Abstract
Lung cancer is the leading cause of cancer death in both males and females in the U.S. and worldwide. Owing to advances in prevention, screening/early detection, and therapy, lung cancer mortality rates are decreasing and survival rates are increasing. These innovations are based on scientific discoveries in imaging, diagnostics, genomics, molecular therapy, and immunotherapy. Outcomes have improved in all histologies and stages. This review provides information on the clinical implications of these innovations that are practical for the practicing physicians, especially oncologists of all specialities who diagnose and treat patients with lung cancer.
Implications for Practice
Lung cancer survival rates have improved because of new prevention, screening, and therapy methods. This work provides a review of current standards for each of these areas, including targeted and immunotherapies. Treatment recommendations are provided for all stages of lung cancer.
Keywords: Lung cancer, Prevention, Screening, Diagnosis and therapy
Short abstract
This article reviews changes in science, pathology, prevention, early detection, and therapy related to lung cancer outcomes that have led to some triumphs and a brighter outlook for patients with lung cancer.
Introduction
Lung cancer has been the leading cause of cancer death in the U.S. and worldwide for many decades [1]. Most patients present with stage IV, metastatic disease that cannot be cured by past and current therapies. And most patients are current or former smokers, which was responsible for causing their disease [2, 3, 4]. These facts led to considerable pessimism and guilt among patients and physicians in the past. Fortunately, science is beginning to triumph over this past pessimism and the outlook is much brighter for patients as incidence and mortality rates fall [5]. In addition, attitudes are changing among physicians, scientists, patients, and advocates. In this article, we will review changes in science, pathology, prevention, early detection, and therapy.
Etiology, Pathogenesis, and Prevention
Early studies identified the fact the carcinogens in the airway caused most lung cancers. Tobacco smoke from active cigarette smoking was identified as the primary cause of lung cancer especially from the studies of Peto and Doll and documented in the U.S. Surgeon General's reports [3, 4, 6]. Later, passive smoke exposure was also shown to cause lung cancer, and this recognition changed attitudes and laws that regulated smoke exposure in public spaces as well as cigarette advertising, taxes, and laws [6, 7]. During World War II, uranium mining became frequent, and Saccomanno and others showed that the uranium decays to radon, a colorless and odorless gas emitting alpha particles that radiate the bronchial epithelium, leading to dysplasia and malignancy [8]. Subsequently, there have been declines in cigarette smoking and radon exposure that have led to declines in lung cancer incidence. However, smoking remains the leading preventable cause of lung cancer, especially in underdeveloped nations where smoking has not declined or increased. In developed countries, declines in cigarette smoking have led to marked declines in the incidence and mortality of lung cancer [1, 9] (Fig. 1).
Figure 1.
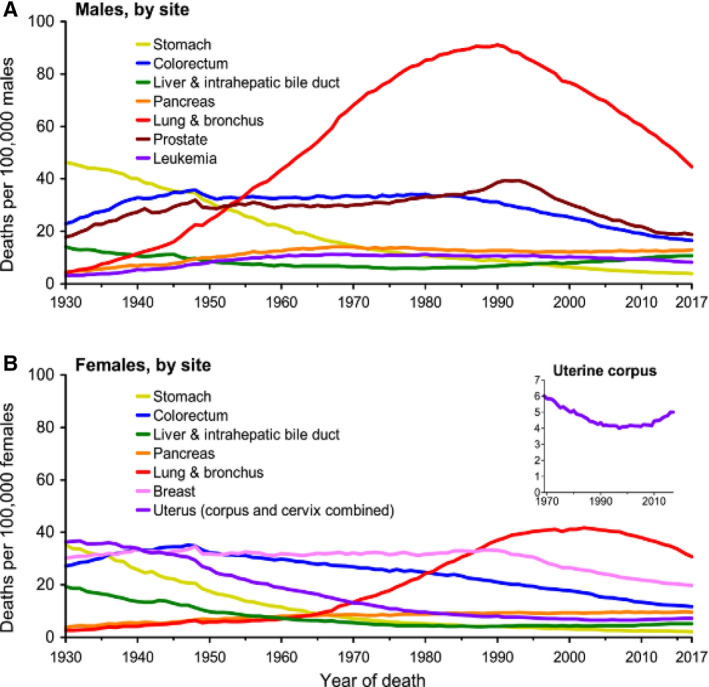
Lung cancer mortality rates in males and females in the U.S. by year [1]. Deaths per 100,000 males (A) and females (B) in the U.S. Although lung cancer has the highest mortality rates in both males and females, mortality rates are declining in both sexes.
In addition to causing pathologic changes in the bronchial epithelium, tobacco carcinogens produce molecular changes that become clonal at early times. The tumor suppressor gene, p53, was among the first to be characterized as an early event in lung cancer pathogenesis and remains the most frequently mutated gene in lung cancer [10]. Clonal p53 mutations can be detected in premalignant lesions and are among the most frequent clonal truncal alterations. Loss of other tumor suppressor genes is also frequent, and the combined loss of p53 and Rb is almost universal in small cell lung cancers, while genetic alterations define subsets of small cell cancers and large cell neuroendocrine cancers [11, 12]. These alterations may also identify new therapeutic strategies [13, 14]. Mutations in dominant oncogenes were recognized in many lung cancer specimens and patient‐derived cell lines. Mutations in myc family genes was reported in many small cell lung cancers, whereas KRAS proto‐oncogene mutations were frequently reported in lung adenocarcinomas [11, 14, 15]. The types of KRAS mutations were shown to differ in smoking‐related and non–smoking‐related adenocarcinomas. Studies of non‐small cell lung cancer (NSCLC) cell lines identified overexpression and amplification of human epidermal growth receptor (HER) family genes in many cancers, leading to development of inhibitors of these pathways and ultimately predictive biomarkers [16, 17]. More recently, whole genome studies, through the cancer genome project and other large efforts, have identified many recurring mutations in lung cancer that have become targets for therapy [18, 19, 20, 21].
Prevention, Screening, and Early Detection
After cigarette smoking was established as the major cause of lung cancer, the major goal worldwide was to reduce consumption of cigarettes and other tobacco products. Although this goal has been elusive, progress has been made, especially in developed countries, through a number of means including increased tax rates, counter advertising, educational promotions, laws restricting public smoking, and others [6]. These prevention efforts have had a major effect on reducing lung cancer incidence and mortality as illustrated in the U.S. (Figs. 1, 2). Prevention efforts in reducing radon exposure, therapeutic cancer radiation exposure, and others have had some effect, although air pollution efforts have largely failed to date. As industry attempts to addict new youth through vaping and other strategies, new prevention efforts remain a priority.
Figure 2.
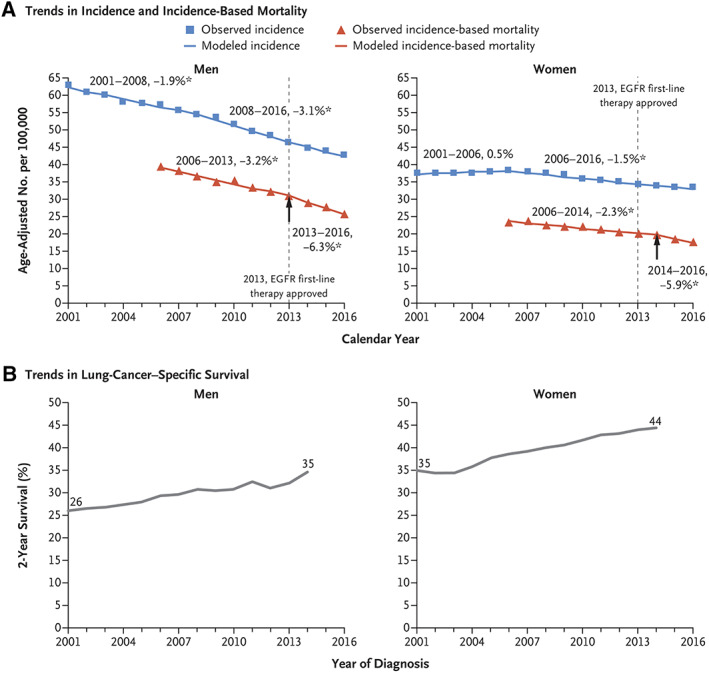
Trends in incidence and incidence‐based mortality (A) and lung cancer–specific survival (B) in men and women in the U.S. [5].Abbreviation: EGFR, epidermal growth factor receptor.
Early detection efforts focused on sputum cytology and chest x‐rays. Unfortunately, routine use of these modalities failed to improve lung cancer detection and survival rates [22, 23, 24]. Spiral chest computed tomography (CT) scans were shown to be considerably more sensitive for detecting small nodules [25], and the National Cancer Institute (NCI)‐sponsored National Lung Screening Trial randomizing high‐risk individuals to annual chest x‐ray or CT screening demonstrated that the CT scanning detected more small lesions and led to a 20% reduction in lung cancer mortality [26]. This reduction in lung cancer mortality was confirmed in the large Nederlands–Leuvens Longkanker Screenings Onderzoek (NELSON) trial in Europe, which also showed an even larger decrease in lung cancer mortality among females [27]. Multiple guidelines now recommend annual CT screening for high‐risk individuals between the ages of 55 and 80 who have smoked for more than 30 pack‐years and quit smoking less than 10 years previously [28, 29, 30]. The United States Preventive Services Task Force is currently considering revising their screening recommendations by lowering the pack‐year threshold for screening to 20 pack‐years and reducing the lower limit of the screening age to 50 years old [31]. Many nodules that are detected on chest CT scans are not malignant, and there are CT properties that can identify risk associated with the nodules to determine if biopsies, resection, or continued follow‐up scans are the best option. There are several computer‐assisted programs using clinical features and CT features to identify risk [32, 33].
Annual chest CT scans are best for detection of peripheral tumors including adenocarcinomas. Mortality from small cell lung cancer was not reduced in the screening studies, and central squamous tumors were less frequently detected. Although not used for routine screening, sputum cytology and bronchoscopy with cytology and biopsy still have a role in early detection [34, 35]. Carcinoma in situ and high‐risk dysplasia require serial follow‐up. Several systemic approaches including iloprost and immune checkpoint inhibitors may be able to reverse high‐risk lesions [36]. Three‐dimensional bronchial progenitor cell cultures and genomic analyses may help with future early detection efforts [37, 38].
More recent efforts evaluating molecular changes in circulating tumor DNA, exosomes, and other blood elements have detected tumor‐specific alterations in many patients, but their sensitivity is not sufficient for routine early detection protocols at present.
Pathology and Molecular Pathology
Lung cancers are classified according to the International Association for the Study of Lung Cancer (IASLC) pathologic system as adenocarcinomas (with varying subtypes), squamous carcinomas, large cell carcinomas with or without neuroendocrine features, and small cell carcinomas [39, 40, 41]. There also mixed tumors such as adenosquamous carcinomas and other rare tumors such as carcinoids. A variety of immunohistochemical markers may be used to help identify the specific histology in difficult cases. Among these are immunohistochemical testing of transcription termination factor 1 (TTF1) for adenocarcinomas, p40 and p63 for squamous carcinomas, and neuroendocrine markers for small cell and large cell carcinomas [42, 43].
The discovery that some lung adenocarcinomas have mutations in the epidermal growth factor receptor (EGFR) gene in 2004 and that there was a correlation between the presence of these mutations and sensitivity to EGFR tyrosine kinase inhibitors (TKIs) created the molecular targeted therapy era and the need to characterize the molecular alterations in individual lung cancers [44, 45, 46, 47]. Subsequent studies including the Cancer Genome Atlas studies and others identified a number of recurring oncogene changes caused by mutation, gene fusion, amplification, and copy number changes. Many of these alterations were shown to be drivers of lung cancer growth and could serve as predictive biomarkers for specific TKI therapy [48, 49, 50]. Among the alterations that serve as drivers as of 2020 are KRAS, EGFR, human epidermal growth receptor 2 (HER2), B‐Raf proto‐oncogene, serine/threonine kinase (BRAF), and MET proto‐oncogene (MET), receptor tyrosine kinase mutations, ALK receptor tyrosine kinase (ALK), ROS proto‐oncogene 1, receptor tyrosine kinase (ROS1), ret proto‐oncogene (RET), and neuregulin 1 (NRG1) fusions. The therapeutic implications of these alterations are discussed below.
Pathologists and oncologists must decide whether to look for these alterations in tissue biopsies, blood, or other fluids. In 2020, it is clear that testing for multiple alterations simultaneously is the only practical and efficient way to do this. Next‐generation sequencing (NGS) testing of DNA and RNA is the preferred method for tissue testing owing to the multiple types of mutations, fusions, and amplifications [47, 48, 49, 50]. There are many commercial and local platforms that are Clinical Laboratory Improvement Amendments approved and test for all alterations for which there is a specific therapy. There also many guidelines that indicate the need for these tests at diagnosis and at the time of progression for those with a driver alteration. At present, guidelines indicate the need for these tests in all advanced lung adenocarcinomas and in other histologies with light or no smoking histories [48, 49, 50]. Many institutions now routinely test for these alterations in earlier‐stage patients as well. As randomized trials of molecular and immunotherapy approaches are completed in earlier stages, it is likely that this panel testing will become mandatory in earlier stages as well. For clinicians, it is imperative that these tests be conducted as soon as a diagnosis is made so as not to delay therapeutic decisions.
Staging
The IASLC, Union for International Cancer Control, and American Joint Committee on Cancer staging classifications are updated every 8 years, and we are currently in the eighth edition, which should be used to stage all patients at diagnosis [51]. The details of this tumor node metastasis classification are shown in Figure 3. The clinical stage is based on the results of CT and CT positron emission tomography (PET) scans as well as brain magnetic resonance imaging (MRI) scans that are indicated in all patients at diagnosis. Patients with enlarged and/or PET‐positive hilar and mediastinal lymph nodes should have an endobronchial ultrasound procedure with node biopsies or mediastinoscopy prior to resection to complete the staging. Stage IV is now divided into stage IVA and IVB based on whether there is pleural‐only disease or metastatic disease in a single site. The 5‐year survival rates for stage IA1 (T1A, N0, M0) exceed 90%, and those for stage IA2 and IA3 exceed 80%. These patients are currently excluded from adjuvant and neoadjuvant trials. However, it is possible that immunotherapies could be studied in stage IA2 and IA3.
Figure 3.
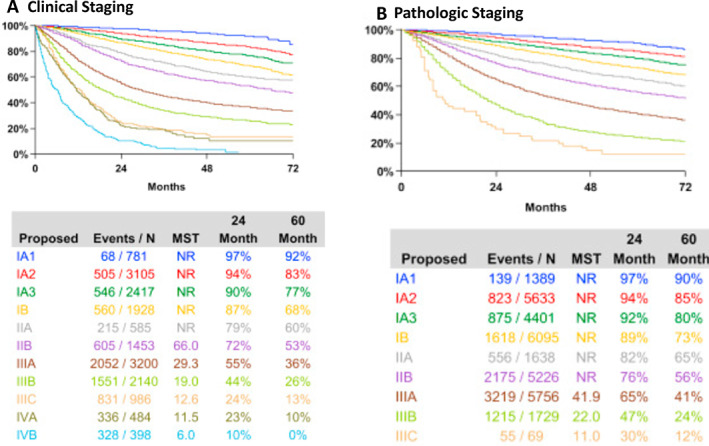
Stage groupings and survival in the eighth edition TNM staging classification [51].Abbreviations: MST, median survival time; N, number; NR, not reached.
Treatment of Early‐Stage Disease (I–IIIA)
Patients with early‐stage disease will most often undergo a surgical resection, so a determination of resectability is usually undertaken consisting of evaluations of pulmonary and cardiac function. Patients with stage I disease (T1a, T1b, T1c, N0, M0) usually undergo resection alone. Resection is most often lobectomy, but wedge resection, segmentectomy, bilobectomy, and pneumonectomy may be performed depending on size and location. The procedures are most often done by video‐assisted thoracoscopic surgery or robotic resection, but conversion to an open thoracotomy occurs in about 10% of cases [52, 53]. Dissection of multiple lymph node stations is always performed.
Cases that are not eligible for surgical resection are most often treated with stereotactic body irradiation (SBRT) [54, 55]. Most recurrences after surgical resection or primary irradiation occur in distant sites, so systemic therapy is usually delivered before (neoadjuvant), after (adjuvant), or before and after (neo and adjuvant) the primary surgery or radiation therapy.
Neoadjuvant Therapy
Neoadjuvant platinum doublet‐based chemotherapy was proven to improve survival in patients with resectable stage II and IIIA NSCLC [56]. For patients with an adenocarcinoma histology, the doublet is most often cisplatin combined with pemetrexed or paclitaxel, but vinorelbine and gemcitabine may also be used. For squamous histology, cisplatin is most often combined with paclitaxel, gemcitabine, or vinorelbine. The number of preoperative cycles has varied from two to four. A meta‐analysis of neoadjuvant randomized trials showed an overall survival (OS) improvement of 5% at 5 years with a survival hazard ratio (HR) of 0.82 [56]. For patients with NSCLC with a single‐station N2 involvement (stage IIIA), a few studies showed a survival advantage for adding nodal irradiation to the preoperative chemotherapy [57]. After neoadjuvant chemotherapy, at the time of surgery, the pathologic complete response rate was very low (<5%) whereas the major pathologic response rate (<10% viable tumor cells in the resection specimen) was 20% [58].
More recently, checkpoint immunotherapeutic antibodies alone or with chemotherapy have been studied in the neoadjuvant setting [58]. Table 1 summarizes results of trials where a single‐agent checkpoint inhibitor produced a major pathologic response (MPR) rate of 27% with pathologic complete response (CR) rates averaging 9% [59, 60, 61, 62, 63]. Pathologic response rates have been higher in the single‐arm trials using combined chemotherapy with checkpoint inhibitor antibodies [64, 65, 66, 67]. Although patient numbers are small, MPR rates in these trials averaged 61% and pathologic CR rates averaged 27%. Long‐term survival has not been reported in any of these trials but is expected to be higher than that achieved with chemotherapy alone. For that reason, there are multiple randomized phase III trials ongoing that compare neoadjuvant chemotherapy alone with combined chemoimmunotherapy (Table 2) [68]. After surgery, all of these neoadjuvant chemoimmunotherapy trials discontinue the chemotherapy but continue the immunotherapy for various periods, which is usually 1 year. Patients with driver molecular alterations are generally excluded from these trials because the response rates to immunotherapy are often lower in these patients. Recently, neoadjuvant studies of various molecular TKIs have been instituted, but there are few results to date.
Table 1.
Results of neoadjuvant immunotherapy and combined chemotherapy/immunotherapy trials in resectable non‐small cell lung cancer
| Rx | n | mPR, n (%) | pCR, n (%) | Ref |
|---|---|---|---|---|
| Nivolumab | 21 | 9 (43) | 3 | 60 |
| Nivolumab or nivolumab + ipilimumab | 44 | 11/41 (25) | 8 (18) | 61 |
| Atezolizumab | 82 | 15 (18) | 4 (5) | 62 |
| Sintilimab | 22 | 10 (45) | 4 (18) | 63 |
| Pembrolizumab | 10 | 4 (40) | 0 | 64 |
| Total I/O alone | 179 | 49 (27) | 19/176 (11) | |
| Nivolumab + CT | 30 | 24 (80) | 13 (60) | 65 |
| Atezolizumab + CT | 30 | 17 (57) | 10 (33) | 66 |
| Nivolumab + CT | 13 | 11 (85) | 5 (38) | 67 |
| Durvalumab + CT | 67 | 33 (60) | 10 (18) | 68 |
| Total I/O + CT | 140 | 85 (61) | 38 (27) |
Abbreviations: CT, chemotherapy; I/O, immunotherapeutic; mPR, major pathologic response; n, number; NR, not reported; pCR, pathologic complete response; Ref, reference; Rx, treatment.
Table 2.
Ongoing clinical trials with neoadjuvant and adjuvant immune checkpoint inhibitors with or without chemotherapy adopted from neoadjuvant and adjuvant trials
| Trial | NCT # | Therapy | Stages | n | Phase | Primary endpoint |
|---|---|---|---|---|---|---|
| Neoadjuvant | ||||||
| CANOPY N | 03968419 | Canakinumab or pembrolizumab (200 mg) or canakinumab + pembrolizumab × 2 cycles ➔ S | IB–IIIA | 110 | II | MPR |
| KEYNOTE 617 | 03425643 | CT + pembrolizumab (200 mg) / placebo × 4 cycles ➔ S ➔ pembrolizumab / placebo × 13 cycles | IIB–IIIA | 786 | III | EFS, OS |
| CheckMate 816 | 02998528 |
CT + nivolumab (360 mg) × 3 cycles ➔ S vs. CT × 3 cycles ➔ S |
IB–IIIA | 350 | III | EFS, MPR |
| IMpower 030 | 03456063 | CT + atezolizumab (1200 mg) / placebo × 4 cycles ➔ S ➔ atezolizumab / placebo × 16 cycles | II–IIIB (cT3N2) | 374 | III | MPR |
| AEGEAN | 03800134 | CT + durvalumab (1,500 mg) / placebo Q3W × 4 cycles ➔ S ➔ durvalumab / placebo Q4W × 12 cycles | IIA–IIIB | 300 | III | MPR |
| Adjuvant | ||||||
| PEARLS | 02504372 | Pembrolizumab vs. placebo | IB–IIIA | 1,080 | III | DFS |
| BR31 | 02273375 | Durvalumab vs. placebo | IB–IIIA | 1,360 | III | DFS in PD‐L1+ and all |
| ANVIL | 02595944 | Nivolumab vs. observation | IB–IIIA | 903 | III | DFS and OS |
| Impower 010 | 02486718 | Atezolizumab vs. observation | IB–IIIA | 1,280 | III | DFS in II–IIIA, DFS in PD‐L1+, DFS in ITT |
| CANOPY‐A | 03447769 | Canakinumab vs. observation | IB–IIIA | 1,500 | III | DFS |
Abbreviations: CT, chemotherapy; DFS, disease‐free survival; EFS, event free survival; ITT, intention to treat; MPR, major pathologic response; n, number; OS, overall survival; PD‐L1, programmed death‐ligand 1; Q3W, every 3 weeks; Q4W, every 4 weeks; S, surgery.
A recent cardiovascular prevention trial with an anti–interleukin‐1β antibody, canakinumab, found a marked decrease in the incidence of lung cancer in subjects randomized to canakinumab [69, 70]. This trial raises many questions including whether this is a tumor microenvironment effect or a direct effect on malignant cells [70]. Based on these results and questions, there is an ongoing phase II randomized neoadjuvant trial comparing single‐agent pembrolizumab, single‐agent canakinumab, and the combination (Table 2).
The IASLC and Food and Drug Administration held a joint conference on neoadjuvant therapy that concluded that there are insufficient data at present to conclude that pathologic response rate correlates with overall survival or cure rates. However, it is hoped that a meta‐analysis of these ongoing randomized trials could establish pathologic response as a proven surrogate endpoint [71]. A key aspect of this effort will be uniform pathologic assessments. The IASLC has published recommendations for scoring pathologic response and is undertaking a comparative review [72].
Adjuvant Therapy After Surgical Resection
For patients with resected stage II and IIIA NSCLC, postoperative platinum doublet–based chemotherapy was shown to improve 5‐year survival rates by about 5%, which was associated with a hazard ratio of 0.82 in a meta‐analysis of these trials [73, 74]. No OS benefit was seen in patients with tumors <3 cm without nodal involvement. These trials used several different cisplatin‐based combinations. The recent Eastern Cooperative Oncology Group (ECOG) 1505 randomized trial allowed four different drugs to be added to cisplatin with randomization to the addition of bevacizumab or placebo. Overall, bevacizumab did not improve outcomes [75]. Although not a primary endpoint, there were no differences in outcomes between platinum doublets. An important aspect of this trial was the excellent survival in all arms, which exceeded 50% at 5 years. These survival results were better than the outcomes previously reported in the meta‐analyses. This is likely due at least in part to better staging through the routine use of PET and brain MRI scans.
Most adjuvant chemotherapy trials planned on four cycles of chemotherapy, but subsequent analyses did not find a difference in survival between those receiving three or four cycles. There was no benefit of postoperative radiation therapy, with the possible exception of N2 disease, where some benefit was observed in most metanalyses of postoperative radiation trials [76, 77]. However, the recently presented randomized phase III LUNG ART trial suggested no improvement in disease‐free survival (DFS) or OS for postoperative radiation in patients with pathologically identified N2 disease [78].
Based on data showing superiority of combined chemo/immunotherapy over chemotherapy alone in stage IV disease, large, randomized studies with the addition of immunotherapy to chemotherapy in the postoperative setting are being conducted. Table 2 shows these ongoing trials, although the results of these trials have not been reported yet [68]. The NCI's Adjuvant Nivolumab in Resected Lung Cancers (ANVIL) trial compares nivolumab with chemotherapy, whereas most other trials compare chemoimmunotherapy with chemotherapy alone. These trials have largely excluded patients with actionable molecular alterations.
Adjuvant trials in patients with EGFR mutations using various EGFR TKIs have been reported while others are ongoing. The early phase II, nonrandomized SELECT trial had a DFS endpoint based on historical controls treated with chemotherapy [79]. The study reported a 90% DFS at 2 years, which exceeded the historical benchmark. The EVAN trial was a randomized phase II study that compared 2 years of erlotinib with vinorelbine/cisplatin (VP) chemotherapy in patients with surgically resected stage IIIA NSCLC with EGFR mutation [80]. The ADJUVANT trial was a randomized phase III study that compared 2 years of adjuvant gefitinib with VP chemotherapy in patients with surgically resected stage II to IIIA (N1–N2) NSCLC with EGFR mutation [81]. Both trials demonstrated significantly longer DFS in favor of adjuvant EGFR‐TKI monotherapy compared with VP chemotherapy. However, the DFS curves came together at 3 years and there were no OS differences.
The RADIANT randomized phase III trial studied the role of adjuvant erlotinib after surgery and chemotherapy in patients with and without EGFR mutations [82]. Patients were randomized to erlotinib or placebo for 2 years. There was a significant prolongation of DFS in the patients with EGFR mutations who received erlotinib. However, OS did not differ between therapies in the total population or in the EGFR mutant patients. Patients who relapsed were treated with erlotinib with high response rates. As expected, just over half of the patients in both arms had not recurred, and clearly, patients cured by surgery and chemotherapy will have toxicity but no benefit from the added TKI. Unfortunately, it is currently not possible to know who is cured and who is not.
The randomized phase III trial termed CTONG 114 randomized EGFR mutant patients following surgical resection to receive gefitinib or chemotherapy [83]. As in the RADIANT trial, there was improved DFS in the TKI group; however, there was no difference in OS. The likely explanation for these results is that just as complete responses are uncommon in advanced disease, there is sufficient cell kill to prolong recurrence but insufficient cell kill to lead to cure.
Most recently, early results of the ADAURA trial were presented and published [84, 85]. In this trial, postsurgical patients with EGFR mutations were randomized to receive “standard therapy” plus either placebo or osimertinib for 3 years after standard therapy. “Standard therapy” could include adjuvant chemotherapy, but radiation was not allowed even in stage III disease. There was a highly statistically significant prolongation of DFS. OS was not different, although the trial had very short follow‐up. The DFS in the control group was shorter than that observed in prior chemotherapy trials such as ECOG 1505, perhaps because many patients in control group did not receive chemotherapy. Long‐term survival results of this trial are eagerly awaited.
There are several other ongoing randomized trials whose results have not been reported including the NCI cooperative group ALCHEMIST trial that included subgroups with EGFR mutation who were randomized to erlotinib or placebo and those with ALK mutations who were randomized to crizotinib or placebo after surgery and standard chemotherapy [86]. There are other ongoing randomized trials evaluating other EGFR TKIs in the adjuvant setting .
Combined Modality Therapy for Unresectable Stage III Disease
Many years ago, randomized trials demonstrated that concurrent platinum doublet chemotherapy and chest radiotherapy provided longer survival than either modality delivered alone or sequentially [87]. Several different platinum doublet combinations appeared to give equivalent results, and additional chemotherapy after completion of the radiation did not improve outcomes [88].
More recent studies evaluated the optimal dose of chest radiation, comparing 60 Gy with 74 Gy. Patients receiving the 60 Gy schedule had superior outcomes compared with the higher dose [89]. This was attributed, at least in part, to increased cardiac toxicity of the higher dose. Other trials using higher radiation doses with newer techniques or using proton beam therapy are ongoing [90].
The results of trials in patients with stage IV disease showing benefit for checkpoint inhibitor immunotherapy led to the randomized PACIFIC trial that compared concurrent chemoradiation therapy followed by up to 1 year of placebo or durvalumab immunotherapy [91]. Patients who did not progress after the initial chemoradiation and who did not have pneumonitis were randomized. The trial demonstrated a highly statistically significant improvement in progression‐free survival (PFS) and OS in patients randomized to maintenance durvalumab. The study included patients with any programmed death‐ligand 1 (PD‐L1) levels. At the protocol‐defined cutoffs, there was some benefit in all patients, but a post hoc evaluation did not find benefit in patients with no PD‐L1 expression. At present, this regimen can be considered as a standard, although ongoing trials are evaluating the earlier start of the immunotherapy before or concurrent with the chemoradiotherapy.
There is mounting evidence that SBRT to oligometastatic sites can improve survival when used after initial response to TKIs for those with molecular drivers and after response to chemoimmunotherapy for those without molecular drivers [92, 93]. There are several ongoing trials to address this question.
Therapy of Stage IV NSCLC
Chemotherapy
Chemotherapy has been the backbone of therapy for patients with stage IV lung cancer for many decades [94]. Many years ago, metanalyses of randomized trials of platinum‐based doublet chemotherapy showed that the doublets improved survival compared with single‐agent chemotherapy, including elderly patients and patients with performance status 2 [95, 96]. Later cooperative group trials showed equivalent results with several different platinum doublets with either cisplatin or carboplatin combined with vinorelbine, paclitaxel, docetaxel, or gemcitabine [97, 98, 99]. Still other trials showed that pemetrexed‐based platinum doublets also produced equivalent survival with less toxicity in those with nonsquamous carcinomas [100]. Shortly after platinum doublets became standard, the EGFR and the vascular endothelial growth factor (VEGF) receptor became new targets for agents to add to platinum doublets. Anti‐EGFR monoclonal antibodies such as cetuximab and necitumumab produced low response rates and little or no overall benefit when added to chemotherapy in the absence of biomarker selection. And although there was some evidence that biomarkers such as gene amplification could select patients most likely to benefit, use of these agents was largely abandoned [101, 102, 103, 104, 105].
Results with anti‐VEGF agents were more promising. The anti‐VEGF antibody bevacizumab showed promise, but early studies demonstrated bleeding toxicities in patients with central squamous cancers. Thus, subsequent bevacizumab trials were limited to nonsquamous histology. Randomized trials in these nonsquamous populations demonstrated that the addition of bevacizumab to a taxane/platinum doublet improved survival [106]. Trials in the nonsquamous population showed that pemetrexed/platinum combinations produced similar outcomes as taxane‐based combinations and that there was survival benefit for maintenance pemetrexed [107]. This observation raised the question of whether maintenance pemetrexed, bevacizumab, or both would be optimal after a pemetrexed/carboplatin bevacizumab induction. Several comparative randomized trials showed no additional benefit of maintenance with combined pemetrexed and bevacizumab over either alone [108, 109, 110, 111]. Recent guidelines define optimal histology‐based platinum doublets with maintenance in advanced lung cancer [112].
Because of the considerable toxicity of doublet chemotherapy, it is generally limited to patients with performance status of 0–1. Analyses of U.S. Surveillance, Epidemiology, and End Results data suggest that as many as 40% of patients with newly diagnosed lung cancer are never referred to an oncologist and receive no therapy, presumably because of poor performance status and comorbid disease [113]. As our supportive care measures have improved and as new therapies described below have fewer toxicities, it will be increasingly important to educate primary care physicians and change attitudes so that all patients have access to the best therapies.
Although platinum doublet chemotherapy remains a major part of first‐line therapy in most patients, an increasing number may receive TKIs or immunotherapies alone in the first line. When these patients progress, a platinum doublet remains the major therapeutic choice if the performance status is adequate. When patients progress on a platinum doublet chemotherapy and immunotherapy or a TKI when indicated, second‐ and third‐line single‐agent chemotherapy remains the major treatment option.
Molecular Therapy
Early in the 21st century, there was increasing evidence that alterations in the HER family of receptors played a role in the pathogenesis of lung and other cancers. Thus, pharmaceutical companies developed TKIs for these receptors and their intracellular adenosine triphosphate (ATP) binding pockets. Early TKIs such as gefitinib and erlotinib produced low response rates (10% or so) in unselected patients, but these responses were often dramatic and long lasting [114]. Early studies suggested that high receptor expression or EGFR gene amplification were marginally useful predictive biomarkers [115]. A phase III trial comparing erlotinib with chemotherapy in the second‐ or third‐line setting demonstrated erlotinib was superior to chemotherapy in overall response rate (ORR) and OS, but the ORR was only 9% [116]. The best predictive marker in this trial was not established [117]. Then, in 2004, three groups identified the fact that activating mutations in the EGFR gene predicted TKI benefit and that these EGFR TKIs preferentially bound to the mutated EGFR ATP binding pocket [44, 45, 46]. This finding revolutionized lung cancer therapy. These results led to a number of trials randomizing EGFR mutant patients to receive an EGFR TKI or chemotherapy (Table 3).
Table 3.
Phase III randomized trials of first‐line EGFR TKI versus platinum doublet chemotherapy in patients with EGFR mutant non‐small cell lung cancer
| First author | Study | TKI | # Pts | ORR % | Median PFS (mo) | Median OS (mo) |
|---|---|---|---|---|---|---|
| Mok [118] | IPASS | Gefitinib | 261 | 71 vs 47 | 9.8 vs 6.4 | 21.6 vs. 21.9 |
|
Maemondo [119] Inoue [120] |
NEJGSG002 | Gefitinib | 228 | 73 vs 31 | 10.8 vs 5.4 | 27.7 vs 26.6 |
|
Mitsudomi [121] Yoshioka [122] |
WJTOG3405 | Gefitinib | 177 | 62 vs 32 | 9.2 vs 6.3 | 34.9 vs 37.3 |
|
Zhou [123] Zhou [124] |
Optimal | Erlotinib | 154 | 83 vs 36 | 13.1 vs 4.6 | 22.6 vs. 28.8 |
| Han [125] | First Signal | Gefitinib | 309 | 55 vs 46 | 5.8 vs 6.4 | 22.3 vs 22.9 |
| Rosell [126] | EURTAC | Erlotinib | 154 | 58 vs 15 | 9.7 vs 5.2 | 19.3 vs. 19.5 |
|
Sequist [127] Yang [128] |
LUX‐Lung 3 | Afatinib | 345 | 56 vs 23 | 11.1 vs 6.9 | 28.2 vs 28.2 |
| Wu [129] | LUX‐Lung 6 | Afatinib | 364 | 67 vs 23 | 11.0 vs 5.6 | 23.6 vs 23.5 |
Abbreviations: EGFR, epidermal growth factor receptor; EURTAC, European randomized trial of tarceva versus chemotherapy; First Signal, first‐line single‐agent iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung; IPASS, Iressa Pan‐Asia study; LUX‐Lung 3, phase III trial of afatinib vs. pemetrexed/cisplatin in locally advanced or metastatic patients; LUX‐Lung 6, equivalent to LUX‐Lung 3 but with gemcitabine/cisplatin chemotherapy; NEJGSG002, North East Japan Gefitinib Study Group 002 trial; Optimal, erlotinib versus standard chemotherapy in the first‐line treatment of patients with advanced EGFR mutation–positive non‐small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TKI, tyrosine kinase inhibitor; WJTOG, West Japan Thoracic Oncology Group.
Recognizing the critical role of EGFR mutations in EGFR TKI sensitivity, the IPASS study randomized patients to receive first‐line carboplatin/paclitaxel chemotherapy or the first‐generation EGFR TKI gefitinib [118]. Those patients with activating EGFR mutations treated with gefitinib had higher response rates, longer PFS, reduced toxicity, and superior quality of life compared with those receiving first‐line chemotherapy. The OS, however, was equivalent in part because second‐line TKI therapy was also effective. The other randomized trials comparing gefitinib, erlotinib, afatinib, and icotinib with chemotherapy showed similar results, with the TKI producing higher response rates, longer PFS, and reduced toxicity compared with chemotherapy, leading to guidelines recommending first‐line TKI for EGFR mutant patients [118, 119, 120, 121, 123, 125, 126, 127, 129] (Table 3).
As was seen with the EGFR TKIs, several randomized trials showed that ALK TKIs were superior to platinum doublet chemotherapy in patients with ALK fusions [131, 132, 133]. Soon, other activating genetic mutations including BRAF, HER2, and MET and genetic activating fusions including ALK, ROS1, neurotrophic receptor tyrosine kinase (NTRK) and RET fusions were identified. Specific TKIs were developed and produced similar high response rates, long PFS, and low toxicity rates in patients with these alterations. A group called the Lung Cancer Mutation Consortium showed that molecular studies could easily identify these patients and that those treated with gene‐specific TKIs lived longer than those that did not receive a gene‐specific TKI [130].
First‐generation TKIs had poor central nervous system (CNS) penetration, and progression in the CNS was frequent. This observation led to the development of second‐ and third‐generation TKIs with better CNS penetration. First‐line randomized trials comparing these newer agents with platinum doublet therapy also demonstrated superiority for the TKI [131, 132, 133]. Other randomized trials compared first‐line use of second‐ or third‐generation TKIs with first generation TKIs. These trials demonstrated superiority of the newer agents in PFS, in reduced CNS metastases and sometimes in OS [134, 135, 136, 137, 138, 139, 140]. Like the studies showing that EGFR TKIs produced higher response rates, longer PFS, and decreased toxicity compared with platinum doublet chemotherapy, randomized trials showed that ALK TKIs were superior to platinum chemotherapy [131, 132, 133].
The frequency of activating genetic alterations in advanced NSCLC is illustrated in Figure 4 [50]. The frequency of these alterations is higher in adenocarcinomas and in light or never‐smokers, and patients with these characteristics should always be tested prior to institution of therapy. However, patients with other histologies, especially light or never‐smokers, should also be tested. There are some unique characteristics of patients with each molecular abnormality, but these are not sufficiently specific to treat without molecular characterization [15, 141, 142, 143, 144]. Secondary mutations may be present as well. The most frequent of these is a mutation in p53 that may associate with a slightly worse prognosis [144]. Therapy should, in general, not start until the results of these tests are known [145]. However, patients who require repeat biopsy or are too ill to wait may receive first‐line chemotherapy and switch to a TKI if actionable alterations are subsequently found. However, sequential therapy can increase toxicity if it includes immunotherapy and a TKI [146].
Figure 4.
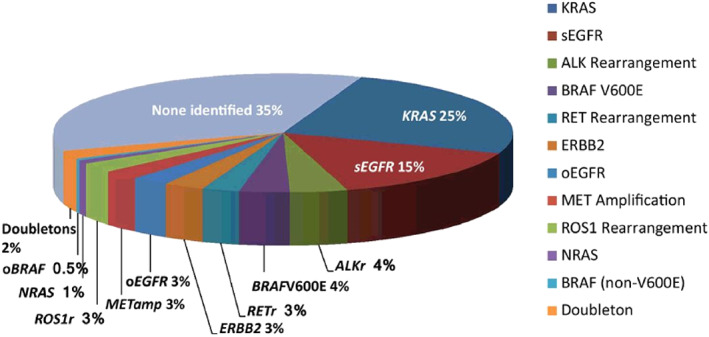
Frequency of various genetic alterations in advanced non‐small cell lung cancer [50].Abbreviation: EGFR, epidermal growth factor receptor.
Recently, several NGS panels conducted on blood samples have been approved, and these results are generally available in shorter time frames (5–14 days) [147, 148, 149]. Although these tests are not as sensitive as tissue tests, they are highly specific, so TKI therapy can be instituted when positive results are received.
Because first‐line therapy with a TKI is standard and the best option, routine NGS testing for multiple genetic alterations is now recommended in multiple guidelines with first‐line use of a TKI when actionable alterations are found [48, 150, 151]. Optimal NGS testing is conducted on tissue DNA and RNA because of the large number and types of alterations. A list of the molecular alterations associated with approved TKIs and other molecular alterations for which other TKIs are under investigation is shown in Table 4. Approved TKIs for EGFR, BRAF, and MET mutations and ALK, ROS1, RET, and TRK fusions are best given in the first line [134, 135, 136, 137, 138, 139, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162].
Table 4.
TKIs approved and under investigation for various genetic driver alterations in non‐small cell lung cancer in 2020
| Oncogene driver | Approved agents | Investigational agents |
|---|---|---|
| EGFR | Gefitinib, erlotinib a , afatinib, dacomitinib b , osimertinib c | EA1045, JND3229 |
|
EGFR exon 20 insertion Uncommon EGFR mutations, e.g., G719X, L861X, S7681V |
Afatinib | Mobocertinib, TAS6417, BDTX‐189, DS2087B, CLN081, osimertinib d |
| ALK | Crizotinib a , certitinib, alectinib, brigatinib, lorlatinib b | Ensartinib b |
| ROS1 | Crizotinib a , certinib b , entrectinib c | Repotrectinib, lorlatinib d , taletrectinib |
| RET | Selpercatinib b , pralsetinib b | Brigatinib d , ensartinib b |
| NTRK | Larotrectinib b , entrectinib b | Taletrectinib |
| MET | Capmatinib | Crizotinib d , tepotinib, savolitinib |
| BRAF V600E | Dabrafenib plus trametinib | Encorafenib |
| KRAS G12C | None | AMG 510, MRTX 849 |
| HER2 exon 20 insertions | None |
Trastuzumab emtansine d trastuzumab deruxtecan d mobocertinib, TAS6417, BDTX‐189, DS2087B, DZD9008, osimertinib d |
| NRG1 fusions | None | tarloxotinib, HER3 antibodies |
First‐generation TKI with poor CNS penetration.
Second‐generation TKI with good CNS penetration.
Third‐generation TKI with good CNS penetration, leads to resistance mutations like C797s.
Approved for other indications.
Vandetanib, cabozantinib, and lenvatinib are multi‐TKIs approved in other indications.
Abbreviations: CNS, central nervous system; EGFR, epidermal growth factor receptor; HER, human epidermal growth receptor; TKI, tyrosine kinase inhibitor.
The most frequent molecular alteration is a KRAS mutation. At present, specific TKIs are only available for KRAS G12C mutations, and these are investigational only [163, 164]. Other KRAS‐specific drugs as well as drug combinations are under investigation. In patients with KRAS mutations, secondary mutations including p53 mutations, serine/threonine kinase 11 mutations, KEAP 1 mutations, and others may influence the response to different therapies [165]. At present, these secondary mutations do not alter therapy selection. Studies of combinations of these KRAS G12C inhibitors with SHP2 inhibitors, MEK inhibitors checkpoint inhibitors, CDK4/6 inhibitors and other agents are also in progress. There are several investigational TKIs that are under study for atypical EGFR and HER2 insertional mutations, but none are approved for these mutations to date [166].
Two antibody drug conjugates have been studied in patients with NSCLC with HER2 mutations: trastuzumab emtansine [167] and trastuzumab deruxtecan [168]. The latter produced an objective response rate of 63% with a median PFS of 14 months. These results are the best reported to date for HER2 mutations in lung cancer, and additional trials are ongoing. There are several agents under study for NRG1 fusions including both TKIs and monoclonal antibodies, but none are approved [169]. It is likely that other activating and recurring mutations may be found in the future, but these are likely to be quite rare in frequency.
In spite of the effectiveness of specific TKI therapy, these drugs rarely induce complete remissions and do not cure stage IV disease. Although several logs of cell kill may occur, the vast majority of cells are not killed by the treatments and undergo a senescent phase where they neither grow nor die. Ultimately, they develop resistance to the TKI and progression occurs. The etiology of the progression has been studied in detail, and there are many similarities across different TKIs. Secondary mutations in the affected oncogene that create changes in the structure of the ATP binding pocket and decrease the affinity of TKI binding are a frequent cause of resistance [170, 171, 172, 173, 174]. The first example of this type of resistance was the T790M EGFR mutation that occurred in many patients treated with the first‐generation EGFR TKIs gefitinib and erlotinib [171, 172, 173]. A third‐generation EGFR TKI, osimertinib, is able to bind to the tumor cells with this secondary mutation and produce ORR >50% with median PFS around 10 months with superior outcomes compared with chemotherapy [171]. Patients treated with osimertinib in the second line frequently develop another resistance mutation, C797S preventing osimertinib binding [172, 173]. To date, no TKI has been approved that can bind to this C797S solvent front mutation. In a similar manner, resistance mutations occur with other oncogenes and new TKIs may bind to these resistance mutations and produce high response rates [170, 174, 175, 176]. A good example of this is the G1202R ALK resistance mutation that occurs in many patients treated with crizotinib or alectinib. Lorlatinib has excellent binding to this resistance mutation, produces high response rates in patients with G1202 ALK resistance mutations, and is approved for use in the setting [139].
Another frequent cause of resistance is the emergence of clones driven by other genetic driver alterations. For example, patients with EGFR mutations treated with osimertinib may develop mutations in BRAF, KRAS or MET, or MET amplification [175]. There are well‐described cases where treatment with the appropriate second TKI (and osimertinib) produce responses in these instances. Patients with other oncogene drivers may also progress with new genetic alterations sensitive to inhibitors of the new alteration [174, 175, 176, 177]. Histologic transformation to small cell lung cancer is another resistance mechanism [178]. Most often the transformed cells have p53 and Rb mutations and respond to the etoposide/carboplatin combination [179].
It is well known that patients with activating genetic alterations have a high propensity for brain metastases. These metastases may occur in about 25% of patients at diagnosis, with as many as another 25% developing new metastases in each subsequent year [136, 137, 138, 139, 180, 181]. Most of the first‐generation TKIs such as erlotinib and gefitinib for EGFR mutations and crizotinib for ALK fusions do not cross the blood–brain barrier in high enough concentrations to inhibit these CNS metastases. Second‐ and third‐generation TKIs such as the EGFR TKI osimertinib or the ALK TKIs alectinib, brigatinib, or lorlatinib are better able to cross the blood–brain barrier. These agents frequently treat patients with brain metastases and prevent the development of brain metastases in those without brain metastases [136, 137, 138, 139]. These newer agents are thus usually preferred to be given first owing to a combination of longer PFS and prevention of brain metastases. Many of the newer TKIs for other genetic alterations such as lorlatinib or repotrectinib for ROS1 fusions [157, 158], selpercatinib or pralsetinib for RET fusions [159, 160], entrectinib for ROS1 and NTRK fusions [161], larotrectinib for NTRK fusions [156], and capmatinib [154] or tepotinib [155] for MET alterations cross the blood–brain barrier and can be used to treat and prevent brain metastases. These TKIs with or without stereotatic brain radiation serve as an alternatives to whole brain radiotherapy in patients with driver alterations who develop brain metastases [182].
It is unlikely that single‐agent TKIs will remain the primary first‐line therapy indefinitely, as CRs are rare and resistance is inevitable. What is needed are rational combinations that can prevent or treat the inevitable senescence that accompanies early treatment with any TKI. Early combination studies focused on the addition of VEGF receptor inhibitors or combination chemotherapy to EGFR TKI therapy. The addition of bevacizumab or ramucirumab to first‐generation EGFR TKIs showed PFS benefit, but any advantage in OS benefit was less certain [183, 184]. It is also not clear that these results would be superior to those produced by osimertinib alone, so new randomized trials comparing osimertinib with the combination of osimertinib and bevacizumab or ramucirumab are ongoing. Similarly, chemotherapy added to first‐generation EGFR TKIs showed PFS and sometimes OS benefit for the combination [185, 186]. Newer studies of the combination of third‐generation agents and chemotherapy are ongoing.
Although rational combinations are urgently needed, it is not known whether the mechanisms preventing cell death from TKIs are similar or different across different genetic alterations. Thus, it is not clear whether new rational combinations will be the same or different across different driver alterations. One way to determine whether these mechanisms are the same or different is to conduct neoadjuvant trials that collect tissues at diagnosis and just prior to surgery after treatment for 6–8 weeks. RNA sequencing and other analyses of the tissue changes may allow for identification of alterations associated with senescence. Several groups are attempting to conduct such trials on patients with several different genetic alterations and several companies are conducting neoadjuvant trials with specific TKIs to answer this question [187].
Immunotherapy
The development of immunologic therapies has been a priority for many years. Early studies focused on soluble factors such as thymosin and interferons with little success [188]. More recent vaccine studies of Bacillus Calmette—Guérin and then other vaccines failed to show improved survival [188, 189]. After certain checkpoint inhibitors were shown to produce responses in experimental animals and melanomas, the Cytotoxic T‐lymphocyte antigen 4 inhibitor ipilimumab was studied in both small cell and non‐small cell lung cancers [189, 190]. Unfortunately, single‐agent ipilimumab failed to improve survival alone or combined with platinum doublet chemotherapy.
Checkpoint Inhibitors in Second‐ and/or Third‐Line Therapy
Exciting results in melanoma with programmed death receptor 1 (PD‐1) checkpoint inhibitors such as nivolumab and pembrolizumab led to trials in lung cancer that initially evaluated their effectiveness in the second‐line‐or‐later setting [193]. Various biomarkers were also evaluated for their predictive value, most often evaluation of the target PD‐L1 protein expression. These early studies of PD‐1 and PD‐L1 monoclonal antibodies showed activity leading to randomized studies in the second‐ and/or third‐line setting comparing these antibodies with chemotherapy. In these trials, pembrolizumab, nivolumab, durvalumab, and atezolizumab were all shown to be superior to chemotherapy in ORR, PFS, and OS [193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203].
Predictive Biomarkers
The early pembrolizumab trials used the anti–PD‐L1 22C3 antibody as a biomarker and reported that higher expression levels correlated with higher response rates [194]. The investigators in these studies set cutoff levels of PD‐L1 expression of 0, 1%–49%, and ≥50%, demonstrating higher response rates with higher expression levels. Long‐term follow‐up of these trials also showed that survival was related to the fraction of cells expressing the PD‐L1 protein, which was called the tumor proportion score (TPS). Patients with high TPS scores (≥50) had longer OS than patients with lower scores, and for the highest levels, 5‐year OS rates were around 30% after treatment in the first, second, or later lines of therapy [194].
These early immunotherapy studies used a variety of anti–PD‐L1 antibodies and different cutoff values, but there was a relationship between PD‐L1 expression and outcome in all of these studies. The Blueprint study compared the performance and interagreement of these antibodies [204, 205]. There was a good agreement between assayed tumor PD‐L1 expression for three of the antibodies (22C3, 28‐8, S263), although one antibody (SP142) had a lower sensitivity. In the studies using SP142, both tumor cells and inflammatory cells were scored for expression and then were grouped with high tumor (TC) or inflammatory cell (IC) scores of 0, 1, 2, or 3. Higher scores correlated with higher ORR and longer PFS and OS [198, 199, 200]. Because subsequent randomized trials first established PD‐L1 biomarker cutoffs with the 22C3 antibody, it has been the most frequently used predictive antibody and is approved for this use. However, subsequent comparative analyses demonstrated that there was good concordance between the 22C3 and SP263 assays and that both correlated with response to checkpoint inhibitors [206]. Objective responses are concentrated in, but not exclusive to, patients with high TPS, and not all patients with high TPS experience a response to immunotherapy. Thus, an ongoing effort is working to identify better predictive immune‐oncology biomarkers. Nevertheless, the clinical trials described below show the value of therapy selection based on TPS score. Routine assessment of TPS score is therefore recommended in all patients with metastatic NSCLC according to most clinical guidelines [112].
One potential biomarker for immunotherapy response lies within the tumor genome and the number of mutations it possesses. In theory, more mutations would result in a tumor expressing neoantigens that may be more readily recognized and eliminated by effector T cells in patients receiving immunotherapy. Several groups began to estimate neoantigen burden through tumor mutation burden (TMB) using whole genome or whole exome sequencing [207]. Several studies evaluating TMB using whole exome sequencing demonstrated a correlation between the number of mutations and outcome from checkpoint inhibitors. Subsequent studies using smaller numbers of genes sequenced in tissue and blood showed some but inconsistent correlations with varying cutoffs [208]. Some of these studies indicated that the markers might be more prognostic than predictive [209]. To date, the role of TMB as a biomarker for immunotherapy response has not been established. This in part may be due to the heterogeneity in platforms used to quantify TMB and the variable cutoffs used to define TMB‐low versus ‐high patients. Additionally, many of the mutations analyzed are not translated into proteins, and not all mutations result in neoantigens that ultimately activate the patient's T‐cell repertoires [210]. Ongoing studies are working to refine TMB and potential neoantigen expression, but none are ready for routine use.
Furthermore, preclinical and early translational studies suggest that immune infiltration within the tumor microenvironment and systemic immunity may also influence patient responsiveness to immunotherapy [211]. Other areas of investigation include changes in levels of circulating tumor DNA and the role of the microbiome as biomarkers for response to immunotherapy. How these potential markers compare with PD‐L1 or may be incorporated into current clinical care are unknown and remain investigational.
First‐Line Immune Therapy for Patients with High PD‐L1 (TPS ≥50%)
Single‐Agent Checkpoint Inhibitor Versus Chemotherapy
Once the value of pembrolizumab in the refractory setting was established and there was a known relationship with TPS score, two large randomized trials were conducted comparing pembrolizumab monotherapy with platinum doublet chemotherapy in patients with a high TPS score (≥50% in KEYNOTE‐024) [212] or any TPS score (≥1% in KEYNOTE‐042) [210]. Both trials included patients with NSCLC of any histology with a performance status of 0 or 1. Both trials met their primary endpoint of improved OS with pembrolizumab monotherapy. In KEYNOTE‐042, OS was superior in prespecified analyses of patients with a TPS score of ≥50% and all evaluable patients enrolled. In an unplanned analysis of patients with a TPS score of 1%–49%, no difference in overall survival was observed between the two arms, suggesting that the patients with TPS ≥50% were driving the improved survival observed in the entire study population.
A phase III trial comparing nivolumab with chemotherapy in the first line failed to show an advantage for nivolumab in patients with a PD‐L1 score >5%. Subsequent studies produced different results (see below) [214]. For nivolumab and nivolumab plus ipilimumab, the CheckMate 227 trial included patients with a PD‐L1 score of 1% or higher with a subset analysis reporting on those with a PD‐L1 score ≥50% [215]. In these high‐PD‐L1‐positive patients, both immunotherapy arms produced a survival superior to chemotherapy alone, with the nivolumab plus ipilimumab combination producing significantly longer OS than chemotherapy although the trial was not powered to compare the two immunotherapy arms.
For atezolizumab, the IMpower110 study randomized patients with any histology to receive atezolizumab alone or chemotherapy with carboplatin and nab‐paclitaxel [216]. In patients with the highest PD‐L1 expression (IC3/TC3 by SP142 assessment), atezolizumab alone produced a significant improvement in OS with a median of 20.2 months versus 13.1 months for chemotherapy (HR, 0.595).
For durvalumab, in the MYSTIC trial among 488 patients with 25% or more of tumor cells expressing PD‐L1, median OS was 16.3 months (95% confidence interval [CI], 12.2–20.8) with durvalumab versus 12.9 months (95% CI, 10.5–15.0) with chemotherapy (HR, 0.76; 97.54% CI, 0.56–1.02; p = .04 [nonsignificant]) [217].
More recently, the results of a trial adding an anti‐T Cell Immunoreceptor with Ig and ITIM Domains antibody to atezolizumab were presented showing a better ORR and PFS with the combination compared with atezolizumab alone in patients with high PD‐L1 (≥50%) [218]. These results led to an ongoing phase III trial.
In summary, patients with PD‐L1 TPS ≥50% could be treated with single‐agent pembrolizumab, single‐agent atezolizumab, nivolumab plus ipilimumab, or a combination of either pembrolizumab or atezolizumab with chemotherapy, with the most data on pembrolizumab alone.
Checkpoint Inhibitor plus Chemotherapy Versus Chemotherapy in High PD‐L1
A number of first‐line randomized trials comparing a checkpoint inhibitor plus chemotherapy with the same chemotherapy alone were conducted in patients with any PD‐L1 expression including those with the highest expression. Some trials included patients with only nonsquamous histology (KEYNOTE‐189 [219], IMpower150 [220], and IMpower130 [218]) or only squamous carcinoma (KEYNOTE‐407 [222] and IMpower131 [223]), whereas other trials included patients of any NSCLC histology (CheckMate 227 and CheckMate 9LA) [213, 224].
Two randomized phase III trials compared pembrolizumab combined with platinum doublet chemotherapy versus platinum doublet chemotherapy alone in patients with any TPS score who had nonsquamous histology (KEYNOTE‐189) or squamous histology (KEYNOTE‐407) [219, 222]. The chemotherapy doublet in KEYNOTE‐189 was pemetrexed combined with cis‐ or carboplatin, and in KEYNOTE‐407, the chemotherapy was carboplatin combined with paclitaxel or nab‐paclitaxel. In both trials, patients with a TPS score ≥50% experienced a statistically significant prolongation of both PFS and OS when treated with combined chemoimmunotherapy. In cross‐trial comparisons, the combined chemoimmunotherapy trials demonstrated a higher ORR compared with pembrolizumab monotherapy responses in KEYNOTE‐024 and 042 at the cost of higher toxicity with similar PFS and OS.
In the IMpower150 and 130 trials in nonsquamous carcinomas and in the IMpower131 trial in squamous carcinomas, the combination of atezolizumab plus chemotherapy was superior to chemotherapy alone in patients with TC3/IC3, although the hazard ratios were not as favorable as in the KEYNOTE trials. Similarly, in CheckMate 9LA, two cycles of chemotherapy plus nivolumab and ipilimumab was superior to chemotherapy alone. In CheckMate 227, nivolumab plus ipilimumab was superior to chemotherapy alone in the high PD‐L1 group.
In summary, for high PD‐L1, several immunotherapies alone are superior to a platinum doublet chemotherapy for PFS and OS with less toxicity. Although the combination of chemotherapy and immunotherapy has higher ORR than immunotherapy alone, it has more toxicity and similar PFS and OS. Thus, many clinicians prefer the chemoimmunotherapy combination for patients with rapidly progressing cancers who need a rapid response and single‐agent pembrolizumab atezolizumab or nivolumab plus ipilimumab for those with less aggressive tumors.
First‐Line Immune Therapy for Patients with Negative or Intermediate PD‐L1 (TPS <50%)
As noted above, the KEYNOTE‐189 and 407 studies compared the combination of pembrolizumab and chemotherapy with chemotherapy alone in patients with any PD‐L1 expression. The results favored the combined therapy in all TPS categories, including TPS 0. These trials established the combination of pembrolizumab with platinum doublet as the standard first‐line therapy for patients of any histology and a TPS score of 0%–49%. Other phase III randomized trials evaluated different checkpoint inhibitor antibodies and systemic therapy combinations but with less success. For atezolizumab, the IMpower150 trial randomized patients with nonsquamous carcinoma to receive chemotherapy with paclitaxel plus carboplatin and bevacizumab, the same chemotherapy plus atezolizumab and bevacizumab, or the same chemotherapy–atezolizumab therapy without bevacizumab. The best results were in the four‐drug regimen arm, although this arm was the most toxic for patients. In cross‐trial comparisons, the hazard ratios for OS were not as striking as in the KEYNOTE trials discussed above. Similarly, the IMpower130 trial, which compared atezolizumab plus nab‐paclitaxel/carboplatin with the same chemotherapy alone, showed advantage for the combination with less impressive hazard ratios for OS when compared with KEYNOTE 189. For patients with squamous carcinomas, IMpower131 compared nab‐paclitaxel/carboplatin with the same chemotherapy combined with atezolizumab or with the combination of atezolizumab with paclitaxel/carboplatin. There was no survival advantage to the addition of atezolizumab in patients with a PD‐L1 score of 0 to TC/IC 2.
The data for the use of nivolumab or the combination of nivolumab and ipilimumab with or without a chemotherapy doublet are a little more nuanced. The CheckMate 227 trial had three arms for patients of any histology with TPS <1% and three arms for patients of any histology with TPS ≥1%. In patients with TPS <1%, both the combinations of nivolumab with ipilimumab or nivoluamb with chemotherapy were superior in OS to chemotherapy alone, with the best survival in the nivolumab plus ipilimumab arm. In patients with TPS ≥1%, the combination of nivolumab plus ipilimumab and nivolumab plus chemotherapy were superior to chemotherapy alone. The Checkmate 9LA study compared four cycles of platinum doublet chemotherapy with two cycles of the same chemotherapy plus both nivolumab and ipilimumab. The combination of chemotherapy plus dual immune checkpoint blockade was superior to chemotherapy alone.
In conclusion, there are multiple options for first‐line therapy in stage IV NSCLC without targetable driver alterations. For patients with the highest levels of PD‐L1 expression, both a single‐agent checkpoint inhibitor such as pembrolizumab and combination chemoimmunotherapy produce long survival. Combinations of a checkpoint inhibitor and chemotherapy produce higher ORR but no increase in OS. Thus, the combination may be best suited to patients needing a rapid response. Patients with intermediate, low, and no PD‐L1 expression are most often treated with a combination of a checkpoint inhibitor and a platinum doublet with or without bevacizumab.
Conclusion
Within the last few years, the decades‐long efforts in clinical trials, translational research, and basic science laboratories have resulted in new therapeutic approaches that have transformed the treatment options available to patients with lung cancer and reshaped our expectations for patient outcomes. For patients with targetable oncogene drivers and patients with high levels of PD‐L1 expression, the expectation of benefit from first‐line therapy and survival is measured in years. Although these advances are unprecedented, multiple opportunities exist to improve outcomes from prevention of lung cancer to improved therapies in metastatic disease.
The fundamental tenet of prevention is finding new ways to reduce tobacco use in all forms, including vaping. Furthermore, we need to capitalize on current efforts and develop new strategies to reduce the number of patients who present with advanced or metastatic NSCLC. More work is needed on new approaches for reversing premalignant airway lesions including high‐risk nodules. For early detection, we must continue to work with patients and primary care providers to increase the fraction of patients who are screened for lung cancer according to guidelines. In conjunction with lung cancer screening, we also need to refine radiographic algorithms that better identify which small lung nodules will become malignant. The potential role for blood tests that detect circulating tumor DNA is critical in developing improved screening.
For patients with early stage, establishing surrogate markers such as pathologic response by correlating this readout with DFS and OS is necessary to more rapidly obtain approval of new therapies. We need to establish early studies of novel approaches in these patients, not just in patients with stage IV disease.
For all patients with stage IV disease, continued studies are needed to establish whether local consolidative therapy with radiation or surgery for residual disease or oligometastatic sites can improve survival in both patients with molecular drivers and those receiving immunotherapy with or without chemotherapy.
In patients with metastatic NSCLC and targetable molecular alterations, the foremost need is development of rational combinations that can produce greater log kill and thereby prevent or delay the development of resistance. Neoadjuvant studies may be the quickest and most rational way to discover and test such combinations. Additionally, investigations must establish whether adjuvant or neoadjuvant use of targeted therapy improves OS for early‐stage patients with molecular drivers.
For patients without molecular drivers, improving biomarkers to identify which patients will and will not respond to checkpoint inhibitor immunotherapy is a major unmet clinical need. For those patients who do not respond to clinically available immunotherapy, new immunotherapeutic approaches are needed as alternatives or to augment efficacy with current checkpoint inhibitors.
The marked advances in lung cancer outcomes can be attributed to consistent pursuit of science and clinical trials. If we continue these pursuits, the future is bright for patients with lung cancer.
Author Contributions
Conception/design: Erin L. Schenk, Tejas Patil, Jose Pacheco, Paul A. Bunn, Jr.
Collection and/or assembly of data: Erin L. Schenk, Tejas Patil, Jose Pacheco, Paul A. Bunn, Jr.
Data analysis and interpretation: Erin L. Schenk, Tejas Patil, Jose Pacheco, Paul A. Bunn, Jr.
Manuscript writing: Erin L. Schenk, Tejas Patil, Jose Pacheco, Paul A. Bunn, Jr.
Final approval of manuscript: Erin L. Schenk, Tejas Patil, Jose Pacheco, Paul A. Bunn, Jr.
Disclosures
Erin L. Schenk: Takeda (RF), American Lung Association, Physicians Education Resource, Takeda, Roche, AbbVie (C/A); Tejas Patil: Roche/Genentech, PRIME Oncology, Aptitude Health LLC, Guidepoint, Takeda (C/A); Jose Pacheco: Blueprint Medicines, Jazz Pharmaceuticals, AstraZeneca, Genentech, Gerson Lehrman Group, Hengrui Pharmaceuticals, Novartis, Research America, Patient Centered Outcomes Research Institute (C/A); Paul A. Bunn, Jr.: C‐Stone, Ascentage, Ipsen (SAB), Celgene, AstraZeneca, Bristol‐Myers Squibb, Eisai, Genentech/Roche, Merck, Takeda (C/A).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Kelly Connolly for editorial assistance, the nurses at the University of Colorado Cancer Center for providing outstanding patient care, and the study coordinators for assisting with our clinical trials. E.L.S is supported by K12 CA086913.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Schroeder SA, Koh HK. Tobacco control 50 years after the 1964 Surgeon General's report. JAMA 2014;311:141–143. [DOI] [PubMed] [Google Scholar]
- 3. Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General's report: Commemorating the 50th anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol 2014;179:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doll R, Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. Br Med J 1976;2:1525–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howlader N, Forjaz G, Mooradian MJ et al. The effect of advances in lung‐cancer treatment on population mortality. N Engl J Med 2020;383:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wellmann KF. Smoking and health. On the report of the Advisory Committee to the Surgeon General of the Public Health Service [in German]. Dtsch Med Wochenschr 1964;89:1085–1086. [PubMed] [Google Scholar]
- 7. National Center for Chronic Disease Prevention and Health Promotion , Office on Smoking and Health. E‐Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2016. [PubMed]
- 8. Saccomanno G, Yale C, Dixon W et al. An epidemiological analysis of the relationship between exposure to Rn progeny, smoking and bronchogenic carcinoma in the U‐mining population of the Colorado Plateau–1960‐1980. Health Phys 1986;50:605–618. [DOI] [PubMed] [Google Scholar]
- 9. Allemani C, Matsuda T, Di Carlo V et al. Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernandez‐Boussard TM, Hainaut P. A specific spectrum of p53 mutations in lung cancer from smokers: Review of mutations compiled in the IARC p53 database. Environ Health Perspect 1998;106:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudin CM, Poirier JT, Byers LA et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat Rev Cancer 2019;19:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. George J, Walter V, Peifer M et al. Integrative genomic profiling of large‐cell neuroendocrine carcinomas reveals distinct subtypes of high‐grade neuroendocrine lung tumors. Nat Commun 2018;9:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiorentino FP, Tokgün E, Solé‐Sánchez S et al. Growth suppression by MYC inhibition in small cell lung cancer cells with TP53 and RB1 inactivation. Oncotarget 2016;7:31014–31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mollaoglu G, Guthrie MR, Böhm S et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to Aurora kinase inhibition. Cancer Cell 2017;31:270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Osta B, Behera M, Kim S et al. Characteristics and outcomes of patients with metastatic KRAS‐mutant lung adenocarcinomas: The Lung Cancer Mutation Consortium experience. J Thorac Oncol 2019;14:876–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch FR, Scagliotti GV, Langer CJ et al. Epidermal growth factor family of receptors in preneoplasia and lung cancer: Perspectives for targeted therapies. Lung Cancer 2003;41(suppl 1):S29–S42. [DOI] [PubMed] [Google Scholar]
- 17. Bunn PA Jr, Helfrich B, Soriano AF et al. Expression of Her‐2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin Cancer Res 2001;7:3239–3250. [PubMed] [Google Scholar]
- 18. Cancer Genome Atlas Research Network . Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. George J, Lim JS, Jang SJ et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clinical Lung Cancer Genome Project, Network Genomic Medicine. A genomics‐based classification of human lung tumors. Sci Transl Med 2013;5:209ra153. [DOI] [PMC free article] [PubMed]
- 22. Tammemagi CM, Pinsky PF, Caporaso NE et al. Lung cancer risk prediction: Prostate, lung, colorectal and ovarian cancer screening trial models and validation. J Natl Cancer Inst 2011;103:1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Usman Ali M, Miller J, Peirson L et al. Screening for lung cancer: A systematic review and meta‐analysis. Prev Med 2016;89:301–314. [DOI] [PubMed] [Google Scholar]
- 24. Kennedy TC, Miller Y, Prindiville S. Screening for lung cancer revisited and the role of sputum cytology and fluorescence bronchoscopy in a high‐risk group. Chest 2000;117(4 suppl 1):72S–79S. [DOI] [PubMed] [Google Scholar]
- 25. Henschke CI, Yankelevitz DF, Libby DM et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–1771. [DOI] [PubMed] [Google Scholar]
- 26. Aberle DR, Adams AM, Berg CD et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Koning HJ, van der Aalst CM, de Jong PA et al. Reduced lung‐cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–513. [DOI] [PubMed] [Google Scholar]
- 28. Wood DE, Kazerooni E, Baum SL et al. Lung cancer screening, version 1.2015: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2015;13:23–34; quiz 34. [DOI] [PubMed] [Google Scholar]
- 29. Donnelly EF, Kazerooni EA, Lee E et al. ACR Appropriateness Criteria(®) Lung Cancer Screening. J Am Coll Radiol 2018;15(suppl 11):S341–S346. [DOI] [PubMed] [Google Scholar]
- 30. Mazzone PJ, Silvestri GA, Patel S et al. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report. Chest 2018;153:954–985. [DOI] [PubMed] [Google Scholar]
- 31. Moyer VA, U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330–338. [DOI] [PubMed] [Google Scholar]
- 32. Gould MK, Fletcher J, Iannettoni MD et al. Evaluation of patients with pulmonary nodules: When is it lung cancer?: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest 2007;132(suppl 3):108S–130S. [DOI] [PubMed] [Google Scholar]
- 33. van Riel SJ, Ciompi F, Jacobs C et al. Malignancy risk estimation of screen‐detected nodules at baseline CT: Comparison of the PanCan model, Lung‐RADS and NCCN guidelines. Eur Radiol 2017;27:4019–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kennedy TC, Franklin WA, Prindiville SA et al. High prevalence of occult endobronchial malignancy in high risk patients with moderate sputum atypia. Lung Cancer 2005;49:187–191. [DOI] [PubMed] [Google Scholar]
- 35. Jonsson S, Varella‐Garcia M, Miller YE et al. Chromosomal aneusomy in bronchial high‐grade lesions is associated with invasive lung cancer. Am J Respir Crit Care Med 2008;177:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keith RL, Blatchford PJ, Kittelson J et al. Oral iloprost improves endobronchial dysplasia in former smokers. Cancer Prev Res (Phila) 2011;4:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghosh M, Miller YE, Nakachi I et al. Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018;197:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chabon JJ, Hamilton EG, Kurtz DM et al. Integrating genomic features for non‐invasive early lung cancer detection. Nature 2020;580:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang ER, Schreiner AM, Pua BB. Advances in lung adenocarcinoma classification: A summary of the new international multidisciplinary classification system (IASLC/ATS/ERS). J Thorac Dis 2014;6(suppl 5):S489–S501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669–692. [DOI] [PubMed] [Google Scholar]
- 41. Travis WD, Brambilla E, Nicholson AG et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–1260. [DOI] [PubMed] [Google Scholar]
- 42. Kriegsmann K, Cremer M, Zgorzelski C et al. Agreement of CK5/6, p40, and p63 immunoreactivity in non‐small cell lung cancer. Pathology 2019;51:240–245. [DOI] [PubMed] [Google Scholar]
- 43. Yatabe Y, Dacic S, Borczuk AC et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol 2019;14:377–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004;304:1497–1500. [DOI] [PubMed] [Google Scholar]
- 45. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 46. Pao W, Miller V, Zakowski M et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sequist LV, Joshi VA, Jänne PA et al. Response to treatment and survival of patients with non‐small cell lung cancer undergoing somatic EGFR mutation testing. The Oncologist 2007;12:90–98. [DOI] [PubMed] [Google Scholar]
- 48. Lindeman NI, Cagle PT, Beasley MB et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drilon A, Wang L, Arcila ME et al. Broad, hybrid capture‐based next‐generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin Cancer Res 2015;21:3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jordan EJ, Kim HR, Arcila ME et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov 2017;7:596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goldstraw P, Chansky K, Crowley J et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 52. Cheng AM, Wood DE. VATS versus open surgery for lung cancer resection: Moving beyond the incision. J Natl Compr Canc Netw 2015;13:166–170. [DOI] [PubMed] [Google Scholar]
- 53. Chai T, Lin Y, Shen Z et al. Comparison between video‐assisted thoracoscopic lung cancer resection and robot‐assisted lung cancer resection: Protocol for a systematic review and meta‐analysis. Medicine (Baltimore) 2019;98:e14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tamura M, Matsumoto I, Tanaka Y et al. Comparison between stereotactic radiotherapy and sublobar resection for non‐small cell lung cancer. Ann Thorac Surg 2019;107:1544–1550. [DOI] [PubMed] [Google Scholar]
- 55. Stokes WA, Bronsert MR, Meguid RA et al. Post‐treatment mortality after surgery and stereotactic body radiotherapy for early‐stage non‐small‐cell lung cancer. J Clin Oncol 2018;36:642–651. [DOI] [PubMed] [Google Scholar]
- 56. NSCLC Meta‐analysis Collaborative Group . Preoperative chemotherapy for non‐small‐cell lung cancer: A systematic review and meta‐analysis of individual participant data. Lancet 2014;383:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen D, Wang H, Song X et al. Preoperative radiation may improve the outcomes of resectable IIIA/N2 non‐small‐cell lung cancer patients: A propensity score matching‐based analysis from Surveillance, Epidemiology, and End Results database. Cancer Med 2018;7:4354–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bunn PA Jr, Schenk E, Pacheco J et al. New developments in neoadjuvant therapy for lung cancer. Oncology (Williston Park) 2019;33:101–106, 109. [PubMed] [Google Scholar]
- 59. Forde PM, Chaft JE, Smith KN et al. Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cascone T, William WN, Weissferdt A et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non‐small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol 2019;37(suppl 15):8504a. [Google Scholar]
- 61. Kwiatkowski DJ, Rusch VW, Chaft JE. Neoadjuvant atezolizumab in resectable non‐small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 37(suppl 15):8503a. [Google Scholar]
- 62. Li N, Ying J, Tao X et al. Efficacy and safety of neoadjuvant PD‐1 blockade with sintilimab in resectable squamous non‐small cell lung cancer (sqNSCLC). J Clin Oncol 2019;37(suppl 15):8531a. [Google Scholar]
- 63. Bar J, Urban D, Ofek E et al. Neoadjuvant pembrolizumab (Pembro) for early stage non‐small cell lung cancer (NSCLC): Updated report of a phase I study, MK3475‐223. J Clin Oncol 2019;37(suppl 15):8534a. [Google Scholar]
- 64. Provencio‐Pulla M, Nadal‐Alforja E, Cobo M et al. Neoadjuvant chemo/immunotherapy for the treatment of stages IIIA resectable non‐small cell lung cancer (NSCLC): A phase II multicenter exploratory study—NADIM study‐SLCG. J Clin Oncol 2018;36(suppl 15):8521a. [Google Scholar]
- 65. Shu CA, Gainor JF, Awad MM et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non‐small‐cell lung cancer: An open‐label, multicentre, single‐arm, phase 2 trial. Lancet Oncol 2020;21:786–795. [DOI] [PubMed] [Google Scholar]
- 66. Zinner R, Axelrod R, Solomides CC et al. Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin /gemcitabine (G) in resectable NSCLC. J Clin Oncol 2020;38(suppl 15):9051a. [Google Scholar]
- 67. Rothschild S, Zippelius A, Savic S et al. SAKK 16/14: Anti‐PD‐L1 antibody durvalumab (MEDI4736) in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non‐small cell lung cancer (NSCLC)—A multicenter single‐arm phase II trial. J Clin Oncology 2018;36(suppl 15):TPS8584a. [DOI] [PubMed] [Google Scholar]
- 68. Remon J, Passiglia F, Ahn M‐J et al. Immune checkpoint inhibitors in thoracic malignancies: Review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol 2020;15:914–947. [DOI] [PubMed] [Google Scholar]
- 69. Ridker PM, MacFadyen JG, Thuren T et al. Effect of interleukin‐1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 70. Chabner BA, Nabel CS. Canakinumab and lung cancer: Intriguing, but is it real? The Oncologist 2018;23:637–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blumenthal GM, Bunn PA Jr, Chaft JE et al. Current status and future perspectives on neoadjuvant therapy in lung cancer. J Thorac Oncol 2018;13:1818–1831. [DOI] [PubMed] [Google Scholar]
- 72. Travis WD, Dacic S, Wistuba I et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol 2020;15:709–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Burdett S, Pignon JP, Tierney J et al. Adjuvant chemotherapy for resected early‐stage non‐small cell lung cancer. Cochrane Database Syst Rev 2015:CD011430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Arriagada R, Auperin A, Burdett S et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non‐small‐cell lung cancer: Two meta‐analyses of individual patient data. Lancet 2010;375:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wakelee HA, Dahlberg SE, Keller SM et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non‐small‐cell lung cancer (E1505): An open‐label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017;18:1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Le Péchoux C. Role of postoperative radiotherapy in resected non‐small cell lung cancer: A reassessment based on new data. The Oncologist 2011;16:672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Burdett S, Rydzewska L, Tierney J et al. Postoperative radiotherapy for non‐small cell lung cancer. Cochrane Database Syst Rev 2016;10:CD002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Le Pechoux C, Pourel N, Barlesi F et al. LBA3_PR An international randomized trial, comparing post‐operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non‐small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end‐point analysis of LungART (IFCT‐0503, UK NCRI, SAKK) NCT00410683. Ann Oncol 2020;31(suppl 4):S1178. [Google Scholar]
- 79. Pennell NA, Neal JW, Chaft JE et al. SELECT: A phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor‐mutant non‐small‐cell lung cancer. J Clin Oncol 2019;37:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yue D, Xu S, Wang Q et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation‐positive non‐small‐cell lung cancer (EVAN): A randomised, open‐label, phase 2 trial. Lancet Respir Med 2018;6:863–873. [DOI] [PubMed] [Google Scholar]
- 81. Feng S, Wang Y, Cai K et al. Randomized adjuvant chemotherapy of EGFR‐mutated non‐small cell lung cancer patients with or without icotinib consolidation therapy. PLoS One 2015;10:e0140794–e0140794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kelly K, Altorki NK, Eberhardt WEE et al. Adjuvant erlotinib versus placebo in patients with stage IB‐IIIA non‐small‐cell lung cancer (RADIANT): A randomized, double‐blind, phase III trial. J Clin Oncol 2015;33:4007–4014. [DOI] [PubMed] [Google Scholar]
- 83. Wu YL, Zhong W, Wang Q et al. CTONG1104: Adjuvant gefitinib versus chemotherapy for resected N1‐N2 NSCLC with EGFR mutation—Final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. J Clin Oncol 2020;38(suppl 15):9005a. [Google Scholar]
- 84. Wu Y‐L, Tsuboi M, He J et al. Osimertinib in resected EGFR‐mutated non–small‐cell lung cancer. N Engl J Med 2020;383:1711–1723. [DOI] [PubMed] [Google Scholar]
- 85.Osimertinib called “home run” for EGFR‐mutant NSCLC. Cancer Discov 2020;10:896. [DOI] [PubMed] [Google Scholar]
- 86. Govindan R, Mandrekar SJ, Gerber DE et al. ALCHEMIST trials: A golden opportunity to transform outcomes in early‐stage non‐small cell lung cancer. Clin Cancer Res 2015;21:5439–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Curran WJ Jr, Paulus R, Langer CJ et al. Sequential vs. concurrent chemoradiation for stage III non‐small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang X, Ding X, Kong D et al. The effect of consolidation chemotherapy after concurrent chemoradiotherapy on the survival of patients with locally advanced non‐small cell lung cancer: A meta‐analysis. Int J Clin Oncol 2017;22:229–236. [DOI] [PubMed] [Google Scholar]
- 89. Bradley JD, Paulus R, Komaki R et al. Standard‐dose versus high‐dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‐small‐cell lung cancer (RTOG 0617): A randomised, two‐by‐two factorial phase 3 study. Lancet Oncol 2015;16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chang JY, Jabbour SK, De Ruysscher D et al. Consensus statement on proton therapy in early‐stage and locally advanced non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 2016;95:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Antonia SJ, Villegas A, Daniel D et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 92. Bansal P, Rusthoven C, Boumber Y et al. The role of local ablative therapy in oligometastatic non‐small‐cell lung cancer: hype or hope. Future Oncol 2016;12:2713–2727. [DOI] [PubMed] [Google Scholar]
- 93. Tsao MN, Ven LI, Cheung P et al. Stereotactic body radiation therapy for extracranial oligometastatic non‐small‐cell lung cancer: A systematic review. Clin Lung Cancer 2020;21:95–105.e1. [DOI] [PubMed] [Google Scholar]
- 94. Bunn PA Jr. Chemotherapy for advanced non‐small‐cell lung cancer: Who, what, when, why? J Clin Oncol 2002;20(suppl 18):23S–33S. [PubMed] [Google Scholar]
- 95. Des Guetz G, Uzzan B, Nicolas P et al. Comparison of the efficacy and safety of single‐agent and doublet chemotherapy in advanced non‐small cell lung cancer in the elderly: A meta‐analysis. Crit Rev Oncol Hematol 2012;84:340–349. [DOI] [PubMed] [Google Scholar]
- 96. Luo L, Hu Q, Jiang J et al. Comparing single‐agent with doublet chemotherapy in first‐line treatment of advanced non‐small cell lung cancer with performance status 2: A meta‐analysis. Asia Pac J Clin Oncol 2015;11:253–261. [DOI] [PubMed] [Google Scholar]
- 97. Scagliotti GV, Parikh P, von Pawel J et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008;26:3543–3551. [DOI] [PubMed] [Google Scholar]
- 98. Kelly K, Crowley J, Bunn PA Jr et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small‐cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol 2001;19:3210–3218. [DOI] [PubMed] [Google Scholar]
- 99. Schiller JH, Harrington D, Belani C et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med 2002;346:92–98. [DOI] [PubMed] [Google Scholar]
- 100. Xiao HQ, Tian RH, Zhang ZH et al. Efficacy of pemetrexed plus platinum doublet chemotherapy as first‐line treatment for advanced nonsquamous non‐small‐cell‐lung cancer: A systematic review and meta‐analysis. Onco Targets Ther 2016;9:1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yang ZY, Liu L, Mao C et al. Chemotherapy with cetuximab versus chemotherapy alone for chemotherapy‐naive advanced non‐small cell lung cancer. Cochrane Database Syst Rev 2014:CD009948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pirker R, Pereira JR, Szczesna A et al. Cetuximab plus chemotherapy in patients with advanced non‐small‐cell lung cancer (FLEX): An open‐label randomised phase III trial. Lancet 2009;373:1525–1531. [DOI] [PubMed] [Google Scholar]
- 103. Paz‐Ares L, Socinski MA, Shahidi J et al. Correlation of EGFR‐expression with safety and efficacy outcomes in SQUIRE: A randomized, multicenter, open‐label, phase III study of gemcitabine‐cisplatin plus necitumumab versus gemcitabine‐cisplatin alone in the first‐line treatment of patients with stage IV squamous non‐small‐cell lung cancer. Ann Oncol 2016;27:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Thatcher N, Hirsch FR, Luft AV et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first‐line therapy in patients with stage IV squamous non‐small‐cell lung cancer (SQUIRE): An open‐label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763–774. [DOI] [PubMed] [Google Scholar]
- 105. Paz‐Ares L, Mezger J, Ciuleanu TE et al. Necitumumab plus pemetrexed and cisplatin as first‐line therapy in patients with stage IV non‐squamous non‐small‐cell lung cancer (INSPIRE): An open‐label, randomised, controlled phase 3 study. Lancet Oncol 2015;16:328–337. [DOI] [PubMed] [Google Scholar]
- 106. Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006;355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 107. Paz‐Ares LG, de Marinis F, Dediu M et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2013;31:2895–2902. [DOI] [PubMed] [Google Scholar]
- 108. Ramalingam SS, Dahlberg SE, Belani CP et al. Pemetrexed, bevacizumab, or the combination as maintenance therapy for advanced nonsquamous non‐small‐cell lung cancer: ECOG‐ACRIN 5508. J Clin Oncol 2019;37:2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gautschi O, Rothschild SI, Li Q et al. Bevacizumab plus pemetrexed versus pemetrexed alone as maintenance therapy for patients with advanced nonsquamous non‐small‐cell lung cancer: Update from the Swiss Group for Clinical Cancer Research (SAKK) 19/09 trial. Clin Lung Cancer 2017;18:303–309. [DOI] [PubMed] [Google Scholar]
- 110. Barlesi F, Scherpereel A, Rittmeyer A et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first‐line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non‐small‐cell lung cancer: AVAPERL (MO22089). J Clin Oncol 2013;31:3004–3011. [DOI] [PubMed] [Google Scholar]
- 111. Patel JD, Socinski MA, Garon EB et al. PointBreak: A randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2013;31:4349–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hanna NH, Schneider BJ, Temin S et al. Therapy for stage IV non‐small‐cell lung cancer without driver alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol 2020;38:1608–1632. [DOI] [PubMed] [Google Scholar]
- 113. Ganti AK, Hirsch FR, Wynes MW et al. Access to cancer specialist care and treatment in patients with advanced stage lung cancer. Clin Lung Cancer 2017;18:640–650.e2. [DOI] [PubMed] [Google Scholar]
- 114. Kris MG, Natale RB, Herbst RS et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non‐small cell lung cancer: A randomized trial. JAMA 2003;290:2149–2158. [DOI] [PubMed] [Google Scholar]
- 115. Hirsch FR, Varella‐Garcia M, McCoy J et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: A Southwest Oncology Group Study. J Clin Oncol 2005;23:6838–6845. [DOI] [PubMed] [Google Scholar]
- 116. Shepherd FA, Pereira JR, Ciuleanu T et al. Erlotinib in previously treated non‐small‐cell lung cancer. N Engl J Med 2005;353:123–132. [DOI] [PubMed] [Google Scholar]
- 117. Tsao MS, Sakurada A, Cutz JC et al. Erlotinib in lung cancer ‐ Molecular and clinical predictors of outcome. N Engl J Med 2005;353:133–144. [DOI] [PubMed] [Google Scholar]
- 118. Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 119. Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non–small‐cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 120. Inoue A, Kobayashi K, Maemondo M et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin‐paclitaxel for chemo‐naïve non‐small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–59. [DOI] [PubMed] [Google Scholar]
- 121. Mitsudomi T, Morita S, Yatabe Y et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–128. [DOI] [PubMed] [Google Scholar]
- 122. Yoshioka H, Shimokawa M, Seto T et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first‐line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation‐positive non‐small‐cell lung cancer. Ann Oncol 2019;30:1978–1984. [DOI] [PubMed] [Google Scholar]
- 123. Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 124. Zhou C, Wu YL, Chen G et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802). Ann Oncol 2015;26:1877–1883. [DOI] [PubMed] [Google Scholar]
- 125. Han JY, Park K, Kim SW et al. First‐SIGNAL: First‐line single‐agent iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–1128. [DOI] [PubMed] [Google Scholar]
- 126. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 127. Sequist LV, Yang JCH, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 128. Yang JC, Wu YL, Schuler M et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–151. [DOI] [PubMed] [Google Scholar]
- 129. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 130. Kris MG, Johnson BE, Berry LD et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 132. Soria JC, Tan DSW, Chiari R et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): A randomised, open‐label, phase 3 study. Lancet 2017;389:917–929. [DOI] [PubMed] [Google Scholar]
- 133. Solomon BJ, Kim DW, Wu YL et al. Final overall survival analysis from a study comparing first‐line crizotinib versus chemotherapy in ALK‐mutation‐positive non‐small‐cell lung cancer. J Clin Oncol 2018;36:2251–2258. [DOI] [PubMed] [Google Scholar]
- 134. Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 135. Ramalingam SS, Vansteenkiste J, Planchard D et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 136. Peters S, Camidge DR, Shaw AT et al. Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med 2017;377:829–838. [DOI] [PubMed] [Google Scholar]
- 137. Hida T, Nokihara H, Kondo M et al. Alectinib versus crizotinib in patients with ALK‐positive non‐small‐cell lung cancer (J‐ALEX): An open‐label, randomised phase 3 trial. Lancet 2017;390:29–39. [DOI] [PubMed] [Google Scholar]
- 138. Camidge DR, Kim HR, Ahn MJ et al. Brigatinib versus crizotinib in ALK‐positive non‐small‐cell lung cancer. N Engl J Med 2018;379:2027–2039. [DOI] [PubMed] [Google Scholar]
- 139. Solomon BJ, Besse B, Bauer TM et al. Lorlatinib in patients with ALK‐positive non‐small‐cell lung cancer: Results from a global phase 2 study. Lancet Oncol 2018;19:1654–1667. [DOI] [PubMed] [Google Scholar]
- 140. Horn L, Wu YL, Reck M et al. eXalt3: Phase III randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase positive non‐small cell lung cancer patients. Ann Oncol 2018;29(suppl 8):viii543. [Google Scholar]
- 141. Pillai RN, Behera M, Berry LD et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 2017;123:4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Villaruz LC, Socinski MA, Abberbock S et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 2015;121:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Patil T, Mushtaq R, Marsh S et al. Clinicopathologic characteristics, treatment outcomes, and acquired resistance patterns of atypical EGFR Mutations and HER2 alterations in stage IV non‐small‐cell lung cancer. Clin Lung Cancer 2020;21:e191–e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Aisner DL, Sholl LM, Berry LD et al. The Impact of smoking and TP53 mutations in lung adenocarcinoma patients with targetable mutations‐The Lung Cancer Mutation Consortium (LCMC2). Clin Cancer Res 2018;24:1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bunn PA Jr, Aisner DL. Broad‐based molecular testing for lung cancer: Precisely the time for precision. JAMA 2018;320:445–446. [DOI] [PubMed] [Google Scholar]
- 146. Schoenfeld AJ, Arbour KC, Rizvi H et al. Severe immune‐related adverse events are common with sequential PD‐L1 blockade and osimertinib. Ann Oncol 2019;30:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yeung KT, More S, Woodward B et al. Circulating tumor DNA for mutation detection and identification of mechanisms of resistance in non‐small cell lung cancer. Mol Diagn Ther 2017;21:375–384. [DOI] [PubMed] [Google Scholar]
- 148. Rolfo C, Mack PC, Scagliotti GV et al. Liquid biopsy for advanced non‐small cell lung cancer (NSCLC): A statement paper from the IASLC. J Thorac Oncol 2018;13:1248–1268. [DOI] [PubMed] [Google Scholar]
- 149. Laufer‐Geva S, Rozenblum AB, Twito T et al. The clinical impact of comprehensive genomic testing of circulating cell‐free DNA in advanced lung cancer. J Thorac Oncol 2018;13:1705–1716. [DOI] [PubMed] [Google Scholar]
- 150. Hanna N, Johnson D, Temin S et al. Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:3484–3515. [DOI] [PubMed] [Google Scholar]
- 151. Ettinger DS, Wood DE, Aggarwal C et al. NCCN guidelines insights: Non‐small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 2019;17:1464–1472. [DOI] [PubMed] [Google Scholar]
- 152. Planchard D, Kim TM, Mazieres J et al. Dabrafenib in patients with BRAF(V600E)‐positive advanced non‐small‐cell lung cancer: a single‐arm, multicentre, open‐label, phase 2 trial. Lancet Oncol 2016;17:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Planchard D, Besse B, Groen HJM et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)‐mutant metastatic non‐small cell lung cancer: An open‐label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wolf J, Seto T, Han JY et al. Capmatinib in MET exon 14‐mutated or MET‐amplified non‐small‐cell lung cancer. N Engl J Med 2020;383:944–957. [DOI] [PubMed] [Google Scholar]
- 155. Paik PK, Felip E, Veillon R et al. Tepotinib in non‐small‐cell lung cancer with MET exon 14 skipping mutations. N Engl J Med 2020;383:931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shaw AT, Ou SHI, Bang YJ et al. Crizotinib in ROS1‐rearranged non–small‐cell lung cancer. N Engl J Med 2014;371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Shaw AT, Solomon BJ, Chiari R et al. Lorlatinib in advanced ROS1‐positive non‐small‐cell lung cancer: A multicentre, open‐label, single‐arm, phase 1‐2 trial. Lancet Oncol 2019;20:1691–1701. [DOI] [PubMed] [Google Scholar]
- 158. Yun MR, Kim DH, Kim SY et al. Repotrectinib exhibits potent antitumor activity in treatment‐naïve and solvent‐front‐mutant ROS1‐rearranged non‐small cell lung cancer. Clin Cancer Res 2020;26:3287–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Drilon A, Oxnard GR, Tan DSW et al. Efficacy of selpercatinib in RET fusion‐positive non‐small‐cell lung cancer. N Engl J Med 2020;383:813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Subbiah V, Gainor JF, Rahal R et al. Precision targeted therapy with BLU‐667 for RET‐driven cancers. Cancer Discov 2018;8:836–849. [DOI] [PubMed] [Google Scholar]
- 161. Doebele RC, Drilon A, Paz‐Ares L et al. Entrectinib in patients with advanced or metastatic NTRK fusion‐positive solid tumours: Integrated analysis of three phase 1‐2 trials. Lancet Oncol 2020;21:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Drilon A. TRK inhibitors in TRK fusion‐positive cancers. Ann Oncol 2019;30(suppl 8):viii23–viii30. [DOI] [PubMed] [Google Scholar]
- 163. Canon J, Rex K, Saiki AY et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti‐tumour immunity. Nature 2019;575:217–223. [DOI] [PubMed] [Google Scholar]
- 164. Hallin J, Engstrom LD, Hargis L et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS‐mutant cancers in mouse models and patients. Cancer Discov 2020;10:54–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Skoulidis F, Heymach JV. Co‐occurring genomic alterations in non‐small‐cell lung cancer biology and therapy. Nat Rev Cancer 2019;19:495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang Y, Jiang T, Qin Z et al. HER2 exon 20 insertions in non‐small‐cell lung cancer are sensitive to the irreversible pan‐HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 2019;30:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ohashi K, Hotta K, Hirata T et al. Trastuzumab emtansine in HER2+ recurrent metastatic non‐small‐cell lung cancer: Study protocol. Clin Lung Cancer 2017;18:92–95. [DOI] [PubMed] [Google Scholar]
- 168. Smit EF, Nakagawa K, Nagasaka M et al. Trastuzumab deruxtecan (T‐DXd; DS‐8201) in patients with HER2‐mutated metastatic non‐small cell lung cancer (NSCLC): Interim results of DESTINY‐Lung01. J Clin Oncol 2020;38(suppl 15):9504a.
- 169. Drilon A, Somwar R, Mangatt BP et al. Response to ERBB3‐directed targeted therapy in NRG1‐rearranged cancers. Cancer Discov 2018;8:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Suda K, Rivard CJ, Mitsudomi T et al. Overcoming resistance to EGFR tyrosine kinase inhibitors in lung cancer, focusing on non‐T790M mechanisms. Expert Rev Anticancer Ther 2017;17:779–786. [DOI] [PubMed] [Google Scholar]
- 171. Odogwu L, Mathieu L, Goldberg KB et al. FDA benefit‐risk assessment of osimertinib for the treatment of metastatic non‐small cell lung cancer harboring epidermal growth factor receptor T790M mutation. The Oncologist 2018;23:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Jia Y, Yun CH, Park E et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant‐selective allosteric inhibitors. Nature 2016;534:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Thress KS, Paweletz CP, Felip E et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non‐small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. McCoach CE, Le AT, Gowan K et al. Resistance mechanisms to targeted therapies in ROS1(+) and ALK(+) non‐small cell lung cancer. Clin Cancer Res 2018;24:3334–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Suzawa K, Offin M, Lu D et al. Activation of KRAS mediates resistance to targeted therapy in MET exon 14‐mutant non‐small cell lung cancer. Clin Cancer Res 2019;25:1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637–658. [DOI] [PubMed] [Google Scholar]
- 177. Nakagawa T, Takeuchi S, Yamada T et al. Combined therapy with mutant‐selective EGFR inhibitor and Met kinase inhibitor for overcoming erlotinib resistance in EGFR‐mutant lung cancer. Mol Cancer Ther 2012;11:2149–2157. [DOI] [PubMed] [Google Scholar]
- 178. Oser MG, Niderst MJ, Sequist LV et al. Transformation from non‐small‐cell lung cancer to small‐cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol 2015;16:e165–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Offin M, Chan JM, Tenet M et al. Concurrent RB1 and TP53 alterations define a subset of EGFR‐mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol 2019;14:1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Martínez P, Mak RH, Oxnard GR. Targeted therapy as an alternative to whole‐brain radiotherapy in EGFR‐mutant or ALK‐positive non‐small‐cell lung cancer with brain metastases. JAMA Oncol 2017;3:1274–1275. [DOI] [PubMed] [Google Scholar]
- 181. Reungwetwattana T, Nakagawa K, Cho BC et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR‐mutated advanced non‐small‐cell lung cancer. J Clin Oncol 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 182. Patil T, Smith DE, Bunn PA et al. The incidence of brain metastases in stage IV ROS1‐rearranged non‐small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol 2018;13:1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Xu JL, Jin B, Ren ZH et al. Chemotherapy plus erlotinib versus chemotherapy alone for treating advanced non‐small cell lung cancer: A meta‐analysis. PLoS One 2015;10:e0131278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zhang S, Mao X, Wang H et al. Efficacy and safety of bevacizumab plus erlotinib versus bevacizumab or erlotinib alone in the treatment of non‐small‐cell lung cancer: A systematic review and meta‐analysis. BMJ Open 2016;6:e011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nakagawa K, Garon EB, Seto T et al. Ramucirumab plus erlotinib in patients with untreated, EGFR‐mutated, advanced non‐small‐cell lung cancer (RELAY): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2019;20:1655–1669. [DOI] [PubMed] [Google Scholar]
- 186. Noronha V, Patil VM, Joshi A et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR‐mutated lung cancer. J Clin Oncol 2020;38:124–136. [DOI] [PubMed] [Google Scholar]
- 187. McCoach CE, Bivona TG, Blakely CM et al. Neoadjuvant oncogene‐targeted therapy in early stage non‐small‐cell lung cancer as a strategy to improve clinical outcome and identify early mechanisms of resistance. Clin Lung Cancer 2016;17:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Bradley JD, Scott CB, Paris KJ et al. A phase III comparison of radiation therapy with or without recombinant beta‐interferon for poor‐risk patients with locally advanced non‐small‐cell lung cancer (RTOG 93‐04). Int J Radiat Oncol Biol Phys 2002;52:1173–1179. [DOI] [PubMed] [Google Scholar]
- 189. O'Mahony D, Kummar S, Gutierrez ME. Non‐small‐cell lung cancer vaccine therapy: A concise review. J Clin Oncol 2005;23:9022–9028. [DOI] [PubMed] [Google Scholar]
- 190. Zhu J, Li R, Tiselius E et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non‐small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev 2017;12:CD011300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Govindan R, Szczesna A, Ahn MJ et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non‐small‐cell lung cancer. J Clin Oncol 2017;35:3449–3457. [DOI] [PubMed] [Google Scholar]
- 192. Reck M, Luft A, Szczesna A et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive‐stage small‐cell lung cancer. J Clin Oncol 2016;34:3740–3748. [DOI] [PubMed] [Google Scholar]
- 193. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 194. Garon EB, Hellmann MD, Rizvi NA et al. Five‐year overall survival for patients with advanced non–small‐cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE‐001 study. J Clin Oncol 2019;37:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Xu Z, Yi F, Yu D et al. Nivolumab provides improved effectiveness and safety compared with docetaxel as a second‐line treatment for advanced non‐small cell lung cancer: A systematic review and meta‐analysis. Cancer Med 2019;8:629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Vokes EE, Ready N, Felip E et al. Nivolumab versus docetaxel in previously treated advanced non‐small‐cell lung cancer (CheckMate 017 and CheckMate 057): 3‐year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959–965. [DOI] [PubMed] [Google Scholar]
- 197. Kazandjian D, Suzman DL, Blumenthal G et al. FDA approval summary: Nivolumab for the treatment of metastatic non‐small cell lung cancer with progression on or after platinum‐based chemotherapy. The Oncologist 2016;21:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Fehrenbacher L, Spira A, Ballinger M et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 201. Spigel DR, Chaft JE, Gettinger S et al. FIR: Efficacy, safety, and biomarker analysis of a phase II open‐label study of atezolizumab in PD‐L1‐selected patients with NSCLC. J Thorac Oncol 2018;13:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Garassino MC, Cho BC, Kim JH et al. Durvalumab as third‐line or later treatment for advanced non‐small‐cell lung cancer (ATLANTIC): An open‐label, single‐arm, phase 2 study. Lancet Oncol 2018;19:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Planchard D, Reinmuth N, Orlov S et al. ARCTIC: Durvalumab with or without tremelimumab as third‐line or later treatment of metastatic non‐small‐cell lung cancer. Ann Oncol 2020;31:609–618. [DOI] [PubMed] [Google Scholar]
- 204. Hirsch FR, McElhinny A, Stanforth D et al. PD‐L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the Blueprint PD‐L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208–222. [DOI] [PubMed] [Google Scholar]
- 205. Tsao MS, Kerr KM, Kockx M et al. PD‐L1 immunohistochemistry comparability study in real‐life clinical samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Koomen BM, Badrising SK, van den Heuvel MM et al. Comparability of PD‐L1 immunohistochemistry assays for non‐small‐cell lung cancer: A systematic review. Histopathology 2020;76:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gandara DR, Paul SM, Kowanetz M et al. Blood‐based tumor mutational burden as a predictor of clinical benefit in non‐small‐cell lung cancer patients treated with atezolizumab. Nat Med 2018;24:1441–1448. [DOI] [PubMed] [Google Scholar]
- 209. Devarakonda S, Rotolo F, Tsao MS et al. Tumor mutation burden as a biomarker in resected non‐small‐cell lung cancer. J Clin Oncol 2018;36:2995–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Shim JH, Kim HS, Cha H et al. HLA‐corrected tumor mutation burden and homologous recombination deficiency for the prediction of response to PD‐(L)1 blockade in advanced non‐small‐cell lung cancer patients. Ann Oncol 2020;31:902–911. [DOI] [PubMed] [Google Scholar]
- 211. Nixon AB, Schalper KA, Jacobs I et al. Peripheral immune‐based biomarkers in cancer immunotherapy: Can we realize their predictive potential? J Immunother Cancer 2019;7:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Reck M, Rodriguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 213. Mok TSK, Wu YL, Kudaba I et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): A randomised, open‐label, controlled, phase 3 trial. Lancet 2019;393:1819–1830. [DOI] [PubMed] [Google Scholar]
- 214. Carbone DP, Reck M, Paz‐Ares L et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017;376:2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Hellmann MD, Paz‐Ares L, Caro RB et al. Nivolumab plus ipilimumab in advanced non‐small‐cell lung cancer. N Engl J Med 2019;381:2020–2031. [DOI] [PubMed] [Google Scholar]
- 216. Jassem J, et al. IMpower110: Phase III trial of 1L atezolizumab in PD‐L1–selected chemotherapy‐naive NSCLC. Ann Oncol 2017;28:ii50.
- 217. Rizvi NA, Cho BC, Reinmuth N et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first‐line treatment of metastatic non‐small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020;6:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Rodriguez‐Abreu D, Johnson ML, Hussein MA et al. Primary analysis of a randomized, double‐blind, phase II study of the anti‐TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first‐line (1L) treatment in patients with PD‐L1‐selected NSCLC (CITYSCAPE). J Clin Oncol 2020;38(suppl 15):9503a.
- 219. Gandhi L, Rodriguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 220. Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 221. West H, McCleod M, Hussein M et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2019;20:924–937. [DOI] [PubMed] [Google Scholar]
- 222. Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 223. Jotte R, Cappuzzo F, Vynnychenko I et al. Atezolizumab in combination with carboplatin and nab‐paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J Thorac Oncol 2020;15:1351–1360. [DOI] [PubMed] [Google Scholar]
- 224. Reck M, Ciuleanu TE, Dols MC et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum‐doublet chemotherapy (chemo) vs 4 cycles chemo as first‐line (1L) treatment (tx) for stage IV/recurrent non‐small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 2020;38(suppl 15):9501a. [Google Scholar]


