Abstract
In the present research, inter and intra genetic variability of 77 accessions belonging to 11 Thymus species were assessed using eight SRAP primer combinations. High polymorphism (98.3%) was observed in the studied species. The cluster analysis classified Thymus species into five main groups. According to molecular variance (AMOVA) analysis, 63.14% of total genetic variation was obtained within the species, while 36.86% of variation was observed among species. STRUCTURE analysis was also performed to estimate the admixture of species. For instance, T. carmanicus and T. transcaspicus revealed high admixtures. HPLC analysis also demonstrated the presence of rosmarinic acid (32.3–150.7 mg/100 g DW), salvianolic acid (8–90 mg/100 g DW), and cinnamic acid (1.7–32.3 mg/100 g DW) as major phenolic acids, as well as apigenin, epicatechin, and naringenin as the major flavonoids. The highest phenolic and flavonoid contents were detected in T. transcaspicus (37.62 mg gallic acid equivalents (GAE) g−1 DW) and T. vulgaris (8.72 mg quercetin equivalents (QE) g−1 DW), respectively. The antioxidant properties and total phenolic of Thymus species were examined using DPPH and β-carotene-linoleic acid model systems and consequently T. vulgaris and T. pubescens were detected with the highest and the lowest antioxidant activities respectively. Cluster and principal Components Analysis (PCA) of the components classified the species in to three groups. Finally, similarity within some species was observed comparing molecular and phytochemical markers. For instance, T. vulgaris separated from other species according to major polyphenolic profiles and molecular analyses, as well as T. transcaspicus, T. carmanicus, and T. fedtschenkoi that were clustered in the same groups.
Subject terms: Genetic markers, Chemical ecology
Introduction
Phenolic and flavonoid compounds have been known as natural products with high antioxidant activity. Nowadays, such components are frequently used and emphasized in food and some industrial products because of their health properties1. These compounds also have a crucial role in scavenging of the free radicals that are considered as serious risk factors for human health. Therefore, polyphenolic components extracted from natural sources of plant species are of great importance and thence, there is a growing interest to use the natural sources of antioxidants instead of synthetic ones.
Thyme (Thymus spp.) has been used as one of the most substantial medicinal plants for food and pharmaceutical purposes2. Thymus is considered as one of the most important genera of the Lamiaceae family with more than 100 species3. It is a perennial medicinal plant wildly grown in different regions of the world. Mediterranean regions have been assumed as the origin of this plant4. Thymus species has been applied in food industries for different purposes including flavoring agent, herbal tea and new products with low antibacterial activities. Furthermore, thyme has been used as antifungal, antiviral, anti-inflammatory, anti-parasitic, and spasmolytic5. Most of the studies on the Thymus species have focused on essential oil components of the species in different countries, including Iran2,3, Serbia6, and Ethiopia7. However, there are limited reports in respect to polyphenolic profiles of Thymus species, including T. vulgaris8, T. satureioides9, T. pannonicus10, and T. praecox11 using chromatography-based analysis. Phenolic acids as well as flavonoids and their derivatives display high variations in different Thymus species such as T. algeriensis (rosmarinic acid and kaempferol-O-glucuronide)12, T. pulegioides (rosmarinic acid and luteolin derivatives)13, and T. capitatus (rosmarinic acid, salvianolic acid)14. In Iran, 14 Thymus species have been identified out of which four of are known to be endemic15. However, there are no reports regarding phenolic and flavonoid components of Iranian Thymus species based on HPLC analysis. Furthermore, most of the previous researches have focused on polyphenolic profiles of one or few limited species and there are no comprehensive and comparative study focusing on phenolics, flavonoids and their derivatives in Thymus species.
Besides the polyphenolic profiles, molecular studies can provide new insights for further classification and breeding programs in this genus. SRAP (Sequence Related Amplified Polymorphism) is a PCR-based dominant marker that amplifies the fragments selectively by targeting the functional genome regions. This marker has been used to amplify the Open Reading Frame (ORF)16. This method is simple and the bands can be easily scored and interpreted17. Previous reports displayed higher polymorphism nature of SRAP markers in comparison with other dominant markers such as ISSR and RAPD18,19. Finally, SRAP has been considered as a high-throughput marker for studying genetic diversity, which has not yet been studied among and within Thymus species. SRAP marker has been applied for the assessment of the genetic diversity in many Lamiaceae plants including Origanum genus20, Salvia aristata21, and Satureja species22. There are limited reports on genetic diversity of Thymus species and most of researches focused on one species including T. daenensis15,23,24, T. sibthorpii25, T. carmanicus26, T. kotschyanus and T. vulgaris27. Moreover, there are limited reports in respect to HPLC analysis of Thymus species including T. serpyllum and T. argaeus28,29. In the present study, the natural compounds of several Thymus species were introduced for the first time. Moreover, as the previous studies mostly reported one species and the extraction methods were different, so another importance of this research was the comparison of 11 Thymus species in one experiment using a similar extraction method that increase the validity of comparison. Therefore, this is the first report to introduce and compare the phenolic and flavonoid compounds of several Thymus species based on HPLC analysis. Finally, comparing phytochemical and molecular markers can contribute to the improvement of the components as well as breeding of studied Thymus species.
The objectives of the present study are: (1) to compare total phenolic, flavonoid and antioxidant capacity of 11 Thymus species viz. Thymus migricus Klokov & Des. -Shost., T. fallax Fisch. & C. A. Mey., T. serpyllum L., T. trautvetteri Klokov & Desj. -Shost., T. transcaspicus Klokov, T. carmanicus Jalas, T. fedtschenkoi Ronneger, T. daenensis Celak subsp. daenensis, T. pubescens Boiss. & Kotschy ex Celak, T. kotschyanus Boiss. & Hohen., and T. vulgaris L, (2) to determine the polyphenolic profiles of studied species based on HPLC analysis, (3) to determine the inter- and intra-genic relationships of the species based on SRAP molecular marker, and (4) to classify the species based on molecular and phytochemical markers using multivariate analyses.
Results and discussion
Phytochemical analysis
Quantitative determination of phenolic acids, flavonoid compounds, and their derivatives were performed using HPLC analysis (Table 1, Fig. 1). HPLC results showed high variation among studied species. Rosmarinic acid, salvianolic acid, cinnamic acid, ferulic acid, caffeic acid apigenin were the major polyphenolic components of Thymus methanolic extract in 11 assessed species. Among phenolic acids, rosmarinic acid possessed the highest content, while among flavonoids apigenin revealed the highest values. Rosmarinic acid has been reported as the major phenolic acid in most of the previous studies in several species9,10,30, while different classes of flavonoids have been reported in Thymus species. Rosmarinic acid content ranged from 32.2 mg/100 g DW in T. daenensis to 150.7 mg/100 g DW in T. serpyllum (Table 1). Similarly, Zengin et al.29 reported a higher content of rosmarinic acid in T. argaeus as compared to our studied species by using similar methanolic extraction method, column (C18) and standard compounds. However, the extraction method and the harvesting time are reported as two most determining factors that can highly affect the chromatography output31. Rosmarinic acid can be found in some other Lamiaceae plants such as Origanum vulgare, Salvia officinalis28, Ocimum basilicum32 and Agastache rugosa33. It has valuable properties such as anti-viral, anti-thrombotic, anti-inflammatory and antiglycative activities34,35. Salvianolic acid also showed high amounts compared to other phenolic acids. The highest and the lowest amounts of salvianolic acid were obtained in T. vulgaris (90.0 mg/100 g DW) and T. migricus (8.0 mg/100 g DW), respectively. This compound is also considered as one of the major phenolic acids in other reported Thymus species, including T. capitatus14, T. carnosus36 and T. pannonicus10. Various pharmaceutical properties have been reported for salvianolic acid including anti-inflammatory37, anti-diabetic activity38, as well as cardiovascular effects. This compound has also been found in other Lamiaceae species, as is considered in the main phenolic acid component of Salvia miltiorrhiza38.
Table 1.
Major phenolic and flavonoid compounds of the studied Thymus species based on HPLC analysis.
| Standards | RTa | T. carmanicus | T. daenensis | T. fallax | T. fedtschenkoi | T. kotschyanus | T. migricus | T. pubesence | T. trautvetteri | T. serpyllum | T. transcaspicus | T. vulgaris |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic acid | 5.5 | 3.0 | 3.6 | ndb | 2.8 | 0.0 | 2.4 | 0.8 | 0.3 | 2.0 | 1.3 | 5.5 |
| Epicatechin | 7.41 | 1.7 | 1.7 | 1.5 | 1.9 | 1.7 | 1.1 | 1.1 | 0.9 | 0.5 | 1.7 | 2.3 |
| Chlorogenic acid | 13.35 | 6.0 | 3.3 | ndb | ndb | ndb | ndb | ndb | 2.9 | ndb | 4.2 | ndb |
| Caffeic acid | 14.68 | 27.4 | 13.1 | 12.9 | 14.0 | 14.2 | 14.0 | 15.8 | 15.8 | 16.0 | 13.7 | 11.8 |
| Luteolin-7-o-glucoside | 22.56 | 7.9 | 3.3 | 1.9 | 43.9 | 12.7 | 4.5 | 6.1 | 11.0 | 2.4 | 1.7 | 0.8 |
| p-Coumaric acid | 26.5 | 3.6 | 5.7 | 7.5 | 4.4 | 8.8 | 6.1 | 9.0 | 6.9 | 6.3 | ndb | 2.9 |
| Ferulic acid | 29.74 | 14.2 | 16.3 | 15.1 | 10.3 | 19.6 | 12.9 | 16.7 | 15.2 | 13.2 | 12.2 | 25.6 |
| Cinnamic acid | 37.96 | 1.7 | 30.7 | 10.7 | 12.1 | 7.6 | 6.8 | 5.4 | 4.3 | 7.9 | 1.7 | 32.3 |
| Rosmarinic acid | 39.04 | 88.0 | 32.3 | 84.8 | 33.3 | 139.5 | 36.5 | 70.4 | 118.0 | 150.7 | 79.8 | 87.4 |
| Salvianolic acid | 42.9 | 23.7 | 9.1 | 18.6 | 11.0 | 27.8 | 8.0 | 19.3 | 28.7 | 27.5 | 29.3 | 90.0 |
| Apigenin | 56.27 | 9.3 | 10.8 | 38.3 | 9.7 | 10.4 | 10.3 | 9.3 | 10.3 | 9.1 | 9.3 | 12.2 |
| Naringenin | 58.13 | 1.7 | 3.3 | 4.3 | 1.2 | 6.7 | 3.5 | 3.1 | 4.7 | 2.9 | 1.6 | 0.6 |
| Kaempferol | 60.14 | 0.9 | 1.3 | 1.7 | 0.9 | 1.5 | 0.7 | ndb | 3.1 | 0.9 | 0.7 | 1.1 |
The values are expressed in mg/100 g of sample dry weight.
aThe data were sorted based on the retention time (RT) of components.
b(nd): Not detected.
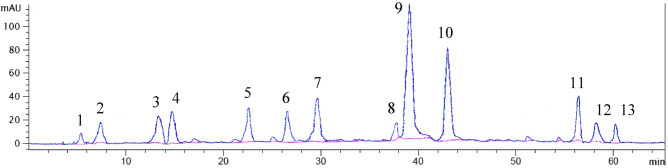
Figure 1.
HPLC chromatogram of Thymus trautvetteri. The polyphenolic compounds were described in footnote. Footnote: 1: gallic acid, 2: epicatechin, 3: chlorogenic acid, 4: caffeic acid, 5: luteolin-7-o-glucoside, 6: p-coumaric acid, 7: ferulic acid, 8: cinnamic acid, 9: rosmarinic acid, 10: salvianolic acid, 11: apigenin, 12: naringenin, 13: kaempferol.
Among the flavonoids, apigenin was the most abundant one followed by naringenin and epicatechin (Table 1). Apigenin content varied from 9.1 mg/100 g DW in T. serpyllum to 38.3 mg/100 g DW in T. fallax. Similar range has also been reported in T. pannonicus6. Several therapeutic properties have been reported for this valuable flavonoid, including cardiovascular, antitumor and bone metabolism effects39. On the other hand, rutin has been found as the most abundant flavonoid in T. glabrescens, T.pannonicus, T. praecox, T. pulegioides, and T. serpyllum30, and high amount of quercetin has also been reported in T. capitatus40.
Flavonoids are considered as the major group of polyphenolic components with different health benefits. Flavonoids activities are mostly related to their hydroxyl groups as well as the presence of double bounds and their methylation and glycosylation1. Flavonoids with an ortho-dihydroxy pattern in the B-ring, such as luteolin and quercetin, are more potent than mono-hydroxylated ones such as apigenin1. In the present study, the combination of luteolin-7-glucoside was observed as a derivative. The previous studies showed that activities of glycosylated flavonoids are less than those of aglycons1.
Total phenols and total flavonoid content
Thymus transcaspicus possessed the highest phenolic content (37.62 mg gallic acid (GAE) g−1 DW), while T. serpyllum displayed the lowest amount (22.14 mg GAE g−1 DW) (Table 2). Similar range for T. pulegioides was obtained using gallic acid as standard for phenolics13. However, lower amount (8.1 mg gallic acid equivalents (GAE) g−1 DW) was also reported by Roby et al.8 in T. vulgaris. The use of different standards and extraction conditions can highly affect the amount of phenolic content and the extraction yield in different studies3,31.
Table 2.
Total phenolic and flavonoid content of studied Thymus species.
| Species | TPCa (mg GAEb/g DW) | TFCc (mg QEd/g DW) |
|---|---|---|
| T. carmanicus | 28.31 ± 0.09 | 3.81 ± 0.01 |
| T. daenensis | 26.12 ± 0.06 | 3.97 ± 0.01 |
| T. fallax | 31.22 ± 0.05 | 7.56 ± 0.02 |
| T. fedtschenkoi | 27.23 ± 0.09 | 4.34 ± 0.03 |
| T. kotschyanus | 30.42 ± 0.08 | 2.97 ± 0.02 |
| T. migricus | 24.81 ± 0.06 | 3.91 ± 0.02 |
| T. pubescens | 30.12 ± 0.07 | 4.62 ± 0.01 |
| T. serpyllum | 22.14 ± 0.06 | 4.36 ± 0.02 |
| T. transcaspicus | 37.62 ± 0.09 | 4.75 ± 0.01 |
| T. trautvetteri | 34.34 ± 0.08 | 1.77 ± 0.01 |
| T. vulgaris | 35.73 ± 0.05 | 8.70 ± 0.01 |
Means with different letter are statistically significant at 5% level probability.
aTotal phenolic content.
bGallic acid equivalents.
cTotal flavonoid content.
dQuercetin equivalents.
High variation was found in respect to total flavonoid content (TFC) of 11 studied Thymus species. The TFC ranged from 1.77 to 8.72 mg quercetin equivalents (QE) g−1 DW. Therefore, the highest and the lowest amounts were obtained in T. vulgaris (8.72 mg QE g−1 DW) and T. trautvetteri (1.77 mg QE g−1 DW) species, respectively. Tohidi et al.45 also reported similar range for TFC in T. kotschyanus (2.11 mg QE g−1 DW to 8.14 mg QE g−1 DW). Different plant species might have different mechanisms to distribute flavonoids among their subcellular compartments such as accumulation of soluble carbohydrates and the balance between carbohydrate sources and sinks41.
DPPH scavenging test
DPPH is one of the most relevant antioxidant activity tests42. The IC50 values were found to be in range from 273.8 to 679.3 μg/ml. T. vulgaris extract displayed the highest antioxidant activity (the lowest IC50 value) (273.8 μg/ml) compared with other species and BHT as synthetic antioxidant, while T. pubescens revealed the weakest activity (679.3 μg/ml). The IC50 values in studied extracts recorded from 273.8 to 679.3 μg/ml for T. vulgaris and T. pubescens, respectively (Table 3). IC50 differences among extracts could be attributed to various polyphenolic compounds of studied extracts.
Table 3.
Antioxidant activity of 11 Thymus species based on DPPH and β-carotene-linoleic acid model systems.
| Species | DPPH scavenging (IC50) | Inhibition of β-carotene bleaching (%) | |||
|---|---|---|---|---|---|
| 50 ppm | 100 ppm | 300 pmm | 500 ppm | ||
| T. carmanicus | 392.3 ± 0.1 | 4.14 ± 0.03 | 11.45 ± 0.09 | 16.54 ± 0.05 | 22.28 ± 0.09 |
| T. daenensis | 371.4 ± 0.1 | 4.43 ± 0.02 | 10.88 ± 0.06 | 16.36 ± 0.04 | 21.81 ± 0.07 |
| T. fallax | 369.8 ± 0.1 | 4.56 ± 0.01 | 11.81 ± 0.05 | 17.44 ± 0.06 | 22.78 ± 0.05 |
| T. fedtschenkoi | 351.4 ± 0.1 | 4.67 ± 0.02 | 13.31 ± 0.04 | 18.54 ± 0.05 | 24.49 ± 0.01 |
| T. kotschyanus | 355.2 ± 0.1 | 5.66 ± 0.01 | 13.26 ± 0.06 | 18.51 ± 0.08 | 34.48 ± 0.06 |
| T. migricus | 411.7 ± 0.1 | 4.39 ± 0.03 | 7.74 ± 0.08 | 11.60 ± 0.06 | 19.03 ± 0.04 |
| T. pubescens | 679.3 ± 0.1 | 1.23 ± 0.02 | 1.59 ± 0.05 | 2.15 ± 0.07 | 5.50 ± 0.04 |
| T. serpyllum | 548.9 ± 0.2 | 1.99 ± 0.03 | 6.27 ± 0.09 | 12.24 ± 0.04 | 27.42 ± 0.06 |
| T. transcaspicus | 473.1 ± 0.1 | 2.85 ± 0.03 | 7.61 ± 0.07 | 10.91 ± 0.09 | 18.25 ± 0.08 |
| T. trautvetteri | 373.8 ± 0.1 | 4.55 ± 0.02 | 11.23 ± 0.05 | 16.38 ± 0.07 | 22.20 ± 0.06 |
| T. vulgaris | 273.8 ± 0.1 | 7.07 ± 0.04 | 18.49 ± 0.05 | 23.68 ± 0.06 | 43.29 ± 0.07 |
| BHT | 172.4 | 8.21 | 22.6 | 30.98 | 51.34 |
Means with different letter are statistically significant at 5% level probability.
Inhibition of β-carotene bleaching
The antioxidant capacity of Thymus species based on β-carotene/linoleic acid model system is presented in Table 3. Oxidation of the linoleic acid was inhibited by the extract of Thymus species at the concentrations of 500, 300, 100 and 50 ppm (P < 0.05). In this assay, T. vulgaris and T. kotschyanus demonstrated higher activities to prevent fatty acid oxidation than other species. The presence of unsaturated fatty acids and their oxidation process is of great importance in food products and diets43. Therefore, it is crucial to have a test based on unsaturated fatty acid oxidation in the assessment of antioxidant activity. The mechanism of this analysis can be explained by the phenomenon of “polar paradox”, in which polar antioxidants can be found in the aqueous phase of the emulsion that are more diluted in the lipid phase than aqueous phase and are thus less effective in protecting the linoleic acid44.
Cluster and PCA analyses of polyphenolic compounds
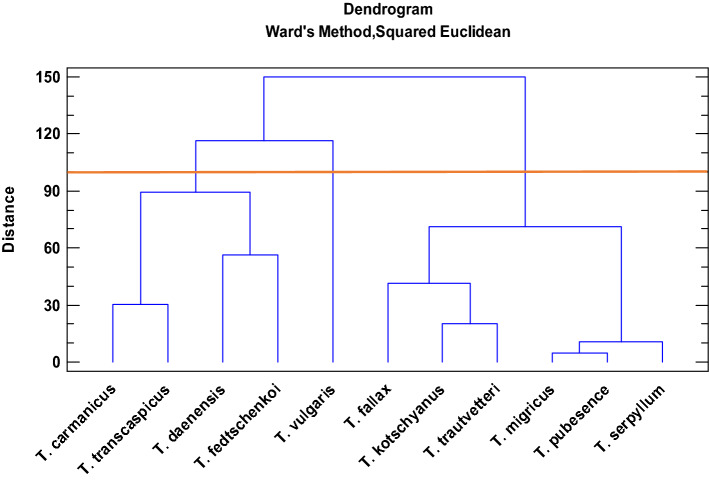
Cluster analysis was performed to distinguish possible groups among the species using Ward Method (Fig. 2). The hierarchical cluster analysis (HCA) allows subdivision of 11 species into three major groups. Although rosmarinic acid is the most abundant component in most of the species, the cluster analysis did not show a trend for this compound. According to cluster analysis, the first group (T. carmanicus, T. transcaspicus, T. fedtschenkoi, and T. daenensis) included medium to high caffeic acid (13.1–27.4 mg/100 g DW) and epicatechin (1.7–1.9 mg/100 g DW). Group 2 (T. vulgaris) consisted of high salvianolic acid (90 mg/100 g DW) and low naringenin (0.6 mg/100 g DW), and group 3 (T. serpyllum, T. migricus, T. trautvetteri, T. fallax, and T. kotschyanus) had high p-coumaric acid (6.3–9 mg/100 g DW) and medium cinnamic acid (4.3–10.7 mg/100 g DW) (Fig. 2). In the present research, groups 1, 2 and 3 were consisted of four, one and six species, respectively (Fig. 2). T. vulgaris was grouped in a separate cluster. Therefore, it might be suggested that this species has a different profile in respect to polyphenolic compounds in comparison to other species. In a similar study, Tohidi et al.45, compared the essential oil composition of Thymus species and the cluster analysis for essential oil components, also displayed the similar trend for this species.
Figure 2.
Cluster analysis of studied Thymus species according to major polyphenolic profiles.
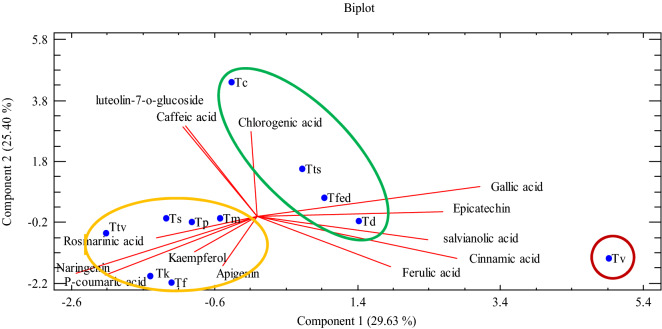
According to PCA, the first three principal components (PC1, PC2, and PC3) explained most of the variation (69.65%). The first PC (PC1) explained 29.63% of total variation and possessed high positive correlation with gallic acid (0.44) and cinnamic acid (0.39) contents, and high negative correlation with naringenin (− 0.36) and p-coumaric acid (− 0.30) contents. PC2 showed 25.40% of total variance and had positive correlation with luteolin-7-o-glucoside (0.44) and caffeic acid (0.44), and high negative correlation with p-coumaric acid (− 0.29) and naringenin (− 0.28) (Fig. 3).
Figure 3.
Classification of studied Thymus species according to major polyphenolic profiles.
PCA analysis of classified the species into three groups. Group 1 consisted of high rosmarinic acid, naringenin, and p-coumaric acid, while group 2 included species with high contents of chlorogenic acid. T. vulgaris was separated from other species in group 3 and was consisted of different components such as salvianolic acid, ferulic acid, cinnamic acid, gallic acid, and epicatechine (Fig. 3).
Correlations of the compounds
The highest correlation coefficient was obtained between salvianolic acid and ferulic acid (0.79) as well as naringenin and p-coumaric acid (0.76) followed by cinnamic acid and gallic acid (0.69) (Table 4). The highest negative correlation was between naringenin and gallic acid (− 0.74) followed by p-coumaric acid and chlorogenic acid (− 0.52). Most of the compounds showed negative correlation with chlorogenic acid (Table 4).
Table 4.
Correlation coefficients between phenolic compounds using HPLC on studied Thymus species.
| Standards | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galliccacid | 1 | ||||||||||||
| Epicatechin | 0.470ns | 1 | |||||||||||
| Chlorogenic acid | 0.092ns | 0.129ns | 1 | ||||||||||
| Caffeic acid | 0.023ns | − 0.127ns | 0.635* | 1 | |||||||||
| Luteolin-7-o-glucoside | 0.002ns | 0.222ns | − 0.170ns | 0.018ns | 1 | ||||||||
| P-coumaric acid | − 0.530ns | − 0.471ns | − 0.521ns | − 0.086ns | 0.018ns | 1 | |||||||
| Ferulic acid | 0.336ns | 0.402ns | − 0.228ns | − 0.239ns | − 0.399ns | 0.119ns | 1 | ||||||
| Cinnamic acid | 0.696* | 0.505ns | − 0.210ns | − 0.480ns | − 0.107ns | − 0.109ns | 0.606* | 1 | |||||
| Rosmarinic acid | − 0.398ns | − 0.387ns | − 0.066ns | 0.161ns | − 0.280ns | 0.244ns | 0.260ns | − 0.312ns | 1 | ||||
| Salvianolic acid | 0.484ns | 0.398ns | − 0.117ns | − 0.172ns | − 0.294ns | − 0.339ns | 0.798** | 0.457ns | 0.336ns | 1 | |||
| Apigenin | − 0.333ns | 0.101ns | − 0.248ns | − 0.242ns | − 0.202ns | 0.231ns | 0.042ns | 0.074ns | 0.004ns | 0.051ns | 1 | ||
| Naringenin | − 0.746 ** | − 0.386ns | − 0.218ns | − 0.141ns | − 0.121ns | 0.763** | 0.058ns | − 0.267ns | 0.415ns | − 0.364ns | 0.225ns | 1 | |
| Kaempherol | − 0.287ns | − 0.123ns | 0.147ns | − 0.116ns | 0.031ns | 0.163ns | 0.118ns | 0.049ns | 0.338ns | 0.094ns | 0.249ns | 0.454ns | 1 |
*Correlation is significant at the 0.05 level.
**Correlation is significant at the 0.01 level.
ns. Not significant.
Several researches have highlighted the role of phenolic compounds in reducing the risk of many diseases, including neurodegenerative disorders, heart disease and arthritis46. However, different kinds of polyphenolic components were determined in studied Thymus species in the present analysis. Previous researches have also demonstrated the synergistic or antagonistic effects of flavonoids and phenolic acids with different antixidative activities31. Since the mixtures of different polyphenolic compounds are present, as in extracts, the balance between flavonoids and phenolic acids in each species can highly affect its final antioxidant activity. Furthermore, each flavonoid’s concentration can highly affect its final cellular activity47. Finally, these modifications and interactions of applied extracts can lead to reduce or increase the final antioxidant activity of food products.
Among studied species, T. vulgaris displayed the highest antioxidant activity. This species possessed high amounts of salvianolic acid, ferulic acid, epicatechin and gallic acid. Previous reports emphasized the role of polyphenolic compounds to improve the antioxidative activity of the extracts48. In this species, the presence of salvianolic acid and epicatechin might lead to an increased antioxidant activity. High antioxidant activities of flavonoids are reported to attribute to the structure of their rings. Flavonoids possessed three rings, viz A, B and C in which their activity is mostly attributed to the ring B. Furthermore, the presence of hydroxyl group, glycosylation, methylation and the position of double bounds can also affect their activity1. In the present study, major flavonoids were epicatechin, apigenin and narenginin (Table 1). Epicatechin belongs to flavanols with two hydroxyl groups in the B-ring, while apigenin has one hydroxyl group. The previous reports illustrated that glycosylated flavonoids, such as luteolin-7-O-glucoside is less potent to scavenge free radicals than aglycons1. The highest amount of luteolin-7-O-glucoside was found in T. fedtschenkoi (Table 1).
Previous reports have also displayed different antioxidant capacities for phenolic acids and flavonoids. Accordingly, phenolic acids have been introduced as more potent radical scavengers in comparison with flavonoids41. Moreover, each model system can be practically different in respect to antioxidant activity in the final product in food industry. For instance, β-carotene-linoleic acid model systems have mostly been used in lipid phase, while DPPH method has been used in non-lipid phases49.
SRAP amplification and levels of polymorphism
The present research evaluated the inter- and intragenic diversity of 11 Thymus species for the first time. The primer polymorphisms’ percentage was between 88.88 and 100, representing high diversity among and within Thymus species. The mean polymorphic band percentage for the study was 93.83%. The total number of amplified bands per primer ranged from 10 to 19 and averaged 14.57 bands per primer. Amplified products varied from 100 to 800 bp. Me5-em4 marker with 17 bands showed the highest polymorphic band, and Me4-em3 marker with 10 bands revealed the lowest polymorphism. The average polymorphic information content (PIC) value for the amplification products was 0.34. This value was in the range of 0.47 > PIC > 0.23 (Table 5) and the primers displayed a moderate polymorphism according to the previous literature23.
Table 5.
Polymorphism number, annealing temperature and PIC of studied Thymus species.
| No | Primer | Sequence (5′ → 3′) | Annealing tempa | primer combinations | No. total bands | No.PBb | PPBc (%) | PIC |
|---|---|---|---|---|---|---|---|---|
| 1 | Me1 | 5′-TGAGTCCAAACCGGATA-3′ | 50 | Me1 + em4 | 15 | 14 | 93.33 | 0.38 |
| 2 | Me2 | 5′-TGAGTCCAAACCGGAGC-3′ | 50 | Me2 + em1 | 18 | 16 | 88.88 | 0.32 |
| 3 | Me3 | 5′-TGAGTCCAAACCGGAAT-3′ | 50 | Me2 + em3 | 13 | 13 | 100 | 0.47 |
| 4 | Me4 | 5′-TGAGTCCAAACCGGACC-3′ | 50 | Me3 + em1 | 13 | 12 | 92.30 | 0.33 |
| 5 | Me5 | 5′-TGAGTCCAAACCGGAAG-3′ | 50 | Me4 + em3 | 10 | 10 | 100 | 0.23 |
| 6 | Em1 | 5′-GACTGCGTACGAATTAAT-3′ | 50 | Me5 + em3 | 14 | 13 | 92.85 | 0.28 |
| 7 | Em3 | 5′-GACTGCGTACGAATTGAC-3′ | 50 | Me5 + em4 | 19 | 17 | 89.47 | 0.38 |
| 8 | Em4 | 5′-GACTGCGTACGAATTTGA-3′ | 50 | |||||
| Total | 102 | 95 | – | – | ||||
| Average | 14.57 | 13.57 | 93.83 | 0.34 |
aDegrees celsius (°C).
bNumber of polymorphic band.
cPercentage of polymorphic band.
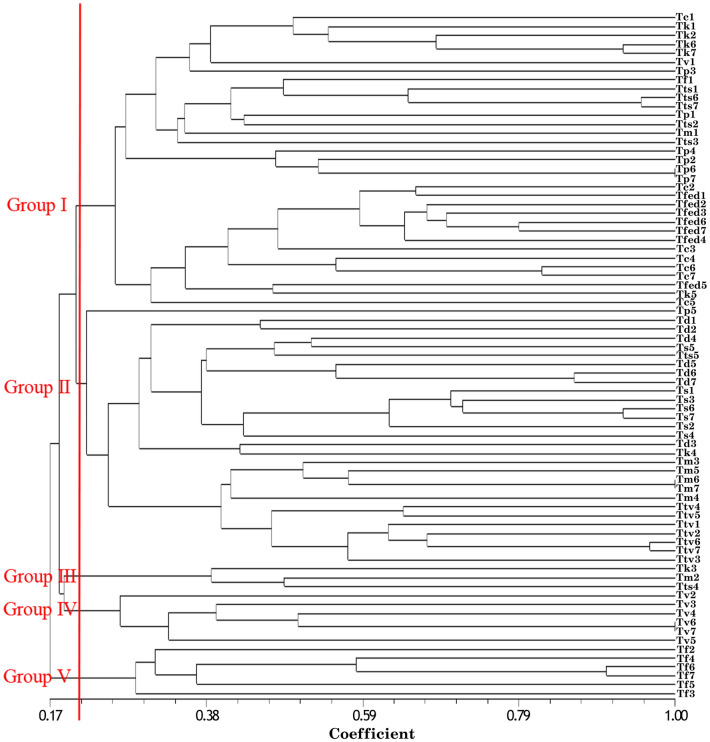
Cluster and PCA molecular analyses
The data were also used for cluster analysis. A high cophenetic correlation coefficient of 0.81 between the Jaccard (J) similarity and the cophenetic matrix was obtained accordingly, indicating a good compatibility between the dendrogram and the similarity matrices. Therefore, the Jaccard Method (Fig. 4) determined the dendrogram of genetic relationships among species. This cluster grouped Thymus species into five main clusters. The first main cluster (Group I) contained four species, including T. carmanicus, T. transcaspicus, T. pubescens, and T. fedtschenkoi (Fig. 4). The second main cluster consisted of T. daenensis, T. serpyllum, T. migricus, T. trautvetteri (Group II). The third cluster (Group III) included different species (Fig. 4). The fourth main cluster (Group IV) consisted of T. vulgaris accessions (Fig. 4). The last main cluster (Group V) included T. fallax (Fig. 4), though the classification did not distinctly separate the species. For instance, T. carmanicus, T. fedtschenkoi, T. daenensis, and T. serpyllum were classified in one group. This kind of discrepancy in clusters might be interpreted by ploidy level, cross pollination, high gene flow and natural hybridization50.
Figure 4.
Dendrogram of 77 studied Thymus genotypes based on SRAP markers according to the Unweighted Pair Group Mean Algorithm (UPGMA) with the Jaccard similarity index.
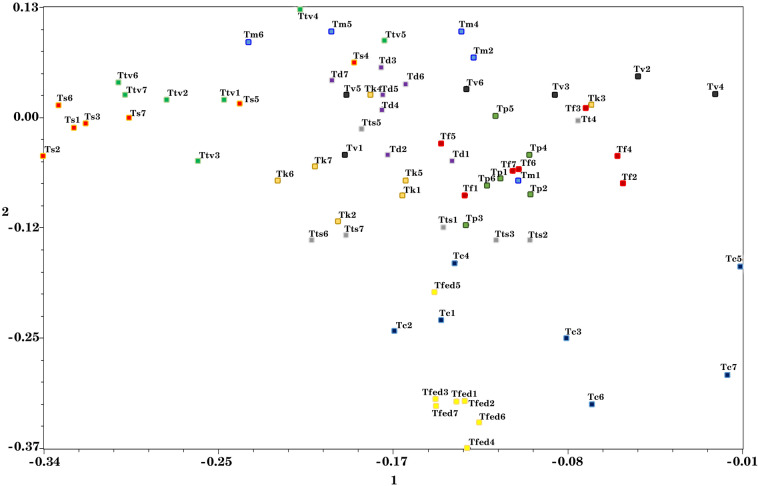
The PCoA results of the SRAP were also performed to confirm the cluster analysis. The PcoA results were largely in line with those obtained by cluster. They revealed that the first three principal coordinates (PCo) accounted for 37.8% of the total variation (Fig. 5) which suggests the relatively high distribution of markers throughout the genome.
Figure 5.
Plot of Thymus samples by principal coordinate analysis using the Jaccard’s similarity coefficients. Footnote:  T. migricus;
T. migricus;  T. fallax;
T. fallax;  T. carmanicus;
T. carmanicus;  T. kotschyanus;
T. kotschyanus;  T. pubescens;
T. pubescens;  T. daenensis,
T. daenensis,  T. trautvetteri;
T. trautvetteri;  T. transcaspicus;
T. transcaspicus;  T. fedtschenkoi;
T. fedtschenkoi;  T. vulgaris;
T. vulgaris;  T. serpyllum.
T. serpyllum.
Analysis of molecular variance (AMOVA) and admixture of species
AMOVA was also used to estimate the percentage of intra and inter species genetic variations (Table 6). AMOVA results demonstrated significant variations among the species (P < 0.001), so that 63.14% of total genetic variation occurs in SRAP analysis within the species. In addition, the total genetic variation among the species was equal to 36.86% in the SRAP analysis. The analysis also revealed a relatively acceptable differentiation in allele frequencies (Fst = 0.36) and a relatively high gene flow (Nm = 0.63) among the studied species. Furthermore, most of genetic variation was within the species (Table 6). Table 6 illustrates the species level of genetic diversity indices of genetic flow (Nm), and diversity among species (gene differentiation factor) (Gst).
Table 6.
Analysis of molecular variance (AMOVA) among and within Thymus species using SRAP markers.
| Source of variation | dfa | Mean of squares | Percentage of variation | P value | Fst | Nmb | Gstc |
|---|---|---|---|---|---|---|---|
| Among species | 10 | 22.24 | 36.86 | < 0.001 | |||
| Within species | 66 | 4.37 | 63.14 | ||||
| Total | 76 | 6.72 | 0.36 | 0.63 | 0.44 |
aDegrees of freedom.
bGene flow. Nm = estimate of gene flow from Gst or Gcs. E.g., Nm = 0.5(1—Gst)/Gst; McDermott and McDonald58.
cDiversity among species.
Moreover, the high variation within the studied Thymus species could be attributed to different factors, including the recombination of genes and gene mutation51. In addition, some environmental factors can affect the variability of the species. For example, the genetic exchange among the species can be a result of the mobility degree of the pollinator16.
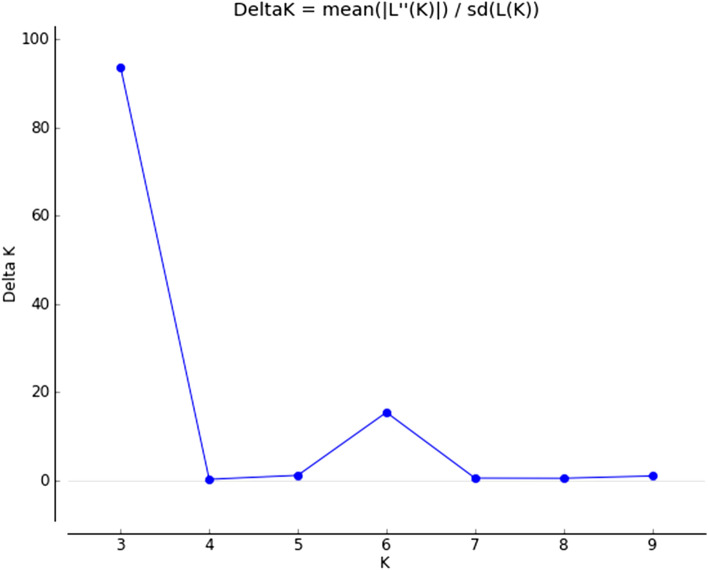
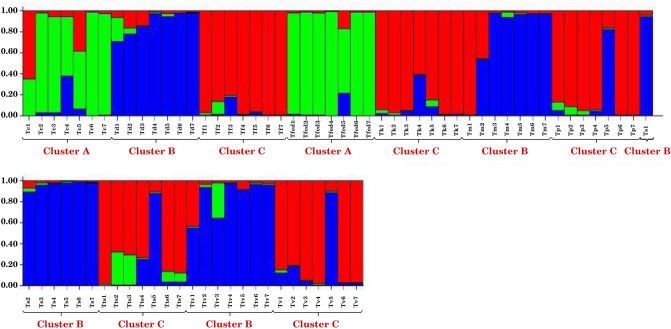
STRUCTURE analysis was also carried out for a better interpretation of admixture in some species obtained through cluster and PCA. The value of K was calculated by posterior probability of the data for a given K, Pr (X|K)52. Accordingly, the highest K value was estimated as K = 3 (Fig. 6). Therefore, analysis was done based on three colors viz. green (A), blue (B) and red (C) for studied Thymus species as shown in Fig. 7 Cluster A in Fig. 7 contained the species including T. carmanicus and T. fedtschenkoi. Cluster B included species such as T. daenensis, T. migricus, T. serpyllum, and T. trautvetteri. The remaining species containing T. fallax, T. kotschyanus, T. pubescens, T. transcaspicus, and T. vulgaris were classified in cluster C. Low to moderate admixtures were observed in the analysis. However, some species revealed higher admixture in comparison with others. For instance, T. carmanicus and T. transcaspicus showed high admixture in this research. One probable reason for this admixture might be a result of natural hybridization in some Thymus species due to the lack of incompatibility mechanism and other self-pollination strategies.
Figure 6.
Estimation of ΔK value obtained with STRUCTURE analysis.
Figure 7.
Genetic structure of 77 Thymus genotypes as inferred by STRUCTURE software with eight SRAP markers data set. Single vertical line represents an individual accessions and different colors represent genetic stocks/gene pools. Segments of each vertical line show extent of admixture in an individual (for interpretation of the references to colour in the text, the reader is referred to the web version of this article).
Comparing molecular and phytochemical clusters revealed some similarities in two dendrograms. For instance, T. vulgaris, separated from other species according to major polyphenolic profiles and molecular analysis, as well as T. transcaspicus, T. carmanicus, and T. fedtschenkoi, were clustered in the same groups. Consequently, this similarity would lead to another hypothesis that the evolution of some phenolic components in Thymus species can be caused by the effect of genetic and epigenetic factors simultaneously.
Overall, based on the objectives of the research, phenolic and flavonoid compounds of many Thymus species were determined and compared with SRAP molecular classifications for the first time. Furthermore, in the present research, the classifications based on molecular and phytochemical revealed similar trend in some species.
Conclusion
A high variation was found among Thymus species in respect to both molecular and phytochemical aspects consequently. High inter and intra genetic variation was obtained in studied species using SRAP markers. Among the species, T. vulgaris had the highest amount of salvianolic acid and antioxidant activity. Moreover, the phenolic compounds and flavonoids in studied species can introduce elite species for pharmaceutical and food purposes. The results of antioxidants study demonstrated that T. vulgaris, T. fedtschenkoi, and T. kotschyanus had the strongest antioxidant activity among the 11 species, while T. transcaspicus, T. vulgaris, and T. trautvetteri were found to be rich in total phenolic content. Overall, T. vulgaris and T. fallax may be recommended as good sources of phenolic compounds and flavonoid content with a high antioxidant activity. Finally, based on molecular and phytochemical analyses, similar trends were observed in T. transcaspicus, T. carmanicus, and T. fedtschenkoi species.
Materials and method
Plant material
The seeds of 11 Thymus species (T. vulgaris, T. fallax, T. trautvetteri, T. transcaspicus, T. fedtschenkoi, T. daenensis, T. carmanicus, T. pubescens, T. serpyllum, T. kotschyanus, and T. migricus) were obtained from gene bank of Research Institute of Forests and Rangeland, Tehran. The species were identified using Flora Iranica53 by Dr. Mozzafarian. The accession codes and their collection sites are illustrated in Table 7. The seeds were grown in a randomized block design (RCBD) with three replications.
Table 7.
Gene bank codes, accession codes and origins of Thymus species.
| Accession no | Gene bank codes | Accession codes | Species | Origin |
|---|---|---|---|---|
| 1 | 18316 | Tc | T. carmanicus | Zarand, Kerman, Iran |
| 2 | 10126 | Td | T. daenensis | Fereydonshahr, Isfahan, Iran |
| 3 | 33916 | Tf | T. fallax | Shahedieh, Yazd, Iran |
| 4 | 18063 | Tfed | T. fedtschenkoi | Naqadeh, Azarbaijan Gharbi, Iran |
| 5 | 17090 | Tk | T. kotschyanus | Qazvin, Qazvin, Iran |
| 6 | 33918 | Tm | T. migricus | Shahedieh, Yazd, Iran |
| 7 | 39341 | Tp | T. pubescens | Shahedieh, Yazd, Iran |
| 8 | 33920 | Ts | T. serpyllum | Shahedieh, Yazd, Iran |
| 9 | 3507 | Tts | T. transcaspicus | Khorasan Razavi, Hezar Masjed, Iran |
| 10 | 33921 | Ttv | T. trautvetteri | Shahedieh, Yazd, Iran |
| 11 | 14287 | Tv | T. vulgaris | London, England, United Kingdom |
Preparation of the methanolic extract
The aerial parts Thymus species were harvested at flowering stage. The harvested parts were shade dried at 25 °C, and the Plant materials were ground and prepared for extraction. Methanol was extracted using 50 g of the dried sample. The extract was shaken using 500 mL of methanol with 150 rpm at 25 ºC for 72 h. Then, a three-layer cheesecloth to remove any solid contamination filtered the extract. Finally, the extracts were evaporated at room temperature (25˚C) and dried in desiccators under vacuum to a constant weight.
High performance liquid chromatography (HPLC)
The studied Thymus extracts were analyzed by the HPLC system (model Agilent 1090). The HPLC elution method has been used previously by Gharibi et al.1. Solutions of pure known compounds available to the study were used for chromatography as external standards. All standards (gallic acid, epicatechin, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, cinnamic acid, rosmarinic acid, salvianolic acid, apigenin, naringenin, and kaempferol) were dissolved in HPLC grade methanol before injection to the analytical HPLC system. A 0.22 μm nylon acro-disk filter and 20 μL of the extract were used for injection. The stationary phase had a 250 mm × 4.6 mm (5 μm) symmetry C18 column (Waters Crop., Milford, MA, USA) (10 mm × 4 mm I.D.), and the mobile phase included solvent A and B with a flow rate of 0.8 mL min−1 and the detection was performed between 200 and 400 nm through UV detector. The column was 25 °C. 0.1% of water-formic acid was applied as solvent A, while 0.1% of formic acid in acetonitrile was used as solvent B in the mobile phase. The gradient conditions were also performed as follows: a linear step from 10 to 26% solvent B (v/v) for 40 min, 65% solvent B for 70 min, and finally to 100% solvent B for 75 min. The phenolic compounds were calculated comparing the peak areas and their retention times. Finally, the results were reported as mg/100 g of the sample dry weight.
Determination of total phenolic and flavonoid contents
Total phenolic content (TPC) was evaluated using Folin-Ciocalteu method. In this procedure, the methanolic extract (0.5 ml) was mixed with tenfold-diluted reagent (2.5 ml) and 7.5% sodium carbonate (2 ml) and then heated at 45 °C for 15 min. Then, absorption was measured using spectrophotometer (Hitachi U-1800) at 765 nm against a blank and the phenolic content was obtained as mg of equivalent tannic acid per gram of dry weight. Total flavonoid content (TFC) of the methanolic extract was determined by Gharibi et al.31 procedure, using the aluminum chloride colorimetric method. In the present study, sodium hydroxide 4% solutions, sodium nitrate 5%, and aluminum chloride 10% were applied. The extraction yield was evaluated as the ratio of the extract weight and the dry weight of each species43.
DPPH scavenging activity
According to the radical scavenging effect on the DPPH free radicals, the plant leaf extracts’ antioxidant activity, as well as standard antioxidants were assessed (Table 3). Different concentrations of Thymus extracts (equivalent to 50, 100, 300 and 500 ppm) were provided in methanol. BHT was used as the synthetic antioxidant (of 0.1 ml sample solution) with five milliliters of 0.1 mM methanolic solution of DPPH that were mixed separately. The mixtures were protected in dark condition for 30 min and the optical density was also evaluated by spectrophotometer (Hitachi U-1800) at 517 nm. The antioxidant activity was calculated based on radical-scavenging activity41.
Antioxidant activity using β-carotene-linoleic acid model system
This study is carried out based on the method explained by Salami et al.43. For this purpose, 0.5 mg β-carotene is dissolved in 1 ml of chloroform and 200 mg Tween 80, and 25 µl linoleic acid was added to provide the stock solution. The solvent evaporated and 100 ml ddH2O (double-distilled water) was added. Afterwards, BHT was applied as synthetic antioxidant in the concentration of (0.5–5 mg per 1 ml) at 50 °C. The absorbance of the sample extract was calculated using spectrophotometer (Hitachi U-1800) at zero time (t = 0) at 490 nm. Absorbance was read at an interval of 25 min (t = 125 min). Antioxidant capacity was stated as the percent of inhibition based on54 reports.
DNA extraction
In the present research, 77 accessions belonging to 11 Thymus species (seven individual plants from each species) were used. DNA extraction was carried out using modified CTAB procedure55. For the estimation of DNA concentration, spectrophotometer UV visible (Hitachi U-1800), as well as gel electrophoresis were applied.
SRAP analysis
PCR reaction was performed by eight selected primer combinations in thyme. For this purpose, a reaction of 15 μl was used. 7 μl of Master Mix Red (Ampliqon, Finland) was used in reaction along with 4 mM MgCl2, 1× PCR buffer, 15 ng DNA, 10 pM of each primer (Me and Em combinations), and 1.5 μl of ddH2O. After selecting the best annealing temperatures, PCR was performed as follows: initial denaturation (94 °C for 1 min) was followed by 35 cycles of 94 °C for 1 min, 50 °C for 1 min, and 72 °C for 1 min. Finally, five minutes at 72° was used as final extension. DNA products were separated in a 1× TBE (100 mMTris–Borate, pH = 8.0 and 2 mM EDTA), 7% acrylamide gel at 220 V for three hours and finally stained by silver nitrate.
Data analysis
The cluster and PCA were performed by NTSYSpc, Ver. 2.0256. Genetic relationships of samples were calculated by the Jaccard’s Similarity Index57 through the Simqual routine. The genetic structure parameters were assessed using Pop Gene. ver. 3258 (Table 8). Variation among and within species were evaluated using Arlequin ver. 3 software. Admixture of Thymus species was assessed using Structure Software based on Evanno’s Method59 The K value was computed with the probability Posterior of a K value, Pr (X | K)52. Variance analysis was performed using SAS ver.9.2. Statgraphic ver.16.2.04 also was applied for PCA and dendrogram production.
Table 8.
Summary of genetic variation statistics for SRAP markers used in Thymus species.
| Species | Sample size | Naa | Neb | Hc | Id | NPLe | PPLf (%) | Heg |
|---|---|---|---|---|---|---|---|---|
| T. carmanicus | 7 | 1.66 | 1.34 | 0.21 | 0.33 | 68 | 66.67 | 0.25 |
| T. daenensis | 7 | 1.43 | 1.20 | 0.13 | 0.20 | 44 | 43.14 | 0.15 |
| T. fallax | 7 | 1.42 | 1.17 | 0.12 | 0.19 | 43 | 42.16 | 0.14 |
| T. fedtschenkoi | 7 | 1.45 | 1.18 | 0.12 | 0.20 | 46 | 45.10 | 0.15 |
| T. kotschyanus | 7 | 1.46 | 1.26 | 0.16 | 0.24 | 47 | 46.08 | 0.19 |
| T. migricus | 7 | 1.58 | 1.28 | 0.18 | 0.29 | 60 | 58.82 | 0.21 |
| T. pubescens | 7 | 1.45 | 1.23 | 0.14 | 0.22 | 46 | 45.10 | 0.17 |
| T. serpyllum | 7 | 1.43 | 1.21 | 0.14 | 0.21 | 44 | 43.14 | 0.16 |
| T. transcaspicus | 7 | 1.57 | 1.29 | 0.18 | 0.28 | 59 | 57.84 | 0.21 |
| T. trautvetteri | 7 | 1.48 | 1.23 | 0.15 | 0.23 | 49 | 48.04 | 0.17 |
| T. vulgaris | 7 | 1.53 | 1.23 | 0.15 | 0.24 | 55 | 53.92 | 0.18 |
| Multi population | 77 | 2 | 1.45 | 0.28 | 0.44 | – | – | 0.28 |
aObserved number of alleles.
bEffective number of alleles.
cGene diversity.
dShannon’s information index.
eNumber of polymorphic loci.
fPercentage of polymorphic loci.
gExpected heterozygosity.
Author contributions
Each of authors contributed to this study as following: D.S. performed experiment and contributed to analysis and interpretation of data, and writing the manuscript. M.R. and G.S. contributed to study conception and project design and critically revised the manuscript for important intellectual content.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gharibi S, Tabatabaei BES, Saeidi G, Talebi M, Matkowski A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. f. Phytochemistry. 2019;162:90–98. doi: 10.1016/j.phytochem.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Tohidi B, Rahimmalek M, Trindade H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crops Prod. 2019;134:89–99. doi: 10.1016/j.indcrop.2019.02.038. [DOI] [Google Scholar]
- 3.Tohidi B, Rahimmalek M, Arzani A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017;220:153–161. doi: 10.1016/j.foodchem.2016.09.203. [DOI] [PubMed] [Google Scholar]
- 4.Ghasemi Pirbalouti A, Hashemi M, Ghahfarokhi FT. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013;48:43–48. doi: 10.1016/j.indcrop.2013.04.004. [DOI] [Google Scholar]
- 5.Rahimmalek M, Goli SAH. Evaluation of six drying treatments with respect to essential oil yield, composition and color characteristics of Thymus daenensis subsp. daenensis Celak leaves. Ind. Crops Prod. 2013;42:613–619. doi: 10.1016/j.indcrop.2012.06.012. [DOI] [Google Scholar]
- 6.Arsenijević J, Drobac M, Šoštarić I, Jevđović R, Živković J, Ražić S, Moravčević Đ, Maksimović Z. Comparison of essential oils and hydromethanol extracts of cultivated and wild growing Thymus pannonicus All. Ind. Crops Prod. 2019;130:162–169. doi: 10.1016/j.indcrop.2018.12.055. [DOI] [Google Scholar]
- 7.Damtie D, Braunberger C, Conrad J, Mekonnen Y, Beifuss U. Composition and hepatoprotective activity of essential oils from Ethiopian thyme species (Thymus serrulatus and Thymus schimperi) J. Essent. Oil. Res. 2019;31:120–128. doi: 10.1080/10412905.2018.1512907. [DOI] [Google Scholar]
- 8.Roby MHH, Sarhan MA, Selim KAH, Khalel KI. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013;43:827–831. doi: 10.1016/j.indcrop.2012.08.029. [DOI] [Google Scholar]
- 9.Khouya T, Ramchoun M, Hmidani A, Amrani S, Harnafi H, Benlyas M, Filali Zegzouti Y, Nazihd EH, Ouguerram K, Alem C. Acute toxicity and antiproliferative and procoagulant activities of fractions derived from Thymus satureioides of the Moroccan High Atlas. S. Afr. J. Bot. 2019;121:568–576. doi: 10.1016/j.sajb.2019.01.005. [DOI] [Google Scholar]
- 10.Ćebović T, Arsenijević J, Drobac M, Živković J, Šoštarić I, Maksimović Z. Potential use of deodorised water extracts: Polyphenol-rich extract of Thymus pannonicus All. as a chemopreventive agent. J. Food Sci. Technol. 2018;55:560–567. doi: 10.1007/s13197-017-2965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taşkın T, Çam ME, Taşkın D, Rayaman E. In vitro and In vivo biological activities and phenolic characterization of Thymus praecox subsp. skorpilii var. skorpilii. J. Food Meas Charact. 2019;13:536–544. doi: 10.1007/s11694-018-9967-1. [DOI] [Google Scholar]
- 12.Ziani BE, Heleno SA, Bachari K, Dias MI, Alves MJ, Barros L, Ferreira IC. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019;116:312–319. doi: 10.1016/j.foodres.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 13.Taghouti M, Martins-Gomes C, Schäfer J, Félix LM, Santos JA, Bunzel M, Nunes FM, Silva AM. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018;9:3617–3629. doi: 10.1039/C8FO00456K. [DOI] [PubMed] [Google Scholar]
- 14.Jaouadi R, Cardoso SM, Silva AM, Yahia IBH, Boussaid M, Zaouali Y. Variation of phenolic constituents of Tunisian Thymus capitatus (L.) Hoff. ET Link. Populations. Biochem. Syst. Ecol. 2018;77:10–15. doi: 10.1016/j.bse.2017.12.009. [DOI] [Google Scholar]
- 15.Rahimmalek M, Bahreininejad B, Khorrami M, Tabatabaei BES. Genetic variability and geographic differentiation in Thymus daenensis subsp. daenensis, an endangered medicinal plant, as revealed by inter simple sequence repeat (ISSR) markers. Biochem. Genet. 2009;47:831. doi: 10.1007/s10528-009-9281-z. [DOI] [PubMed] [Google Scholar]
- 16.Ghafouri F, Rahimmalek M. Genetic structure and variation in different Iranian myrtle (Myrtus communis L.) populations based on morphological, phytochemical and molecular markers. Ind. Crops Prod. 2018;123:489–499. doi: 10.1016/j.indcrop.2018.06.086. [DOI] [Google Scholar]
- 17.Kumar J, Heikrujam M, Sharma K, Agrawal V. SRAP and SSR marker-assisted genetic diversity, population structure analysis and sex identification in Jojoba (Simmondsia chinensis) Ind. Crops Prod. 2019;133:118–132. doi: 10.1016/j.indcrop.2019.03.006. [DOI] [Google Scholar]
- 18.Liu, L., Zhu, X., Gong, Y., Song, X., Wang, Y., Zhao, L. & Wang, L. Genetic diversity analysis of radish germplasm with RAPD, AFLP and SRAP markers. In XXVII International Horticultural Congress-IHC2006: II International Symposium on Plant Genetic Resources of Horticultural Vol. 760, 125–130 (2006).
- 19.Liu LW, Zhao LP, Gong YQ, Wang MX, Chen LM, Yang JL, Wang Y, Yu FM, Wang LZ. DNA fingerprinting and genetic diversity analysis of late-bolting radish cultivars with RAPD, ISSR and SRAP markers. Sci. Hortic. 2008;116:240–247. doi: 10.1016/j.scienta.2007.12.011. [DOI] [Google Scholar]
- 20.Taşcıoğlu T, Sadıkoğlu N, Doğanlar S, Frary A. Molecular genetic diversity in the Origanum genus: EST-SSR and SRAP marker analyses of the 22 species in eight sections that naturally occur in Turkey. Ind. crops Prod. 2018;123:746–761. doi: 10.1016/j.indcrop.2018.07.027. [DOI] [Google Scholar]
- 21.Moein F, Jamzad Z, Rahiminejad M. An integrating study of genetic diversity and ecological niche modelling in Salvia aristata (Lamiaceae) Acta. Bot. Hung. 2019;61:185–204. doi: 10.1556/034.61.2019.1-2.10. [DOI] [Google Scholar]
- 22.Namayandeh A, Mokhtari N, Kamelmanesh MM, Bedaf MT. Genetic diversity in Satureja species determined by sequence-related amplified polymorphism markers. J. Genet. 2017;96:5–11. doi: 10.1007/s12041-017-0752-4. [DOI] [PubMed] [Google Scholar]
- 23.Talebi M, Rahimmalek M, Norouzi M. Genetic diversity of Thymus daenensis subsp. daenensis using SRAP markers. Biologia. 2015;70:453–459. doi: 10.1515/biolog-2015-0059. [DOI] [Google Scholar]
- 24.Heydari A, Hadian J, Esmaeili H, Kanani MR, Mirjalili MH, Sarkhosh A. Introduction of Thymus daenensis into cultivation: Analysis of agro-morphological, phytochemical and genetic diversity of cultivated clones. Ind. Crops Prod. 2019;131:14–24. doi: 10.1016/j.indcrop.2019.01.033. [DOI] [Google Scholar]
- 25.Abraham EM, Aftzalanidou A, Ganopoulos I, Osathanunkul M, Xanthopoulou A, Avramidou E, Sarrou E, Aravanopoulos F, Madesis P. Genetic diversity of Thymus sibthorpii Bentham in mountainous natural grasslands of Northern Greece as related to local factors and plant community structure. Ind. Crops Prod. 2018;111:651–659. doi: 10.1016/j.indcrop.2017.11.038. [DOI] [Google Scholar]
- 26.Hadian J, Bigdeloo M, Nazeri V, Khadivi-Khub A. Assessment of genetic and chemical variability in Thymus caramanicus. Mol. Biol. Rep. 2014;41:3201–3210. doi: 10.1007/s11033-014-3180-z. [DOI] [PubMed] [Google Scholar]
- 27.Yousefi V, Najaphy A, Zebarjadi A, Safari H. Molecular characterization of Thymus species using ISSR markers. J. Anim. Plant Sci. 2015;8:55. [Google Scholar]
- 28.Milevskaya VV, Temerdashev ZA, Butyl’skaya TS, Kiseleva NV. Determination of phenolic compounds in medicinal plants from the Lamiaceae family. J Anal. Chem. 2017;72:342–348. doi: 10.1134/S1061934817030091. [DOI] [Google Scholar]
- 29.Zengin G, Atasagun B, Aumeeruddy MZ, Saleem H, Mollica A, Bahadori MB, Mahomoodally MF. Phenolic profiling and in vitro biological properties of two Lamiaceae species (Salvia modesta and Thymus argaeus): A comprehensive evaluation. Ind. Crops Prod. 2019;128:308–314. doi: 10.1016/j.indcrop.2018.11.027. [DOI] [Google Scholar]
- 30.Boros B, Jakabová S, Dörnyei Á, Horváth G, Pluhár Z, Kilár F, Felinger A. Determination of polyphenolic compounds by liquid chromatography–mass spectrometry in Thymus species. J. Chromatogr. A. 2010;1217:7972–7980. doi: 10.1016/j.chroma.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Gharibi S, Tabatabaei BES, Saeidi G, Goli SAH, Talebi M. Total phenolic content and antioxidant activity of three Iranian endemic Achillea species. Ind. Crops Prod. 2013;50:154–158. doi: 10.1016/j.indcrop.2013.07.038. [DOI] [Google Scholar]
- 32.Scagel CF, Lee J, Mitchell JN. Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind. Crops Prod. 2019;127:119–128. doi: 10.1016/j.indcrop.2018.10.048. [DOI] [Google Scholar]
- 33.Zielińska S, Dryś A, Piątczak E, Kolniak-Ostek J, Podgórska M, Oszmiański J, Matkowski A. Effect of LED illumination and amino acid supplementation on phenolic compounds profile in Agastache rugosa in vitro cultures. Phytochem. Lett. 2019;31:12–19. doi: 10.1016/j.phytol.2019.02.029. [DOI] [Google Scholar]
- 34.Ou J, Huang J, Zhao D, Du B, Wang M. Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food Funct. 2018;9:851–860. doi: 10.1039/C7FO01508A. [DOI] [PubMed] [Google Scholar]
- 35.Taamalli A, Arráez-Román D, Abaza L, Iswaldi I, Fernández-Gutiérrez A, Zarrouk M, Segura-Carretero A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015;26:320–330. doi: 10.1002/pca.2566. [DOI] [PubMed] [Google Scholar]
- 36.Martins-Gomes C, Taghouti M, Schäfer J, Bunzel M, Silva AM, Nunes FM. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods. 2018;43:154–164. doi: 10.1016/j.jff.2018.02.012. [DOI] [Google Scholar]
- 37.Xia ZB, Yuan YJ, Zhang QH, Li H, Dai JL, Min JK. Salvianolic acid B suppresses inflammatory mediator levels by downregulating NF-κB in a rat model of rheumatoid arthritis. Med. Sci. Monit. 2018;24:2524. doi: 10.12659/MSM.907084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang M, Wang P, Xu S, Xu W, Xu W, Chu K, Lu J. Biological activities of salvianolic acid B from Salvia miltiorrhiza on type 2 diabetes induced by high-fat diet and streptozotocin. Pharm. Biol. 2015;53:1058–1065. doi: 10.3109/13880209.2014.959611. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Wang F, Zhou R, Song X, Xie M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017;41:e12376. doi: 10.1111/jfbc.12376. [DOI] [Google Scholar]
- 40.Jabri-Karoui I, Bettaieb I, Msaada K, Hammami M, Marzouk B. Research on the phenolic compounds and antioxidant activities of Tunisian Thymus capitatus. J. Funct. Foods. 2012;4:661–669. doi: 10.1016/j.jff.2012.04.007. [DOI] [Google Scholar]
- 41.Gharibi S, Tabatabaei BES, Saeidi G, Goli SAH. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016;178:796–809. doi: 10.1007/s12010-015-1909-3. [DOI] [PubMed] [Google Scholar]
- 42.Baharfar R, Azimi R, Mohseni M. Antioxidant and antibacterial activity of flavonoid-, polyphenol-and anthocyanin-rich extracts from Thymus kotschyanus boiss & hohen aerial parts. J. Food Sci. Technol. 2015;52:6777–6783. doi: 10.1007/s13197-015-1752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salami M, Rahimmalek M, Ehtemam MH. Inhibitory effect of different fennel (Foeniculum vulgare) samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem. 2016;213:196–205. doi: 10.1016/j.foodchem.2016.06.070. [DOI] [PubMed] [Google Scholar]
- 44.Goli SAH, Mokhtari F, Rahimmalek M. Phenolic compounds and antioxidant activity from saffron (Crocus sativus L.) petal. J. Agric. Sci. 2012;4:175. [Google Scholar]
- 45.Tohidi B, Rahimmalek M, Arzani A. Variations in chemical composition and bioactive compounds of Thymus kotschyanus Boiss. & Hohen populations originated from different collection sites. J. Essent. Oil. Bear. Pl. 2018;21:1272–1283. doi: 10.1080/0972060X.2018.1533435. [DOI] [Google Scholar]
- 46.Aguilera Y, Martin-Cabrejas MA, de Mejia EG. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016;15:405–423. doi: 10.1007/s11101-015-9443-z. [DOI] [Google Scholar]
- 47.Pacifico S, Piccolella S, Papale F, Nocera P, Lettieri A, Catauro M. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods. 2016;20:253–266. doi: 10.1016/j.jff.2015.11.008. [DOI] [Google Scholar]
- 48.Afshari M, Rahimmalek M, Miroliaei M. Variation in polyphenolic profiles, antioxidant and antimicrobial activity of different Achillea species as natural sources of antiglycative compounds. Chem. Biodivers. 2018;15:e1800075. doi: 10.1002/cbdv.201800075. [DOI] [PubMed] [Google Scholar]
- 49.Akbarian A, Rahimmalek M, Sabzalian MR. Variation in essential oil yield and composition of Dorema aucheri Boiss., an endemic medicinal plant collected from wild populations in natural habitats. Chem. Biodivers. 2016;13:1756–1766. doi: 10.1002/cbdv.201600160. [DOI] [PubMed] [Google Scholar]
- 50.Heidari EF, Rahimmalek M, Mohammadi S, Ehtemam MH. Genetic structure and diversity of ajowan (Trachyspermum ammi) populations based on molecular, morphological markers, and volatile oil content. Ind. Crops. Prod. 2016;92:186–196. doi: 10.1016/j.indcrop.2016.08.014. [DOI] [Google Scholar]
- 51.Rahimi BS, Rahimmalek M. Genetic structure and variation of moshgak (Ducrosia anethifolia boiss.) populations based on morphological and molecular markers. Sci. Hortic. 2019;257:108668. doi: 10.1016/j.scienta.2019.108668. [DOI] [Google Scholar]
- 52.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rechinger, K. H. Flora iranica, Vol. 158, Verlagsanstalt Wien Austria: Akademische Druke-U 49–71 (1963).
- 54.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- 55.Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4326. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rohlf FJ. NTSYS-pc Version 2.0. Numerical Taxonomy and Multivariate Analysis System. Exeter software. New York: Setauket; 1988. [Google Scholar]
- 57.Jaccard P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908;44:223–270. [Google Scholar]
- 58.McDermott JM, McDonald BA. Gene flow in plant pathosystems. Annu. Rev. Phytopathol. 1993;31:353–373. doi: 10.1146/annurev.py.31.090193.002033. [DOI] [Google Scholar]
- 59.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]