Abstract
During oviposition, ectoparasitoid wasps not only inject their eggs but also a complex mixture of proteins and peptides (venom) in order to regulate the host physiology to benefit their progeny. Although several endoparasitoid venom proteins have been identified, little is known about the components of ectoparasitoid venom. To characterize the protein composition of Torymus sinensis Kamijo (Hymenoptera: Torymidae) venom, we used an integrated transcriptomic and proteomic approach and identified 143 venom proteins. Moreover, focusing on venom gland transcriptome, we selected additional 52 transcripts encoding putative venom proteins. As in other parasitoid venoms, hydrolases, including proteases, phosphatases, esterases, and nucleases, constitute the most abundant families in T. sinensis venom, followed by protease inhibitors. These proteins are potentially involved in the complex parasitic syndrome, with different effects on the immune system, physiological processes and development of the host, and contribute to provide nutrients to the parasitoid progeny. Although additional in vivo studies are needed, initial findings offer important information about venom factors and their putative host effects, which are essential to ensure the success of parasitism.
Subject terms: Proteomics, Sequencing, Entomology
Introduction
Insects are characterized by the highest level of biodiversity of all organisms, with around 1 million described species1,2. A molecular and functional understanding of major associations between insects and other organisms may result in the identification of useful genes and molecules for the development of new strategies to control harmful insects3,4, and also for biomedical and industrial applications through biotechnologies5,6. Phytophagous insects are responsible for massive negative impacts on agricultural production, inducing directly and indirectly plant damage, i.e. through the transmission of plant pathogens7. However, insects can be attacked by a wide range of natural antagonists, which are commonly known as entomophages and categorized as predators or parasitoids8. Predators attack and consume their prey directly and immediately8. Parasitoids, instead, preserve the victim for their own progeny by injecting secretions into the hosts that can regulate their physiology, transforming the host into a suitable environment for the development of the new-born larvae9. Hymenopteran parasitoids show the most sophisticated strategies. Whereas ectoparasitoid wasps lay their eggs on the surface of hosts or in the environment close to the host, and their larvae develop outside the host body, endoparasitoid wasps oviposit and their progeny develop inside the host hemocoel10.
The main alterations observed in the parasitized host are induced by secretions (venom and ovarian calyx fluid) injected by the adult female during oviposition10. Venom of parasitoid insects is produced by glands attached to the female reproductive system and consists of a complex mixture of proteins, peptides, and some unidentified compounds11. Bioactive components in venom are responsible for alterations of host development and metabolism, in order to optimize nutrient supply for parasitoid offspring12. Unlike endoparasitoids whose venom can induce various effects ranging from the regulation/alteration of host physiology13,14 to transiently paralyzing even lethal effects15,16, ectoparasitoid venom paralyze or rapidly kill the host12,17. To better understand the pathological syndrome observed in parasitized hosts, including alterations of host physiology, development, and reproduction, it is essential to identify and characterize the components of venom and ovarian fluid.
To date, several venom proteins have been identified both in endo- and ectoparasitoid wasps using different approaches18–20. Only after candidate venom protein identification, they can be functionally characterized to understand how they alter the host physiological processes. These alterations, in combination with other parasitic factors, induce changes in the immune system, both humoral (suppression of melanization processes) and cellular (inhibition of the encapsulation of foreign bodies by the hemocytes), in reproductive processes and in the digestive system (host tissues provide suitable nutrients for the parasitoid offspring)13,21–23. Moreover, some of the venom protein components can also play a paralyzing role, preserving the host tissues in response to the nutritional needs of the parasitoid progeny24,25.
Although several venom proteins from endoparasitoids have been identified18,26–29, proteins from ectoparasitoid venom are still largely unknown20,30–32. The most reliable strategy for the identification of these proteins consists of an integrated bottom-up proteomic and transcriptomic approach, using a combination of high-throughput next-generation transcriptome sequencing and mass spectrometry18,20,22,26,28,30,32,33.
Torymus sinensis Kamijo (Hymenoptera: Torymidae), is the parasitoid of the Asian chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae), a globally invasive pest of Castanea species. T. sinensis, a univoltine ectoparasitoid, is considered as the main biocontrol agent of D. kuriphilus, and its biological cycle is perfectly synchronized with its host34,35. The adult female inserts its ovipositor in the newly formed galls of D. kuriphilus and lays eggs in the inner wall of the gall or on the surface of the D. kuriphilus larva (Supplementary Fig. S1). Adults of T. sinensis emerge from the gall in early spring and mate, starting the biological cycle again34; the lack of a host may cause up to a 12-month diapause36. For these reasons, T. sinensis, native to China, has been introduced into several countries of Asia, North America and Europe to control populations of Asian chestnut gall wasps37–40.
Here, we employed an effective approach that combined next-generation transcriptome sequencing and proteomics to identify the major protein components of T. sinensis venom. The transcriptome of the T. sinensis venom gland was built by using a high-throughput nucleic acid sequencing method. Transcriptomic information provided an overall picture of the putative proteins of the venom gland and on their molecular functions, biological processes, and putative cellular compartments. Proteomic analysis was carried out on the components of the venom, fractionated by SDS-PAGE electrophoresis, and analyzed by mass spectrometry (nanoLC-MS/MS). The comparison between translated transcriptomic and proteomic data allowed us to identify the expressed venom proteins. Based on similarities in databases, we obtained a number of functional annotated proteins and a group of novel proteins (without any similarities in databases).
By understanding the role of venom in parasitized hosts, we hope to apply these molecules as bioinsecticides in integrated pest control41,42.
Results
Transcriptome assembly and functional analysis by gene ontology
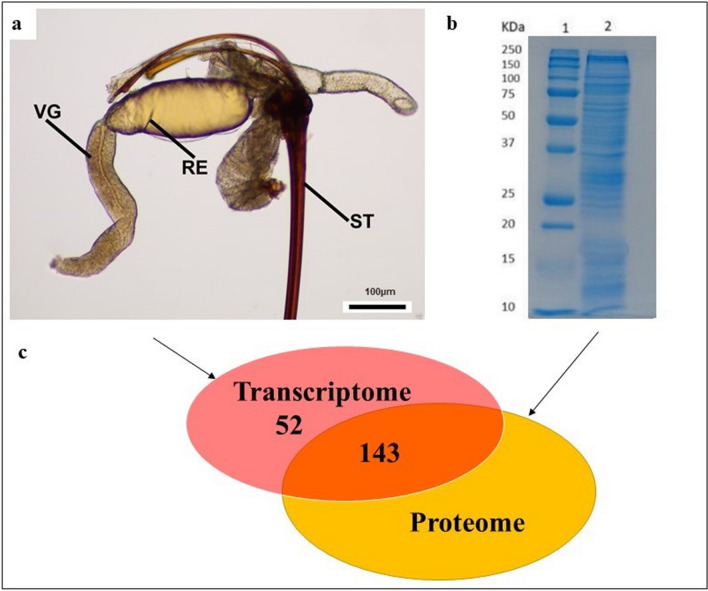
Next-generation sequencing (RNAseq) performed with RNA isolated from the venom glands of Torymus sinensis (Fig. 1a) allowed us to generate a de novo transcriptome assembly, which contained 22,875 contigs, with a maximum contig length of 19,306 bp. The six reading frames of the 22,875 nucleotide sequences were translated into their corresponding amino acid sequences, resulting in 137,250 predicted proteins (“T. sinensis protein database”).
Figure 1.
Identification of putative venom proteins in T. sinensis (Hymenoptera: Torymidae) combining transcriptomic and proteomic approach. (a) Overview of venom gland, reservoir, and sting of the female of T. sinensis at the optical microscope. Scale bar 100 µm. RE reservoir, ST sting, VG venom gland. (b) SDS-PAGE of crude venom extract from T. sinensis. Venom proteins were separated on a 12.5% SDS-PAGE gel (Sigma, St. Louis, MO, USA) and stained with colloidal Coomassie Brilliant Blue G250 (Sigma, St. Louis, MO, USA) (lane 2). Selected protein bands were excised from the gel and processed for LC/MS–MS analysis. Lane 1: molecular marker “All Blue Standards Biorad” (Biorad, Hercules, California, USA) (c) venom protein number identified with transcriptomic and combined proteomic and transcriptomic approach: 143 venom proteins were identified through an integrated transcriptomic and proteomic approach and 52 additional transcripts encoding putative venom proteins were identified in venom gland transcriptome through a “venom” keyword research.
To define similarities with annotated proteins, the contig sequences of the de novo transcriptome of T. sinensis venom glands were searched using the BLASTx algorithm43 against a non-redundant (nr) NCBI protein database with an E-value cut-off of 10–5 identifying 7,466 contigs (33%) matching entries. The species distribution of the top BLAST hit against the nr database for the T. sinensis venom gland transcriptome showed that the majority of obtained top hits matched N. vitripennis (Fig. 2).
Figure 2.
Distribution of top BLAST hit species for the T. sinensis transcriptome assembly. Top BLAST hits were obtained from BLASTx analysis against the NCBI non-redundant (nr) protein database. The number of top BLAST hits per species is shown on the x-axis. The highest number of matches were obtained for the ectoparasitoid wasp Nasonia vitripennis.
For functional annotation, all sequences were subjected to gene ontology (GO) analysis in Blast2GO, with 12,714 (56%) of the 22,875 contigs sharing significant similarity to proteins with assigned molecular functions in the GO database43, whereas 44% of the total transcripts did not match any annotated sequences in the nr database indicating a consistent group of noncoding transcript and species-specific putative proteins or more probably transcript not annotated yet.
The 12,714 annotated contigs were classified into three GO categories: biological processes, cellular components and molecular functions. The most prominent GO Cellular Component categories (Level 3) were organelles (51%) (Fig. 3a). The most prominent Molecular Function (Level 3) were different kinds of protein with binding activity (74%), followed by hydrolases (10%) and transferases (9%) (Fig. 3b). The most prominent category of GO Biological Processes (Level 2-3-4) was composed of proteins involved in metabolic and cellular processes (Fig. 3c–e). This result was linked to the very large number of general GO terms, terms that include basic processes for a living organism.
Figure 3.
Gene Ontology sequence annotation. Functional classification of all nr-matched transcripts from the T. sinensis venom gland. (a) Cellular component; (b) molecular function; (c–e) biological process. Data are presented as level 2 GO category for Biological Process (c), level 3 GO category for cellular component (a), molecular function (b) and biological process (d) and level 4 GO category for biological process (e). Classified gene objects are displayed as total contig number and percentages of the total number of gene objects with GO assignments. Percentages below 2% are not shown.
A GO analysis was performed on the identified 195 venom proteins (Fig. 4). The most abundant categories of Biological Processes (Level 4) were macromolecules, proteins and organonitrogen metabolic processes (Fig. 4a). Four macro-categories were identified through Molecular Function (Level 4) analysis: peptidases, serine proteases, hydrolases and cation binding activity proteins (Fig. 4b). A further "Enrichment Analysis" highlighted that proteins with proteolytic and serine-type endopeptidase activity are the most abundant in T. sinensis venom, comparing venom protein components and total T. sinensis transcripts (Fig. 4c).
Figure 4.
Gene Ontology sequence annotation of T. sinensis 195 venom proteins. Gene Ontology (GO) assignments for the T. sinensis venom proteins. GO assignments as predicted for their involvement in (a) biological processes and (b) molecular functions. All data are presented at level 4 GO categorization. Classified gene objects are depicted as absolute numbers and percentages (in brackets) of the total number of gene objects with GO assignments. In (c) enriched distribution of Gene Ontology (GO) terms in T. sinensis venom proteins were identified. Bar charts show the GO terms that were significantly (false discovery rate (FDR) < 0.05) enriched in the group of venom proteins compared to the complete T. sinensis gland transcriptome. The GO terms are sorted in an ascending order according to their FDR value, starting with the most significantly enriched. Only the most specific GO terms are displayed. Differences are shown as the percentage of sequences associated with a specific GO category in the test set (venom protein-encoding contigs) versus the reference set (transcriptome backbone assembly) using Fisher’s exact test in OmicsBox.
As previously reported, contigs not matching any known sequences in the nr database are relevant. The relatively high number of contigs without significant BLAST matches is quite common in the case of de novo transcriptome assemblies obtained from RNA-Seq data, and could be due to fragmented assembly, resulting in larger numbers of contigs corresponding to untranslated (UTR) regions of cDNAs, noncoding transcripts or species-specific un-annotated orphan genes. In T. sinensis venom transcriptome, the enzyme code distribution shows that the most abundant families of enzymes were transferases and hydrolases (Fig. 5).
Figure 5.
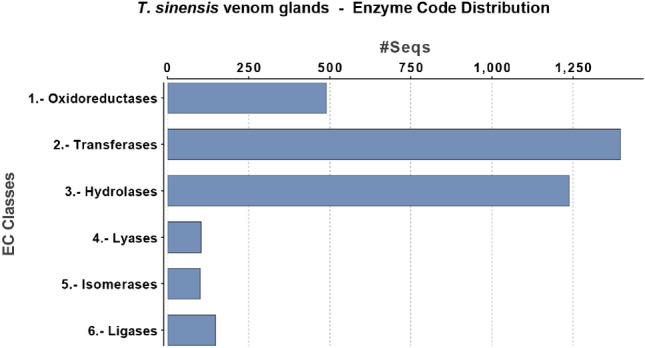
Enzyme Code (EC) Classes of the T. sinensis contigs encoding enzymes. The most abundant families of enzymes found in the transcriptome of T. sinensis venom glands are shown.
A quantitative RNA-Seq analysis of the transcripts encoding for putative venom proteins based on the Reads Per Kilobase of transcript method, per Million mapped reads (RPKM) showed large differences in expression levels; the most expressed transcripts in the venom glands are reported in Table 1.
Table 1.
Most expressed T. sinensis venom proteins.
| Contig | Protein name | RPKM |
|---|---|---|
| 445 | Alpha-amylase-like | 9,77118 |
| 4 | Serine protease 33 isoform X2 | 9,71077 |
| 270 | Trypsin-like | 9,66998 |
| 1650 | Peptidyl-prolyl cis–trans isomerase 5 | 9,54057 |
| 157 | Venom protein U precursor | 9,49706 |
| 1105 | Serine protease inhibitor 3/4 isoform X16 | 9,17066 |
| 293 | Cathepsin L | 8,64858 |
| 2095 | Carboxylesterase clade B, member 2 precursor | 8,59989 |
| 289 | Low-density lipoprotein receptor-related protein 2-like | 8,58940 |
| 428 | Lysosomal aspartic protease-like | 8,45276 |
| 940 | Heat shock protein 70 | 8,39405 |
| 1194 | Ferritin precursor | 8,33117 |
| 1001 | Calreticulin precursor | 8,31852 |
| 1747 | Endoplasmin | 8,09155 |
| 1971 | Chymotrypsin-1-like | 7,94740 |
| 392 | Lipase 3-like | 7,90375 |
| 181 | Protein disulfide-isomerase A3 | 7,84263 |
| 495 | Pancreatic triacylglycerol lipase | 7,75945 |
| 4156 | Ribonuclease Oy-like | 7,67279 |
| 3120 | Venom allergen 5-like | 7,52232 |
| 874 | Adipocyte plasma membrane-associated protein-like | 7,17361 |
| 2736 | Chitotriosidase-1 isoform X1 | 7,14344 |
| 128 | Pancreatic triacylglycerol lipase | 7,03636 |
| 1330 | Alpha-glucosidase-like | 6,95432 |
| 1835 | Serine protease homolog 21 precursor | 6,76623 |
| 1890 | Ovomucoid-like | 6,61221 |
| 3708 | Trypsin beta-like | 6,55134 |
| 2538 | Apolipoprotein D-like | 6,40265 |
| 575 | Mesencephalic astrocyte-derived neurotrophic factor homolog | 6,31104 |
| 2298 | Dipeptidase 1 isoform X1 | 6,29161 |
| 69 | Trypsin-3-like | 6,28669 |
| 18 | Serine protease inhibitor 28Dc isoform X2 | 6,21690 |
| 1034 | Vitellogenin | 6,21393 |
| 2025 | Serpin 5 precursor | 6,17429 |
| 5412 | Calnexin isoform X2 | 6,02715 |
| 3815 | Inosine-uridine preferring nucleoside hydrolase-like precursor | 6,02358 |
Proteins identified by SDS-PAGE and LC–MS/MS of venom extract, ordered by descending RPKM values. Expression levels are reported as log2-transformed RPKM values.
Gene expression levels in Torymus sinensis venom gland
To confirm the specific expression of genes in T. sinensis venom gland, a quantitative real time PCR (qPCR) experiment was performed on RNA extracted from venom glands and females deprived of the venom gland, focusing on a sample set of 10 genes selected among transcripts reported in Table 1. Female body without venom glands was used as calibrator. A significantly higher transcription level in venom glands was observed for all the selected genes (Fig. 6).
Figure 6.
Relative expression level of ten selected genes of T. sinensis venom gland and T. sinensis female body deprived of venom gland. Gene expression levels were quantified by quantitative real time PCR (qPCR). Data represent the mean of three independent replicates ± SEM. Samples were compared by the Unpaired t-test and statistically significant differences between samples are indicated with asterisk (*p = 0.015, **p = 0.011, ***p = 0.0009, ****p < 0.0001). Reference genes: GAPDH and beta-tubulin. Calibrator sample: female body deprived of venom gland.
SDS-PAGE and mass spectrometry
The one-dimensional SDS-PAGE electrophoretic profile showed that the T. sinensis venom consists of a complex protein mixture whose molecular weights range from 10 to 250 KDa (Fig. 1b). The sample lane containing the venom proteins was cut into thin slices, and the protein bands were excised, destained and subjected to in situ proteolytic digestion with trypsin. Protein identification was performed by direct nanoLC–MS/MS analysis of the corresponding peptide mixtures. Mass spectral data were processed with the Andromeda44 search engine and then compared with the “T. sinensis protein database”, leading to the identification of the proteins expressed in venom. Mass spectrometry analysis provided a list of 15,381 peptide sequences.
Proteomic and transcriptomic data analysis
The comparison between proteomic and transcriptomic data sets led to the identification of 1322 contig. The surprisingly large number of proteins identified by mass spectrometry is common20,27,28,45. Further analyses were performed with the software Signal P 5.0 (http://www.cbs.dtu.dk/services/SignalP/) in order to select only proteins bearing the signal peptide and therefore are likely to be secreted. This in silico prediction allowed us to select a total of 195 secretory proteins.
To further confirm the venomous nature of these 195 proteins, their sequences were aligned and compared to venom proteins from Nasonia vitripennis, described in de Graaf et al.30, obtaining 74 matches reported in Supplementary Tables S1, S2 and S3.
Among the 195 T. sinensis venom proteins, 121 proteins with signal peptides did not match any N. vitripennis venom protein. Although two contigs (12 and 2618) are not annotated, as they have no similarity with other known proteins, 69 proteins were found to show significant similarity with venom protein components of other parasitoid wasps or venomous animals or were uncharacterized proteins.
The 143 putative venom protein identified with the proteomic approach are listed in Supplementary Table S4 (Fig. 1c). The remaining 50 proteins without any similarity to known components of insect or other animal venom were considered either ‘venom trace elements’ with a limited function in the venom duct or in the reservoir, or contaminants of the venom gland released during the dissection of the gland tissue30,46. In addition to the proteomic approach, a key-word approach was used to identify a further group of putative venom protein in the venom gland transcriptome: all putative proteins annotated with the word “venom” were selected. 52 contigs annotated with “venom” keyword were identified (Supplementary Tables S5, S6; Fig. 1c). In addition, 2 contigs annotated with the “toxin” keyword were also identified (Supplementary Tables S5, S6). 18 of these contigs showed the signal peptide while this signature was missing in 34 contigs (Supplementary Table S5). Among the contigs missing the signal peptide, 18 had an incomplete sequence at 5 ' end, 14 had a complete sequence and 2 had an open reading frame (ORF) too small to give information about signal peptide (Supplementary Table S6).
To further confirm that the 52 putative proteins annotated using “venom” as a keyword belonged to the mixture of T. sinensis venom protein components, the nucleotide sequences of these proteins were translated using Expasy-translate tool software (https://web.expasy.org/translate/), and the corresponding protein sequences were compared to those of N. vitripennis, reported in de Graaf et al.30, obtaining 46 matches reported in Supplementary Tables S7, S8, S9. 9 T. sinensis contigs did not match any of the N. vitripennis venom proteins but were similar to other venom protein components, including carboxylesterases, Kazal-type serine protease inhibitors, acid phosphatases and carboxypeptidases (Supplementary Table S10).
All T. sinensis venom proteins, identified by the proteomic approach or exclusively by using the transcriptomic approach, can be classified in different groups, according to their functional annotation (Table 2).
Table 2.
T. sinensis venom proteins, identified by the transcriptomic or proteomic approach, categorized according to their role.
| Protein category | Contig number | Approach |
|---|---|---|
| Hydrolases | ||
| Serine proteases | 1, 4, 25, 69, 70, 87, 125, 235, 270, 368, 378, 706, 753, 775, 789, 991, 1058, 1059, 1089, 1143, 1233, 1310, 1617, 1636, 1794, 1835, 1855, 1907, 1971, 2101, 2283, 2399, 3012, 3708, 5058, 6126, 6382, 11,004, 11,592 | Proteomic |
| 7259, 8948 | Transcriptomic | |
| Metalloproteases | 8199 | Proteomic |
| Dipeptidases | 2298, 6303, 11,199 | Proteomic |
| 7070 | Transcriptomic | |
| Aminopeptidases | 5399, 7646, 18,029 | Proteomic |
| Lysosomal proteases | 293 | Proteomic |
| Carboxypeptidases | 1049, 12,519 | Proteomic |
| 7893, 15,076 | Transcriptomic | |
| Venom acid phosphatases | 3591, 3878 | Proteomic |
| 3070, 7321, 7343, 7836, 8187, 8691, 8857, 10,928, 13,041, 13,453, 14,872, 14,873, 16,876, 16,269, 18,015, 18,733, 18,844, 20,332 | Transcriptomic | |
| Carboxylesterases | 106, 1651, 1652, 2095, 2872, 3239, 9844 | Proteomic |
| 4090, 7684, 11,671, 12,240, 15,429, 19,895, 21,975 | Transcriptomic | |
| Lipases | 128, 392, 495 | Proteomic |
| Glucosidases | 1330, 2201, 5291, 8029, 10,216, 10,716 | Proteomic |
| Galactosidases | 7966, 4813 | Proteomic |
| Amylases | 445, 5249 | Proteomic |
| Trehalase | 4724 | Proteomic |
| Chitinases | 2736 | Proteomic |
| Nucleases | 2219, 3815, 4156, 4479, 6082, | Proteomic |
| 1587, 1953 | Transcriptomic | |
| Protease inhibitors | ||
| Serine protease inhibitors | 18, 1105, 2025 | Proteomic |
| Kazal type serine protease inhibition-like venom proteins | 1890 | Proteomic |
| 1535, 11,921 | Transcriptomic | |
| Cysteine-rich proteins | 5346, 21,875 | Transcriptomic |
| Immune related proteins | ||
| Calreticulin | 1001 | Proteomic |
| Calnexin | 5412 | Proteomic |
| Nucleobindin | 4917 | Proteomic |
| C1q-like venom protein | 9506 | Transcriptomic |
| Recognition/binding proteins | ||
| Odorant binding proteins | 281, 590, 1303, 1372, 4531, 4663, 7398, 8693, 9106, 10,799, 14,598 | Proteomic |
| Chemosensory proteins | 5573 | Proteomic |
| Low-density lipoprotein receptors | 289, 2622 | Proteomic |
| 1256 | Transcriptomic | |
| Apolipophorines | 784, 2538 | Proteomic |
| Insulin-like growth factor-binding protein | 2057 | Proteomic |
| Glutathione metabolism | ||
| γ-Glutamyl cyclotransferase | 5910 | Transcriptomic |
| Oxidase | ||
| Laccase | 17,081 | Transcriptomic |
| Dehydrogenases | ||
| Alcohol dehydrogenase | 2176 | Proteomic |
| Isomerases | ||
| Peptidyl-prolyl cis/trans isomerase | 1650 | Proteomic |
| FK506-binding protein | 3257 | Proteomic |
| Protein disulfide-isomerase A3 | 181 | Proteomic |
| Heat shock proteins | ||
| Endoplasmin | 1747 | Proteomic |
| Heat shock 70 kDa | 940 | Proteomic |
| Other proteins | ||
| Allergens 3 and 5 | 3120, 8578 | Proteomic |
| 1427, 7940, 12,687, 12,783 | Transcriptomic | |
| Major royal jelly protein | 11,185 | Proteomic |
| Adipocyte plasma membrane-associated protein | 874 | Proteomic |
| Ferritin | 1194, 1432 | Proteomic |
| Lachesin | 3847 | Proteomic |
| Mesencephalic astrocyte-derived neurotrophic factor | 575 | Proteomic |
| Vitellogenin | 1034 | Proteomic |
| Agatoxin | 12,568 | Transcriptomic |
| Unknown and hypothetical proteins | ||
| Venom protein D | 3227 | Transcriptomic |
| Venom protein F | 1029 | Transcriptomic |
| Venom protein L | 2092 | Transcriptomic |
| Venom protein N | 10,580 | Transcriptomic |
| Venom protein O | 19,579 | Transcriptomic |
| Venom protein Q | 472 | Transcriptomic |
| Venom protein R | 2910 | Proteomic |
| Venom protein T | 2487 | Transcriptomic |
| Venom protein U | 157, 1301 | Proteomic |
| Venom protein V | 4647 | Transcriptomic |
| Uncharacterized proteins | 158, 266, 1114, 1951, 3464, 3779, 5267, 5502, 6782, 7280, 7912, 7916, 8096, 8212, 9897, 10,521 | Proteomic |
| Hypothetical proteins | 529, 990, 1109 | Proteomic |
The table contains the contig number and the approach by which each protein was identified.
Among the identified proteins, hydrolases constituted the most abundant family followed by protease inhibitors, recognition and binding proteins, isomerases and dehydrogenases. These findings are in agreement with data reported for the venom of other parasitoids18,28,29,47. Among the hydrolases in the T. sinensis venom glands, we identified proteases and, in particular, serine proteases, metalloproteases, different kinds of peptidases, proteins of carbohydrate metabolism, lipases, phosphatases and nucleases.
Discussion
In order to identify the venom proteins of the ectoparasitoid Torymus sinensis, a combined proteomic and transcriptomic approach was used (hereafter defined as the proteomic approach). The transcriptomic analysis provided general information about putative proteins of the venom gland, focusing on their molecular functions, biological processes, and cellular compartments. This first level of analysis allowed us to select putative candidate proteins, annotated as “venom/toxin”. The proteomic analysis was performed on the venom extract using mass spectrometry, and the expressed proteins were identified when their sequences matched with the translated transcripts (contigs) of the venom gland transcriptome. Proteins containing a putative signal peptide for extracellular localization and predicted cleavage sites were reported as venom-expressed components. All putative (predicted from the transcriptome) or expressed (confirmed by the proteomic approach) proteins identified as venom protein components were further compared to Nasonia vitripennis venom proteins described by de Graaf et al.30, as both ectoparasitoids belong to the Chalcidoidea superfamily. The comparison with N. vitripennis venom proteins further confirmed the venomous nature of T. sinensis predicted and expressed proteins. We also identified putative or expressed T. sinensis venom proteins showing similarity to venom protein components of other parasitoids or animals. Lastly, a group of T. sinensis venom proteins was identified with sequence similarities to unknown, hypothetical or uncharacterized proteins. All the identified proteins were grouped according to their functions, and their possible role in the complex parasitic syndrome after envenomation was discussed.
Hydrolases
Hydrolases have been found in several endo- and ectoparasitoid venoms48. The hydrolases identified in T. sinensis venom can be grouped in different classes: proteases (serine proteases, metalloproteases, dipeptidases, amino- and carboxypeptidases), phosphatases, esterases, lipases, glucosidases, galactosidases, amylases, trehalases, nucleases. Some of these proteins belong to carbohydrate metabolism, including glucosidases, galactosidases, amylases and trehalases.
Proteases have been reported as abundant components in numerous parasitoid venoms18,20,27–29,49.
Among proteases, serine proteases are the most abundant family, including trypsins, chymotrypsins and serine protease homologues. Serine proteases and serine protease homologues were found in different parasitoid species, such as the endoparasitoids Aphidius ervi49, Microplitis mediator47, Pimpla hypochondriaca24, Pteromalus puparum29, Toxoneuron nigriceps18 and Cotesia rubecula50. Serine proteases can play a crucial role in regulating the immune system by inhibiting melanization in the host hemolymph blocking the phenoloxidase cascade51,52. Generally, serine protease homologues can act as co-enzymes for prophenoloxidase-activating proteinases and are important for the activation of prophenoloxidase and melanization53. In C. rubecula, a particular protease named Vn50 was structurally similar to other serine protease homologues but showed a different function. Vn50 inhibits melanization presumably by competing with host serine protease homologs for binding the prophenoloxidase that remains un-cleaved in the hemolymph50,51. Besides the action of serine protease homologues, serine protease may also play a role in down-regulating prophenoloxidase20,54. A different model for the involvement of venom serine protease in the melanization response was proposed by Choo et al.55 In the venom of Apis mellifera and Bombus spp., serine proteases seemed to be involved in the hyperactivation of prophenoloxidases, resulting in an excessive melanization response leading the target insects (Bombyx mori, Spodoptera exigua and Pieris rapae) to death. A similar poisoning effect may be induced by injecting ectoparasitoid venoms into the host. In addition to its involvement in host immunity disruption, a serine protease in N. vitripennis venom showed a putative cytotoxic function in assays with a Spodoptera frugiperda cell line56, whereas a trypsin-like enzyme found in salivary secretions of the ectoparasitoid Euplectrus separatae larva seemed to be able to digest host tissues57, prompting the hypothesis that serine proteases are extensively involved in the parasitic syndrome. In T. sinensis venom, many serine proteases were identified: trypsins (peptides identified by the proteomic approach matching to 25 contigs), chymotrypsins (peptides identified by the proteomic approach matching to 6 contigs), general serine proteases (peptides identified by the proteomic approach matching to 7 contigs, and 2 contigs identified by the transcriptomic approach) and a serine protease homologue (identified by the proteomic approach). Seven trypsins, and two general serine proteases identified in T. sinensis venom did not show the classic serine protease catalytic site, composed of serine, histidine and aspartic acid.
In the predicted serine proteases identified from the T. sinensis data, the relative positions of the amino acids of catalytic triad are mostly conserved: histidine at position 70, aspartic acid at positions 125 and 130, and serine at positions 240 and 245. A few anomalies have to be mentioned: in three contigs, the catalytic triad showed slight differences; in a trypsin, the serine residue is substituted by arginine, and in the serine protease homologue, the histidine residue is substituted by glycine. For some contig-derived amino acid sequences, the absence of the catalytic triad can be explained by the incompleteness of the contig sequence.
The high number of serine proteases identified as part of the complex T. sinensis venom and the absence of clear one-to-one orthology to other insect proteases (Supplementary Fig. S2), including N. vitripennis venom proteins, indicates species-specific gene duplication events in T. sinensis. Such large-scale gene duplication events of the serine protease gene family is a frequent phenomenon29,58 and we suspect that a greatly expanded set of proteases is associated with complex venom function.
Metalloproteases are involved in several biological and disease-related processes, such as intracellular signalling, matrix degradation, inflammation, and coagulation disorders59,60. In insect, metalloproteases are related to the immune response; indeed zinc-dependent proteases were highly expressed in Manduca sexta larvae infected with bacteria, and one of these proteases was quite similar to human neutral endopeptidase NEP 24.11, which is involved in the immune response61. In addition, metalloproteases were found in the venom of P. hypochondriaca24 and M. mediator endoparasitoids62, and Eulophus pennicornis ectoparasitoid63. In the latter cases, metalloproteases were shown to be responsible for the alteration of host development, reducing and even blocking host larval growth and metamorphosis, and promoting parasitoid development47,63. Some of M. mediator metalloproteases belong to the M12B subfamily, a member of which was also identified in the venom of endoparasitoid T. nigriceps18. According to the MEROPS database (http://merops.sanger.ac.uk), the T. sinensis venom metalloprotease, identified by the proteomic approach, belongs to the M12B subfamily, whose components are able to regulate processes related to neoplastic progression in mammals, such as immune response evasion, matrix degradation, metastasis and inflammation processes64.
Dipeptidases including Dipeptidyl peptidase IV, dipeptidase 1 and the Angiotensin-converting enzyme, are exopeptidases that catalyze hydrolysis of dipeptides65.
Dipeptidyl peptidase IV is a very common enzyme in snake venom66 and in the venom of some Hymenoptera, such as A. mellifera67, Vespa basalis68 and Polistes dominula69. Although its function is not completely known, it seems to be involved in the processing of precursors of venom protein components70. This enzyme is a serine protease that cleaves dipeptides from the N-terminus of peptides with proline or alanine in the penultimate position71, and its function could be related to the maturation of toxic peptides, as proposed for mastoparan B, the major toxin in the venom of V. basalis68. Mastoparan B is indeed synthesized as pro-peptide and then activated through an enzymatic cleavage by a dipeptidyl peptidase IV able to generate a consecutive release of dipeptides68. Dipeptidyl peptidase IV cleavage activity was also related to other functions, such as the regulation of inflammatory and immunological responses, signal transduction and apoptosis by degrading physiological substances67. The angiotensin-converting enzyme is a peptidyl dipeptidase that removes dipeptides from the C-terminus of short oligopeptides. In mammals, angiotensin I is converted into angiotensin II and bradykinin is activated, thus regulating blood pressure and electrolyte homeostasis. This enzyme was identified in the venom of the endoparasitic wasp P. hypochondriaca5, in Thalassophryne nattereri and Scorpaena plumieri, venomous fishes typical of the north-eastern coast of Brazil72. Although its specific function remains unknown, it is probably involved in processing peptide precursors.
In T. sinensis venom, two contigs encoding for dipeptidyl peptidase IV proteins were identified, one of them using the proteomic approach and the other one by the transcriptomic approach. A dipeptidase 1 and one Angiotensin-converting enzyme were identified using the proteomic approach.
Aminopeptidases remove one or more specific N-terminal residues from target proteins or peptides, and are common in venom of snakes73,74, in the venom of the predatory ant Pachycondyla striata75, in the venom of the velvet spider Stegodyphus mimosarum76 and in the venom of the genus Thoracobombus (Hymenoptera)77, even if its function has not been understood yet. Mammalian aminopeptidases, which cleave brain angiotensin II to angiotensin III, are implicated in the control of arterial blood pressure78. This suggests that aminopeptidases might be involved, in synergy with angiotensin-converting enzymes, in angiogenic mechanisms, such as in regulating blood vessel formation and blood pressure79. However, the most probable functions in venoms are 1) to help degrade the host tissues extracellular matrix in order to increase its permeability to venom protein components74 and 2) to contribute in transforming host tissues into nutrients for parasitoid progeny. In T. sinensis venom, three aminopeptidases were identified using the proteomic approach.
Lysosomal proteases belong to the aspartic, cysteine, or serine endoprotease family; despite the adjective “lysosomal,” they are usually detected within all vesicles of the endocytic pathway. They are also known as cathepsins80. Lysosomal aspartic proteases are enzymes whose catalytic sites consist of two aspartate residues81. A similar protein, a cathepsin D, was found in the venom of the endoparasitoids Leptopilina heterotoma82 and Chouioia cunea83. A putative lysosomal aspartic protease was also upregulated in the pupal transcriptome of Sarcophaga crassipalpis 25 h after envenomation by N. vitripennis, supporting the hypothesis that this protease plays a key role in the success of parasitism84. Lysosomal proteases, such as cathepsins, are activated in apoptotic and necrotic cells and during autophagy phenomena81. The regulation of autophagy, which is associated with starvation, nutrient recycling and cell cycle arrest, can be a strategy used by parasitoids to manipulate host development and metabolism for its progeny84. Although the specific role of lysosomal proteases in Hymenoptera parasitoids is still unclear, they may be involved in the production of venom protein components or in blocking host immunity, contributing to their offspring development82. In T. sinensis venom, one lysosomal aspartic protease was identified using the proteomic approach. Cathepsin L is a lysosomal cysteine protease with a catalytic dyad consisting of cysteine and histidine85. Lysosomal cysteine proteases are involved in extra- and intra-cellular protein degradation, antigen presentation and cellular development86, and in various orders of insects are considered important digestive enzymes87. In parasitoid venom, cathepsin L was first found in T. nigriceps venom18. A large quantity of this protein was found in fat body/hemocytes complex of a Spodoptera littoralis larva parasitized by the ectoparasitoid Bracon nigricans88; in this latter case it was hypothesized that cathepsin L induced the formation of these complexes, because of the rapid degradation of fat body required to mobilize the stored nutrients in favor of the parasitoid offspring88. In T. sinensis venom, one cathepsin L was identified using the proteomic approach. Moreover, a protein annotated as “uncharacterized”, identified by the proteomic approach, might also be included in the Lysosomal protease category as it contains a cysteine protease domain and a region similar to cathepsin L.
Carboxypeptidases cleave peptide bonds at the C-terminal of a protein89. Putative venom serine carboxypeptidases were found in the venom of the ants Odontomachus monticola90 and Tetramorium bicarinatum91, the snake Crotalus durissus terrificus92, the Hymenoptera of the Apidae family77 and in the venom gland of the ectoparasitoid Anisopteromalus calandrae31. A serine carboxypeptidase was also found in the venom of the endoparasitoids Psyttalia concolor93 and T. nigriceps18. Although the specific role of carboxypeptidases in parasitoid venom is not clear yet, this enzyme could be involved in the degradation of the host tissues, most likely as the aminopeptidase enzymes. In T. sinensis venom, four carboxypeptidases were identified: three using the proteomic approach, and one using the transcriptomic one.
Venom acid phosphatases have been identified in the venom of some hymenopteran species, such as A. mellifera and Apis cerana94,95, the endoparasitoids P. hypochondriaca96, Pimpla turionellae45, P. puparum97 and the ectoparasitoids A. calandrae31 and Bracon hebetor98. Venom acid phosphatases were also identified in the venom gland of the endoparasitoid M. pulchricornis99. They are characterized by a conserved catalytic core containing a histidine residue which is phosphorylated during catalysis. In venom, they have a neurotoxic, myotoxic, anticoagulant and inflammatory effect100. In T. sinensis, two acid phosphatases were identified by the proteomic approach, and eighteen acid phosphatases were identified by the transcriptomic approach.
Carboxylesterases are hydrolases containing a catalytic apparatus consisting of three residues, serine, glutamate or aspartate and histidine, and the mechanism involves a nucleophilic attack on a carbonyl carbon atom101. Although their function in venoms has not been identified yet, they could be allergen proteins; indeed, carboxylesterase-6 is one of the main allergens in honeybee venom102. In N. vitripennis venom, two types of esterases have been found: an arylsulfatases B isoform X1 and a carboxylesterase clade B member 2 precursor30. In T. sinensis venom, seven carboxylesterases were identified by the proteomic approach and seven by the transcriptomic one.
Lipases, which act on carboxylic esters, have the same catalytic triad as esterases. In general, lipases have essential roles in the digestion, transport, and processing of dietary lipids in most living organisms103. It has been demonstrated that N. vitripennis venom induces alterations in the host lipid metabolism, although the specific role of lipases has not been clarified yet104. Recently, lipase activity has been found in the venom of the endoparasitoid wasps P. hypochondriaca96, Psyttalia lounsburyi and P. concolor93, Microctonus aethiopoides105, Ooencyrtus telenomicida106 and the ectoparasitoid B. nigricans32. An interesting example is reported in the endoparasitoid Cotesia kariyai: several days after parasitization, the total amount of lipid from the fat body of the parasitized hosts decreased as the lipase activity of parasitoid larvae increased. Although in this case lipase was not annotated as a component of the endoparasitoid venom, this observation could support the hypothesis that, in general, lipases can digest host lipids and provide nutrients to the parasitoid larvae54,107. Lysis of fat body cells, with the increase in the hemolymphatic lipid content, was also observed in the lepidoptera Pseudaletia separata after it was parasitized by ectoparasitoid wasps of the genus Euplectrus108. Lipases can then be considered involved, at least partially, in increasing the suitability of the host environment in favor of parasitoid progeny. In T. sinensis venom three lipases were identified by the proteomic approach.
Glucosidases hydrolyze glycosidic bonds from glycosides and oligosaccharides and remove non-reducing terminal glucosyl residues releasing glucose as product109. Among parasitoids, β-glucosidases were detected in the venom of the endoparasitoids P. hypochondriaca96, Microplitis demolitor110, P. lounsburyi and P. concolor93. The release of glucose, deriving from host hemolymph carbohydrates, may increase the amount of energetic nutrients available for the developing parasitoid larvae, suggesting that glucosidases are involved in modifying host metabolic pathways in favor of parasitoid development. In T. sinensis venom six glucosidases were identified by the proteomic approach.
Galactosidases catalyze the hydrolysis of galactoside molecules by breaking glycosidic bonds111. Among parasitoids, galactosidases were identified in the venom of the endoparasitoid P. hypochondriaca96. Like glucosidases, galactosidases could also release carbohydrates into the host hemolymph for supply of parasitoid larvae. In T. sinensis venom, two galactosidases were identified by the proteomic approach.
Amylases are enzymes catalyzing the hydrolysis of alpha-1,4 glycosidic bonds of starch and glycogen into sugars, such as maltose, maltotriose and residual branched maltodextrins112. Most amylases have been identified in insect salivary glands or digestive tracts; few examples have been reported in the venom of parasitoid wasps, such as Nasonia species and P. puparum113,114. As carbohydrates are essential for metabolism, it is proposed that amylases expressed in the venom of P. puparum and secreted in the host (P. rapae) hemolymph are involved in the degradation of host polysaccharides for energy intake and parasitoid larvae development114. In T. sinensis venom, two amylases were identified by the proteomic approach.
Trehalases, enzymes which catalyze the conversion of trehalose to glucose, have been found in the venom of wasps such as Cerceris rybyensis115, the endoparasitoids Ampulex compressa116, P. hypochondriaca117 and the ectoparasitoid N. vitripennis118. Trehalases may convert the high concentration of trehalose in the host hemolymph to glucose in order to provide a source of energy for the parasitoid larva development118,119. In T. sinensis venom, one trehalase was identified by the proteomic approach.
Collectively glucosidases, galactosidases, amylases and trehalases may play a key role in metabolic pathways, providing nutrients to the parasitoid offspring.
Chitinases are able to disrupt and digest chitin, one of the components of the exoskeleton of arthropods120. This enzyme is vital for animals during ecdysis and metamorphosis, as well as for animals that feed on organisms whose structures are composed of chitin. Although this enzyme has been detected in the venom of spiders121 and scorpions122, it is also important in the physiology of different endoparasitoids, like T. nigriceps, where teratocytes release chitinases to support the emergence of larvae by degrading the host cuticle123. A similar process may be performed by chitinase in the venom of the endoparasitoid wasp Chelonus inanitus, helping the parasitoid larva to hydrolyze the host embryonic cuticle to reach host embryo124. In T. sinensis venom, one chitotriosidase was identified by the proteomic approach. Moreover, a protein annotated as “uncharacterized protein”, found by the proteomic approach was included in this group as it contains chitin-binding domains in its structure.
Nucleases are capable of cleaving the phosphodiester bonds among nucleotides. Nucleases are very common in snake125 and Cnidaria venoms126. It was predicted that these enzymes play a central role in strategies of prey immobilization, as free adenosine molecules may induce the inhibition of neurotransmitter release. Moreover, these enzymes may induce renal failure and cardiac arrest and increase vascular permeability, thereby helping the spread of toxins in host tissue125. Endonuclease-like venom proteins are characterized by a DNA/RNA non-specific endonuclease conserved domain that gives the protein the ability to cut double-stranded and single-stranded nucleic acids127. They were found in the venom of the ectoparasitoid A. calandrae31 and the endoparasitoid Cotesia chilonis28. A ribonuclease Oy-like found in the venom of the ectoparasitoid Pachycrepoideus vindemmiae may be related to the cleavage of host RNA to face host defensive reactions20. Inosine uridine-preferring nucleoside hydrolase-like is an enzyme capable of hydrolyzing nucleotides in nucleosides, with a preference for inosine and uridine128. It was found in the venom of the seed-parasitic wasp, Megastigmus spermotrophus129 and in the venom of the endoparasitoids Leptopilina boulardi and L. heterotoma26. However, its role in venom is still unknown. Another kind of deoxyribonuclease with predicted function in DNA degradation found in T. sinensis venom is plancitoxin. Plancitoxin, first detected in the venom of the starfish commonly known as crown-of-thorns, Acanthaster planci, is a DNAse II able to reduce cellular antioxidant level in response to high oxidative stress and induce hepatotoxic damage130. It was found in the venom gland of the endoparasitoids M. demolitor109 and Lysiphlebus fabarum131, although its role in parasitoid venom remains unknown. In T. sinensis, several nucleases were found by the proteomic approach: one ribonuclease oy-like, two endonuclease-like venom protein precursors, one inosine uridine-preferring nucleoside hydrolase-like precursor, one poly(U)-specific endoribonuclease homolog. Moreover, one endonuclease-like venom protein precursor and one plancitoxin were identified by the transcriptomic approach. Finally, one protein annotated as “uncharacterized protein” found by the proteomic approach can also be included in this group, as in its structure it contains a DDE_Tnp_4 domain, belonging to the endonuclease family.
Protease inhibitors
Serine protease inhibitors, known also as serpin proteins, are common in the venom of different species, such as snakes, scorpions and wasps. Serpin proteins were found in the venom of endoparasitoids L. boulardi132, A. ervi49, A. calandrae31, T. nigriceps18 and P. puparum133 and in the venom gland of the endoparasitoid Meteorus pulchricornis99. Serpins can form permanent covalent complexes with target serine proteases134 and are involved in regulating the prophenoleoxidase cascade as well as blocking melanization process133,135. In T. sinensis venom, one serpin 5, a serine protease inhibitor 28Dc isoform X2 and a serine protease inhibitor 3/4 isoform X16 were identified using the proteomic approach. Moreover, a further protein annotated as “uncharacterized protein” found by the proteomic approach, was also included in this group, as it contains domains belonging to the Serpin superfamily.
Kazal-type serine protease inhibition-like venom proteins are involved in the inhibition of serine proteases, such as trypsin, chymotrypsin, and elastases. The inhibitory domain contains a specific peptide bond, which serves as a substrate for the cognate enzyme. The reactive site peptide bond is located within a loop whose conformation is identical in all Kazal inhibitors and all enzyme/inhibitor complexes. Similar domains, which are also present in follistatin and follistatin-like family members, play an important role in regulating specific tissues136. Four Kazal proteins are significantly expressed in the venom gland of the endoparasitoid P. puparum29, and two types were identified in the venom gland of the ectoparasitoid A. calandrae31, all with protease inhibitory activity. Kazal proteins may be involved in the immune response. Indeed, in B. mori, Kazal inhibitor proteins showed antimicrobial activity playing a putative protective role against invading pathogenic microorganisms137. Moreover, Kazal inhibitor proteins were also found in the saliva of blood-sucking insects, indicating a putative anticoagulant role138. Few studies on these proteins have been carried out in parasitoid wasps, unlike in other insects. Studies on Kazal-type serine protease inhibition-like proteins of N. vitripennis venom showed an inhibition of prophenoloxidase activation in parasitized Musca domestica hemolymph139. The same activity was observed in the endoparasitoid wasps Venturia canescens140. The prophenoloxidase system, one of the main components of the immune system in arthropods, activates specific humoral immune responses to non-self organisms through the melanization and damages their tissues. This process is mediated by the enzyme phenoloxidase, which is synthesized as the zymogen prophenoloxidase. The activation of prophenoloxidase in phenoloxidase is tightly regulated by the serine proteases cascade and by serpins141. Accordingly, it was proposed that Kazal-type serine protease inhibition-like venom proteins may play a role in suppressing host melanization139. One protein belonging to Kazal-type serine protease inhibition-like venom protein-1 and one protein belonging to Kazal-type serine protease inhibition-like venom protein-2 were identified in T. sinensis venom, using the transcriptomic approach.
Finally, an ovomucoid-like protein was identified by the proteomic approach. Ovomucoids, proteins found in the whites of eggs, are composed of three Kazal-type domains142. This protein, found in the venom of the snake Bothriechis schlegelii, has a putative function of a serine protease inhibitor, according to Kazal-type serine protease inhibitor role142.
Cysteine-rich proteins are protease inhibitors showing a distribution of cysteine residues similar to toxin proteins and serine protease inhibitors of insects and crustaceans30. Specifically, the Kunitz (KU) type motif was found in toxins from amphibians, snakes, spiders, cone snails and sea anemones143. In addition to the classical function of serine proteases inhibition, these cysteine-rich/KU venom proteins can block ion channels, which are essential for regulating various physiological processes such as blood coagulation, fibrinolysis and host defense, favoring the spread of parasitization events30,45. Moreover, cysteine-rich protease inhibitors could be involved in disrupting host immunity by inactivating the prophenoloxidase cascade, as the Kazal-type serine protease inhibition-like venom protein of the endoparasitoids P. hypochondriaca and P. turionellae and the ectoparasitoids A. calandrae and N. vitripennis31,45,118,144. In T. sinensis venom, using the transcriptomic approach, one cysteine-rich/KU venom protein and one cysteine-rich/pacifastin venom protein-2 were identified.
Immune-related proteins
Several studies report that N. vitripennis venom inhibits host cellular immune response145. Venom proteins involved in the suppression of host defense are collectively named “Immune-related proteins”.
Calreticulin is a Ca2+-binding chaperone that was found in endoparasitoid C. rubecola venom, where it may compete with host calreticulin on the surface of hemocytes, acting as an antagonist of hemocyte activation in early encapsulation reactions. Calreticulin, indeed, seems to play an important role in encapsulation and phagocytosis, inhibiting hemocytes diffusion and suppressing host immune reaction146,147. Calreticulin has also been identified in the venom of other endoparasitoids such as P. puparum148 and T. nigriceps18, suggesting a role similar to that of C. rubecola calreticulin in hemocyte encapsulation. Beside that, by altering the intracellular calcium balance, calreticulin might affect the biological processes in which Ca2+ is involved, such as apoptosis, inflammation and the activation of hydrolytic enzymes. In T. sinensis venom, a calreticulin was identified by the proteomic approach.
Calnexin is another Ca2+-binding protein found in the venom of snake Bothrops colombiensis; together with calreticulin, calnexin may be involved in the process of toxin secretion149. In T. sinensis venom, calnexin was identified by the proteomic approach.
Nucleobindin-2 is another protein that can be included in the Ca2+-binding protein group. In mammals, nucleobindin 2 is the precursor of a DNA- and calcium-binding protein, nesfatin-1, that seems to be associated with brain changes in stress situations. Nucleobindin-2 was reported in the venom of A. mellifera102, in the venom of Ornithorhynchus anatinus platypus, a venomous monotreme150, and in the venom of the snake Bothrops jararaca151, in which it could induce excitotoxicity, that is the alteration of nerve cells by excessive neurotransmitter stimulation151. In T. sinensis venom, Nucleobindin-2 was identified by the proteomic approach.
Complement component 1q (C1q) is a key protein in the classical complement pathway and represents the joining link between the acquired and the innate immune response152. As the human C1q domain directly interacts with lipopolysaccharides from gram-negative bacteria153, it is possible that this protein plays a role in opsonizing molecules30. This protein was also found in A. mellifera venom154. In T. sinensis venom, one C1q-like venom protein was identified through the transcriptomic approach.
Recognition/binding proteins
Odorant binding proteins (OBPs) are a group of small globular and soluble polypeptides highly concentrated in olfactory organs as nasal mucus and tears in vertebrates155 and sensillar lymph in insect sensilla156. In invertebrates, they are characterized by a pattern of six conserved cysteine residues, paired in three disulphide bridges157. Beside the general OBP-like (GOBP-like) proteins in N. vitripennis venom30, OBPs were found in the venom of the endoparasitoids P. puparum158, L. heterotoma82, C. inanitus124, in the fire ant Solenopsis invicta159 and in the giant ant Dinoponera quadriceps. In the giant ant, OBP-like proteins induced IgE antibody production in prey (insects, small birds, and mammals) and acted as a powerful allergy-inducing molecule160. Their possible role in Hymenoptera venom and in host-parasitoid interactions is not known yet, but OBPs were supposed to be involved in host selection and in the search for an appropriate substrate to oviposit. In addition to this classical chemosensory function, OBP-like proteins in venom may play a role in transporting hydrophobic molecules, such as components of the nourishment, ensuring nutrients to the parasitoid rather than the host31,156. In T. sinensis venom, eleven general OBP-like proteins were identified by the proteomic approach.
Chemosensory proteins, similarly to OBPs, are small soluble proteins mediating sensory perception in insects. Their function in parasitoid venom could be very similar to OBPs mediating host selection and carrying hydrophobic feed molecules for the parasitoid offspring31,124. Chemosensory proteins were found in the venom of the endoparasitoid C. inanitus124 and the ectoparasitoid A. calandrae31. In T. sinensis venom, one chemosensory protein was identified using the proteomic approach.
Low-density lipoprotein receptors have a central role in cholesterol and other lipoprotein metabolism161, even though they are uncommon among venom proteins and their role in envenomation is still unknown. Low-density lipoprotein receptors were described in the venom of parasitoids, such as in N. vitripennis30 and P. puparum27 and the spider Latrodectus hesperus162. An example of this protein is PH-4 (neuropeptide prohormone-4) of Profundiconus cone snail genus. This protein, found also in the venom of other sea snails, has a mature sequence and a precursor-related peptide containing low-density lipoprotein receptor A domain163. Another example is PS1 (peptidase S1) from the crustacean Xibalbanus tulumensis, which also contains a low-density lipoprotein receptor A domain. This domain allows PS1 to bind lipoproteins, while the PS1 domain facilitates their digestion. These proteins are hypothesized to facilitate the interaction with lipoproteins of the prey/host to create a substrate for predators and parasitoids164. In T. sinensis venom, two low-density lipoprotein receptors were identified using the proteomic approach and one using the transcriptomic one.
Apolipophorins, which belong to apolipoprotein family and are involved in lipid transport processes in the insect hemolymph165, play also an important role in immunity, programmed cell death and the detoxification of lipopolysaccharide endotoxins166. They were found in venom of A. mellifera and B. pascuorum wasps and in predatory ant O. monticola90,165,167. In T. sinensis venom, two apolipophorins were identified using the proteomic approach. Moreover, this group of apolipophorins may include one protein annotated as “hypothetical protein”, as it contains an apolipophorin domain in its structure.
Insulin-like growth factor-binding proteins are able to bind insulin-like growth factors and allow their transport to target tissues, where they promote cell growth, proliferation, differentiation and survival168. These proteins were identified in the venom of the scorpion Tityus stigmurus169, in venom glands of the spider Cupiennius salei170 and in cobra venom, in which it seems to be related to apoptosis induction171. In T. sinensis venom, an insulin-like growth-factor-binding protein was identified using the proteomic approach.
Glutathione metabolism
γ-Glutamyl cyclotransferase proteins are involved in glutathione metabolism. This enzyme was found in transcripts of the venom gland in the ectoparasitoid A. calandrae31 and in the venom of M. spermotrophus129. The function of this protein in the parasitoid venoms is still unknown. However, glutathione is fundamental in regulating homeostasis in the cell, and its alteration could result in oxidative stress and apoptosis, as reported for the γ-Glutamyl transpeptidase like venom protein identified in A. ervi venom13,49. In T. sinensis venom, one γ-glutamyl cyclotransferase-like venom protein was identified using the transcriptomic approach.
Oxidases
Laccases, which belong to a group of proteins collectively known as multicopper oxidases, were supposed to play an important role in insect cuticle sclerotization172. During this extracellular process, cuticular proteins are cross-linked into a matrix as result of oxidative and nucleophilic reactions of catechols to their corresponding quinones173. This enzyme was found also in the venom of both the ectoparasitoid N. vitripennis30 and the endoparasitoid P. hypochondriaca118. In T. sinensis venom, one laccase was identified by the transcriptomic approach.
Dehydrogenases
Alcohol dehydrogenases are oxidoreductase enzymes that catabolize otherwise toxic alcohols. They are not common in venom; to the best of our knowledge, this enzyme was previously identified exclusively in the venom of A. mellifera174 and in the venom of scorpion species Leiurus abdullahbayrami175. In T. sinensis venom, one alcohol dehydrogenase was identified by the proteomic approach.
Isomerases
Peptidyl-prolyl cis/trans isomerases are enzymes that catalyze the cis-to-trans isomerization around proline, allowing proteins to fold into their correct conformation. In nature, proline is the only amino acid existing in cis and trans isomerization form; correct protein folding is often not possible when a proline peptide bond is in the incorrect configuration, and proper isomerization is necessary176. Peptidyl-prolyl cis/trans isomerases were identified in the venom gland of predatory marine cone snails Conus novaehollandiae, where they facilitate the in vitro folding of conotoxins177. This enzyme was also identified in the venom of the endoparasitoids C. chilonis28 and T. nigriceps18, in the venom of the ectoparasitoid B. nigricans32 and in the venom of the predatory ant O. monticola90 and the snake C. durissus terrificus92. Although the specific function of these enzymes in parasitoid venom remains to be determined, they may be involved in the folding of toxin peptides, as it occurs in other venomous organisms177. In T. sinensis venom, an isomerase, annotated as peptidyl-prolyl cis–trans isomerase 5-like, was identified by the proteomic approach.
FK506-binding proteins are immunophilins that bind immunosuppressive drugs such as FK506, rapamycin and cyclosporin A178. They often have peptidyl-prolyl cis–trans isomerase activity179. This enzyme was found in venom of the endoparasitoid C. chilonis28. In T. sinensis venom, one isomerase, annotated as FK506-binding protein, was identified by the proteomic approach.
Protein disulfide-isomerase A3 is a molecular chaperone, supporting folding and processing of glycoprotein after their synthesis in the endoplasmic reticulum180. It was identified in the venom of the endoparasitoid P. puparum27, although its role in venom is still unknown. In T. sinensis venom, one isomerase, annotated as protein disulfide-isomerase A3, was identified by the proteomic approach.
Heat shock proteins (HSPs)
Endoplasmins are molecular chaperones belonging to the HSP 90 family and involved in the final processing and export of secreted proteins. They may also play a role in the stabilization of other proteins. They were found in the venom of the endoparasitoid A. ervi, and, according to HSP functions, they may help protect parasitoid proteins during secretion and transport in host cells49. The same protein was identified in venom of the endoparasitoids P. lounsburyi, P. concolor93 and C. cunea83 and snake Crotalus adamanteus181. In T. sinensis venom, a heat shock protein, annotated as endoplasmin, was identified by the proteomic approach.
Heat shock 70 kDa, molecular chaperones involved in the folding of other proteins, were found in venom of the Hymenoptera wasps Apoica pallens182 and Polybia paulista183, and in venom of the ant Neoponera villosa184. These proteins also occur in the venom glands of bees165 and in venom of the endoparasitoid T. nigriceps18. In T. sinensis venom a heat shock protein, annotated as generic heat shock 70 kDa, was identified by the proteomic approach.
Other proteins
In addition to the previously described categories, other proteins were identified, difficult to categorize but similar to known proteins.
Allergens 3 and 5 are allergen proteins. Mostly studied and characterized in humans, they are also found in several wasp and ant species130,185. Venom allergen 3-like and allergen 5 were found in the venom of the ectoparasitoid A. calandrae31, allergen 3 in the venom of the endoparasitoid P. puparum27, allergen 5 in the venom of the endoparasitoids T. nigriceps18, C. inanitus124 and the ectoparasitoid B. nigricans32. T. nigriceps venom allergen 5-protein contains a sperm-coating protein (SCP)-like extracellular protein domain, that may function as endopeptidase. This protein might be involved in protein proteolysis and tissue degradation by the parasitoid18. In T. sinensis venom, six allergen proteins annotated as allergens 3 and 5 have been identified, two of which by the proteomic approach and four by the transcriptomic one. These proteins are very similar to N. vitripennis antigen 5-like proteins, which is also one of three major allergenic proteins found in the venom of Vespula, Vespa and Dolichovespula100.
Major royal jelly proteins are proteins involved in the development of bee larvae. MRJP7 is highly expressed in nurse bees and bees that feed the worker and the queen with jelly secreted from specific glands186. Moreover, proteins very similar to MRJP8 and 9 were also identified as components of honeybee venom165,186 and of the venom of parasitoids, such as the endoparasitoid P. puparum27 and the ectoparasitoid P. vindemmiae20, although their function in parasitoid venom is still unknown165,187. Recently it was hypothesized that they could be allergens188 or proteins related to storage of nutrients20. In T. sinensis venom, one protein annotated as major royal jelly protein was identified by the proteomic approach. Moreover, a protein annotated as “Uncharacterized protein” found by the proteomic approach could also be included in this group, as it contains a major royal jelly protein domain in its structure. The same protein was found in the ectoparasitoid P. vindemmiae venom20.
Adipocyte plasma membrane-associated protein was previously identified in the endoparasitoid Tetrastichus brontispae. In this study, the protein was found on the surface of the parasitoid egg and it was supposed to be involved in evading the host immunity response and protecting the egg during the early parasitoid stages, in association with a lipophorin protein189. The adipocyte plasma membrane-associated protein was very similar to hemomucin, an O-glycosylated surface mucin found on the extraembryonic membrane of many parasitoid eggs that may allow the embryo to evade the host encapsulation reaction190. Therefore, this protein could have protective properties in host-parasitoid systems. In T. sinensis venom, one protein annotated as adipocyte plasma membrane-associated protein was identified by the proteomic approach.
Ferritin is an intracellular protein that carries and stores iron. This protein is found in the venom of scorpion Centruroides vittatus191 and the endoparasitoids M. aethiopoides and Microctonus hyperodae105 and in venom gland of the endoparasitoid M. pulchricornis99, but its role is still unknown. In T. sinensis venom two proteins annotated as ferritin were identified by the proteomic approach.
Lachesins belong to the disintegrin family, typically of viper venoms, and act as potent inhibitors of platelet aggregation and integrin-dependent cell adhesion192. Lachesin was characterized in snake Lachesis muta venom193. Although this protein is not found in parasitoid venom, it could be involved in defence against host immunity responses. In T. sinensis venom one protein annotated as lachesin was identified by the proteomic approach.
Mesencephalic astrocyte-derived neurotrophic factor is an endoplasmic reticulum stress-inducible protein, originally identified as protein protecting rat dopaminergic neurons in vitro and prevents neuron degeneration in Parkinson's disease194. It was found in venom of endoparasitoid C. chilonis, in which probably it is involved in cell protection from endoplasmic reticulum stress28. In T. sinensis venom one protein, annotated as mesencephalic astrocyte-derived neurotrophic factor, was identified by the proteomic approach.
Vitellogenin is a protein involved in lipid transport from ovarian follicle cells to oocytes, providing nutrition during embryogenesis and playing a role as egg yolk protein precursor in the ovaries195. Despite its main role, it is also component of venom of Hymenoptera, such as A. mellifera and Vespula vulgaris in which it represents one of the allergens, with its IgE-reactive allergenic properties196, and A. cerana in which it was hypothesized that could be involved in response to microbial infection and oxidative stress, ensuring protection to DNA against ROS197. Indeed, it could be also considered an antimicrobial and antioxidant agent197. It was also found in venom of the ant O. monticola134 and in venom of the ectoparasitoid A. calandrae31. In T. sinensis venom a protein annotated as vitellogenin was identified by the proteomic approach.
Agatoxins are polyamine and peptide toxins isolated from spider and scorpion venoms170,198. Their mechanism of action led to the inactivation of several ion channels, causing neurotoxic effects199,200. Agatoxin was found also in the venom of the giant ant D. quadriceps201 and in the venom gland transcriptome of solitary and social wasps, such as Vespa velutina202. In T. sinensis one u8-agatoxin-ao1a-like isoform 1 was identified exclusively by the transcriptomic approach. To the best of our knowledge, the T. sinensis venom is the only one containing a putative agatoxin among parasitoid venoms. Because agatoxins are strongly involved in blocking ion channels, their action in parasitic syndrome could be strictly related to prey paralysis, that is one of the main effects of ectoparasitoid wasps attack12.
Unknown and hypothetical proteins
We found different proteins that are not associated with known proteins, but they are very similar to the same unknown N. vitripennis proteins; they are named from “venom protein A” to “venom protein Z”.
In T. sinensis we found:
venom protein D (one protein) identified by the transcriptomic approach, also found in the venom of P. puparum27 and A. calandrae31;
venom protein F (one protein) identified by the transcriptomic approach, also found in the venom of the wasps M. spermotrophus129, Megaphragma amalphitanum, Ceratosolen solmsi, the endoparasitoid Trichogramma pretiosum203, and A. calandrae, in this case with a putative role in actin polymerization and in transcription regulation of cholesterol and fatty acid homeostasis31;
venom protein L (one protein) identified by the transcriptomic approach, also found in the venom of P. puparum27, A. calandrae31 and P. vindemmiae20;
venom protein N (one protein) identified by the transcriptomic approach, also found in the venom of A. calandrae31 and P. vindemmiae20;
venom protein O (one protein) identified by the transcriptomic approach also found in the venom of P. puparum27 and A. calandrae, in this case with a putative role of OBP31;
venom protein Q (one protein) identified by the transcriptomic approach, also found in the venom of Nasonia giraulti114 and A. calandrae, in which a seryl-tRNA synthetase domain was detected31;
venom protein R (one protein) identified by the proteomic approach, also found in the venom of P. puparum27, N. giraulti114, M. amalphitanum, C. solmsi, T. pretiosum203, Tetrastichus brontispae204,205 and M. spermotrophus129. RNA sequencing performed on abdomen tissue in Ischnura elegans also revealed a venom protein r-like, a toxin and hemolymph juvenile hormone binding protein, that regulates embryogenesis, larva development and reproductive maturation206.
venom protein T (one protein) identified by the transcriptomic approach, also found in the venom of A. calandrae31 and N. giraulti114.
venom protein U (two proteins) identified by the proteomic approach, also found in the venom of P. puparum27, A. calandrae31 and P. vindemmiae20;
venom protein V (one protein) identified by the transcriptomic approach, also found in the venom of P. vindemmiae20 and A. calandrae, in which a chaperone_ClpB domain was detected31. Chaperone ClpBs from several microorganisms are essential for survival under severe stress conditions207.
In addition to the above-mentioned proteins, a group of “uncharacterized proteins” and a group of “hypothetical proteins” were also found by the proteomic approach. A hypothetical protein is a protein whose existence has been predicted since it derives from an ORF, but there is no experimental evidence of translation208. The identification of these proteins in venom by the proteomic approach is an experimental evidence of their expression, but their characterization and their role remain unknown. In T. sinensis venom, we identified two proteins similar to hypothetical protein LOC100679659 isoform 1 and uncharacterized protein LOC100118367 of N. vitripennis, also found in the venom of P. puparum27. The analysis of the structure of uncharacterized protein LOC100113619 led to the identification of a SWVC domain (single-domain von Willebrand factor type C). Proteins characterized by this domain were also identified in the venom of the spider Pamphobeteus verdolaga209 and in the venom gland transcriptome of the scorpions Hadrurus spadix208, Centruroides hentzi211 and Paravaejovis schwenkmeyeri212. In the structures of uncharacterized protein LOC106783674 isoform X2 and uncharacterized protein LOC108911535 isoform X1, a DUF4803 domain (domain of unknown function) was identified. Some molecules containing this domain were found in venom of the endoparasitoids P. lounsburyi and P. concolor93 and the ectoparasitoid B. nigricans32. DUF protein families are still functionally uncharacterized. Overall, in T. sinensis venom, sixteen uncharacterized proteins and three hypothetical proteins were identified by the proteomic approach. According to the functional domains identified through the BLASTp software (https://blast.ncbi.nlm.nih.gov/Blast.cgi), some of the uncharacterized/hypothetical proteins can be included in the previous groups, as already described.
Further investigation will provide more information about these proteins.
Conclusions
The integrated transcriptomic and proteomic approach used to analyze Torymus sinensis venom and the accompanying analysis, using a transcriptomic approach, provides an overview of venom’s major protein components, in order to understand the mechanism underlying the complex host/parasitoid interaction. The study of endo- and ectoparasitoid venoms, using these approaches, is the starting point for detailed knowledge of the molecular biology, evolution and effects of venom proteins in the host/parasitoid interactions. Indeed, although general physiological effects of Hymenoptera parasitoid venoms have been recorded, their exact composition is not completely known, also considering the high number of species and the differences among them. Specifically, in T. sinensis venom, a large number of proteins was identified, involved both in inhibition of the host immune system and in providing nutrients to the parasitoid progeny. Although additional in vivo studies are needed, this first view of venom protein components confirms how venom, combined with other maternal factors, helps ensure the success of parasitism.
Materials and methods
Insect rearing
Torymus sinensis adults were reared from galls of Dryocosmus kuriphilus collected in 26 sites within Cuneo province (NW Italy), where 40% of the forestry area is covered by sweet chestnut, Castanea sativa Mill. (Eudicots: Fagaceae) and nearly 10% by Quercus spp.213. This area has been widely infested by D. kuriphilus and includes the initial sites of T. sinensis release in Europe, performed in 2005. Chestnut trees were sampled from both mixed forests and chestnut orchards. Galls were randomly collected by hand from low branches and with the aid of lopping shears from the medium–high canopy, according to a previously described method36,214. They were separated from any non-gall plant material to avoid contamination by other insects not associated with the galls and then stored in containers outdoors in cardboard rearing boxes. Up to 2000 galls were kept in a container. Rearing boxes were checked once per week until the emergence of the first parasitoid wasp, then parasitoids were collected daily and their date of emergence recorded. All T. sinensis wasps were removed using an entomological pooter, then stored in 99% ethanol. All specimens were divided by sex by observing their morphological characters and then sent to the University of Basilicata’s laboratory for the subsequent analysis.
Venom gland collection
Before venom glands were collected, T. sinensis adult females were briefly (a few minutes) anesthetized on ice and subsequently placed in a phosphate-buffered saline solution (1X PBS) (Sigma, St. Louis, MO, USA) in a Petri dish (Sigma, St. Louis, MO, USA). A stereo microscope (Nikon, Tokyo, Japan) was used for dissections. The entire reproductive apparatus was removed with a pair of forceps and placed in a drop of 1X PBS solution. Subsequently, the venom glands were isolated and placed in a centrifuge tube (Eppendorf, Hamburg, Germany) containing TRI Reagent (Sigma, St. Louis, MO, USA) for the following RNA extraction and on slides for the subsequent microscope observations (Sigma, St. Louis, MO, USA).
Light microscopy
Venom glands were carefully transferred onto slides and fixed at room temperature for 10 min with 4% formaldehyde pH 6.9 (Carlo Erba, Milano, Italy). Then samples were observed using a Nikon Eclipse 80i (Instruments Europe, Amsterdam, The Netherlands) at 10X magnification. Images were recorded by a Nikon Digital Sight Ds-Fil camera (Nikon Instruments Europe).
RNA extraction
Total RNA was extracted from 360 venom glands, collected as previously described, using TRI Reagent following the manufacturer's instructions (Sigma, St. Louis, MO, USA). A Turbo DNase (Ambion Austin, TX, USA) treatment was carried out to eliminate any contaminating DNA. The DNase enzyme was then removed and the RNA was further purified using the RNeasy MinElute Clean up Kit (Qiagen, Venlo, The Netherlands) following the manufacturer's protocol and eluted in 20 µl of RNAse free water (Ambion Austin, TX, USA). RNA integrity was verified on an Agilent 2100 Bioanalyzer using the RNA Nano chips (Agilent Technologies, Palo Alto, CA), and RNA quantity was determined by a Nanodrop ND1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
RNAseq data generation and de novo transcriptome assembly
Tissue-specific transcriptome sequencing of the RNA sample was performed with poly(A) + enriched mRNA (New England Biolabs, Ipswich, MA, USA) fragmented to an average of 240 nucleotides. Sequencing was carried out by the Max Planck Genome Center (http://mpgc.mpipz.mpg.de/home/) using standard TruSeq procedures on an Illumina HiSeq2500 sequencer (Illumina., San Diego, CA, USA), generating ~ 42 Mio paired-end (2 × 100 bp) reads for the venom tissue sample. Quality control measures, including the filtering of high-quality reads based on the score given in fastq files, removal of reads containing primer/adaptor sequences and the trimming of read length, were carried out using CLC Genomics Workbench v9 (http://www.clcbio.com). The de novo transcriptome assembly was carried out using CLC Genomics Workbench v9 with standard settings and two additional CLC-based assemblies with different parameters and then selecting the presumed optimal consensus transcriptome, as previously described215. The transcriptome was annotated using BLAST, Gene Ontology and InterProScan with Blast2GO Pro version 4.1216. For BLASTx searches against the non-redundant NCBI protein database (nr database), up to 20 best NR hits per transcript were retained, with an E-value cutoff of ≤ 1E-3 and a minimum match length of 15 amino acids. To optimize annotation of the obtained data, we used GO slim, a subset of GO terms that provides a high level of annotations and allows a global view of the result. The functions "Gene Ontology Graphs" and "Enrichment Analysis" (Fisher's exact test) was used as part of OmicsBox (1.4.11) to identify the distribution of gene ontology (GO) terms as well as overrepresentation of GO terms among the T. sinensis venom protein dataset relative to the complete reference dataset (T. sinensis transcriptome assembly). The GO-enriched bar charts were reduced to display only the most specific GO terms by removing parent terms representing existing child terms using the function “Reduce to most specific terms” implemented in OmicsBox. A GO term was considered significantly enriched if the p-value corrected by false discovery rate control (FDR) was less than 0.05. To assess transcriptome completeness, we performed a BUSCO (Benchmarking Universal Single-Copy Orthologs; http://busco.ezlab.org) analysis by comparing our assembled transcriptome against a set of highly conserved single-copy orthologs. This was accomplished using the BUSCO v3 pipeline217, comparing the predicted proteins of the T. sinensis transcriptome to the predefined set of 1658 Insecta single-copy orthologs from the OrthoDB v9.1 database. This resulted in 91.9% complete and 5% missing BUSCO genes for the venom gland transcriptome assembly. The assembled and annotated venom gland transcriptome was used to generate a custom-made protein database. The six reading frames of the 22,874 nucleotide sequences were translated in their corresponding amino acid sequences by SEQtools software (http://www.seqtools.dk/).
Digital gene expression analysis
Digital gene expression analysis was carried out by using CLC Genomics workbench v9 (http://www.clcbio.com) to generate BAM (mapping) files and QSeq Software (DNAStar Inc., Madison, WI, USA) to remap the Illumina reads onto the reference transcriptome, and finally by counting the sequences to estimate expression levels, using previously described parameters for read-mapping and normalization218. In particular, the expression abundance of each contig was calculated based on the reads per kilobase per million mapped reads (RPKM) method218, using the formula: RPKM (A) ¼ (10,00,000 _ C _ 1000)/(N _ L), where RPKM (A) is the abundance of gene A, C is the number of reads that uniquely aligned to gene A, N is the total number of reads that uniquely aligned to all genes, and L is the number of bases in gene A.
Quantitative real time PCR (qPCR)
The relative expression in T. sinensis venom gland and female body deprived of venom gland of 10 genes selected among those reported in Table 1 was evaluated by a quantitative real time PCR (qPCR) (ABI PRISM R 7500 Fast Real-Time PCR System Thermal Cycler—Applied Biosystems, Foster City, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Beta-tubulin have been chosen as reference genes for normalization of qPCR data. Primers were designed using Primer3web (version 4.1.0) (Supplementary Table S11). PCR amplifications, with 30 ng of cDNA, were carried out using GoTaq qPCR Master Mix (Promega, Madison, WI, USA), following the manufacturer's instructions. Cycling programme was: 2 min at 95 °C, 40 cycles of 15 s at 95 °C and 1 min at 58 °C. Three technical replicates and three biological replicates were performed for each reaction. To evaluate gene expression levels, relative quantification was performed using equations described by Liu et al.219, based on PCR amplification efficiencies of reference and target genes. Amplification efficiency of each gene was calculated according to the equation E = 10–1/S—1 (S is the slope of the curve derived from three serial tenfold cDNA dilutions)220. The efficiencies of the amplicons were approximately equal. Quantification analysis of amplification was performed using the comparative ΔΔCt method221. Data were expressed as mean ± SEM (standard error of mean) of independent biological replicates and were compared by the Unpaired t-test using GraphPad Prism 6.00 software for Windows (GraphPad Software, La Jolla, CA, USA).
Phylogenetic analyses of T. sinensis trypsins
We inferred the species-specific diversification patterns of putative trypsins identified in the T. sinensis venom gland transcriptome in phylogenetic analyses. We used all predicted serine protease sequences from T. sinensis as query to search for homologs in the NCBI nr protein database using Blastp (E-value threshold of 10–5), identified the top 50 best Blast hits and removed redundant entries. Next, we removed partial sequences with less than 50% of the typical protease length. The corresponding protein sequences were aligned using MAFFT implemented in Geneious (v11.0.4) with FFT-NS-i × 1000 algorithm and BLOSUM62 scoring matrix. The alignments were trimmed manually. Maximum-likelihood phylogenetic trees were constructed in FastTree implemented in Geneious (v11.0.4) with 1000 ultrafast bootstrap replicates for the full dataset. The tree was visualized and processed in Figtree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree). Numbers next to the tree branches indicate the support values.
Collection of venom and SDS-PAGE electrophoresis
Wasps previously anesthetized on ice were submerged in 1 × PBS solution (Sigma, St. Louis, MO, USA) and their venom apparatus (venom glands and reservoir) was isolated. Each reservoir was gently opened with a dissecting needle in a drop of water (ratio 1 µl of water: 1 reservoir). The resulting crude extract was centrifuged at 5000g for 5 min at 4 °C, and the supernatant was used for electrophoretic analysis. For the proteome analysis, the venom from 30 T. sinensis females was collected for a total of 150 µg of protein. Protein quantity was measured using the Bradford method, with bovine serum albumin as standard222. An aliquot of venom proteins was loaded on a 12,5% polyacrylamide running gel on a Bio Rad Electrophoresis Cell Mini Protean II (Biorad, Hercules, CA, USA). After the run, the gel was stained with colloidal Coomassie Blue G-250 (Sigma, St. Louis, MO, USA) for 1 h and the excess dye was removed by washing in deionized water for 12 h.
In situ protein digestion
After electrophoresis and staining, whole lanes were cut in 35 bands. The bands were in situ hydrolysed by trypsin as reported in Medugno et al.223. Briefly, gel bands were destained by washes in acetonitrile (ACN) (Honeywell, Charlotte, NC, USA), and 50 mM ammonium bicarbonate (NH4HCO3). Cysteine residues involved in disulphide bridges were reduced by 10 mM of dithiothreitol (Sigma, St. Louis, MO, USA) in NH4HCO3 for 45 min at 56 °C and then alkylated in 55 mM iodoacetamide (Sigma, St. Louis, MO, USA) for 30 min. The excess reagents were finally removed by washing with ACN and 50 mM NH4HCO3 alternatively. The dehydrated gel bands were then treated overnight with trypsin and peptide mixtures extracted in 0.2% HCOOH and ACN. The obtained mixtures were vacuum dried by a Savant SpeedVac System (Thermo Fisher Scientific, Waltham, MA, USA).
LC–MS/MS and protein identification
Each peptide mixture was resuspended in 10 μl of 0.2% HCOOH (Sigma, St. Louis, Missouri, USA) and analyzed by nanoLC–MS/MS on a LTQ Orbitrap mass spectrometer equipped with a nano HPLC system (Thermo Fisher Scientific, Waltham, MA, USA). After loading, the peptide mixture was first concentrated and desalted in the precolumn (C18 Easy Column L = 2 cm, ID = 100 mm, Thermo Fisher Scientific Waltham, MA, USA). Each peptide sample was then fractionated on a C18 reverse-phase capillary column (C18 Easy Column L = 20 cm, ID = 7.5 µm, 3 µm, (Thermo Fisher Scientific Waltham, MA, USA) working at a flow rate of 250 nl/min. The gradient used for peptide elution ranged from 5 to 95% of buffer B. Buffers A and B have the following composition:—buffer A, 2% ACN LC–MS grade and 0.2% HCOOH;—buffer B, 95% ACN LC–MS grade and 0.2% HCOOH. The MS/MS method was set up in a data-dependent acquisition mode, with a full scan ranging from 400 to 1800 m/z range, followed by fragmentation in CID modality of the top 10 ions (MS/MS scan) selected on the basis of intensity and charge state (+ 2, + 3, + 4 charges). An exclusion time of 40 s was applied to avoid the repetitive fragmentation of the same signals over a 40 s interval and to increase the number of fragmented peptides and, therefore, the number of available protein identification sequences. The raw files obtained from this analysis were used as inputs in the Andromeda search engine. The peak list generated was uploaded in Andromeda software and a research was performed using the “T. sinensis protein database.” These parameters were fixed: “trypsin” as an enzyme allowing up to 2 missed cleavages, carbamidomethyl as a fixed modification, oxidation of M, pyroGlu N-term Q, as variable modifications, 0.5 Da MS/MS tolerance, 10 ppm peptide tolerance. Scores used to evaluate the quality of matches for MS/MS data were higher than 10 for unmodified peptides, otherwise 40.
Transcriptomic and proteomic data analysis
Putative venom proteins in the venom gland transcriptome were identified using a key-word approach: all proteins annotated with the word “venom” or “toxin” were selected. Proteins identified with the proteomic and the transcriptomic approach were analyzed using the Signal P 5.0 software (http://www.cbs.dtu.dk/services/SignalP/) in order to pick out those with signal peptide. Then, a second filter was applied, consisting of the alignment of the amino acid sequences of these proteins with venom proteins of Nasonia vitripennis identified by de Graaf et al. 201030. The N. vitripennis database was used as reference because the top BLAST Hit Species Distribution showed a high level of matching with T. sinensis. The alignments were made using the software BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins). Most of these proteins have been updated in recent years, so the final amino acid sequences were identified in the NCBI protein database by using accession numbers or amino acid sequences.
Supplementary Information
Acknowledgements
We would like to thank Emily Wheeler for editorial assistance.
Author contributions
Conceptualization, P.F.; data curation, C.S. and R.S.; methodology, C.S., R.S., A.F., F.C., C.F., M.P., H.V. and P.F.; project administration, P.F.; supervision, P.F.; validation, H.V., P.P., A.A. and P.F.; writing—original draft, P.F.; writing—review and editing, C.S., R.S., A.F., M.P., F.C., M.C., Ch.Sc., H.V., M.M., C.F., P.P., A.A. and P.P.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Carmen Scieuzo and Rosanna Salvia.
Contributor Information
Heiko Vogel, Email: hvogel@ice.mpg.de.
Patrizia Falabella, Email: patrizia.falabella@unibas.it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84385-5.
References
- 1.Scudder, G.G.E. The Importance of Insects in Insect Biodiversity: Science and Society. (eds. Foottit, R.G. and Adler, P.H.) (Wiley Blackwell, 2017).
- 2.Stork NE. How many species of insects and other terrestrial arthropods are there on earth? Annu. Rev. Entomol. 2018;63:31–45. doi: 10.1146/annurev-ento-020117-043348. [DOI] [PubMed] [Google Scholar]
- 3.Vogel E, Santos D, Mingels L, Verdonckt TW, Broeck JV. RNA Interference in insects: Protecting beneficials and controlling pests. Front. Physiol. 2019;9:1912. doi: 10.3389/fphys.2018.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZZ, Liu YQ, Shi M, Huang JH, Chen XX. Parasitoid wasps as effective biological control agents. J. Integr. Agr. 2019;18:705–715. doi: 10.1016/S2095-3119(18)62078-7. [DOI] [Google Scholar]
- 5.Dani MP, Richards EH, Isaac RE, Edwards JP. Antibacterial and proteolytic activity in venom from the endoparasitic wasp Pimpla hypochondriaca (Hymenoptera: Ichneumonidae) J. Insect Physiol. 2003;49:945–954. doi: 10.1016/S0022-1910(03)00163-X. [DOI] [PubMed] [Google Scholar]
- 6.Bordon KC, Wiezel GA, Amorim FG, Arantes EC. Arthropod venom Hyaluronidases: biochemical properties and potential applications in medicine and biotechnology. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2015;21:43. doi: 10.1186/s40409-015-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas AE. Strategies for enhanced crop resistance to insect pests. Annu. Rev. Plant. Biol. 2018;69:637–660. doi: 10.1146/annurev-arplant-042817-040248. [DOI] [PubMed] [Google Scholar]
- 8.Hoy, M.A. Natural enemies important in biological control in Encyclopedia of Entomology (ed. Capinera, J.L.) 2555–2564 (Springer, 2008).
- 9.Kalyanasundaram, M. & Merlin Kamala. Parasitoids in Ecofriendly Pest Management for Food Security. (ed. Omkar) 109–138 (Academic Press, Elsevier Inc 2016).
- 10.Pennacchio F, Strand MR. Evolution of developmental strategies in parasitic hymenoptera. Ann. Rev. Entomol. 2006;51:233–258. doi: 10.1146/annurev.ento.51.110104.151029. [DOI] [PubMed] [Google Scholar]
- 11.Moreau SJ, Asgari S. Venom proteins from parasitoid wasps and their biological functions. Toxins. 2015;7:2385–2412. doi: 10.3390/toxins7072385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamatsu, Y. & Tanaka, T. Venom of ectoparasitoid, Euplectrus sp. near plathypenae (Hymenoptera: Eulophidae) regulates the physiological state of Pseudaletia separata (Lepidoptera: Noctuidae) host as a food resource. J. Insect Physiol.49, 149–159 (2003). [DOI] [PubMed]
- 13.Falabella P, et al. A Γ-glutamil transpeptidase of Aphidius ervi, induces apoptosis in the ovaries of host aphids. Insect Biochem. Mol. Biol. 2007;37:453–465. doi: 10.1016/j.ibmb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhu JY, Ye GY, Dong SZ, Fang Q, Hu C. Venom of Pteromalus puparum (Hymenoptera: Pteromalidae) induced endocrine changes in the hemolymph of its host, Pieris rapae (Lepidoptera: Pieridae) Arch. Insect Biochem. Physiol. 2009;71:45–53. doi: 10.1002/arch.20304. [DOI] [PubMed] [Google Scholar]
- 15.Desneux N, Barta RJ, Delebecque CJ, Heimpel GE. Transient host paralysis as a means of reducing self-superparasitism in koinobiont endoparasitoids. J. Insect Physiol. 2009;55:321–327. doi: 10.1016/j.jinsphys.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Zhu, J. Y., Ye, G. Y., Dong, S. Z., & Hu, C. Venom of the endoparasitoid wasp pteromalus puparum: An overview. Psyche: J. Entomol. 2011, 520926 (2011)
- 17.Quistad GB, Nguyen Q, Bernasconi P, Leisy DJ. Purification and characterization of insecticidal toxins from venom glands of the parasitic wasp Bracon hebetor. Insect Biochem. Mol. Biol. 1994;24:955–961. doi: 10.1016/0965-1748(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 18.Laurino S, et al. Identification of major Toxoneuron nigriceps venom proteins using an integrated transcriptomic/proteomic approach. Insect Biochem. Mol. Biol. 2016;76:49–61. doi: 10.1016/j.ibmb.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Colinet D, Schmitz A, Depoix D, Crochard D, Poirie M. Convergent use of RhoGAP toxins by eukaryotic parasites and bacterial pathogens. PLoS Pathog. 2007;3:203. doi: 10.1371/journal.ppat.0030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, et al. Identification and comparative analysis of venom proteins in a pupal ectoparasitoid Pachycrepoideus vindemmiae. Front. Physiol. 2020;11:9. doi: 10.3389/fphys.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battaglia D, et al. The effect of Leptomastix dactylopii parasitism and venom injection on host Planococcus citri. Invert. Surviv. J. 2014;11:273–285. [Google Scholar]
- 22.Labella C, et al. Identification of two Arginine Kinase forms of endparasitoid Leptomastix dactylopii venom by bottom up-sequence tag approach. J. Mass Spectrom. 2015;50:756–765. doi: 10.1002/jms.3585. [DOI] [PubMed] [Google Scholar]
- 23.Falabella P. The mechanism utilized by Toxoneuron nigriceps in inhibiting the host immune system. Invert. Surviv. J. 2018;15:240–255. [Google Scholar]
- 24.Parkinson N, Conyers C, Smith I. A venom protein from the endoparasitoid wasp Pimpla hypochondriaca is similar to snake venom reprolysin-type metalloproteases. J. Invertebr. Pathol. 2002;79:129–131. doi: 10.1016/S0022-2011(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 25.Moore EL, Haspel G, Libersat F, Adams ME. Parasitoid wasp sting: A cocktail of GABA, taurine, and beta-alanine opens chloride channels for central synaptic block and transient paralysis of a cockroach host. J. Neurobiol. 2006;66:811–820. doi: 10.1002/neu.20254. [DOI] [PubMed] [Google Scholar]
- 26.Goecks J, et al. Integrative approach reveals composition of endoparasitoid wasp venoms. PLoS ONE. 2013;8:e64125. doi: 10.1371/journal.pone.0064125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan ZC, et al. Into the venom composition and evolution of an endoparasitoid wasp by combining proteomic and transcriptomic analyses. Sci. Rep. 2016;6:19604. doi: 10.1038/srep19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng ZW, et al. Protein discovery: Combined transcriptomic and proteomic analyses of venom from the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae) Toxins. 2017;9:135. doi: 10.3390/toxins9040135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, et al. Identification and characterization of serine protease inhibitors in a parasitic wasp Pteromalus puparum. Sci. Rep. 2017;7:15755. doi: 10.1038/s41598-017-16000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Graaf DC, et al. Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. Insect Mol. Biol. 2010;19:11–26. doi: 10.1111/j.1365-2583.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkin LC, Friesen KS, Flinn PW, Oppert B. Venom gland components of the ectoparasitoid wasp Anisopteromalus calandrae. J. Venom Res. 2015;6:19–37. [PMC free article] [PubMed] [Google Scholar]
- 32.Becchimanzi A, et al. Venomics of the ectoparasitoid wasp Bracon nigricans. BMC Genomics. 2020;21:34. doi: 10.1186/s12864-019-6396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duretre, S. et al. Venoms-based drug discovery: proteomic and transcriptomic approaches in Venoms to Drugs (ed. Glenn, K.F) 80–96 (Royal Society of Chemistry, 2015).
- 34.Moriya, S., Shiga, M. & Adachi, I. Classical biological control of the chestnut gall wasp in Japan in Proceedings of the 1st international symposium on biological control of arthropods (ed. Van Driesche RG) 407–415 (USDA Forest Service) (2003).
- 35.Alma A, Ferracini C, Sartor C, Ferrari E, Botta R. Il cinipide orientale del castagno: lotta biologica e sensibilità varietale. Italus Hortus. 2014;21:15–29. [Google Scholar]
- 36.Ferracini C, et al. Non-target host risk assessment for the parasitoid Torymus sinensis. Biocontrol. 2015;60:583–594. doi: 10.1007/s10526-015-9676-1. [DOI] [Google Scholar]
- 37.Cooper WR, Rieske LR. Community associates of an exotic gallmarker, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), in eastern north America. Ann. Entomol. Soc. Am. 2007;100:236–244. doi: 10.1603/0013-8746(2007)100[236:CAOAEG]2.0.CO;2. [DOI] [Google Scholar]
- 38.Quacchia A, Moriya S, Bosio G, Scapin I, Alma A. Rearing, release and settlement prospect in Italy of Torymus sinensis, the biological control agent of the chestnut gall wasp Dryocosmus kuriphilus. Biocontrol. 2008;53:829–839. doi: 10.1007/s10526-007-9139-4. [DOI] [Google Scholar]
- 39.Matošević DN, et al. Success of classical biocontrol agent Torymus sinensis within its expanding range in Europe. J. Appl. Entomol. 2017 doi: 10.1111/jen.12388. [DOI] [Google Scholar]
- 40.Ferracini C, Ferrari E, Pontini M, Saladini MA, Alma A. Effectiveness of Torymus sinensis: A successful long-term control of the Asian chestnut gall wasp in Italy. J. Pest. Sci. 2019;92:353–359. doi: 10.1007/s10340-018-0989-6. [DOI] [Google Scholar]
- 41.Johnson, J.H., Kral, R.M. & Krapcho, K Jr. Insecticidal toxins from Bracon hebetor nucleic acid encoding said toxin and methods of use. US Patent No. 5874298A (1999).
- 42.Ullah MI, et al. Arthropods venom used as bio-pesticides: A new challenge to manage insect pests. Int. J. Agric. Appl. Sci. 2017;9:122–131. [Google Scholar]
- 43.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic. Acids. Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox J, et al. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 45.Özbek R, et al. Proteo-transcriptomic characterization of the venom from the endoparasitoid wasp Pimpla turionellae with aspects on its biology and evolution. Toxins. 2019;11:721. doi: 10.3390/toxins11120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Oliveira, U. C., et al. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PLoS One.13, e0193739 (2018). [DOI] [PMC free article] [PubMed]
- 47.Lin Z, et al. Insights into the venom protein components of Microplitis mediator, an endoparasitoid wasp. Insect Biochem. Mol. Biol. 2019;105:33–42. doi: 10.1016/j.ibmb.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Moreau SJM, Guillot S. Advances and prospects on biosynthesis, structures and functions of venom proteins from parasitic wasps. Insect Biochem. Molec. Biol. 2005;35:1209–1223. doi: 10.1016/j.ibmb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Colinet D, et al. Identification of the main venom protein components of Aphidius ervi, a parasitoid wasp of the aphid model Acyrthosiphon pisum. BMC Genomics. 2014;15:342. doi: 10.1186/1471-2164-15-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asgari S, Zhang G, Zareie R, Schmidt O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect. Biochem. Mol. Biol. 2003;33:1017–1012. doi: 10.1016/S0965-1748(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 51.Zhang G, Lu ZQ, Jiang H, Asgari S. Negative regulation of prophenoloxidase (proPO) activation by a clip-domain serine proteinase homolog (SPH) from endoparasitoid venom. Insect Biochem. Mol. Biol. 2004;34:477–483. doi: 10.1016/j.ibmb.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Thomas P, Asgari S. Inhibition of melanization by a parasitoid serine protease homolog venom protein requires both the clip and the non-catalytic protease-like domains. Insects. 2011;2:509–514. doi: 10.3390/insects2040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu XQ, Jiang H, Wang Y, Kanost MR. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm Manduca sexta. Insect Biochem. Mol. Biol. 2003;33:197–208. doi: 10.1016/S0965-1748(02)00191-1. [DOI] [PubMed] [Google Scholar]
- 54.Danneels EL, Rivers DB, de Graaf DC. Venom proteins of the parasitoid wasp Nasonia vitripennis: Recent discovery of an untapped pharmacopee. Toxins. 2010;2:494–516. doi: 10.3390/toxins2040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choo YM, et al. Dual function of a bee venom serine protease: Prophenoloxidase-activating factor in arthropods and fibrin(ogen)olytic enzyme in mammals. PLoS ONE. 2010;5:e10393. doi: 10.1371/journal.pone.0010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Formesyn EM, Heyninck K, de Graaf DC. The role of serine- and metalloproteases in Nasonia vitripennis venom in cell death related processes towards a Spodoptera frugiperda Sf21 cell line. J. Insect Physiol. 2013;59:795–803. doi: 10.1016/j.jinsphys.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Nakamatsu Y, Tanaka T. The function of a trypsin-like enzyme in the saliva of Euplectrus separatae larvae. J. Insect Physiol. 2004;50:847–854. doi: 10.1016/j.jinsphys.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Bao YY, et al. Genomic insights into the serine protease gene family and expression profile analysis in the planthopper Nilaparvata lugens. BMC Genomics. 2014;21:507. doi: 10.1186/1471-2164-15-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kini RM, Koh CY. Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins. 2016;8:284. doi: 10.3390/toxins8100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fingleton B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta. Mol. Cell. Res. 2017;1864:2036–2042. doi: 10.1016/j.bbamcr.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Willott E, Tran HQ. Zinc and Manduca sexta hemocyte functions. J. Insect Sci. 2002;2:6. doi: 10.1673/031.002.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Z, et al. A metalloprotease homolog venom protein from a parasitoid wasp suppresses the toll pathway in host hemocytes. Front. Immunol. 2018;9:2301. doi: 10.3389/fimmu.2018.02301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Price DRG, et al. A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae) Insect Mol. Biol. 2009;18:195–202. doi: 10.1111/j.1365-2583.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- 64.Zadka L, Kulus MJ, Piatek K. ADAM protein family: Its role in tumorigenesis, mechanisms of chemoresistance and potential as diagnostic and prognostic factors. Neoplasma. 2018;65:823–839. doi: 10.4149/neo_2018_171220N832. [DOI] [PubMed] [Google Scholar]
- 65.Rawlings, N.D. & Salvesen, G. Handbook of Proteolytic Enzymes. (eds. Rawlings, N.D. & Salvesen, G) e1-e39 (Academic Press, 2013).
- 66.Aird SD. Snake venom dipeptidyl peptidase IV: Taxonomic distribution and quantitative variation. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2008;150:222–228. doi: 10.1016/j.cbpb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Blank S, et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J. Immunol. 2010;184:5403–5413. doi: 10.4049/jimmunol.0803709. [DOI] [PubMed] [Google Scholar]
- 68.Hsieh SK, et al. Functional expression and characterization of dipeptidyl peptidase IV from the black-bellied hornet Vespa basalis in Sf21 insect cells. Biosci. Biotechnol. Biochem. 2011;75:2371–2375. doi: 10.1271/bbb.110571. [DOI] [PubMed] [Google Scholar]
- 69.Schiener M, et al. The high molecular weight dipeptidyl peptidase IV Pol d 3 is a major allergen of Polistes dominula venom. Sci. Rep. 2018;8:1318. doi: 10.1038/s41598-018-19666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreil L, Haiml L, Suchanek G. Stepwise cleavage of the pro part of promelittin bydipeptidylpeptidase-IV – evidence for a new type of precusor-product conversion. Eur. J. Biochem. 1980;111:49–58. doi: 10.1111/j.1432-1033.1980.tb06073.x. [DOI] [PubMed] [Google Scholar]
- 71.Borloo M, De Meester I. Dipeptidyl peptidase IV: development, design, synthesis and biological evaluation of inhibitors. Verh. K. Acad. Geneeskd. Belg. 1994;56:57–88. [PubMed] [Google Scholar]
- 72.Dos Santos DM, de Souza CB, Pereira HJ. Angiotensin converting enzymes in fish venom. Toxicon. 2017;131:63–67. doi: 10.1016/j.toxicon.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 73.Ogawa Y, Murayama N, Fujita Y, Yanoshita R. Characterization and cDNA cloning of aminopeptidase A from the venom of Gloydius blomhoffi brevicaudus. Toxicon. 2007;49:1172–1181. doi: 10.1016/j.toxicon.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Vaiyapuri S, et al. Purification and functional characterisation of rhiminopeptidase A, a novel aminopeptidase from the venom of Bitis gabonicarhinoceros. PLoS Negl. Trop. Dis. 2010;4:e796. doi: 10.1371/journal.pntd.0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santos PP, et al. Proteomic analysis of the venom of the predatory ant Pachycondyla striata (Hymenoptera: Formicidae) Arch. Insect Biochem. Physiol. 2017;96:e21424. doi: 10.1002/arch.21424. [DOI] [PubMed] [Google Scholar]
- 76.Sanggaard KW, et al. Spider genomes provide insight into composition and evolution of venom and silk. Nat. Commun. 2014;5:3765. doi: 10.1038/ncomms4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barkan NP, Bayazit MB, Ozel Demiralp D. Proteomic Characterization of the Venom of Five Bombus (Thoracobombus) Species. Toxins. 2017;9:362. doi: 10.3390/toxins9110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reaux A, et al. Aminopeptidase A, which generates one of the main effector peptides of the brain renin-angiotensin system, angiotensin III, has a key role in central control of arterial blood pressure. Biochem. Soc. Trans. 2000;28:435–440. [PubMed] [Google Scholar]
- 79.Marchiò S, et al. Aminopeptidase A is a functional target in angiogenic blood vessels. Cancer Cell. 2004;5:151–162. doi: 10.1016/S1535-6108(04)00025-X. [DOI] [PubMed] [Google Scholar]
- 80.Brix, K. Lysosomal Proteases: Revival of the Sleeping Beauty in Lysosomes (ed. Saftig, P.) (Springer, 2005).
- 81.Benes P, Vetvicka V, Fusek M. Cathepsin D-many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heavner ME, et al. Partial venom gland transcriptome of a Drosophila parasitoid wasp, Leptopilina heterotoma, reveals novel and shared bioactive profiles with stinging Hymenoptera. Gene. 2013;526:195–204. doi: 10.1016/j.gene.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xin B, Liu P, Xu X, Zhang S, Zheng Y. Identification of venom proteins of the indigenous endoparasitoid Chouioia cunea (Hymenoptera: Eulophidae) J. Econ. Entomol. 2017;110:2022–2030. doi: 10.1093/jee/tox200. [DOI] [PubMed] [Google Scholar]
- 84.Danneels EL, et al. Early changes in the pupal transcriptome of the flesh fly Sarcophagha crassipalpis to parasitization by the ectoparasitic wasp Nasonia vitripennis. Insect Biochem. Mol. Biol. 2013;43:1189–1200. doi: 10.1016/j.ibmb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Stoka V, Turk B, Turk V. Lysosomal cysteine proteases: structural features and their role in apoptosis. IUBMB Life. 2005;57:347–353. doi: 10.1080/15216540500154920. [DOI] [PubMed] [Google Scholar]
- 86.Zhou J, Zhang YY, Li QY, Cai ZH. Evolutionary History of Cathepsin L (L-like) Family Genes in Vertebrates. Int. J. Biol. Sci. 2015;11:1016–1025. doi: 10.7150/ijbs.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cristofoletti PT, Ribeiro AF, Deraison C, Rahbe Y, Terra WR. Midgut adaptation and digestive enzyme distribution in a phloem feeding insect, the pea aphid Acythosiphon pisum. J. Insect Physiol. 2003;49:11–24. doi: 10.1016/S0022-1910(02)00222-6. [DOI] [PubMed] [Google Scholar]
- 88.Becchimanzi A, et al. Host regulation by the ectophagous parasitoid wasp Bracon nigricans. J. Insect Physiol. 2017;101:73–81. doi: 10.1016/j.jinsphys.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 89.Appel, W. Carboxypeptidases in Methods of enzymatic analysis (ed. Bergermeyer H.U.) 986–988 (Academic Press, 1974).
- 90.Tani N, et al. Mass spectrometry analysis and biological characterization of the predatory ant odontomachus monticola venom and venom sac components. Toxins. 2019;11:50. doi: 10.3390/toxins11010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouzid W, et al. De Novo sequencing and transcriptome analysis for Tetramorium bicarinatum: A comprehensive venom gland transcriptome analysis from an ant species. BMC Genomics. 2014;15:987. doi: 10.1186/1471-2164-15-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melani RD, et al. Seeing beyond the tip of the iceberg: a deep analysis of the venome of the Brazilian rattlesnake Crotalus durissus terrificus. EuPA Open Proteom. 2015;8:144–156. doi: 10.1016/j.euprot.2015.05.006. [DOI] [Google Scholar]
- 93.Hubert HM, et al. Comparative venomics of Psyttalia lounsburyi and P. concolor, two olive fruit fly parasitoids: a hypothetical role for a GH1 β-glucosidase. Sci. Rep. 2016;6:35873. doi: 10.1038/srep35873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim BY, Jin BR. Molecular characterization of a venom acid phosphatase Acph-1-like protein from the Asiatic honeybee Apis cerana. J. Asia-Pacific Entomol. 2014;17:695–700. doi: 10.1016/j.aspen.2014.07.002. [DOI] [Google Scholar]
- 95.Hossen MS, Shapla UM, Gan SH, Khalil MI. Impact of bee venom enzymes on diseases and immune responses. Molecules. 2016;22:25. doi: 10.3390/molecules22010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dani MP, Edwards JP, Richards EH. Hydrolase activity in the venom of the pupal endoparasitic wasp, Pimpla hypochondriaca. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2005;141:373–381. doi: 10.1016/j.cbpc.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Zhu JY, Ye GY, Hu C. Molecular cloning and characterization of acid phosphatase in venom of the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae) Toxicon. 2008;51:1391–1399. doi: 10.1016/j.toxicon.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 98.Manzoor, A., UlAbdin, Z., Webb, B. A., Arif, M. J. & Jamil, A. De novo sequencing and transcriptome analysis of female venom glands of ectoparasitoid Bracon hebetor (Say.) (Hymenoptera: Braconidae). Comp. Biochem. Physiol. Part D Genomics Proteomics.20, 101–110 (2016). [DOI] [PubMed]
- 99.Yokoi K, et al. The major constituents of the venom gland of a braconid endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae) Appl. Entomol. Zool. 2017;52:271–285. doi: 10.1007/s13355-016-0476-6. [DOI] [Google Scholar]
- 100.Hoffman DR. Hymenoptera venom allergens. Clin. Rev. Allergy Immunol. 2006;30:109–128. doi: 10.1385/CRIAI:30:2:109. [DOI] [PubMed] [Google Scholar]
- 101.Aranda J, et al. The catalytic mechanism of carboxylesterases: A computational study. Biochemistry. 2014;53:5820–5829. doi: 10.1021/bi500934j. [DOI] [PubMed] [Google Scholar]
- 102.Matysiak J, Hajduk J, Pietrzak L, Schmelzer CE, Kokot ZJ. Shotgun proteome analysis of honeybee venom using targeted enrichment strategies. Toxicon. 2014;90:255–264. doi: 10.1016/j.toxicon.2014.08.069. [DOI] [PubMed] [Google Scholar]
- 103.Sahu, A. & Birner-Gruenberger, R. Lipases in Encyclopedia of Metalloproteins (eds. Kretsinger, R.H., Uversky, V.N. & Permyakov, E.A.) (Springer, 2013).
- 104.Rivers DB, Denlinger DL. Venom-induced alterations in fly lipid-metabolism and its impact on larval development of the ectoparasitoid Nasonia vitripennis (Walker) (Hymenoptera, Pteromalidae) J. Invertebr. Pathol. 1995;66:104–110. doi: 10.1006/jipa.1995.1071. [DOI] [Google Scholar]
- 105.Crawford, A. M. et al. The constituents of Microctonus sp. parasitoid venoms. Insect. Mol. Biol.17, 313–324 (2008). [DOI] [PubMed]
- 106.Cusumano A, et al. First extensive characterization of the venom gland from an egg parasitoid: Structure, transcriptome and functional role. J. Insect Physiol. 2018;107:68–80. doi: 10.1016/j.jinsphys.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Nakamatsu Y, Fujii S, Tanaka T. Larvae of an endoparasitoid, Cotesia kariyai (Hymenoptera: Braconidae), feed on the host fat body directly in the second stadium with the help of teratocytes. J. Insect Physiol. 2002;48:1041–1052. doi: 10.1016/S0022-1910(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 108.Nakamatsu Y, Tanaka T. Venom of Euplectrus separatae causes hyperlipidemia by lysis of host fat body cells. J. Insect Physiol. 2004;50:267–275. doi: 10.1016/j.jinsphys.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Ketudat Cairns JR, Esen A. β-glucosidases. Cell. Mol. Life Sci. 2010;67:3389–3405. doi: 10.1007/s00018-010-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burke GR, Strand MR. Systematic analysis of a wasp parasitism arsenal. Mol. Ecol. 2014;23:890–901. doi: 10.1111/mec.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Juers DH, Matthews BW, Huber RE. LacZ β-galactosidase: structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 2012;21:1792–1807. doi: 10.1002/pro.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Da Lage JL. The amylases of insects. Int. J. Insect Sci. 2018;10:1–14. doi: 10.1177/1179543318804783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Werren JH, et al. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2010;327:343–348. doi: 10.1126/science.1178028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang B, et al. Venom α-amylase of the endoparasitic wasp Pteromalus puparum influences host metabolism. Pest. Manag. Sci. 2020;76:2180–2189. doi: 10.1002/ps.5755. [DOI] [PubMed] [Google Scholar]
- 115.Kote S, et al. Analysis of venom sac constituents from the solitary, aculeate wasp Cerceris rybyensis. Toxicon. 2019;169:1–4. doi: 10.1016/j.toxicon.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 116.Arvidson R, et al. Parasitoid jewel wasp mounts multipronged neurochemical attack to hijack a host brain. Mol. Cell. Proteomics. 2019;18:99–114. doi: 10.1074/mcp.RA118.000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parkinson NM, et al. cDNAs encoding large venom proteins from the parasitoid wasp Pimpla hypochondriaca identified by random sequence analysis. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2003;134:513–520. doi: 10.1016/S1532-0456(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 118.Sim AD, Wheeler D. The venom gland transcriptome of the parasitoid wasp Nasonia vitripennis highlights the importance of novel genes in venom function. BMC Genomics. 2016;17:571. doi: 10.1186/s12864-016-2924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dongol, Y., Dhananjaya, D.L., Shrestha, R.K. & Aryal, G. Pharmacological and immunological properties of wasp venom in Pharmacology and Therapeutics (ed. Sivakumar J.T.G.) (47–81) (IntechOpen, 2014).
- 120.Kramer KJ, Koga D. Insect chitin: Physical state, synthesis, degradation and metabolic regulation. Insect Biochem. 1986;16:851–877. doi: 10.1016/0020-1790(86)90059-4. [DOI] [Google Scholar]
- 121.Liberato T, Troncone LRP, Yamashiro ET, Serrano SMT, Zelanis A. High-resolution proteomic profiling of spider venom: Expanding the toxin diversity of Phoneutria nigriventer venom. Amino Acids. 2016;48:901–906. doi: 10.1007/s00726-015-2151-6. [DOI] [PubMed] [Google Scholar]
- 122.Fuzita FJ, et al. Biochemical, transcriptomic and proteomic analyses of digestion in the scorpion Tityus serrulatus: Insights into function and evolution of digestion in an ancient arthropod. PLoS ONE. 2015;10:e0123841. doi: 10.1371/journal.pone.0123841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Consoli FL, Lewis D, Keeley L, Vinson SB. Characterization of a cDNA encoding a putative chitinase from teratocytes of the endoparasitoid Toxoneuron nigriceps. Entomol. Exp Appl. 2007;122:271–278. doi: 10.1111/j.1570-7458.2006.00514.x. [DOI] [Google Scholar]
- 124.Vincent B, et al. The venom composition of the parasitic wasp Chelonus inanitus resolved by combined expressed sequence tags analysis and proteomic approach. BMC Genomics. 2010;11:693. doi: 10.1186/1471-2164-11-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dhananjaya BL, D’Souza CJ. An overview on nucleases (DNase, RNase, and phosphodiesterase) in snake venoms. Biochemistry (Mosc). 2010;75:1–6. doi: 10.1134/S0006297910010013. [DOI] [PubMed] [Google Scholar]
- 126.Neeman I, Calton GJ, Burnett JW. Purification and characterization of the endonuclease present in Physalia physalis venom. Comp. Biochem. Phys. B. 1980;67:155–158. doi: 10.1016/0305-0491(80)90286-2. [DOI] [Google Scholar]
- 127.Miller MD, Tanner J, Alpaugh M, Benedik MJ, Krause KL. A structure of Serratia endonuclease suggests a mechanism for binding to double-stranded DNA. Nat. Struct. Biol. 1994;1:461–468. doi: 10.1038/nsb0794-461. [DOI] [PubMed] [Google Scholar]
- 128.Gopaul DN, Meyer SL, Degano M, Sacchettini JC, Schramm VL. Inosine-uridine nucleoside hydrolase from Crithidia fasciculata. Genetic characterization, crystallization, and identification of histidine 241 as a catalytic site residue. Biochemistry. 1996;35:5963–5970. doi: 10.1021/bi952998u. [DOI] [PubMed] [Google Scholar]
- 129.Paulson AR, et al. Transcriptome analysis provides insight into venom evolution in a seed-parasitic wasp Megastigmus spermotrophus. Insect Mol. Biol. 2016;25:604–616. doi: 10.1111/imb.12247. [DOI] [PubMed] [Google Scholar]
- 130.Lee, C. C., Hsieh, H. J., Hsieh, C. H. & Hwang, D. F. Plancitoxin I from the venom of crown-of-thorns starfish (Acanthaster planci) induces oxidative and endoplasmic reticulum stress associated cytotoxicity in A375.S2 cells. Exp. Mol. Pathol.99, 7–15 (2015). [DOI] [PubMed]
- 131.Dennis AB, Patel V, Oliver KM, Vorburger C. Parasitoid gene expression changes after adaptation to symbiont-protected hosts. Evolution. 2017;71:2599–2617. doi: 10.1111/evo.13333. [DOI] [PubMed] [Google Scholar]
- 132.Colinet D, et al. A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Dev. Comp Immunol. 2009;33:681–689. doi: 10.1016/j.dci.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 133.Yan Z, et al. A venom serpin splicing isoform of the endoparasitoid wasp Pteromalus puparum suppresses host prophenoloxidase cascade by forming complexes with host hemolymph proteinases. J. Biol. Chem. 2017;292:1038–1051. doi: 10.1074/jbc.M116.739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gettins PG. Serpin structure, mechanism, and function. Chem. Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 135.Kanost, M.R. & Gorman, M.J. Phenoloxidases in Insect immunology (ed. Beckage, N.E) 69–96 (Academic Press, 2008).
- 136.Niimi T, Yokoyama H, Goto A, Beck K, Kitagawa Y. A Drosophila gene encoding multiple splice variants of Kazal-type serine protease inhibitor-like proteins with potential destinations of mitochondria, cytosol and the secretory pathway. Eur. J. Biochem. 1999;266:282–292. doi: 10.1046/j.1432-1327.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 137.Zheng, Q. L. et al. Expression, purification and characterization of a three-domain Kazal-type inhibitor from silkworm pupae (Bombyx mori). Comp. Biochem. Physiol. B Biochem. Mol. Biol.146, 234–240 (2007). [DOI] [PubMed]
- 138.Campos IT, Tanaka-Azevedo AM, Tanaka AS. Identification and characterization of a novel factor XIIa inhibitor in the hematophagous insect, Triatoma infestans (Hemiptera: Reduviidae) FEBS Lett. 2004;577:512–516. doi: 10.1016/j.febslet.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 139.Qian C, Fang Q, Wang L, Ye GY. Molecular cloning and functional studies of two kazal-type serine protease inhibitors specifically expressed by Nasonia vitripennis venom apparatus. Toxins. 2015;7:2888–2905. doi: 10.3390/toxins7082888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beck M, Theopold U, Schmidt O. Evidence for serine protease inhibitor activity in the ovarian calyx fluid of the endoparasitoid Venturia canescens. J. Insect Physiol. 2000;46:1275–1283. doi: 10.1016/S0022-1910(00)00048-2. [DOI] [PubMed] [Google Scholar]
- 141.Lu A, et al. Insect prophenoloxidase: the view beyond immunity. Front. Physiol. 2014;5:252. doi: 10.3389/fphys.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fernández J, Gutiérrez JM, Calvete JJ, Sanz L, Lomonte B. Characterization of a novel snake venom component: Kazal-type inhibitor-like protein from the arboreal pitviper Bothriechis schlegelii. Biochimie. 2016;125:83–90. doi: 10.1016/j.biochi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Morjen M, et al. PIVL, a snake venom Kunitz-type serine protease inhibitor, inhibits in vitro and in vivo angiogenesis. Microvasc. Res. 2014;95:149–156. doi: 10.1016/j.mvr.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 144.Parkinson NM, et al. Towards a comprehensive view of the primary structure of venom proteins from the parasitoid wasp Pimpla hypochondriaca. Insect Biochem. Mol. Biol. 2004;34:565–571. doi: 10.1016/j.ibmb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 145.Rivers DB, Ruggiero L, Hayes M. The ectoparasitic wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) differentially affects cells mediating the immune response of its flesh fly host, Sarcophaga bullata Parker (Diptera: Sarcophagidae) J. Insect Physiol. 2002;48:1053–1064. doi: 10.1016/S0022-1910(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 146.Choi JY, et al. Calreticulin enriched as an early-stage encapsulation protein in wax moth Galleria mellonella larvae. Dev. Comp. Immunol. 2002;26:335–343. doi: 10.1016/S0145-305X(01)00081-7. [DOI] [PubMed] [Google Scholar]
- 147.Asgari S, Schmidt O. Is cell surface calreticulin involved in phagocytosis by insect hemocytes? J. Insect. Physiol. 2003;49:545–550. doi: 10.1016/S0022-1910(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 148.Zhu JY, Fang Q, Wang L, Hu C, Ye GY. Proteomic analysis of the venom from the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae) Arch. Insect Biochem. Physiol. 2010;75:28–44. doi: 10.1002/arch.20380. [DOI] [PubMed] [Google Scholar]
- 149.Suntravat M, et al. Gene expression profiling of the venom gland from the Venezuelan mapanare (Bothrops colombiensis) using expressed sequence tags (ESTs) BMC Mol. Biol. 2016;17:7. doi: 10.1186/s12867-016-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wong ES, et al. Proteomics and deep sequencing comparison of seasonally active venom glands in the platypus reveals novel venom peptides and distinct expression profiles. Mol. Cell. Proteomics. 2012;11:1354–1364. doi: 10.1074/mcp.M112.017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nicolau CA. An in-depth snake venom proteopeptidome characterization: Benchmarking Bothrops jararaca. J. Proteomics. 2017;151:214–231. doi: 10.1016/j.jprot.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 152.de Graaf DC, et al. Two novel proteins expressed by the venom glands of Apis mellifera and Nasonia vitripennis share an ancient C1q-like domain. Insect Mol. Biol. 2010;19:1–10. doi: 10.1111/j.1365-2583.2009.00913.x. [DOI] [PubMed] [Google Scholar]
- 153.Roumenina LT, et al. Interaction of the globular domain of human C1q with Salmonella typhimurium lipopolysaccharide. Biochem. Biophys. Acta. 2008;1784:1271–1276. doi: 10.1016/j.bbapap.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 154.Russkamp D, et al. Characterization of the honeybee venom proteins C1q-like protein and PVF1 and their allergenic potential. Toxicon. 2018;150:198–206. doi: 10.1016/j.toxicon.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 155.Bignetti E, et al. Purification and characterisation of an odorant-binding protein from cow nasal tissue. Eur. J. Biochem. 1985;149:227–231. doi: 10.1111/j.1432-1033.1985.tb08916.x. [DOI] [PubMed] [Google Scholar]
- 156.Pelosi P, Iovinella I, Zhu J, Wang G, Dani FR. Beyond chemoreception: diverse tasks of soluble olfactory proteins in insects. Biol. Rev. Camb. Philos. Soc. 2018;93:184–200. doi: 10.1111/brv.12339. [DOI] [PubMed] [Google Scholar]
- 157.Scaloni A, Monti M, Angeli S, Pelosi P. Structural analysis and disulphide-bridge pairing of two odorant-binding proteins from Bombyx mori. Biochem. Biophys. Res. Comm. 1999;266:386–391. doi: 10.1006/bbrc.1999.1791. [DOI] [PubMed] [Google Scholar]
- 158.Wang L, Zhu JY, Qian C, Fang Q, Ye GY. Venom of the parasitoid wasp Pteromalus puparum contains an odorant binding protein. Arch. Insect Biochem. Physiol. 2015;88:101–110. doi: 10.1002/arch.21206. [DOI] [PubMed] [Google Scholar]
- 159.Das T, et al. Major venom proteins of the fire ant Solenopsis invicta: Insights into possible pheromone-binding function from mass spectrometric analysis. Insect. Mol. Biol. 2018;27:505–511. doi: 10.1111/imb.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ceolin Mariano DO, et al. Bottom-up proteomic analysis of polypeptide venom components of the giant ant Dinoponera quadriceps. Toxins. 2019;11:448. doi: 10.3390/toxins11080448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rodenburg, K.W. & van der Horst, D.J. Lipoprotein-mediated lipid transport in insects: Analogy to the mammalian lipid carrier system and novel concepts for the functioning of LDL receptor family members. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids.1736, 10–29 (2005). [DOI] [PubMed]
- 162.Haney RA, Ayoub NA, Clarke TH, Hayashi CY, Garb JE. Dramatic expansion of the black widow toxin arsenal uncovered by multi-tissue transcriptomics and venom proteomics. BMC Genomics. 2014;15:366. doi: 10.1186/1471-2164-15-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fassio G, et al. Venom diversity and evolution in the most divergent cone snail genus Profundiconus. Toxins. 2019;11:623. doi: 10.3390/toxins11110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.von Reumont BM, Undheim E, Jauss RT, Jenner RA. Venomics of remipede crustaceans reveals novel peptide diversity and illuminates the Venom's Biological role. Toxins. 2017;9:234. doi: 10.3390/toxins9080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peiren N, et al. Proteomic analysis of the honey bee worker venom gland focusing on the mechanisms of protection against tissue damage. Toxicon. 2008;52:72–83. doi: 10.1016/j.toxicon.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 166.Whitten MMA, Tew IF, Lee BL, Ratcliffe NA. A novel role for an insect apolipoprotein (apolipophorin III) in beta-1,3-glucan pattern recognition and cellular encapsulation reactions. J. Immunol. 2004;172:2177–2185. doi: 10.4049/jimmunol.172.4.2177. [DOI] [PubMed] [Google Scholar]
- 167.Barkan NP, Chevalier M, Pradervand JN, Guisan A. Alteration of bumblebee venom composition toward higher elevation. Toxins. 2020;12:4. doi: 10.3390/toxins12010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Forbes BE, McCarthy P, Norton RS. Insulin-like growth factor binding proteins: A structural perspective. Front. Endocrinol. 2012;3:38. doi: 10.3389/fendo.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Almeida DD, et al. Profiling the resting venom gland of the scorpion Tityus stigmurus through a transcriptomic survey. BMC Genomics. 2012;13:362. doi: 10.1186/1471-2164-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kuhn-Nentwig L, Langenegger N, Heller M, Koua D, Nentwig W. The dual prey-inactivation strategy of spiders-in-depth venomic analysis of Cupiennius salei. Toxins. 2019;11:167. doi: 10.3390/toxins11030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zainal Abidin SA, Lee YQ, Othman I, Naidu R. Malaysian cobra venom: A potential source of anti-cancer therapeutic agents. Toxins. 2019;11:75. doi: 10.3390/toxins11020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dittmer NT, Kanost MR. Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem. Mol. Biol. 2010;40:179–188. doi: 10.1016/j.ibmb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 173.Suderman RJ, Dittmer NT, Kanost MR, Kramer KJ. Model reactions for insect cuticle sclerotization: Cross-linking of recombinant cuticular proteins upon their laccase- catalyzed oxidative conjugation with catechols. Insect Biochem. Mol. Biol. 2006;36:353–365. doi: 10.1016/j.ibmb.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 174.Li R, et al. Proteome and phosphoproteome analysis of honeybee (Apis mellifera) venom collected from electrical stimulation and manual extraction of the venom gland. BMC Genomics. 2013;14:766. doi: 10.1186/1471-2164-14-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ozkan, O., Yagmur, E. A. & Ark, M. A newly described scorpion species, Leiurus abdullahbayrami (Scorpion: Buthidae), and the lethal potency and in vivo effects of its venom. J. Venom. Anim. Toxins incl. Trop. Dis. 17, 414–421 (2011).
- 176.Wilkinson B, Gilbert HF. Protein disulfide isomerase. BBA Proteins Proteom. 2004;1699:35–44. doi: 10.1016/S1570-9639(04)00063-9. [DOI] [PubMed] [Google Scholar]
- 177.Safavi-Hemami H, Bulaj G, Olivera BM, Williamson NA, Purcell AW. Identification of Conus peptidylprolyl cis-trans isomerases (PPIases) and assessment of their role in the oxidative folding of conotoxins. J. Biol. Chem. 2010;285:12735–12746. doi: 10.1074/jbc.M109.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tong M, Jiang Y. FK506-binding proteins and their diverse functions. Curr. Mol. Pharmacol. 2015;9:48–65. doi: 10.2174/1874467208666150519113541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Barik S. Immunophilins: For the love of proteins. Cell. Mol. Life Sci. 2006;63:2889–2900. doi: 10.1007/s00018-006-6215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Turano C, Gaucci E, Grillo C, Chichiarelli S. ERp57/GRP58: A protein with multiple functions. Cell. Mol. Biol. Lett. 2011;16:539–563. doi: 10.2478/s11658-011-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rokyta DR, Lemmon AR, Margres MJ, Aronow K. The venom-gland transcriptome of the eastern diamondback rattlesnake (Crotalus adamanteus) BMC Genomics. 2012;13:312. doi: 10.1186/1471-2164-13-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mendonça A, et al. Proteomic analysis of the venom of the social wasp Apoica pallens (Hymenoptera: Vespidae) Rev. Bras. Entomol. 2019;63:322–330. doi: 10.1016/j.rbe.2019.10.001. [DOI] [Google Scholar]
- 183.Dos Santos LD, et al. Profiling the proteome of the venom from the social wasp Polybia paulista: A clue to understand the envenoming mechanism. J. Proteome Res. 2010;9:3867–3877. doi: 10.1021/pr1000829. [DOI] [PubMed] [Google Scholar]
- 184.Pessoa WF, et al. Analysis of protein composition and bioactivity of Neoponera villosa venom (Hymenoptera: Formicidae) Int. J. Mol. Sci. 2016;17:513. doi: 10.3390/ijms17040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yoon KA, et al. Comparative functional venomics of social hornets Vespa crabro and Vespa analis. J. Asia Pac. Entomol. 2015;18:815–823. doi: 10.1016/j.aspen.2015.10.005. [DOI] [Google Scholar]
- 186.Liu H, et al. Transcriptome differences in the hypopharyngeal gland between Western Honeybees (Apis mellifera) and Eastern Honeybees (Apis cerana) BMC Genomics. 2014;15:744. doi: 10.1186/1471-2164-15-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peiren N, et al. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim. Biophys. Acta. 2005;1752:1–5. doi: 10.1016/j.bbapap.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 188.Blank S, Bantleon FI, McIntyre M, Ollert M, Spillner E. The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin. Exp. Allergy. 2012;42:976–985. doi: 10.1111/j.1365-2222.2012.03966.x. [DOI] [PubMed] [Google Scholar]
- 189.Meng E, et al. Effects of ovarian fluid, venom and egg surface characteristics of Tetrastichus brontispae (Hymenoptera: Eulophidae) on the immune response of Octodonta nipae (Coleoptera: Chrysomelidae) J. Insect Physiol. 2018;109:125–137. doi: 10.1016/j.jinsphys.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 190.Hu J, et al. Hemomucin, an O-glycosylated protein on embryos of the wasp Macrocentrus cingulum that protects it against encapsulation by hemocytes of the host Ostrinia furnacalis. J. Innate. Immun. 2014;6:663–675. doi: 10.1159/000360819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McElroy T, et al. Differential toxicity and venom gland gene expression in Centruroides vittatus. PLoS ONE. 2017;12:e0184695. doi: 10.1371/journal.pone.0184695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chakrabarty, D. & Chanda, C. Snake Venom Disintegrins in Toxinology (eds. Gopalakrishnakone, P., Inagaki, H., Vogel, C.W., Mukherjee, A. & Rahmy, T.) 437–449 (Springer, 2017).
- 193.Junqueira-de-Azevedo IL, et al. Lachesis muta (Viperidae) cDNAs reveal diverging pit viper molecules and scaffolds typical of cobra (Elapidae) venoms: Implications for snake toxin repertoire evolution. Genetics. 2006;173:877–889. doi: 10.1534/genetics.106.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu H, Tang X, Gong L. Mesencephalic astrocyte-derived neurotrophic factor and cerebral dopamine neurotrophic factor: New endoplasmic reticulum stress response proteins. Eur. J. Pharmacol. 2015;750:118–122. doi: 10.1016/j.ejphar.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 195.Carducci F, Biscotti MA, Canapa C. Vitellogenin gene family in vertebrates: Evolution and functions. Eur. Zool. J. 2019;86:233–240. doi: 10.1080/24750263.2019.1631398. [DOI] [Google Scholar]
- 196.Blank S, et al. Vitellogenins are new high molecular weight components and allergens (Api m 12 and Ves v 6) of Apis mellifera and Vespula vulgaris venom. PLoS ONE. 2013;8:e62009. doi: 10.1371/journal.pone.0062009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Park HG, et al. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev. Comp. Immunol. 2018;85:51–60. doi: 10.1016/j.dci.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 198.Luna-Ramírez K, Quintero-Hernández V, Juárez-González VR, Possani LD. Whole transcriptome of the venom gland from Urodacus yaschenkoi scorpion. PLoS ONE. 2015;10:e0127883. doi: 10.1371/journal.pone.0127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Adams ME. Agatoxins: ion channel specific toxins from the American funnel web spider Agelenopsis aperta. Toxicon. 2004;43:509–525. doi: 10.1016/j.toxicon.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 200.Durertre S, Lewis RJ. Use of venom peptides to probe ion channel structure and function. J. Biol. Chem. 2010;285:13315–13320. doi: 10.1074/jbc.R109.076596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Torres AF, et al. Transcriptome analysis in venom gland of the predatory giant ant Dinoponera quadriceps: insights into the polypeptide toxin arsenal of hymenopterans. PLoS ONE. 2014;9:e87556. doi: 10.1371/journal.pone.0087556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee SH, Baek JH, Yoon K. Differential properties of venom peptides and proteins in solitary vs social hunting wasps. Toxins. 2016;8:32. doi: 10.3390/toxins8020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sharko FS, et al. A partial genome assembly of the miniature parasitoid wasp Megaphragma amalphitanum. PLoS ONE. 2019;14:e0226485. doi: 10.1371/journal.pone.0226485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tang BZ, et al. Combination of label-free quantitative proteomics and transcriptomics reveals intraspecific venom variation between the two strains of Tetrastichus brontispae, a parasitoid of two invasive beetles. J. Proteomics. 2019;192:37–53. doi: 10.1016/j.jprot.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 205.Zhang XM, et al. Cloning and Immunosuppressive Properties of an Acyl-Activating Enzyme from the Venom Apparatus of Tetrastichus brontispae (Hymenoptera: Eulophidae) Toxins. 2019;11:672. doi: 10.3390/toxins11110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chauhan P, et al. De novo transcriptome of Ischnura elegans provides insights into sensory biology, colour and vision genes. BMC Genomics. 2014;15:808. doi: 10.1186/1471-2164-15-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang T, et al. Aggregate-reactivation activity of the molecular chaperone ClpB from Ehrlichia chaffeensis. PLoS ONE. 2013;8:e62454. doi: 10.1371/journal.pone.0062454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lubec G, Afjehi-Sadat L, Yang JW, John JP. Searching for hypothetical proteins: Theory and practice based upon original data and literature. Prog. Neurobiol. 2005;77:90–127. doi: 10.1016/j.pneurobio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 209.Estrada-Gomez S, et al. Venomic, transcriptomic, and bioactivity analyses of pamphobeteus verdolaga venom reveal complex disulfide-rich peptides that modulate calcium channels. Toxins. 2019;11:496. doi: 10.3390/toxins11090496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rokyta DR, Ward MJ. Venom-gland transcriptomics and venom proteomics of the black-back scorpion (Hadrurus spadix) reveal detectability challenges and an unexplored realm of animal toxin diversity. Toxicon. 2017;128:23–37. doi: 10.1016/j.toxicon.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 211.Ward MJ, Ellsworth SA, Rokyta DR. Venom-gland transcriptomics and venom proteomics of the Hentz striped scorpion (Centruroides hentzi; Buthidae) reveal high toxin diversity in a harmless member of a lethal family. Toxicon. 2018;142:14–29. doi: 10.1016/j.toxicon.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 212.Cid-Uribe JI, et al. The diversity of venom components of the scorpion species Paravaejovis schwenkmeyeri (Scorpiones: Vaejovidae) revealed by transcriptome and proteome analyses. Toxicon. 2018;151:47–62. doi: 10.1016/j.toxicon.2018.06.085. [DOI] [PubMed] [Google Scholar]
- 213.IPLA. Regione Piemonte. Collana Manuali Tecnico-divulgativi di Selvicoltura. Tipi forestali del Piemonte. (Blu Edizioni, 2004)
- 214.Ferracini C, et al. Do Torymus sinensis (Hymenoptera: Torymidae) and agroforestry system affect native parasitoids associated with the Asian chestnut gall wasp? Biol. Control. 2018;121:36–43. doi: 10.1016/j.biocontrol.2018.01.009. [DOI] [Google Scholar]
- 215.Vogel H, Badapanda C, Knorr E, Vilcinskas A. RNA-sequencing analysis reveals abundant developmental stage-specific and immunity-related genes in the pollen beetle Meligethes aeneus. Insect Mol. Biol. 2014;23:98–112. doi: 10.1111/imb.12067. [DOI] [PubMed] [Google Scholar]
- 216.Gotz S, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Waterhouse RM, et al. BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol. Boil. Evol. 2018;35:543–548. doi: 10.1093/molbev/msx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA. Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 219.Liu W, Saint DA. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal. Biochem. 2002;302:52–59. doi: 10.1006/abio.2001.5530. [DOI] [PubMed] [Google Scholar]
- 220.Lee C, Kim J, Shin SG, Hwang S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 2006;123:273–280. doi: 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 221.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 222.Bradford MM. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 223.Medugno L, et al. A novel zinc finger transcriptional repressor, ZNF224, interacts with the negative regulatory element (AldA-NRE) and inhibits gene expression. FEBS Lett. 2003;534:93–100. doi: 10.1016/S0014-5793(02)03783-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.