Abstract
Varicose veins are the most common vascular disease in humans. Veins have valves that help the blood return gradually to the heart without leaking blood. When these valves become weak, blood and fluid collect and pool by pressing against the walls of the veins, causing varicose veins. In the cardiovascular system, mechanical forces are important determinants of vascular homeostasis and pathological processes. Blood vessels are constantly exposed to a variety of hemodynamic forces, including shear stress and environmental strains caused by the blood flow. In varicose veins within the leg, venous blood pressure rises in the vein of the lower extremities due to prolonged standing, creating a peripheral tension in the vessel wall thereby causing mechanical stimulation of endothelial cells and vascular smooth muscle. Studies have shown that long-term increased exposure to vascular wall tension is associated with the overexpression of HIF-1α and HIF-2α and increased levels of MMP-2 and MMP-9, thereby reducing venous contraction and progressive venous dilatation, which is involved in the development of varicose veins. Following the expression of metalloproteinase, the expression of type 1 collagen increases, and the amount of type 3 collagen decreases. Therefore, collagen imbalance will cause the varicose veins to not stretch. Loss of structural proteins (type 3 collagen and elastin) in the vessel wall causes the loss of the biophysical properties of the varicose vein wall. This review article tries to elaborate on the effect of mechanical forces and sensors of these forces on the vascular wall in creating the mechanism of mechanosignaling, as well as the role of the onset of molecular signaling cascades in the pathology of varicose veins.
Keywords: Varicose, Veins, Mechanosignaling
Varicose veins
A diverse array of cellular receptors and binding proteins exist in the walls of blood vessels so that changes in the lumen (interior of the blood vessel tube) can be transmitted to the vascular tissue and its surrounding supporting structures by these proteins/receptors via intracellular signaling. Changes in blood pressure distort the vein wall as blood travels through the vein, and this pressure can trigger changes in the intracellular signaling pathway (Norouzpour et al. 2013) (Fig. 1). Varicose veins are alternatively called dilations or swellings of the veins. They constitute the most common human vascular disease, affecting about 10–20% of the population (Raffetto and Khalil 2008a). Varicose veins are long and dilated, and are often found on the inner surfaces of the lower extremities (Metcalfe et al. 2007). The return of the blood from the vein to the heart is facilitated by a series of valves in the veins, and varicose veins occur whenever these valves have a problem (Hamann et al. 2019; Birdina et al. 2017). Any factor that disrupts the work of veins can be a cause of varicose veins. For example, obesity (especially around the abdomen) can put extra pressure on the leg veins (Sadick 1992; Jacobs et al. 2017; Cavallini 2019). In this study, we try to elaborate on the effect of mechanical forces and sensors of these forces on the vascular wall in creating the mechanism of mechanosignaling, and also the role of the onset of molecular signaling cascades in the pathology of varicose veins. Table 1 shows comparison of blood pressure and characteristics among of arteries, veins, and capillaries.
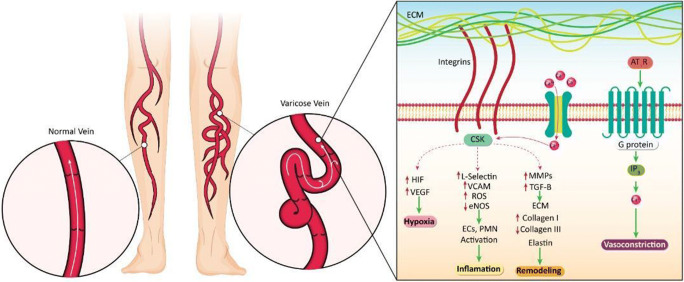
Fig. 1.
Intracellular signaling due to changes in blood pressure in the varicose vein. Changes in mechanical stress in the varicose vein can be transmitted into the cell through an extracellular matrix dependent on integrins, ion channels, or AT1R. The resulting changes can eventually cause remodeling in vein structure, inflammation, hypoxia, and vasoconstriction
Table 1.
Comparison of blood pressure and characteristics among of arteries, veins, and capillaries (McGhee and Bridges 2002; Williams et al. 1988; Ma et al. 2018)
| Type of vessel | Blood pressure (mmHg) | Lumen diameter | Wall thickness | Wall layer | Valves |
|---|---|---|---|---|---|
| Veins | 5–10 | Low | Thin | Three | Yes |
| Capillaries | 10.5–22.5 | Extremely narrow | Extremely thin | Three | No |
| Arteries | Systolic 65–95 Diastolic 30–60 | Narrow | Thick | One | No |
Genetic bases
The hereditary basis of varicose veins is controversial. Cornu-Thenard (1994) found that children with both parents with varicose veins had a 90% risk of developing varicose veins (Krysa et al. 2012; Fukaya et al. 2018). Further investigations revealed an autosomal dominant inheritance pattern with variable permeability (Bradbury and Pappas 2006) suggesting that there is a strong genetic component to primary venous insufficiency (Kim et al. 2005). The FOXC2 gene was the first one identified in connection with venous valve insufficiency in both superficial and deep vessels of the lower extremities (Ng et al. 2005). Another gene involved with demonstrated involvement is thrombomodulin (TM), a glycoprotein receptor on the surface of endothelial cells that binds to thrombin. Further to this point, it was found that 19% of people with varicose veins suffered from venous thrombosis (Segiet et al. 2015). The C677T methylenetetrahydrofolate reductase (MTHFR) functional polymorphism, which has been consistently associated with arterial disease, has also been linked to the development of varicose veins (Karathanos et al. 2013). The MTHFR gene encodes the enzyme methyl hydro folate reductase, which functions to help regulate homocysteine levels in the body. This gene is critical for the uptake of folic acid and other forms of folate by cells. In this gene, the most important polymorphisms are C677T and A1298C (Hiraoka and Kagawa 2017), with the former being 40–60% incapable of producing methyl folate, which itself is a vital and effective nutrient for the production of cardiovascular carriers. Sverdlova et al. found that people with at least one C677T MTHFR allele had an increased risk for varicose veins as compared to the general population (Wilmanns et al. 2015).
Stretching changes and its effect on the pathology of venous varicose veins
Mechanical forces play an important role at every stage of development and in every part of the body, from the early growth to the physiology and pathology of adults (Raffetto and Khalil 2008a). During development and physical formation of a growing fetus, vascular development and morphogenesis of all organs are regulated by mechanical forces (Xu and Shi 2014). Similarly, mechanical forces in adulthood are involved in several physiological processes, including the senses of touch, hearing, and the production of respiratory-induced pulmonary surfactant (Atta 2012). Mechanical forces also play an important role in the etiology of pathological conditions, such as tumor metastasis and atherosclerosis (Shyu 2009). In the cardiovascular system, mechanical forces are important determinants of vascular homeostasis and pathological processes, such as varicose veins (Atta 2012).
Blood vessels are constantly exposed to various types of hemodynamic forces, including shear stress and environmental strains due to the blood flow (Atta 2012). Shear stress is the force of blood friction on the endothelial layer, while peripheral tension is caused by total pressure within the vein (Fitts et al. 2014). The effect of these forces on endothelial cells, internal smooth muscle cells, fibroblasts, and extracellular matrix is the release of biochemical reagents which assist in maintaining physiological function (Wang et al. 2010). The pathological process of varicose veins can be due to the mechanical process of “vascular traction” which contributes to the synthesis of natural collagen and blood circulation, but can also lead to the development of atherosclerosis (Raffetto and Khalil 2008a). In addition, increased cardiac load (read blood pressure) due to exercise or high blood pressure can lead to cardiac hypertrophy (Kockx et al. 1998). In varicose veins, venous blood pressure rises in the vein of the lower extremities due to prolonged standing and creates the peripheral tension in the vessel wall and the mechanical stimulation of the EC and SMC (Kucukguven and Khalil 2013; Atta 2012). Among the sensors that help respond to this mechanical force are integrins, ion channels, and G protein receptors (Martinac 2014; Atta 2012). We will describe each of these in turn.
Integrins
Integrins are a large family of cell surface receptors that attach cells to the extracellular matrix of the ECM and surrounding cells (Cario-Toumaniantz et al. 2007). The ability of integrin’s to act as a bridge between the extracellular environment and intracellular protein kinases enables them to transmit extracellular signals into the cell (Giancotti and Tarone 2003). There are 24 known integrins, 16 of which are involved in the vascular system (Martinez-Lemus et al. 2003). During stress on the cell, integrins bind to adhesive proteins via an extracellular dimer that binds to the ECM. This binding causes the intracellular skeleton to tighten and the formation of a second intracellular message that causes the phosphorylation of tyrosine kinase bound to CSK, resulting in the transfer of mechanical forces within the membrane (Han et al. 2004).
Vascular muscle-related integrins
Smooth muscle cells are in a three-dimensional network of ECM proteins including collagen, fibronectin, elastin, vitronectin, and laminin (Laronha and Caldeira 2020). The ECM interacts with the integrins during high blood pressure, in which the ECM itself, in conjunction with the integrins, will transfer the pressure to the underlying vascular layers. Therefore, an acute increase in vascular pressure increases both the peripheral wall tension and the longitudinal stress, all of which are micromechanical changes mediated by integrins (Zhu et al. 2019). The mechanism of integrin regulation is based on the binding of peptides containing specific amino acid sequences that bind within integrins. There are specific sites in the vascular wall that can detect Asp-Gly-Asp amino acid (RGD) sequences. These diagnostic sites are commonly found in extracellular proteins, such as collagen and fibronectin, and an antibody against the outer part of the vascular wall integrin heterodimer can cause decreased vascular myogenic dilatation by the above mechanism (Cario-Toumaniantz et al. 2007).
Regulation of calcium in integrin-dependent myogenic responses
Another important role in signaling transmission is the overlap between both calcium and integrin signaling pathways in vascular smooth muscle (Kucukguven and Khalil 2013). Interaction between vascular cell integrins and their appropriate ligands can induce calcium entry, after which integrin is absorbed into the ECM by calcium channel kinases, such as FAK, and other calcium-dependent signaling proteins, such as Rho GTPase, paxillin, and vinculin (Ward et al. 2004), followed by the beginning of phosphorylation cascades. This process can be inhibited by protein tyrosine kinase inhibitors (Lim 2010).
Role of CSK in integrin-dependent myogenic responses
CSK acts as an interface between extracellular and intracellular environments in the generation of the myogenic response through signaling pathways (Sharif-Naeini et al. 2008). In terms of its location, Csk is part of an organized three-dimensional network suspended between elastin and actin filaments (Wang et al. 1993). After exposure to external mechanical stress, CSK undergoes partial deformation which then causes a change in function that affects a series of reactions, including microtubule polymerization, thereby producing cellular pressure tolerance, which significantly increases vascular muscle myogenesis (Martinac 2014).
Flow-sensitive ion channels
The activation of ion channels of calcium, potassium, and sodium is one of the fastest ways to respond to changes in vascular flow velocities. Ionic channels act in response to different types of flow profiles (Raffetto et al. 2007). Potassium channels have been shown to be activated by low and oscillating blood pressures, while calcium channels are activated by stable, non-oscillating currents (Raffetto et al. 2007), which indicates the differentiation power of types of currents in ion channels (Raffetto and Khalil 2008b).
G protein-coupled receptors (GPCRs)
Receptors belonging to the G family of proteins are expressed in most organs. One important member of this family is the angiotensin II type1 receptor (AT1R), which is abundantly expressed in blood vessels. AT1R can be activated in the absence of ligands by the stimulation of peripheral traction (Atta 2012). Self-activation of AT1R can cause vasoconstriction. The downstream signaling pathways of AT1R include the activation of Rac and PI3 proteins (Yokota et al. 2016). Another mechanism in the renal and cerebral arteries is the cooperation of AT1Rs with transient canonical potential receptor (TRPC) channels. GPCRs are activated when they enter the vascular wall and then activate TRPC channels (Asghar and Törnquist 2020). TRPC itself activates phospholipase C. Studies have shown that the three main processes of hypoxia, inflammation, and stretching, play major roles in the initiation of signaling pathways responsible for the pathophysiology of varicose veins (Asghar and Törnquist 2020; Stennett et al. 2009).
Mechanosignaling in the pathophysiology of varicose veins
Integrin-stimulated signaling
Integrins stimulate intracellular signaling pathways interacting with the ECM to induce calcium-dependent intracellular signaling. Calcium-sensitive protein kinases, including MAPK and calcium-rich Rho, are activated in this pathway (Schlaepfer et al. 1998). These kinases are located in the path of phosphorylation cascades. The signaling pathway associated with the CSK structure is dependent on association between extracellular matrix and integrins that trigger the myogenic response of vascular muscles (Ingber 2002). The role of the MAPK molecule in causing arterial smooth muscle contractions in different parts of the body has been partially investigated, with further studies needed to understand the role of this molecule in causing myogenic responses; however so far, studies have shown that it is active in this pathway (Ortega et al. 2018). Signaling molecules are synthesized/released upon stimulating integrin junctions with the ECM, but other studies in the cerebral artery show that the activity of this kinase is dependent on the activity of upstream kinases of the tyrosine kinases of the src kinase family, which react in cerebral arteries in response to biomechanical stress (Kowalewski et al. 2009; Atta 2012; Hu et al. 2020).
Inflammatory changes in the pathology of varicose veins
Inflammation changes the pathology of varicose veins causing the activation of leukocytes and the release of inflammatory factors (Korkmaz et al. 2018). During previous studies, inflammatory infiltration in varicose veins was compared with normal control groups, which showed the infiltration of inflammatory cells, such as mast cells, macrophages, monocytes, and T lymphocytes (Korkmaz et al. 2018; GOSHCHYNSKY and MIGENKO 2019). Evidence suggests a role of inflammation in regulating the expression of MMPs in varicose veins similar to that which happens in other diseases, such as atherosclerosis, in response to a series of cytokines and inflammatory cells, such as macrophages (Ghaderian et al. 2010; Sayer and Smith 2004; Raffetto and Khalil 2008b).
Hypoxia changes in the pathology of varicose veins
Lower extremity venous wall hypoxia has been shown to play a role in the formation of varicose veins (Lim and Davies 2009). Both venous hypertension and ischemia are involved in vascular wall hypoxia (Birdina et al. 2017). Oxygen and nutrients are transported through the lumen of the vessel to the endothelium and other layers of the vessel (Birdina et al. 2017). Secondary hydrostatic pressure causes the compression of the outer layers of the vascular wall (Atta 2012). Intravenous blood pressure causes the vessel wall to stretch, which increases the oxygen demand of the vessel wall and the compression of the vessel wall, resulting in hypoxia of the vessel wall (Lim 2010). Histological examination of varicose veins can be used to determine the number of vasa vasorums due to hypoxia and decreased oxygen supply to the cells of the vascular wall. Among these changes include differences in progenitor cytokines, vascular endothelial growth factor (VEGF), and its upstream transcription factor, hypoxia-inducible factor-1alpha (HIF-1α), which all play an important role in the formation of oxygen-supplying vessels of the vasa vasorum in the vessel wall.
Remodeling in the pathology of varicose veins
In varicose veins, MMPs have a negative effect on the vascular wall (Kucukguven and Khalil 2013). These MMP enzymes are the cause of vascular wall relaxation in varicose veins (Lim and Davies 2009). The spread of varicose veins will increase the expression of metalloproteinase, which further causes the vascular wall to relax and not contract (MacColl et al. n.d.). A relationship has been shown between increased size and duration of wall tension with decreased vasoconstriction and overexpression of MMPs 2 and 9 (Raffetto et al. 2008). On the other hand, further studies on the expression of HIF-1 and HIF-2 showed that the expression of HIF-1 and HIF-2 increased during the stretching and dilation of varicose veins, along with elevated expression of MMPs 2 and 9. This association led to increased progressive vasodilation, which is involved in the formation of varicose veins (Raffetto et al. 2008).
Mechanical traction of the wall can increase the level of TGF-β1 in the ECM (Pascual et al. 2007). This cytokine is involved in the proliferation and expansion of the ECM (Sprague and Khalil 2009). Signaling studies in varicose veins have shown increases in the expression of TGF-β1 and the downstream effectors of this signaling pathway. Various studies show the hypertrophy in the layers of the wall of varicose veins compared to normal vessels (Wali and Eid 2001). Biochemical studies concerning the wall of varicose veins show a decrease in the density and size of elastin minerals (Görmüs et al. 2014). According to studies, a reduction of elastin in the vascular wall will reduce the level of lysine oxidase (LOXL1) (Behmoaras et al. 2008), which is a cross-linking enzyme for elastin that will be responsible for the deposition of elastin polymer (Liu et al. 2004). In addition to elastin, a disorder has also been observed in the regulation of collagen levels. In the case of collagen, the expression of type 1 collagen increases and the amount of type 3 collagen decreases due to the production of more metalloproteinases, hence the collagen imbalance will cause the varicose veins not to stretch (Sansilvestri-Morel et al. 2005). Studies on the loss of structural protein in the walls of elastin and collagen type 3 cause the loss of biophysical properties of the wall of varicose veins. Elastin, which plays an important role in regulating vascular diameter (Travers et al. 1996), can play an important role in blood pressure and thus the spread of varicose veins by losing elastin (Atta 2012).
Conclusions
Studies show that genetics play a role in the development of varicose veins. However, it should be noted that venous varicose veins are a multifactorial disease determined by a number of genetic and environmental factors (Raffetto and Khalil 2008a; Lim and Davies 2009). Varicose veins can be a secondary manifestation of other vascular diseases; hence, there are many limitations despite many techniques available for determining genetic factors. In addition, determining the contribution of a genetic factor found can be difficult (Kucukguven and Khalil 2013; Shadrina et al. 2019). Therefore, determination of a genetic factor requires a complete examination of the patient. First of all, the patient’s family history, age, sex, and pregnancy should be taken into consideration (Evans et al. 1999). There are various methods to determine the role of genetics in the development of venous varicose veins. One of these methods is to verify the gene expression profile by examining FOXC2, Desmoglein, thrombomodulin, and methylene tetrahydrofolate reductase genes (Krysa et al. 2012; Cario-Toumaniantz et al. 2007). Another technique could be to study the relationship between genome, metabolism, and microRNA analysis in patients with venous varicose veins (Lee et al. 2005). Contrasting clinical diagnosis with a molecular understanding of the pathology of varicose veins is the next major research step (Kim et al. 2005).
Abbreviations
- MMP
Matrix metallopeptidases
- HIF
Hypoxia-inducible factor-1alpha
- FOXC2
Forkhead box protein C2
- MTHFR
Methylene tetrahydrofolate reductase
- CSK
C-terminal Src kinase
- ROS
Reactive oxygen species
- eNOS
Endothelial NOS
- ECM
Extracellular matrix
- RGD
Arg-Gly-Asp
- LOXL1
Lysyl oxidase-like 1
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Asghar MY, Törnquist K. Transient receptor potential canonical (TRPC) channels as modulators of migration and invasion. Int J Mol Sci. 2020;21:1739. doi: 10.3390/ijms21051739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta HM (2012, 2012) Varicose veins: role of mechanotransduction of venous hypertension. J Vasc Med. 10.1155/2012/538627 [DOI] [PMC free article] [PubMed]
- Behmoaras J, Slove S, Seve S, Vranckx R, Sommer P, Jacob M-P. Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta. Rejuvenation Res. 2008;11:883–889. doi: 10.1089/rej.2008.0760. [DOI] [PubMed] [Google Scholar]
- Birdina J, Pilmane M, Ligers A. The morphofunctional changes in the wall of varicose veins. Ann Vasc Surg. 2017;42:274–284. doi: 10.1016/j.avsg.2016.10.064. [DOI] [PubMed] [Google Scholar]
- Bradbury AW, Pappas PJ (2006) ‘Chronic venous insufficiency, varicose veins, lymphedema, and arteriovenous fistulas.’ In, Vascular Surgery (Springer). 10.1007/1-84628-008-7_10
- Cario-Toumaniantz C, Boularan C, Schurgers LJ, Heymann M-F, Le Cunff M, Léger J, Loirand G, Pacaud P. Identification of differentially expressed genes in human varicose veins: involvement of matrix gla protein in extracellular matrix remodeling. J Vasc Res. 2007;44:444–459. doi: 10.1159/000106189. [DOI] [PubMed] [Google Scholar]
- Cavallini, A (2019) Doctor, why do I have varicose veins?. Veins Lymph 8(1). 10.4081/vl.2019.7937
- Cornu-Thenard A, Boivin P, Baud JM, de Vincenzi I, Carpentier PH (1994) Importance of the familial factor in varicose disease: clinical study of 134 families. J Dermatol Surg Oncol 20(5):318–326 [DOI] [PubMed]
- Evans CJ, Fowkes FGR, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999;53:149–153. doi: 10.1016/j.jvsv.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts MK, Pike DB, Anderson K, Shiu Y-T. Hemodynamic shear stress and endothelial dysfunction in hemodialysis access. Open Urol Nephrol J. 2014;7:33. doi: 10.2174/1874303X01407010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya E, Flores AM, Lindholm D, Gustafsson S, Zanetti D, Ingelsson E, Leeper NJ. Clinical and genetic determinants of varicose veins: prospective, community-based study of ≈ 500 000 individuals. Circulation. 2018;138:2869–2880. doi: 10.1161/CIRCULATIONAHA.118.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderian SMH, Lindsey NJ, Graham AM, Homer-Vanniasinkam S, Najar RA. Pathogenic mechanisms in varicose vein disease: the role of hypoxia and inflammation. Pathology. 2010;42:446–453. doi: 10.3109/00313025.2010.493865. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- Görmüs U, Timirci-Kahraman Ö, Arzu E, Kunt AT, Selim İ, Burak Dalan A, İsbir T. Expression levels of elastin and related genes in human varicose veins. Folia Biol. 2014;60:68. doi: 10.14712/fb2014060020068. [DOI] [PubMed] [Google Scholar]
- Goshchynsky VB, Migenko BO (2019) Pathophysiological and pathomorphological aspects of relapse of varicose veins after endovascular laser vein coagulation. Perspect Sci Educ 155 [PubMed]
- Hamann SAS, Mik L T-d, Fritschy WM, Kuiters GRR, Nijsten TEC, van den Bos RR. Randomized clinical trial of endovenous laser ablation versus direct and indirect radiofrequency ablation for the treatment of great saphenous varicose veins. Br J Surg. 2019;106:998–1004. doi: 10.1002/bjs.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Bai X-H, Lodyga M, Xu J, Yang BB, Keshavjee S, Post M, Liu M. Conversion of mechanical force into biochemical signaling. J Biol Chem. 2004;279:54793–54801. doi: 10.1074/jbc.M406880200. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Kagawa Y. Genetic polymorphisms and folate status. Congenital Anomal. 2017;57:142–149. doi: 10.1111/cga.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Lu K, Liu W (2020) Exendin-4 attenuates inflammation-mediated endothelial cell apoptosis in varicose veins through inhibiting the MAPK-JNK signaling pathway. J Recept Signal Transduct. 10.1080/10799893.2020.1756326 [DOI] [PubMed]
- Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- Jacobs BN, Andraska EA, Obi AT, Wakefield TW. Pathophysiology of varicose veins. J Vasc Surg: Venous Lymphatic Disord. 2017;5:460–467. doi: 10.1016/j.jvsv.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Karathanos C, Exarchou M, Tsezou A, Kyriakou D, Wittens C, Giannoukas A. Factors associated with the development of superficial vein thrombosis in patients with varicose veins. Thromb Res. 2013;132:47–50. doi: 10.1016/j.thromres.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Kim D-I, Eo H-S, Joh J-H. Identification of differentially expressed genes in primary varicose veins. J Surg Res. 2005;123:222–226. doi: 10.1016/j.jss.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Kockx MM, Knaapen MWM, Bortier HE, Cromheeke KM, Boutherin-Falson O, Finet M. Vascular remodeling in varicose veins. Angiology. 1998;49:871–877. doi: 10.1177/000331979804901101. [DOI] [PubMed] [Google Scholar]
- Korkmaz Ö, Göksel S, Gül M, Başçil H, Yildir Y, Berkan Ö. Does the use of N-butyl-2 cyanoacrylate in the treatment of lower extremity superficial varicose veins cause acute systemic inflammation and allergic reactions. Cardiovasc J Africa. 2018;29:213–217. doi: 10.5830/CVJA-2018-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewski R, Malkowski A, Sobolewski K, Gacko M. Evaluation of aFGF/bFGF and FGF signaling pathway in the wall of varicose veins. J Surg Res. 2009;155:165–172. doi: 10.1016/j.jss.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Krysa J, Jones GT, Van Rij AM. Evidence for a genetic role in varicose veins and chronic venous insufficiency. Phlebology. 2012;27:329–335. doi: 10.1258/phleb.2011.011030. [DOI] [PubMed] [Google Scholar]
- Kucukguven A, Khalil RA. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets. 2013;14:287–324. [PMC free article] [PubMed] [Google Scholar]
- Laronha H, Caldeira J. Structure and function of human matrix metalloproteinases. Cells. 2020;9:1076. doi: 10.3390/cells9051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee W, Choe Y, Kim D, Na G, Kim J, Kim M, Kim J, Cho J. Gene expression profiles in varicose veins using complementary DNA microarray. Dermatol Surg. 2005;31:391–395. doi: 10.1111/j.1524-4725.2005.31103. [DOI] [PubMed] [Google Scholar]
- Lim CS (2010) The hypoxia-inducible factor (HIF) pathway in varicose veins [DOI] [PubMed]
- Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96:1231–1242. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase–like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Ma Y, Choi J, Hourlier-Fargette A, Xue Y, Chung HU, Lee JY, Wang X, Xie Z, Kang D, Wang H. Relation between blood pressure and pulse wave velocity for human arteries. Proc Natl Acad Sci. 2018;115:11144–11149. doi: 10.1073/pnas.1814392115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacColl E, Khalil RA et al Matrix metalloproteinases as regulators of vein structure and function: implications in chronic venous disease. J Pharmacol Exp Ther 355:410–428. 10.1124/jpet.115.227330 [DOI] [PMC free article] [PubMed]
- Martinac B. The ion channels to cytoskeleton connection as potential mechanism of mechanosensitivity. Biochim Biophys Acta (BBA)-Biomembr. 2014;1838:682–691. doi: 10.1016/j.bbamem.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, Meininger GA. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–233. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- McGhee BH, Bridges EJ. Monitoring arterial blood pressure: what you may not know. Crit Care Nurse. 2002;22:60–79. doi: 10.4037/ccn2002.22.2.60. [DOI] [PubMed] [Google Scholar]
- Metcalfe MJ, Baker DM, Turmaine M, Burnstock G. Alterations in purinoceptor expression in human long saphenous vein during varicose disease. Eur J Vasc Endovasc Surg. 2007;33:239–250. doi: 10.1016/j.ejvs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Ng MYM, Andrew T, Spector TD, Jeffery S. Linkage to the FOXC2 region of chromosome 16 for varicose veins in otherwise healthy, unselected sibling pairs. J Med Genet. 2005;42:235–239. doi: 10.1136/jmg.2004.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norouzpour A, Hooshyar Z, Mehdizadeh A. Autoregulation of blood flow: vessel diameter changes in response to different temperatures. J Biomed Phys Eng. 2013;3:63. [PMC free article] [PubMed] [Google Scholar]
- Ortega MA, Romero B, Asúnsolo Á, Sainz F, Martinez-Vivero C, Álvarez-Mon M, Buján J, García-Honduvilla N (2018) 'Behavior of smooth muscle cells under hypoxic conditions: possible implications on the varicose vein endothelium. Biomed Res Int. 10.1155/2018/7156150 [DOI] [PMC free article] [PubMed]
- Pascual G, Mendieta C, García-Honduvilla N, Corrales C, Bellón JM, Buján J. TGF-β1 upregulation in the aging varicose vein. J Vasc Res. 2007;44:192–201. doi: 10.1159/000100375. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23:85–98. doi: 10.1258/phleb.2007.007027. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr Vasc Pharmacol. 2008;6:158–172. doi: 10.2174/157016108784911957. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2–induced venous dilation via hyperpolarization and activation of K+ channels: Relevance to varicose vein formation. J Vasc Surg. 2007;45:373–380. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: potential implications in varicose veins. J Vasc Surg. 2008;48:447–456. doi: 10.1016/j.jvs.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick NS. Predisposing factors of varicose and telangiectatic leg veins. J Dermatol Surg Oncol. 1992;18:883–886. doi: 10.1111/j.1524-4725.1992.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Sansilvestri-Morel P, Rupin A, Jullien ND, Lembrez N, Mestries-Dubois P, Fabiani JN, Verbeuren TJ. Decreased production of collagen Type III in cultured smooth muscle cells from varicose vein patients is due to a degradation by MMPs: possible implication of MMP-3. J Vasc Res. 2005;42:388–398. doi: 10.1159/000087314. [DOI] [PubMed] [Google Scholar]
- Sayer GL, Smith PDC. 'Immunocytochemical characterisation of the inflammatory cell infiltrate of varicose veins. Eur J Vasc Endovasc Surg. 2004;28:479–483. doi: 10.1016/j.ejvs.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src-and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/MCB.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segiet OA, Brzozowa-Zasada M, Piecuch A, Dudek D, Reichman-Warmusz E, Wojnicz R. Biomolecular mechanisms in varicose veins development. Ann Vasc Surg. 2015;29:377–384. doi: 10.1016/j.avsg.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Shadrina AS, Sharapov SZ, Shashkova TI, Tsepilov YA. Varicose veins of lower extremities: Insights from the first large-scale genetic study. PLoS Genet. 2019;15:e1008110. doi: 10.1371/journal.pgen.1008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Naeini R, Dedman A, Folgering JHA, Duprat F, Patel A, Nilius B, Honoré E. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflügers Archiv-Eur J Physiol. 2008;456:529–540. doi: 10.1007/s00424-007-0432-y. [DOI] [PubMed] [Google Scholar]
- Shyu K-G. Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin Sci. 2009;116:377–389. doi: 10.1042/CS20080163. [DOI] [PubMed] [Google Scholar]
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennett AK, Qiao X, Falone AE, Koledova VV, Khalil RA. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am J Phys Heart Circ Phys. 2009;296:H745–HH55. doi: 10.1152/ajpheart.00861.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JP, Brookes CE, Evans J, Baker DM, Kent C, Makin GS, Mayhew TM. Assessment of wall structure and composition of varicose veins with reference to collagen, elastin and smooth muscle content. Eur J Vasc Endovasc Surg. 1996;11:230–237. doi: 10.1016/s1078-5884(96)80058-x. [DOI] [PubMed] [Google Scholar]
- Wali MA, Eid RA. Smooth muscle changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2001;37:123–135. doi: 10.1540/jsmr.37.123. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Yan Z-Q, Qi Y-X, Cheng B-B, Wang X-D, Zhao D, Shen B-R, Jiang Z-L. Normal shear stress and vascular smooth muscle cells modulate migration of endothelial cells through histone deacetylase 6 activation and tubulin acetylation. Ann Biomed Eng. 2010;38:729–737. doi: 10.1007/s10439-009-9896-6. [DOI] [PubMed] [Google Scholar]
- Ward JPT, Knock GA, Snetkov VA, Aaronson PI. Protein kinases in vascular smooth muscle tone—role in the pulmonary vasculature and hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2004;104:207–231. doi: 10.1016/j.pharmthera.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Williams SA, Wasserman S, Rawlinson DW, Kitney RI, Smaje LH, Tooke JE. Dynamic measurement of human capillary blood pressure. Clin Sci. 1988;74:507–512. doi: 10.1042/cs0740507. [DOI] [PubMed] [Google Scholar]
- Wilmanns C, Cooper A, Wockner L, Katsandris S, Glaser N, Meyer A, Bartsch O, Binder H, Walter PK, Zechner U. Morphology and progression in primary varicose vein disorder due to 677C > T and 1298A > C variants of MTHFR. EBioMedicine. 2015;2:158–164. doi: 10.1016/j.ebiom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shi G-P (2014) Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta (BBA)-Molec Basis Dis:1842, 2106–1819. 10.1016/j.bbadis.2014.07.008 [DOI] [PMC free article] [PubMed]
- Yokota A, Gamoh S, Tanaka-Totoribe N, Shiba T, Kuwabara M, Nakamura E, Hayase T, Hisa H, Nakamura K, Yamamoto R. Angiotensin II, as well as 5-hydroxytriptamine, is a potent vasospasm inducer of saphenous vein graft for coronary artery bypass grafting in patients with diabetes mellitus. Biochem Biophys Rep. 2016;6:82–87. doi: 10.1016/j.bbrep.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Niu H, Yin N, Wu T, Zhao Y. Analysis of varicose veins of lower extremities based on vascular endothelial cell inflammation images and multi-scale deep learning. IEEE Access. 2019;7:174345–174358. doi: 10.1109/ACCESS.2019.2954708. [DOI] [Google Scholar]