Abstract
Background
Prior comparisons of chemotherapy adverse events (AEs) by age and performance status (PS) are limited by the traditional maximum grade approach, which ignores low‐grade AEs and longitudinal changes.
Materials and Methods
To compare fatigue and neuropathy longitudinally by age (<65, ≥65 years) and PS (0–1, 2), we analyzed data from a large phase III trial of carboplatin and paclitaxel versus paclitaxel for advanced non‐small cell lung cancer (CALGB 9730, n = 529). We performed multivariable (a) linear mixed models to estimate mean AE grade over time, (b) linear regression to estimate area under the curve (AUC), and (c) proportional hazards models to estimate the hazard ratio of developing grade ≥2 AE, as well as traditional maximum grade analyses.
Results
Older patients had on average a 0.17‐point (95% confidence interval [CI], 0.00–0.34; p = .049) higher mean fatigue grade longitudinally compared with younger patients. PS 2 was associated with earlier development of grade ≥2 fatigue (hazard ratio [HR], 1.56; 95% CI, 1.07–2.27; p = .02). For neuropathy, older age was associated with earlier development of grade ≥2 neuropathy (HR, 1.41; 95% CI, 1.00–1.97; p = .049). Patients with PS 2 had a 1.30 point lower neuropathy AUC (95% CI, −2.36 to −0.25; p = .02) compared with PS 0–1. In contrast, maximum grade analyses only detected a higher percentage of older adults with grade ≥3 fatigue and neuropathy at some point during treatment.
Conclusion
Our comparison of complementary but distinct aspects of chemotherapy toxicity identified important longitudinal differences in fatigue and neuropathy by age and PS that are missed by the traditional maximum grade approach. Clinical trial identification number: NCT00003117 (CALGB 9730)
Implications for Practice
The traditional maximum grade approach ignores persistent low‐grade adverse events (AEs) and changes over time. This toxicity over time analysis of fatigue and neuropathy during chemotherapy for advanced non‐small cell lung cancer demonstrates how to use longitudinal methods to comprehensively characterize AEs over time by age and performance status (PS). We identified important longitudinal differences in fatigue and neuropathy that are missed by the maximum grade approach. This new information about how older adults and patients with PS 2 experience these toxicities longitudinally may be used clinically to improve discussions about treatment options and what to expect to inform shared decision making and symptom management.
Keywords: Geriatric oncology, Chemotherapy, Lung cancer, Fatigue, Neuropathy
Short abstract
To expand beyond the traditional maximum grade approach to comparing chemotherapy adverse events, this article describes a toxicity‐over‐time approach to more comprehensively assess treatment toxicity.
Introduction
With an anticipated 67% increase in cancer incidence for older adults by 2030 [1], understanding treatment safety and tolerability [2] among older adults is increasingly important. For non‐small cell lung cancer (NSCLC) in particular, treatment tolerability is especially pertinent among older adults and vulnerable patients with a poor performance status (PS) given the high symptom burden and prevalent functional impairments caused by NSCLC [3]. Furthermore, older adults with NSCLC are more likely to discontinue treatment for adverse events (AEs) [4]. However, traditional AE reporting focuses on the maximum AE grade experienced anytime during treatment without providing any information on low‐grade AEs, their duration, or the complexity of how AEs change over time. Although toxicity tables reporting the percentage of patients with grade ≥3 AEs are sufficient to evaluate safety and easy to standardize, the one‐dimensional maximum grade approach misses important details of how patients experience the longitudinal cumulative burden of toxicity, which contributes to overall treatment tolerability and quality of life (QOL).
Although targeted therapies and immunotherapy have revolutionized care for a subset of patients [5, 6], the majority of patients with advanced NSCLC still receive platinum‐based chemotherapy with or without immunotherapy during the course of their treatment (e.g., first‐line carboplatin, (nab)‐paclitaxel, pembrolizumab for squamous histology [7]). Thus, understanding a patient's risk of chemotherapy AEs remains a common clinical challenge.
Prior comparisons of chemotherapy toxicity in NSCLC trials by age have found mixed results using traditional maximum grade approaches [8, 9, 10, 11, 12]. In a phase III trial of carboplatin plus paclitaxel (four cycles vs. until disease progression), there were no age‐related differences in grade 3–4 AEs using the maximum grade approach [8]. In contrast, a meta‐analysis of five phase III chemotherapy trials found that 35% of older patients experienced a grade 3–4 AE compared with only 26% of younger patients [9]. However, even when age‐related differences in toxicity are detected using the maximum grade approach, whether the time profiles of AEs differ by age or other important patient characteristics, such as PS, remains unknown. Trials often exclude patients with a PS of 2 or, if allowed, do not enroll sufficient numbers to allow for subgroup comparisons by PS so even less is known about how these vulnerable patients experience toxicities longitudinally.
To expand beyond the traditional maximum grade approach, the toxicity over time (ToxT) [13] approach uses multiple longitudinal methods and illustrative graphs to more comprehensively assess treatment toxicity to capture the overall burden of toxicity, analyzing mean AE grade, area under the curve (AUC), and time to development of AEs [14]. However, no studies to date have applied ToxT to longitudinal AE analysis to evaluate differences in toxicity by age or PS. Therefore, we used ToxT to examine differences in the severity and time to development of two common, clinically relevant symptomatic AEs (fatigue and neuropathy) by age and PS among patients with advanced NSCLC receiving chemotherapy in the Cancer and Leukemia Group B (CALGB) 9730 (Alliance) trial [10]. Additionally, we compared our ToxT findings to results from traditional maximum grade analyses.
Materials and Methods
Study Design
We used AE data from the randomized phase III CALGB 9730 cooperative group trial (NCT00003117) [10], which enrolled 584 patients with PS 0–2 age ≥18 with stage IIIB (malignant effusion) or IV NSCLC. Overall, 561 eligible patients were randomized to receive first‐line carboplatin plus paclitaxel versus paclitaxel every three weeks until disease progression or intolerance (maximum of six cycles). Randomization was stratified by stage (IIIB, IV, vs. recurrent), PS (0–1 vs. 2), and age (<70 vs. ≥70). AEs were assessed according to CALGB Expanded Common Toxicity Criteria (supplemental online Table 1). We selected CALGB 9730 because the trial collected data on AEs of all grades and enrolled a relatively higher percentage of older (48% age ≥65 years) and patients with PS 2 (18%). The University of California, San Francisco Institutional Review Board determined that the current study qualified as exempt due to the use of existing, deidentified data.
Statistical Analysis
Patient characteristics were compared by age (<65 vs. ≥65 years) using χ2 tests. Although CALGB 9730 stratified randomization by age 70 years, we used age 65 years to define age groups to increase the older group size for our multiple longitudinal approaches. Sensitivity analyses were performed by repeating all analyses described below using an alternative age cutoff of 70 to define groups. Two‐sided tests with a p value <.05 were considered statistically significant. Study data were frozen on January 22, 2017; data collection and analyses were conducted using SAS (version 9.4; SAS Institute, Cary, NC) in accordance with Alliance policies.
For this analysis, we selected two of the most common symptomatic AEs in CALGB 9730, fatigue and neuropathy, based on their prevalence in the trial [10], clinical relevance, and impact on QOL in older adults [15, 16, 17, 18]. Fatigue is one of the most common and distressing symptoms among older patients with NSCLC and is associated with functional decline with chemotherapy [17, 19]. Neuropathy can interfere with daily activities and is associated with increased fall risk and lower QOL [16, 18, 20, 21]. In CALGB 9730, grade ≥3 fatigue was reported among 7% and 5% of patients in the carboplatin/paclitaxel and paclitaxel arms, respectively. Grade ≥3 neuropathy was reported among 15% and 14% of patients in the carboplatin plus paclitaxel and paclitaxel arms, respectively.
For fatigue and neuropathy, the following three ToxT analyses were performed as well as a traditional maximum grade analysis:
Linear Mixed Models
Mean AE grade was first plotted across six cycles by age and PS, stratified by treatment arm to visualize group changes in toxicity over time. For example, if 1% of patients had a grade 4 AE at cycle 1, 5% had grade 3, 15% had grade 2, and 30% had grade 1, the resulting mean AE grade for cycle one would be plotted as 0.79. We then performed multivariable linear mixed effects models, which capture within‐ and between‐patient effects longitudinally, to estimate mean AE grade over six cycles. AE grade was modeled as a continuous outcome to facilitate interpretation of results. Analyses were adjusted for sex, race, stage, histology, treatment arm, and cycle number. Missing AE grades were imputed using last observation carried forward (LOCF). LOCF is a widely used single imputation method that assumes AE grade remains the same as the last observed value and has been advocated in some regulatory guidelines [22, 23].
Area Under the Curve Models
The AUC for AE grade over six cycles was calculated for each patient. The AUC provides a single number to summarize each patient's overall burden of AE over the entire course of treatment, capturing both toxicities of all grades and their duration. For example, if a patient experienced a grade 3 AE with cycle 2 that then completely resolved and did not recur, the AUC would be 3. In contrast, if a patient experienced a grade 1 AE throughout all six cycles, the AUC would be 6, reflecting a higher overall burden of toxicity over time. To allow for comparisons with patients who did not complete all six cycles, their AUC was prorated by multiplying the mean AUC for completed cycles by 6. Differences in AUC by age and PS were examined using multivariable linear regression adjusting for sex, race, stage, histology, and treatment arm.
Time to Development of a Grade ≥2 AE
Cumulative incidence functions for grade ≥2 AE were plotted by age and PS, stratified by treatment arm. To estimate the hazard ratio of developing a grade ≥2 AE, we performed multivariable Cox proportional hazards models with death as a competing risk adjusting for sex, race, stage, histology, and treatment arm.
Additional detailed information on ToxT longitudinal statistical and graphical methods are described elsewhere [13, 24].
Traditional Maximum Grade Analysis
For each AE, the percentage of patients with and without a grade ≥3 AE anytime during their treatment course were compared by age and PS using χ2 tests. As is typical of the maximum grade approach, this analysis does not provide any information about when the grade ≥3 AE was experienced during the treatment course, how long it lasted, or if it occurred only once versus multiple times.
Results
Of the 561 randomized patients in CALGB 9730, 529 patients had available AE data and were included. Overall, 52.4% were age <65 whereas 47.6% were age ≥65; 67.9% were men and 82.1% were white (Table 1). There were no statistically significant differences in PS by age (p = .28); 16.6% of younger and 17.1% of older patients had a PS of 2. Approximately half in each age group received carboplatin/paclitaxel (49.8% younger patients, 54.4% older patients) whereas the remainder received paclitaxel (p = .30).
Table 1.
Patient characteristics according to age group (n = 529)
| Characteristic | Age <65 (n = 277), n (%) | Age ≥65 (n = 252), n (%) | p value |
|---|---|---|---|
| Age, median (range) | 56 (31–64) | 71 (65–86) | <.0001 |
| <45 yr | 18 (6.5) | ||
| 45–54 yr | 99 (35.7) | ||
| 55–64 yr | 160 (57.8) | ||
| 65–74 yr | 203 (80.6) | ||
| ≥75 yr | 49 (19.4) | ||
| ECOG performance status | .28 | ||
| 0 | 75 (27.1) | 83 (32.9) | |
| 1 | 156 (56.3) | 126 (50.0) | |
| 2 | 46 (16.6) | 43 (17.1) | |
| Sex | .71 | ||
| Male | 190 (68.6) | 169 (67.1) | |
| Female | 87 (31.4) | 83 (32.9) | |
| Race a | .16 | ||
| White | 205 (79.8) | 203 (84.6) | |
| Other | 52 (20.2) | 37 (15.7) | |
| Stage IV NSCLC | 192 (69.8) | 378 (72.3) | .19 |
| Histology | .26 | ||
| Adenocarcinoma | 156 (56.3) | 125 (49.6) | |
| Squamous | 51 (18.4) | 58 (23.0) | |
| Other histology | 70 (25.3) | 69 (27.4) | |
| Treatment arm | .30 | ||
| Carboplatin, paclitaxel | 138 (49.8) | 137 (54.4) | |
| Paclitaxel | 139 (50.2) | 115 (45.6) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NSCLC, non‐small cell lung cancer.
Race information was missing for 20 younger adults and 12 older adults.
Fatigue
Mean fatigue grade over six cycles was plotted stratified by treatment arm according to age (Fig. 1A, B) and PS (Fig. 1C, D). In the multivariable linear mixed model for fatigue, there was a statistically significant association between older age group and higher fatigue grade (Table 2). Older patients age ≥65 with advanced NSCLC had on average a 0.17‐point (95% confidence interval [CI], 0.00–0.34; p = .049) higher mean fatigue grade overall compared with younger patients. Increasing cycle number was also associated with higher mean fatigue grade compared with cycle one. There were no differences in mean fatigue grade over time according to PS or treatment arm. In a sensitivity analysis using age 70 to define groups, there was no longer a statistically significant association between older age and higher mean fatigue grade overall (data not shown).
Figure 1.
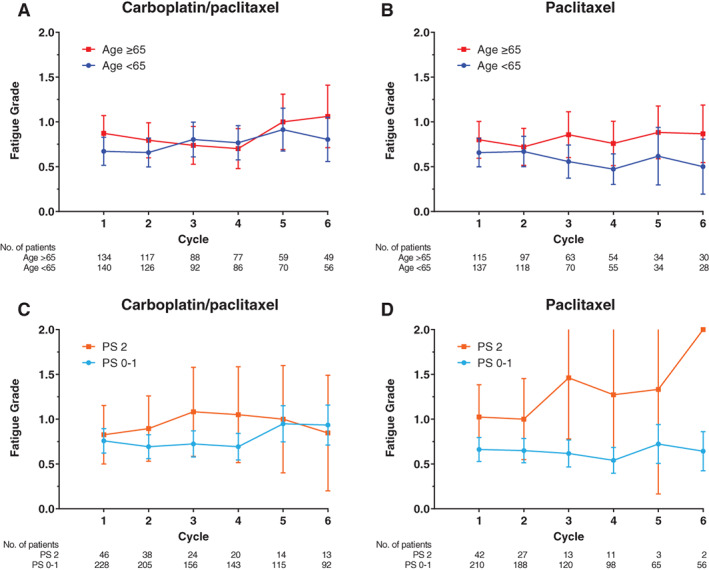
Mean fatigue grade over time. Mean fatigue grade plotted by cycle number for (A) carboplatin plus paclitaxel and (B) paclitaxel alone according to age group and for (C) carboplatin plus paclitaxel and (D) paclitaxel alone according to performance status group with error bars representing 95% confidence intervals.Abbreviation: PS, performance status.
Table 2.
Associations between patient characteristics and toxicity over time for fatigue and neuropathy using AE grade, AUC, and time to grade ≥ 2 multivariable models
| Characteristic | Fatigue | Neuropathy | ||||
|---|---|---|---|---|---|---|
| AE grade a : beta coefficient (95% CI) | AUC model b : beta coefficient (95% CI) | Time to grade ≥2 c : HR (95% CI) | AE grade a : beta coefficient (95% CI) | AUC model b : beta coefficient (95% CI) | Time to grade ≥2 c : HR (95% CI) | |
| Age ≥65 years | 0.17 (0.00–0.34) | 0.82 (−0.14 to 1.78) | 1.33 (0.99–1.79) | 0.12 (−0.01 to 0.25) | 0.61 (−0.18 to 1.39) | 1.41 (1.00–1.97) |
| ECOG PS 2 | 0.21 (−0.02 to 0.44) | 0.64 (−0.64 to 1.92) | 1.56 (1.07–2.27) | −0.17 (−0.35 to 0.01) | −1.30 (−2.36 to − 0.25) | 0.63 (0.35–1.12) |
| Female sex | −0.11 (−0.29 to 0.07) | −0.61 (−1.64 to 0.42) | 1.01 (0.73–1.38) | 0.00 (−0.14 to 0.14) | 0.25 (−0.59 to 1.09) | 0.86 (0.60–1.23) |
| Nonwhite race (ref: white) | −0.14 (−0.35 to 0.06) | −0.78 (−1.92 to 0.36) | 0.72 (0.49–1.05) | 0.08 (−0.08 to 0.23) | 0.45 (−0.48 to 1.39) | 1.14 (0.77–1.70) |
| Stage IV NSCLC | 0.04 (−0.15 to 0.23) | 0.24 (−0.84 to 1.32) | 1.08 (0.77–1.52) | −0.10 (−0.25 to 0.05) | −0.46 (−1.34 to 0.42) | 0.87 (0.60–1.25) |
| Squamous histology (ref: adeno) | −0.02 (−0.24 to 0.20) | −0.16 (−1.30 to 0.98) | 0.89 (0.60–1.30) | 0.04 (−0.13 to 0.21) | 0.12 (−0.82 to 1.05) | 1.02 (0.68–1.52) |
| Other histology (ref: adeno) | 0.00 (−0.20 to 0.20) | −0.26 (−1.51 to 0.99) | 0.88 (0.62–1.26) | 0.01 (−0.15 to 0.16) | 0.36 (−0.66 to 1.38) | 0.90 (0.57–1.40) |
| Carboplatin, paclitaxel (ref: paclitaxel) | 0.08 (−0.09 to 0.25) | 0.72 (−0.24 to 1.67) | 1.34 (0.99–1.80) | 0.04 (−0.09 to 0.17) | 0.57 (−0.21 to 1.35) | 1.11 (0.79–1.57) |
| Cycle 2 (all ref: cycle 1) | −0.02 (−0.09 to 0.06) | 0.02 (−0.05 to 0.08) | ||||
| Cycle 3 | 0.12 (0.04–0.21) | 0.32 (0.24–0.4) | ||||
| Cycle 4 | 0.09 (0.01–0.18) | 0.30 (0.21–0.38) | ||||
| Cycle 5 | 0.33 (0.23–0.43) | 0.67 (0.58–0.77) | ||||
| Cycle 6 | 0.29 (0.18–0.4) | 0.61 (0.51–0.71) | ||||
Bold text indicates a statistically significant difference with a p value <.05.
Linear mixed model clustered by individual patient.
Linear regression model.
Competing risk proportional hazard model with death as competing risk.
Abbreviations: adeno, adenocarcinoma; AE, adverse event; AUC, area under the curve; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NSCLC, non‐small cell lung cancer; PS, performance status; ref, reference.
In the AUC model of fatigue grade, mean AUC overall was 7.98 (95% CI, 7.40–8.57). There were no statistically significant associations between age, PS, treatment arm, or any of the other covariates and AUC for fatigue grade (Table 2).
Cumulative incidence functions for time to development of grade ≥2 fatigue were plotted stratified by treatment arm according to age (Fig. 2A, B) and PS (Fig. 2C, D). Overall, the median time to grade ≥2 fatigue was 240 days. In the multivariable Cox proportional hazards model (Table 2), PS 2 was associated with shorter time to development of grade ≥2 fatigue (hazard ratio [HR], 1.56; 95% CI, 1.07–2.27; p = .02). Treatment arm and the other covariates were not associated with time to grade ≥2 fatigue.
Figure 2.
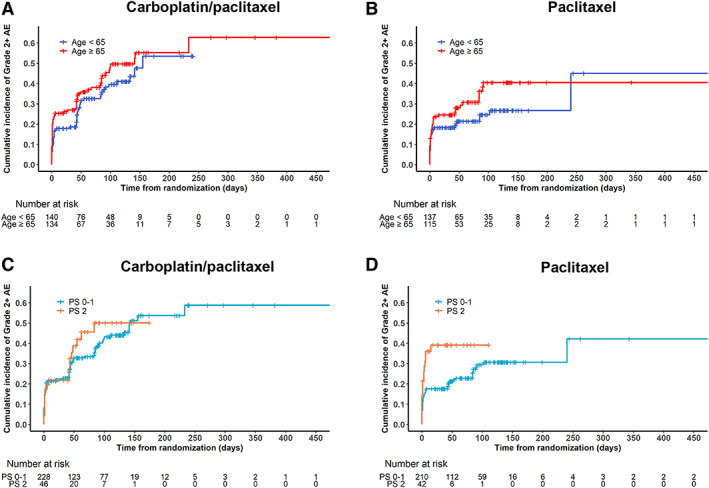
Time to development of grade ≥2 fatigue. Cumulative incidence functions for time to development of grade ≥2 fatigue for (A) carboplatin plus paclitaxel and (B) paclitaxel alone according to age group and for (C) carboplatin plus paclitaxel and (D) paclitaxel alone according to performance status group.Abbreviations: AE, adverse event; PS, performance status.
In the traditional maximum grade analysis (Table 3), older adults were more likely to experience grade ≥3 fatigue at some point during their treatment course compared with younger patients (17.3% vs. 9.7%, p = .01). In a sensitivity analysis using age 70 to define groups, there was no longer a statistically significant difference in grade ≥3 fatigue by age (data not shown). There was also no difference in grade ≥3 fatigue by PS.
Table 3.
Traditional maximum grade AE analysis comparing the proportion of patients with and without grade ≥ 3 AE
| Adverse event, characteristic | Grade <3 AE, n (%) | Grade ≥3 AE, n (%) | p value |
|---|---|---|---|
| Fatigue | |||
| Age <65 | 250 (90.3) | 27 (9.7) | .01 |
| Age ≥65 | 206 (82.7) | 43 (17.3) | |
| PS 0–1 | 382 (87.2) | 56 (12.8) | .43 |
| PS 2 | 74 (84.1) | 14 (15.9) | |
| Neuropathy | |||
| Age <65 | 254 (91.4) | 24 (8.6) | .03 |
| Age ≥65 | 211 (85.4) | 36 (14.6) | |
| PS 0–1 | 386 (87.9) | 53 (12.1) | .29 |
| PS 2 | 79 (91.9) | 7 (8.1) |
Abbreviations: AE, adverse event; PS, performance status.
Neuropathy
Mean neuropathy grade over six cycles was plotted and stratified by treatment arm according to age (Fig. 3A, B) and PS (Fig. 3C, D). In the multivariable linear mixed model for neuropathy grade over time, there were no statistically significant associations with age or PS group (Table 2). Only cycle number was associated with neuropathy grade, such that cycle 6 was associated with a 0.61‐point (95% CI, 0.51–0.71; p < .0001) higher mean neuropathy grade compared with cycle one.
Figure 3.
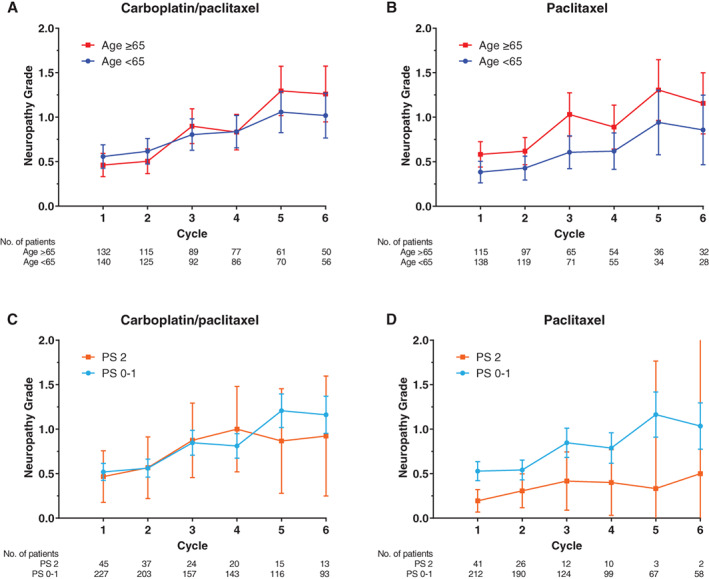
Mean neuropathy grade over time. Mean neuropathy grade plotted by cycle number for (A) carboplatin plus paclitaxel and (B) paclitaxel alone by age group and for (C) carboplatin plus paclitaxel and (D) paclitaxel alone by performance status group with error bars representing 95% confidence intervals.Abbreviation: PS, performance status.
In the AUC model of neuropathy grade, mean AUC overall was 3.70 (95% CI, 3.31–4.09). Patients with PS 2 had a 1.30‐point lower neuropathy AUC (95% CI, −2.36 to −0.25; p = .02) compared with patients with PS 0–1 (Table 2). There were no statistically significant associations between age, treatment arm, or any of the other covariates and AUC for neuropathy grade.
Cumulative incidence functions for time to development of grade ≥2 neuropathy were plotted stratified by treatment arm according to age (Fig. 4A, B) and PS (Fig. 4C, D) groups. Overall, the median time to grade ≥2 neuropathy was 411 days. In the multivariable Cox proportional hazards model (Table 2), older age was associated with shorter time to development of grade ≥2 neuropathy (HR, 1.41; 95% CI, 1.00–1.97; p = .049). In a sensitivity analysis using age 70 to define groups, this association remained statistically significant (p = .04).
Figure 4.
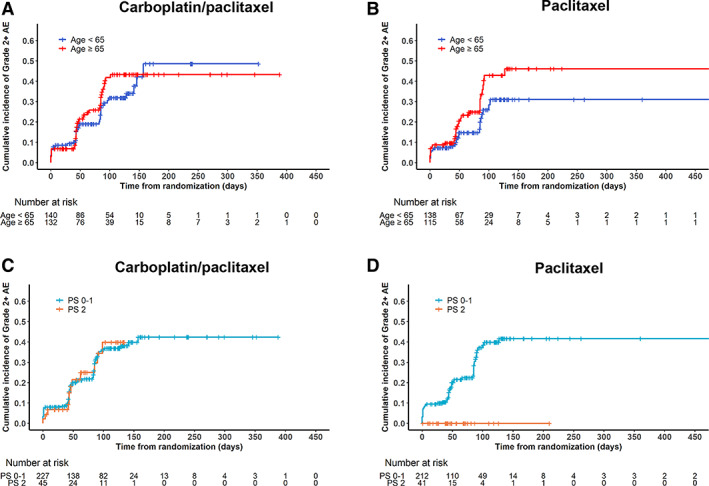
Time to development of grade ≥2 neuropathy. Cumulative incidence functions for time to development of grade ≥2 neuropathy for (A) carboplatin plus paclitaxel and (B) paclitaxel alone according to age group and for (C) carboplatin plus paclitaxel and (D) paclitaxel alone according to performance status group.
Abbreviations: AE, adverse event; PS, performance status.
In the traditional maximum grade analysis (Table 3), older adults were more likely to experience grade ≥3 neuropathy at some point during their treatment course (14.6% vs. 8.6%, p = 0.03). In a sensitivity analysis using age 70 to define groups, there was no longer a statistically significant difference in grade ≥3 neuropathy by age (data not shown). There was no difference in grade ≥3 neuropathy by PS.
Discussion
By expanding beyond the traditional maximum grade approach, we identified important differences in chemotherapy toxicity over time in older adults and patients with PS 2 in a large phase III NSCLC trial. Whereas the maximum grade analysis identified a higher percentage of patients age ≥65 with grade ≥3 fatigue at some point during their treatment course, the more comprehensive ToxT approach was able to further characterize that difference by detecting a higher mean fatigue grade longitudinally for older adults and a shorter time to development of grade ≥2 fatigue for patients with PS 2 compared with younger and patients with PS 0–1, respectively. Similarly, whereas the maximum grade analysis for neuropathy identified a higher percentage of older adults with grade ≥3 neuropathy at some point during their treatment course, we further characterized that difference by detecting a shorter time to development of grade >2 neuropathy for older adults. Interestingly, ToxT also identified an inverse association between PS 2 and neuropathy AUC that the maximum grade analysis did not; patients with PS 2 experienced a statistically significant lower AUC for neuropathy grade than patients with PS 1.
As one of the most common chemotherapy AEs, fatigue is a highly distressing symptom [17] and is associated with depression in patients with lung cancer [25]. By performing both the maximum grade and longitudinal ToxT analyses, we found that older adults with NSCLC experience a higher burden of fatigue during chemotherapy in two ways. Older adults are more likely to experience grade ≥3 fatigue at some point during treatment and higher mean grade throughout treatment. The additional longitudinal toxicity information can inform chemotherapy decision making and education for older adults to provide more detailed anticipatory guidance on what to expect during treatment and how to best manage it. For example, clinicians and patients can anticipate that the overall burden of fatigue is higher in older patients, which can help clinicians screen for treatment‐related fatigue early in the course of chemotherapy and help patients manage it with physical activity, energy conservation, and psychosocial interventions [26]. Similarly, earlier development of grade ≥2 fatigue among patients with PS 2, which was only detected by the ToxT approach and missed by the traditional maximum grade approach, may lead to earlier symptom management referrals among these vulnerable patients. Of note, adding carboplatin to paclitaxel did not increase mean fatigue grade or AUC or shorten time to development of grade ≥ 2 fatigue, which is clinically useful information when discussing combination chemotherapy versus single agent treatment options with patients.
Although prior studies have demonstrated an increased risk of chemotherapy‐induced peripheral neuropathy with older age [27, 28], we identified both an age‐related difference in experiencing grade ≥3 neuropathy at some point during the treatment course in the maximum grade analysis and earlier development of grade ≥2 neuropathy among older adults. These complementary but individually important results emphasize the value of using multiple approaches, both maximum grade and longitudinal analyses, to examine different aspects of the toxicity experience.
The inverse association between PS and neuropathy AUC identified by the ToxT approach was unexpected. We hypothesize that patients with PS 2 experienced a lower AUC for neuropathy grade because clinicians may have dose reduced chemotherapy once patients reported the development of mild neuropathy in order to avoid falls in this more vulnerable population. The association between chemotherapy‐induced peripheral neuropathy and falls is well documented [29], and poor PS represents an additional independent risk factor [30]. For patients with PS 0–1, moderate neuropathy may have been more readily tolerated, in essence traded off, to maximize treatment efficacy. Our finding of a lower neuropathy AUC among patients with PS 2 without PS‐related difference in the mixed effects model of neuropathy grade over time highlights the added value of examining longitudinal toxicity using multiple distinct approaches.
This ToxT analysis demonstrates how to use multiple longitudinal methods to more comprehensively characterize chemotherapy toxicity over time among older adults and patients with PS 2. Our study serves as a proof of concept to advance how cancer treatment toxicity and tolerability is evaluated in the field of geriatric oncology by expanding beyond the one‐dimensional maximum grade approach. Clinical implications for understanding these longitudinal differences in AEs according to age and PS span the cancer care continuum from treatment decision making, patient education and anticipatory guidance, through symptom management and survivorship.
To inform shared decision making, routine implementation of ToxT analyses of various treatment options (e.g., chemotherapy, immunotherapy, chemoimmunotherapy) may help patients and clinicians compare the longitudinal treatment experience of each option. For example, if an older adult is considering carboplatin plus paclitaxel versus paclitaxel alone, our results show that older patients are more likely to experience higher mean fatigue grade with either regimen, but fatigue and neuropathy are similar between the regimens. In addition, with the increasing use of immunotherapy and targeted therapy in NSCLC and many other cancer types, persistent lower grade AEs (e.g., fatigue) may become increasingly relevant as the rates of grade ≥ 3 AEs decrease compared with more toxic chemotherapy options. Information on the timing of development of specific AEs can inform anticipatory guidance to plan supportive care measures for older or frail patients. As advances in cancer treatments result in improved survival for many cancer types, management of persistent AEs or delayed toxicities are critical components of survivorship care.
We recognize several study limitations. First, we only focused on two common symptomatic AEs in CALGB 9730. Unlike the maximum grade approach in which AEs are often grouped, the ToxT approach evaluates the time profile of individual AEs. Therefore, we selected symptomatic AEs based on their prevalence and potential impact on patient QOL and did not include asymptomatic AEs (e.g., neutropenia). Provider‐reported AEs may also underestimate toxicity compared with patient‐reported AEs [31, 32]. In addition, although CALBG 9730 enrolled a relatively large proportion of older adults and patients with PS 2, the sample size in each subgroup was too small to allow evaluation of interaction effects (e.g., older and with PS 2 compared with younger and with PS 0–1). Furthermore, we were unable to adjust for chemotherapy dose adjustments or supportive care measures during treatment because complete detailed information on these factors and the timing of dose adjustments were not available in this legacy CALGB trial.
Finally, the effect size of ToxT results may be challenging to interpret clinically because we are accustomed to reviewing toxicity tables with percentages of patients with severe AEs. For example, our result of an average 0.17‐point higher mean fatigue grade overall among older patients with NSCLC does not immediately translate into percentages of patients or familiar discrete AE grades. A 0.17‐point increase in mean fatigue grade can result from an additional 5.7% of older patients experiencing grade 3 fatigue, or an additional 8.5% experiencing grade 2 fatigue, or an additional 17% experiencing grade 1 fatigue, or a comparable combination of increased toxicity. Despite these challenges of interpretation, our ToxT analysis identified important longitudinal differences in fatigue and neuropathy according to age and PS in a large phase III trial that are missed by the traditional maximum grade approach.
Conclusion
Expanding beyond the basic toxicity information provided by the maximum grade approach can identify clinically important longitudinal differences in AEs according to age and PS. For older adults with cancer and younger patients with a poor performance status, this more nuanced toxicity information is vital to shared decision making and identification of patients at higher risk for different types of AEs over time. The AE data required for these ToxT analyses are readily available from existing trials, and a ToxT SAS macro [13] is also available. The increasing use of patient‐reported AEs [33] in trials provides additional valuable AE data for longitudinal analysis to further inform tolerability. Furthermore, future research should study the applicability of the ToxT approach to visualizing toxicity longitudinally in real‐world clinical practice outside of clinical trials, particularly as real‐time symptom monitoring using patient‐reported outcomes is increasingly implemented [34].
Rather than reduce rich cancer treatment toxicity data collected from both clinicians and patients down to a summary maximum grade table, ToxT analyses provide an opportunity to utilize these data to their fullest to improve patient‐centered cancer care. Adding these enhanced longitudinal analyses to trials will improve our ability to detect important differences in toxicity and patient and clinician understanding of treatment tolerability beyond the maximum grade approach.
Author Contributions
Conception/design: Melisa L. Wong, Junheng Gao, Gita Thanarajasingam, Jeff A. Sloan, Amylou C. Dueck, Paul J. Novotny, Aminah Jatoi, Arti Hurria, Louise C. Walter, Christine Miaskowski, Harvey J. Cohen, William A. Wood, Josephine L. Feliciano, Thomas E. Stinchcombe, Xiaofei Wang
Provision of study material or patients: Junheng Gao, Gita Thanarajasingam, Jeff A. Sloan, Amylou C. Dueck, Paul J. Novotny, Aminah Jatoi, Arti Hurria, Xiaofei Wang
Collection and/or assembly of data: Melisa L. Wong, Junheng Gao, Gita Thanarajasingam, Jeff A. Sloan, Amylou C. Dueck, Paul J. Novotny, Xiaofei Wang
Data analysis and interpretation: Melisa L. Wong, Junheng Gao, Gita Thanarajasingam, Jeff A. Sloan, Amylou C. Dueck, Paul J. Novotny, Aminah Jatoi, Arti Hurria, Louise C. Walter, Christine Miaskowski, Harvey J. Cohen, William A. Wood, Josephine L. Feliciano, Thomas E. Stinchcombe, Xiaofei Wang
Manuscript writing: Melisa L. Wong, Junheng Gao, Gita Thanarajasingam, Jeff A. Sloan, Amylou C. Dueck, Paul J. Novotny, Aminah Jatoi, Arti Hurria, Louise C. Walter, Christine Miaskowski, Harvey J. Cohen, William A. Wood, Josephine L. Feliciano, Thomas E. Stinchcombe, Xiaofei Wang
Final approval of manuscript: Melisa L. Wong, Junheng Gao, Gita Thanarajasingam, Jeff A. Sloan, Amylou C. Dueck, Paul J. Novotny, Aminah Jatoi, Arti Hurria, Louise C. Walter, Christine Miaskowski, Harvey J. Cohen, William A. Wood, Josephine L. Feliciano, Thomas E. Stinchcombe, Xiaofei Wang
Disclosures
Arti Hurria: Celgene, Novartis, GlaxoSmithKline (RF), Boehringer Ingelheim Pharmaceuticals, Carevive, Sanofi, GTx Inc, Pierian Biosciences, MJH Healthcare Holdings, LLC (C/A); Melisa L. Wong: Genentech (family‐E, family‐OI); William Wood: Koneksa Health, Elektra Labs (C/A, OI), Genentech, Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1. CALGB Expanded Common Toxicity Criteria utilized in CALGB 9730
Acknowledgments
The findings were previously presented in part at the 2018 International Society of Geriatric Oncology Annual Conference in Amsterdam, The Netherlands.
This work was supported by the National Institutes of Health National Cancer Institute under the award number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA232760, P30CA033572, U10CA180838, U10CA180802; National Institute on Aging (R03AG056439, K76AG064431, P30AG044281); and National Center for Advancing Translational Sciences (KL2TR001870) and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Smith BD, Smith GL, Hurria A et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 2. Basch E, Campbell A, Hudgens S et al. Broadening the definition of tolerability in cancer clinical trials to better measure the patient experience. 2018. Friends of Cancer Research. Available at https://www.focr.org/sites/default/files/pdf/Comparative%20Tolerability%20Whitepaper_FINAL.pdf. Accessed March 6, 2020.
- 3. Given CW, Given B, Azzouz F et al. Comparison of changes in physical functioning of elderly patients with new diagnoses of cancer. Med Care 2000;38:482–493. [DOI] [PubMed] [Google Scholar]
- 4. Gajra A, Zemla TJ, Jatoi A et al. Time‐to‐treatment‐failure and related outcomes among 1000+ advanced non‐small cell lung cancer patients: Comparisons between older versus younger patients (Alliance A151711). J Thorac Oncol 2018;13:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barlesi F, Mazieres J, Merlio JP et al. Routine molecular profiling of patients with advanced non‐small‐cell lung cancer: Results of a 1‐year nationwide programme of the french cooperative thoracic intergroup (IFCT). Lancet 2016;387:1415–1426. [DOI] [PubMed] [Google Scholar]
- 6. Mok TSKWu Y‐LKudaba I et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): A randomised, open‐label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 7. Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 8. Hensing TA, Peterman AH, Schell MJ et al. The impact of age on toxicity, response rate, quality of life, and survival in patients with advanced, stage IIIB or IV nonsmall cell lung carcinoma treated with carboplatin and paclitaxel. Cancer 2003;98:779–788. [DOI] [PubMed] [Google Scholar]
- 9. Pallis AG, Karampeazis A, Vamvakas L et al. Efficacy and treatment tolerance in older patients with NSCLC: A meta‐analysis of five phase III randomized trials conducted by the hellenic oncology research group. Ann Oncol 2011;22:2448–2455. [DOI] [PubMed] [Google Scholar]
- 10. Lilenbaum RC, Herndon JE 2nd, List MA et al. Single‐agent versus combination chemotherapy in advanced non‐small‐cell lung cancer: The cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190–196. [DOI] [PubMed] [Google Scholar]
- 11. Begg CB, Carbone PP. Clinical trials and drug toxicity in the elderly. The experience of the eastern cooperative oncology group. Cancer 1983;52:1986–1992. [DOI] [PubMed] [Google Scholar]
- 12. Giovanazzi‐Bannon S, Rademaker A, Lai G et al. Treatment tolerance of elderly cancer patients entered onto phase II clinical trials: An Illinois cancer center study. J Clin Oncol 1994;12:2447–2452. [DOI] [PubMed] [Google Scholar]
- 13. Thanarajasingam G, Atherton PJ, Novotny PJ et al. Longitudinal adverse event assessment in oncology clinical trials: The toxicity over time (ToxT) analysis of Alliance trials NCCTG N9741 and 979254. Lancet Oncol 2016;17:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thanarajasingam G, Minasian LM, Baron F et al. Beyond maximum grade: Modernising the assessment and reporting of adverse events in haematological malignancies. Lancet Haematol 2018;5:e563–e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luciani A, Jacobsen PB, Extermann M et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31:424–430. [DOI] [PubMed] [Google Scholar]
- 16. Wong ML, Cooper BA, Paul SM et al. Age‐related differences in patient‐reported and objective measures of chemotherapy‐induced peripheral neuropathy among cancer survivors. Support Care Cancer 2019;27:3905–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong ML, Paul SM, Cooper BA et al. Predictors of the multidimensional symptom experience of lung cancer patients receiving chemotherapy. Support Care Cancer 2017;25:1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mols F, Beijers T, Vreugdenhil G et al. Chemotherapy‐induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer 2014;22:2261–2269. [DOI] [PubMed] [Google Scholar]
- 19. Wong ML, Paul SM, Mastick J et al. Characteristics associated with physical function trajectories in older adults with cancer during chemotherapy. J Pain Symptom Manage 2018;56:678–688.e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miaskowski C, Mastick J, Paul SM et al. Chemotherapy‐induced neuropathy in cancer survivors. J Pain Symptom Manage 2017;54:204–218.e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy‐induced peripheral neuropathy. Support Care Cancer 2012;20:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. European Medicines Agency . Guideline on missing data in confirmatory clinical trials; 2010. Available at https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐missing‐data‐confirmatory‐clinical‐trials_en.pdf. Accessed May 2, 2020.
- 23.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry developing products for weight management; 2007. Available at https://www.fda.gov/media/71252/download. Accessed May 5, 2020.
- 24. Thanarajasingam G, Leonard JP, Witzig TE et al. Longitudinal toxicity over time (ToxT) analysis to evaluate tolerability: A case study of lenalidomide in CALGB 50401 (Alliance). Lancet Haematol 2020;7:e490–e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopwood P, Stephens RJ. Depression in patients with lung cancer: Prevalence and risk factors derived from quality‐of‐life data. J Clin Oncol 2000;18:893–903. [DOI] [PubMed] [Google Scholar]
- 26. National Comprehensive Cancer Network . Clinical practice guidelines in oncology: Cancer‐related fatigue. (Version 2.2020). Available at https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. Accessed May 4 2020.
- 27. Hershman DL, Till C, Wright JD et al. Comorbidities and risk of chemotherapy‐induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group clinical trials. J Clin Oncol 2016;34:3014–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karavasilis V, Papadimitriou C, Gogas H et al. Safety and tolerability of anthracycline‐containing adjuvant chemotherapy in elderly high‐risk breast cancer patients. Clin Breast Cancer 2016;16:291–298. [DOI] [PubMed] [Google Scholar]
- 29. Kolb NA, Smith AG, Singleton JR et al. The association of chemotherapy‐induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol 2016;73:860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wildes TM, Maggiore RJ, Tew WP et al. Factors associated with falls in older adults with cancer: A validated model from the Cancer and Aging Research Group. Support Care Cancer 2018;26:3563–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atkinson TM, Dueck AC, Satele DV et al. Clinician vs patient reporting of baseline and postbaseline symptoms for adverse event assessment in cancer clinical trials. JAMA Oncol 2019;6:437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Atkinson TM, Ryan SJ, Bennett AV et al. The association between clinician‐based common terminology criteria for adverse events (CTCAE) and patient‐reported outcomes (PRO): A systematic review. Support Care Cancer 2016;24:3669–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Cancer Institute Healthcare Delivery Research Program . Patient‐reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE). Available at https://healthcaredelivery.cancer.gov/pro‐ctcae/. Accessed December 12, 2019.
- 34. Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1. CALGB Expanded Common Toxicity Criteria utilized in CALGB 9730


