Abstract
Medicines derived from plants are preferred over synthetic therapeutic agents in treating different diseases. Ziziphus oxyphylla (a member of Rhamnaceae family) is a medicinal plant used as a remedy of different diseases in Greek and Ayurveda medical systems. Z. oxyphylla roots were shade dried and then subjected to extraction of bioactive compounds using different solvent systems and silica gel. From ethyl acetate fraction, three compounds viz., p-coumaric acid (V), 3,4-dimethoxy benzoic acid (VI), and 4-heptyloxy benzoic acid (VII) were isolated in pure form. The selection of ethyl acetate fraction for isolation was based on HPLC profiling of crude extract and different fractions. These compounds were characterized by different spectroscopic techniques and evaluated for their in vitro antioxidant, anticholinesterase, α–glucosidase, and α-amylase inhibitory potentials. To find out possible binding interactions of V with AChE and BChE crystals, in-silico docking studies were also carried out. Compound V showed maximum scavenging capabilities of DPPH and ABTS free radicals with IC50 values of 69 and 62 μg/mL respectively. Excellent percent inhibition (83.4 ± 0.5% at highest concentration 1000 μg/mL) of acetylcholinesterase (AChE) was exhibited by compound V (IC50 = 80 μg/mL); whereas, for the mentioned concentration, 83.2 ± 1.1% inhibition (IC50 = 90 μg/mL) of butyrylcholinesterase (BChE) was observed as well. The compound VI exhibited highest % inhibition against α-glucosidase (IC50 = 84 μg/mL) whereas α-amylase was more potently inhibited by compound V (% inhibition = 86.8 % and IC50 = 85 μg/mL). Docking scores of -1.391 Kcal/mol (BChE) and –6.253 Kcal/mol (AChE) were recorded using molecular docking software. Compound V exhibited strong free radical scavenging and anticholinesterase potentials suggesting that it can be effectively used to treat oxidative stress and dementia in human.
Keywords: Ziziphus oxyphylla, Bioactive compounds, Antioxidant, Anti-cholinesterase, Molecular docking
Ziziphus oxyphylla, bioactive compounds, antioxidant, anti-cholinesterase, molecular docking.
1. Introduction
Ziziphus oxyphylla that belongs to family Rhamnaceae (genus Ziziphus), is a medicinal plant used to treat a number of human diseases in different parts of the world. Rhamnaceae family is a large family with 58 genera and about 900 species. Out of them, 21 species presenting 6 genera of Rhamnaceae family have been reported from Pakistan (Ahmad et al., 2013; Kaleem et al., 2014; Ali et al., 2015). Ziziphus oxyphylla grows in subtropical and tropical regions of the world. In Pakistan, this plant is found in district Dir, Kohistan, Swat, Buner, Hazara, and Malakand. Members of the genus Ziziphus are very rich in biologically active secondary metabolites like nummularine, zizynummin, lotusanine, adouctine, mauritine, dachuine, franganine, frangufoline etc., (Kaleem et al., 2014; Ali et al., 2015; Ahmad et al., 2016; Lakache et al., 2016; Ahmad2017). Devi et al. (1987) isolated nummularine-K, nummularine-R, amphibine sorbitol H, and frangufoline from Z. nummularia and Ziziphus jujuba. Singh and Pandey (1995) isolated nummularine-T from Zizyphus nummularia,. Ghedira et al. (1995) isolated lutisine from Ziziphus lotus. Jubanine-C, a new alkaloid was isolated by Tripathi et al. (2001) from Zizyphus jujube. Another alkaloid lotusine G was isolated from Zizyphus lotus by Le Crouéour et al. (2002).
In human body, free radicals are produced during normal metabolic activities which are immediately detoxified by the body's defense mechanisms. Their increased synthesis under certain pathological conditions leads to a number of physiological disorders/complications. Natural organic compounds, especially those having phenolic rings in their structure are good scavenger of these free radicals. The main sources of phenolic compounds are vegetable and fruits; as they are included in the category of antioxidants and their intake in food relieves the harmful effects produced by reactive oxygen species in the body. Herbal products are generally associated with limited or no side effects while a number of side effects including genotoxic effect have been reported about the synthetic antioxidants used in food industry (Lakache et al., 2016; Mustapha et al., 2016; Russo et al., 2015).
Alzheimer disease (AD) is the most prevalent type of dementia characterized by loss in memory, cognition and changes in behavior (Ovais et al., 2018; Zahoor2018; Bari et al., 2019). In clinical practice, the medicines which are prescribed for symptomatic treatment of AD act by inhibition of Cholinesterases. Among the currently available medications of AD, two are derived from plant sources (galantamine and rivastigmine), both of these are inhibitors of Acetylcholine (AChE) and butyrylcholine (BChE) esterase. ACHE and BCHE are responsible for the hydrolysis of neurotransmitters acetyl and butyrylcholine (ACh and BCh) after they successfully transmit the nerve signal. AD is usually associated with the depleted level of ACh in the body, thus the inhibition of AChE and BChE in the condition could allow preservation of ACh levels in the body by slowing down its hydrolysis (Dastjerdi2015; Ovais et al., 2018; Zahoor2018; Bari et al., 2019; Khan et al., 2020a, b). Moreover, the inhibition of both ACHE and BCHE are equally important in AD, as in circumstances when the ACHE levels are lowered down through medications, BCHE could act instead and deficiency brought about is compensated. The medicines in use are either associated with multiple side effects or could only inhibit either ACHE or BCHE. Therefore, there is a need to explore other plants for finding an efficient and potent inhibitor that would be able to successfully inhibit both of the Cholinesterases and has lesser/no side effects.
The most prevailing disease of carbohydrate metabolism is diabetes mellitus which is considered to be the most highly prevalent chronic condition after cancer. The condition is caused either by deficient/no production of insulin (Type-1) or by insulin resistance (Type-2). A number of approaches are used to treat type-2 diabetes mellitus including inhibition of the major enzymes of carbohydrate metabolism viz., alpha amylase and glucosidase. Alpha amylase causes hydrolysis of starch into oligo and disaccharides while the later causes the release of free glucose from the products formed by amylases (Nazir et al., 2018; Khan et al., 2020a, b). Inhibition of any of the two enzymes would result in delayed release of glucose in the blood preventing hyperglycemia.
To best of our knowledge, the roots of Z. oxyphylla have not been subjected to isolation of biologically active compounds by any researcher. In the current research, three compounds viz., p-coumaric acid (V), 3,4 dimethoxy benzoic acid (VI), and 4-heptyloxy benzoic acid (VII) were isolated from the ethyl acetate fraction in pure form. The isolated compounds were identified using various spectroscopic techniques. Although the first two are reported compounds but new from this genus while compound VII is reported for the first time from any medicinal plant. These compounds were evaluated for their in-vitro antioxidant, anticholinesterase, α-amylase, and α–glucosidase inhibitory potentials. To study the interactions between the most active compound and AChE/BChE, in-silico molecular docking studies were also performed.
2. Methods
2.1. Plant
Ziziphus oxyphylla roots sample were collected from village Barimkai, Dir Lower, KPK, Pakistan in April, 2016. The selected plant was authenticated by Dr. Mohammad Nisar, Department of Botany, University of Malakand. As the collected plant is a wild variety, therefore the guidelines for collecting the wild plants devised by Herbarium, Malakand University (UOM/HU/Eth/Collect.0321) were followed. The collected plant specimen was deposited at the herbarium of the university (voucher specimen; 1022HU).
The collected root samples were washed with tap water to eliminate dust particles and other impurities, which were then shade dried. The root samples (5 kg) were grounded into fine powder using a mechanical grinder. The powder was then macerated for 75 h in 95:5% v/v methanol/water solvent system (12 L). The mixture was filtered through a porous cloth. The residue left was re-macerated in the mentioned solvent system for additional 75 h. Filtrates of both the steps were mixed up and evaporated at 40 °C under reduced pressure, using rotary evaporator (Switzerland, Modal R-200 Buchi, Rotavapor). Fractionation of the crude extract was carried out using different solvent systems (in the order of increasing polarity). For the purpose, 700 g of the extract was dissolved in 1.5 L water and was fractionated using solvent-solvent extraction method. After drying, the fractions were kept in the labeled flasks, within a refrigerator.
After HPLC profiling (agilent 1260 system), ethyl acetate fraction (38 g) was subjected to the isolation of biologically active compounds using silica gel columns (mesh size 70–230). The effluents from the column were collected in glass viols. Silica gel 60 PF254 (Merck) was used in the preparative TLC, whereas, the isolated compounds were visualized at 254 and 266 nm using iodine (solid) and cerium (IV) sulfate spray. On the basis of TLC profiling, some of the viols were recombined and thus a number of combined fractions (from column) were obtained. Fraction no: A-6 was further fractionated into a number of sub-fractions out of which Q-3 and Q-10 fractions were subjected to pencil column, resulting in the isolation of compound V and VI having masses 8 (% yield = 0.021) and 7 mg (% yield = 0.018) respectively. Fraction A-10, resulted in the isolation of compound VII having a mass of 10 mg (% yield = 0.026).
Bruker Spectrometers operated at 600 MHz was used to record 13C and 1H NMR spectra. Coupling constants (J) and chemical shifts (δ) were measured in Hertz (Hz) and parts per million (ppm), respectively.
2.2. Antioxidant assays
2.2.1. DPPH assay
DPPH assay was used to determine the antioxidant potential of isolated compounds. About 3 mL of the DPPH standard solution (20 mg DPPH/100 mL methanol) was used as blank. Its absorbance was adjusted to 0.75 at 515 nm. For the development of free radicals, the stock solution was placed in a dark cabinet for 24 h. Stock solutions of the respective isolated compounds were prepared in methanol (5 mL) while the working standards of each were prepared in the range of 1000-62.5 μg/mL. About 2 mL from each dilution was mixed with 2 mL of DPPH solution and incubated in dark for 15 min. Ascorbic acid (1 mg/mL) was used as a standard. The antioxidant potential of the isolated compounds was calculated using Eq. (1);
| (1) |
Where: A represents the absorbance of DPPH in oxidized form and B is the sample absorbance after incubation with free radical.
2.2.2. ABTS assay
The isolated compounds were also assessed for the antioxidant potentials by the ABTS (2, 2-azinobis [3-ethylbenzthiazoline]-6-sulfonic acid) assay using method of Re et al. (1999). The specified quantities of ABTS and potassium persulphate (7 and 2.45 mM dissolved in 100 ml methanol respectively) were mixed and incubated in dark for 12 h (time required to develop free radical in mixture). Each of the working dilutions of the compounds (300 μL) was mixed with ABTS (3 mL) and incubated at room temperature for 15 min. After incubation, absorbance of each reaction mixture was recorded at 745 nm Eq. (1) was used to calculate the free radical scavenging potential of the isolated compounds.
2.3. Anticholinesterase assay
The isolated compounds were evaluated for their AChE and BChE inhibitory potentials using Ellman's assay (Ovais et al., 2018). In Ellman's assay, acetylthiocholine iodide hydrolysis results in the formation of thiocholine which make complex with an anion formed from DTNB (5-thio-2-nitrobenzoate) resulting in a coloured complex. From different dilutions of each compound, 1 mL was incubated at 25 °C for 15 min with 100 μL of DNTB and selected enzymes (AChE and BChE) and were mixed thoroughly. After incubation, the substrate-acetylcholine/butyrylcholine iodide (100 μL) was added to each reaction mixture and allowed to react for 15 min. The absorbance of reaction mixtures was recorded at 412 nm using spectrophotometer. Eqs. (2), (3), and (4) were used to calculate enzyme inhibition:
| (2) |
| (3) |
| (4) |
Whereas, Ea = Enzyme activity, Ei = Enzyme inhibition, A = Absorbance, V = reaction rate in the presence of inhibitor, and Vmax = reaction rate without inhibitor.
2.4. Inhibitory assay of α - glucosidase
The α -glucosidase (20.0 μL having concentration of 0.500 unit/mL) solution was prepared in 120.0 μL phosphate buffer (0.1 M, pH 6.9). The substrate (p-nitrophenyl- α -D-glucopyranoside) solution was also prepared in phosphate buffer. Solutions of the isolated compound were prepared in the concentration range of 125–1000 μg/mL. Solutions of the compound and enzyme were mixed in proper ratio and were incubated at 37 °C for 15 min. Then 20 μL of the substrate was added to reaction mixtures and incubated for additional 15 min. Then, 80 μL of 0.2 M Na2CO3 was added to reaction mixture to stop the reaction. The absorbance of the final mixture was recorded using UV/visible spectrophotometer (Germany, UV- 3000 O.R.I.) at 405 nm. The following equation was used to determine the percent α -glucosidase inhibition;
| (5) |
2.5. Inhibitory assay of α-amylase
The % inhibition of α-amylase can be estimated from the amount of reducing sugar (maltose equivalent) liberated during the reaction of substrate and enzyme. To determine the alpha amylase inhibition potential of isolated compounds, dinitrosalicylic acid (DNS) assay was used with little modification. About 1 mL from each compound dilution was mixed with α-amylase (1 U/mL) and pre-incubated for 30 min. Then 1 mL starch solution was added to this mixture and incubated at 37 °C for 10 min; the reaction was stopped by adding 1 mL DNS reagent. In a boiling water bath, the mixture was heated up for 5 min. A blank solution not having any of the compounds was prepared and its absorbance was recorded at 540 nm. The following formula was used to estimate the enzyme inhibition;
| (6) |
2.6. Molecular docking studies
Based on the results of biological activities, the most pharmacologically active compound (V) was subjected to molecular docking simulation studies using chemical structure of V and crystal structure of the AChE and BChE (PDB: 1ACL, 4BOP) from the RCSB Protein Data Bank. Preferably, the neutral configuration of ligands prepared by Schrödinger Lig: was used. The receptor grid box was considered to be a 20 Å box with an active site having the water molecule in the center. The simulation was made with Glide (Schrödinger) using XP extra precision with evasion settings and glide scoring function, for 15 top ranked poses of each ligand. The process was visually observed (binding poses) while the images were taken through Schrödinger Maestro (Pintus et al., 2017).
3. Results
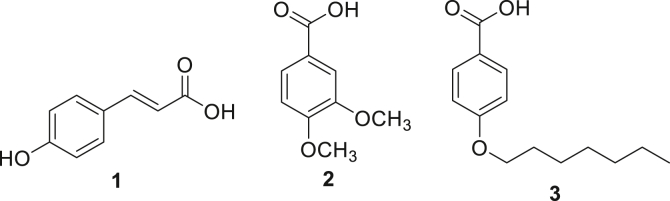
This study was conducted keeping in view the medicinal importance of the selected plant in the Greek medicines system. Three compounds viz., p-coumaric acid (V), 3,4-dimethoxy benzoic acid (VI), and 4-heptyloxy benzoic acid (VII) were isolated for the first time from this genus (Figure 1).
Figure 1.
Chemical structures of compounds V-VII, isolated from roots of Z. oxyphylla.
3.1. Isolated compounds
3.1.1. p-Coumaric acid (V)
Compound V is an amorphous solid, having molecular formula is C9H8O3, soluble in organic solvents with melting point from 310 to 313 °C.
1H NMR (600 MHz, DMSO-d6): δ 12.61 (1H, s), 7.36 (2H, d, J = 9.0 Hz, H-2/6), 7.34 (1H, d, J = 15.6 Hz, H-7), 6.75 (2H, d, J = 9.6 Hz, H-3/5), 6.30 (1H, d, J = 15.6 Hz, H-8).
13C NMR (150 MHz, DMSO-d6): δ 165.0 (C-9), 124.6 (C-1), 158.5 (C-4), 142.8 (C-7), 128.4 (C-2/6), 129.7 (C-5), 114.3 (C-3/5), 113.9 (C-8) (Kuroda et al., 2016; Ji et al., 2005).
3.1.2. 3,4-dimethoxy benzoic acid (VI)
Compound VI has molecular formula C9H10O4, soluble in organic solvents with melting point of 181 °C.
1H NMR (600 MHz, DMSO-d6): δ 12.67 (1H. s), 7.48 (1H, m, H-6), 7.43 (1H, d, 9.6 Hz, H-5), 6.44 (1H, d, J = 4.2 Hz), 3.67 (6H, s, -OCH3).
13C NMR (151 MHz, DMSO-d6): δ 168.73 (C-7), 122.5 (C-1), 113.5 (C-2), 148.4 (C-3), 155.1 (C-4), 121.5 (C-6), 111.3 (C-5), 55.2 (-OCH3) (Sakhuja et al., 2014; Scott, 1972; Awad et al., 2017).
3.1.3. 4-Heptyloxy benzoic acid (VII)
Compound VII has molecular formula C14H20O3, soluble in organic solvents with melting point of 146 °C. This compound has isolated for the first time from this medicinal plant.
1H NMR (600 MHz, DMSO-d6): δ 12.60 (s, 1H, H-1), 7.87 (m, 2H, d, J = 8.6 Hz, H-2/6), 6.90 (2H, d, J = 8.6 Hz, H-3/5), 4.01 (2H,t, H-1′), 1.71 (m, 2H, H-2′), 1.29 (m, 2H, H-3′), 1.39 (m, 6H, H-4′,5′,6′), 0.86 (3H, t, H-7′).
13C NMR (151 MHz, DMSO-d6): δ 166.9 (C-7), 122.7 (C-1), 131.2 (C-3/5), 114.1 (C-2/6), 162.2 (C-4), 67.7 (C-1′), 31.1 (C-5′), 28.5 (C-2′), 28.3 (C-3′), 25.3 (C-6′), 22.0 (C-4′), 13.9 (C-7′) (Muniprasad et al., 2012).
3.2. Antioxidant activities
The DPPH and ABTS assay are usually used to evaluate the free radical scavenging abilities of biologically active substances. If a compound has the ability to scavenge free radicals produced by DPPH and ABTS, then it is believed that compound has an antioxidant potential. The isolated p-coumaric acid showed highest scavenging activity of 85.8 ± 0.3% against DPPH free radical with IC50 value of 59 μg/mL followed by 3,4-dimethoxy benzoic acid with percent inhibition of 82.1 ± 1.2 and IC50 value of 67 μg/mL (Table 1). Against the ABTS free radical again p-coumaric acid was a potent inhibitor with percent inhibition of 84.4 ± 1.0 (IC50 = 62 μg/mL). Ascorbic acid used positive control had IC50 value of 38 μg/mL.
Table 1.
Free radical scavenging ability of the compounds isolated from Ziziphus oxyphylla.
| Compound | Concentrations (μg/mL) | DPPH Percent inhibition (mean ± S.E.M) | DPPH IC50 (μg/mL) | ABTS percent inhibition (mean ± S.E.M) | ABTS IC50 (μg/mL) |
|---|---|---|---|---|---|
| V | 1000.0 | 85.8 ± 0.3∗∗∗∗ | 59 | 84.4 ± 1.0∗∗∗∗ | 62 |
| 500.0 | 76.1 ± 1.2∗∗∗∗ | 75.5 ± 1.2∗∗∗∗ | |||
| 250.0 | 67.6 ± 0.5∗∗∗∗ | 66.6 ± 1.2∗∗∗ | |||
| 125.0 | 59.5 ± 0.4∗∗ | 57.3 ± 1.6∗∗∗∗ | |||
| 62.5 | 51.8 ± 1.2ns | 50.2 ± 1.3∗∗ | |||
| VI | 1000.0 | 82.1 ± 1.2∗∗∗∗ | 67 | 81.2 ± 1.0∗∗∗∗ | 67 |
| 500.0 | 72.3 ± 0.3∗∗∗∗ | 71.0 ± 1.6∗∗∗∗ | |||
| 250.0 | 63.7 ± 0.8∗∗∗∗ | 61.1 ± 1.4∗∗∗ | |||
| 125.0 | 54.7 ± 2.1∗∗ | 56 .1 ± 1.7∗∗ | |||
| 62.5 | 48.2 ± 2.3 ns | 48.2 ± 1.3∗∗ | |||
| VII | 1000.0 | 80.4 ± 1.1∗∗∗∗ | 118 | 80.1 ± 1.0∗∗∗∗ | 115 |
| 500.0 | 71.2 ± 1.3∗∗∗∗ | 71.2 ± 2.1∗∗∗∗ | |||
| 250.0 | 62.5 ± 1.1∗∗∗∗ | 62.1 ± 1.3∗∗∗ | |||
| 125.0 | 51.9 ± 1.3∗∗ | 51 .4 ± 1.4∗∗ | |||
| 62.5 | 46.7 ± 2.1∗∗ | 46.1 ± 1.9∗∗ | |||
| Ascorbic acid | 1000.0 | 93.2 ± 0.1 | 38 | 93.0 ± 0.7 | 39 |
| 500.0 | 84.1 ± 0.4 | 83.1 ± 1.1 | |||
| 250.0 | 73.8 ± 2.2 | 72.1 ± 0.4 | |||
| 125.0 | 63.2 ± 1.1 | 62 .1 ± 1.0 | |||
| 62.5 | 52.2 ± 2.3 | 52.1 ± 1.1 |
Data presented as mean ± SEM; values differ significantly compared to the positive control, ns: P > 0.05∗: P < 0.05, ∗∗∗: P < 0.001,. n = 3.
3.3. Anticholinesterase activities
The depleted amounts of acetylcholine and butyrylcholine as usually happen in AD and other forms of dementia can be controlled if AChE and BChE are inhibited in the brain to prevent their hydrolysis. The activities of AChE and BChE were inhibited by all isolated compounds (Table 2). The compounds V and VI showed very good percent inhibition with IC50 values of 80 and 90 μg/mL and percent inhibitions of 83.4 ± 0.5 and 80.7 ± 1.1 respectively against AChE.
Table 2.
Anticholinesterase activities of the isolated compounds from Ziziphus oxyphylla.
| Compound | Concentrations (μg/mL) | Percent AChE (mean ± SEM) | AChE IC50 (μg/mL) | Percent BChE (mean ± SEM) | BChE IC50 (μg/mL) |
|---|---|---|---|---|---|
| V | 1000 | 83.4 ± 0.5∗∗∗ | 80 | 83.2 ± 1.1∗∗∗ | 80 |
| 500 | 74.1 ± 0.9∗∗∗ | 72.6 ± 1.3∗∗ | |||
| 250 | 65.9 ± 1.4∗∗∗ | 64.9 ± 0.1∗∗∗ | |||
| 125 | 56.1. ± 1.4∗∗ | 55.9 ± 1.0∗∗ | |||
| 62.5 | 48.2 ± 1.4∗∗∗ | 48.1 ± 1.9∗ | |||
| VI | 1000 | 80.7 ± 1.1∗∗ | 90 | 79.8 ± 1.4∗∗ | 91 |
| 500 | 71.2 ± 1.8∗∗ | 71.1 ± 1.6∗∗ | |||
| 250 | 62.1 ± 2.0∗∗ | 61.4 ± 0.3∗∗∗ | |||
| 125 | 54.7. ± 1.6∗∗∗ | 53.5 ± 1.6∗∗ | |||
| 62.5 | 47.2 ± 1.5∗∗∗ | 47.0 ± 1.1∗∗ | |||
| VII | 1000 | 80.1 ± 1.2∗∗ | 89 | 80.0 ± 1.1∗∗ | 90 |
| 500 | 71.5 ± 1.6∗∗ | 71.1 ± 0.8∗∗ | |||
| 250 | 62.3 ± 1.1∗∗ | 62.1 ± 0.0∗∗∗ | |||
| 125 | 54.1. ± 1.2∗∗∗ | 53.4 ± 1.1∗∗ | |||
| 62.5 | 47.8 ± 1.3∗∗∗ | 47.2 ± 1.2∗∗ | |||
| Galantamine | 1000 | 92.1 ± 1.0 | 40 | 92.0 ± 0.8 | 43 |
| 500 | 82.3 ± 1.2 | 81.8 ± 1.4 | |||
| 250 | 73.4 ± 0.5 | 71.8 ± 1.5 | |||
| 125 | 64.2. ± 1.2 | 64 .0 ± 1.2 | |||
| 62.5 | 55.6 ± 2.6 | 54.1 ± 1.0 |
Data presented as mean ± SEM; Values differ significantly compared to the positive control, ns: P > 0.05∗: P < 0.05, ∗∗∗: P < 0.001, n = 3.
The isolated compounds also showed substantial inhibition of the BChE as presented in Table 2. The compounds V and VI more potently inhibited BChE with IC50 value of 80 and 90 μg/mL and percent inhibition of 83.2 ± 1.1 and 80.0 ± 1.1 respectively. Galantamine was used as positive control.
3.4. Alpha-amylase and alpha-glucosidase inhibitory activities
The isolated compounds were assessed for their inhibitory potentials of alpha-glucosidase and amylase at different concentrations. The compound VI exhibited maximum inhibition of alpha-glucosidase, showing % inhibition of 84.6 % with IC50 value of 84 μg/mL. The lowest percent inhibition was recorded for compound V (IC50 = 96 μg/mL), and VII (IC50 = 108 μg/mL) as presented in Table 3. For acarbose (positive control), the IC50 value recorded was 70 μg/mL.
Table 3.
Alpha-glucosidase activities of the isolated compounds.
| Compounds | Concentration (μg/mL) | %inhibition (mean ± SEM) | IC50 (μg/mL) |
|---|---|---|---|
| V | 1000.0 | 79.3 ± 2.3 | 96 |
| 500.0 | 73.2 ± 1.5 | ||
| 250.0 | 63.5 ± 2.3 | ||
| 125.0 | 53.4 ± 2.7 | ||
| VI | 1000.0 | 84.6 ± 1.5∗∗∗∗ | 84 |
| 500.0 | 68.2 ± 1.7∗∗ | ||
| 250.0 | 62.5 ± 2.4∗∗∗ | ||
| 125.0 | 54.3 ± 1.5∗∗∗ | ||
| V | 1000.0 | 74.6 ± 1.3∗∗∗∗ | 108 |
| 500.0 | 65.6 ± 1.5∗∗ | ||
| 250.0 | 57.5 ± 1.9∗ | ||
| 125.0 | 51.1 ± 2.4∗∗∗ | ||
| Acarbose | 1000.0 | 93.7 ± 1.0 | 70 |
| 500.0 | 78.6 ± 1.1 | ||
| 250.0 | 67.2 ± 1.6 | ||
| 125.0 | 58.7 ± 1.1 |
Data is represented as (mean ± SEM) Values notably different in comparison to the positive control, ∗: P < 0.05, ∗∗:P < 0.01, ∗∗∗:P < 0.001, ns: P > 0.05. n = 3.
The isolated bioactive compounds were also tested to determine their inhibitory potentials against alpha-amylase (Table 4). The maximum percent inhibition was recorded for compound V showing an inhibition of 86.8% with IC50 value of 85 μg/mL. The high numerical values of IC50 were noted for compound VI (IC50 = 112 μg/mL) followed by VII (IC50 = 110 μg/mL). The IC50 value of acarbose (positive control) was 75 μg/mL.
Table 4.
Alpha-amylase activities of the isolated compounds.
| Compounds | Concentration (μg/mL) | % inhibition (mean ± SEM) | IC50 (μg/mL) |
|---|---|---|---|
| V | 1000.0 | 86.8 ± 1.8∗∗∗∗ | 85 |
| 500.0 | 76.5 ± 2.8∗∗∗ | ||
| 250.0 | 64.5 ± 1.8∗∗∗∗ | ||
| 125.0 | 59.2 ± 2.5∗∗∗ | ||
| VI | 1000.0 | 79.0 ± 3.2∗∗∗∗ | 112 |
| 500.0 | 71.3 ± 1.7∗∗∗∗ | ||
| 250.0 | 59.1 ± 1.9∗∗∗∗ | ||
| 125.0 | 50.3 ± 2.4∗∗ | ||
| VII | 1000.0 | 73.3 ± 1.1∗∗∗∗ | 110 |
| 500.0 | 64.5 ± 2.8∗∗ | ||
| 250.0 | 61.2 ± 2.2∗ | ||
| 125.0 | 51.4 ± 1.5∗∗∗ | ||
| Acarbose | 1000.0 | 92.1 ± 0.8 | 75 |
| 500.0 | 77.1 ± 1.4 | ||
| 250.0 | 66.4 ± 1.2 | ||
| 125.0 | 57.0 ± 1.2 |
Table represent data as (mean ± SEM) Values differs notably in comparison to positive control, ∗: P < 0.05, ∗∗:P < 0.01, ∗∗∗:P < 0.001, ns: P > 0.05. n = 3.
3.5. Molecular docking studies
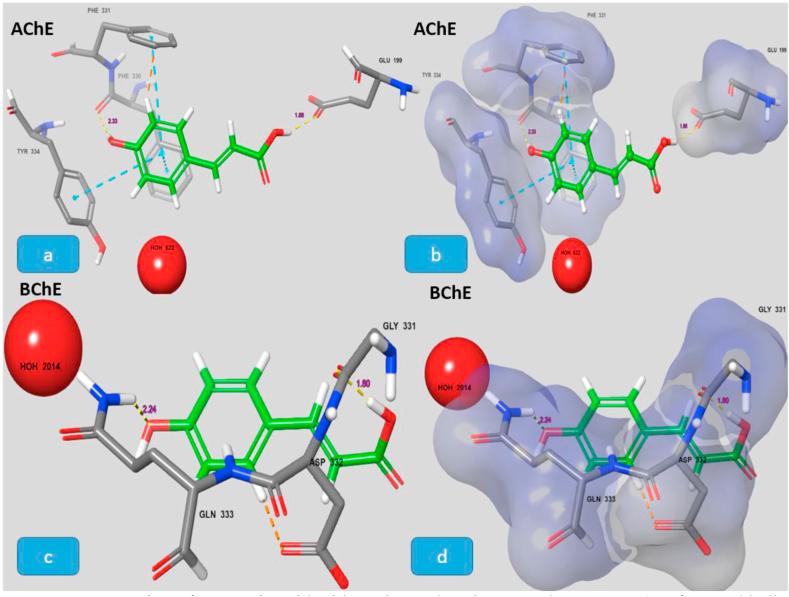
To confirm the anticholinesterase potential of most potent bioactive compound, in-silico studies were performed (Figure 2). Coumaric acid properly bound in the enzyme's binding pocket with docking score of –6.253 kcal/mol (AChE) and -1.391 Kcal/mol (BChE). The carbonyl group of compounds was in interaction with GLU-199 through H-bonding (1.86 Ao). A hydrogen bonding (2.33 Ao) was also there between the phenolic OH of the ligand and PHE-330 of AChE. Along with this interaction there are pi-stacking between the ligand and GLY- 331/TYR-334 of AChE active site (Figure 2, a and b). Whereas, some good interactions between p-coumaric acid (ligand) and BChE's active site was also observed through hydrogen bonding with amine group of GLN-333 and GLY-331having bond length of 2.24 and 1.80 Ao respectively (Figure 2c and d).
Figure 2.
Interaction of coumaric acid with AChE and BChE crystal structures (Surface and ball and stick representation of the docking pose in the active site of AChE and BChE (1 ACL, 4BOP), showing the highly ranked pose for the active compound, p-coumaric acid, shown as green. The inhibitor predicted to bind in the active site in the space close to the water molecule. Residue, in stick representation, that forming the active site of the enzyme are labelled). (a). 2D diagram of ligand and AChE protein residues in contact. (b). Docked ligand in the active binding pocket of AChE (c). 2D diagram of ligand and BChE protein residues in contact. (d). Docked ligand in the active binding pocket of BChE.
4. Discussion
Based on the ethno-pharmacological uses (Banu and Cathrine, 2015) of Z. oxyphylla, this study was designed to isolate bioactive compounds from this plant. Initially, different extracts and their fractions were prepared. Ethyl acetate fraction was selected for the isolation of the pure compounds; the selection was based on HPLC finger printing results. Three compounds viz., coumaric acid, 3,4-dimethoxy benzoic acid, and 4-heptyloxy benzoic acid were isolated in pure state. The structural elucidation of the isolated compounds was carried out using spectroscopic techniques.
The metabolic processes in the body produces free radicals that could trigger a number of complications like heart diseases, neurodegenerative disorders, suppression of immune system etc., (Ovais et al., 2018). Human body has its own defense mechanisms against the generated free radical. Antioxidant system of human body prevents the generation of free radical through chain breaking thereby preventing human from its hazardous effects. Disturbance in the balance between the detoxification of free radical by defense system and their generation rate leads to serious complications in human (Jacob, 1995). This balance must be maintained within a certain limit to avoid health complications. Certain exogenous substances are supplied in food when this balance disturbs. A number of plant-based herbal medicines have been reported to have capabilities of scavenging free radicals. The isolated compounds showed substantial free radical scavenging activities against the DPPH and ABTS free radicals. The smallest IC50 value, 59 μg/mL (85.8 ± 0.3 percent inhibition at 1000 μg/mL) against DPPH was recorded for coumaric acid. The compound 3,4-dimethoxy benzoic acid with IC50 value of 67 μg/mL and percent inhibition of 82.1 ± 1.2 (against DPPH) was ranked as second potent compound that effectively scavenged the radical. Against ABTS free radical, again coumaric acid was the most potent compound that excellently scavenged the free radical with percent inhibition of 84.4 ± 1.0 (IC50 = 62 μg/mL). The other compounds also exhibited good to satisfactory degree of inhibitory activities against ABTS free radical.
ACHE and BCHE enzymes are responsible for the over hydrolysis of acetyl and butyryl choline in many neurological diseases. In such uneven situations the inhibition of these enzymes is needed (Nawaz and Choudary, 2004). One such example is AD where decrease in the amount of ACh due to hyper activity of AChE and BChE enzymes (Xiao et al., 2017). The isolated compounds showed remarkable anti AChE and BChE inhibitory activities. Against the AChE, coumaric acid was the most potent inhibitor that showed promising percent inhibition of 83.4 ± 0.5 with IC50 value of 80 μg/mL, followed by 4-heptyloxy benzoic acid with percent inhibition of 80.1 ± 1.2 (IC50 = 89 μg/mL). The compounds; 3,4-dimethoxy benzoic acid acid with the IC50 of 90 μg/mL was ranked as a moderate inhibitor of the AChE.
Against the BChE, the isolated compounds also exhibited promising inhibitions. P-Coumaric acid with IC50 value of 80 μg/mL and 83.2 ± 1.1 percent inhibition was the most potent inhibitor of BChE followed by 3,4-dimethoxy benzoic acid with excellent percent inhibition of 80.0 ± 1.1 and IC50 value of 90 μg/mL. The 4-heptyloxy benzoic acid with IC50 value of 91 μg/mL was ranked as third most potent inhibitor. Galantamine, a compound from plant origin was used as positive control. The molecular docking studies also supported the binding ability of coumaric acid with the target enzymes.
Alpha amylase and glucosidase are the important enzymes of glucose metabolic pathways (Nazir et al., 2018). To control hyperglycemia, it is necessary that the activities of these enzymes are kept within control. The isolated compound 3,4-dimethoxy benzoic acid showed maximum inhibition of alpha-glucosidase with a percent inhibition of 84.6 % (IC50 = 84 μg/mL). A moderate percent inhibition was recorded for coumaric acid (IC50 = 96 μg/mL) while lowest for 4-heptyloxy benzoic acid (IC50 = 108 μg/mL). As positive control acarbose was used (IC50 = 70 μg/mL).
Against alpha-amylase, the maximum percent inhibition amongst the isolated compounds was recorded for coumaric acid which showed an inhibition of 86.8% with IC50 value 85 μg/mL followed by 3,4-dimethoxy benzoic acid (IC50 = 112 μg/mL) and 4-heptyloxy benzoic acid (IC50 = 110 μg/mL).
5. Conclusion
The isolated bioactive compounds in in vitro studies showed that compound V is a potent inhibitor of Cholinesterases (AChE and BChE), DPPH/ABTS free radicals, α-amylase and α-glucosidase. Docking studies support the potential use of coumaric acid as effective drug in relieving symptoms associated with dementia. Further studies are needed to confirm the observed effects in animal models.
Declarations
Author contribution statement
Muhammad Zahoora: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Irfan Khanb: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Alam Zeba; Wasim Ul Barib: Performed the experiments; Wrote the paper.
Muhammad Umar Khayam Sahibzadac: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sumaira Naza; Abdul Wahid Kamranb: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Higher Education commission of Pakistan (Project No: 20-2515/R&D/HEC).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ahmad R., Ahmad N., Naqvi A.A. Ziziphus oxyphylla”: ethnobotanical, ethnopharmacological and phytochemical review. Biomed. Pharmacother. 2017;91:970–998. doi: 10.1016/j.biopha.2017.04.129. [DOI] [PubMed] [Google Scholar]
- Ahmad R., Ahmad N., Naqvi A.A., Cos P., Maes L., Apers S., Hermans N., Pieters L. Anti-infective, cytotoxic and antioxidant activity of Ziziphus oxyphylla and Cedrela serrata. Asian Pac J Trop Biomed. 2016;6(8):671–676. [Google Scholar]
- Ahmad R., Upadhyay A., Ahmad M., Pieters L. Antioxidant, antliglycation and antimicrobial activities of Ziziphus oxyphylla and Cedrela serrata extracts. Eur. J. Med. Plants. 2013;3(4):520–529. [Google Scholar]
- Ali R., Shah H.U., Ullah I., Anwar J., Numan M., Humaira K., Awan A., Sohail S.R. Analgesic, anti-inflammatory and antipyretic activities of stem extract of Ziziphus oxyphylla edgew. World J. Zool. 2015;10(2):107–111. [Google Scholar]
- Awad B.M., Habib E.S., Ibrahim A.K., Wanas A.S., Radwan M.M., Helal M.A., ElSohly M.A., Ahmed S.A. Cytotoxic activity evaluation and molecular docking study of phenolic derivatives from Achillea fragrantissima (Forssk.) growing in Egypt. Med. Chem. Res. 2017;26(9):2065–2073. [Google Scholar]
- Banu K.S., Cathrine L. General techniques involved in phytochemical analysis. Int.l J. Adv. Res. Chem. Sci. 2015;2(4):25–32. [Google Scholar]
- Bari W.U., Zahoor M., Zeb A., Khan I., Nazir Y., Khan A., Rehman N.U., Ullah R., Shahat A.A., Mahmood H.M. Anticholinesterase, antioxidant potentials, and molecular docking studies of isolated bioactive compounds from Grewia optiva. Int. J. Food Prop. 2019;22(1):1386–1396. [Google Scholar]
- Dastjerdi Z.M., Namjoyan F., Azemi M.E. Alpha amylase inhibition activity of some plants extract of Teucrium species. Eur. Biolog. Sci. 2015;7(1):26–31. [Google Scholar]
- Devi S., Pandey V., Singh J., Shah A. Peptide alkaloids from Zizyphus species. Phytochemistry (Oxf.) 1987;26(12):3374–3375. [Google Scholar]
- Ghedira K., Chemli R., Caron C., Nuzilard J.-M., Zeches M., Le Men-Olivier L. Four cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry (Oxf.) 1995;38(3):767–772. [Google Scholar]
- Jacob R.A. The integrated antioxidant system. Nutr. Res. 1995;15(5):755–766. [Google Scholar]
- Ji R., Chen Z., Corvini P.F., Kappler A., Brune A., Haider K., Schäffer A. Synthesis of [13C]-and [14C]-labeled phenolic humus and lignin monomers. Chemosphere. 2005;60(9):1169–1181. doi: 10.1016/j.chemosphere.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Kaleem W.A., Muhammad N., Khan H., Rauf A. Pharmacological and phytochemical studies of genus Ziziphus. Middle East J. Sci. Res. 2014;21(8):1243–1263. [Google Scholar]
- Khan I., Zahoor M., Zeb A., Ul Bari W. Vitro antioxidant, antidiabetic, and anticholinesterase, and in vivo toxicological, hypoglycemic, and antilipidemic potentials of Ziziphus oxyphylla. Lat. Am. J. Pharm. 2020;39(1):7–21. [Google Scholar]
- Khan I., Zahoor M., Zeb A., Sahibzada M.U.K., Bari W.U., Naz S. Isolation, characterization, pharmacological evaluation and in silico modeling of bioactive secondary metabolites from Ziziphus oxyphylla a member of Rhamnaceae family. Trop. J. Pharmaceut. Res. 2020;19(2):351–359. [Google Scholar]
- Kuroda M., Ohshima T., Kan C., Mimaki Y. Chemical constituents of the leaves of tussilago farfara and their aldose reductase inhibitory activity. Nat. prod. commun. 2016;11(11) 1934578X1601101109. [PubMed] [Google Scholar]
- Lakache Z., Tigrine-Kordjani N., Tigrine C., Aliboudhar H., Kameli A. Phytochemical screening and antioxidant properties of methanolic extract and different fractions of Crataegus azarolus leaves and flowers from Algeria. Int. Food Res. J. 2016;23(4) [Google Scholar]
- Le Crouéour G., Thépenier P., Richard B., Petermann C., Ghédira K., Zèches-Hanrot M., Lotusine G. A new cyclopeptide alkaloid from Zizyphus lotus. Fitoterapia. 2002;73(1):63–68. doi: 10.1016/s0367-326x(01)00363-x. [DOI] [PubMed] [Google Scholar]
- Muniprasad M., Srinivasulu M., Chalapathi P.V., Potukuchi D.M. Influence of chemical moieties and the flexible chain for the tilted smectic phases in linear hydrogen bonded liquid crystals with Schiff based pyridene derivatives. J. Mol. Struct. 2012;1015:181–191. [Google Scholar]
- Mustapha B., Kubmarawa D., Shagal M., Ardo B. Preliminary phytochemical screening of medicinal plants found in the vicinity of quarry site in demsa, adamawa state, Nigeria. Am. Chem. Sci. J. 2016;11(2):1–7. [Google Scholar]
- Nawaz S.A., Choudhary M.I. New cholinesterase inhibiting bisbenzylisoquinoline alkaloids from Cocculus pendulus. Chem. Pharm. Bull. 2004;52(7):802–806. doi: 10.1248/cpb.52.802. [DOI] [PubMed] [Google Scholar]
- Nazir N., Zahoor M., Nisar M., Khan I., Karim N., Abdel-Halim H., Ali A. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach. BMC Compl. Alternative Med. 2018;18(1):332. doi: 10.1186/s12906-018-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovais M., Ayaz M., Khalil A.T., Shah S.A., Jan M.S., Raza A., Shahid M., Shinwari Z.K. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Compl. Alternative Med. 2018;18(1):1. doi: 10.1186/s12906-017-2057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintus F., Matos M.J., Vilar S., Hripcsak G., Varela C., Uriarte E., Santana L., Borges F., Medda R., Di Petrillo A., Era B. New insights into highly potent tyrosinase inhibitors based on 3-heteroarylcoumarins: anti-melanogenesis and antioxidant activities, and computational molecular modeling studies. Bioorg. Med. Chem. 2017;25(5):1687–1695. doi: 10.1016/j.bmc.2017.01.037. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Russo D., Valentão P., Andrade P., Fernandez E., Milella L. Evaluation of antioxidant, antidiabetic and anticholinesterase activities of Smallanthus sonchifolius landraces and correlation with their phytochemical profiles. Int. J. Mol. Sci. 2015;16(8):17696–17718. doi: 10.3390/ijms160817696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhuja R., Vashist M., Bhoon Y.K., Jain S.C. Phytochemical investigation of Tabebuia palmeri. Chem. Nat. Compd. 2014;49(6):1039–1042. [Google Scholar]
- Scott K.N. Carbon-13 nuclear magnetic resonance of biologically important aromatic acids. I. Chemical shifts of benzoic acid and derivatives. J. Am. Chem. Soc. 1972;94(24):8564–8568. doi: 10.1021/ja00779a045. [DOI] [PubMed] [Google Scholar]
- Singh B., Pandey V. An N-formyl cyclopeptide alkaloid from Zizyphus nummularia bark. Phytochemistry (Oxf.) 1995;38(1):271–273. doi: 10.1016/0031-9422(94)00548-8. [DOI] [PubMed] [Google Scholar]
- Tripathi M., Pandey M., Jha R., Pandey V., Tripathi P., Singh J. Cyclopeptide alkaloids from Zizyphus jujuba. Fitoterapia. 2001;72(5):507–510. doi: 10.1016/s0367-326x(01)00278-7. [DOI] [PubMed] [Google Scholar]
- Xiao S., Wang T., Ma X., Qin Y., Li X., Zhao Z., Liu X., Wang X., Xie H., Jiang Q., Sun L. Efficacy and safety of a novel acetylcholinesterase inhibitor octohydroaminoacridine in mild-to-moderate Alzheimer's disease: a phase II multicenter randomised controlled trial. Age Ageing. 2017;46(5):767–773. doi: 10.1093/ageing/afx045. [DOI] [PubMed] [Google Scholar]
- Zahoor M., Zafar R., Rahman N.U. Isolation and identification of phenolic antioxidants from Pistacia integerrima gall and their anticholine esterase activities. Heliyon. 2018;4(12) doi: 10.1016/j.heliyon.2018.e01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.