Abstract
The symptoms of functional neurological disorder (FND) are a product of its pathophysiology. The pathophysiology of FND is reflective of dysfunction within and across different brain circuits that, in turn, affects specific constructs. In this perspective article, we briefly review five constructs that are affected in FND: emotion processing (including salience), agency, attention, interoception, and predictive processing/inference. Examples of underlying neural circuits include salience, multimodal integration, and attention networks. The symptoms of each patient can be described as a combination of dysfunction in several of these networks and related processes. While we have gained a considerable understanding of FND, there is more work to be done, including determining how pathophysiological abnormalities arise as a consequence of etiologic biopsychosocial factors of FND. To facilitate advances in this underserved and important area, we propose a pathophysiology-focused research agenda to engage government-sponsored funding agencies and foundations.
Keywords: functional neurological disorder, psychogenic, conversion disorder, emotion, agency, attention, interoception, predictive coding, multimodal integration, biopsychosocial model, circuits, networks, frontoparietal, salience
Introduction
Functional neurological disorder (FND) is a common and disabling condition at the intersection of neurology and psychiatry that until recently has been largely neglected by the clinical neuroscience community. Over the past two decades, significant advances have been made in understanding the pathophysiology of this condition, revealing evidence of neural mechanisms underlying the development of functional neurological symptoms.1,2 The growth in FND research has been catalyzed by an emphasis on diagnosing patients based on physical examination signs and semiological features.3 The start of a new international professional society, the Functional Neurological Disorder Society (www.fndsociety.org), and a published authoritative textbook have further established this as a valid field.4 Additionally, there is an increasing appreciation of the value of a transdiagnostic approach to conceptualize FND across its various subtypes (e.g., functional movement disorder vs. functional [psychogenic nonepileptic] seizures), given that many individuals present with mixed symptoms at onset or develop distinct symptoms over the course of their illness.5 Obtaining a better understanding of the pathophysiology generating symptoms is particularly valuable when discussing the diagnosis with patients. Academic research interest in comprehensively characterizing FND is growing rapidly, yet researchers are currently faced with a lack of funding opportunities across government-sponsored agencies and foundations. Bridging this gap is essential to understand the neurobiology of this disorder, aid the development of biologically-informed treatments,6 and address the growing public health need. As such, this perspective article defines a framework for understanding candidate constructs and neural circuits underlying the pathophysiology of FND. We also propose a research agenda highlighting areas of inquiry likely to yield high impact advances.
Constructs and Neural Circuits
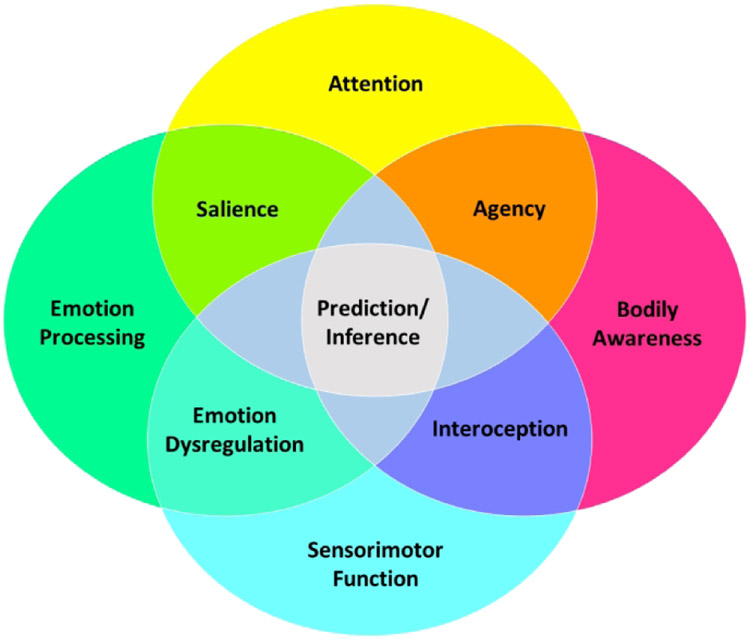
The brain operates in neural circuits, and symptoms in different disorders can be understood as mapping onto alterations within and across these circuits (Figure 1a). The different symptoms of FND arise from one or a combination of specific abnormal constructs. For example, paroxysmal functional movements are perceived as involuntary by patients due to abnormalities in the construct of agency (Figure 1b). Other constructs in FND include impairments in emotion processing, attention, interoception, multimodal integration, and their interactions. The implicated neural circuits can be explored using in vivo techniques including connectivity-based neuroimaging metrics, functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI), nuclear imaging, electroencephalography (EEG), and transcranial magnetic stimulation (TMS). Abnormal constructs can be mapped onto specific brain circuits. A diminished sense of agency, for example, is mediated by dysfunction involving a multimodal integration network, including the right temporoparietal junction (TPJ).7 Informed by phenomenological, neurobiological and treatment research in FND to date, this article focuses on several candidate constructs and their neural circuits. Abnormalities of these brain circuits (and constructs) interact in different ways to produce the signs and symptoms of FND (Figure 2).
Figure 1:
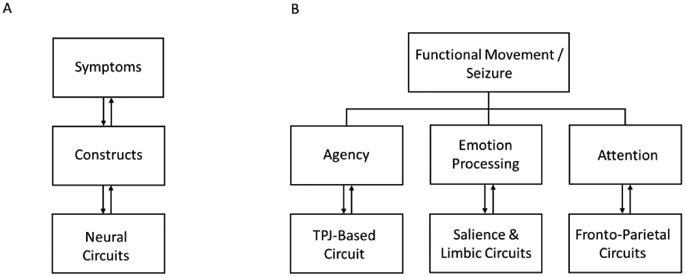
A) Illustration of the relationship between symptoms, constructs and neural circuits underlying functional neurological disorder (FND). Symptoms can be understood as mapping onto alterations of different constructs, which are generated by neural circuit abnormalities. B) Examples of how different symptoms or observable manifestations of FND can be understood as arising from one or a combination of specific abnormal constructs. For example, paroxysmal movements can be perceived as involuntary by an individual with FND due to a dysfunction of the construct of agency, which is driven by abnormalities of a TPJ-based circuit. TPJ indicates temporo-parietal junction.
Figure 2:
Abnormalities of several constructs (and their associated neural circuits) can interact in different ways to produce symptoms and observable signs of functional neurological disorder.
Emotion Processing
Emotion processing deficits are core features in some patients with FND. Evidence supports increased emotional reactivity, heightened arousal and avoidance, impaired top-down emotion regulation, amplification of functional neurological symptoms during negatively valenced or psychologically-threatening mood states (e.g., panic, shame), deficits in emotional awareness (e.g., physiological arousal in the absence of emotional arousal, alexithymia), aberrant salience processing, and errant activation of learned / innate defensive responses.8 At the circuit-level, many of these interrelated emotion processing functions map onto salience and other limbic/paralimbic (e.g., ventromedial and orbitofrontal prefrontal cortex, parahippocampus, hippocampus) circuits. The salience circuit, used as the primary example here, comprises the dorsal anterior cingulate cortex, anterior insula, dorsal amygdala, periaqueductal gray (PAG), and hypothalamus, and is implicated in detecting and responding to homeostatic demands.9 Heightened emotional reactivity, arousal and defensive responses occur from increased bottom-up amygdala and PAG activations. For example, studies have found reduced amygdala habituation and increased sensitization during negative emotion processing in patients with FND.10,11 Conversely, insufficient prefrontal control (regulation) of amygdala and PAG activations also promotes heightened emotional responses. This under-regulation of emotional response is relevant to deficits in fear extinction, while over-regulation is linked to dissociative responses.12
Task and resting-state neuroimaging studies in FND show increased functional connectivity between salience/limbic/paralimbic and motor control circuits (e.g. precentral gyrus, supplementary motor area).1,11 Connectivity strength between cingulo-insular and motor control areas correlates with patient-reported symptom severity,13,14 and modulation of anterior cingulate activity has been linked to favorable cognitive behavioral psychotherapy response in FND.6 Increased limbic-motor circuit connectivity is theorized to represent heightened limbic influence over motor behavior in patients with FND.10 This is seen clinically when FND patients report that negative emotions worsen their motor symptoms. Deficits in putting emotions into words (alexithymia)15 and the experience of autonomic arousal in the absence of perceived negative affect have also been described in FND.16 This is notable given that the cognitive-affective neuroscience literature implicates the anterior insula (and its related connectivity) to emotional and self-awareness.17 Regarding the PAG, increased activation to negatively-valenced stimuli and heightened laterobasal amygdala-PAG functional connectivity have been described in FND cohorts,13,18 implicating abnormalities of defensive behaviors (e.g. tonic immobility) in functional neurological symptom expression. Conceptually, it is important to highlight that the salience network overlaps with the central pain matrix19 and the multimodal integration network,20 underscoring the importance of these overlapping circuits not only in emotion processing but also in other interrelated constructs.
Agency
Self-agency reflects a person’s belief that he or she is the agent of the action or thought—this is the sense of volition or free will that characterizes voluntary movement.21 Two events must occur to produce self-agency: i) the person must have the sense of willing the movement and ii) the movement (congruent with what has been willed) has to happen. Movements deemed as voluntary are produced by the primary motor cortex that is activated by a network of structures, most proximally the premotor and supplementary motor cortices. When a movement is generated, the rest of the brain is notified by a feedforward signal. When movements happen, there is feedback through various sensory experiences about the movement. If the feedback matches the feedforward, then there is a sense of causality and self-agency. The networks involved in this process include cortico-cortical pathways from motor structures and sensory pathways to multimodal sensory areas where perceptions are generated. A primary site of matching of feedforward and feedback is the right TPJ. When there is a mismatch between willing and movement, the TPJ becomes activated. In studies of agency, the TPJ is one node of the multimodal integration brain network.20
Patients with FND have movements which lack self-agency and are experienced as involuntary. There are many examples in neurology of involuntary movements that are produced by pathological processes such as tremor in Parkinson disease. In hyperkinetic functional movement disorders such as tremor, and likely major motor functional seizures, the brain areas generating movements are the same as with voluntary movement, and typically operate normally.22 Patients with these disorders generally do not have an intrinsic sensory deficit that would make feedback incorrect. Yet, the movements are perceived to be involuntary. A number of studies have shown right TPJ dysfunction in patients with hyperkinetic movement disorders, as was first demonstrated in functional tremor.23,24 Hence, the agency network is not working properly in at least some patients with FND.
While more work is needed to clarify this process, there are at least two possibilities: either the TPJ agency circuit is impaired or the feedforward signal is abnormal due to abnormal influences on the motor apparatus. Moreover, it will be important to understand relationships across networks (e.g., TPJ and insula interactions)13,24.
Attention
Impairments in attention have been characterized in FND.1 These disruptions manifest as attentional perseveration—that is, a tendency to focus on a particular physiological system to the neglect of other systems and an impaired ability to adaptively, volitionally shift attention. Attending to unaffected body parts is effortful and difficult in FND; this attentional rigidity is analogous to hemineglect syndromes.25 Attentional abnormalities have been well characterized using neuropsychological testing, with disruptions in sustained and selective attention observed in some individuals with FND.26-29 Further, there is preferential allocation of attentional resources to threatrelevant stimuli (angry faces) in FND populations, particularly those with functional seizures.30-33
Inefficient or impaired attentional shifting, as well as involuntary attentional biases in FND, emerge from abnormal connections in both goal-directed and stimulus-driven neural networks. Certain FND symptoms emerge from an explicit and excessive focus on physiological states, whereas in others the process is more implicit and involuntary. Decreased fronto-parietal network responses have been observed in FND patients.34,35 However, meta-analytic evidence indicates overall greater activation in fronto-parietal networks, as well as in limbic regions such as the amygdala, in FND patients versus controls.36 This underscores the complex relationships between emotion processing and attention regulation in FND. Further, the network effects of psychiatric and neurologic comorbidities, as well as medication side effects impeding attentional mechanisms in this patient population (e.g., psychotropics, opioids, and analgesics) must also be considered. In sum, attentional control deficits are found in FND, but there are likely multiple ways that these deficits are represented in neural circuits. More research exploring relationships between FND symptoms and attentional processes in both neutral and emotional, implicit and explicit contexts, is needed to identify common and distinct features of attentional disruptions in patients with FND.
Interoception
Interoception refers to the process by which the nervous system senses, interprets, and integrates internal bodily signals, providing a moment-by-moment mapping of the body’s internal landscape across conscious and unconscious levels.37 It is important for monitoring the internal state of the body, predicting future bodily states, and informing self-regulatory actions. Abnormal interoceptive awareness has been identified in FND via reduced perceptual accuracy for the resting heartbeat.38,39 However, some individuals with FND actually show intact perceptual discrimination during homeostatic perturbation of interoceptive states, and instead exhibit a dissociation characterized by heightened symptom intensity during the peri-stimulation time periods.40 This suggests that i) aspects of FND symptoms might be explained by disrupted internal models of the body, ii) the ability to modulate physiological states and contextual cues is important to gaining insight into this process38, and iii) our knowledge of interoceptive awareness deficits in FND is incomplete.40,41
Mechanistically, interoception is conceptualized as a bidirectional process between the brain and body, with feedback and feedforward loops leading to an internal representation of the body. Interoceptive abnormalities can contribute to the generation of the FND ‘symptom scaffold’.42 For example, abnormal interoceptively-focused attention in FND may preferentially influence the weighting of top-down or bottom-up information streams in the central nervous system,43 leading to abnormally enhanced or diminished sensory perceptions (e.g. attenuated visual, auditory, or skin sensitivity) or movements (tremor, dystonia, weakness, seizures). Neural circuits of interoception include those mapping autonomic, chemosensory, endocrine, and immune systems. They include afferent signals from the lamina I spinothalamic system, and the vagus and glossopharyngeal cranial nerves through the brainstem (e.g., nucleus tractus solitarius), that synapse onto the thalamus and subsequent cortical areas, including the posterior insula and somatosensory cortices. The neural circuitry contributing to the amplification of bodily signals in FND cuts across frontolimbic, subcortical, and brainstem structures.44
Perceptual Inference and Predictive Processing
While distinct from interoception, inference is an important overlapping construct that refers to the process by which a person generates beliefs (or explanations) about the causes and effects of events occurring within and outside the body.45 Perceptual inferences are strongly influenced by expectations (including suggestibility), either explicit or implicit, and can rapidly change depending on the environmental context. FND is a condition that can be characterized by the development of erroneous perceptual inference—about sensorimotor and emotionally valenced phenomena (Figure 2). Because they reflect beliefs, it is natural that these inferences are experienced as ‘real’ by the patient.
Computational neuroscience has provided mechanistic insights into the underpinnings of causal inference in the nervous system. Unlike the classical hierarchical feedforward model of perception that involves a mostly linear filtering and translation of sensory signals to arrive at higher-order perception, a new argument has emerged that explains perception as arising from predictive processing.46 In this scenario, neurons transmitting predictions about sensory states communicate with neurons detecting deviations from those predictions (so-called ‘prediction errors’) to develop an explanation for the perceptual information received (called a ‘generative model’).47 Over time, when the observed information deviates from what is predicted, the generative model is updated through learning. In addition, the metacognitive evaluation of perceptual content plays a role in generating awareness states,48 and it is conceivable that abnormalities in the neural circuitry underlying metacognition underpin aspects of the FND symptom scaffold.49
The predictive coding framework is one computational approach to predictive processing which uses the application of Bayesian mathematical principles to develop models of causal inference. Using computational modeling of behavior on a timed-decision task,50 individuals with motor FND showed deficits in decision-making and sensory processing. From a predictive coding perspective, the authors interpreted this as evidence that predictions were overemphasized in FND relative to the sensory information. Since prior experiences influence predictions, it was argued essentially that the FND patients’ prior history dominated their ongoing perceptions. Thus, someone who always expects the weather to be cold—and leaves home wearing a winter jacket on a sunny day in the middle of summer could represent a similar example. Applying predictive processing and other computational methods to sensory and motor domains (for example, with a focus on the role of agency and/or emotional awareness in predictive coding errors), will inform the pathophysiology of FND.
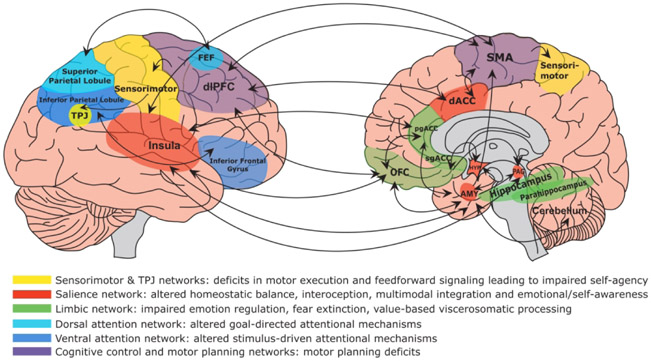
See Figure 3 for a display of brain circuits and constructs described above that are important in the pathophysiology of FND.
Figure 3:
Display of brain circuits (and related constructs) that are emerging as important in the pathophysiology of functional neurological disorder (FND). As depicted, FND is a multi-network disorder involving abnormalities within and across brain circuits implicated in self-agency, emotion processing, attention, homeostatic balance, interoception, multimodal integration, and cognitive/motor control among other functions. Circuits are described by their related dysfunction in the pathophysiology of FND. It should also be noted that several areas cut across multiple networks; for example, the dorsal anterior insula is most strongly interconnected with the dorsal anterior cingulate cortex (dACC), while the posterior insula receives afferent projections from the lamina I spinothalamocortical pathway and somatosensory cortices. Similarly, the amygdala is part of both the salience and limbic networks. Prefrontal brain regions are interconnected with striatal-thalamic areas (not shown), and these pathways should also be factored into the neural circuitry of FND. TPJ indicates temporoparietal junction; FEF, frontal eye fields; dlPFC, dorsolateral prefrontal cortex; pgACC, perigenual anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; OFC, orbitofrontal cortex; SMA, supplementary motor area; AMY, amygdala; HYP, hypothalamus; PAG, periaqueductal gray.
Bridging Pathophysiology and Etiology in FND
The pathophysiology of FND unfolds within the context of developmental trajectories, life experiences, sex differences, social-cultural norms and many other factors within a biopsychosocial model. While it is useful to consider mechanisms (how) and etiology (why) separately, from a research perspective it is also important to bridge the two, as these processes are interrelated. In this section, we contextualize pathophysiological mechanisms within the framework of predisposing vulnerabilities, acute precipitants, and perpetuating factors.
Through the lens of inference models, socio-emotional-perceptual processes that are compromised in FND are shaped by prior experiences at the neural circuit level. These processes are refined through interactions between epigenetic substrates, the psychophysiological matrix, and the environment. In other words, daily life experiences are not just encoded as external events, but also require internal integration that shapes the brain’s predictions through neuroplastic mechanisms. To illustrate, beginning in infancy, a consistent experience of caregiver affectionate touch has the potential to shape development of interoceptive perceptions and the sense of (physical) self, and can facilitate sensorimotor integration as well.51 In this way, attachment is not just a theoretical construct; it reflects the ‘real’ (embodied) way that caregivers create a socioemotional context in which appropriate recognition and delineation of physical neural signals can develop. Throughout the lifespan, expectations about the body and its signals, which are also shaped by sociocultural beliefs and context (e.g., religion, economic circumstances, language, gender norms), further refine sensorimotor perceptions. In less favorable circumstances, these factors, coupled with genetic vulnerabilities and environmental demands, lead to compromised integration, resulting in FND symptoms.
Adverse early life events are one example (see Ludwig et al. 2018 for a systematic review and meta-analysis).52 It is well-established that trauma impacts the developing brain, with evidence that childhood maltreatment affects salience, emotion processing and sensorimotor circuits;53 this, in turn, explains their role in predisposing the central nervous system to the development of FND symptoms. Furthermore, childhood trauma in the context of a specific genetic profile can lead to epigenetic changes and delayed-onset symptoms.54 In patients with FND, the magnitude of experienced childhood abuse correlates with symptom severity,55 insecure attachment,56 poor prognosis,57 limbic-motor circuit connectivity58 and neuro-endocrine abnormalities.30 It is important, however, to consider pathophysiological similarities and differences between FND patients with and without prominent adverse early-life experiences, as it is unclear if disease mechanisms are uniform across populations.
In FND, it is not only the occurrence of stressful events and elevation of biological stress markers, but also the addition of (1) greater perceived stress and/or (2) lack of awareness of this stress (i.e., a mismatch in perception/interpretation/felt experience) that appears to contribute to and result from the underlying pathophysiology. Studying these processes teaches us more about how and why FND develops, while revealing in a fundamental way how these processes work, and where targeted treatments may be possible.
Limitations of Pathophysiology Research to Date
While a detailed description of pathophysiology-related research limitations in FND is beyond the scope of this article, several concerns are important to highlight. Across neurobiological research in FND, sample sizes have been modest (no studies with N > 100) limiting statistical power to adjust for variables that may also influence brain circuit profiles such as psychiatric comorbidities, chronic pain disorders, medication effects, and illness (and developmental) trajectories. For example, only a subset of studies controlled for antidepressant use, which is notable given that amygdala activity is modulated by serotonergic medications;59 this may help explain inconsistent limbic profiles across studies.10,60 In addition to healthy subjects, the use of neurologic and psychiatric control groups to understand the specificity of disease findings has also been exceedingly limited.58 Studies examining psychiatric comorbidities and etiologies, such as depression, anxiety and trauma-related disorders, do find that these account for many of the non-specific findings, such as poor emotion regulation in those with functional seizures; however, the processes highlighted here, such as sense of agency, interoceptive awareness, attention to somatic symptoms, and their intersection, are points of differentiation in FND, even accounting for medication use and comorbidities. Additionally, few studies have included multimodal neuroimaging techniques, or combined neuroimaging data with autonomic, neuroendocrine or genetic/epigenetic information.54,60 The potential role of sex differences in a condition that has a well-established tendency to be more common in women has also been minimally investigated.61 Lastly, longitudinal data, including but not limited to post vs. pretreatment, have only been collected in small pilot studies – suggesting that more work is needed to understand the relationship between disease pathophysiology and prognostic neural mechanisms.6
Pathophysiology-Focused Research Agenda
Key functional neurological symptoms have been characterized and much knowledge has been gained about their underlying pathophysiology, but the detailed biochemistry, structural/functional anatomy and electrophysiological connectivity of these neural circuits and their interactions remain incompletely understood. Moreover, while a number of common FND symptoms and features are well recognized (e.g., paroxysmal functional movement disorder, emotional dysregulation, alexithymia), gaps exist in our ability to reliably measure symptom presence and severity in order to optimally characterize their nature, extent, and resolution in a systematic manner. Some of the socio-emotional, cognitive, and awareness-based deficits and risk factors observed in FND are also common in other neuropsychiatric conditions (e.g., epilepsy, post-traumatic stress disorder, dissociative disorders, schizophrenia); a mechanistic investigation of these symptoms will benefit from the synergy of methods and concepts available across distinct patient populations while simultaneously leading to gains in all areas (e.g., studying socio-emotional processing in a patient undergoing stereoEEG for localization of seizures provides us with simultaneous in vivo neural information during task performance). In addition to understanding the pathophysiology underlying FND symptoms, rigorous studies are needed to comprehensively determine predisposing, precipitating, and perpetuating variables that contribute to their development and maintenance, and to characterize the nature of intervening variables which may serve to strengthen resilience (i.e., Why do some trauma-exposed individuals develop FND symptoms and others do not?). Behavioral, autonomic, neuroendocrine, epigenetic, genetic and social-environmental factors and developmental trajectories likely hold answers.60,62
It is expected that additional research will allow us to understand and manage psychosocial/cultural/spiritual/environmental variables that influence FND symptom development and maintenance, and to identify and/or develop diagnostic and prognostic biomarkers and biologically-informed therapeutics, including rehabilitative, psychopharmacologic and neuromodulatory strategies. As such, five core broadly-defined research agendas at the pathophysiology level that would move the field forward should be considered. Below each research agenda is an illustrative example.
-
Identify how functional neurological symptoms, cognitive-affective-bodily-sensorimotor processing constructs and brain circuits relate to one another, including but not limited to how interactions across circuits relate to symptoms and constructs.
e.g., Do disturbances in self-agency map onto the right TPJ-related network across the hyperkinetic and hypokinetic motor spectrum of FND (including functional seizures)?
-
Characterize relationships between pathophysiology (how), etiological factors (why) and/or treatment responses (e.g., physical therapy, psychotherapy, etc.) across constructs and brain circuits in FND.
e.g., Do patients with and without prominent childhood maltreatment have the same neural mechanisms for their functional neurological symptoms, and if differences are present, do they have prognostic (and treatment response) implications?
-
Identify variables that bridge relationships between symptoms and circuits, including characterization of neuroendocrine, autonomic, cellular/molecular and genetic/epigenetic factors.
e.g., Do stress hormones (e.g., salivary cortisol, amylase), sex hormones and autonomic profiles (e.g., heart rate variability) provide additional differentiating clinical-pathologic insights, and if so, might composite biomarkers of FND be important?
-
Describe the common (transdiagnostic) mechanistic pathways across FND, as well as subtype specific disruptions; this includes the investigation of individual differences, sex differences and potential biologically-informed subtypes.
e.g., Do men and women with similar functional neurological symptoms recruit the same neural circuits for symptom generation and maintenance?
-
Identify the specificity of constructs and brain circuits implicated in FND, by incorporating not only healthy subjects, but also neurologic, psychiatric and medical control groups (e.g., major depression, PTSD, functional somatic disorders).
e.g., Are sensorimotor and agency network interactions with limbic and salience brain circuits distinguishing characteristics of FND when compared to other psychiatric and neurological conditions comorbid in FND.
Overall, while there are other fruitful research directions not addressed by this article, we have detailed important constructs and circuits implicated in the pathophysiology of FND that would benefit from additional rigorous research inquiry to move the field forward.
Acknowledgements:
The authors thank Mark and Barbara Klein for their support.
Funding:
Daniel L. Drane. is funded by the National Institute of Neurological Disorders and Stroke (NINDS) grant R01NS088748. Mark Hallett is supported by the NINDS intramural program. Sahib S. Khalsa is supported by National Institute of Mental Health (NIMH) grant K23MH112949, National Institute of General Medical Sciences (NIGMS) Center grant 1P20GM121312, and The William K. Warren Foundation. David L. Perez. is funded by the NIMH grant K23MH111983, the Massachusetts General Hospital Physician- Scientist Development Award, and the Sidney R. Baer Jr. Foundation. Nicole A. Roberts received support from the Arizona Institute for Mental Health Research and the Arizona State University Institute for Social Science Research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures:
David L. Perez has received honoraria for continuing medical education lectures in functional neurological disorder from Harvard Medical School, American Academy of Neurology, Movement Disorder Society, Toronto Western Hospital and Newton Wellesley Hospital. Daniel Drane, Negar Fani, Mark Hallett, Sahib Khalsa, and Nicole A. Roberts do not report any disclosures or competing interests.
References
- 1.Baizabal-Carvallo JF, Hallett M, Jankovic J. Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis. 2019; 127:32–44. [DOI] [PubMed] [Google Scholar]
- 2.Begue I, Adams C, Stone J, et al. Structural alterations in functional neurological disorder and related conditions: a software and hardware problem? Neuroimage Clin. 2019; 22:101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espay AJ, Aybek S, Carson A, et al. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurology. 2018; 75(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallett M, Stone J, Carson AJ, ed Functional Neurological Disorders. Academic Press; 2016; No. 139. [Google Scholar]
- 5.McKenzie PS, Oto M, Graham CD, et al. Do patients whose psychogenic non-epileptic seizures resolve, ‘replace’ them with other medically unexplained symptoms? Medically unexplained symptoms arising after a diagnosis of psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2011; 82(9):967–969. [DOI] [PubMed] [Google Scholar]
- 6.Espay AJ, Ries S, Maloney T, et al. Clinical and neural responses to cognitive behavioral therapy for functional tremor. Neurology. 2019; 93(19):e1787–e1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zito GA, Wiest R, Aybek S. Neural correlates of sense of agency in motor control: A neuroimaging meta-analysis. PLoS One. 2020; 15(6):e0234321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pick S, Goldstein LH, Perez DL, et al. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry. 2019; 90(6):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeley WW. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J Neurosci. 2019; 39(50):9878–9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voon V, Brezing C, Gallea C, et al. Emotional stimuli and motor conversion disorder. Brain. 2010; 133(Pt 5):1526–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aybek S, Nicholson TR, Zelaya F, et al. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry. 2014; 71(1):52–60. [DOI] [PubMed] [Google Scholar]
- 12.Lanius RA, Vermetten E, Loewenstein RJ, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010; 167(6):640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez I, Ortiz-Terán L, Williams B, et al. Corticolimbic fast-tracking: enhanced multimodal integration in functional neurological disorder. J Neurology Neurosurg Psychiatry. 2019; 90(8):929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R, Li Y, An D, et al. Altered regional activity and inter-regional functional connectivity in psychogenic non-epileptic seizures. Sci Rep. 2015; 5:11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demartini B, Petrochilos P, Ricciardi L, et al. The role of alexithymia in the development of functional motor symptoms (conversion disorder). J Neurol Neurosurg Psychiatry. 2014; 85(10):1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indranada AM, Mullen SA, Duncan R, et al. The association of panic and hyperventilation with psychogenic non-epileptic seizures: A systematic review and meta-analysis. Seizure. 2018; 59:108–115. [DOI] [PubMed] [Google Scholar]
- 17.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009; 10(1):59–70. [DOI] [PubMed] [Google Scholar]
- 18.Aybek S, Nicholson TR, O'Daly O, et al. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015; 10(4):e0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014; 17(2):192–200. [DOI] [PubMed] [Google Scholar]
- 20.Sepulcre J, Sabuncu MR, Yeo TB, et al. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci. 2012; 32(31): 10649–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallett M Physiology of free will. Annals of Neurology. 2016; 80(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallett M Neurophysiologic studies of functional neurologic disorders. Handbook of Clinical Neurology. 2016; 139:61–71. [DOI] [PubMed] [Google Scholar]
- 23.Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010; 74(3):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer CW, LaFaver K, Ameli R, et al. Impaired self-agency in functional movement disorders: A resting-state fMRI study. Neurology. 2016; 87(6):564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez DL, Barsky AJ, Daffner K, et al. Motor and somatosensory conversion disorder: a functional unawareness syndrome? J Neuropsychiatry Clin Neurosci. 2012; 24(2):141–151. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien FM, Fortune GM, Dicker P, et al. Psychiatric and neuropsychological profiles of people with psychogenic nonepileptic seizures. Epilepsy Behav. 2015; 43:39–45. [DOI] [PubMed] [Google Scholar]
- 27.Simani L, Roozbeh M, Rostami M, et al. Attention and inhibitory control deficits in patients with genetic generalized epilepsy and psychogenic nonepileptic seizure. Epilepsy Behav. 2020; 102:106672. [DOI] [PubMed] [Google Scholar]
- 28.Strutt AM, Hill SW, Scott BM, et al. A comprehensive neuropsychological profile of women with psychogenic nonepileptic seizures. Epilepsy Behav. 2011; 20(1):24–28. [DOI] [PubMed] [Google Scholar]
- 29.Alluri PR, Solit J, Leveroni CL, et al. Cognitive Complaints in Motor Functional Neurological (Conversion) Disorders: A Focused Review and Clinical Perspective. Cogn Behav Neurol. 2020; 33(2):77–89. [DOI] [PubMed] [Google Scholar]
- 30.Bakvis P, Roelofs K, Kuyk J, et al. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia. 2009; 50(5): 1001–1011. [DOI] [PubMed] [Google Scholar]
- 31.Bakvis P, Spinhoven P, Roelofs K. Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav. 2009; 16(3):558–560. [DOI] [PubMed] [Google Scholar]
- 32.Pick S, Mellers JDC, Goldstein LH. Implicit attentional bias for facial emotion in dissociative seizures: Additional evidence. Epilepsy Behav. 2018; 80:296–302. [DOI] [PubMed] [Google Scholar]
- 33.Roelofs K, van Galen GP, Eling P, et al. Endogenous and exogenous attention in patients with conversion paresis. Cognitive Neuropsychology. 2003; 20(8):733–745. [DOI] [PubMed] [Google Scholar]
- 34.Spence SA, Crimlisk HL, Cope H, et al. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000; 355(9211):1243–1244. [DOI] [PubMed] [Google Scholar]
- 35.Morris LS, To B, Baek K, et al. Disrupted avoidance learning in functional neurological disorder: Implications for harm avoidance theories. Neuroimage Clin. 2017; 16:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boeckle M, Liegl G, Jank R, et al. Neural correlates of conversion disorder: overview and meta-analysis of neuroimaging studies on motor conversion disorder. BMC Psychiatry. 2016; 16:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalsa SS, Adolphs R, Cameron OG, et al. Interoception and Mental Health: A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018; 3(6):501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricciardi L, Demartini B, Crucianelli L, et al. Interoceptive awareness in patients with functional neurological symptoms. Biological Psychology. 2016; 113:68–74. [DOI] [PubMed] [Google Scholar]
- 39.Koreki A, Garfkinel SN, Mula M, et al. Trait and state interoceptive abnormalities are associated with dissociation and seizure frequency in patients with functional seizures. Epilepsia. 2020; 61 (6):1156–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogaerts K, Van Eylen L, Li W, et al. Distorted symptom perception in patients with medically unexplained symptoms. Journal of Abnormal Psychology. 2010; 119(1):226–234. [DOI] [PubMed] [Google Scholar]
- 41.Jungilligens J, Wellmer J, Schlegel U, et al. Impaired emotional and behavioural awareness and control in patients with dissociative seizures. Psychol Med. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 42.Brown RJ, Reuber M. Towards an integrative theory of psychogenic non-epileptic seizures (PNES). Clinical Psychology Review. 2016; 47:55–70. [DOI] [PubMed] [Google Scholar]
- 43.Edwards MJ, Adams RA, Brown H, et al. A Bayesian account of ‘hysteria’. Brain. 2012; 135(Pt 11):3495–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015; 27(1):e40–50. [DOI] [PubMed] [Google Scholar]
- 45.Teufel C, Fletcher PC. Forms of prediction in the nervous system. Nat Rev Neurosci. 2020; 21(4):231–242. [DOI] [PubMed] [Google Scholar]
- 46.Keller GB, Mrsic-Flogel TD. Predictive Processing: A Canonical Cortical Computation. Neuron. 2018; 100(2):424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh KS, McGovern DP, Clark A, et al. Evaluating the neurophysiological evidence for predictive processing as a model of perception. Annals of the New York Academy of Sciences. 2020; 1464(1):242–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleming SM. Awareness as inference in a higher-order state space. Neuroscience of Consciousness. 2020; 2020(1):niz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bègue I, Blakemore R, Klug J, et al. Metacognition of visuomotor decisions in conversion disorder. Neuropsychologia. 2018; 114:251–265. [DOI] [PubMed] [Google Scholar]
- 50.Sadnicka A, Daum C, Meppelink AM, et al. Reduced drift rate: a biomarker of impaired information processing in functional movement disorders. Brain. 2020; 143(2):674–683. [DOI] [PubMed] [Google Scholar]
- 51.Burleson MH, Quigley KS. Social interoception and social allostasis through touch: Legacy of the Somatovisceral Afference Model of Emotion. Social Neuroscience. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ludwig L, Pasman JA, Nicholson T, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. 2018; 5(4):307–320. [DOI] [PubMed] [Google Scholar]
- 53.Teicher MH, Samson JA, Anderson CM, et al. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016; 17(10):652–666. [DOI] [PubMed] [Google Scholar]
- 54.Spagnolo PA, Norato G, Maurer CW, et al. Effects of TPH2 gene variation and childhood trauma on the clinical and circuit-level phenotype of functional movement disorders. J Neurol Neurosurg Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roelofs K, Keijsers GP, Hoogduin KA, et al. Childhood abuse in patients with conversion disorder. Am J Psychiatry. 2002; 159(11):1908–1913. [DOI] [PubMed] [Google Scholar]
- 56.Williams B, Ospina JP, Jalilianhasanpour R, et al. Fearful Attachment Linked to Childhood Abuse, Alexithymia, and Depression in Motor Functional Neurological Disorders. J Neuropsychiatry Clin Neurosci. 2019; 31(1):65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hailes HP, Yu R, Danese A, et al. Long-term outcomes of childhood sexual abuse: an umbrella review. Lancet Psychiatry. 2019; 6(10):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diez I, Larson AG, Nakhate V, et al. Early-life trauma endophenotypes and brain circuit-gene expression relationships in functional neurological (conversion) disorder. Mol Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Godlewska BR, Norbury R, Selvaraj S, et al. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med. 2012; 42(12):2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allendorfer JB, Nenert R, Hernando KA, et al. FMRI response to acute psychological stress differentiates patients with psychogenic non-epileptic seizures from healthy controls - A biochemical and neuroimaging biomarker study. Neuroimage Clin. 2019; 24:101967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez DL, Matin N, Barsky A, et al. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J Neurol Neurosurg Psychiatry. 2017; 88(6):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kozlowska K, Griffiths KR, Foster SL, et al. Grey matter abnormalities in children and adolescents with functional neurological symptom disorder. NeuroImage Clin. 2017; 15:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]