Abstract
Background
The management of severe adverse events (AEs) is important in safely and effectively providing chemotherapy to older adults with diffuse large B‐cell lymphoma (DLBCL). However, reports on simple and DLBCL‐specific predictive models for treatment‐related toxicity in elderly individuals are scarce. The aim of this study was to examine the usefulness of Geriatric 8 (G8) in predicting treatment‐related severe AEs, nonhematological toxicity, and febrile neutropenia in older adults with DLBCL in real‐world practice.
Materials and Methods
We conducted a multicenter, retrospective study on 398 consecutive patients with DLBCL (aged ≥65 years) who received standard therapy at three centers in Japan (University of Fukui Hospital, the Fukui Prefectural Hospital, and the Japanese Red Cross Fukui Hospital), between 2007 and 2017.
Result
Multivariate logistic analysis demonstrated that the G8 score was an independent predictive factor for severe AEs. Moreover, a logistic regression model with restricted cubic spline showed a nonlinear association between the incidence of severe AEs and the G8 score. According to receiver operating characteristic analysis, the most discriminative cutoff value of the G8 for the incidence of severe AEs was 11, with an area under the curve value of 0.670. AEs occurred most often in the first course of chemotherapy and decreased as the course progressed.
Conclusion
The G8 score, an easy‐to‐use geriatric assessment tool, can be a useful prediction model of treatment‐related severe AEs during standard therapy in older adults with DLBCL.
Implications for Practice
In older patients with diffuse large B‐cell lymphoma (DLBCL), to accurately predict the risk of severe adverse events (AEs) in advance is essential for safe and effective treatment. This study demonstrated that the Geriatric 8 score, a simple and established geriatric assessment tool, indicated a high predictive ability for occurrence of therapy‐related severe AEs in elderly patients with DLBCL who were treated with standard treatment.
Keywords: Geriatric 8, Prediction, Severe adverse events, Elderly, Diffuse large B‐cell lymphoma
Short abstract
To achieve a relative dose intensity of multidrug chemotherapy sufficient to cure diffuse large B‐cell lymphoma, an accurate prediction model for adverse events is essential. This article examines whether the Geriatric 8 (G8) score could be a predictor of severe adverse events, nonhematological toxicity, and febrile neutropenia in 398 patients aged 65 years and older.
Introduction
Diffuse large B‐cell lymphoma (DLBCL) is the most common type of non‐Hodgkin lymphoma, accounting for approximately 25% of all cases of non‐Hodgkin lymphoma [1]. Because the elderly population has been increasing worldwide, the use of chemotherapy treatment has been growing as well [2]. With a sufficient relative dose intensity (RDI) of multidrug chemotherapy, DLBCL has been curable even in the elderly population [3]. In order to achieve a high RDI to cure DLBCL, an accurate prediction model for adverse events (AEs) is essential [4]. Moreover, in real‐world practice, it is important for meticulous management of DLBCL to predict AEs not only during the entire treatment period but also during each course of chemotherapy. Because of the paucity of detailed evidence of AEs focused on each course of chemotherapy, the development of a therapy‐related toxicity prediction model in DLBCL applicable to every course is necessary [5, 6, 7].
DLBCL is a unique disease with clinical features different from those of common solid cancer. Thus, the toxicity prediction models mainly used in solid cancers, such as the Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score and the Cancer and Aging Research Group (CARG) toxicity score, cannot be completely applicable to DLBCL [8, 9, 10]. Moreover, the fact that the majority of eligible patients in the studies for both the CRASH and the CARG toxicity scores were those with solid cancers (not lymphoma) is also a reason for the unsuitability of these prediction models for DLBCL [8, 9, 10]. Comprehensive geriatric assessment (CGA) might potentially predict severe AEs in older adults with DLBCL [4]; however, CGA is time consuming [11, 12]. The Geriatric 8 (G8) score is a simple geriatric screening tool that can be used to assess patient frailty and only takes a few minutes; thus, it may be useful in a busy clinical practice [13]. An abnormal G8 is associated not only with poor prognosis in patients with malignancies, including hematological malignancy [14, 15, 16, 17], but also with a higher incidence of severe chemotherapy toxicity in older patients with solid cancers [18]. However, little has been reported on the utility of G8 for the prediction of severe AEs in standard therapy of older adults with DLBCL [4, 19, 20, 21].
The aims of this study were to examine whether the G8 score could be a predictor of severe AEs, nonhematological toxicity, and febrile neutropenia (FN) in patients with DLBCL aged 65 years and older. We also assessed the detailed AEs and the utility of the G8 score for toxicity prediction for each treatment course as well as for the entire treatment period.
Materials and Methods
Patient Selection
This retrospective and multicenter cohort study was conducted at three tertiary institutions in Japan: the University of Fukui Hospital, the Fukui Prefectural Hospital, and the Japanese Red Cross Fukui Hospital. Clinical features and treatment records of patients aged ≥65 years who were newly diagnosed with DLBCL between 2007 and 2017 were electronically collected. Histological diagnoses were performed according to the World Health Organization classification [22, 23]. Patients treated with the standard regimen as first‐line therapy were enrolled in this study. The standard regimen was defined as (R‐) CHOP regimen (rituximab 375 mg/m2, cyclophosphamide [CPA] 750 mg/m2 intravenously on day 1, adriamycin [ADR] 50 mg/m2 intravenously on day 1, vincristine [VCR] 1.4 mg/m2 [maximum 2 mg/body] intravenously on day 1, and prednisolone 100 mg/body orally or intravenously on days 1–5; every three weeks), (R‐) THP‐COP regimen (the same as CHOP including the doses, except tetrahydropyranyl adriamycin [THP] 50 mg/m2 intravenously on day 1 replaced ADR) [24, 25], and radiation therapy after three courses of standard therapy for limited stage DLBCL. The exclusion criteria were transformed DLBCL, methotrexate‐associated lymphoproliferative disorders, central nervous system involvement, or undergoing treatment besides CHOP/THP‐COP therapy. Patients with human immunodeficiency virus infection were also excluded. The present study was conducted in accordance with the principles of the Declaration of Helsinki, and the protocols were approved by the respective institutional review boards. The requirement for written informed consent was waived because this study used retrospective data obtained from hospital records.
Clinical Information
In addition to patient characteristics and baseline parameters, the intensity of treatment and the occurrence of severe AEs were also assessed. Specifically, the Eastern Cooperative Oncology Group (ECOG) performance status (PS), the number of extranodal sites, Ann Arbor stage, elevated lactate dehydrogenase, serum albumin (Alb) [26, 27], International Prognostic Index (IPI), bulky mass (>7.5 cm), B symptoms, and comorbidities at diagnosis were extracted from the medical records. Frailty and comorbidities before treatment were evaluated by the G8 score [13, 14, 15, 16] and the Charlson Comorbidity Index (CCI) [28, 29], respectively. The G8 scores were obtained on the basis of information from the patients’ medical records including nursing records, pharmacists’ records, and records of nutritionists and physical therapists [30, 31, 32, 33]. AEs were assessed using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. In this study, the total NCI CTCAE score was dichotomized into categories: “low” (0–2) and “severe” (3–5) toxicity [9]. The severe AEs in the present cohort were defined as FN or nonhematological toxicity (grade ≥3).
Calculation of RDI
The RDI is the ratio of actual dose intensity (DI) and planned DI and expressed as a percentage.
The DI is the index of a planned dose per specific period.
The average relative dose intensity (ARDI) was defined as the average delivered RDI of each chemotherapeutic agent (ADR or THP, CPA, and VCR) in each cycle. The total average relative dose intensity (tARDI) was also defined as the average delivered ARDI of each cycle in the total treatment duration. The maximum value of tARDI 100% refers to six cycles of standard regimens without any reduction or delay. If treatment is interrupted due to disease progression, AEs, or death within six courses, it is calculated as 100% up to that course.
Outcome Measures
The primary outcome was whether the pretreatment G8 score could predict the occurrence of severe AEs associated with chemotherapy. When evaluating the AEs through the whole treatment period, events were defined as severe AEs at least once during this time, which is equal to the number of patients who experienced severe AEs in the entire treatment period. We also counted severe AEs in each course. When a patient experienced more than one severe AE in same treatment course, the first event was counted. When a patient experienced severe AEs over multiple treatment courses, the events in those courses were counted separately. As for irreversible AEs, such as peripheral neuropathy, they were counted as an event only in the first course that they appeared.
Statistical Analysis
Continuous variables were expressed as median values and range; these were compared using the Mann‐Whitney U test. Categorical variables were expressed as numbers and percentages; these were compared using the chi‐square test or Fisher's exact test, as appropriate. A multivariate logistic regression analysis was performed to identify factors related to severe AEs. Factors examined included sex, IPI score, serum albumin, bulky mass, total ARDI, G8 score and CCI score, based on previous knowledge [3, 26, 29, 34]. The model construction and variable selections were based on the published DLBCL risk algorithm (e.g., the IPI), substantive knowledge about DLBCL prognosis to guide variable selection. Covariates determined a priori after our review of the literature and organized focus group meetings with our research staff included sex, the IPI, serum Alb, bulky mass, total ARDI, the G8, and the CCI in order to avoid consequences of overfitting [35]. Nonlinear regression with restricted cubic spline (RCS) of four knots was used to assess the presence of a nonlinear relationship between the G8 score and the frequency of severe AEs. The performance of the G8 screening tool to predict AEs was evaluated using receiver operating characteristic (ROC) analysis, and the cutoff value was determined using the Youden's index. To assess the impact of the patient's self‐perception of health item on the G8 score, we conducted a sensitivity analysis using the modified G8 score, which excludes the patient's self‐perception of health items from the G8 score, and E‐value, which is an indicator related to the robustness of associations to potential unmeasured or residual confounding factors [36, 37]. Two‐sided values of p < .05 were considered significant. Statistical analyses were performed with R (version 3.4.1, The R Foundation for Statistical Computing, Vienna, Austria) or EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan, version 1.37), which is a graphical user interface for R [38].
Results
Patient Characteristics
In three participating institutions, 499 patients aged ≥65 years were initially diagnosed with DLBCL from 2007 to 2017. A total of 101 patients were excluded owing to the exclusion criteria, and the remaining 398 patients were enrolled in the present cohort study (Fig. 1). A total of 298 patients (74.9%) received (R‐) CHOP, 96 patients (24.1%) received (R‐) THP‐COP, and 4 patients (1.0%) received CHOP and switched to THP‐COP midway through treatment. Nine patients (2.3%) received three courses of standard regimens followed by involved field radiotherapy for limited stage disease.
Figure 1.
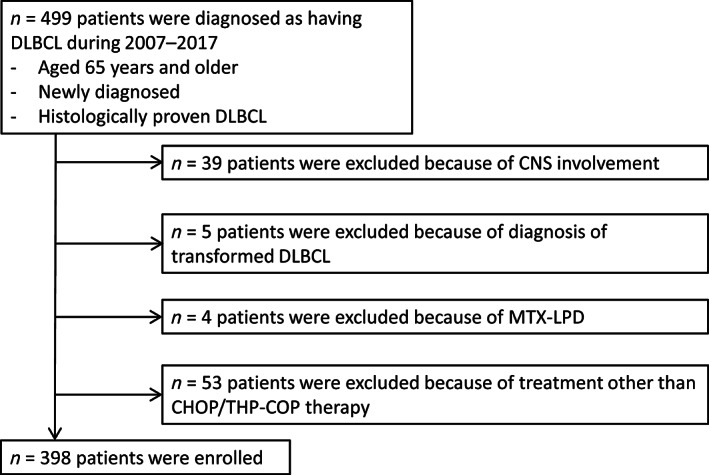
Flow chart of patient selection.Abbreviations: CHOP, cyclophosphamide, adriamycin, vincristine and prednisolone; CNS, central nervous system; DLBCL, diffuse large B‐cell lymphoma; MTX‐LPD, methotrexate‐associated lymphoproliferative disorders; THP‐COP, cyclophosphamide, tetrahydropyranyl adriamycin, vincristine, and prednisolone.
Table 1 shows the baseline characteristics at the diagnosis of DLBCL. A total of 241 patients (54.8%) had severe AEs. Patients who experienced severe AEs during the whole treatment period had significantly worse IPI, CCI, and G8 scores than patients who did not experience severe AEs. There was no significant difference in tARDI between the two groups separated by the presence or absence of severe AEs. The number of patients who received prophylaxis with granulocyte colony‐stimulating factor (G‐CSF), prophylactic oral antibiotics, and both prophylactic G‐CSF and oral antibiotics were 340 (85.4%), 56 (14.1%), and 342 (85.9%), respectively. The median percentage of patients hospitalized among all treatment periods was 60.4% (range 0%–100%).
Table 1.
Patient characteristics at diagnosis
| Characteristic | All patients (n = 398), n (%) | With severe AEs (n = 241), n (%) | Without severe AEs (n = 157), n (%) | p value |
|---|---|---|---|---|
| Age, median (range), years | 77 (65–96) | 77 (65–96) | 76 (65–93) | .548 |
| Male sex | 194 (48.7) | 119 (49.4) | 75 (47.8) | .759 |
| ECOG PS ≥2 | 133 (33.4) | 105 (43.6) | 28 (17.8) | <.001 |
| Extranodal sites ≥2 | 142 (35.7) | 104 (43.2) | 38 (24.2) | <.001 |
| Ann Arbor stage III/IV | 272 (68.3) | 180 (74.7) | 92 (58.6) | <.001 |
| Elevated LDH (>ULN) | 265 (66.6) | 167 (69.3) | 98 (62.4) | .159 |
| Serum albumin, median (range), g/dL | 3.4 (0.6–5.1) | 3.3 (0.6–5.1) | 3.7 (1.8–4.7) | <.001 |
| IPI | ||||
| Low (0, 1) | 69 (17.3) | 31 (12.9) | 38 (24.2) | |
| Low intermediate (2) | 70 (17.6) | 34 (14.1) | 36 (23.0) | <.001 |
| High intermediate (3) | 88 (21.1) | 49 (20.3) | 39 (24.8) | |
| High (4, 5) | 171 (43.0) | 127 (52.7) | 44 (28.0) | |
| Bulky mass | 82 (20.6) | 63 (26.1) | 19 (12.1) | <.001 |
| B symptoms | 141 (35.4) | 103 (42.7) | 38 (24.2) | <.001 |
| CCI | ||||
| 0 | 137 (34.4) | 75 (31.1) | 62 (39.5) | |
| 1, 2 | 171 (43.0) | 100 (41.5) | 71 (45.2) | .034 |
| 3, 4 | 68 (17.1) | 49 (20.3) | 19 (12.1) | |
| ≥5 | 22 (5.5) | 17 (7.1) | 5 (3.2) | |
| G8 score, median (range) | 11 (2–17) | 10 (2–17) | 12 (3–17) | <.001 |
| Total ARDI, median (range), % | 81.9 (6.6–140.1) | 80.8 (6.6–140.1) | 82.3 (11.4–135.2) | .568 |
| Prephase prednisolone | 93 (23.4) | 67 (27.8) | 26 (16.5) | .015 |
| Prophylactic oral antibiotics | 56 (14.1) | 39 (16.2) | 17 (10.8) | .184 |
| Prophylactic G‐CSF | 340 (85.4) | 221 (91.7) | 119 (75.8) | <.001 |
Abbreviations: AE, adverse event; ARDI, average relative dose intensity; CCI, Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group performance status; G8, Geriatric 8; G‐CSF, granulocyte colony‐stimulating factor; IPI, International Prognostic Index; LDH, lactate dehydrogenase; ULN, upper limit of normal.
The details of severe AEs that occurred in each course were investigated and are shown in Table 2. The most frequent severe AE during all courses was FN. In fact, about half of the patients developed FN in the first course of the standard regimen. As far as nonhematological toxicity was concerned, anorexia and infection were common, but those percentages decreased in later courses.
Table 2.
Occurrence of severe nonhematological toxicity (grade ≥3) and febrile neutropenia
| Adverse event | All duration (n = 398), n (%) | Course 1 (n = 398), n (%) | Course 2 (n = 384), n (%) | Course 3 (n = 360), n (%) | Course 4 (n = 303), n (%) | Course 5 (n = 284), n (%) | Course 6 (n = 268), n (%) | Course 7 (n = 129), n (%) | Course 8 (n = 120), n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Any nonhematological toxicity | 76 (19.1) | 28 (7.3) | 29 (8.1) | 16 (5.3) | 15 (5.3) | 7 (2.6) | 4 (3.1) | 4 (3.3) | |
| Anorexia | 28 (7.0) | 17 (4.3) | 4 (1.0) | 3 (0.8) | 3 (1.0) | 1 (0.4) | |||
| Lung infection | 26 (6.5) | 6 (1.5) | 6 (1.6) | 6 (1.7) | 2 (0.7) | 6 (2.1) | 1 (0.8) | 2 (1.7) | |
| Sepsis | 24 (6.0) | 12 (3.0) | 2 (0.5) | 3 (0.8) | 3 (1.0) | 2 (0.7) | 1 (0.4) | 1 (0.8) | |
| Tumor lysis syndrome | 9 (2.3) | 9 (2.3) | |||||||
| Heart failure | 8 (2.0) | 4 (1.0) | 3 (0.8) | 1 (0.3) | |||||
| Skin infection | 6 (1.5) | 4 (1.0) | 1 (0.3) | 1 (0.4) | |||||
| Urinary tract infection | 6 (1.5) | 2 (0.5) | 1 (0.3) | 1 (0.3) | 1 (0.4) | 1 (0.8) | |||
| Hyperglycemia | 6 (1.5) | 4 (1.0) | 1 (0.3) | 1 (0.4) | |||||
| Delirium | 6 (1.5) | 2 (0.5) | 1 (0.3) | 1 (0.3) | 1 (0.4) | 1 (0.4) | |||
| Malaise | 6 (1.5) | 1 (0.3) | 3 (0.8) | 2 (0.6) | |||||
| Febrile neutropenia | 183 (46.0) | 108 (27.1) | 48 (12.5) | 36 (10.0) | 43 (14.2) | 42 (14.8) | 30 (11.2) | 15 (11.6) | 20 (16.7) |
| Treatment‐related mortality | 5 (1.3) | 4 (1.1) | 1 (0.4) |
Analyses of Predictors of Severe AEs Using Logistic Model and ROC Curve
A multivariate logistic regression analysis for clinical factors associated with the occurrence of severe AEs was performed (Table 3). Independent predictive factors of severe AEs were the G8 score (p = .015) and bulky mass (p = .023). Moreover, supplemental online Tables 1 and 2 showed that the G8 score also had significant predictive impact on the occurrence of FN and nonhematological toxicities, respectively (p for FN = .008, p for nonhematological toxicities = .041).
Table 3.
Multivariate logistic regression analysis for clinical factors associated with the occurrence of severe adverse events
| Factor | IPI | IPI factors | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Male sex | 1.260 (0.807–1.950) | .314 | 1.190 (0.756–1.870) | .455 |
| IPI score | 1.170 (0.951–1.450) | .136 | ||
| Age | 0.975 (0.937–1.010) | .198 | ||
| ECOG PS ≥2 | 1.840 (0.999–3.390) | .050 | ||
| LDH >ULN | 0.669 (0.391–1.140) | .142 | ||
| Stage ≥3 | 1.010 (0.573–1.770) | .983 | ||
| Extranodal sites ≥2 | 1.860 (1.090–3.170) | .023 | ||
| Serum albumin (g/dL) | 0.737 (0.511–1.060) | .101 | 0.736 (0.507–1.070) | .106 |
| Bulky mass | 2.010 (1.100–3.680) | .023 | 1.950 (1.050–3.620) | .035 |
| Total ARDI | 1.000 (0.995–1.010) | .474 | 1.000 (0.995–1.010) | .514 |
| G8 score (/point) | 0.900 (0.827–0.979) | .015 | 0.887 (0.808–0.975) | .013 |
| CCI score (/point) | 1.140 (0.979–1.320) | .091 | 1.120 (0.961–1.300) | .147 |
Abbreviations: ARDI, average relative dose intensity; CCI, Charlson Comorbidity Index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; G8, Geriatric 8; IPI, International Prognostic Index; LDH, lactate dehydrogenase; OR, odds ratio; ULN, upper limit of normal.
The association between the frequency of severe AEs and the G8 score was also examined in the logistic regression model with RCS. As shown in Figure 2, the association between the risk of incidence of severe AEs and the G8 score was not linear, but an almost inverted S‐shaped relationship. In those with a G8 score < 8, the log odds of the risk of severe AEs are obviously higher than 0 but plateau. Between G8 scores 8 and 11, the curve of the risk of severe AEs drops rapidly as the G8 score increases. For G8 scores ≥11, the y‐axis that indicates the log odds of the risk for severe AEs gradually falls below 0, and the curve of the risk of severe AEs approaches a plateau as the G8 score increases. Moreover, according to the ROC curve as shown in Figure 3, the most discriminative cutoff value of the G8 for the incidence of severe AEs was 11 (sensitivity 62.4%, specificity 66.4%), with an area under the curve (AUC) value of 0.670 (95% confidence interval, 0.616–0.724).
Figure 2.
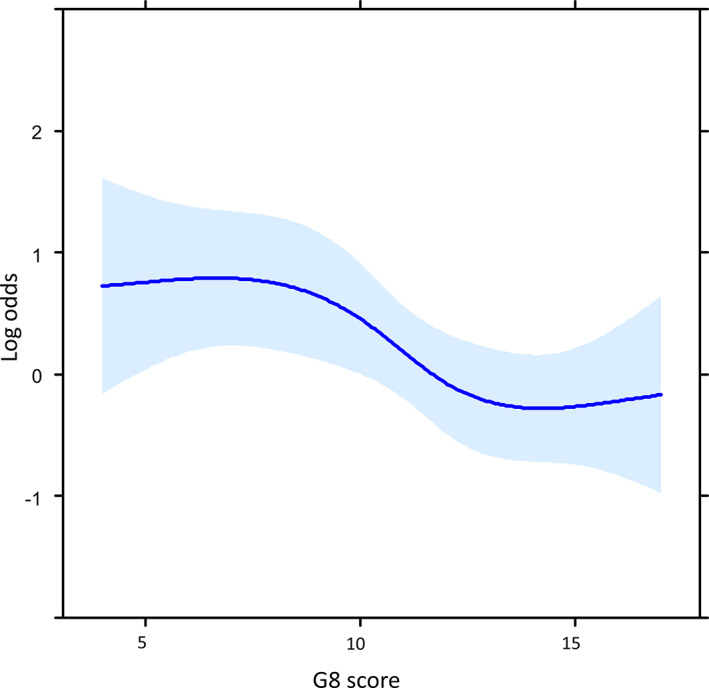
Associations between the incidence of severe adverse events and G8 score (n = 398) using a model with restricted cubic spline with four knots. The solid line represents the log hazard ratio, and the shaded area is the 95% confidence interval.Abbreviation: G8, Geriatric 8.
Figure 3.
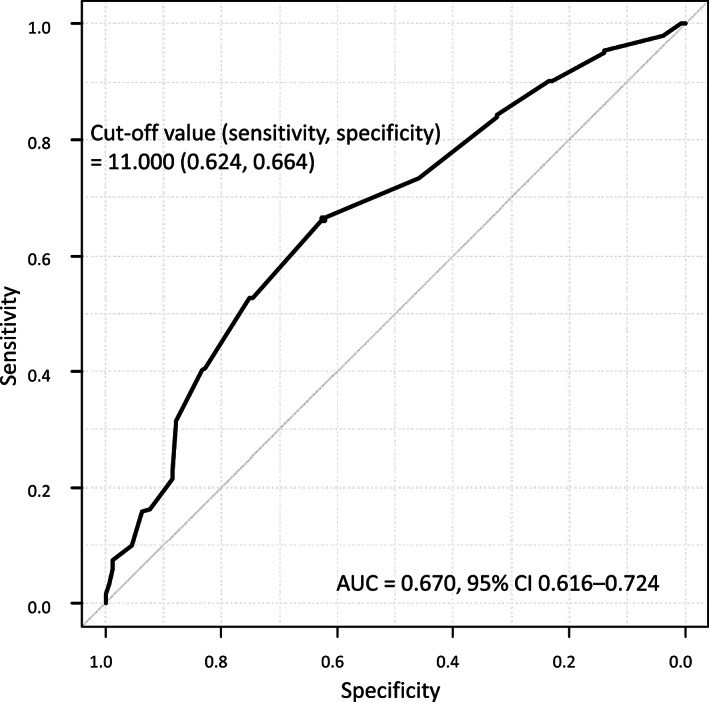
Receiver operating characteristic curve showing the performance of the Geriatric 8 score for predicting the incidence of severe adverse events in 398 patients.Abbreviations: AE, adverse event; AUC, area under the curve; CI, confidence interval.
Evaluation of Severe AEs in Each Course Depending on the G8 Score
Based on the cutoff value of the G8 score from the ROC analysis, the frequency of severe AEs in each chemotherapy course was evaluated and is shown as a bar graph in Figure 4. The frequency of severe AEs was highest in the first course of chemotherapy in both groups divided by the G8 score, and its incidence was more than 50% in patients with a G8 score ≤ 11. The frequency of severe AEs decreased in later courses. Moreover, the frequency of severe AEs was apparently higher in the G8 score ≤ 11 group than in the G8 score > 11 group in all courses.
Figure 4.
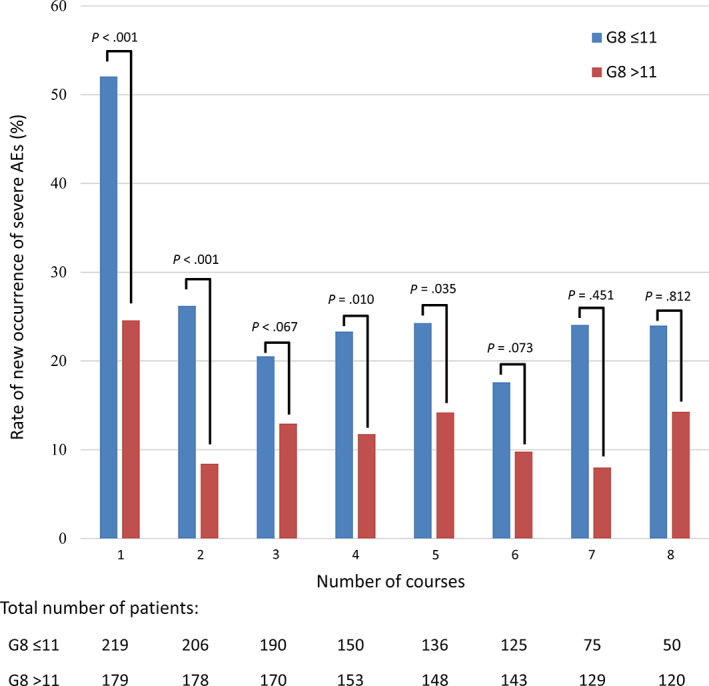
Bar graph showing the rate of new occurrences of severe AEs in each course according to the G8 score. The blue bar represents the rate of new occurrence of severe AEs in the G8 ≤ 11 group, and the red bar represents the rate of new occurrence of severe AEs in the G8 > 11 group.Abbreviations: AE, adverse event; G8, Geriatric 8.
Sensitivity Analyses for the Predictive Accuracy of the G8 Score for Severe AEs
The sensitivity analyses using modified G8 score, which was the G8 screening without item of the patient's self‐perception of health, showed the consistency of the predictive ability of the G8 score for severe AEs (supplemental online Fig. 1 and supplemental online Table 3). The E‐value of modified G8 score was 1.28.
Discussion
The present study showed that the G8 score was a significant predictor of severe AEs, nonhematological toxicities, and FN in older adults with DLBCL. A logistic regression model with RCS confirmed apparent nonlinear association between the G8 score and the risk of severe AEs. The frequency of severe AEs was the highest in the first chemotherapy course through the entire treatment period and decreased gradually as the course of chemotherapy progressed. The patients with low G8 scores (especially ≤11) had a higher incidence of severe AEs during the entire treatment period. In addition, the frequency of severe AEs in patients who had low G8 scores were apparently higher in each course.
Firstly, we found that the G8 score can predict the risk of severe AEs in older adults undergoing standard therapy for DLBCL. Currently, using the several measures such as the G8 score for the routine measurement of frailty in hematological practice has been recommended [21]. The G8 score has the highest sensitivity in prognostic prediction among simple geriatric assessment tools indicating frailty and relates to survival in various malignancies [15, 39]. A major advantage of the G8 score is that it is an easier‐to‐use and less time‐consuming geriatric assessment tool than CGA, the validated DLBCL‐specific prediction model for AEs [4]. We previously reported that keeping the highest possible RDI in standard regimens of DLBCL can provide a better prognosis, even in the very elderly population [3]. Of note, RDI did not increase the risk of severe AEs in our previous cohort [3]. Besides, a previous study also showed that the risk for treatment‐related mortality is associated with poor PS in older adults with DLBCL treated using multidrug chemotherapy [40].
The CRASH score and the CARG toxicity score are, indeed, reasonable predictive models for chemotherapy‐related toxicity mainly in older adults with solid cancer, but we cannot adopt these models directly for DLBCL [8, 9, 10]. Only 78 patients with lymphoma (15.1% of the entire study population) were included in a previously reported study population focusing the CRASH score [8]. Owing to its significantly higher chemosensitivity, lymphoma (especially DLBCL) has clinical features that are very different from those of solid cancer. It is not possible to directly compare DLBCL with common solid cancers because the former is curable by chemotherapy with a high RDI. The overall sensitivity and specificity for prediction of severe AEs were relatively moderate of the G8 score with AUC of 0.670 in the present cohort. This result is probably due to a small percentage of the items related to motor function in the G8 score. Recently, the association between the grip strength and gait speed (measured, e.g., by the 4‐meter walk test) and survival outcome in hematological malignancies has been reported [41]. The moderate prediction accuracy in ROC curve in the present study might be improved by adding the G8 score to another prediction model using assessment of motor function.
In the present cohort, we showed not only the utility of the G8 score in predicting severe AEs in older adults with DLBCL but also the visual relationship between the G8 score and the risk of AEs by a logistic regression model with RCS. In this study, the relationship between the G8 score and the risk of severe AEs was apparently not linear in a logistic regression model with RCS, and the value range of the G8 score close to the log odds of severe AEs ≤0 was 11–12. This G8 value range obtained from an RCS analysis, 11–12, was nearly the same as the cutoff value, 11, obtained from the ROC curve. Both results of the logistic regression models with RCS and ROC are comparable, and this fact showed the robustness of our findings. These results suggested that the cutoff value predicting the incidence of severe AEs in older adults with DLBCL may be lower than 14, which was the previously reported cutoff value for survival in older patients with solid cancer or hematological malignancies [13, 14, 15, 16].
The number of new severe AEs was highest during the first course of treatment and gradually decreased as the chemotherapy course progressed. Therapy‐related deaths, in particular, are most frequent in the first course, and the frequency decreased with each course according to a previous report [7]. However, little has been reported about AEs other than mortality depending on the number of chemotherapy courses [7]. In Japan, older adults are usually treated as inpatients in the first few courses. The findings in our cohort indicated that the physicians’ judgement of hospitalization was appropriate. In our cohort, mortality during treatment was considerably low at 1.3%, similar to what is documented in previous reports [7, 42]. This fact suggested adequate management of older adults in participating institutions. In Japan, all expensive medical care including hospitalization is supported by the Japanese universal insurance system. Most patients who undergo the standard regimens for DLBCL are usually treated as inpatients and are provided generous supportive care at least during the initial courses for the sake of safety. Because of the risk of severe AE especially during earlier courses, there may be consideration for more careful monitoring or inpatient treatment during the initial cycle.
The patient with low G8 score needs more intensive supportive care during chemotherapy, such as G‐CSF or prophylactic oral antibiotics, than those with high G8 score. Because the risk of severe AEs is declined in later courses, it might be better to treat this vulnerable population as inpatient during the earlier courses, at least during the first course of chemotherapy. How the G8 score affects the treatment selection, including determination of RDI, was unclear in the present cohort. Reduction of RDI regimen such as R‐miniCHOP might be suitable for the patients with low G8 [43]. But reduction of RDI <50% apparently worsens prognosis even in very older adults with DLBCL; thus, we should pay attention to the risk of undertreatment [3]. Further studies are required to understand how the G8 may be incorporated into treatment decision‐making for optimal treatment doses and duration.
Several limitations of this study should be acknowledged. First, this was a retrospective study. There was a treatment bias in supportive care including the prevention of AEs owing to physicians’ descriptions and assessments of patient conditions. Physicians may reduce and/or delay chemotherapy without consensus criteria to avoid AEs because of a retrospective cohort study. Second, there was a survival bias because only patients who can tolerate the treatment persist; thus, the incidence of severe AEs may decrease with each course. Moreover, (R‐) CHOP or THP‐COP regimens usually take six or eight courses, and patients with limited stage DLBCL receive three courses of standard regimens before radiation therapy. Patients who received seven or more courses of standard regimens were limited in number. Third, the impact of the G8 score on survival was not evaluated in the present study [31, 44]. Fourth, E‐value was comparable to PS and bulky mass, and it is unlikely that the conclusions of this study would be overturned by the absence of information on the questionnaire assessing one's health status in consideration of the weighting of this questionnaire in the previous meta‐analysis [45]. Finally, Japan is the most advanced superaged society. In addition to the normal life expectancy, Japan has also the highest healthy life expectancy in the world, at 65 years [46]. Older adults in Japan might be healthier than those in other countries. Thus, we cannot simply apply the results of our cohort to the patient population of other countries.
Conclusion
In summary, the G8 score before initial treatment can predict the incidence of severe AEs, FN, or nonhematological toxicity, not only during the entire treatment period but also in each course of standard therapy in older adults with DLBCL. The frequency of severe AEs was highest during the first course and decreased gradually as the courses progressed.
Author Contributions
Conception/design: Kana Oiwa, Kei Fujita, Shin Lee, Tetsuji Morishita
Provision of study material or patients: Eiju Negoro, Takanori Ueda, Takahiro Yamauchi
Collection and/or assembly of data: Kana Oiwa, Kei Fujita, Shin Lee, Hikaru Tsukasaki
Data analysis and interpretation: Kei Fujita, Shin Lee, Tetsuji Morishita
Manuscript writing: Kana Oiwa, Shin Lee
Final approval of manuscript: Kana Oiwa, Kei Fujita, Shin Lee, Tetsuji Morishita, Hikaru Tsukasaki, Eiju Negoro, Takanori Ueda, Takahiro Yamauchi
Disclosures
Takahiro Yamauchi: Chugai, Ono, Solasia, Astellas, Daiichi‐Sankyo, Nihon‐Shinyaku, Teijin, Merck Sharp & Dohme, Takeda, Dainihon, Otsuka, Taiho, Kyowa‐Kirin, Tanabe‐Mitsubishi, Asahi‐Kasei (RF), Abbvie, Janssen, Pfizer (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables
Acknowledgments
The authors would like to thank Nami Fujita for her technical support; Dr. Yuya Steven Otake for his language support; and Dr. Keiichi Kinoshita, Dr. Yasukazu Kawai, and Dr. Shin Imamura for allowing patient data disclosure. The first author, K.O., would like to thank Dr. Shinsuke Iida for his valuable suggestions.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. van Leeuwen MT, Turner JJ, Joske DJ et al. Lymphoid neoplasm incidence by WHO subtype in Australia 1982–2006. Int J Cancer 2014;135:2146–2156. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Lee S, Fujita K, Negoro E et al. Impact of relative dose intensity of standard regimens on survival in elderly patients aged 80 years and older with diffuse large B cell lymphoma. Haematologica 2020;105;e415–e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin RJ, Behera M, Diefenbach CS et al. Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B‐cell lymphoma. Blood 2017;130:2180–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lyman GH, Kuderer NM. Moving beyond febrile neutropenia. Support Cancer Ther 2005;2:95–97. [DOI] [PubMed] [Google Scholar]
- 6. Lyman GH, Morrison VA, Dale DC et al. Risk of febrile neutropenia among patients with intermediate‐grade non‐Hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 2003;44:2069–2076. [DOI] [PubMed] [Google Scholar]
- 7. Pfreundschuh M, Trumper L, Kloess M et al. Two‐weekly or 3‐weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: Results of the NHL‐B2 trial of the DSHNHL. Blood 2004;104:634–641. [DOI] [PubMed] [Google Scholar]
- 8. Extermann M, Boler I, Reich RR et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 9. Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurria A, Mohile S, Gajra A et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 2016;34:2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wildiers H, Heeren P, Puts M et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Extermann M, Aapro M, Bernabei R et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241–252. [DOI] [PubMed] [Google Scholar]
- 13. Soubeyran P, Bellera C, Goyard J et al. Validation of the G8 screening tool in geriatric oncology: The ONCODAGE project. J Clin Oncol 2011;29(suppl 15):9001a. [Google Scholar]
- 14. Bellera CA, Rainfray M, Mathoulin‐Pelissier S et al. Screening older cancer patients: First evaluation of the G‐8 geriatric screening tool. Ann Oncol 2012;23:2166–2172. [DOI] [PubMed] [Google Scholar]
- 15. Kenis C, Decoster L, Van Puyvelde K et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol 2014;32:19–26. [DOI] [PubMed] [Google Scholar]
- 16. Velghe A, Petrovic M, De Buyser S et al. Validation of the G8 screening tool in older patients with aggressive haematological malignancies. Eur J Oncol Nurs 2014;18:645–648. [DOI] [PubMed] [Google Scholar]
- 17. Decoster L, Van Puyvelde K, Mohile S et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations. Ann Oncol 2015;26:288–300. [DOI] [PubMed] [Google Scholar]
- 18. Stokoe JM, Pearce J, Sinha R et al. G8 and VES‐13 scores predict chemotherapy toxicity in older patients with cancer. J Geriatr Oncol 2012;3(suppl 1)P95a. [Google Scholar]
- 19. Dottorini L, Catena L, Sarno I et al. The role of geriatric screening tool (G8) in predicting side effect in older patients during therapy with aromatase inhibitor. J Geriatr Oncol 2019;10:356–358. [DOI] [PubMed] [Google Scholar]
- 20. Kotzerke D, Moritz F, Mantovani L et al. The performance of three oncogeriatric screening tools ‐ G8, optimised G8 and CARG ‐ in predicting chemotherapy‐related toxicity in older patients with cancer. A prospective clinical study. J Geriatr Oncol 2019;10:937–943. [DOI] [PubMed] [Google Scholar]
- 21. Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood 2018;131:515–524. [DOI] [PubMed] [Google Scholar]
- 22. Swerdlow S, Campo E, Harris NL et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th edition. Lyon, France: IARC, 2017. [Google Scholar]
- 23. Swerdlow SH, Campo E, Pileri SA et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hara T, Yoshikawa T, Goto H et al. R‐THP‐COP versus R‐CHOP in patients younger than 70 years with untreated diffuse large B cell lymphoma: A randomized, open‐label, noninferiority phase 3 trial. Hematol Oncol 2018;36:638–644. [DOI] [PubMed] [Google Scholar]
- 25. Kitamura K, Takaku F. Pirarubicin, a novel derivative of doxorubicin. THP‐COP therapy for non‐Hodgkin's lymphoma in the elderly. Am J Clin Oncol 1990;13(suppl 1):S15–S19. [DOI] [PubMed] [Google Scholar]
- 26. Dalia S, Chavez J, Little B et al. Serum albumin retains independent prognostic significance in diffuse large B‐cell lymphoma in the post‐rituximab era. Ann Hematol 2014;93:1305–1312. [DOI] [PubMed] [Google Scholar]
- 27. Eatrides J, Thompson Z, Lee JH et al. Serum albumin as a stable predictor of prognosis during initial treatment in patients with diffuse large B cell lymphoma. Ann Hematol 2015;94:357–358. [DOI] [PubMed] [Google Scholar]
- 28. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 29. Saygin C, Jia X, Hill B et al. Impact of comorbidities on outcomes of elderly patients with diffuse large B‐cell lymphoma. Am J Hematol 2017;92:989–996. [DOI] [PubMed] [Google Scholar]
- 30. Liuu E, Canouï‐Poitrine F, Tournigand C et al. Accuracy of the G‐8 geriatric‐oncology screening tool for identifying vulnerable elderly patients with cancer according to tumour site: The ELCAPA‐02 study. J Geriatr Oncol 2014;5:11–19. [DOI] [PubMed] [Google Scholar]
- 31. Sakurai M, Karigane D, Kasahara H et al. Geriatric screening tools predict survival outcomes in older patients with diffuse large B cell lymphoma. Ann Hematol 2019;98:669–678. [DOI] [PubMed] [Google Scholar]
- 32. Takahashi M, Takahashi M, Komine K et al. The G8 screening tool enhances prognostic value to ECOG performance status in elderly cancer patients: A retrospective, single institutional study. PLoS One 2017;12:e0179694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamada SI, Hasegawa T, Okuyama K et al. Clinical significance of the G8 screening tool in elderly patients with oral squamous cell carcinoma. Clin Oral Investig 2020;24:1953–1961. [DOI] [PubMed] [Google Scholar]
- 34. International Non‐Hodgkin's Lymphoma Prognostic Factors Project . A predictive model for aggressive non‐Hodgkin's lymphoma. N Engl J Med 1993;329:987–994. [DOI] [PubMed] [Google Scholar]
- 35. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Cham, Switzerland: Springer, 2015. [Google Scholar]
- 36. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E‐value. Ann Intern Med 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 37. Kubo T, Watanabe H, Ninomiya K et al. Immune checkpoint inhibitor efficacy and safety in older non‐small cell lung cancer patients. Jpn J Clin Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 38. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kenig J, Zychiewicz B, Olszewska U et al. Screening for frailty among older patients with cancer that qualify for abdominal surgery. J Geriatr Oncol 2015;6:52–59. [DOI] [PubMed] [Google Scholar]
- 40. Gomez H, Hidalgo M, Casanova L et al. Risk factors for treatment‐related death in elderly patients with aggressive non‐Hodgkin's lymphoma: Results of a multivariate analysis. J Clin Oncol 1998;16:2065–2069. [DOI] [PubMed] [Google Scholar]
- 41. Liu MA, DuMontier C, Murillo A et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019;134:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuhnl A, Cunningham D, Counsell N et al. Outcome of elderly patients with diffuse large B‐cell lymphoma treated with R‐CHOP: Results from the UK NCRI R‐CHOP14v21 trial with combined analysis of molecular characteristics with the DSHNHL RICOVER‐60 trial. Ann Oncol 2017;28:1540–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peyrade F, Jardin F, Thieblemont C et al. Attenuated immunochemotherapy regimen (R‐miniCHOP) in elderly patients older than 80 years with diffuse large B‐cell lymphoma: a multicentre, single‐arm, phase 2 trial. Lancet Oncol 2011;12:460–468. [DOI] [PubMed] [Google Scholar]
- 44. Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann Hematol 2014;93:1031–1040. [DOI] [PubMed] [Google Scholar]
- 45. Hamaker ME, Jonker JM, de Rooij SE et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol 2012;13:e437–444. [DOI] [PubMed] [Google Scholar]
- 46. GBD 2016 DALYs and HALE Collaborators . Global, regional, and national disability‐adjusted life‐years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Tables


