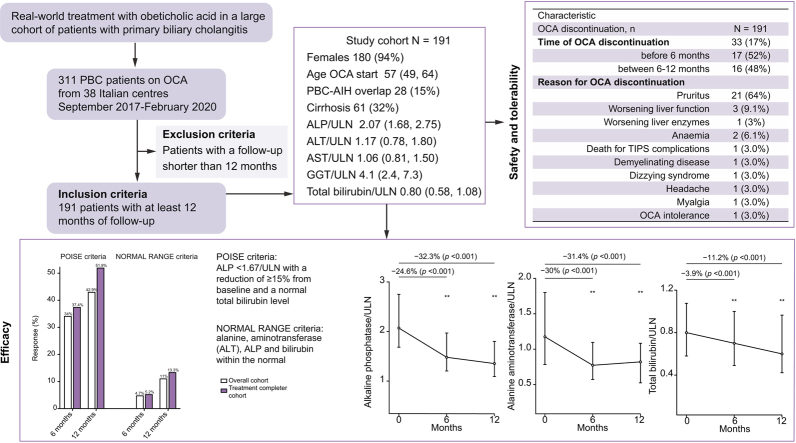
Abstract
Background & aims
Obeticholic acid (OCA) is the second-line treatment approved for patients with primary biliary cholangitis (PBC) and an inadequate response or intolerance to ursodeoxycholic acid. We aimed to evaluate the effectiveness and safety of OCA under real-world conditions.
Methods
Patients were recruited into the Italian PBC Registry, a multicentre, observational cohort study that monitors patients with PBC at national level. The primary endpoint was the biochemical response according to Poise criteria; the secondary endpoint was the biochemical response according to normal range criteria, defined as normal levels of bilirubin, alkaline phosphatase (ALP), and alanine aminotransferase (ALT) at 12 months. Safety and tolerability were also assessed.
Results
We analysed 191 patients until at least 12 months of follow-up. Median age was 57 years, 94% female, 61 (32%) had cirrhosis, 28 (15%) had histologically proven overlap with autoimmune hepatitis (PBC-AIH). At 12 months, significant median reductions of ALP (-32.3%), ALT (-31.4%), and bilirubin (-11.2%) were observed. Response rates were 42.9% according to Poise criteria, and 11% by normal range criteria. Patients with cirrhosis had lower response than patients without cirrhosis (29.5% vs. 49.2%, p = 0.01), owing to a higher rate of OCA discontinuation (30% vs. 12%, p = 0.004), although with similar ALP reduction (29.4% vs. 34%, p = 0.53). Overlap PBC-AIH had a similar response to pure PBC (46.4% vs. 42.3%, p = 0.68), with higher ALT reduction at 6 months (-38% vs. -29%, p = 0.04). Thirty-three patients (17%) prematurely discontinued OCA because of adverse events, of whom 11 experienced serious adverse events. Treatment-induced pruritus was the leading cause of OCA discontinuation (67%).
Conclusions
Effectiveness and safety of OCA under real-world conditions mirror those in the Poise trial. Patients with cirrhosis had lower tolerability. Overlap PBC-AIH showed higher ALT reduction at 6 months compared with patients with pure PBC.
Lay summary
Obeticholic acid (OCA) was shown to be effective in more than one-third of patients not responding to ursodeoxycholic acid in a real-world context in Italy. Patients with cirrhosis had more side effects with OCA, and this led to suspension of the drug in one-third of patients. OCA was also effective in patients who had overlap between autoimmune hepatitis and primary biliary cholangitis.
Keywords: Cholestasis, Cirrhosis, Overlap PBC-AIH, Autoimmunity
Abbreviations: AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine transferase; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; aRR, adjusted risk ratio; AST, aspartate transferase; CRFs, case record forms; EDC, electronic data capture; GGT, gamma-glutamyl transferase; OC, Overall cohort; OCA, obeticholic acid; PBC, primary biliary cholangitis; QC, quality control; RCT, randomised controlled trial; RR, risk ratio; TCC, Treatment Completer Cohort; TIPS, transjugular intrahepatic portosystemic shunt; UDCA, ursodeoxycholic acid; ULN, upper limit of normal
Graphical abstract
Highlights
-
•
Under real-world conditions, OCA was effective in ~43% of patients who were non-responders to UDCA, according to Poise criteria.
-
•
Patients with cirrhosis showed lower efficacy (29.5%), mainly attributed to reduced tolerability and higher discontinuation rate.
-
•
Patients with overlap AIH-PBC showed a comparable efficacy to pure PBC, with a higher ALT reduction at 6 months.
-
•
Most patients with PBC are still in need of additional therapy if aiming to normalise liver biochemistry.
Introduction
Primary biliary cholangitis (PBC) is an autoimmune liver disease characterised by chronic inflammation of the small bile ducts and cholestasis which, if under-treated, leads to fibrosis progression culminating in end-stage liver disease.1 First-line therapy in PBC is ursodeoxycholic acid (UDCA) at a dose of 13–15 mg/kg per day,2 but it is not always effective.3,4 In 2016, obeticholic acid (OCA) received conditional approval as second-line therapy for patients with inadequate response or intolerance to UDCA.1
Data from the randomised controlled trial (RCT) POISE and its open-label extension demonstrated that with 48 months of OCA treatment there was a significant reduction of alkaline phosphatase (ALP) and stabilisation of total bilirubin,5,6 which are both surrogate markers of survival. There are no real-world data on OCA to date, excluding those reported very recently from a small series in Canada.7 Real-world data are crucial for understanding treatment effectiveness and safety in everyday clinical practice, particularly in patient populations that may be under-represented or excluded from clinical trials, such as those with cirrhosis and mixed phenotypes (e.g. overlap autoimmune hepatitis [AIH]-PBC).
The aim of this study was to evaluate the efficacy, safety, and tolerability of OCA under real-world conditions in a large cohort of patients with PBC treated in secondary and tertiary centres across Italy.
Patients and methods
Study design and cohort
This is a retrospective study of prospectively collected data within the Italian PBC Registry, an ongoing, non-interventional, multicentre, observational cohort study that monitors patients with PBC in Italy. All adult patients who had received a diagnosis of PBC consecutively starting OCA treatment from 38 Italian centres between September 2017 and February 2020 were screened for the study. All patients who had taken at least 1 dose of OCA, and with an overall follow-up of at least 12 months (therefore, having started OCA not later than February 2019), were included in the study. Patients who had been previously enrolled in a sponsored trial with OCA were excluded. Patients receiving off-label fibrate therapy on stable treatment for at least 6 months at the time of starting OCA were not excluded. Hospitals with a dedicated autoimmune liver diseases outpatient clinic were defined as ’tertiary centres’; those with a general hepatology outpatient clinic were defined as ‘secondary centres’.
Diagnosis of PBC and ‘definite’ PBC-AIH overlap syndrome were defined according to European Association for the Study of the Liver (EASL) guidelines.1 The diagnosis of PBC-AIH overlap syndrome was histologically confirmed in all cases and all patients were on a stable immunosuppressive treatment for at least 6 months. Diagnosis of liver cirrhosis was based on clinical presentation (at ultrasound: surface nodularity, caudate hypertrophy, splenomegaly; at upper endoscopy: gastroesophageal varices), or histological evaluation.
Indications to OCA treatment are reported in Appendix S1.
We defined the Overall Cohort (OC) as all patients who had received at least 1 dose of OCA and had at least 12 months of follow-up. The Treatment Completer Cohort (TCC) was defined as all patients who completed the treatment period of 6 or 12 months for the analysis at 6 or 12 months, respectively. The primary efficacy analysis was carried out on both the OC and TCC populations. Safety and tolerability were analysed in the OC population only.
The study was conducted in accordance with the Declaration of Helsinki guidelines and the principles of good clinical practice. All participants to the Italian PBC Registry provided written informed consent. The study was approved by the University of Milan-Bicocca research ethics committee (Study name: PBC322), coordinator of the Italian National Registry and by the Research and Development Department of each collaborating hospital.
Data source
Data were captured using baseline and follow-up case record forms (CRFs), completed by physicians in each collaborating centre. Demographic, clinical, and biochemical data were collected at baseline (immediately before starting OCA therapy), and at 6 and 12 months of treatment during follow-up visits. Management of OCA therapy was tailored for each patient and clinical decisions were taken independently by physicians based on the drug package insert. Data on OCA dose adjustment and OCA discontinuation were systematically collected.
Pruritus was systematically assessed at baseline and at every follow-up visit. Pruritus was classified as mild, moderate, or severe as follows: mild, pruritus of mild intensity or localised; moderate, pruritus of moderate intensity or diffuse but intermittent; severe, pruritus of severe intensity or diffuse and continuous. Moreover, pruritus was classified as ‘de novo’ if it occurred after the start of OCA treatment, or as ‘worsening’ if present at baseline but increased on OCA therapy. Other adverse events were not systematically assessed but registered when they led to permanent drug discontinuation.
Completed CRFs underwent quality control (QC) for completeness and accuracy at the University of Milan-Bicocca, Milan and University Campus Bio Medico, Rome. Missing, inaccurate, or implausible data were systematically queried with the treating physicians. Data that passed QC were uploaded into a bespoke database, collecting clinical and biochemical data at each follow-up time point. The database is an electronic data capture (EDC) system with an e-CRF developed for the purpose of this study and other projects on the Italian PBC Registry. The EDC system runs on a server maintained by a dedicated Clinical Research Organisation. The EDC system allows research staff in collaborating centres to log in from any National Health Service computer to view information about participants recruited from their own centres and to complete e-CRFs and upload the results of medical investigations directly into the database.
Study endpoints
The ‘primary endpoint’ was the biochemical response at 6 and 12 months of OCA therapy using the Poise definition of biochemical response: (1) ALP<1.67/upper limit of normal (ULN) with a reduction of ≥15% from baseline and a normal total bilirubin level, as applied in the registrative trial of OCA (Poise criteria).5
The ‘secondary endpoint’ was the biochemical response at 6 and 12 months of OCA therapy according to the following criteria: alanine aminotransferase (ALT), ALP, and bilirubin within the normal (normal range criteria), as normalisation of liver biochemistry has been recently proposed as the new therapeutic target in PBC.8
Other efficacy endpoints included variation of ALP, gamma-glutamyltransferase (GGT), ALT, aspartate aminotransferase (AST), and total bilirubin levels at 6 and 12 months of OCA therapy.
We defined missed up-titrations when patients were taking 5 mg/day at 12 months even though they had an ALP ≥1.5/UNL at 6 months and no pruritus.
Assessment of safety and side effects included systematic evaluation of pruritus, and collection of adverse events and laboratory abnormalities that led to treatment discontinuation.
Statistical analysis
Continuous variables were described by mean, standard deviation, and as median and IQRs in case they showed a skewed distribution with significant departure from the normal distribution. To account for inter-laboratory variability, ALP, GGT, ALT, AST, and total bilirubin were expressed as ratios of their respective ULN. Categorical variables were described by absolute frequencies and percentages. To compare groups, we used the χ2 test for categorical variables (or Fisher exact test in the case of sparse data) and the Student t test for continuous variables (or Wilcoxon test when a significant departure from normality was detected). The analysis of factors associated with an increased risk of no response after 12 months of OCA therapy was carried out by reporting risk ratios (RR) with 95% CIs, and performed by means of Poisson regression models with robust error variance, as described by Zou et al.9 Age at OCA start (which was collinear with age and age at PBC diagnosis), and all variables associated at univariate analysis with a value of p <0.10 entered the multivariate model. All analyses were undertaken using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/).
Results
Characteristics of the study cohort
From a total population of 311 patients from 38 Italian centres who had received at least 1 dose of OCA between September 1st 2017 and February 1st 2020 (date of data-lock), we finally included 191 patients with at least 12 months of follow-up, e.g. who had started OCA not later than February 14, 2019. Notably, none of the patients who began OCA treatment was lost to follow-up within the 12 months. Characteristics of the cohort are reported in Table 1. Of note, 61 patients (32%) had cirrhosis, of whom 59 patients were in Child-Pugh class A, and 2 in class B. Fifty patients (26%), of whom 27 had cirrhosis, had abnormal bilirubin levels at baseline. Twenty-eight patients (15%) had histologically-proven PBC-AIH overlap syndrome; 11 of these also had cirrhosis. Forty-eight patients (25%) had a history of pruritus, including 25 on medications for pruritus before starting OCA.
Table 1.
General characteristics of the study cohort.
| Characteristic | N = 191 |
|---|---|
| Sex, female | 180 (94%) |
| Age at diagnosis, years | 49 (41, 56) |
| Age at OCA start, years | 57 (49, 64) |
| AMA positivity | 163 (85%) |
| ANA positivity | 62 (32%) |
| PBC-AIH overlap | 28 (15%) |
| Cirrhosis | 61 (32%) |
| Oesophageal varices, presence | 12 (6.3%) |
| UDCA use | 186 (97%) |
| UDCA dose, mg/kg | 15.0 (15.0, 17.3) |
| Duration of disease before OCA start, years | 7.0 (3.0, 11.0) |
| Indication to OCA start | |
| Intolerance to UDCA | 0 (0%) |
| Inadequate response to UDCA | 191 (100%) |
| Acc. to Paris I criteria | 95 (49.7%) |
| Acc. to Paris II criteria | 181 (94.8%) |
| Acc. to Toronto criteria | 144 (75.4%) |
| OCA dose | |
| <5 mg daily∗ | 10 (5%) |
| 5 mg daily | 115 (60%) |
| 5 mg up-titrated to 10 mg daily | 66 (35%) |
| ALP/ULN at baseline | 2.07 (1.68, 2.75) |
| ALT/ULN at baseline | 1.17 (0.78, 1.80) |
| AST/ULN at baseline | 1.06 (0.81, 1.50) |
| GGT/ULN at baseline | 4.1 (2.4, 7.3) |
| Total bilirubin/ULN at baseline | 0.80 (0.58, 1.08) |
| OCA started after fibrates | 6 (3.1%) |
Paris I criteria: ALP <3x ULN, ALT <2x ULN and bilirubin <1 mg/dl. Paris II criteria: ALP <1.5x ULN, ALT <1.5x ULN and bilirubin <1 mg/dl. Toronto criteria: ALP <1.67x ULN. Data expressed as median (IQR) or number (percentage).
Acc, according; AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine transferase; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; AST, aspartate transferase; GGT, gamma-glutamyl transferase; PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Includes 10 mg twice/week (n = 3), 5 mg weekly (n = 2), 5 mg, 4 times/week (n = 5).
For the majority (n = 186, 97.4%), OCA therapy was indicated by persistently elevated ALP (i.e. ≥1.5/ULN after at least 12 months of UDCA); 5 patients started OCA monotherapy because of intolerance to UDCA. Consistently, at the time of starting OCA, almost 95% of patients were UDCA non-responders according to Paris I criteria. In 10 patients, 6 of whom had cirrhosis, OCA was started and maintained at a dose of <5 mg/day; in the majority (n = 115), OCA was started at the dose of 5 mg/day and maintained unchanged; in 66 patients, OCA was started at 5 mg/day and up-titrated to 10 mg/day after 6 months. Among the latter, at 6 months, 18 had an ALP ≥1.5/UNL at 6 months and no pruritus, whereas the others up-titrated notwithstanding missing 1 or both of these conditions.
Response rate at 6 and 12 months
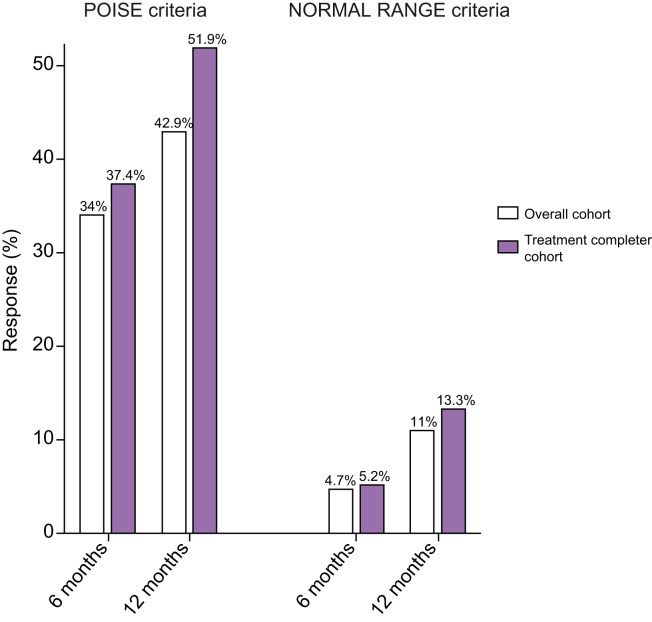
According to the Poise criteria, 34% and 42.9% of patients achieved a response at 6 and 12 months, respectively, in the OC population; and 37.4% and 51.9% at 6 and 12 months, respectively, in the TCC population (Fig. 1). According to the normal range criteria, 4.7% and 11% of patients achieved a response at 6 and 12 months, respectively, in the OC population; and 5.2% and 13.3% at 6 and 12 months, respectively, in the TCC population (Fig. 1).
Fig. 1.
Rates of response to OCA therapy according to the POISE (left panel) and the normal range criteria (right panel) in the overall cohort and the treatment completer cohort. Data are expressed as number (percentage). OCA, obeticholic acid.
Considering only the 144 patients with a baseline ALP ≥1.67 times the ULN, 31.9% and 40.3% of patients achieved a response at 6 and 12 months, respectively, in the OC population; and 35.4% and 49.6% at 6 and 12 months, respectively, in the TCC population (Fig. S1).
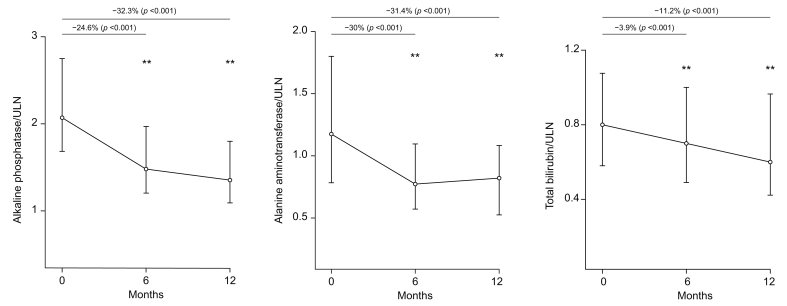
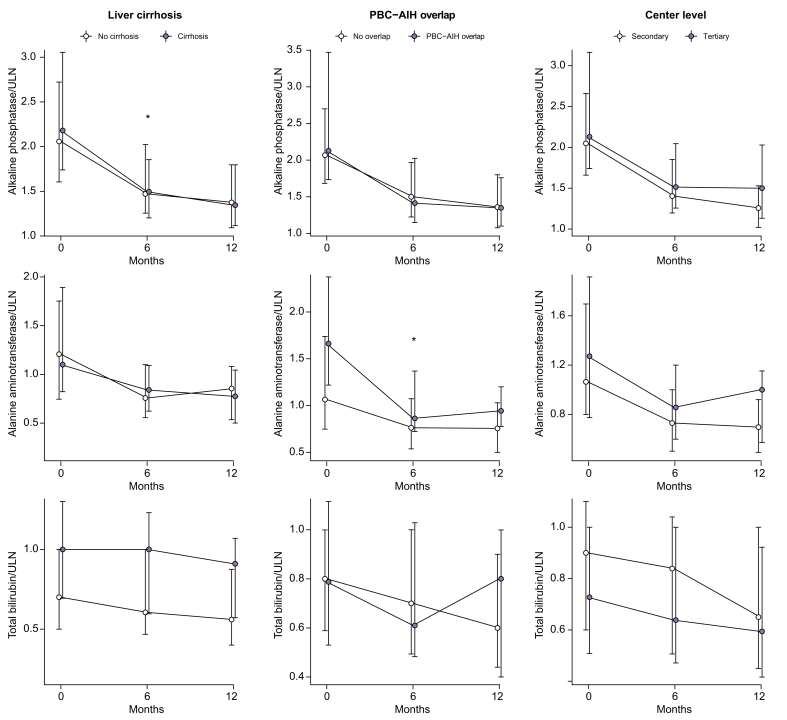
Progressive reduction of the median value was observed at 6 and 12 months for ALP (-24.6% and -32.3%), bilirubin (-3.9% and -11.2%), and ALT (30% and -31.4%; Fig. 2).
Fig. 2.
Variation of alkaline phosphatase, alanine aminotransferase and total bilirubin during OCA treatment.
Median values with interquartile range at different time point, and percentage reduction compared to baseline values, are presented. The p values are related to the Wilcoxon test. OCA, obeticholic acid; ULN, upper limit of normal.
Compared with patients on a stable OCA dose of 5 mg/day, patients up-titrating to 10 mg/day achieved a lower reduction of ALP values at 6 months (-20% vs. -28%, p = 0.006), which was substantially reversed after dose adjustment at 12 months (-31% vs. -35%, p = 0.16).
Eighty-two patients experienced a reduction of bilirubin below 0.6/ULN, a result which has been recently associated with a better prognosis,8 corresponding to 42% of the OC and 51% of the TCC.
Among 47 patients eligible for up-titration, 29 (62%) did not up-titrate (Appendix S2).
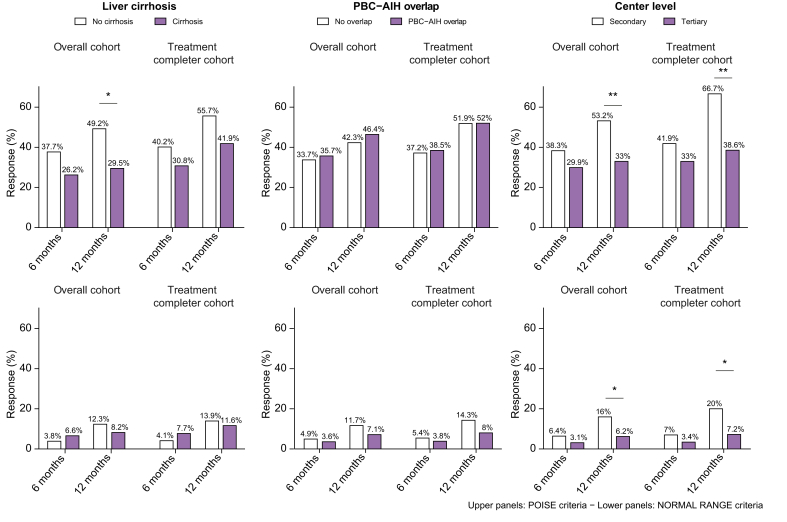
Treatment response in patients with cirrhosis
No significant differences in response rates were found between patients with and without cirrhosis at 6 months (Fig. 3). At 12 months, a significant lower rate of response was observed in patients with cirrhosis only in the OC population (29.5% vs. 49.2%, p = 0.01, in patients with cirrhosis vs. patients without cirrhosis, according to Poise criteria; and 8.2% vs. 12.3 %, p = 0.53, according to normal range criteria). In the TCC population at 12 months, these trends were confirmed despite no statistical significance (Fig. 3).
Fig. 3.
Rates of response to OCA therapy stratified according to the presence of liver cirrhosis (left panel), PBC-AIH overlap (mid panel) and centre level (right panel).
Response estimated based on POISE (upper panels) and normal range criteria (lower panels) in the overall cohort and treatment completer cohort. ∗p <0.05 and ∗∗p <0.01 using the χ2 test. AIH, autoimmune hepatitis; OCA, obeticholic acid; PBC, primary biliary cholangitis.
Out of 43 patients with cirrhosis who did not achieve response at 12 months, 12 had a bilirubin ≥1/ULN, 16 an ALP ≥1.67/ULN, and 11 did not achieve the ≥15% ALP reduction. Patients with cirrhosis had more frequently abnormal baseline bilirubin levels (≥1/ULN) compared with those without cirrhosis [27 (44.3%) vs. 23 (17.7%), p <0.001]. Twelve patients with cirrhosis (19.7%), and 3 patients without cirrhosis (2.3%), had abnormal bilirubin and discontinued OCA within the 12 months. Four out of the 15 patients with cirrhosis with abnormal bilirubin (26.7%), and 11 out of the 20 patients without cirrhosis (55%), completing 12 months of treatment, had normalised bilirubin at the final follow-up.
Notably, the response in terms of ALP, ALT, and bilirubin reduction was not different between patients with and without cirrhosis (Fig. 4). The biochemical response was obtained despite the fact that patients with cirrhosis had taken significantly lower doses of OCA therapy than those without cirrhosis (patients with cirrhosis: 11.0% <5 mg/day, 75.0% 5 mg/day, 13.0% 5 mg up-titrated to 10 mg/day vs. patients without cirrhosis: 2.3% <5 mg/day, 53.0% 5 mg/day, 45.0% 5 mg up-titrated to 10 mg/day, p <0.001; Table S1).
Fig. 4.
Variation of alkaline phosphatase, alanine aminotransferase and total bilirubin during OCA treatment stratified according to the presence of liver cirrhosis (left panel), PBC-AIH overlap (mid panel) and centre level (right panel).
Median values with IQR at different time points are presented. ∗p <0.05 and ∗∗p <0.01 using the Wilcoxon test compared with baseline values. AIH, autoimmune hepatitis; OCA, obeticholic acid; PBC, primary biliary cholangitis; ULN, upper limit of normal.
Among 14 patients eligible for up-titration, 12 (86%) did not up-titrate (Appendix S2).
Treatment response in PBC-AIH overlap patients
Patients with PBC-AIH overlap were on stable (at least 6 months before OCA therapy) immunosuppression with prednisone and azathioprine. Twenty-three patients (82%) had elevated transaminases before starting OCA (median ALT was 1.66 vs. 1.06 in pure PBC, p = 0.003; Table S2), and 11 (39%) patients had cirrhosis. No difference was observed in the response rate at 6 and 12 months between patients with PBC-AIH overlap and pure PBC in either the OC and TCC population (Fig. 3). However, the reduction of ALT levels in the first 6 months of OCA therapy was significantly higher in patients with PBC-AIH overlap than in those with pure PBC (38% vs. 29%, p = 0.037; Fig. 4 and Fig. S2). An improvement of the other liver tests also occurred, which was not significantly different between the 2 groups (Fig. 4).
Among 5 patients eligible for up-titration, 3 did not up-titrate (Appendix S2).
Secondary and tertiary centres
Patients treated in secondary centres showed significantly higher response rates at 12 months than those in tertiary centres (53.2% vs. 33% in the OC population p = 0.004, 66.7% vs. 38.6% in the TCC population, p = 0.0004; Fig. 3). Notably, patients treated in tertiary centres had a higher level of ALP at baseline, although not statistically significant, were younger at diagnosis (median age: 47 vs. 51 years, p = 0.03; Table S3). Six patients followed at tertiary centres were on fibrate off-label therapy before starting OCA, of whom 2 and none responded according to Poise and normal range criteria. There was an overall improvement of liver tests, in both subgroups of patients (Fig. 4).
Safety and side effects
We explored tolerability focusing on the side effects or adverse events that necessitated treatment discontinuation. Thirty-three patients (17%) discontinued OCA treatment, 17 (52%) of whom were in the first 6 months of treatment, and 16 (48%) between 6 and 12 months of treatment (Table 2). Pruritus was the most common adverse event, which occurred in 52 (27.3%) patients; it was mild in 16, moderate in 19 and severe in 17, and caused treatment discontinuation in 21 patients (66% discontinuation). In particular, of the 52 patients experiencing pruritus on OCA, 40 patients had de novo pruritus and 12 had worsening of pre-existing pruritus. The discontinuation rate in patients with de novo pruritus was 37.5% compared with 50% discontinuation rate in patients with worsening pruritus (p = 0.43).
Table 2.
Occurrence of treatment discontinuation.
| Characteristic | N = 191 |
|---|---|
| OCA discontinuation, n | 33 (17%) |
| Time of OCA discontinuation | |
| Before 6 months | 17 (52%) |
| Between 6 and 12 months | 16 (48%) |
| Reason for OCA discontinuation | |
| Pruritus | 21 (64%) |
| Worsening liver function | 3 (9.1%) |
| Worsening liver enzymes | 1 (3%) |
| Anaemia | 2 (6.1%) |
| Death from TIPS complications | 1 (3.0%) |
| Demyelinating disease | 1 (3.0%) |
| Dizzying syndrome | 1 (3.0%) |
| Headache | 1 (3.0%) |
| Myalgia | 1 (3.0%) |
| OCA intolerance | 1 (3.0%) |
Data are expressed as number (percentage). OCA, obeticholic acid; TIPS, transjugular intrahepatic portosystemic shunt.
Other adverse events that caused discontinuation are reported in Table 2. Serious adverse events within the study period were worsening of liver function observed in 3 patients with cirrhosis and refractory bleeding from severe portal hypertension requiring transjugular intrahepatic portosystemic shunt (TIPS) placement (for further details see Appendix S2).
Predictors of OCA treatment failure
At univariate analysis, factors significantly or nearly significantly associated with non-response at 12 months were age at PBC diagnosis and at OCA start, liver cirrhosis, OCA started after fibrates, pre-treatment values of ALP/ULN, AST/ULN, GGT/ULN, and of total bilirubin. Only OCA started after fibrates and pre-treatment values of ALP/ULN and of total bilirubin were confirmed to be associated in the multivariate model after correction for OCA discontinuation (Table 3).
Table 3.
Factors associated with lack of response to obeticholic acid at 12 months.
| Variable | Univariate |
Multivariate |
|
|---|---|---|---|
| RR (95% CI), p | aRR (95% CI), p | aRR∗ (95% CI), p | |
| Age, years | 1.01 (1.00–1.02), 0.088 | ||
| Age at PBC diagnosis, years | 1.01 (1.00–1.03), 0.020 | ||
| Age at OCA start, years | 1.01 (1.00–1.02), 0.089 | 1.01 (1–1.02), 0.068 | 1.01 (1.00–1.02), 0.253 |
| Male sex | 0.79 (0.41–1.52), 0.476 | ||
| ANA positivity | 1.16 (0.90–1.49), 0.243 | ||
| AMA positivity | 0.81 (0.61–1.09), 0.163 | ||
| Liver cirrhosis | 1.39 (1.10–1.76), 0.006 | 1.26 (0.98–1.62), 0.077 | 1.11 (0.86–1.43), 0.418 |
| PBC-AIH overlap | 0.93 (0.64–1.34), 0.696 | ||
| Triple therapy with UDCA, OCA, and fibrates | 1.26 (0.78–2.05), 0.345 | ||
| OCA started after fibrates | 1.48 (1.01–2.17), 0.042 | 1.81 (1.10–3.00), 0.021 | 2.04 (1.23–3.38), 0.006 |
| Duration of PBC, years | 0.99 (0.97–1.01), 0.374 | ||
| ALP/ULN at baseline | 1.12 (1.05–1.20), <0.001 | 1.16 (1.06–1.27), 0.001 | 1.14 (1.04–1.26), 0.005 |
| ALT/ULN at baseline | 1.05 (0.95–1.17), 0.339 | ||
| AST/ULN at baseline | 1.13 (1.02–1.24), 0.017 | 0.92 (0.78–1.09), 0.328 | 0.87 (0.73–1.04), 0.134 |
| GGT/ULN at baseline | 1.01 (1.00–1.03), 0.069 | 0.99 (0.97–1.02), 0.604 | 1.01 (0.98–1.03), 0.549 |
| Total bilirubin/ULN at baseline | 1.18 (1.06–1.32), 0.003 | 1.20 (1.03–1.40), 0.021 | 1.15 (1.00–1.34), 0.040 |
Response to OCA evaluated according to Poise criteria in the overall cohort (OC). Risk ratios with 95% confidence intervals were from Poisson regression models with robust error variance. Age at OCA start (which was collinear with age and age at PBC diagnosis), sex and all variables associated at univariate analysis with a p < 0.10 entered the multivariate model. A second multivariable model (∗) was fitted additionally correcting for OCA discontinuation. AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine transferase; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; aRR, adjusted risk ratio; AST, aspartate transferase; GGT, gamma-glutamyl transferase; OCA, obeticholic acid; PBC, primary biliary cholangitis; RR, risk ratio; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Additionally corrected for OCA discontinuation.
Discussion
Real-world studies enable evaluation of treatment effectiveness, safety, and prescribing patterns in routine clinical practice and represent an important complement to the results obtained from clinical trials. Our data, obtained in a large cohort of patients with a follow-up of 12 months, confirm in a real-world context that OCA is an effective and well-tolerated treatment option for patients with PBC who are non-responding to or non-tolerating UDCA. More importantly, the present study provides important novel evidence concerning response rates and side effects in patients with cirrhosis and those with overlap PBC-AIH, who had been either under-represented or excluded, respectively, from RCTs with OCA.
In the phase III registrative trial, 12-month treatment of PBC patients who were non-responders to UDCA[10], [11], [12], [13], [14], [15], [16] led to biochemical response in 46%, 47%, and 10% in the OCA titration, OCA 10 mg fixed-dose and placebo arms, respectively.5 Pruritus was the main side effect and the principal cause of OCA discontinuation. The 3-year interim data from the open-label extension demonstrated that prolonged OCA treatment is associated with durable improvements in markers of cholestasis without new safety signals.6 Nevertheless, to date, there have been no sizeable cohort studies reporting data concerning OCA therapy in a real-world context, where patient characteristics are more heterogeneous with respect to subphenotypes (e.g. overlap PBC-AIH, cirrhosis) and drug schedule (e.g. starting dose, criteria for up-titration or discontinuation) may be less rigid and more personalised by each treating physician. Indeed, only 1 real-world experience with OCA has been reported very recently, carried out in a small cohort of 64 patients, of which only 36 with at least 12 months of observation.7
The present study analysed 191 patients from 38 Italian secondary and tertiary centres. The population enrolled was similar to that described in the Poise trial5 with the following exceptions: (1) 61 patients (32%) in our cohort had cirrhosis (clinically and/or histologically), compared with an estimated 20% in the Poise trial based on transient elastography, which was, however, performed only in 43% of the study population; (2) 28 patients (15%) had a histologically confirmed diagnosis of overlap PBC-AIH, which was an exclusion criterion in the POISE study;5 (3) 50 patients (26%) in our cohort had abnormal bilirubin levels at baseline, compared with 18 patients (8%) in the Poise cohort.5 Because liver cirrhosis, overlap PBC-AIH, and abnormal bilirubin are well-known predictors of worse prognosis in PBC,8,9,17 the present study population is more difficult to treat, as expected in an early post-marketing real-world context, where the most severely affected patients3,18 are frequently granted the new therapeutic option first.
In our study, the benefit of OCA on liver biochemistry was evident in the whole cohort, with an overall reduction of ≅30% for ALP and ALT levels, and ≅10% reduction of bilirubin levels, at 12 months. We reported results for the cut-off ALP <1.67/ULN, as this is how UDCA response has been defined in the RCT Poise. This cut-off is, however, debatable. Given the strong correlation between ALP and histological features of biliary injury,4,13,19 it might be argued that the threshold should be the complete biochemical remission. Indeed, this has already been used in the Bezurso trial.20 Recognising this on-going debate, we provide results also for the normal range criteria, which highlighted an enormous unmet clinical need with only 11% of patients normalising ALP, ALT, and bilirubin in the OC analysis.21 Given limited healthcare budgets and the limited evidence of efficacy of this drug in some patients, with the assumption that patient-centred care must be fair and cost effective, these data should prompt cost-effective analysis.
One-third of patients in our cohort had cirrhosis, including 2 with Child-Pugh B function and 12 with gastro-oesophageal varices. The response rate was lower in the cirrhosis group in the OC analysis, owing to higher rate of drop-out and higher baseline bilirubin levels. Indeed, cirrhosis was not associated with non-response in the multivariate model corrected also for OCA discontinuation. Indeed, the degree of reduction of the liver biochemistry was comparable between patients with and without cirrhosis (12-month reduction was 29.4% and 34% for ALP, 30% and 31.4% for ALT, and 3.7% and 14.3% in patients with cirrhosis vs. those without cirrhosis, respectively, p = n.s. for all comparisons). Three patients with cirrhosis (2 with Child-Pugh B) experienced worsening liver function during OCA treatment. We believe OCA is effective in patients with cirrhosis providing the disease class is Child-Pugh A (97% of our cohort with cirrhosis), the treatment regimen is correct, and pruritus (the major cause for drop-out in this population) is monitored and appropriately prevented and/or managed.
In the majority of patients (60%), OCA was started and continued until the 12th month at 5 mg/day, and in only 35% it was up-titrated to 10 mg/day. Notably, in 10 patients, including 5 Child-Pugh A patients with cirrhosis, OCA was maintained at the dose of <5 mg/day22 (5 mg/every other day, every 3 days) and 86% of patients with cirrhosis on 5 mg/day did not up-titrate despite preserved liver function and no adverse events. This choice is likely the result of a prudent real-world approach after the FDA warning about serious liver injury with OCA when the drug was incorrectly dosed in patients with moderate-to-decreased liver function.23
Inadequate response was the reason for OCA up-titration at 6 months, and increasing to 10 mg partially recovered the suboptimal result on ALP levels in these patients. Of note, beyond the majority of patients with cirrhosis, also 45% of patients without cirrhosis eligible for up-titration did not up-titrate. Hopefully, more experience and continuing medical education will tackle this clinical gap.
Among the 28 patients with PBC-AIH overlap syndrome,24 82% had incomplete response to immunosuppression therapy with elevated transaminase (and ALP) despite stable immunosuppression and UDCA. After treatment with OCA, they showed a median ALT reduction of 38%, and 16 patients normalised the ALT levels at 6 months and maintained normalised ALT levels at 12 months (with a median 33% reduction of ALP). This might suggest an anti-inflammatory and/or immunomodulatory effect of OCA on the hepatitis component of the overlap syndrome. In alternative, OCA might improve the hepatitis component secondary to cholestasis. However, this deserves confirmation, as we cannot certainly exclude that the cholestasis and hepatitic activity non-responding to immunosuppression was surrogate of an aggressive phenotype of PBC.
The lower rate of OCA response observed in tertiary centres is not surprising, considering that tertiary centres included ounger patients, with more elevated liver biochemistry, and more likely to be on triple therapy with UDCA, fibrates, and OCA (rate of response ≅30%).25 However, owing to the limited number of patients on triple therapy, further studies are needed to make conclusions on this category of patients.
Pruritus was the most common adverse event affecting one-quarter of the cohort, compared with 18% in the titration arm and 30% in the 10 mg arm in the Poise trial. Pruritus was classified as mild, moderate, or severe, rather than using a visual-analogue scale to detect clinically significant pruritus. The rate of OCA permanent discontinuation was slightly higher than that observed in the Poise trial (17% vs. 9%). The other side effects leading to treatment discontinuation did not have any clustering.
This study has some limitations. We did not collect all side effects experienced under OCA treatment and all the medications of pruritus adopted. This approach was aimed at reducing the burden of workload for clinicians therefore maximising the adhesion of many (secondary) centres. Consistent with this, we did not include the biochemical variables which were not routinely assessed at every control by all centres, such as platelets, serum albumin, and immunoglobulins in the database. We included patients who were already on off-label fibrate therapy and with overlap PBC-AIH syndrome, who can bias the rate of response of a ‘pure’ second-line PBC population. However, this allows to have a full understanding of the treatment burden in a real-world context.
In conclusion, the present study suggests that the results obtained by OCA in the registrative RCTs can be substantially reproduced in a real-world context in efficacy, safety, and tolerability, even if including patients with cirrhosis and overlap PBC-AIH. A careful approach is required in patients with cirrhosis who might need optimisation of pruritus control before starting OCA and over the treatment. Patients with PBC-AIH overlap might experience additional benefit in terms of improvement of hepatitis activity. If complete normalisation of the liver biochemistry is the new therapeutic target in PBC, a significant population of patients will likely require an adjunctive tertiary treatment.
Financial support
Ministero della Salute, PE-2016-02363915 (PI); Ministero della Salute, GR-2018-12367794 (MC). The funders had no role in the study design, data collection, analysis, or interpretation, preparation of the report, or the decision to publish. The corresponding author had full access to the raw data and had final responsibility for the decision to submit for publication.
Authors’ contributions
Study concept and design: MC, UVG, PI, DD, AD. Acquisition of data: all authors. Analysis and interpretation of data: MC, UVG, DD, AD. Drafting of the manuscript: MC, UVG, DD, AD. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: DD, AD. Obtained funding: MC, UVG. Technical or material support: DD, AD. Study supervision: MC, UVG
Data availability statement
All the data are available in the biorepository of the University of Milano-Bicocca upon request.
Conflicts of interest
The authors have no conflicts of interest to declare related to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found at https://doi.org/10.1016/j.jhepr.2021.100248.
Supplementary data
References
- 1.Hirschfield G.M., Beuers U., Corpechot C., Invernizzi P., Jones D.E., Marzioni M. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Poupon R.E., Poupon R., Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. N Engl J Med. 1994;330:1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 3.Carbone M., Mells G.F., Pells G., Dawwas M.F., Newton J.L., Heneghan M.A. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560–569. doi: 10.1053/j.gastro.2012.12.005. e7. [DOI] [PubMed] [Google Scholar]
- 4.Carbone M., Nardi A., Flack S., Carpino G., Varvaropoulou N., Gavrila C. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: development and validation of the UDCA Response Score. Lancet Gastroenterol Hepatol. 2018;3:626–634. doi: 10.1016/S2468-1253(18)30163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nevens F., Andreone P., Mazzella G., Strasser S.I., Bowlus C., Invernizzi P. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 6.Trauner M., Nevens F., Shiffman M.L., Drenth J.P.H., Bowlus C.L., Vargas V. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4:445–453. doi: 10.1016/S2468-1253(19)30094-9. [DOI] [PubMed] [Google Scholar]
- 7.Roberts S.B., Ismail M., Kanagalingam G., Mason A.L., Swain M.G., Vincent C. Real-world effectiveness of obeticholic acid in patients with primary biliary cholangitis. Hepatol Commun. 2020;4:1332–1345. doi: 10.1002/hep4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murillo Perez C.F., Harms M.H., Lindor K.D., van Buuren H.R., Hirschfield G.M., Corpechot C. Goals of treatment for improved survival in primary biliary cholangitis: treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol. 2020;115:1066–1074. doi: 10.14309/ajg.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 9.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 10.Parés A., Caballería L., Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C., Abenavoli L., Rabahi N., Chrétien Y., Andréani T., Johanet C. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871–877. doi: 10.1002/hep.22428. [DOI] [PubMed] [Google Scholar]
- 12.Kuiper E.M.M., Hansen B.E., de Vries R.A., den Ouden-Muller J.W., van Ditzhuijsen T.J., Haagsma E.B. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281–1287. doi: 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Kumagi T., Guindi M., Fischer S.E., Arenovich T., Abdalian R., Coltescu C. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:2186–2194. doi: 10.1038/ajg.2010.216. [DOI] [PubMed] [Google Scholar]
- 14.Corpechot C., Chazouillères O., Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55:1361–1367. doi: 10.1016/j.jhep.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Lammers W.J., Hirschfield G.M., Corpechot C., Nevens F., Lindor K.D., Janssen H.L. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804–1812. doi: 10.1053/j.gastro.2015.07.061. e4. [DOI] [PubMed] [Google Scholar]
- 16.Carbone M., Sharp S.J., Flack S., Paximadas D., Spiess K., Adgey C. The UK-PBC risk scores: derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016;63:930–950. doi: 10.1002/hep.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey E.J., Ali A.H., Lindor K.D. Primary biliary cirrhosis. Lancet. 2015;386:1565–1575. doi: 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 18.Cristoferi L., Nardi A., Ronca V., Invernizzi P., Mells G., Carbone M. Prognostic models in primary biliary cholangitis. J Autoimmun. 2018;95:171–178. doi: 10.1016/j.jaut.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Carbone M., D’Amato D., Hirschfield G.M., Jones D.E.J., Mells G.F. Letter: histology is relevant for risk stratification in primary biliary cholangitis. Aliment Pharmacol Ther. 2020;51:192–193. doi: 10.1111/apt.15583. [DOI] [PubMed] [Google Scholar]
- 20.Corpechot C., Chazouillères O., Rousseau A., Le Gruyer A., Habersetzer F., Mathurin P. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378:2171–2181. doi: 10.1056/NEJMoa1714519. [DOI] [PubMed] [Google Scholar]
- 21.Gerussi A., D’Amato D., Cristoferi L., O’Donnell S.E., Carbone M., Invernizzi P. Multiple therapeutic targets in rare cholestatic liver diseases: time to redefine treatment strategies. Ann Hepatol. 2020;19:5–16. doi: 10.1016/j.aohep.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency. https://www.ema.europa.eu/en/documents/assessment-report/ocaliva-epar-public-assessment-report_en.pdf. Accessed on February, 2 2021.
- 23.FDA. Drug Safety Communication. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-highlight-correct-dosing-ocaliva-obeticholic-acid-patients-rare-chronic-liver. Accessed on February, 2 2021.
- 24.Chazouillères O., Wendum D., Serfaty L., Rosmorduc O., Poupon R. Long term outcome and response to therapy of primary biliary cirrhosis–autoimmune hepatitis overlap syndrome. J Hepatol. 2006;44:400–406. doi: 10.1016/j.jhep.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Vespasiani-Gentilucci U., Rosina F., Pace-Palitti V., Sacco R., Pellicelli A., Chessa L. Rate of non-response to ursodeoxycholic acid in a large real-world cohort of primary biliary cholangitis patients in Italy. Scand J Gastroenterol. 2019;54:1274–1282. doi: 10.1080/00365521.2019.1669702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are available in the biorepository of the University of Milano-Bicocca upon request.