eyes Imaged With Swept-Source Optical Coherence Tomography Angiography. Am. J. Ophthalmol 2019;200:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv. Ophthalmol 1999;44:S10–S32. [DOI] [PubMed] [Google Scholar]
- 37.Marsh-Armstrong B, Migacz J, Jonnal R, Werner JS. Automated quantification of choriocapillaris anatomical features in ultrahigh-speed optical coherence tomography angiograms. Biomed. Opt. Express 2019;10:(10):5337–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


