Abstract
Sterilization is the process of killing all microorganisms, while disinfection is the process of killing or removing all kinds of pathogenic microorganisms except bacterial spores. Biomaterials involved in cell experiments, animal experiments, and clinical applications need to be in the aseptic state, but their physical and chemical properties as well as biological activities can be affected by sterilization or disinfection. Decellularized matrix (dECM) is the low immunogenicity material obtained by removing cells from tissues, which retains many inherent components in tissues such as proteins and proteoglycans. But there are few studies concerning the effects of sterilization or disinfection on dECM, and the systematic introduction of sterilization or disinfection for dECM is even less. Therefore, this review systematically introduces and analyzes the mechanism, advantages, disadvantages, and applications of various sterilization and disinfection methods, discusses the factors influencing the selection of sterilization and disinfection methods, summarizes the sterilization and disinfection methods for various common dECM, and finally proposes a graphical route for selecting an appropriate sterilization or disinfection method for dECM and a technical route for validating the selected method, so as to provide the reference and basis for choosing more appropriate sterilization or disinfection methods of various dECM.
Keywords: Decellularized matrix, Sterilization, Disinfection, Irradiation, Ethylene oxide, Supercritical carbon dioxide, Peracetic acid, Hydrogen peroxide, Alcohol, Antibiotic
Graphical abstract
Highlights
-
•
Asepsis is the prerequisite for the experiment and application of biomaterials.
-
•
Sterilization or disinfection affects physic-chemical properties of biomaterials.
-
•
Mechanism, advantages and disadvantages of sterilization or disinfection methods.
-
•
Factors influencing the selection of sterilization or disinfection methods.
-
•
Selection of sterilization or disinfection methods for decellularized matrix.
1. Introduction
Sterilization is the process of killing or removing all microorganisms, including bacterial spores [1]. Disinfection is the process of killing or removing all kinds of pathogenic microorganisms except bacterial spores [2]. Asepsis is no living microorganism in the object, which is the premise for biomaterials to be used in cell experiments, animal experiments and clinical applications. Implants such as artificial blood vessels and heart valves must be sterilized before clinical applications [3]. The most common sterilization methods are irradiation and ethylene oxide (EO). However, if biomaterials are only used in experimental research rather than clinical application, and the aseptic state of biomaterials meets the needs of cell experiments and animal experiments, there are many other sterilization and disinfection methods, such as peracetic acid, alcohol, and ultraviolet, etc., could be used besides irradiation and EO. The sterilization and disinfection often affect the physic-chemical properties and biological activity of biomaterials [[4], [5], [6]], for example high temperature [7] or high-dose irradiation [8,9] can induce protein denaturation [2], remained EO is toxic, etc.
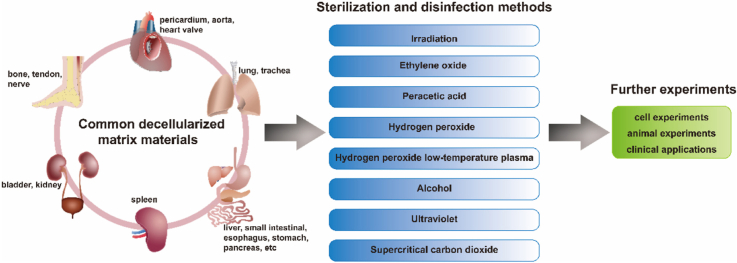
Decellularized matrix (dECM) is a low immunogenicity scaffold material obtained by removing cells from tissues with physical or chemical methods. DECM is a complex network of macromolecules interacting with each other and has complex components. It retains many inherent components in tissues, such as collagen, elastin, proteoglycan, hyaluronic acid and other macromolecules, as well as growth factors [10]. With its complex composition and structure close to natural tissues, good biocompatibility and low immunogenicity, dECM has gradually become a research hotspot of biomaterials in the past two decades, such as decellularized pericardium [11], decellularized blood vessels [12], decellularized corneas [13], decellularized bone [14], decellularized lung [15], and decellularized nerve [10].
There are various final states of dECM. Some decellularized matrix materials maintain the natural morphology of tissues, such as decellularized trachea prepared by M Den Hondt [16] and decellularized kidney prepared by Jeremy J Song [17]. Some decellularized matrix materials are further processed to obtain new forms, such as decellularized pericardial matrix hydrogel [18], decellularized perivascular matrix hydrogel [19], bovine cancellous bone granules [20], and human placental derived extracellular matrix sponge [21], as well as decellularized matrix as coating for 3D printing polycaprolactone (PCL) tubes [22].
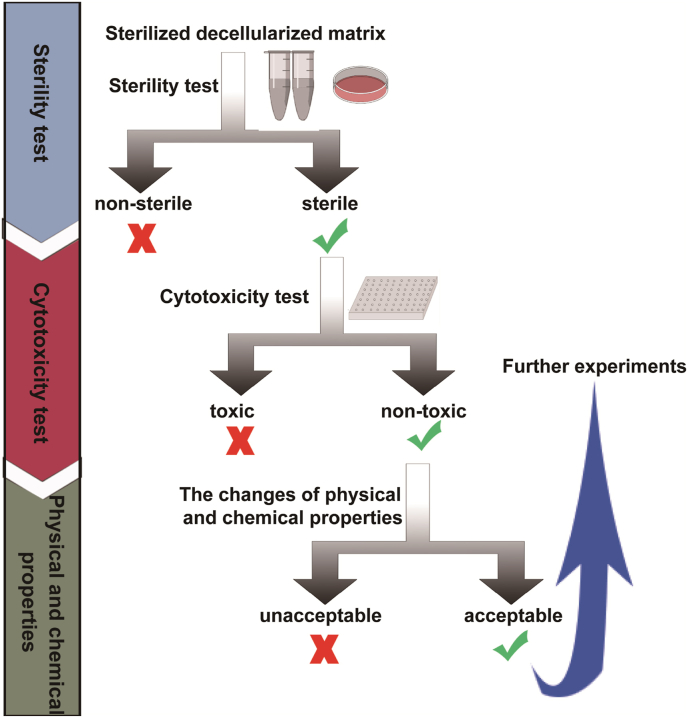
The ideal sterilization or disinfection for dECM can not only effectively remove microorganisms, but also ensure that the sterilized material is non-toxic, and maintain the physical and chemical properties and biological activity of the biomaterial. Therefore, the selection of sterilization or disinfection method is very important. The technical route to verify an appropriate sterilization or disinfection method for dECM is as follows. First of all, to determine whether the dECM is sterile with the sterility test; after effective sterilization or disinfection, to test whether there are toxic and harmful substances in the biomaterials with the cytotoxicity test; finally, after ensuring the materials are non-toxic and harmless, to evaluate whether the changes of physical and chemical properties of biomaterials after sterilization or disinfection affect the desired function of dECM, such as whether the materials used to promote tissue repair effectively retain growth factors, and whether the membranes used to wrap wounds have appropriate hardness and elasticity. After the above conditions are satisfied, the sterilized dECM can be used for further experiments and applications (Fig. 1).
Fig. 1.
The technical route for validating the sterilization or disinfection method for dECM.
Although being in the aseptic state is very important for dECM, there are few studies concerning the effects of sterilization or disinfection on dECM, and the systematic introduction to the sterilization or disinfection for dECM is even less.
In the present paper, the mechanism, advantages, disadvantages and applications of various sterilization or disinfection methods are systematically introduced and analyzed, then the factors influencing the selection of sterilization and disinfection methods are discussed, and the sterilization and disinfection methods for various common dECM are summarized. Finally, the graphical route for selecting the sterilization or disinfection method for dECM is proposed. This review aims to provide the reference basis for choosing appropriate sterilization or disinfection methods of various dECM.
2. Sterilization and disinfection methods of dECM
2.1. Irradiation
Irradiation is a physical sterilization method. The common irradiation sterilization mainly includes gamma ray (GI) produced by 60Co device and electron beam (E‐beam) produced by electron accelerator. Comparing between them, the penetrability of gamma ray is stronger than that of electron beam, and gamma ray is more often used for sterilization of biomaterials [2]. According to the different dose rate of radiation source, the required time of radiation sterilization is different, and usually the sterilization can be completed within hours to days.
The effect of irradiation on materials is mainly related to radiation dose [23,24]. For the same material, at relatively low dose, radiation-induced crosslinking increases tensile strength and stiffness of dECM; while at relatively high dose, tissue denaturation and degradation decrease tensile strength and stiffness [24]. Moreover, due to the differences in shape and size of various biomaterials, the appropriate dose is set according to the specific conditions.
In addition to the radiation dose, the gas state around the materials (such as nitrogen, oxygen, or air) [25,26] and water content [27] may affect the effectiveness of irradiation sterilization and the physic-chemical properties of materials. However, the comparative studies about the effects of above factors on the irradiation sterilization of dECM have not been found, which need to be further studied.
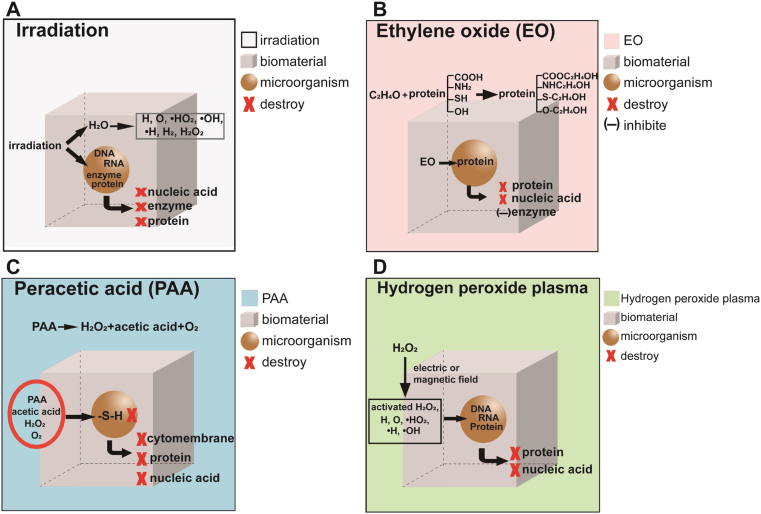
Sterilization mechanism: Irradiation directly destroys the nucleic acids, proteins and enzymes of microorganisms. At the same time, the water molecules within the organism are radiated to produce peroxides and free radicals, which destroy the nucleic acids, enzymes and proteins of the microorganisms and make the microorganisms lose their metabolic function [2,28] (Fig. 2A).
Fig. 2.
Sterilization and disinfection mechanisms of irradiation (A), ethylene oxide (B), peracetic acid (C), and hydrogen peroxide plasma (D).
Advantages: Irradiation sterilization is carried out at room temperature, with no residual toxicity, and its strong permeability could result in thorough sterilization.
Disadvantages: Irradiation may lead to changes in physical and chemical properties and biocompatibility, such as protein denaturation, and changes in material color and mechanical strength [29].
Applications: Irradiation is a widely used sterilization method. It is often used for sterilization of disposable medical supplies, drugs, food and daily necessities. The dECM sterilized by irradiation mentioned in the existing literature are mainly derived from valve [30], blood vessel [31], liver [32], stomach [33], pancreas [34,35], esophagus [36], small intestine [37,38], lung [15,39], trachea [[40], [41], [42]], bladder [[43], [44], [45], [46]], kidney [23,47,48], bone [14,[49], [50], [51], [52], [53]], tendon [27,[54], [55], [56]] and nerve [[57], [58], [59]].
2.2. Ethylene oxide
Ethylene oxide (EO) sterilization is a mature sterilization method. EO is a toxic organic compound. It is a colorless and transparent liquid below 10.8 °C and a colorless gas with ether-like pungent odor at room temperature. Because dECM has the strong adsorption capacity for EO, it is necessary to extend the time of removing EO residue after sterilization, so EO sterilization often takes several weeks.
Sterilization mechanism: EO can alkylate with sulfhydryl, amino and carboxyl groups in proteins and nucleic acid molecules, which make these macromolecules of microorganisms lose their activity and result in sterilization [60]. For example, EO can inhibit the activities of various enzymes, hinder the normal metabolism and then lead to the death of microorganisms [2] (Fig. 2B).
Advantages: EO sterilization is carried out at room temperature which has strong permeability and cause no damage to most of materials.
Disadvantages:
-
(1)
EO is toxic and its residues cause adverse reactions [61]. Therefore, EO is not recommended for sterilization of materials which are easy to absorb EO such as loose and porous lyophilized decellularized matrix materials, unless EO can be effectively removed.
-
(2)
EO is easily soluble in water to form toxic ethylene glycol and harmful chloroethanol is produced in the presence of chlorine. Therefore, the materials containing water and chlorine should not be sterilized with EO.
Applications: EO is usually used in sterilization of medical devices, including high polymer materials such as catheters of medical instruments, instruments with high sharpness, etc. The dECM materials sterilized by EO mentioned in the existing literature are mainly derived from blood vessel [62], stomach [63], esophagus [64,65], bladder [[66], [67], [68], [69], [70], [71], [72], [73], [74], [75]], kidney [76], bone [77] and tendon [[78], [79], [80]].
2.3. Peroxide
Peroxide is a strong oxidant containing the peroxy group "-O-O-" in the chemical molecular structure. Common peroxides include peracetic acid and hydrogen peroxide.
2.3.1. Peracetic acid
Peracetic acid (PAA) is a common disinfectant, but it can achieve sterilization effect under certain conditions, so it is also used as a chemical sterilization agent. For example, 1% PAA can kill the spores of Bacillus anthracis in 30 min [81]. It is an organic peroxide with strong oxygenation. The pH value of PAA is less than 5. The stronger the acidity, the better is the sterilization effect. In addition, the experiment of Jason Hodde showed that exposure to 0.18% (v/v) PAA/4.8% (v/v) ethanol aqueous solution for more than 0.5 h could effectively inactivate the virus of porcine small intestine [82]. The sterilization and disinfection of PAA is usually to soak the material in PAA solution for dozens of minutes [4].
Sterilization and disinfection mechanism: Peracetic acid is produced by the reaction of hydrogen peroxide and acetic acid, which has poor stability and is easy to decompose. The strong oxidation of PAA and the synergistic effect of hydrogen peroxide with acetic acid destroy the cytoderm of microorganisms, oxidize the sulfhydryl (-S-H) in proteins and enzymes, and destroy the enzyme system [2,83] (Fig. 2C).
Advantages: The decomposition products of PAA are acetic acid, water and oxygen, which are non-toxic.
Disadvantages: PAA has strong oxidation and acidity which may affect the physical and chemical properties of some materials. For example, the young's modulus of the decellularized porcine bladder sterilized by PAA was decreased [66]; sterilization of the decellularized small intestine with PAA resulted in the fraying and flattening of the edge at the villous portions [5].
Applications: PAA is mainly used for sterilization and disinfection of non-metal objects and environment. The dECM materials sterilized by PAA mentioned in the existing literature are mainly derived from valve [84], liver [85,86], small intestine [5,87,88], spleen [89,90], lung [91], bladder [66,92,93], kidney [48,94], tendon [95,96] and nerve [97]. In addition, the decellularized matrix materials sterilized with PAA/ethanol solution are mainly derived from blood vessel [19], esophagus [65,98], liver [4,99], small intestine [38,100], trachea [40,42,101], lung [15,102,103], bladder [[104], [105], [106], [107], [108], [109]], kidney [48,110], spleen [[111], [112], [113]] and nerve [114].
2.3.2. Hydrogen peroxide and hydrogen peroxide low-temperature plasma
Hydrogen peroxide (H2O2) is a strong oxidant, and usually used as a disinfectant. Under certain conditions, hydrogen peroxide can also kill bacterial spores to achieve sterilization effect, for example exposure to liquid H2O2 at 30 °C for 20 min can effectively inactivate the Bacillus anthracis spores [115]. Hydrogen peroxide low-temperature plasma (HPLP) sterilization is a commonly used low-temperature sterilization method in hospitals. In HPLP devices, the working temperature of sterilization is generally 45–55 °C and does not exceed 60 °C. Both H2O2 and HPLP need dozens of minutes to achieve the effects of disinfection or sterilization [116].
Sterilization and disinfection mechanism: H2O2 and its active derivatives produced after decomposition have strong oxidation effects which directly cause the destruction of cytomembrane and the denaturation of nucleic acids and proteins [117].
The sterilization mechanism of HPLP is that H2O2 generates a large number of active oxygen and high-energy free radicals through magnetic field excitation, such as hydroxyl radical (•OH), hydroxyl peroxide radical (•HO2), activated oxygen atom (O), activated hydrogen atom (H) and activated H2O2 molecule, which can denature nucleic acids and proteins in microorganisms and destroy the balance of potential inside and outside of microbial cytomembrane [2] (Fig. 2D).
Advantages: Both of H2O2 and HPLP can be decomposed into nontoxic water and oxygen.
Disadvantages:
-
(1)
The most serious problem of H2O2 and HPLP is that H2O2 can produce oxygen under the catalysis of catalase in vivo which is easy to cause gas embolism [118]. Therefore, whether they are suitable for application in the sterilization of dECM needs further exploration. It is suggested that H2O2 residue detection should be carried out before further experiments.
-
(2)
They have strong oxidation which may affect the physical and chemical properties of some materials, such as changes in the tertiary structure of proteins [119].
-
(3)
In addition, because HPLP sterilization needs to be in vacuum and has poor permeability, the object material is required to be in a dry state. Moreover, HPLP is not suitable for the sterilization of materials which cannot bear vacuum or with one end blocked.
Applications: H2O2 and HPLP are mainly used for the disinfection and sterilization of nonmetal objects and environment, but rarely used for disinfection and sterilization of dECM. Occasionally, H2O2 was used in combination with other sterilization methods in dECM sterilization [120].
2.4. Alcohol
Alcohol is a common disinfectant, which cannot kill spores. And the most commonly used alcohol disinfectants are ethanol and isopropanol. Alcohol disinfection method is to soak the materials in alcohol solution for dozens of minutes [11,121].
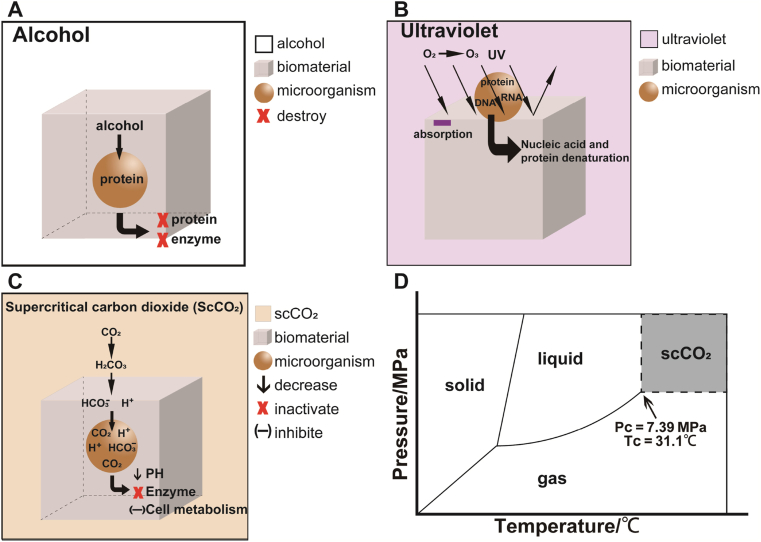
Disinfection mechanism: Alcohols can denature protein and destroy the enzyme system of microorganisms (Fig. 3A).
Fig. 3.
Sterilization and disinfection mechanisms of alcohol (A), ultraviolet (B), and supercritical carbon dioxide (C). Three-phase diagram of supercritical carbon dioxide (D).
Advantages: Alcohol has less damage to the tissue structure of dECM materials. For example, after 1 h of alcohol disinfection, the structure of renal tubule and glomerulus of pig kidney had no obvious change [48].
Disadvantages: Alcohol has no killing effect on bacterial spores and can only be used for disinfection, but not for sterilization [122]. And alcohol can denature protein and significantly decrease the content of collagen [4].
Applications: Alcohol is commonly used in disinfection of skin and precision instruments, etc. There are many reports on the use of alcohol to disinfect dECM, which are mainly derived from pericardium [11], valve [123,124], blood vessel [12,121,125,126], liver [[127], [128], [129]], pancreas [130], trachea [131], bladder [130,[132], [133], [134], [135]], kidney [48,136], bone [137,138], tendon [[139], [140], [141]] and nerve [142].
2.5. Ultraviolet ray
Ultraviolet ray (UV) is mainly used for physical disinfection. Generally, the UV wavelength is 200–300 nm and the strongest disinfection effect is at 253.7 nm for dozens of minutes. UV is transmitted along a straight line and can be reflected or absorbed by the surface of an object. The penetrability of UV is weak, but it is easy to penetrate air and pure water.
Disinfection mechanism: UV can not only denature nucleic acids and proteins directly, but also make oxygen in the air produce ozone, and ozone also has bactericidal effects. Therefore, UV could complete disinfection together with ozone [28,143] (Fig. 3B).
Advantages: UV is a simple and easy method to disinfect the surface and environment.
Disadvantages: Because of weak penetrability of UV, it is not suitable for liquid medicine disinfection and internal disinfection of solid objects.
Applications: UV ray is mainly used for the disinfection of object surface and environment. The dECM materials disinfected with UV usually has a thin shape and large specific surface area, such as decellularized small intestine [5], liver slice [144] and bladder [145]. Chen et al. sliced the decellularized liver into 250 μm thickness and disinfected it with UV rays for 2 h [144]. UV rays are rarely used alone for disinfection, and are often used in combination with other disinfection methods. For example, Selcan Guler immersed the decellularized sheep aorta in 3% (w/v) AA (antibiotic/antifungal) solution for 10 min, and then exposed to UV for 30 min [146].
2.6. Supercritical carbon dioxide
In the field of disinfection or sterilization, supercritical carbon dioxide (ScCO2) technology is still in the new stage, and its killing effect on spores remains to be further studied, so it is uncertain whether it is sterilization or disinfection.
There are three kinds of phase states: gas phase, liquid phase and solid phase. The point of equilibrium between liquid phase and gas phase is called the critical point (PC, TC). Fluid is the general term of liquid and gas. When the temperature and pressure of the fluid is higher than the critical point, it is in supercritical state. The properties of supercritical fluid are between liquid and gas, with similar density of liquid, viscosity between liquid and gas, and diffusivity similar to gas, so that it has strong permeability and solubility. Carbon dioxide (CO2) has a lower critical temperature (TC = 31.1 °C) and a critical pressure (PC = 7.39 MPa) (Fig. 3D). ScCO2 technology is not only widely used in extraction separation, but also a friendly green disinfection and sterilization method. In ScCO2 devices, it takes dozens of minutes to achieve disinfection or sterilization.
Sterilization and disinfection mechanism: The mechanism of ScCO2 is not completely clear, and many theories have been proposed, among which acidification is the main mechanism [147]. CO2 dissolves and transforms into carbonic acid, and then decomposes into bicarbonate ions (HCO3−) and hydrogen ions (H+), which increases the permeability of cytomembrane. CO2, H+ and HCO3− penetrate the cytomembrane and accumulate in cells. Hence, the intracellular pH decreases which results in the inactivation of enzymes, inhibition of cell metabolism, and final death of microorganisms (Fig. 3C).
Advantages: ScCO2 has no toxicity and no residue, and it also has the function of decellularization [148].
Disadvantages: There are few studies on ScCO2 for disinfection and sterilization. ScCO2 may have effects on the physical and chemical properties of the materials. For example, after ScCO2 sterilization, stiffness of human tendon was significantly reduced [74], and the tensile strength of decellularized porcine pericardium was significantly increased [149]. Moreover, ScCO2 can cause acidification, which may lead to the partial dissolution of proteins.
Applications: ScCO2 is rarely used for disinfection and sterilization. The decellularized matrix materials sterilized by ScCO2 mentioned in the existing literature are mainly derived from pericardium [149], blood vessel [148], lung [150] and tendon [55].
Table 1 summarizes the advantage, disadvantage, and accessibility of the sterilization and disinfection methods of dECM, in order to benefit researchers to compare various methods and choose the appropriate method for their materials.
Table 1.
Summary of sterilization and disinfection methods of dECM.
| Methods | Sterilization/Disinfection | Advantages | Disadvantages | Time required and accessibility |
|---|---|---|---|---|
| Irradiation | sterilization |
|
|
|
| EO | sterilization |
|
|
|
| PAA | disinfection; sterilization (under certain conditions) |
|
|
|
| H2O2 | disinfection; sterilization (under certain conditions) |
|
|
|
| HPLP | sterilization |
|
|
|
| Alcohol | disinfection |
|
|
|
| UV | disinfection |
|
|
|
| ScCO2 | disinfection/sterilization (uncertain) |
|
|
|
2.7. Antibiotics
Although the use of antibiotics is not the typical method of disinfection or sterilization, antibiotics are also used alone for the treatment of dECM, without combining with other sterilization methods, in order to achieve the aseptic state [[151], [152], [153], [154], [155]]. Therefore, this paper introduces and analyzes the mechanism, advantages and disadvantages of antibiotics acting on microorganisms and their applications for dECM, so as to provide a possible selection for sterilization methods of dECM.
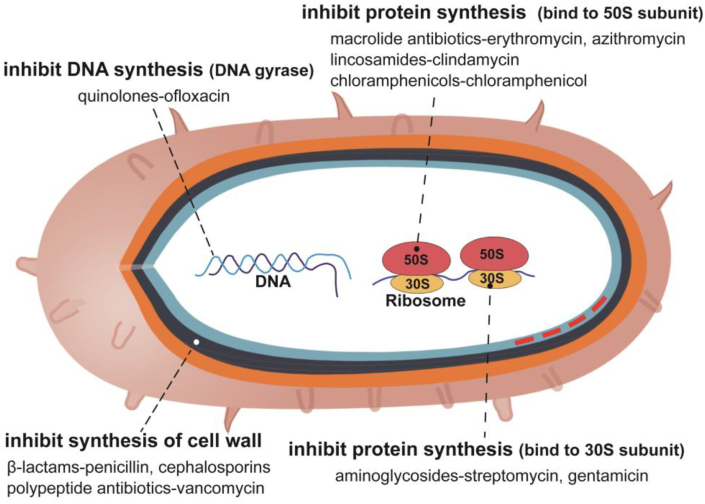
Action mechanism: Antibiotics have a variety of action mechanisms [156]. Antibiotics inhibit or kill microorganisms through the following mechanisms: ①β-lactams such as penicillin, and polypeptide antibiotics such as vancomycin inhibit the synthesis of bacterial cell wall; ②Aminoglycosides such as streptomycin and gentamicin can bind to the 30S subunit of ribosome, so as to prevent protein translation of bacteria; ③Macrolide antibiotics such as erythromycin and azithromycin, and lincosamides such as clindamycin can bind to the 50S subunit of the ribosome to interfere with bacterial protein synthesis; ④Quinolones such as ofloxacin can interfere with DNA gyrase and inhibit DNA synthesis of bacteria; ⑤Amphotericin B and other polyene antifungal antibiotics can affect the permeability of cell membrane and inhibit the growth of fungi [157] (Fig. 4).
Fig. 4.
The action mechanism of antibiotics.
Advantages: Antibiotics have no obvious effect on mechanical properties, structure and components of dECM.
Disadvantages:
-
(1)
The antimicrobial spectrum of each antibiotic is limited. Even if multiple antibiotics are used in combination, some microorganisms are not sensitive to these antibiotics, such as mycoplasma, chlamydia, anaerobic bacteria, viruses, and so on. Therefore, it is suggested to carry out sterility test after treatment of dECM with antibiotics.
-
(2)
Some antibiotics have adverse effects on cells. For example, Gilda Kalinec evaluated the response of HEI-OC1 cells to several antibiotics, and found that tobramycin could induce significant cell death, gentamicin could stimulate autophagy, and penicillin and streptomycin could reduce cell viability and increase senescence [158]. Some antibiotics may cause adverse reactions such as hypersensitivity reactions (HSRs) and toxic reactions. The most common antibiotics causing HSRs are β - lactams, followed by quinolones, and the clinical manifestations of HSRs are maculopapular rash and even anaphylactic shock [159]. In addition, some antibiotics can cause toxic reactions in the human body, such as streptomycin and other aminoglycosides have ototoxicity and nephrotoxicity. Therefore, in view of the possible toxic and side effects of antibiotics on cells and human, it is suggested to detect antibiotic residues and conduct the cytotoxicity test.
Applications: The decellularized matrix materials incubated in antibiotics without other sterilization methods mentioned in the existing literature are mainly derived from pericardium [151,160], valve [152], blood vessel [161,162], liver [163,164], stomach [154], pancreas [165,166], esophagus [167], small intestine [168], lung [[169], [170], [171], [172], [173]], trachea [16,[174], [175], [176]], kidney [17,177,178], bone [20], tendon [153,179] and nerve [155].
3. Factors influencing the selection of sterilization and disinfection methods
3.1. Application purpose of materials
When selecting a sterilization or disinfection method for a material, first of all, we need to determine its application purpose. If it is to be used for clinical application, the material needs to be sterilized, so only sterilization methods should be selected, such as irradiation and EO. If the material is only used for experimental research, all disinfection and sterilization methods can be considered.
3.2. Physical and chemical properties of materials
The chemical properties of biomaterials mainly depend on their composition, and the physical properties mainly include physical state, appearance and size.
3.2.1. Chemical properties
The chemical properties of the main components in the material need to be considered. Most of the dECM materials mainly retain organic substance, in which protein is the main component. Protein is not resistant to high temperature, acid and alkali, strong oxidation and strong irradiation. High temperature and irradiation can denature proteins [8]. PAA and H2O2 have strong oxidation and acidity, which may lead to protein denaturation or dissolution [5]. In addition, a small number of materials mainly retain inorganic components, such as some kinds of bone powders, and irradiation is suitable for their sterilization.
3.2.2. Physical properties
-
(1)
Physical state
Most of the dECM materials are solid, which include dry state and wet state, and a small amount of dECM is hydrogel. EO is easily soluble in water and forms toxic ethylene glycol, so it cannot be used in materials containing water. HPLP sterilization requires the sterilized materials to be in dry state. Due to its weak penetrability, UV is only suitable for the disinfection of the surface of objects and distilled water, but not for the disinfection of liquid medicine and the deep disinfection of solid objects. So, the above methods are not suitable for wet solid materials and hydrogel. In addition, selecting a sterilization or disinfection method for dECM hydrogel still face the following problems: ①irradiation [180] and high temperature [181] tend to reduce the molecular weight, resulting in a decrease in viscosity of hydrogels; ②PAA, alcohol and H2O2 are dissolved in water and difficult to be removed; ③there are almost no research on the sterilization or disinfection for dECM hydrogel with ScCO2, so its effect of sterilization is uncertain. Therefore, for sterilization or disinfection of dECM hydrogels, it is recommended to first sterilize the solid raw materials of dECM hydrogel, and then carry out the subsequent hydrogel preparation following the aseptic operation principle.
-
(2)
Appearance
Freeze dried dECM is loose and porous which makes it having strong adsorption capacity, so it is not suitable to be sterilized by EO, unless the removal time of EO residue is prolonged to remove the residue effectively. In another scene, the materials with one end blocked cannot to be sterilized by HPLP due to its poor permeability.
-
(3)
Size
Considering size, thickness is the main factor involved in affecting sterilization and disinfection. The influence of thickness on the selection of sterilization and disinfection method is related to the penetrability of sterilization and disinfection agent, for example, the penetrability of irradiation and EO is strong, while the penetrability of HPLP and UV is poor.
3.3. Time required and accessibility
Due to the need to extend the removal time of EO residue after EO sterilization of dECM materials, EO sterilization usually takes several weeks. Irradiation sterilization takes hours to days. Sterilization or disinfection by PAA, H2O2, HPLP, alcohol and UV take dozens of minutes (Table 1).
Sterilization with irradiation, EO and HPLP need corresponding devices. UV disinfection requires the UV light source, but UV device is more common. Sterilization or disinfection by PAA, H2O2, and alcohol are to soak the materials directly in their solution (Table 1).
4. Sterilization and disinfection methods of various common decellularized matrix materials
Because of the different composition, structure, volume and desired function of the dECM materials, the sterilization or disinfection methods are various. However, for the same tissue or organ, the sterilization or disinfection methods reported in the current literature are often different, mainly due to the lack of ideal selection criteria, and the literature about the effects of sterilization and disinfection methods on dECM materials is scarce.
4.1. Cardiovascular system
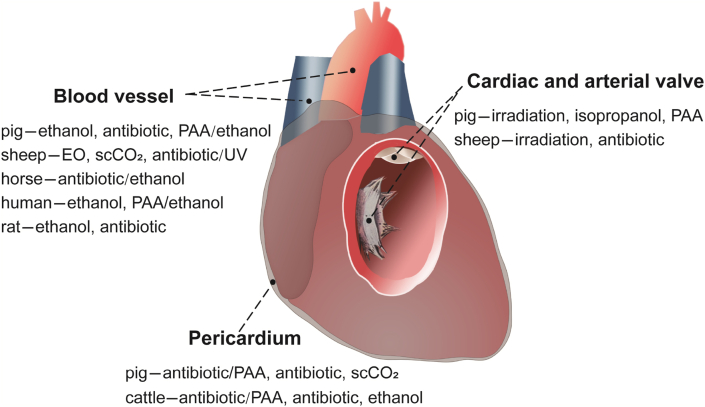
There are many dECM materials in cardiovascular system such as decellularized pericardium, aorta, and heart valve (Fig. 5 and Table 2).
Fig. 5.
Summary of sterilization and disinfection methods for decellularized pericardium, valves, and blood vessels.
Table 2.
Summary of sterilization and disinfection methods for decellularized pericardium, valves and blood vessels.
| Tissue type | Origins | Methods | Appearance and Size | Details and References |
|---|---|---|---|---|
| Pericardium | pig | antibiotic/PAA | piece (12 mm diameter) | antibiotic, 37 °C, 24 h. Then, 0.05% (v/v) or 0.1% (v/v) PAA (pH 7.3), 27 °C, 3 h [182] |
| antibiotic | overall shape | antibiotic, 4 °C, 72 h [160] | ||
| ScCO2 | overall shape | ScCO2 [149] | ||
| bovine | antibiotic/PAA | piece (12 mm diameter) | antibiotic, 37 °C, 24 h. Then, 0.05% (v/v) or 0.1% (v/v) PAA (pH 7.3), 27 °C, 3 h [182] | |
| antibiotic | piece (100 mm × 100 mm) | antibiotic [151] | ||
| overall shape | antibiotic, 4 °C, 72 h [160] | |||
| ethanol | overall shape | ethanol, 20%–75% (v/v), 4 h [11] | ||
| Blood vessel | pig | ethanol | segment | 80% (v/v) ethanol, 3 days [121] |
| antibiotic | segment | antibiotic, 4 °C [161] | ||
| PAA/ethanol | aortic adventitia | 0.1% (v/v) PAA in 4% (v/v) ethanol [19] | ||
| sheep | EO | segment (60 mm length, 3–4 mm diameter) | EO, room temperature [62] | |
| ScCO2 | segment (15 mm length, 10 mm diameter) | ScCO2, 1 h [148] | ||
| antibiotic/UV | segment (10 mm × 40 mm) | antibiotic, 10 min. Then, UV, 30 min [146] | ||
| horse | antibiotic/ethanol | segment | antibiotic. Then, 70% (v/v) ethanol, 20 min [183] | |
| human | ethanol | segment | 70% (v/v) ethanol [125] | |
| PAA/ethanol | segment | 0.1% (v/v) PAA in 4% (v/v) ethanol [19] | ||
| rat | ethanol | segment (30–40 mm length) | ethanol [126] | |
| antibiotic | segment | antibiotic [162] | ||
| Cardiac and arterial valves | pig | irradiation | overall shape | gamma irradiation, 1.5 kGy and 3 kGy [30] |
| overall shape | gamma irradiation, 0.15 kGy [31] | |||
| isopropanol | overall shape | 10% (v/v) isopropanol [123] | ||
| PAA | overall shape | 0.1% (v/v) PAA, 37 °C, 3 h [84] | ||
| sheep | irradiation | overall shape | gamma irradiation, 0.15 kGy [31] | |
| antibiotic | overall shape | antibiotic [152] |
4.1.1. Pericardium
The pericardium is the membrane that surrounds the heart. The decellularized pericardium is the biomaterial widely used in clinic. The main source species of decellularized pericardium are pig and bovine, and the sterilization and disinfection methods mentioned in current literature mainly include incubation in antibiotics [151,160], combination of antibiotics and PAA [182], ethanol [11] and ScCO2 [149].
Cátia Fidalgo [182] sterilized porcine and bovine pericardium with the two-step sterilization, which was antibiotics/antimycotic (AA) cocktail and PAA. It was found that the aseptic scaffolds could be obtained after the two-step sterilization, while the structural integrity and biocompatibility of the tissue were preserved. Joshua A Choe [149] sterilized porcine pericardium with ScCO2, and the compression and tensile strength of the material were improved after sterilization.
4.1.2. Blood vessel
Decellularized vascular materials involve arteries, veins and perivascular tissues. They come from a variety of source species including pig, sheep, horse, human, and rat according to relevant literature. The sterilization and disinfection methods mentioned in current reports mainly include ethanol [121,125,126,183], PAA/ethanol [19], antibiotics [161,162], EO [62], antibiotic/UV [146] and ScCO2 [148], etc.
George R. Fercana [19] incubated the decellularized adventitia in a solution of 0.1% (v/v) PAA/4% (v/v) ethanol. After sterilization, the decellularized arterial adventitia was freeze-dried, ground and prepared into hydrogels to promote angiogenesis. Selcan Guler [148] sterilized the aorta of sheep with ScCO2 for 1 h, with the result showing that ScCO2 could not only get sterilization, but also bring about decellularization. After treatment, the tissue retained its thickness and stiffness, but became drier.
4.1.3. Cardiac and arterial valve
The main source species of valves are pig and sheep, and their sterilization and disinfection methods mainly include irradiation [30,31], isopropanol [123], PAA [84] and antibiotics [152].
Meghana R K Helder [30] irradiated the decellularized porcine heart valves with 1.5 kGy and 3 kGy irradiation, with the result that the valves irradiated with 1.5 kGy were not completely sterilized, while the valves irradiated with 3 kGy showed no signs of infection, and the mechanical strength of the valves decreased with the increase of irradiation dose.
4.2. Digestive system
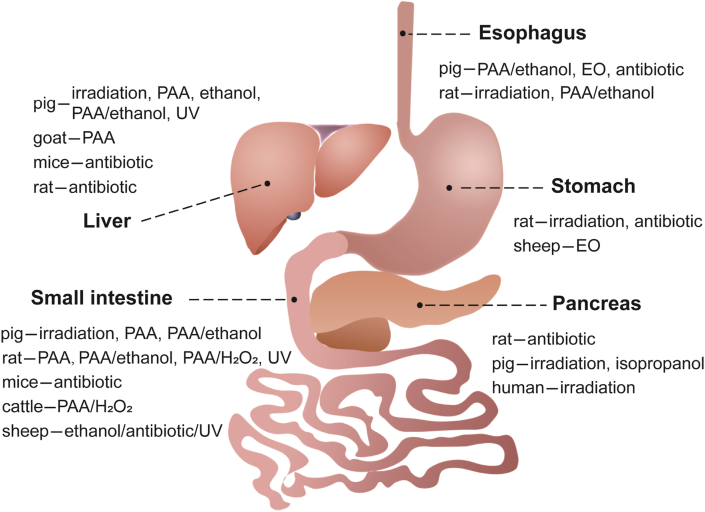
There are many dECM materials of digestive system such as decellularized liver, small intestinal, esophagus, stomach and pancreas, etc. (Fig. 6 and Table 3)
Fig. 6.
Summary of sterilization and disinfection methods for decellularized tissues and organs in digestive system.
Table 3.
Summary of sterilization and disinfection methods for decellularized tissues and organs in digestive system.
| Tissue type | Origins | Methods | Appearance and Size | Details and References |
|---|---|---|---|---|
| Liver | pig | irradiation | slice (1 mm thick) | (placed in PBS) gamma irradiation, 10 kGy [32] |
| PAA | powder (<250 μm) | 0.1% (v/v) PAA, 2 h [85] | ||
| slice (14 mm diameter, 3 mm thick) | 0.1% (v/v) PAA, 2 h [184] | |||
| slice (25 mm × 15 mm × 2 mm) | 0.1% (v/v) PAA [4] | |||
| PAA/ethanol | slice (3 mm thick) | 0.1% (v/v) PAA in 4% (v/v) ethanol for 2 h [99] | ||
| ethanol | powder | 70% (v/v) ethanol [127,128] | ||
| slice (5 mm × 5 mm × 5 mm) | 70% (v/v) ethanol [129] | |||
| UV | slice (4 mm diameter, 0.25 mm thick) | UV, 2 h [144] | ||
| goat | PAA | slice (5 mm × 5 mm × 2 mm) | lyophilized, PAA, 2–3 h [86] | |
| mice | antibiotic | overall shape | antibiotic [163] | |
| rat | antibiotic | overall shape | antibiotic [164] | |
| Small intestine | pig | irradiation | segment (10 cm length) | lyophilized, gamma irradiation, 25 kGy [38] |
| segment | gamma irradiation, 0.15 kGy [185] | |||
| EO | flocculent piece | EO [100] | ||
| PAA | segment (30 cm length) | 0.1% (v/v) PAA, room temperature, 3 h [87] | ||
| rat | PAA | segment | 0.1% (v/v) PAA, 3 h [5,88] | |
| PAA/ethanol | segment | 0.18% (v/v) PAA with 4.8% (v/v) ethanol, 30 min [5] | ||
| PAA/H2O2 | segment | 0.08% (v/v) PAA with 1% (v/v) H2O2, 3 h [5] | ||
| UV | segment | UV at 254 nm, 90 s [5] | ||
| mice | antibiotic | segment | antibiotic [168] | |
| bovine | PAA/H2O2 | segment (10 cm length) | 0.15% (v/v) PAA in 20% (v/v) H2O2, 16 h [120] | |
| Esophagus | pig | EO | segment | EO [65] |
| antibiotic | segment | antibiotic, overnight [167] | ||
| rat | irradiation | segment | gamma irradiation [36] | |
| PAA/ethanol | segment | 0.1% (v/v) PAA in a 4% (v/v) ethanol, 4 °C, 24 h [98] | ||
| Stomach | rat | irradiation | overall shape | gamma irradiation, 12 kGy [33] |
| antibiotic | overall shape | antibiotic [154] | ||
| sheep | EO | uncertain | EO [63,64] | |
| Pancreas | rat | antibiotic | overall shape | antibiotic, 72 h [165,166] |
| pig | irradiation | slice (7 mm diameter, 5 mm thick) | gamma irradiation, 10 kGy [34] | |
| isopropanol | slice (5 mm × 5 mm) | 70% isopropanol, 1 h [130] | ||
| human | irradiation | overall shape | gamma irradiation, 12 kGy [35] |
4.2.1. Liver
Decellularized livers are derived mainly from pigs, and some from goats, rats and mice, etc. The sterilization and disinfection methods of decellularized liver mainly include ethanol [[127], [128], [129]], PAA [85,86,184], PAA/ethanol [4,99], irradiation [32], UV [144] and antibiotics [163,164].
Kamal H Hussein [4] sterilized pig liver slices with PAA and ethanol for 2 h. The results showed that ethanol could not sterilize materials effectively, but PAA could. The content of glycosaminoglycan after ethanol treatment was higher than that of PAA treatment, but the content of collagen after ethanol treatment decreased significantly than that of PAA treatment.
4.2.2. Small intestine
Decellularized submucosa of small intestine is a common decellularized material, and there is a lot of related literature. Decellularized small intestine mainly comes from pigs, some from rats and mice, and a few from bovine and sheep. The common sterilization and disinfection methods include irradiation [37,38,185], PAA/ethanol [5,38,100], PAA [5,87,88], antibiotics [168], UV [5], PAA/H2O2 [5,120] and so on.
Carolyn Gosztylal [5] sterilized the decellularized small intestine of rats with four methods, which mainly includes perfusion with 0.1% (v/v) PAA for 3 h, 0.08% (v/v) PAA/1% (v/v) H2O2 solution for 3 h, 0.18% (v/v) PAA/4.8% (v/v) ethanol solution for 3 h, and placed in the UV cross-linker at 254 nm for 90 s. The results showed that all of these sterilization methods were effective, and the sterilized tissues in each group had similar amount of collagen and glycosaminoglycan, and the similar ability to support cell growth. However, chemical sterilization led to the loss of tissue structure with fraying and flattening of the edge at the villous portions, while UV sterilization could keep the integrity of tissue structure.
4.2.3. Esophagus, stomach and pancreas
There are few reports about decellularized esophagus, stomach and pancreas, and most of this literature does not clearly elucidate sterilization and disinfection methods. The decellularized esophagus mainly comes from pigs and rats. The sterilization and disinfection methods mainly include antibiotics [167], PAA/ethanol [65,98], EO [65] and irradiation [36].
The decellularized gastric matrix mentioned in the existing literature mainly comes from sheep and rats. The sterilization methods mainly include EO [63,64], irradiation [33], and immersion in antibiotic solution [154]. Liang Feng [33] irradiated the decellularized rat stomach with 12 kGy, with the result that the spatial structure and elasticity of the tissue did not change significantly.
Decellularized pancreas in the existing reports is derived from rats, pigs and humans. Most of the decellularized pancreas was obtained with aseptic operation, and then washed and soaked in antibiotic solution. The sterilization and disinfection methods mainly include antibiotic [165,166], isopropanol [130] and irradiation [34,35].
4.3. Respiratory system
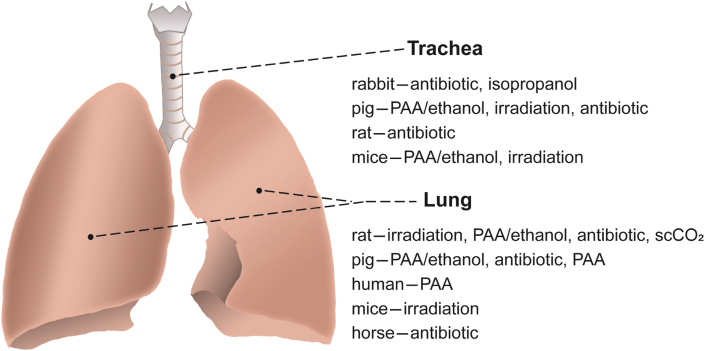
Decellularized lung and trachea are the most common dECM materials in respiratory system (Fig. 7 and Table 4).
Fig. 7.
Summary of sterilization and disinfection methods for decellularized lung and trachea.
Table 4.
Summary of sterilization and disinfection methods for decellularized lung and trachea.
| Tissue type | Origins | Methods | Appearance and Size | Details and References |
|---|---|---|---|---|
| Lung | rat | irradiation | overall shape | irradiation, 5 Gy/min, 12 min [15] |
| PAA/ethanol | overall shape | 0.1% (v/v) PAA in 4% (v/v) ethanol, 2 h [15]; 1 h [102] | ||
| antibiotic | overall shape | antibiotic, 37 °C, 48 h [169]; 37 °C, 72 h [171]; 4 °C [172] | ||
| ScCO2 | overall shape | ScCO2, 1440 psi, 35 °C, 30–45 min [150] | ||
| pig | PAA/ethanol | overall shape | 0.1% (v/v) PAA in 4% (v/v) ethanol [103] | |
| antibiotic | overall shape | antibiotic [173] | ||
| PAA | overall shape | PAA [91] | ||
| human | PAA | overall shape | PAA [91] | |
| mice | irradiation | overall shape | gamma irradiation, 31 kGy (at room temperature; frozen) [39] | |
| horse | antibiotic | overall shape | antibiotic [186] | |
| Trachea | rabbit | antibiotic | segment | antibiotic [16,174,187] |
| isopropanol | segment (0.75 cm length) | isopropanol, overnight [131] | ||
| pig | PAA/ethanol | segment | 0.1% (v/v) PAA in 4% (v/v) ethanol, 6 h [101] | |
| irradiation | segment | gamma irradiation, 20 kGy [40] | ||
| antibiotic | segment | antibiotic, 7 days [188] | ||
| rat | antibiotic | segment | antibiotic [176,187,189] | |
| mice | irradiation | segment | (in PBS) gamma irradiation, 5 kGy, 25 kGy [41] (in 0.9% NaCl) gamma irradiation, 20 kGy [42] |
4.3.1. Lung
The common decellularized lung mainly comes from rats, and a few from pigs, humans, mice and horses. The main method to obtain the aseptic state of decellularized lung is antibiotic rinsing [[169], [170], [171], [172],186]. In addition, there are PAA/ethanol [15,102,103], PAA [91], irradiation [15,39], ScCO2 [150], etc.
Nicholas R Bonenfant [15] compared two sterilization methods of decellularized mouse lungs, one of which was irradiation with 5 Gy/min for 12 min, and the other was to wash the decellularized lung in a mixture of 0.1% (v/v) PAA/4% (v/v) ethanol solution thrice and incubated in the mixture for 2 h. The results showed that PAA/ethanol could dissolve the extracellular matrix, and the overall appearance of PAA/ethanol treated lung was similar to that of natural lung with slight atelectasis in the central region. While in the irradiated lung, alveolar septum thickening, fusion and large emphysema appeared.
Jenna L. Balestrini [150] compared the sterilization effect of ScCO2 for 2 h and 0.1% PAA for 2 h on decellularized lung. The results showed that ScCO2 sterilization had no adverse effect on the mechanical properties and the contents of collagen, elastin and glycosaminoglycan (sGAG) in decellularized lung; while PAA-treated samples had no change in mechanical properties after 24 h, but had higher Young's moduli and lower failure strain after 6 months of storage, and PAA could significantly decrease the contents of elastin and sGAG.
Juan J. Uriarte [39] sterilized the decellularized lung of mice with 31 kGy. It was found that the volume of irradiated lung decreased and the mechanical impedance increased.
4.3.2. Trachea
Decellularized tracheas are mostly from rabbits, while pigs, rats and mice are also relatively common. Most of the decellularized tracheas were obtained in aseptic condition, and incubated in antibiotic solution after decellularization. The sterilization and disinfection methods mainly include antibiotic [16,[174], [175], [176],[187], [188], [189]], PAA/ethanol [40,42,101], irradiation [[40], [41], [42]], and isopropanol [131].
Christopher M. Johnson [41] irradiated the mice trachea with 25 kGy gamma radiation, with the result showing that 25 kGy radiation was enough to effectively sterilize the trachea, but the ultrastructure of tissue would be seriously degraded, such as a large number of empty lacunae and matrix disorganization.
4.4. Urinary system
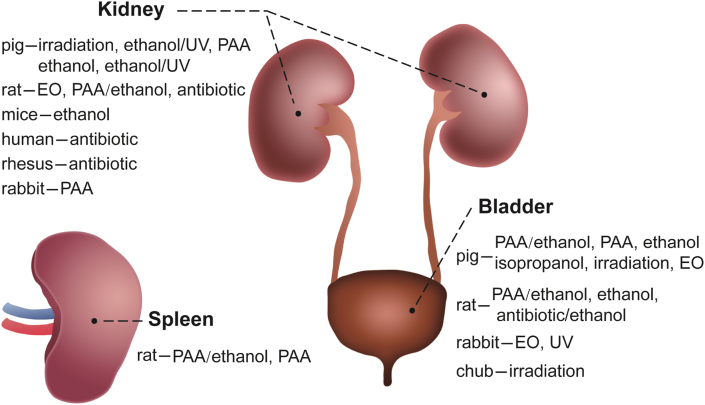
The most common dECM materials of urinary system are decellularized bladder and kidney (Fig. 8 and Table 5).
Fig. 8.
Summary of sterilization and disinfection methods for decellularized bladder, kidney and spleen.
Table 5.
Summary of sterilization and disinfection methods for decellularized bladder, kidney and spleen.
| Tissue type | Origins | Methods | Appearance and Size | Details and References |
|---|---|---|---|---|
| Bladder | pig | ethanol | overall shape | 70% ethanol [132,191] |
| piece (muscle removed) | 75% ethanol [135] | |||
| isopropanol | piece (5 mm × 5 mm) | 70% isopropanol, 1 h [130] | ||
| PAA/ethanol | piece | 0.1% (v/v) PAA in 4% (v/v) ethanol, 2 h [[104], [105], [106],109,190] | ||
| irradiation | piece (basement membrane, lamina propria) | gamma irradiation, 20 kGy [43,45] | ||
| piece (1 cm2) | gamma irradiation, 30 kGy [44] | |||
| EO | piece | EO [[66], [67], [68], [69], [70], [71], [72], [73],134] | ||
| overall shape; piece (8 cm × 10 cm) | EO [67] | |||
| piece (0.2–0.4 mm thick) | EO [70] | |||
| piece (1 cm × 1 cm) | EO [71] | |||
| piece (15 cm × 15 cm) | EO [73] | |||
| PAA | overall shape | 0.1% (v/v) PAA (pH 7.5), 37 °C, 3 h [93] 0.1% (v/v) PAA, room temperature, 3 h or 5 h [66] |
||
| piece (<2 mm thick) | 0.1% (v/v) PAA, 1 h [92] | |||
| rat | antibiotic/ethanol | overall shape | antibiotic; then, 70% ethanol, 10 min [192] | |
| PAA/ethanol | overall shape | 0.1% (v/v) PAA in 4% (v/v) ethanol, 4 °C, 24 h [98] | ||
| ethanol | piece | 70% ethanol [133] | ||
| rabbit | EO | overall shape | EO [75] | |
| UV | piece (submucosa) | UV, 24 h [145] | ||
| chub | irradiation | piece (10 cm2) | gamma irradiation, 25 kGy [46] | |
| Kidney | pig | irradiation | overall shape | gamma irradiation [47]; (placed in PBS), 10 kGy [23] |
| slice (7 mm diameter, 2 mm thick) | gamma irradiation, 1–10 kGy [47] | |||
| ethanol/UV | electrospinning membrane | 70% ethanol; then UV, 30 min/side [193] | ||
| ethanol | slice (7 mm diameter, 2 mm thick) | 70% ethanol (pH 7.4), 0.5 h, 1 h, 2 h, 3 h, 4 h [48] | ||
| PAA/ethanol | slice (7 mm diameter, 2 mm thick) | 0.2% (v/v) PAA in 4% (v/v) ethanol, (pH 3.0), 0.5 h, 1 h, 2 h, 3 h, 4 h [48] | ||
| PAA | slice (7 mm diameter, 2 mm thick) | 0.2% (v/v) PAA (in 1 M NaCl) (pH 3.1), 0.5 h, 1 h, 2 h, 3 h, 4 h [48] | ||
| rat | EO | overall shape | EO [76] | |
| PAA/ethanol | overall shape | 0.2% (v/v) PAA in 4% (v/v) ethanol, 10 min [110] | ||
| antibiotic | overall shape | antibiotic, 96 h [17] | ||
| mice | ethanol | overall shape | 70% ethanol, 30 min [136] | |
| human | antibiotic | slice (2 mm thick) | antibiotic [177] | |
| rhesus | antibiotic | transverse sections | antibiotic, 4 °C [178] | |
| rabbit | PAA | slice (10 mm × 10 mm × 3 mm) | 0.5%, 1%, 1.5% (v/v) PAA (pH 7.2–7.4), 2 h [94] | |
| Spleen | rat | PAA/ethanol | overall shape | 0.1% (v/v) PAA in 4% (v/v) ethanol, 2 h [[111], [112], [113]] |
| PAA | overall shape | 0.1% (v/v) PAA, 3 h [89], 2 h [90] |
4.4.1. Bladder
Decellularized bladder is a common dECM material mainly from pigs, as well as rats, rabbits and chubs. The main sterilization and disinfection methods include PAA/ethanol [[104], [105], [106], [107], [108], [109],190], EO [[66], [67], [68], [69], [70],75], irradiation [[43], [44], [45]], isopropanol [130], ethanol [132,133,191], antibiotic/ethanol [192], PAA [44,66,92] and UV [145].
Derek J Rosario [66] sterilized the decellularized pig bladder with EO and 0.1% (v/v) PAA, with the results showed that the young's modulus of the tissue sterilized by PAA was decreased, while the young's modulus of the tissue sterilized with EO increased.
4.4.2. Kidney
Decellularized kidney has a wide range of tissue sources mainly including pig, rat, mouse, rabbit, human and rhesus monkey in accordance with the relevant literature. In most of the reports, researchers immerse or preserve the tissue in an antibiotic solution after decellularization, without other sterilization or disinfection methods [17,177,178]. In addition, there are ethanol [48,136], EO [76], irradiation [23,47,48,94], PAA/ethanol [48,110], ethanol/UV [193], and PAA [48,94].
Lida Moradi [94] sterilized the decellularized rabbit kidney slices with four sterilization methods. ①The rabbit kidney slices were immersed in 0.5% PAA solution with pH value of 7.2–7.4 for 2 h. The result showed that they could be sterilized effectively and retain the mechanical properties of tissues and the main components of matrix. ②The rabbit kidney slices were incubated in the antibiotic mixture composed of penicillin G, amphotericin B and gentamicin for 30 min, with the result that they were effectively sterilized and preserved the mechanical properties of tissues, but no cell adhesion was observed when rabbit adipose mesenchymal stem cells were seeded on the kidney slices. ③The kidney slices were immersed in sterile PBS and irradiated with UV at 320–480 nm for 2 h, with the result that they could not be sterilized effectively. ④After 3–5 min irradiation with 5 kGy gamma radiation, the mechanical strength of kidney slices decreased and the microstructure changed.
Nafiseh Poornejad [48] obtained the optimal conditions of four common sterilization and disinfection methods for decellularized whole porcine kidneys through sterility test. Specifically, porcine kidneys were exposed respectively to 70% (v/v) ethanol for 1 h, 0.2% (v/v) PAA in 1 M NaCl for 1 h, 0.2% (v/v) PAA in 4% PAA ethanol for 1 h, and gamma irradiation at room temperature with 3 kGy, and the sterile state could be obtained. Further experiments showed that the content of collagen decreased by more than 50% after irradiation, while other methods had no significant change. The porosity of the sample irradiated increases, which makes its swelling ratio the largest of all the methods; while the sample with PAA in 1 M NaCl is closest to the normal tissue. Except 70% ethanol, other methods may further damage the structure of glomerulus and renal tubules.
4.5. Immune system
The most common dECM material in the immune system is decellularized spleen, and almost all of it comes from rats. The main sterilization and disinfection method of decellularized spleen is to infuse 0.1% (v/v) PAA/4% (v/v) ethanol [[111], [112], [113]], and some studies have used PAA alone for sterilization [89,90], e.g., Rui Gao [89] sterilized the spleen of rats in 0.1% (v/v) PAA for 3 h (Fig. 8 and Table 5).
4.6. Motor and nervous system
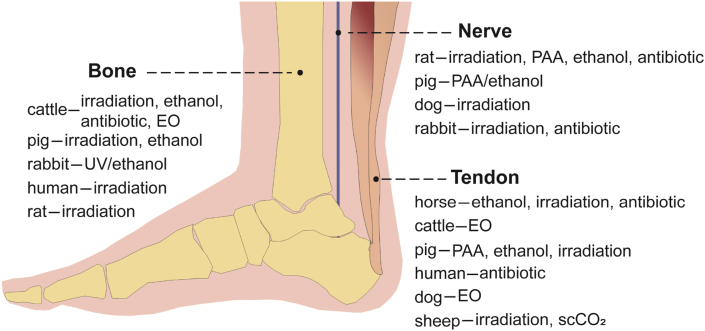
The dECM materials of motor and nervous system mainly include decellularized bone, tendon and nerve (Fig. 9 and Table 6).
Fig. 9.
Summary of sterilization and disinfection methods for decellularized bone, tendon, and nerve.
Table 6.
Summary of sterilization and disinfection methods for decellularized bone, tendon and nerve.
| Tissue type | Origins | Methods | Appearance and Size | Details and References |
|---|---|---|---|---|
| Bone | bovine | irradiation | piece (15 cm × 4 cm × 2 cm) | lyophilized, gamma irradiation, 15 kGy [14] |
| piece (2 mm length, 10 mm diameter) | gamma irradiation [49] | |||
| EO | piece (5.5 cm × 0.5 cm × 0.2 cm) | EO [77] | ||
| ethanol | piece (2 mm length, 4 mm diameter) | lyophilized, ethanol [137] | ||
| antibiotic | granule | antibiotic, 4 °C, 24 h [20] | ||
| pig | irradiation | piece (10 mm × 10 mm × 10 mm) | gamma irradiation [50] | |
| piece (5 mm length, 10 mm diameter) | discs (5 mm thick), gamma irradiation, 20 kGy [51] | |||
| ethanol | piece (5 mm × 5 mm × 3 mm) | 70% ethanol [138] | ||
| rabbit | UV/ethanol | piece | UV, 20 min; then, 70% ethanol [194] | |
| human | irradiation | granule (1–20 μm) | gamma irradiation, 30 kGy [52] | |
| rat | irradiation | piece (8 mm diameter) | gamma irradiation, 10 kGy [53] | |
| Tendon | horse | ethanol | piece (natural thick, or 0.3 mm thick) | aseptic working, washed twice in ethanol [139] |
| piece (20 mm × 10 mm × 1.2 mm) | 70% ethanol, 4 h [139] | |||
| irradiation | piece (100 mm × 15 mm × 3 mm); segment | (placed in PBS) β-irradiation, 15 kGy [54] | ||
| segment | β-irradiation, 15 kGy [56] | |||
| antibiotic | segment | antibiotic, 4 °C, overnight [179] | ||
| bovine | EO | piece (2 mm × 1.5 mm) | lyophilized, EO [78] | |
| pig | PAA | segment | 0.1% (v/v) PAA (pH 7.6), 3 h [95] 0.1% (v/v) PAA [96] 0.1% (v/v) PAA (pH 6.0), 3 h [27] |
|
| ethanol | segment | 70% ethanol, 5 min × three times [141] | ||
| irradiation | segment | gamma irradiation, 15 kGy, 30 kGy, 55 kGy; E‐beam, 15 kGy, 34 kGy, (15 + 15) kGy (fractionated dose) [27] | ||
| human | antibiotic | segment | antibiotic, overnight [153] | |
| dog | EO | piece (300 μm thick) | EO [79,80] | |
| piece (40 mm length) | EO [80] | |||
| sheep | irradiation | piece (40 mm length) | (in dry ice) gamma irradiation, 25 kGy [55] | |
| ScCO2 | piece (40 mm length) | ScCO2, 2 h [55] | ||
| Nerve | rat | irradiation | segment | gamma irradiation, 2.5 kGy [57] |
| PAA | segment | 0.1% (v/v) PAA, room temperature, 1 h [97] | ||
| ethanol | porous scaffold | 75% ethanol, 1 h [142] | ||
| antibiotic | segment | antibiotic, 7 days [155] | ||
| pig | PAA/ethanol | piece (<30 mm length) | 0.1% (v/v) PAA in 4% (v/v) ethanol, 2 h [114] | |
| dog | irradiation | piece (30 mm length; 60 mm length) | gamma irradiation [58] | |
| rabbit | irradiation | piece (30 mm length) | gamma irradiation, 25 kGy [59] | |
| antibiotic | segment | antibiotic, 7 days [155] |
4.6.1. Bone
Bone repair has been a research hotspot for many years, and there are a large number of research studies about it. However, there are not many reports that could clarify the sterilization and disinfection methods of decellularized bone. Decellularized bone mainly comes from bovine and pigs, and a small amount from human, mice and rabbits. Irradiation [14,[49], [50], [51], [52], [53]] is the most common sterilization method for decellularized bone, and ethanol [137,138], EO [77], antibiotics [20], UV/antibiotic [194] can also be used for its sterilization or disinfection.
4.6.2. Tendon
Decellularized tendon matrix is a common decellularized biomaterial. Tendons mainly come from bovine, horse and pig, and a small number of tendons are from human, rabbit, sheep, dog. The sterilization and disinfection methods of decellularized tendon mainly include ethanol [[139], [140], [141]], EO [[78], [79], [80]], PAA [95,96], irradiation [27,[54], [55], [56]], antibiotics [153,179] and ScCO2 [55], etc.
Jennifer H. Edwards [27] studied the sterilization effects of irradiation on decellularized porcine tendon, and the results confirmed that irradiation changed its biomechanical properties including reduction of the ultimate tensile strength and Young's modulus. Yikan Sun [55] compared the sterilization effects of 25 kGy irradiation and ScCO2 for 2 h on sheep tendon, with the results showing that the tendons were dried and contracted after irradiation, while the tissue was dehydrated and transparent to a certain extent after ScCO2. There were obvious holes in the cross section of the irradiated tendon, while the ultrastructure of ScCO2 treated tendon was intact.
4.6.3. Nerve
There are a lot of reports about decellularized neural matrix, but only few studies have clarified the sterilization and disinfection methods. Decellularized nerves are mainly derived from rats and pigs, and a small number is obtained from dogs and rabbits. The sterilization and disinfection methods mainly include irradiation [[57], [58], [59]], PAA [97], ethanol [142], antibiotic [155], PAA/ethanol [114], etc.
5. Discussion
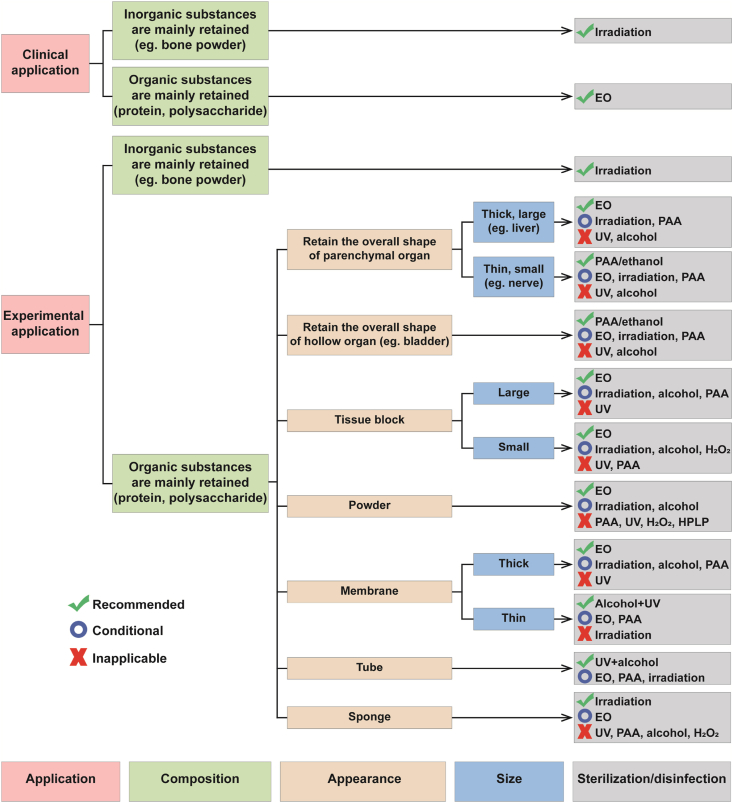
In order to facilitate researchers to have a clear idea on the selection of sterilization or disinfection methods of dECM after reading this review, a graphical route for then to select an appropriate sterilization or disinfection method were proposed, which is based on the application purpose and physic-chemical properties of various dECM, as well as the advantages and disadvantages of common sterilization and disinfection methods (Fig. 10).
Fig. 10.
The graphical route for selecting an appropriate sterilization or disinfection method for dECM.
In Fig. 10, there are three options for the selecting sterilization or disinfection methods, namely “Recommended”, “Conditional” and “Inapplicable”. “Recommended” means “the sterilization or disinfection method has less impact on the physical and chemical properties of materials under the premise of effective sterilization or disinfection”; “Conditional” means “the sterilization or disinfection method is only applicable under certain conditions, such as appropriate exposure time, solution concentration or radiation dose, etc.“; “Inapplicable” refers to “the sterilization or disinfection method has serious problems”.
The application purpose should be considered first, while selecting a sterilization or disinfection method for dECM materials. If the material is prepared for clinical application, only sterilization methods should be chosen, such as irradiation and EO sterilization. Irradiation is suitable for dECM with inorganic retention, such as hydroxyapatite-retained bone powder. EO is better for materials with “organic substances are mainly retained”. If the material is used only for experimental research, both of sterilization and disinfection methods can be considered.
Appearance and size are also the important concerns while selecting a sterilization or disinfection method for dECM materials. For the thick and large parenchymal organs with overall shape, EO is recommended to be used for sterilization due to its strong penetrability and good sterilization effect. Although EO is toxic, it can be solved by prolonging the removal time of EO residue. Both irradiation and PAA are “Conditional” options for sterilization or disinfection of the thick and large parenchymal organs, but the lowest dose of irradiation and the shortest exposure time of PAA need to be explored. The thin and small parenchymal organs and hollow organs are recommended to be sterilized or disinfected with PAA/ethanol, because it can get an effective sterilization and takes less time, and does not require residue test. The tissue block and thick membrane are recommended to be sterilized by EO. Because the exposed collagen in tissue block may be dissolved in acidic PAA, PAA cannot be used for relatively small tissue block. In addition, PAA is adjusted to neutral and then used for sterilization in some studies [93,95], and its mechanism and sterilization effect still need to be further studied. The powder is recommended to be sterilized by EO. PAA, H2O2 and HPLP are not suitable for the sterilization and disinfection of powder. For thin membranes and tubes, it is recommended to combine UV and alcohol immersion to achieve the aseptic effect, especially for tubes prepared by electrospinning, because most electrospinning machines have UV function.
Sponge is a special material with strong adsorption capacity. If toxic EO is used to sterilize the sponge, the removal time of EO residue needs to be extended considerably. Due to the poor penetrability, UV is difficult to effectively sterilize the sponge. After the sponge is sterilized by liquid agents, it is difficult to remove the residues of PAA, alcohols and H2O2. Therefore, irradiation may be the solution for sterilization of sponge at present, but irradiation may reduce the porosity of sponge. Perhaps, it is better to sterilize the raw materials, and then process them into sponges under aseptic conditions.
In addition to solid materials, dECM materials also include hydrogels. As mentioned in the section of “factors influencing the selection of sterilization and disinfection methods”, irradiation, EO, PAA, alcohols, H2O2, HPLP, UV and ScCO2 are not suitable for sterilization and disinfection of dECM hydrogels. Therefore, the sterilization of hydrogels is recommended by sterilizing the solid raw materials of dECM hydrogels, followed by subsequent preparation of hydrogels in a sterile environment.
The aseptic state obtained by effective sterilization and disinfection is the premise of subsequent experiment and application of dECM. In order to achieve more efficient sterilization and disinfection, various methods can be used not only alone, but also in combination. Because of the strong penetrability and stable sterilization effect, irradiation and EO can be used alone to obtain satisfactory sterilization effects. Moreover, PAA, alcohol, H2O2, UV and ScCO2 can be used in combination. For example, “0.1% (v/v) PAA in 4% (v/v) ethanol” has been widely used in various dECM materials [98,114]. There is also the combination of PAA and H2O2 [195], ScCO2 and PAA [196], etc. Due to the convenience of operation, UV can be combined with various sterilization or disinfection methods to disinfect the surface of dECM materials.
There are many researchers who incubated dECM in antibiotic solution to achieve aseptic state. For example, trachea and lung were obtained and decellularized in sterile environment, and washed or soaked the decellularized tissue in antibacterial solution, without using other disinfection methods, and their subsequent cell experiments and animal experiments were found successful [169,174,175]. Due to the limited antibacterial spectrum, antibiotic immersion is not a typical sterilization or disinfection method, but under certain circumstances (e.g., low chance of virus contamination), antibiotics have some advantages, such as not affecting the physical and chemical properties of biomaterials, whereas many other methods do not have the advantage. Considering the limited antimicrobial spectrum of antibiotics and some potential adverse effects of residual antibiotics, it is suggested that researchers should carry out the aseptic tests, cytotoxicity tests, and antibiotic residue tests to ensure the biological safety of dECM after antibiotic treatment. Furthermore, the removal of antibiotic residues may be an important issue which need to be paid more attention by researchers. In addition, if the antibiotic-treated dECM is applied in clinical practice, the cross-species infection would be a potential risk.
6. Conclusion
There are many reports on dECM, but only few of them clarified their sterilization and disinfection methods, and these methods are not necessarily correct or the best. Due to the diversity of dECM materials, the suitable sterilization methods are different, and the specific methods need to be determined according to the specific material and relevant conditions. Therefore, basing on the applications and physic-chemical properties of various dECM, and considering the advantages and disadvantages of common sterilization and disinfection methods, this paper proposes a graphical route for selecting a suitable sterilization or disinfection method for dECM (Fig. 10), and the chosen method could be validated according to “the technical route for validating the sterilization or disinfection method for dECM” (Fig. 1).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2017YFA0105802), the Sichuan Science and Technology Program (No. 2020YFH0008), and the Joint Research Fund Liaoning-Shenyang National Laboratory for Materials Science (2019JH3/30100022).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplement
(1) Sterility test-method 1
According to the European Pharmacopoeia (EP 10.0) [197], the United States Pharmacopoeia (USP 41) [198], and Chinese Pharmacopoeia 2020 (CP 2020) [199], the sterility test and related evaluation indicators are summarized as follows:
The positive control group is the medium containing relative positive bacteria; the negative control group is the medium without materials; and the sample group is the medium with materials.
Fluid Thioglycollate Medium is mainly used for the cultivation of anaerobic bacteria, but can also be used for aerobic bacteria, incubated at 30–35 °C. Soybean-Casein Digest Medium is suitable for the cultivation of fungi and aerobic bacteria, incubated at 20–25 °C.
During the culture period, the turbidity and the microbial growth are observed by visual examination. After 14 days, if the medium is turbid and the presence or absence of microbial growth cannot be determined visually, transfer 1 ml of medium to the fresh tubes of the same medium and continue to incubate for 4 days, then observe the microbial growth.
Results: the positive control group has obvious microbial growth, while the negative control group has no microbial growth, which is the premise of effective sterility test.
If the sample tube is clear or it is turbid but without microbial growth, the sample complies with sterility test. If any tube is turbid and microbial growth is confirmed in the sample tube, it does not comply with sterility test.
(2) Sterility test-method 2
Agar diffusion can be used as an auxiliary method for sterility test. With the agar diffusion, the microbial growth can be observed within 24, 48, and 72 h, so agar diffusion is suitable for the screening of invalid sterilization of biomaterials.
In brief, Luria-Bertani (LB) agar medium dissolved in distilled water is autoclaved at 121 °C for 15 min. The hot medium is poured into the dish and cooled to solidify the agar. The samples are laid flat in the center of these dishes and the dishes are kept upside down. The positive group is the one in which unsterilized samples are lying in the center of the solid medium, and the negative group contains the solid medium without samples. After incubation at 37 °C for 24, 48, and 72 h, the microbial growth on the solid medium is observed [150,200].
References
- 1.Zhao Y., Zhu B., Wang Y., Liu C., Shen C. Effect of different sterilization methods on the properties of commercial biodegradable polyesters for single-use, disposable medical devices, Materials science & engineering. C, Materials for biological applications. 2019;105:110041. doi: 10.1016/j.msec.2019.110041. [DOI] [PubMed] [Google Scholar]
- 2.Xue G. People's Medical Publishing House; 2008. Sterilization, Disinfection, Antisepsis and Preservation; pp. 167–303. [Google Scholar]
- 3.William D.J.W., Rutala A. The healthcare infection control practices advisory committee guideline for disinfection and sterilization in healthcare facilities. 2008. https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf 2019.
- 4.Hussein K.H., Park K.M., Teotia P.K., Hong S.H., Yang S.R., Park S.M., Ahn C., Woo H.M. Sterilization using electrolyzed water highly retains the biological properties in tissue-engineered porcine liver scaffold. Int. J. Artif. Organs. 2013;36(11):781–792. doi: 10.5301/ijao.5000246. [DOI] [PubMed] [Google Scholar]
- 5.Gosztyla C., Ladd M.R., Werts A., Fulton W., Johnson B., Sodhi C., Hackam D.J. A comparison of sterilization techniques for production of decellularized intestine in mice, tissue engineering. Part C, Methods. 2020;26(2):67–79. doi: 10.1089/ten.tec.2019.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pekkarinen T., Hietala O., Lindholm T.S., Jalovaara P. Influence of ethylene oxide sterilization on the activity of native reindeer bone morphogenetic protein. Int. Orthop. 2004;28(2):97–101. doi: 10.1007/s00264-003-0524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rnjak-Kovacina J., DesRochers T.M., Burke K.A., Kaplan D.L. The effect of sterilization on silk fibroin biomaterial properties. Macromol. Biosci. 2015;15(6):861–874. doi: 10.1002/mabi.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton B., Gaspar A., Josey D., Tupy J., Grynpas M.D., Willett T.L. Bone embrittlement and collagen modifications due to high-dose gamma-irradiation sterilization. Bone. 2014;61:71–81. doi: 10.1016/j.bone.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Monaco G., Cholas R., Salvatore L., Madaghiele M., Sannino A. Sterilization of collagen scaffolds designed for peripheral nerve regeneration: effect on microstructure, degradation and cellular colonization, Materials science & engineering. C, Materials for biological applications. 2017;71:335–344. doi: 10.1016/j.msec.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Yu T., Wen L., He J., Xu Y., Li T., Wang W., Ma Y., Ahmad M.A., Tian X., Fan J., Wang X., Hagiwara H., Ao Q. Fabrication and evaluation of an optimized acellular nerve allograft with multiple axial channels. Acta Biomater. 2020;115:235–249. doi: 10.1016/j.actbio.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 11.Wei H.-J., Liang H.-C., Lee M.-H., Huang Y.-C., Chang Y., Sung H.-W. Construction of varying porous structures in acellular bovine pericardia as a tissue-engineering extracellular matrix. Biomaterials. 2005;26(14):1905–1913. doi: 10.1016/j.biomaterials.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Negishi J., Hashimoto Y., Yamashita A., Kimura T., Kishida A., Funamoto S. Histological structure affects recellularization of decellularized arteries, Materials science & engineering. C, Materials for biological applications. 2017;70(Pt 1):450–455. doi: 10.1016/j.msec.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Islam M.M., Sharifi R., Mamodaly S., Islam R., Nahra D., Abusamra D.B., Hui P.C., Adibnia Y., Goulamaly M., Paschalis E.I., Cruzat A., Kong J., Nilsson P.H., Argüeso P., Mollnes T.E., Chodosh J., Dohlman C.H., Gonzalez-Andrades M. Effects of gamma radiation sterilization on the structural and biological properties of decellularized corneal xenografts. Acta Biomater. 2019;96:330–344. doi: 10.1016/j.actbio.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakabadze A., Mardaleishvili K., Loladze G., Karalashvili L., Chutkerashvili G., Chakhunashvili D., Kakabadze Z. Reconstruction of mandibular defects with autogenous bone and decellularized bovine bone grafts with freeze-dried bone marrow stem cell paracrine factors. Oncology letters. 2017;13(3):1811–1818. doi: 10.3892/ol.2017.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonenfant N.R., Sokocevic D., Wagner D.E., Borg Z.D., Lathrop M.J., Lam Y.W., Deng B., Desarno M.J., Ashikaga T., Loi R., Weiss D.J. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials. 2013;34(13):3231–3245. doi: 10.1016/j.biomaterials.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Den Hondt M., Vanaudenaerde B.M., Maughan E.F., Butler C.R., Crowley C., Verbeken E.K., Verleden S.E., Vranckx J.J. An optimized non-destructive protocol for testing mechanical properties in decellularized rabbit trachea. Acta Biomater. 2017;60:291–301. doi: 10.1016/j.actbio.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Song J.J., Guyette J.P., Gilpin S.E., Gonzalez G., Vacanti J.P., Ott H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 2013;19(5):646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seif-Naraghi S.B., Horn D., Schup-Magoffin P.J., Christman K.L. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8(10):3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fercana G.R., Yerneni S., Billaud M., Hill J.C., VanRyzin P., Richards T.D., Sicari B.M., Johnson S.A., Badylak S.F., Campbell P.G., Gleason T.G., Phillippi J.A. Perivascular extracellular matrix hydrogels mimic native matrix microarchitecture and promote angiogenesis via basic fibroblast growth factor. Biomaterials. 2017;123:142–154. doi: 10.1016/j.biomaterials.2017.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alom N., Peto H., Kirkham G.R., Shakesheff K.M., White L.J. Bone extracellular matrix hydrogel enhances osteogenic differentiation of C2C12 myoblasts and mouse primary calvarial cells. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(2):900–908. doi: 10.1002/jbm.b.33894. [DOI] [PubMed] [Google Scholar]
- 21.Rameshbabu A.P., Ghosh P., Subramani E., Bankoti K., Kapat K., Datta S., Maity P.P., Subramanian B., Roy S., Chaudhury K., Dhara S. Investigating the potential of human placenta-derived extracellular matrix sponges coupled with amniotic membrane-derived stem cells for osteochondral tissue engineering. J. Mater. Chem. B. 2016;4(4):613–625. doi: 10.1039/c5tb02321a. [DOI] [PubMed] [Google Scholar]
- 22.Chen C.-C., Yu J., Ng H.-Y., Lee A.K.-X., Chen C.-C., Chen Y.-S., Shie M.-Y. The physicochemical properties of decellularized extracellular matrix-coated 3D printed poly(ε-caprolactone) nerve conduits for promoting schwann cells proliferation and differentiation. Materials. 2018;11(9):1665. doi: 10.3390/ma11091665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan D.C., Mirmalek-Sani S.-H., Deegan D.B., Baptista P.M., Aboushwareb T., Atala A., Yoo J.J. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33(31):7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Sun W.Q., Leung P. Calorimetric study of extracellular tissue matrix degradation and instability after gamma irradiation. Acta Biomater. 2008;4(4):817–826. doi: 10.1016/j.actbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Medel F.J., Kurtz S.M., Hozack W.J., Parvizi J., Purtill J.J., Sharkey P.F., MacDonald D., Kraay M.J., Goldberg V., Rimnac C.M. Gamma inert sterilization: a solution to polyethylene oxidation?, the Journal of bone and joint surgery. American volume. 2009;91(4):839–849. doi: 10.2106/JBJS.H.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchanan F.J., Sim B., Downes S. Influence of packaging conditions on the properties of gamma-irradiated UHMWPE following accelerated ageing and shelf ageing. Biomaterials. 1999;20(9):823–837. doi: 10.1016/s0142-9612(98)00237-3. [DOI] [PubMed] [Google Scholar]
- 27.Edwards J.H., Herbert A., Jones G.L., Manfield I.W., Fisher J., Ingham E. The effects of irradiation on the biological and biomechanical properties of an acellular porcine superflexor tendon graft for cruciate ligament repair. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105(8):2477–2486. doi: 10.1002/jbm.b.33786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Z., Ronholm J., Tian Y., Sethi B., Cao X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016;7 doi: 10.1177/2041731416648810. 2041731416648810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dearth C.L., Keane T.J., Carruthers C.A., Reing J.E., Huleihel L., Ranallo C.A., Kollar E.W., Badylak S.F. The effect of terminal sterilization on the material properties and in vivo remodeling of a porcine dermal biologic scaffold. Acta Biomater. 2016;33:78–87. doi: 10.1016/j.actbio.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Helder M.R.K., Hennessy R.S., Spoon D.B., Tefft B.J., Witt T.A., Marler R.J., Pislaru S.V., Simari R.D., Stulak J.M., Lerman A. Low-dose gamma irradiation of decellularized heart valves results in tissue injury in vitro and in vivo. Ann. Thorac. Surg. 2016;101(2):667–674. doi: 10.1016/j.athoracsur.2015.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goecke T., Theodoridis K., Tudorache I., Ciubotaru A., Cebotari S., Ramm R., Höffler K., Sarikouch S., Vásquez Rivera A., Haverich A., Wolkers W.F., Hilfiker A. In vivo performance of freeze-dried decellularized pulmonary heart valve allo- and xenografts orthotopically implanted into juvenile sheep. Acta Biomater. 2018;68:41–52. doi: 10.1016/j.actbio.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 32.Mirmalek-Sani S.-H., Sullivan D.C., Zimmerman C., Shupe T.D., Petersen B.E. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am. J. Pathol. 2013;183(2):558–565. doi: 10.1016/j.ajpath.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng L., Hu Y.-L., Ma P., Feng Y., Guo Y.-B., Huang H., Li P., Mao Q.-S., Xue W.-J. Decellularized gastric matrix as a mesh for gastric perforation repair. J. Biomed. Mater. Res. B Appl. Biomater. 2020 doi: 10.1002/jbm.b.34713. [DOI] [PubMed] [Google Scholar]
- 34.Mirmalek-Sani S.-H., Orlando G., McQuilling J.P., Pareta R., Mack D.L., Salvatori M., Farney A.C., Stratta R.J., Atala A., Opara E.C., Soker S. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34(22):5488–5495. doi: 10.1016/j.biomaterials.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peloso A., Urbani L., Cravedi P., Katari R., Maghsoudlou P., Fallas M.E.A., Sordi V., Citro A., Purroy C., Niu G., McQuilling J.P., Sittadjody S., Farney A.C., Iskandar S.S., Zambon J.P., Rogers J., Stratta R.J., Opara E.C., Piemonti L., Furdui C.M., Soker S., De Coppi P., Orlando G. The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann. Surg. 2016;264(1):169–179. doi: 10.1097/SLA.0000000000001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbani L., Camilli C., Phylactopoulos D.-E., Crowley C., Natarajan D., Scottoni F., Maghsoudlou P., McCann C.J., Pellegata A.F., Urciuolo A., Deguchi K., Khalaf S., Aruta S.F., Signorelli M.C., Kiely D., Hannon E., Trevisan M., Wong R.R., Baradez M.O., Moulding D., Virasami A., Gjinovci A., Loukogeorgakis S., Mantero S., Thapar N., Sebire N., Eaton S., Lowdell M., Cossu G., Bonfanti P., De Coppi P. Multi-stage bioengineering of a layered oesophagus with in vitro expanded muscle and epithelial adult progenitors. Nat. Commun. 2018;9(1):4286. doi: 10.1038/s41467-018-06385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chai Y., Xu J., Zhang Y., Zhang J., Hu Z., Zhou H. Evaluation of decellularization protocols for production of porcine small intestine submucosa for use in abdominal wall reconstruction. Hernia : the journal of hernias and abdominal wall surgery. 2020;24(6):1221–1231. doi: 10.1007/s10029-019-01954-4. [DOI] [PubMed] [Google Scholar]
- 38.Luo J.-C., Chen W., Chen X.-H., Qin T.-W., Huang Y.-C., Xie H.-Q., Li X.-Q., Qian Z.-Y., Yang Z.-M. A multi-step method for preparation of porcine small intestinal submucosa (SIS) Biomaterials. 2011;32(3):706–713. doi: 10.1016/j.biomaterials.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Uriarte J.J., Nonaka P.N., Campillo N., Palma R.K., Melo E., de Oliveira L.V.F., Navajas D., Farré R. Mechanical properties of acellular mouse lungs after sterilization by gamma irradiation. Journal of the mechanical behavior of biomedical materials. 2014;40:168–177. doi: 10.1016/j.jmbbm.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Remlinger N.T., Czajka C.A., Juhas M.E., Vorp D.A., Stolz D.B., Badylak S.F., Gilbert S., Gilbert T.W. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010;31(13):3520–3526. doi: 10.1016/j.biomaterials.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 41.Johnson C.M., Guo D., Ryals S., Postma G.N., Weinberger P.M. The feasibility of gamma radiation sterilization for decellularized tracheal grafts. Laryngoscope. 2017;127(8):E258–E264. doi: 10.1002/lary.26367. [DOI] [PubMed] [Google Scholar]
- 42.Kutten J.C., McGovern D., Hobson C.M., Luffy S.A., Nieponice A., Tobita K., Francis R.J., Reynolds S.D., Isenberg J.S., Gilbert T.W. Decellularized tracheal extracellular matrix supports epithelial migration, differentiation, and function, Tissue engineering. Part. Accel. 2015;21(1–2):75–84. doi: 10.1089/ten.tea.2014.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang J., San B.H., Turner N.J., White L.J., Faulk D.M., Badylak S.F., Li Y., Yu S.M. Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomater. 2017;53:268–278. doi: 10.1016/j.actbio.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullers S.J., Baker S.C., Ingham E., Southgate J. The human tissue-biomaterial interface: a role for PPARγ-dependent glucocorticoid receptor activation in regulating the CD163+ M2 macrophage phenotype, Tissue engineering. Part. Accel. 2014;20(17–18):2390–2401. doi: 10.1089/ten.tea.2013.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faulk D.M., Carruthers C.A., Warner H.J., Kramer C.R., Reing J.E., Zhang L., D'Amore A., Badylak S.F. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014;10(1):183–193. doi: 10.1016/j.actbio.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q., Zhang F., Wang H., Pan T. Preparation and characterization of a novel acellular swim bladder as dura mater substitute. Neurol. Res. 2019;41(3):242–249. doi: 10.1080/01616412.2018.1550139. [DOI] [PubMed] [Google Scholar]
- 47.Abolbashari M., Agcaoili S.M., Lee M.-K., Ko I.K., Aboushwareb T., Jackson J.D., Yoo J.J., Atala A. Repopulation of porcine kidney scaffold using porcine primary renal cells. Acta Biomater. 2016;29:52–61. doi: 10.1016/j.actbio.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 48.Poornejad N., Nielsen J.J., Morris R.J., Gassman J.R., Reynolds P.R., Roeder B.L., Cook A.D. Comparison of four decontamination treatments on porcine renal decellularized extracellular matrix structure, composition, and support of human renal cortical tubular epithelium cells. J. Biomater. Appl. 2016;30(8):1154–1167. doi: 10.1177/0885328215615760. [DOI] [PubMed] [Google Scholar]
- 49.You L., Weikang X., Lifeng Y., Changyan L., Yongliang L., Xiaohui W., Bin X. In vivo immunogenicity of bovine bone removed by a novel decellularization protocol based on supercritical carbon dioxide. Artificial cells, nanomedicine, and biotechnology. 2018;46(sup2):334–344. doi: 10.1080/21691401.2018.1457044. [DOI] [PubMed] [Google Scholar]
- 50.Gerhardt L.C., Widdows K.L., Erol M.M., Nandakumar A., Roqan I.S., Ansari T., Boccaccini A.R. Neocellularization and neovascularization of nanosized bioactive glass-coated decellularized trabecular bone scaffolds. J. Biomed. Mater. Res. 2013;101(3):827–841. doi: 10.1002/jbm.a.34373. [DOI] [PubMed] [Google Scholar]
- 51.Sun X.-j., Peng W., Yang Z.-l., Ren M.-l., Zhang S.-c., Zhang W.-g., Zhang L.-y., Xiao K., Wang Z.-g., Zhang B., Wang J. Heparin-chitosan-coated acellular bone matrix enhances perfusion of blood and vascularization in bone tissue engineering scaffolds, Tissue engineering. Part. Accel. 2011;17(19–20):2369–2378. doi: 10.1089/ten.TEA.2011.0027. [DOI] [PubMed] [Google Scholar]
- 52.Ni P., Ding Q., Fan M., Liao J., Qian Z., Luo J., Li X., Luo F., Yang Z., Wei Y. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials. 2014;35(1):236–248. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Lee D.J., Padilla R., Zhang H., Hu W.-S., Ko C.-C. Biological assessment of a calcium silicate incorporated hydroxyapatite-gelatin nanocomposite: a comparison to decellularized bone matrix. BioMed Res. Int. 2014;2014:837524. doi: 10.1155/2014/837524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pellegata A.F., Bottagisio M., Boschetti F., Ferroni M., Bortolin M., Drago L., Lovati A.B. Terminal sterilization of equine-derived decellularized tendons for clinical use, Materials science & engineering. C, Materials for biological applications. 2017;75:43–49. doi: 10.1016/j.msec.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Sun Y., Lovric V., Wang T., Oliver R.A., Walsh W.R. Effects of SCCO, gamma irradiation, and sodium dodecyl sulfate treatments on the initial properties of tendon allografts. Int. J. Mol. Sci. 2020;21(5):1565. doi: 10.3390/ijms21051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talò G., D'Arrigo D., Lorenzi S., Moretti M., Lovati A.B. Independent, controllable stretch-perfusion bioreactor chambers to functionalize cell-seeded decellularized tendons. Ann. Biomed. Eng. 2020;48(3):1112–1126. doi: 10.1007/s10439-019-02257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hundepool C.A., Nijhuis T.H.J., Kotsougiani D., Friedrich P.F., Bishop A.T., Shin A.Y. Optimizing decellularization techniques to create a new nerve allograft: an in vitro study using rodent nerve segments. Neurosurg. Focus. 2017;42(3):E4. doi: 10.3171/2017.1.FOCUS16462. [DOI] [PubMed] [Google Scholar]
- 58.Qiu S., Rao Z., He F., Wang T., Xu Y., Du Z., Yao Z., Lin T., Yan L., Quan D., Zhu Q., Liu X. Decellularized nerve matrix hydrogel and glial-derived neurotrophic factor modifications assisted nerve repair with decellularized nerve matrix scaffolds. Journal of tissue engineering and regenerative medicine. 2020;14(7):931–943. doi: 10.1002/term.3050. [DOI] [PubMed] [Google Scholar]
- 59.Li Y.-J., Zhao B.-L., Lv H.-Z., Qin Z.-G., Luo M. Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects: biomechanics and validation of mathematical models. Neural regeneration research. 2016;11(8):1322–1326. doi: 10.4103/1673-5374.189198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendes G.C.C., Brandão T.R.S., Silva C.L.M. Ethylene oxide sterilization of medical devices: a review. American journal of infection control. 2007;35(9):574–581. doi: 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Thier R., Bolt H.M. Carcinogenicity and genotoxicity of ethylene oxide: new aspects and recent advances. Crit. Rev. Toxicol. 2000;30(5):595–608. doi: 10.1080/10408440008951121. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y., Zhang S., Zhou J., Wang J., Zhen M., Liu Y., Chen J., Qi Z. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 2010;31(2):296–307. doi: 10.1016/j.biomaterials.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 63.Dempsey S.G., Miller C.H., Hill R.C., Hansen K.C., May B.C.H. Functional insights from the proteomic inventory of ovine forestomach matrix. J. Proteome Res. 2019;18(4):1657–1668. doi: 10.1021/acs.jproteome.8b00908. [DOI] [PubMed] [Google Scholar]
- 64.Irvine S.M., Cayzer J., Todd E.M., Lun S., Floden E.W., Negron L., Fisher J.N., Dempsey S.G., Alexander A., Hill M.C., O'Rouke A., Gunningham S.P., Knight C., Davis P.F., Ward B.R., May B.C.H. Quantification of in vitro and in vivo angiogenesis stimulated by ovine forestomach matrix biomaterial. Biomaterials. 2011;32(27):6351–6361. doi: 10.1016/j.biomaterials.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 65.Keane T.J., Londono R., Carey R.M., Carruthers C.A., Reing J.E., Dearth C.L., D'Amore A., Medberry C.J., Badylak S.F. Preparation and characterization of a biologic scaffold from esophageal mucosa. Biomaterials. 2013;34(28):6729–6737. doi: 10.1016/j.biomaterials.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosario D.J., Reilly G.C., Ali Salah E., Glover M., Bullock A.J., Macneil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med. 2008;3(2):145–156. doi: 10.2217/17460751.3.2.145. [DOI] [PubMed] [Google Scholar]
- 67.Yang B., Zhang Y., Zhou L., Sun Z., Zheng J., Chen Y., Dai Y. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering, Tissue engineering. Part C, Methods. 2010;16(5):1201–1211. doi: 10.1089/ten.TEC.2009.0311. [DOI] [PubMed] [Google Scholar]
- 68.De Filippo R.E., Kornitzer B.S., Yoo J.J., Atala A. Penile urethra replacement with autologous cell-seeded tubularized collagen matrices. Journal of tissue engineering and regenerative medicine. 2015;9(3):257–264. doi: 10.1002/term.1647. [DOI] [PubMed] [Google Scholar]
- 69.Gilbert T.W., Gilbert S., Madden M., Reynolds S.D., Badylak S.F. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann. Thorac. Surg. 2008;86(3):967–974. doi: 10.1016/j.athoracsur.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 70.Zhao F., Zhou L., Liu J., Xu Z., Ping W., Li H., Xu L., Xu Z., Zhou C., Wang M., Jia R. Construction of a vascularized bladder with autologous adipose-derived stromal vascular fraction cells combined with bladder acellular matrix via tissue engineering. J. Tissue Eng. 2019;10 doi: 10.1177/2041731419891256. 2041731419891256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhe Z., Jun D., Yang Z., Mingxi X., Ke Z., Ming Z., Zhong W., Mujun L. Bladder acellular matrix grafts seeded with adipose-derived stem cells and incubated intraperitoneally promote the regeneration of bladder smooth muscle and nerve in a rat model of bladder augmentation. Stem Cell. Dev. 2016;25(5):405–414. doi: 10.1089/scd.2015.0246. [DOI] [PubMed] [Google Scholar]
- 72.Zhou L., Yang B., Sun C., Qiu X., Sun Z., Chen Y., Zhang Y., Dai Y. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model, Tissue engineering. Part. Accel. 2013;19(1–2):264–276. doi: 10.1089/ten.tea.2011.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Q., Xiao D.-D., Yan H., Zhao Y., Fu S., Zhou J., Wang Z., Zhou Z., Zhang M., Lu M.-J. The morphological regeneration and functional restoration of bladder defects by a novel scaffold and adipose-derived stem cells in a rat augmentation model. Stem Cell Res. Ther. 2017;8(1):149. doi: 10.1186/s13287-017-0597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baldini T., Caperton K., Hawkins M., McCarty E. Effect of a novel sterilization method on biomechanical properties of soft tissue allografts. Knee Surg. Sports Traumatol. Arthrosc. : official journal of the ESSKA. 2016;24(12):3971–3975. doi: 10.1007/s00167-014-3221-0. [DOI] [PubMed] [Google Scholar]
- 75.Zhu W.-D., Xu Y.-M., Feng C., Fu Q., Song L.-J., Cui L. Bladder reconstruction with adipose-derived stem cell-seeded bladder acellular matrix grafts improve morphology composition. World J. Urol. 2010;28(4):493–498. doi: 10.1007/s00345-010-0508-8. [DOI] [PubMed] [Google Scholar]
- 76.Zhou C., Zhou L., Liu J., Xu L., Xu Z., Chen Z., Ge Y., Zhao F., Wu R., Wang X., Jiang N., Mao L., Jia R. Kidney extracellular matrix hydrogel enhances therapeutic potential of adipose-derived mesenchymal stem cells for renal ischemia reperfusion injury. Acta Biomater. 2020;115(1):250–263. doi: 10.1016/j.actbio.2020.07.056. [DOI] [PubMed] [Google Scholar]
- 77.Qing Q., Zhang Y.-J., Yang J.-L., Ning L.-J., Zhang Y.-J., Jiang Y.-L., Zhang Y., Luo J.-C., Qin T.-W. Effects of hydrogen peroxide on biological characteristics and osteoinductivity of decellularized and demineralized bone matrices. J. Biomed. Mater. Res. 2019;107(7):1476–1490. doi: 10.1002/jbm.a.36662. [DOI] [PubMed] [Google Scholar]
- 78.Zhang C.-H., Jiang Y.-L., Ning L.-J., Li Q., Fu W.-L., Zhang Y.-J., Zhang Y.-J., Xia C.-C., Li J., Qin T.-W. Evaluation of decellularized bovine tendon sheets for achilles tendon defect reconstruction in a rabbit model. Am. J. Sports Med. 2018;46(11):2687–2699. doi: 10.1177/0363546518787515. [DOI] [PubMed] [Google Scholar]
- 79.Pan J., Liu G.-M., Ning L.-J., Zhang Y., Luo J.-C., Huang F.-G., Qin T.-W. Rotator cuff repair using a decellularized tendon slices graft: an in vivo study in a rabbit model, Knee surgery, sports traumatology, arthroscopy. official journal of the ESSKA. 2015;23(5):1524–1535. doi: 10.1007/s00167-014-2923-7. [DOI] [PubMed] [Google Scholar]
- 80.Qin T.-W., Sun Y.-L., Thoreson A.R., Steinmann S.P., Amadio P.C., An K.-N., Zhao C. Effect of mechanical stimulation on bone marrow stromal cell-seeded tendon slice constructs: a potential engineered tendon patch for rotator cuff repair. Biomaterials. 2015;51:43–50. doi: 10.1016/j.biomaterials.2015.01.070. [DOI] [PubMed] [Google Scholar]
- 81.Xue G. People's Medical Publishing House; 2008. Sterilization, Disinfection, Antisepsis and Preservation; pp. 288–295. [Google Scholar]
- 82.Hodde J., Hiles M. Virus safety of a porcine-derived medical device: evaluation of a viral inactivation method. Biotechnol. Bioeng. 2002;79(2):211–216. doi: 10.1002/bit.10281. [DOI] [PubMed] [Google Scholar]
- 83.Clapp P.A., Davies M.J., French M.S., Gilbert B.C. The bactericidal action of peroxides; an E.P.R. spin-trapping study. Free Radic. Res. 1994;21(3):147–167. doi: 10.3109/10715769409056566. [DOI] [PubMed] [Google Scholar]
- 84.Luo J., Korossis S.A., Wilshaw S.-P., Jennings L.M., Fisher J., Ingham E. Development and characterization of acellular porcine pulmonary valve scaffolds for tissue engineering, Tissue engineering. Part. Accel. 2014;20(21–22):2963–2974. doi: 10.1089/ten.tea.2013.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun D., Liu Y., Wang H., Deng F., Zhang Y., Zhao S., Ma X., Wu H., Sun G. Novel decellularized liver matrix-alginate hybrid gel beads for the 3D culture of hepatocellular carcinoma cells. Int. J. Biol. Macromol. 2018;109:1154–1163. doi: 10.1016/j.ijbiomac.2017.11.103. [DOI] [PubMed] [Google Scholar]
- 86.Agarwal T., Narayan R., Maji S., Ghosh S.K., Maiti T.K. Decellularized caprine liver extracellular matrix as a 2D substrate coating and 3D hydrogel platform for vascularized liver tissue engineering. Journal of tissue engineering and regenerative medicine. 2018;12(3):e1678–e1690. doi: 10.1002/term.2594. [DOI] [PubMed] [Google Scholar]
- 87.Nowocin A.K., Southgate A., Gabe S.M., Ansari T. Biocompatibility and potential of decellularized porcine small intestine to support cellular attachment and growth. Journal of tissue engineering and regenerative medicine. 2016;10(1):E23–E33. doi: 10.1002/term.1750. [DOI] [PubMed] [Google Scholar]
- 88.Dew L., English W.R., Chong C.K., MacNeil S. Investigating neovascularization in rat decellularized intestine: an in vitro platform for studying angiogenesis, tissue engineering. Part. Accel. 2016;22(23–24):1317–1326. doi: 10.1089/ten.tea.2016.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao R., Wu W., Xiang J., Lv Y., Zheng X., Chen Q., Wang H., Wang B., Liu Z., Ma F. Hepatocyte culture in autologous decellularized spleen matrix. Organogenesis. 2015;11(1):16–29. doi: 10.1080/15476278.2015.1011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu P., Tian B., Yang L., Zheng X., Zhang X., Li J., Liu X., Lv Y., Xiang J. Hemocompatibility improvement of decellularized spleen matrix for constructing transplantable bioartificial liver. Biomed. Mater. 2019;14(2) doi: 10.1088/1748-605X/aaf375. [DOI] [PubMed] [Google Scholar]
- 91.Zvarova B., Uhl F.E., Uriarte J.J., Borg Z.D., Coffey A.L., Bonenfant N.R., Weiss D.J., Wagner D.E. Residual detergent detection method for nondestructive cytocompatibility evaluation of decellularized whole lung scaffolds, tissue engineering. Part C, Methods. 2016;22(5):418–428. doi: 10.1089/ten.tec.2015.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Neill J.D., Freytes D.O., Anandappa A.J., Oliver J.A., Vunjak-Novakovic G.V. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials. 2013;34(38):9830–9841. doi: 10.1016/j.biomaterials.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bolland F., Korossis S., Wilshaw S.-P., Ingham E., Fisher J., Kearney J.N., Southgate J. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials. 2007;28(6):1061–1070. doi: 10.1016/j.biomaterials.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 94.Moradi L., Mohammadi Jobania B., Jafarnezhad-Ansariha F., Ghorbani F., Esmaeil-Pour R., Majidi Zolbina M., Kajbafzadeh A.-M. Evaluation of different sterilization methods for decellularized kidney tissue. Tissue Cell. 2020;66:101396. doi: 10.1016/j.tice.2020.101396. [DOI] [PubMed] [Google Scholar]
- 95.Jones G., Herbert A., Berry H., Edwards J.H., Fisher J., Ingham E. Decellularization and characterization of porcine superflexor tendon: a potential anterior cruciate ligament replacement, tissue engineering. Part. Accel. 2017;23(3–4):124–134. doi: 10.1089/ten.tea.2016.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Whitaker S., Edwards J.H., Guy S., Ingham E., Herbert A. Stratifying the mechanical performance of a decellularized xenogeneic tendon graft for anterior cruciate ligament reconstruction as a function of graft diameter: an animal study. Bone & joint research. 2019;8(11):518–525. doi: 10.1302/2046-3758.811.BJR-2019-0065.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sridharan R., Reilly R.B., Buckley C.T. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. Journal of the mechanical behavior of biomedical materials. 2015;41:124–135. doi: 10.1016/j.jmbbm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 98.Barnes C.A., Brison J., Michel R., Brown B.N., Castner D.G., Badylak S.F., Ratner B.D. The surface molecular functionality of decellularized extracellular matrices. Biomaterials. 2011;32(1):137–143. doi: 10.1016/j.biomaterials.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coronado R.E., Somaraki-Cormier M., Natesan S., Christy R.J., Ong J.L., Halff G.A. Decellularization and solubilization of porcine liver for use as a substrate for porcine hepatocyte culture: method optimization and comparison. Cell Transplant. 2017;26(12):1840–1854. doi: 10.1177/0963689717742157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang W., Zhang X., Chao N.-N., Qin T.-W., Ding W., Zhang Y., Sang J.-W., Luo J.-C. Preparation and characterization of pro-angiogenic gel derived from small intestinal submucosa. Acta Biomater. 2016;29:135–148. doi: 10.1016/j.actbio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 101.Weymann A., Patil N.P., Sabashnikov A., Korkmaz S., Li S., Soos P., Ishtok R., Chaimow N., Pätzold I., Czerny N., Schmack B., Popov A.-F., Simon A.R., Karck M., Szabo G. Perfusion-decellularization of porcine lung and trachea for respiratory bioengineering. Artif. Organs. 2015;39(12):1024–1032. doi: 10.1111/aor.12481. [DOI] [PubMed] [Google Scholar]
- 102.Ma J., Ju Z., Yu J., Qiao Y., Hou C., Wang C., Hei F. Decellularized rat lung scaffolds using sodium lauryl ether sulfate for tissue engineering. Am. Soc. Artif. Intern. Organs J. 2018;64(3):406–414. doi: 10.1097/MAT.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 103.Platz J., Bonenfant N.R., Uhl F.E., Coffey A.L., McKnight T., Parsons C., Sokocevic D., Borg Z.D., Lam Y.-W., Deng B., Fields J.G., DeSarno M., Loi R., Hoffman A.M., Bianchi J., Dacken B., Petersen T., Wagner D.E., Weiss D.J. Comparative decellularization and recellularization of wild-type and alpha 1,3 galactosyltransferase knockout pig lungs: a model for ex vivo xenogeneic lung bioengineering and transplantation, tissue engineering. Part C, Methods. 2016;22(8):725–739. doi: 10.1089/ten.tec.2016.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolf M.T., Daly K.A., Brennan-Pierce E.P., Johnson S.A., Carruthers C.A., D'Amore A., Nagarkar S.P., Velankar S.S., Badylak S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33(29):7028–7038. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu Y., Hideyoshi S., Jiang H., Matsumura Y., Dziki J.L., LoPresti S.T., Huleihel L., Faria G.N.F., Fuhrman L.C., Lodono R., Badylak S.F., Wagner W.R. Injectable, porous, biohybrid hydrogels incorporating decellularized tissue components for soft tissue applications. Acta Biomater. 2018;73:112–126. doi: 10.1016/j.actbio.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Angelozzi M., Penolazzi L., Mazzitelli S., Lambertini E., Lolli A., Piva R., Nastruzzi C. Dedifferentiated chondrocytes in composite microfibers as tool for cartilage repair. Frontiers in bioengineering and biotechnology. 2017;5:35. doi: 10.3389/fbioe.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Y., Bharadwaj S., Lee S.J., Atala A., Zhang Y. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials. 2009;30(23–24):3865–3873. doi: 10.1016/j.biomaterials.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 108.Kitamura M., Hirano S., Kanemaru S.-I., Kitani Y., Ohno S., Kojima T., Nakamura T., Ito J., Rosen C.A., Gilbert T.W. Glottic regeneration with a tissue-engineering technique, using acellular extracellular matrix scaffold in a canine model. Journal of tissue engineering and regenerative medicine. 2016;10(10):825–832. doi: 10.1002/term.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Badylak S.F., Vorp D.A., Spievack A.R., Simmons-Byrd A., Hanke J., Freytes D.O., Thapa A., Gilbert T.W., Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J. Surg. Res. 2005;128(1):87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 110.Fedecostante M., Westphal K.G.C., Buono M.F., Sanchez Romero N., Wilmer M.J., Kerkering J., Baptista P.M., Hoenderop J.G., Masereeuw R. Recellularized native kidney scaffolds as a novel tool in nephrotoxicity screening. Drug Metab. Dispos.: the biological fate of chemicals. 2018;46(9):1338–1350. doi: 10.1124/dmd.118.080721. [DOI] [PubMed] [Google Scholar]
- 111.Xiang J., Zheng X., Liu P., Yang L., Dong D., Wu W., Liu X., Li J., Lv Y. Decellularized spleen matrix for reengineering functional hepatic-like tissue based on bone marrow mesenchymal stem cells. Organogenesis. 2016;12(3):128–142. doi: 10.1080/15476278.2016.1185584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiang J.-X., Zheng X.-L., Gao R., Wu W.-Q., Zhu X.-L., Li J.-H., Lv Y. Liver regeneration using decellularized splenic scaffold: a novel approach in tissue engineering, Hepatobiliary & pancreatic diseases international. HBPD INT. 2015;14(5):502–508. doi: 10.1016/s1499-3872(15)60423-4. [DOI] [PubMed] [Google Scholar]
- 113.Zheng X.-L., Xiang J.-X., Wu W.-Q., Wang B., Liu W.-Y., Gao R., Dong D.-H., Lv Y. Using a decellularized splenic matrix as a 3D scaffold for hepatocyte cultivation in vitro: a preliminary trial. Biomed. Mater. 2015;10(4) doi: 10.1088/1748-6041/10/4/045023. [DOI] [PubMed] [Google Scholar]
- 114.Crapo P.M., Medberry C.J., Reing J.E., Tottey S., van der Merwe Y., Jones K.E., Badylak S.F. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33(13):3539–3547. doi: 10.1016/j.biomaterials.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hayrapetyan H., Nederhoff L., Vollebregt M., Mastwijk H., Nierop Groot M. Inactivation kinetics of Geobacillus stearothermophilus spores by a peracetic acid or hydrogen peroxide fog in comparison to the liquid form. Int. J. Food Microbiol. 2020;316:108418. doi: 10.1016/j.ijfoodmicro.2019.108418. [DOI] [PubMed] [Google Scholar]
- 116.Xue G. People's Medical Publishing House; 2008. Sterilization, Disinfection, Antisepsis and Preservation; p. 191. [Google Scholar]
- 117.Linley E., Denyer S.P., McDonnell G., Simons C., Maillard J.-Y. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. JAC (J. Antimicrob. Chemother.) 2012;67(7):1589–1596. doi: 10.1093/jac/dks129. [DOI] [PubMed] [Google Scholar]
- 118.Akuji M.A., Chambers D.J. Hydrogen peroxide: more harm than good? Br. J. Anaesth. 2017;118(6):958–959. doi: 10.1093/bja/aex151. [DOI] [PubMed] [Google Scholar]
- 119.Shah D.D., Zhang J., Hsieh M.-C., Sundaram S., Maity H., Mallela K.M.G. Effect of peroxide- versus alkoxyl-induced chemical oxidation on the structure, stability, aggregation, and function of a therapeutic monoclonal antibody. J. Pharmaceut. Sci. 2018;107(11):2789–2803. doi: 10.1016/j.xphs.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 120.Parmaksiz M., Elcin A.E., Elcin Y.M. Decellularization of bovine small intestinal submucosa and its use for the healing of a critical-sized full-thickness skin defect, alone and in combination with stem cells, in a small rodent model. Journal of tissue engineering and regenerative medicine. 2017;11(6):1754–1765. doi: 10.1002/term.2071. [DOI] [PubMed] [Google Scholar]
- 121.Negishi J., Funamoto S., Kimura T., Nam K., Higami T., Kishida A. Porcine radial artery decellularization by high hydrostatic pressure. Journal of tissue engineering and regenerative medicine. 2015;9(11):E144–E151. doi: 10.1002/term.1662. [DOI] [PubMed] [Google Scholar]
- 122.Thomas P. Long-term survival of Bacillus spores in alcohol and identification of 90% ethanol as relatively more spori/bactericidal. Curr. Microbiol. 2012;64(2):130–139. doi: 10.1007/s00284-011-0040-0. [DOI] [PubMed] [Google Scholar]
- 123.Naso F., Gandaglia A., Iop L., Spina M., Gerosa G. First quantitative assay of alpha-Gal in soft tissues: presence and distribution of the epitope before and after cell removal from xenogeneic heart valves. Acta Biomater. 2011;7(4):1728–1734. doi: 10.1016/j.actbio.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 124.Dijkman P.E., Driessen-Mol A., Frese L., Hoerstrup S.P., Baaijens F.P.T. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials. 2012;33(18):4545–4554. doi: 10.1016/j.biomaterials.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 125.Mangold S., Schrammel S., Huber G., Niemeyer M., Schmid C., Stangassinger M., Hoenicka M. Evaluation of decellularized human umbilical vein (HUV) for vascular tissue engineering - comparison with endothelium-denuded HUV. Journal of tissue engineering and regenerative medicine. 2015;9(1):13–23. doi: 10.1002/term.1603. [DOI] [PubMed] [Google Scholar]
- 126.Jiang B., Suen R., Wang J.-J., Zhang Z.J., Wertheim J.A., Ameer G.A. Vascular scaffolds with enhanced antioxidant activity inhibit graft calcification. Biomaterials. 2017;144:166–175. doi: 10.1016/j.biomaterials.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim M.K., Jeong W., Lee S.M., Kim J.B., Jin S., Kang H.-W. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication. 2020;12(2) doi: 10.1088/1758-5090/ab5d80. [DOI] [PubMed] [Google Scholar]
- 128.Lewis P.L., Su J., Yan M., Meng F., Glaser S.S., Alpini G.D., Green R.M., Sosa-Pineda B., Shah R.N. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci. Rep. 2018;8(1):12220. doi: 10.1038/s41598-018-30433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu C., Ma X., Zhu W., Wang P., Miller K.L., Stupin J., Koroleva-Maharajh A., Hairabedian A., Chen S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials. 2019;194:1–13. doi: 10.1016/j.biomaterials.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang K., Chaimov D., Patel S.N., Liang J.P., Wiggins S.C., Samojlik M.M., Rubiano A., Simmons C.S., Stabler C.L. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 2019;198:37–48. doi: 10.1016/j.biomaterials.2018.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singh A., Lee D., Jeong H., Yu C., Li J., Fang C.H., Sabnekar P., Liu X., Yoshida T., Sopko N., Bivalacqua T.J. Tissue-Engineered neo-urinary conduit from decellularized trachea, tissue engineering. Part. Accel. 2018;24(19–20):1456–1467. doi: 10.1089/ten.TEA.2017.0436. [DOI] [PubMed] [Google Scholar]
- 132.Evren S., Loai Y., Antoon R., Islam S., Yeger H., Moore K., Wong K., Gorczynski R., Farhat W.A. Urinary bladder tissue engineering using natural scaffolds in a porcine model: role of Toll-like receptors and impact of biomimetic molecules, Cells, tissues. organs. 2010;192(4):250–261. doi: 10.1159/000317332. [DOI] [PubMed] [Google Scholar]
- 133.Pokrywczynska M., Rasmus M., Jundzill A., Balcerczyk D., Adamowicz J., Warda K., Buchholz L., Drewa T. Mesenchymal stromal cells modulate the molecular pattern of healing process in tissue-engineered urinary bladder: the microarray data. Stem Cell Res. Ther. 2019;10(1):176. doi: 10.1186/s13287-019-1266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown A.L., Farhat W., Merguerian P.A., Wilson G.J., Khoury A.E., Woodhouse K.A. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. 2002;23(10):2179–2190. doi: 10.1016/s0142-9612(01)00350-7. [DOI] [PubMed] [Google Scholar]
- 135.Zhao Y., He Y., Guo J.-H., Wu J.-S., Zhou Z., Zhang M., Li W., Zhou J., Xiao D.-D., Wang Z., Sun K., Zhu Y.-J., Lu M.-J. Time-dependent bladder tissue regeneration using bilayer bladder acellular matrix graft-silk fibroin scaffolds in a rat bladder augmentation model. Acta Biomater. 2015;23:91–102. doi: 10.1016/j.actbio.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 136.Rafighdoust A., Shahri N.M., Baharara J. Decellularized kidney in the presence of chondroitin sulfate as a natural 3D scaffold for stem cells. Iranian journal of basic medical sciences. 2015;18(8):788–798. [PMC free article] [PubMed] [Google Scholar]
- 137.Marcos-Campos I., Marolt D., Petridis P., Bhumiratana S., Schmidt D., Vunjak-Novakovic G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials. 2012;33(33):8329–8342. doi: 10.1016/j.biomaterials.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nie Z., Wang X., Ren L., Kang Y. Development of a decellularized porcine bone matrix for potential applications in bone tissue regeneration. Regen. Med. 2020;15(4):1519–1534. doi: 10.2217/rme-2019-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roth S.P., Brehm W., Groß C., Scheibe P., Schubert S., Burk J. Transforming growth factor beta 3-loaded decellularized equine tendon matrix for orthopedic tissue engineering. Int. J. Mol. Sci. 2019;20(21):5474. doi: 10.3390/ijms20215474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aeberhard P.-A., Grognuz A., Peneveyre C., McCallin S., Hirt-Burri N., Antons J., Pioletti D., Raffoul W., Applegate L.A. Efficient decellularization of equine tendon with preserved biomechanical properties and cytocompatibility for human tendon surgery indications. Artif. Organs. 2020;44(4):E161–E171. doi: 10.1111/aor.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lohan A., Stoll C., Albrecht M., Denner A., John T., Krüger K., Ertel W., Schulze-Tanzil G. Human hamstring tenocytes survive when seeded into a decellularized porcine Achilles tendon extracellular matrix. Connect. Tissue Res. 2013;54(4–5):305–312. doi: 10.3109/03008207.2013.820283. [DOI] [PubMed] [Google Scholar]
- 142.Zhu M., Li W., Dong X., Yuan X., Midgley A.C., Chang H., Wang Y., Wang H., Wang K., Ma P.X., Wang H., Kong D. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019;10(1):4620. doi: 10.1038/s41467-019-12545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gerchman Y., Cohen-Yaniv V., Betzalel Y., Yagur-Kroll S., Belkin S., Mamane H. The involvement of superoxide radicals in medium pressure UV derived inactivation. Water Res. 2019;161:119–125. doi: 10.1016/j.watres.2019.05.084. [DOI] [PubMed] [Google Scholar]
- 144.Chen C., Pla-Palacín I., Baptista P.M., Shang P., Oosterhoff L.A., van Wolferen M.E., Penning L.C., Geijsen N., Spee B. Hepatocyte-like cells generated by direct reprogramming from murine somatic cells can repopulate decellularized livers. Biotechnol. Bioeng. 2018;115(11):2807–2816. doi: 10.1002/bit.26784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fu Q., Deng C.-l., Liu W., Cao Y.-l. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007;99(5):1162–1165. doi: 10.1111/j.1464-410X.2006.06691.x. [DOI] [PubMed] [Google Scholar]
- 146.Guler S., Hosseinian P., Aydin H.M. Hybrid aorta constructs via in situ crosslinking of poly(glycerol-sebacate) elastomer within a decellularized matrix, tissue engineering. Part C, Methods. 2017;23(1):21–29. doi: 10.1089/ten.TEC.2016.0375. [DOI] [PubMed] [Google Scholar]
- 147.Kim S.R., Rhee M.S., Kim B.C., Kim K.H. Modeling the inactivation of Escherichia coli O157:H7 and generic Escherichia coli by supercritical carbon dioxide. Int. J. Food Microbiol. 2007;118(1):52–61. doi: 10.1016/j.ijfoodmicro.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 148.Guler S., Aslan B., Hosseinian P., Aydin H.M. Supercritical carbon dioxide-assisted decellularization of aorta and cornea, tissue engineering. Part C, Methods. 2017;23(9):540–547. doi: 10.1089/ten.TEC.2017.0090. [DOI] [PubMed] [Google Scholar]
- 149.Choe J.A., Jana S., Tefft B.J., Hennessy R.S., Go J., Morse D., Lerman A., Young M.D. Biomaterial characterization of off-the-shelf decellularized porcine pericardial tissue for use in prosthetic valvular applications. Journal of tissue engineering and regenerative medicine. 2018;12(7):1608–1620. doi: 10.1002/term.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Balestrini J.L., Liu A., Gard A.L., Huie J., Blatt K.M.S., Schwan J., Zhao L., Broekelmann T.J., Mecham R.P., Wilcox E.C., Niklason L.E. Sterilization of lung matrices by supercritical carbon dioxide, tissue engineering. Part C, Methods. 2016;22(3):260–269. doi: 10.1089/ten.tec.2015.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McDade J.K., Brennan-Pierce E.P., Ariganello M.B., Labow R.S., Michael Lee J. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: influence of crosslinking treatment. Acta Biomater. 2013;9(7):7191–7199. doi: 10.1016/j.actbio.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 152.Lichtenberg A., Tudorache I., Cebotari S., Ringes-Lichtenberg S., Sturz G., Hoeffler K., Hurscheler C., Brandes G., Hilfiker A., Haverich A. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials. 2006;27(23):4221–4229. doi: 10.1016/j.biomaterials.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 153.Long C., Galvez M.G., Legrand A., Joubert L.-M., Wang Z., Chattopadhyay A., Chang J., Fox P.M. Intratendinous injection of hydrogel for reseeding decellularized human flexor tendons. Plast. Reconstr. Surg. 2017;139(6):1305e–1314e. doi: 10.1097/PRS.0000000000003359. [DOI] [PubMed] [Google Scholar]
- 154.Urita Y., Komuro H., Chen G., Shinya M., Kaneko S., Kaneko M., Ushida T. Regeneration of the esophagus using gastric acellular matrix: an experimental study in a rat model. Pediatr. Surg. Int. 2007;23(1):21–26. doi: 10.1007/s00383-006-1799-0. [DOI] [PubMed] [Google Scholar]
- 155.Wang W., Itoh S., Takakuda K. Comparative study of the efficacy of decellularization treatment of allogenic and xenogeneic nerves as nerve conduits. J. Biomed. Mater. Res. 2016;104(2):445–454. doi: 10.1002/jbm.a.35589. [DOI] [PubMed] [Google Scholar]
- 156.Calvo J., Martínez-Martínez L. [Antimicrobial mechanisms of action] Enferm. Infecc. Microbiol. Clín. 2009;27(1):44–52. doi: 10.1016/j.eimc.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 157.Brötz-Oesterhelt H., Brunner N.A. How many modes of action should an antibiotic have? Curr. Opin. Pharmacol. 2008;8(5):564–573. doi: 10.1016/j.coph.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 158.Kalinec G., Thein P., Park C., Kalinec F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear. Res. 2016;335:105–117. doi: 10.1016/j.heares.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 159.Doña I., Moreno E., Pérez-Sánchez N., Andreu I., Hernández Fernandez de Rojas D., Torres M.J. Update on quinolone allergy. Curr. Allergy Asthma Rep. 2017;17(8):56. doi: 10.1007/s11882-017-0725-y. [DOI] [PubMed] [Google Scholar]
- 160.Amadeo F., Boschetti F., Polvani G., Banfi C., Pesce M., Santoro R. Aortic valve cell seeding into decellularized animal pericardium by perfusion-assisted bioreactor. Journal of tissue engineering and regenerative medicine. 2018;12(6):1481–1493. doi: 10.1002/term.2680. [DOI] [PubMed] [Google Scholar]
- 161.Lin C.-H., Kao Y.-C., Lin Y.-H., Ma H., Tsay R.-Y. A fiber-progressive-engagement model to evaluate the composition, microstructure, and nonlinear pseudoelastic behavior of porcine arteries and decellularized derivatives. Acta Biomater. 2016;46:101–111. doi: 10.1016/j.actbio.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 162.Iijima M., Aubin H., Steinbrink M., Schiffer F., Assmann A., Weisel R.D., Matsui Y., Li R.-K., Lichtenberg A., Akhyari P. Bioactive coating of decellularized vascular grafts with a temperature-sensitive VEGF-conjugated hydrogel accelerates autologous endothelialization in vivo. Journal of tissue engineering and regenerative medicine. 2018;12(1):e513–e522. doi: 10.1002/term.2321. [DOI] [PubMed] [Google Scholar]
- 163.Wang X., Cui J., Zhang B.-Q., Zhang H., Bi Y., Kang Q., Wang N., Bie P., Yang Z., Wang H., Liu X., Haydon R.C., Luu H.H., Tang N., Dong J., He T.-C. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J. Biomed. Mater. Res. 2014;102(4):1017–1025. doi: 10.1002/jbm.a.34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Butter A., Aliyev K., Hillebrandt K.-H., Raschzok N., Kluge M., Seiffert N., Tang P., Napierala H., Muhamma A.I., Reutzel-Selke A., Andreou A., Pratschke J., Sauer I.M., Struecker B. Evolution of graft morphology and function after recellularization of decellularized rat livers. Journal of tissue engineering and regenerative medicine. 2018;12(2):e807–e816. doi: 10.1002/term.2383. [DOI] [PubMed] [Google Scholar]
- 165.Citro A., Moser P.T., Dugnani E., Rajab T.K., Ren X., Evangelista-Leite D., Charest J.M., Peloso A., Podesser B.K., Manenti F., Pellegrini S., Piemonti L., Ott H.C. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials. 2019;199:40–51. doi: 10.1016/j.biomaterials.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 166.Wan J., Wang L., Huang Y., Fan H., Chen C., Yuan X., Guo Y., Yin L. Using GRGDSPC peptides to improve re-endothelialization of decellularized pancreatic scaffolds. Artif. Organs. 2020;44(4):E172–E180. doi: 10.1111/aor.13602. [DOI] [PubMed] [Google Scholar]
- 167.Arakelian L., Caille C., Faivre L., Corté L., Bruneval P., Shamdani S., Flageollet C., Albanese P., Domet T., Jarraya M., Setterblad N., Kellouche S., Larghero J., Cattan P., Vanneaux V. A clinical-grade acellular matrix for esophageal replacement. Journal of tissue engineering and regenerative medicine. 2019;13(12):2191–2203. doi: 10.1002/term.2983. [DOI] [PubMed] [Google Scholar]
- 168.Alabi B.R., LaRanger R., Shay J.W. Decellularized mice colons as models to study the contribution of the extracellular matrix to cell behavior and colon cancer progression. Acta Biomater. 2019;100:213–222. doi: 10.1016/j.actbio.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 169.Calle E.A., Hill R.C., Leiby K.L., Le A.V., Gard A.L., Madri J.A., Hansen K.C., Niklason L.E. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater. 2016;46:91–100. doi: 10.1016/j.actbio.2016.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Calle E.A., Mendez J.J., Ghaedi M., Leiby K.L., Bove P.F., Herzog E.L., Sundaram S., Niklason L.E. Fate of distal lung epithelium cultured in a decellularized lung extracellular matrix, Tissue engineering. Part. Accel. 2015;21(11–12):1916–1928. doi: 10.1089/ten.tea.2014.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mishra D.K., Thrall M.J., Baird B.N., Ott H.C., Blackmon S.H., Kurie J.M., Kim M.P. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann. Thorac. Surg. 2012;93(4):1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Doi R., Tsuchiya T., Mitsutake N., Nishimura S., Matsuu-Matsuyama M., Nakazawa Y., Ogi T., Akita S., Yukawa H., Baba Y., Yamasaki N., Matsumoto K., Miyazaki T., Kamohara R., Hatachi G., Sengyoku H., Watanabe H., Obata T., Niklason L.E., Nagayasu T. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci. Rep. 2017;7(1):8447. doi: 10.1038/s41598-017-09115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fernandez-Moure J.S., Van Eps J.L., Rhudy J.R., Cabrera F.J., Acharya G.S., Tasciotti E., Sakamoto J., Nichols J.E. Porcine acellular lung matrix for wound healing and abdominal wall reconstruction: a pilot study. J. Tissue Eng. 2016;7 doi: 10.1177/2041731415626018. 2041731415626018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Batioglu-Karaaltin A., Ovali E., Karaaltin M.V., Yener M., Yılmaz M., Eyüpoğlu F., Yılmaz Y.Z., Bozkurt E.R., Demir N., Konuk E., Bozdağ E.S., Yiğit Ö., Cansiz H. Decellularization of trachea with combined techniques for tissue-engineered trachea transplantation. Clinical and experimental otorhinolaryngology. 2019;12(1):86–94. doi: 10.21053/ceo.2018.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhong Y., Jiang A., Sun F., Xiao Y., Gu Y., Wu L., Zhang Y., Shi H. A comparative study of the effects of different decellularization methods and genipin-cross-linking on the properties of tracheal matrices. Tissue engineering and regenerative medicine. 2019;16(1):39–50. doi: 10.1007/s13770-018-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Haag J., Baiguera S., Jungebluth P., Barale D., Del Gaudio C., Castiglione F., Bianco A., Comin C.E., Ribatti D., Macchiarini P. Biomechanical and angiogenic properties of tissue-engineered rat trachea using genipin cross-linked decellularized tissue. Biomaterials. 2012;33(3):780–789. doi: 10.1016/j.biomaterials.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 177.Bombelli S., Meregalli C., Scalia C., Bovo G., Torsello B., De Marco S., Cadamuro M., Viganò P., Strada G., Cattoretti G., Bianchi C., Perego R.A. Nephrosphere-derived cells are induced to multilineage differentiation when cultured on human decellularized kidney scaffolds. Am. J. Pathol. 2018;188(1):184–195. doi: 10.1016/j.ajpath.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 178.Nakayama K.H., Batchelder C.A., Lee C.I., Tarantal A.F. Renal tissue engineering with decellularized rhesus monkey kidneys: age-related differences, Tissue engineering. Part. Accel. 2011;17(23–24):2891–2901. doi: 10.1089/ten.tea.2010.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Burk J., Plenge A., Brehm W., Heller S., Pfeiffer B., Kasper C. Induction of tenogenic differentiation mediated by extracellular tendon matrix and short-term cyclic stretching. Stem Cell. Int. 2016;2016:7342379. doi: 10.1155/2016/7342379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hodder E., Duin S., Kilian D., Ahlfeld T., Seidel J., Nachtigall C., Bush P., Covill D., Gelinsky M., Lode A. Investigating the effect of sterilisation methods on the physical properties and cytocompatibility of methyl cellulose used in combination with alginate for 3D-bioplotting of chondrocytes. J. Mater. Sci. Mater. Med. 2019;30(1):10. doi: 10.1007/s10856-018-6211-9. [DOI] [PubMed] [Google Scholar]
- 181.Fatimi A., Tassin J.-F., Turczyn R., Axelos M.A.V., Weiss P. Gelation studies of a cellulose-based biohydrogel: the influence of pH, temperature and sterilization. Acta Biomater. 2009;5(9):3423–3432. doi: 10.1016/j.actbio.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 182.Fidalgo C., Iop L., Sciro M., Harder M., Mavrilas D., Korossis S., Bagno A., Palù G., Aguiari P., Gerosa G. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater. 2018;67:282–294. doi: 10.1016/j.actbio.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 183.Böer U., Hurtado-Aguilar L.G., Klingenberg M., Lau S., Jockenhoevel S., Haverich A., Wilhelmi M. Effect of intensified decellularization of equine carotid arteries on scaffold biomechanics and cytotoxicity. Ann. Biomed. Eng. 2015;43(11):2630–2641. doi: 10.1007/s10439-015-1328-1. [DOI] [PubMed] [Google Scholar]
- 184.Mattei G., Di Patria V., Tirella A., Alaimo A., Elia G., Corti A., Paolicchi A., Ahluwalia A. Mechanostructure and composition of highly reproducible decellularized liver matrices. Acta Biomater. 2014;10(2):875–882. doi: 10.1016/j.actbio.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 185.Vukadinovic-Nikolic Z., Andrée B., Dorfman S.E., Pflaum M., Horvath T., Lux M., Venturini L., Bär A., Kensah G., Lara A.R., Tudorache I., Cebotari S., Hilfiker-Kleiner D., Haverich A., Hilfiker A. Generation of bioartificial heart tissue by combining a three-dimensional gel-based cardiac construct with decellularized small intestinal submucosa, Tissue engineering. Part. Accel. 2014;20(3–4):799–809. doi: 10.1089/ten.TEA.2013.0184. [DOI] [PubMed] [Google Scholar]
- 186.da Palma R.K., Fratini P., Schiavo Matias G.S., Cereta A.D., Guimarães L.L., Anunciação A.R.d.A., de Oliveira L.V.F., Farre R., Miglino M.A. Equine lung decellularization: a potential approach for in vitro modeling the role of the extracellular matrix in asthma. J. Tissue Eng. 2018;9 doi: 10.1177/2041731418810164. 2041731418810164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun F., Jiang Y., Xu Y., Shi H., Zhang S., Liu X., Pan S., Ye G., Zhang W., Zhang F., Zhong C. Genipin cross-linked decellularized tracheal tubular matrix for tracheal tissue engineering applications. Sci. Rep. 2016;6:24429. doi: 10.1038/srep24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Melo E., Kasper J.Y., Unger R.E., Farré R., Kirkpatrick C.J. Development of a bronchial wall model: triple culture on a decellularized porcine trachea, tissue engineering. Part C, Methods. 2015;21(9):909–921. doi: 10.1089/ten.TEC.2014.0543. [DOI] [PubMed] [Google Scholar]
- 189.Baiguera S., Del Gaudio C., Kuevda E., Gonfiotti A., Bianco A., Macchiarini P. Dynamic decellularization and cross-linking of rat tracheal matrix. Biomaterials. 2014;35(24):6344–6350. doi: 10.1016/j.biomaterials.2014.04.070. [DOI] [PubMed] [Google Scholar]
- 190.Kobayashi M., Kadota J., Hashimoto Y., Fujisato T., Nakamura N., Kimura T., Kishida A. Elastic modulus of ECM hydrogels derived from decellularized tissue affects capillary network formation in endothelial cells. Int. J. Mol. Sci. 2020;21(17):6304. doi: 10.3390/ijms21176304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Merguerian P.A., Reddy P.P., Barrieras D.J., Wilson G.J., Woodhouse K., Bagli D.J., McLorie G.A., Khoury A.E. Acellular bladder matrix allografts in the regeneration of functional bladders: evaluation of large-segment (> 24 cm) substitution in a porcine model. BJU Int. 2000;85(7):894–898. doi: 10.1046/j.1464-410x.2000.00513.x. [DOI] [PubMed] [Google Scholar]
- 192.Moreno-Manzano V., Mellado-López M., Morera-Esteve M.J., Alastrue-Agudo A., Bisbal-Velasco V., Forteza-Vila J., Serrano-Aroca Á., Vera-Donoso C.D. Human adipose-derived mesenchymal stem cells accelerate decellularized neobladder regeneration. Regenerative biomaterials. 2020;7(2):161–169. doi: 10.1093/rb/rbz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sobreiro-Almeida R., Melica M.E., Lasagni L., Romagnani P., Neves N.M. Co-cultures of renal progenitors and endothelial cells on kidney decellularized matrices replicate the renal tubular environment in vitro. Acta Physiol. 2020;230(1) doi: 10.1111/apha.13491. [DOI] [PubMed] [Google Scholar]
- 194.Pennington E.C., Dionigi B., Gray F.L., Ahmed A., Brazzo J., Dolinko A., Calderon N., Darrah T., Zurakowski D., Nazarian A., Snyder B., Fauza D.O. Limb reconstruction with decellularized, non-demineralized bone in a young leporine model. Biomed. Mater. 2015;10(1) doi: 10.1088/1748-6041/10/1/015021. [DOI] [PubMed] [Google Scholar]
- 195.Alasri A., Valverde M., Roques C., Michel G., Cabassud C., Aptel P. Sporocidal properties of peracetic acid and hydrogen peroxide, alone and in combination, in comparison with chlorine and formaldehyde for ultrafiltration membrane disinfection. Can. J. Microbiol. 1993;39(1):52–60. doi: 10.1139/m93-008. [DOI] [PubMed] [Google Scholar]
- 196.Bednarski D.M., Lantz E.E., Bobst C.E., Eisenhut A.R., Eyles S.J., Fey J.P. Sterilization of epidermal growth factor with supercritical carbon dioxide and peracetic acid; analysis of changes at the amino acid and protein level, Biochimica et biophysica acta. Proteins and proteomics. 2020;1868(2):140334. doi: 10.1016/j.bbapap.2019.140334. [DOI] [PubMed] [Google Scholar]
- 197.Europe C.o. 2019. Sterility Test, European Pharmacopoeia; p. 191. [Google Scholar]
- 198.Convention U.S.P. 2017. Sterility Test, the United States Pharmacopeia; pp. 5984–5991. [Google Scholar]
- 199.T.S.P.C.o.t.p.s.R.o. China . Chemical Industry Press; 2020. Sterility Test, Pharmacopoeia of the People's Republic of China; p. 520. [Google Scholar]
- 200.Łopianiak I., Butruk-Raszeja B.A. Evaluation of sterilization/disinfection methods of fibrous polyurethane scaffolds designed for tissue engineering applications. Int. J. Mol. Sci. 2020;21(21):8092. doi: 10.3390/ijms21218092. [DOI] [PMC free article] [PubMed] [Google Scholar]