Abstract
Purpose:
To investigate if the presence and severity of traction bronchiectasis/bronchiolectasis are associated with poorer survival in subjects with ILA.
Method:
The study included 3,594 subjects (378 subjects with ILA and 3,216 subjects without ILA) in AGES-Reykjavik Study. Chest CT scans of 378 subjects with ILA were evaluated for traction bronchiectasis/bronchiolectasis, defined as dilatation of bronchi/bronchioles within areas demonstrating ILA. Traction bronchiectasis/bronchiolectasis Index (TBI) was assigned as: TBI=0, ILA without traction bronchiectasis/bronchiolectasis: TBI=1, ILA with bronchiolectasis but without bronchiectasis or architectural distortion: TBI=2, ILA with mild to moderate traction bronchiectasis: TBI=3, ILA and severe traction bronchiectasis and/or honeycombing. Overall survival (OS) was compared among the subjects in different TBI groups and those without ILA.
Results:
The median OS was 12.93 years (95%CI; 12.67 – 13.43) in the subjects without ILA; 11.95 years (10.03 – not reached) in TBI-0 group; 8.52 years (7.57 – 9.30) in TBI-1 group; 7.63 years (6.09 – 9.10) in TBI-2 group; 5.40 years (1.85 – 5.98) in TBI-3 group. The multivariable Cox models demonstrated significantly shorter OS of TBI-1, TBI-2, and TBI-3 groups compared to subjects without ILA (P<0.0001), whereas TBI-0 group had no significant OS difference compared to subjects without ILA, after adjusting for age, sex, and smoking status.
Conclusions:
The presence and severity of traction bronchiectasis/bronchiolectasis are associated with shorter survival. The traction bronchiectasis/bronchiolectasis is an important contributor to increased mortality among subjects with ILA.
Keywords: Interstitial lung abnormality, Usual interstitial pneumonia, Pulmonary fibrosis, Traction bronchiectasis, Age Gene/Environment Susceptibility-Reykjavik Study
Introduction
Interstitial lung abnormalities (ILA) have been increasingly recognized as a common set of features on chest computed tomography (CT), occurring in 4% - 9% of smokers and 2% - 7% of nonsmokers, however, clinical evaluation and management of ILA remain to be determined [1–5]. ILA have been defined as nondependent ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, and traction bronchiectasis as previously reported [6, 7] in research participants without a known history of interstitial lung disease. Subjects with ILA have increased symptoms of chronic cough and shortness of breath, decreased total lung capacity, decrease diffusion capacity, reduced exercise capacity, and increased all-cause mortality [1, 2, 4, 5, 7, 8]. The progression of ILA is associated with accelerated lung function decline and an increased rate of mortality [5, 9].
Traction bronchiectasis is known as an important feature of fibrotic lung diseases, which is identified in the early stage of the disease and results in honeycomb lung in the end stage of fibrotic lung diseases. Traction bronchiolectasis is dilatation of bronchioles, which proceeds the development of traction bronchiectasis. Traction bronchiectasis/bronchiolectasis is noted as dilatation of airway (i.e. bronchi and bronchioles) within the lung areas demonstrating ILA on CT. Pathologically, traction bronchiectasis and bronchiolectasis are thought to be the result of contraction of lung tissue surrounding airway because of fibrosis, inflammation, and scarring, which correspond to the radiologic findings of bronchiectasis and bronchiolectasis on chest CT. Several studies have reported that traction bronchiectasis was associated with mortality in patients with idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP) [10, 11]. Recently, we have reported that ILA with architectural distortion, often associated with established traction bronchiectasis, has been associated with poor survival [12]. However, architectural distortion can be difficult to identify, and its reproducibility is unknown. Traction bronchiectasis and bronchiolectasis would probably represent a simpler and more reliable sign of early fibrosis in ILA.
We hypothesized that the presence and severity of traction bronchiectasis and bronchiolectasis in subjects with ILA would be associated with poorer survival. To address the hypothesis, we evaluated CT scans from 3,594 subjects in the Age Gene/Environment Susceptibility (AGES)-Reykjavik Study.
Materials and Methods
Study population
This study was approved by the institutional review boards, and all subjects provided written informed consent. The original cohort consisted of 5,764 subjects in the AGES-Reykjavik Study. The AGES-Reykjavik Study is a longitudinal birth cohort including women and men born in Reykjavik from 1907 to 1935 who are now followed by the Iceland Heart Association [13] The protocols of participant enrollment in the study have been previously reported [5, 12, 14].
The original cohort of 5,764 subjects in the AGES-Reykjavik Study have been previously studied for the presence of ILA on their chest CT scans [5, 12, 14]. ILA are defined radiologically on chest CT scans as increased lung density including nondependent ground-glass or reticular abnormalities, diffuse centrilobular nodularity, non-emphysematous cysts, honeycombing, and traction bronchiectasis as previously described [2, 4, 6, 7]. Of the 5,764 subjects, ILA were present in 378 (7%), were indeterminate in 1,726 (32%), and were absent in 3,216 (61%), as reported in the prior study which demonstrated that the presence of ILA was associated with higher risk of all-cause mortality [5].
The current study focused on the 378 subjects who demonstrated ILA on chest CT, to further evaluate the presence and severity of traction bronchiectasis/bronchiolectasis and to investigate the impact of traction bronchiectasis/bronchiolectasis on survival among subjects with ILA. The group of 3,216 subjects without ILA (and thus without traction bronchiectasis/bronchiolectasis) from the original cohort was used as a reference group in the assessment of survival. Therefore, a total of 3,594 subjects including 378 subjects with ILA and 3,216 subjects without ILA comprised the cohort of the present study.
Traction bronchiectasis on CT images and Traction Bronchiectasis/Bronchiolectasis Index (TBI)
Traction bronchiectasis/bronchiolectasis was defined as dilatation of airway (i.e. bronchi and bronchioles) within areas demonstrating ILA on chest CT. In 378 subjects with ILA, severity of traction bronchiectasis was visually evaluated by comparing the diameter of the airway with the diameter of the adjacent pulmonary artery using a categorical 5-point score: 0) none (no dilatation), 1) minimal (dilatation of bronchioles without obvious bronchiectasis or architectural distortion), 2) mild (dilated bronchi almost same diameters with adjacent pulmonary artery), 3) moderate (between mild and severe), and 4) severe (remarkable dilatation of bronchi including honeycombing), in the lung regions where traction bronchiectasis/bronchiolectasis was most prominent [15, 16]. Two board-certified chest radiologists (T. H. and H. H.) interpreted CT images of each subject and scored traction bronchiectasis/bronchiolectasis by consensus. The radiologists were blinded to the demographical and clinical data of the subjects.
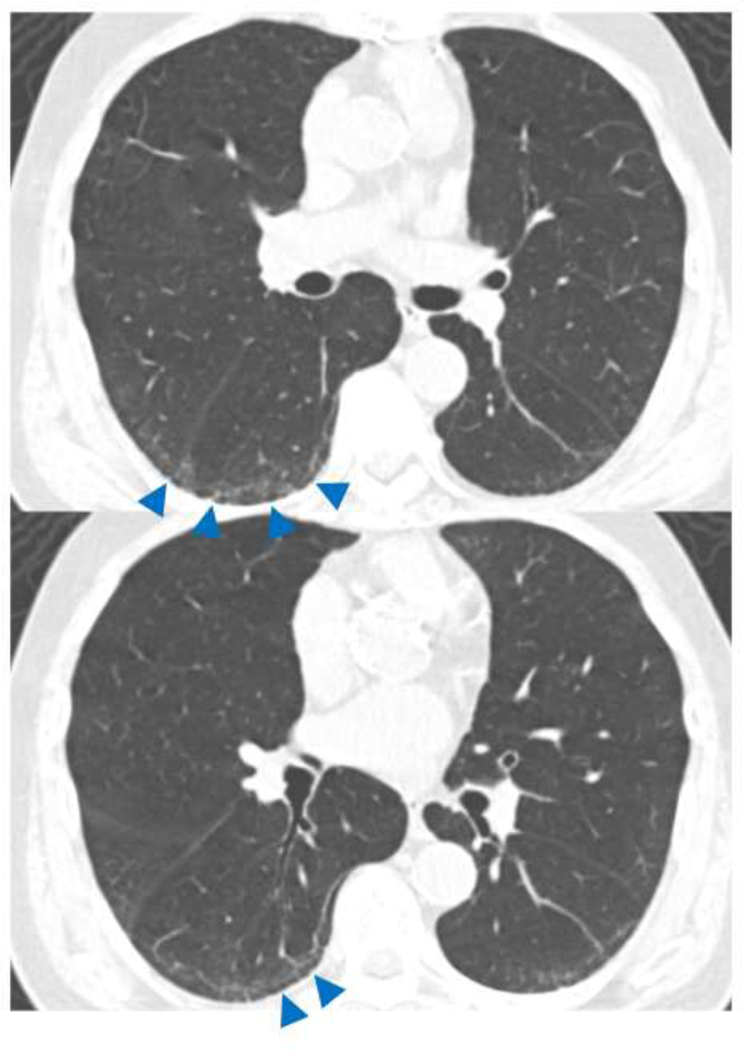
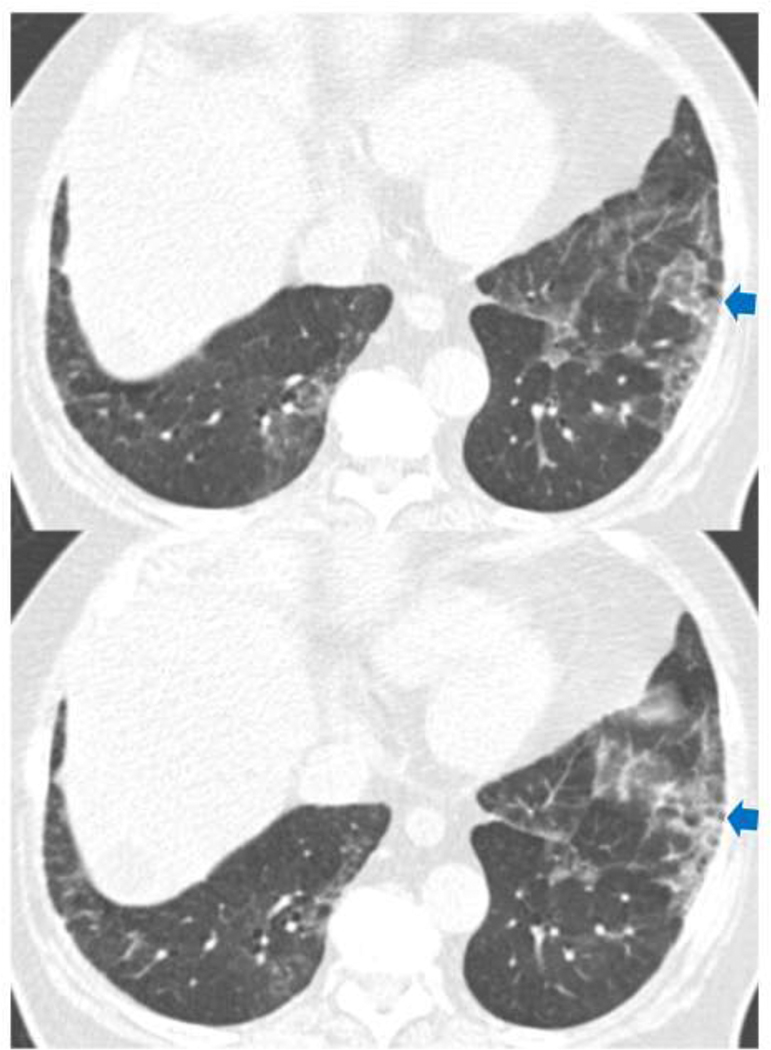
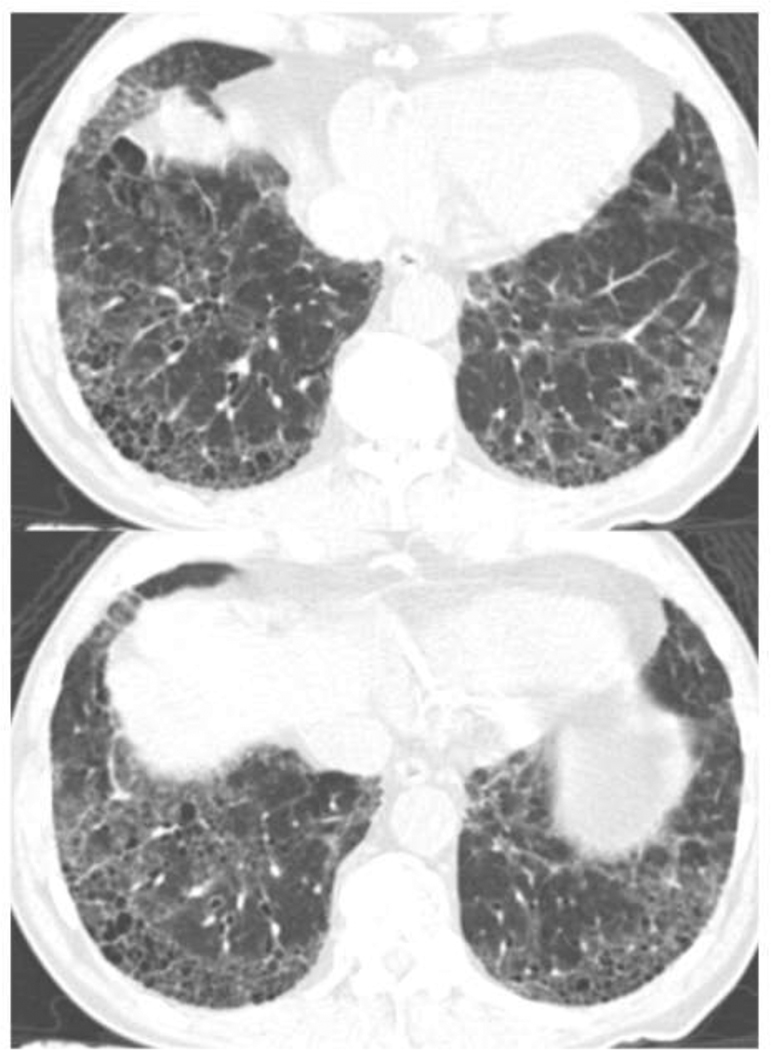
Traction Bronchiectasis/bronchiolectasis Index (TBI) was defined from the above mentioned 5-point traction bronchiectasis score: TBI=0, when traction bronchiectasis score is 0 (ILA without traction bronchiectasis/bronchiolectasis): TBI=1, ILA with bronchiolectasis (score 1) but without bronchiectasis or architectural distortion (Figure 1), TBI=2, ILA with mild or moderate traction bronchiectasis (score 2 or 3) (Figure 2): TBI=3, ILA and severe traction bronchiectasis and/or honeycombing (score 4) (Figure 3).
Figure 1.
TBI=1. CT images demonstrated subpleural ground-glass and reticular opacities indicating ILA. Note is made of dilatation of bronchioles (arrows) without obvious architectural distortion in the area of subpleural opacities of ILA. ILA, interstitial lung abnormalities; TBI, traction bronchiectasis index.
Figure 2.
TBI=2. CT images demonstrated ground-glass and reticular opacities with subpleural and basilar distribution indicating ILA. Note is made of mild bronchiectasis (arrows) associated with architectural distortion in the area of subpleural opacities of ILA. ILA, interstitial lung abnormalities; TBI, traction bronchiectasis index.
Figure 3.
TBI=3. CT images demonstrated ground-glass and reticular opacities with subpleural and basilar distribution indicating ILA. Note is made of severe bronchiectasis associated with architectural distortion as well as honeycombing. ILA, interstitial lung abnormalities; TBI, traction bronchiectasis index.
The image analyses data were sent to Iceland Heart Association after the image review, according to the protocol. The data including survival were then provided to the investigators’ team after deidentification, so that the personal data of each participant were not identifiable.
Statistical analysis
The demographics were compared among the control group and the four groups, using Steel test for age (continuous variables) and Fisher’s exact test for sex and smoking history (categorical variables). Overall survival (OS) was compared among the control group and the four groups according to TBI. OS was defined as the time from the date of recruitment to the AGES-Reykjavik Study (between January 2002 and February 2006) to the date of death of any cause. Patients who were still alive by the time of analyses were censored at the last known date of follow-up. OS of the different groups were estimated using the method of Kaplan-Meier, and the log-rank test with Bonferroni correction was used to assess differences in the OS distributions between groups. The log-rank trend test was utilized to analyze trend between TBI and OS. The Cox proportional hazards models were used to estimate hazard ratios (HRs) for univariate analyses, as well as for multivariable analyses which adjusted for the three available demographic variables including age, sex, and smoking status. Statistical analyses were performed using R version 3.5.3 software (R Foundation for Statistical Computing, Vienna, Austria). All P values were two-sided and P < 0.05 was considered statistically significant.
Results
Table 1 provides the demographics of 3,594 subjects including 378 subjects with ILA who were subcategorized into four TBI groups (TBI-0, TBI-1, TBI-2, and TBI-3), as well as the reference group of 3,216 subjects without ILA. The subjects with ILA and traction bronchiectasis/bronchiolectasis (TBI-1, TBI-2, and TBI-3) were older (all P < 0.001), more likely to be male than those in the group without ILA (TBI-1 and TBI-2, P < 0.001; TBI-3, P = 0.023), whereas subjects with ILA but without traction bronchiectasis/bronchiolectasis (TBI-0 group) showed no significant difference in age and sex from the group without ILA (P = 0.966 and P = 0.915, respectively). The subjects with TBI-0, TBI-1, and TBI-2 were more likely to be current or former smokers compared to the group without ILA (TBI-1 and TBI-3, P < 0.001; TBI-2, P = 0.006).
Table 1.
Baseline characteristics of participants stratified by ILA (Interstitial lung Abnormalities) and TBI (Traction Bronchiectasis Index)
| Demographics | No ILA (n=3216) | Subjects with ILA (n=378) | |||
|---|---|---|---|---|---|
| TBI-0 No TB (n=93) | TBI-1 Bronchiolectasis (n=150) | TBI-2 Mild/moderate TB (n=118*) | TBI-3 Severe TB (n=17) | ||
| Age (years) | |||||
| Median [Range] | 75 [66–96] | 75 [67–94] | 78 [67–92] | 79 [69–91] | 79 [69–92] |
| P value† | - | 0.966 | <0.0001 | <0.0001 | 0.032 |
| Sex | |||||
| Male | 1306 | 37 | 87 | 70 | 12 |
| Female | 1910 | 56 | 63 | 48 | 5 |
| P value† | - | 0.915 | <0.0001 | <0.0001 | 0.023 |
| Smoking History | |||||
| Never | 1463 | 24 | 51 | 26 | 4 |
| Former | 1376 | 45 | 79 | 68 | 10 |
| Current | 374 | 22 | 20 | 24 | 3 |
| Unknown | 3 | 2 | 0 | 0 | 0 |
| P value¶ | - | <0.0001 | 0.006 | <0.0001 | 0.087 |
Include 72 subjects with mild traction bronchiectasis and 46 subjects with moderate traction bronchiectasis
P value is for the comparison to the control group.
P value is for the comparison of never and former/current smokers to those in the control group.
ILA, interstitial lung abnormality; TBI, traction bronchiectasis index; TB, Traction Bronchiectasis
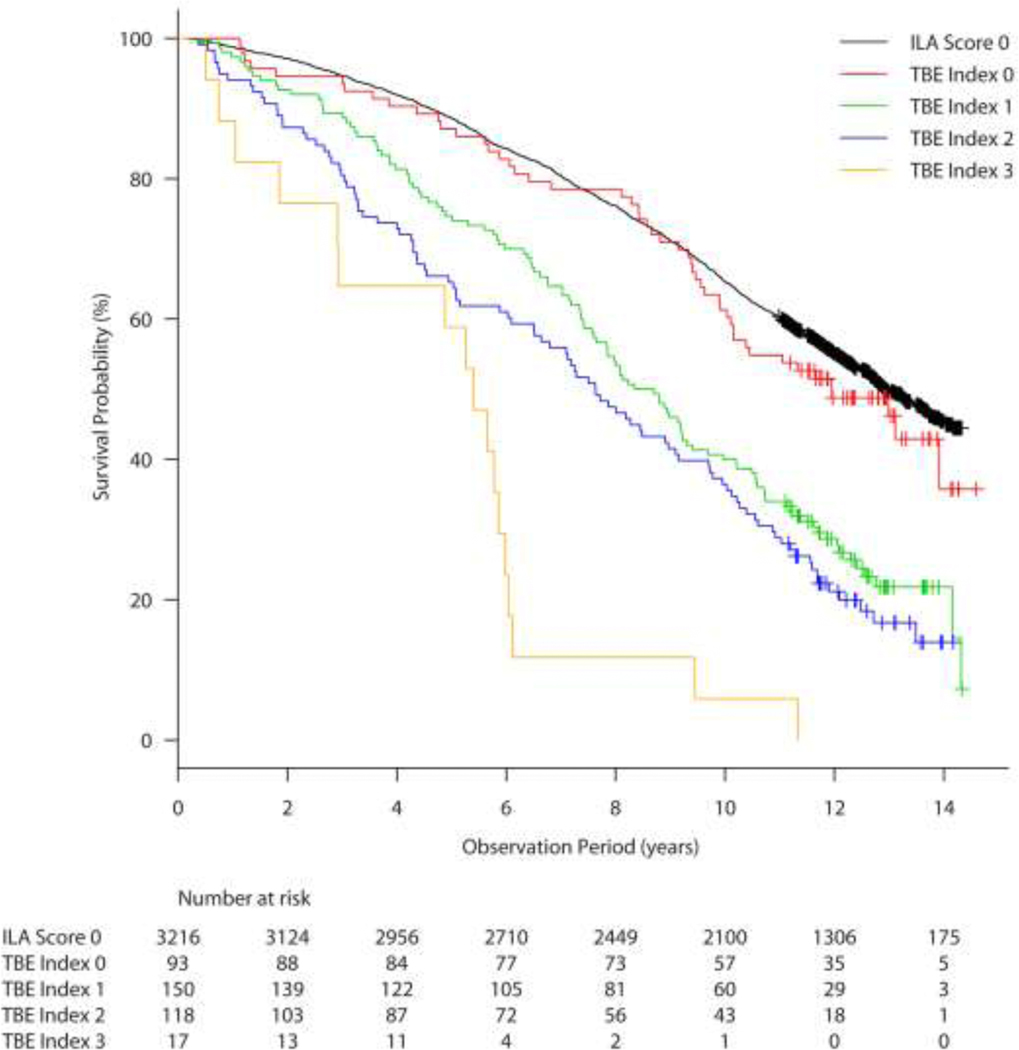
Figure 4 demonstrates the Kaplan-Meier estimates of overall survival (OS) of the group without ILA (3,216 subjects) and 378 subjects with ILA categorized into four TBI groups. The median OS was 12.93 years (95% confidence interval (CI) for the median; 12.67 – 13.43 years) in the group without ILA; 11.95 years (95% CI; 10.03 – not reached) for the subjects in the TBI-0 group (ILA without traction bronchiectasis/bronchiolectasis); 8.52 years (95% CI; 7.57 – 9.30) for the subjects in TBI-1 group (ILA with bronchiolectasis but without obvious bronchiectasis or architectural distortion); 7.63 years (95% CI; 6.09 – 9.10) for the subjects in TBI-2 group (ILA with mild to moderate traction bronchiectasis); and 5.40 years (95% CI; 1.85 – 5.98) for the subjects in TBI-3 group (ILA and severe traction bronchiectasis and/or honeycombing) (Table 2). A clear trend was observed among subjects with ILA that the higher the TBI, the OS is shorter (P = 1.2×10−9). In the univariate Cox model, OS of TBI-1, TBI-2, and TBI-3 groups were significantly shorter compared to the reference group without ILA (TBI-1: hazard ratio (HR) = 2.178; P < 0.0001; TBI-2: HR = 2.647; P < 0.0001; TBI-3: HR = 6.847; P < 0.0001, each compared to the reference group without ILA). In contrast, OS of the TBI-0 group did not differ significantly from the reference group without ILA (HR = 1.136; P = 0.375), in spite of the fact that all subjects in the TBI-0 group did have ILA.
Figure 4.
Kaplan-Meier survival curves showing percent survival provability over time in years among participants with ILA stratified by TBI = 0, 1, 2, and 3 compared with the subjects without ILA. ILA, interstitial lung abnormalities; TBI, traction bronchiectasis index.
Table 2.
Overall Survival stratified by ILA and TBI with Univariate Cox Model using the subjects without ILA as the reference group
| Median OS (years) | 95% CI for the median OS | HR | 95% CI for HR | P value* | |
|---|---|---|---|---|---|
| No ILA (n=3216) | 12.93 | 12.67 – 13.43 | - | - | - |
| TBI-0 ILA without TB (n=93) | 11.95 | 10.03 – NR | 1.136 | 0.857 – 1.505 | 0.375 |
| TBI-1 ILA with traction bronchiolectasis (n=150) | 8.52 | 7.57 – 9.30 | 2.178 | 1.8 – 2.635 | <0.0001 |
| TBI-2 ILA with mild/moderate TB (n=118) | 7.63 | 6.09 – 9.10 | 2.647 | 2.153 – 3.253 | <0.0001 |
| TBI-3 ILA with sever TB/honeycombing (n=17) | 5.40 | 1.85 – 5.98 | 6.847 | 4.237 – 11.07 | <0.0001 |
From univariable Cox models, in comparison with the control group
CI, confidence interval; ILA, interstitial lung abnormality; NR, not reached; TBI, traction bronchiectasis index; TB, Traction Bronchiectasis
The multivariable Cox models also demonstrated significantly shorter OS of TBI-1, TBI-2, and TBI-3 groups compared to the reference group without ILA (TBI-1: HR = 1.628, P < 0.0001; TBI-2: HR = 1.744, P < 0.0001; TBI-3: HR = 4.328, P < 0.0001), whereas TBI-0 group shad no significant OS difference with the reference group without ILA (HR = 1.113, P = 0.466), after adjusting for age (HR = 1.13, P < 0.0001), sex (male vs. female; HR = 1.49, P < 0.0001) and smoking status (former/current vs. never smokers; HR = 1.274, P < 0.0001).
Further comparisons of OS among the different groups according to the ILA and TBI status were performed with the log-rank test with Bonferroni correction (Table 3). OS was significantly shorter in TBI-1, TBI-2, and TBI-3 groups compared to the subjects without ILA, whereas there was no significant difference for OS between TBI-0 group (the subject with ILA but without bronchiectasis/bronchiolectasis) and the subjects without ILA (P = 1.00), further confirming the results in the Cox models. Comparison of OS between TBI-0 group versus TBI-1, TBI-2, and TBI-3 groups showed that each of TBI-1, TBI-2, and TBI-3 groups has significantly shorter OS compared to TBI-0 group (P = 0.001, P = 1.9×10−5, P = 1.5×10−10, respectively). When compared to TBI-1 group, TBI-2 group showed no significant difference for OS (P = 1.00), but TBI-3 group has significantly shorter OS than TBI-1. OS of TBI-3 group was also significantly shorter compared with TBI-2 group (P = 0.0086). The results indicate that the detailed stratification by TBI status can differentiate subgroups of subjects with different prognosis.
Table 3.
Comparisons of overall survival among the subjects in subgroups stratified by ILA and TBI status, using the log-rank test with Bonferroni correction
| No ILA | TBI-0 (ILA without traction bronchiectasis/bronchiolectasis) | TBI-1 (ILA with traction bronchiolectasis) | TBI-2 (ILA with mild/moderate traction bronchiectasis) | TBI-3 (ILA with severe traction bronchiectasis/honeycombing) | |
|---|---|---|---|---|---|
| No ILA | _ | _ | _ | _ | _ |
| TBI-0 (ILA without traction bronchiectasis/ bronchiolectasis) | 1 | _ | _ | _ | _ |
| TBI-1 (ILA with traction bronchiolectasis) | 2.0×10−15 | 0.001 | _ | _ | _ |
| TBI-2 (ILA with mild/moderate traction bronchiectasis) | <2.0×10−16 | 1.9×10−5 | 1 | _ | _ |
| TBI-3 (ILA with severe traction bronchiectasis/ honeycombing) | <2.0×10−16 | 1.5×10−10 | 4.6×10−5 | 0.0086 | _ |
ILA, interstitial lung abnormality; TBI, Traction bronchiectasis index
Discussion
To our knowledge, several studies have shown that traction bronchiectasis is associated with mortality in IPF/UIP (10, 11, 15), and in subjects with ILA (12), however, this is the first report demonstrating the association between traction bronchiectasis/bronchiolectasis and poor survival in subjects with ILA including the subjects with ILA without obvious fibrotic changes. When traction bronchiectasis index (TBI) is higher, overall survival (OS) is shorter. The subjects with TBI-1, 2, or 3 had significantly shorter OS compared to the subjects without ILA, however, in contrast, subjects with ILA but without traction bronchiectasis/bronchiolectasis (TBI-0) had similar OS compared to the reference group without ILA (and thus without bronchiectasis/bronchiolectasis). The results indicate that the presence of traction bronchiectasis/bronchiolectasis may be an important contributor to increased mortality among subjects with ILA, which provide important insights to further understand the clinical impact of ILA [1].
The presence of bronchiectasis among subjects with ILA is associated with older age, male sex, and current and former smoking history. The results are consistent with the prior studies that have also reported these characteristics in subjects with pulmonary fibrosis or ILA [1–5]. Miller et al from our investigational group reported that measurable increase in airway wall thickness is consistently noted in research participants with ILA and in patients with IPF, suggesting that early detectable airway abnormalities may play a role in pathophysiology of fibrotic lung diseases [17].
Traction bronchiectasis has been identified as a prognostic factor predicting poor prognosis in fibrotic lung disease patients. In 2008, Sumikawa et al investigated CT images in 98 patients with pathologically proven UIP, and reported traction bronchiectasis as a predictor of poor prognosis with HR of 1.30 (95% CI; 1.18 – 1.43) [10]. Edey and Hansell et al. independently studied CT images of 146 consecutive individuals with fibrotic idiopathic interstitial pneumonias presented between 2000 and 2004, and found traction bronchiectasis to be a powerful predictor of poor outcome (HR = 1.85; 95% CI; 1.42 – 2.40, P < 0.001) [16]. It has been published that the presence of traction bronchiectasis correlates with profusion of fibroblastic foci [18]. Traction bronchiectasis has been an important CT feature for probable UIP and typical UIP in the recently updated Fleischner and American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Latin America Thoracic Association (ALAT) criteria [19, 20]. Therefore, it is of great interest to study if the severity of traction bronchiectasis may predict OS in subjects with ILA.
Our data demonstrated that median overall survival (OS) with TBI-1 (ILA with bronchiolectasis but without bronchiectasis or architectural distortion), TBI-2 (ILA with mild to moderate traction bronchiectasis), and TBI-3 group (ILA and severe traction bronchiectasis and/or honeycombing) were significantly shorter (P < 0.0001) compared to that of subjects without ILA. The subjects with TBI-1 had significantly shorter OS compared to the subjects without ILA, even though the subjects with TBI-1 had only dilation of bronchioles without bronchiectasis, architectural distortion or definite evidence of fibrotic lung disease yet. It is possible that bronchiolectasis is an earlier sign of fibrotic lung disease, before the development of traction bronchiectasis and architectural distortion. The subjects with TBI-0 (ILA without bronchiectasis/bronchiolectasis) had similar overall survival compared to the subjects in the reference group without ILA (P = 0.375), in spite of the fact that the subjects with TBI-0 did have ILA. There was clear trend observed that the higher the TBI, the shorter the median OS, when tested using the log-rank trend test (P = 1.2×10−9). The differences between every pair of TBI groups comprised of TBI-0, TBI-1, TBI-2, and TBI-3 were statistically significant except for between TBI-1 and TBI-2 as shown in Table 3, when they were analysed using the univariate log-rank test with Bonferroni correction as a supplement to further demonstrate the differences of OS among TBI groups shown in Table 2 and Figure 4 while adjusting for multiple comparisons (Kaplan-Meier survival curves). TBI remained significant in the multivariate Cox model that adjusted for other significant factors including age, sex, and smoking status, demonstrating that TBI can be an independent predictor of shorter survival in subjects with ILA. These findings indicate that TBI may play an important role in determining the clinical outcome of subjects with ILA.
Furthermore, our results demonstrated that presence of traction bronchiolectasis further stratified the subjects with ILA without obvious bronchiectasis or architectural distortion into two groups of TBI-0 and TBI-1, which have different survival (TBI-0, 11.95 years with 95% CI; 10.03 – not reached versus TBI-1, 8.52 years with 95% CI; 7.57 – 9.30; p=0.001). The result is important and unique in that it indicates the potential predictive value of identification of traction bronchiolectasis on imaging for the prognosis of subjects with ILA who do not yet demonstrate definite fibrosis such as architectural distortion or bronchiectasis. Recently, Putman et al reported ILA with definite fibrosis defined by architectural distortion was associated with a 70% increase in the risk of death in participants in the AGES-Reykjavik Study (HR=1.7, 95%CI 1.3–2.1, p<0.0001) [12]. However, this previous study did not stratify subjects with ILA without definite fibrosis, and thus distinct from the present study that carried out the detailed evaluation of subject with ILA without definite fibrosis with a particular focus on presence or absence of bronchiolectasis. We agree that is necessary to investigate if the alteration represented by TBI-1 is an early fibrosis from the histological point of view in the future.
Our study has limitations. The study population of the AGES-Reykjavik, as designed in its original protocol, includes a population of older adults. Further studies with younger population cohorts are needed. The evaluation of traction bronchiectasis was performed qualitatively by visual assessment, without using the quantitative approaches such as texture analysis, machine learning, or artificial intelligence, which is an important area of investigational focus in the future, for which the results of the current study from a large observational cohort can provide important training and validation data sets [20–25]. The traction bronchiectasis was evaluated visually by consensus of two radiologists. It has been reported that many of various CT findings in patients with fibrotic lung diseases have only poor to good interobserver correlations. However, traction bronchiectasis had one of the highest kappa values of 0.75, when two pairs of consensus reading by two radiologists were tested in the report by Sumikawa et al. [10]. The interobserver variability was not examined in this study. It is a potential limitation that this type of approach may require those with significant experience in this area to review images. The overall survival of subjects with TBI-3 is 5.4 years, which are comparable with patients with ILD. This fact indicates that these subjects with TBI-3 probably have ILD. The analysis of survival included all-cause mortality, rather than deaths caused by ILA. It is also important to address whether and how traction bronchiectasis progresses over time and eventually lead to deaths, which will be investigated as the next step from our ongoing study of AGES-Reykjavik cohort with five-year follow-up scans.
In conclusion, among the subjects with ILA in the AGES-Reykjavik Study, the presence and severity of traction bronchiectasis/bronchiolectasis are associated with shorter survival, whereas subjects with ILA without traction bronchiectasis/bronchiolectasis had similar survival with those without ILA. Traction bronchiectasis index (TBI) may serve as a useful imaging marker for the future scheme of initial evaluation, monitoring, and management of subjects with ILA.
Highlights:
Traction bronchiectasis (TB) is noted within interstitial lung abnormalities (ILA) on CT.
TB is associated with shorter survival in ILA.
ILA with dilation of bronchioles (bronchiolectasis) without TB also shows shorter survival.
Bronchiolectasis may be an earlier sign of fibrotic lung disease including ILA.
Traction bronchiectasis/bronchiolectasis index (TBI) predicts shorter survival in ILA.
Acknowledgments
Funding Information:
Dr. Nishino is supported by NIH grant R01 CA203636. Dr. Putman is supported by NIH grant K08 HL140087. Dr. Gudmundsson is supported by project grant 141513-051 from the Icelandic Research Fund. Dr. Washko is supported by NIH grants R01 HL116473 and R01 HL122464. Dr. Christiani is supported by NIH (NCI) grant # U01CA209414. The Age, Gene/Environment Susceptibility-Reykjavik Study was supported by NIH contracts N01-AG-1-2100 and HHSN27120120022C, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Dr. Gudnason is supported by NIA grant: 27120120022C and project grant 141513-051 from the Icelandic Research Fund. Dr. Hunninghake is supported by NIH grants R01 HL111024, R01 HL130974, R01 135142, and project grant 141513-051 from the Icelandic Research Fund.
Conflict of interest
MN reports personal fees from Daiichi Sankyo, personal fees from AstraZeneca, grants from Research grant to the institution from Merck, grants from Research grant to the institution from Canon Medical systems, grants from Research grant to the institution from AstraZeneca, grants from Research grant to the institution from Daiichi Sankyo, personal fees from Roche, grants from NIH, outside the submitted work; RKP reports grants from NIH, during the conduct of the study; RSJE reports grants from Boehringer Ingelheim, personal fees from Boehringer Ingelheim, personal fees from Chiesi, grants from NHBLI, outside the submitted work; and he is also a founder and co-owner of Quantitative Imaging Solutions which is a company that provides image based consulting and develops software to enable data sharing.; RSJE is also a founder and co-owner of Quantitative Imaging Solutions which is a company that provides image based consulting and develops software to enable data sharing.: Dr. Washko reports grants from NIH, grants and other from Boehringer Ingelheim, other from Quantitative Imaging Solutions, other from PulmonX, grants from BTG Interventional Medicine, grants and other from Janssen Pharmaceuticals, other from GlaxoSmithKline, other from Novartis, other from Vertex, outside the submitted work; and Dr. Washko’s spouse works for Biogen.; DAL reports personal fees from Boehringer Ingelheim, personal fees from Parexel, Inc, personal fees from Veracyte, Inc, outside the submitted work; In addition, DAL has a pending patent “Systems and methods for automatic detection and quantification of pathology using dynamic feature classification.”: GMH reports personal fees from Boehringer-Ingelheim, personal fees from Gerson Lehrman Group, personal fees from Mitsubishi Chemical, outside the submitted work; HH reports grants from Canon Medical System Inc, grants from Konica Minolta Inc, other from Mitsubishi Chemical Inc, other from Canon Medical System Inc, outside the submitted work.
Abbreviation
- AGES-Reykjavik Study
Age Gene/Environment Susceptibility-Reykjavik Study
- ILA
interstitial lung abnormality
- TBI
traction bronchiectasis/bronchiolectasis index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hatabu H, Hunninghake GM, Lynch DA, Interstitial Lung Abnormality: Recognition and Perspectives, Radiology 291(1) (2019) 1–3. [DOI] [PubMed] [Google Scholar]
- [2].Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, Misumi S, Kwon KS, Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate, Radiology 268(2) (2013) 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA, A common MUC5B promoter polymorphism and pulmonary fibrosis, The New England journal of medicine 364(16) (2011) 1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, Nishino M, Araki T, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Murphy E, Steele MP, Loyd JE, Schwarz MI, Fingerlin TE, Rosas IO, Washko GR, O’Connor GT, Schwartz DA, MUC5B promoter polymorphism and interstitial lung abnormalities, The New England journal of medicine 368(23) (2013) 2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, El-Chemaly S, Coxson HO, Celli BR, Fernandez IE, Zazueta OE, Ross JC, Harmouche R, Estepar RS, Diaz AA, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Budoff MJ, Kinney GL, Hokanson JE, Williams MC, Murchison JT, MacNee W, Hoffmann U, O’Donnell CJ, Launer LJ, Harrris TB, Gudnason V, Silverman EK, O’Connor GT, Washko GR, Rosas IO, Hunninghake GM, Association Between Interstitial Lung Abnormalities and All-Cause Mortality, Jama 315(7) (2016) 672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San Jose Estepar R, Silverman EK, Rosas IO, Hatabu H, Identification of early interstitial lung disease in smokers from the COPDGene Study, Academic radiology 17(1) (2010) 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D’Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Lung volumes and emphysema in smokers with interstitial lung abnormalities, The New England journal of medicine 364(10) (2011) 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Divo MJ, Celli BR, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Hunninghake GM, Interstitial lung abnormalities and reduced exercise capacity, American journal of respiratory and critical care medicine 185(7) (2012) 756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Schwartz DA, Rosas IO, Washko GR, O’Connor GT, Hunninghake GM, Development and Progression of Interstitial Lung Abnormalities in the Framingham Heart Study, American journal of respiratory and critical care medicine 194(12) (2016) 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sumikawa H, Johkoh T, Colby TV, Ichikado K, Suga M, Taniguchi H, Kondoh Y, Ogura T, Arakawa H, Fujimoto K, Inoue A, Mihara N, Honda O, Tomiyama N, Nakamura H, Muller NL, Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival, American journal of respiratory and critical care medicine 177(4) (2008) 433–9. [DOI] [PubMed] [Google Scholar]
- [11].Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, Walsh SL, Wells AU, Hansell DM, Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures, The European respiratory journal 49(1) (2017). [DOI] [PubMed] [Google Scholar]
- [12].Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, Yanagawa M, Nishino M, Miller ER, Eiriksdottir G, Gudmundsson EF, Tomiyama N, Honda H, Rosas IO, Washko GR, Cho MH, Schwartz DA, Gudnason V, Hatabu H, Hunninghake GM, Imaging Patterns are Associated with Interstitial Lung Abnormality Progression and Mortality, American journal of respiratory and critical care medicine (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V, Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics, American journal of epidemiology 165(9) (2007) 1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Putman RK, Gudmundsson G, Araki T, Nishino M, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Ross JC, San Jose Estepar R, Miller ER, Yamada Y, Yanagawa M, Tomiyama N, Launer LJ, Harris TB, El-Chemaly S, Raby BA, Cho MH, Rosas IO, Washko GR, Schwartz DA, Silverman EK, Gudnason V, Hatabu H, Hunninghake GM, The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes, The European respiratory journal 50(3) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Walsh SL, Sverzellati N, Devaraj A, Wells AU, Hansell DM, Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants, European radiology 22(8) (2012) 1672–9. [DOI] [PubMed] [Google Scholar]
- [16].Edey AJ, Devaraj AA, Barker RP, Nicholson AG, Wells AU, Hansell DM, Fibrotic idiopathic interstitial pneumonias: HRCT findings that predict mortality, European radiology 21(8) (2011) 1586–93. [DOI] [PubMed] [Google Scholar]
- [17].Miller ER, Putman RK, Diaz AA, Xu H, San Jose Estepar R, Araki T, Nishino M, Poli de Frias S, Hida T, Ross J, Coxson H, Dupuis J, O’Connor GT, Silverman EK, Rosas IO, Hatabu H, Washko G, Hunninghake GM, Increased Airway Wall Thickness in Interstitial Lung Abnormalities and Idiopathic Pulmonary Fibrosis, Annals of the American Thoracic Society 16(4) (2019) 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walsh SL, Wells AU, Sverzellati N, Devaraj A, von der Thusen J, Yousem SA, Colby TV, Nicholson AG, Hansell DM, Relationship between fibroblastic foci profusion and high resolution CT morphology in fibrotic lung disease, BMC medicine 13 (2015) 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr., Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ, An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management, American journal of respiratory and critical care medicine 183(6) (2011) 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, Goldin JG, Hansell DM, Inoue Y, Johkoh T, Nicholson AG, Knight SL, Raoof S, Richeldi L, Ryerson CJ, Ryu JH, Wells AU, Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper, The Lancet. Respiratory medicine 6(2) (2018) 138–153. [DOI] [PubMed] [Google Scholar]
- [21].Kim SY, Diggans J, Pankratz D, Huang J, Pagan M, Sindy N, Tom E, Anderson J, Choi Y, Lynch DA, Steele MP, Flaherty KR, Brown KK, Farah H, Bukstein MJ, Pardo A, Selman M, Wolters PJ, Nathan SD, Colby TV, Myers JL, Katzenstein AL, Raghu G, Kennedy GC, Classification of usual interstitial pneumonia in patients with interstitial lung disease: assessment of a machine learning approach using high-dimensional transcriptional data, The Lancet. Respiratory medicine 3(6) (2015) 473–82. [DOI] [PubMed] [Google Scholar]
- [22].Pankratz DG, Choi Y, Imtiaz U, Fedorowicz GM, Anderson JD, Colby TV, Myers JL, Lynch DA, Brown KK, Flaherty KR, Steele MP, Groshong SD, Raghu G, Barth NM, Walsh PS, Huang J, Kennedy GC, Martinez FJ, Usual Interstitial Pneumonia Can Be Detected in Transbronchial Biopsies Using Machine Learning, Annals of the American Thoracic Society 14(11) (2017) 1646–1654. [DOI] [PubMed] [Google Scholar]
- [23].Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, Raghunath SM, Walsh SL, Wells AU, Hansell DM, Automated Quantitative Computed Tomography Versus Visual Computed Tomography Scoring in Idiopathic Pulmonary Fibrosis: Validation Against Pulmonary Function, Journal of thoracic imaging 31(5) (2016) 304–11. [DOI] [PubMed] [Google Scholar]
- [24].Ash SY, Harmouche R, Ross JC, Diaz AA, Rahaghi FN, Vegas Sanchez-Ferrero G, Putman RK, Hunninghake GM, Onieva Onieva J, Martinez FJ, Choi AM, Bowler RP, Lynch DA, Hatabu H, Bhatt SP, Dransfield MT, Wells JM, Rosas IO, San Jose Estepar R, Washko GR, Interstitial Features at Chest CT Enhance the Deleterious Effects of Emphysema in the COPDGene Cohort, Radiology 288(2) (2018) 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Humphries SM, Yagihashi K, Huckleberry J, Rho BH, Schroeder JD, Strand M, Schwarz MI, Flaherty KR, Kazerooni EA, van Beek EJR, Lynch DA, Idiopathic Pulmonary Fibrosis: Data-driven Textural Analysis of Extent of Fibrosis at Baseline and 15-Month Follow-up, Radiology 285(1) (2017) 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]