Abstract
Background
This study investigated the correlation between a history of human papillomavirus (HPV) infection and skin cancer risk.
Materials and Methods
The study cohort comprised 26,919 patients with newly diagnosed HPV infection between 2000 and 2012; with the use of computer‐generated numbers, patients without previous HPV infection were randomly selected as the comparison cohort. The patients in the HPV infection cohort were matched to comparison individuals at a 1:4 ratio by demographic characteristics and comorbidities. All study individuals were followed up until they developed skin cancer, withdrew from the National Health Insurance program, were lost to follow‐up, or until the end of 2013. The primary outcome was subsequent skin cancer development. Cox proportional hazards regression analysis was used to analyze the risk of skin cancer with hazard ratios (HRs) and 95% confidence intervals (CIs) between the HPV and control cohort.
Results
The adjusted HR of skin cancer for patients with HPV relative to controls was 2.45 after adjusting sex, age and comorbidities. (95% CI, 1.44–4.18, p < .01). The subgroup analysis indicated that a patient with HPV infection had a significantly greater risk of skin cancer if they were aged >40 years. Notably, a risk of skin cancer was found in the group diagnosed with HPV within the first 5 years after the index date (adjusted HR, 3.12; with 95% CI, 1.58–5.54). Sensitivity analysis by propensity score, matching with balanced sex, age, and comorbidities, showed consistent results.
Conclusion
A history of HPV infection is associated with the development of subsequent skin cancer in Taiwanese subjects, and the risk wanes 5 years later.
Implications for Practice
In this Taiwan nationwide cohort study, there was a 2.45‐fold increased risk of developing new‐onset skin cancers for patients with incident human papillomavirus (HPV) infection, compared with the matched controls. Furthermore, the risk was noticeably significant among patients aged >40 years. A prominent risk of skin cancers was found in the group diagnosed with HPV within the first 5 years after the index date in this study. The results of this analysis may raise consensus on the effect of HPV infection on the risk of skin cancers. Clinicians are encouraged to implement prudently on the differential diagnosis of skin cancers and HPV prevention and treatment, especially in older patients.
Keywords: Human papillomavirus infection, Skin cancer, Cohort, Nonmelanoma skin cancer (NMSC)
Short abstract
Melanoma and nonmelanoma skin cancer (NMSC) remain common worldwide. This 12‐year retrospective cohort study explored whether people diagnosed with human papillomavirus infection have an increased risk of skin cancers, focusing on melanoma and NMSC.
Introduction
Skin cancers are known to result from genetic factors, environmental carcinogens, and immunity, including ultraviolent (UV) light exposure, immunosuppression, chronic inflammation, family history, and viral infection [1, 2, 3, 4, 5, 6]. Numerous studies have shown evidence supporting an etiologic relationship between skin cancers and human papillomavirus (HPV) infection, especially between beta HPV and squamous cell carcinoma (SCC). A multiplex serology study detected HPV antibodies more frequently in patients with SCC [7]. A population‐based study found no significant difference in HPV prevalence between SCC and basal cell carcinoma (BCC), but SCC lesions were significantly more infected with beta HPV than were BCC [8]. In a meta‐analysis of six case‐control studies, an increased SCC risk associated with beta HPV seropositivity was found [9]. A case‐control study measuring serum antibodies of HPV genres found that beta HPV consistently presented in the SCC tissues [10]. In a meta‐analysis, researchers discovered a positive overall association between HPV and cutaneous SCC [11]. An epidemiological study in Australia also explored that HPV may contribute to the formation of SCC [12]. Notably, in a previous retrospective study, high‐risk mucosal HPV types (type 16, 31, 35, and 51) were detected in patients with nonmelanoma skin cancer (NMSC), suggesting that high‐risk genital HPV‐type infection may also represent a risk factor for NMSC in the nonimmunosuppressed population [13]. A high prevalence of mucosal HPV types in NMSC biopsy specimens was revealed in a Tunisian study, associated with loss of p53 function [14].
The mechanism of carcinogenesis is believed to be associated with the transforming activities of E6 and E7 proteins, especially from beta HPV types. These oncoproteins are responsible for interrupting the cell cycle, causing malignization of HPV‐infected cells. By efficiently deregulating the p53 and pRb pathways, E6 and E7 proteins immortalize human keratinocytes. Increased expression of the E6 and E7 oncoproteins can be observed in replication of HPVs in dividing epithelial cells [15]. An increase of cutaneous HPV replication in the presence of ultraviolet radiation (UVR) may be an explainable reason to the compatible observation. In particular, studies have also demonstrated that the noncoding region promoter activity of the cutaneous type HPV can be stimulated by exposure of ultraviolet radiation [16]. A hit‐and‐run hypothesis was surmised as a necessary part in an early stage of carcinogenesis. By inhibiting the UVR‐induced DNA damage repair process, DNA breaks and mutations are then accumulated; E6 and E7 proteins would not be required for the maintenance of the malignancy phenotype at later stages.
The understanding of the association between melanoma and HPV was rudimentary. A Greek retrospective study evaluated melanoma biopsy specimens and detected high‐risk HPV types from them, although the result was insignificant [17]. Epidemiological evidence suggested that the incidence of cutaneous melanoma increased significantly, especially in European populations, which may result from HPV infection of follicular hair melanocytes found in biopsies [18]. A previous study about uveal melanoma development demonstrated a possible HPV involvement and suggested a downregulation of HPV 18E6/E7 and activation of the p53 and Rb pathways [19].
Melanoma and NMSC remain two of the most common types of cancer in most populations worldwide [20]. In this 12‐year retrospective cohort study, we aimed to identify whether people diagnosed with HPV infection would have an increased risk of skin cancers, including melanoma and NMSC.
Materials and Methods
Data Source
This study was constructed using the data from the National Health Insurance Research Database (NHIRD), including the claims data from Taiwan's National Health Insurance (NHI) program, which was established in 1995 and covers more than 99.99% of Taiwan's 23 million citizens. Insurance benefits include inpatient, ambulatory, emergency, dental, and traditional Chinese medicine services. Quarterly expert reviews on random samples of claims data, with a sampling rate of one in 50 to 100, were performed by the Bureau of NHI for ensuring the accuracy. The NHI records diseases based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM). Before releasing the database for research, the original identification numbers were anonymized to protect patients' privacy. The NHI Registry for Catastrophic Illness provided detailed information for all patients with severe diseases categories under the NHI program. All cancers were included in the category of catastrophic illness. Patients are exempt from copayments where they receive treatment and follow‐up for the specific disease with Catastrophic Illness Certificates (CICs), so nearly all patients with catastrophic disease have CICs. The applications of CICs are reviewed by Bureau of NHI. For approval of CICs for cancer, peer review of histological confirmation of malignancy and associated laboratory and imaging studies is mandatory. Therefore, the CICs are both complete and accurate. This study was approved by the Institutional Review Board of China Medical University Hospital Research Ethics Committee (CMUH104‐REC2–115[AR‐4]).
Study Population
We carried out a retrospective, population‐based cohort study to investigate the association between HPV infection and the risks for developing skin cancers. We first identified patients infected with new onset HPV infection between 2000 and 2012. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes we used were 079.4 (HPV infection in conditions classified elsewhere and of unspecified site), 078.1 (viral warts), 795.05 (cervical high‐risk HPV DNA test positive), 795.09 (other abnormal Papanicolaou smear of cervix and cervical HPV), 795.15 (vaginal high‐risk HPV DNA test positive), 795.19 (other abnormal Papanicolaou smear of vagina and vaginal HPV), 796.75 (anal high‐risk HPV DNA test positive), and 796.79 (other abnormal Papanicolaou smear of anus and anal HPV). Cutaneous types HPV infections are codes 078.10, 078.12, and 078.19, whereas others are classified as mucosal types. We also extracted their data to exclude patients with history of HPV infection or cancers 3 years before their entry into the study. The index date was defined as the first date that HPV was diagnosed. For further ascertainment, only patients with at least one inpatient admission or two outpatient visits within a year after first being diagnosed with HPV were selected. In addition, we confirmed the treatment procedure code (“electrocauterization for condyloma [50005],” “condyloma, excision and electrocauterization [55008],” “CO2 laser operation [62020],” “chemosurgery, condyloma [50015],” “electrocauterization, simple [51005],” “electrocauterization, complicated [51006],” “liquid nitrogen cryosurgery [51017],” “cryotherapy, simple, including CO2 freezing and liquid nitrogen [51021C],” “cryotherapy, complicated, including CO2 freezing and liquid nitrogen [51022]”) during the 3 months after the index date to be the inclusion criteria to increase validity of diagnosing HPV infection.
The control group was randomly selected from inpatients without HPV infection. They were individually‐paired with patients with HPV infection by a 1:4 ratio matched by age, sex, and index year.
Outcome and Relevant Variables
The main outcome of this study was the presence or absence of skin malignancies, including both melanoma (ICD‐9‐CM code 172) and NMSC (ICD‐9‐CM code 173) development. To observe the incidence of skin cancers, follow‐up stopped on December 31, 2012, or whenever the patient dropped out from the NHI program.
To control the effects of potential confounders, we checked the following data from the data set, including age, gender, and medical comorbidities. We classified age into 4 groups: 14–30, 31–40, 40–50, and greater than 50 years. The cancer‐related comorbidities analyzed in this study were hypertension (ICD‐9‐CM codes 401–405), diabetes mellitus (ICD‐9‐CM code 250), hyperlipidemia (ICD‐9‐CM code 272), chronic kidney disease (ICD‐9‐CM code 585), peptic ulcer disease (ICD‐9‐CM codes 531–533), asthma (ICD‐9‐CM code 493), chronic obstructive pulmonary disease (ICD‐9‐CM codes 491, 492, 496), Helicobacter pylori (ICD‐9‐CM code 041.86), hepatitis B (ICD‐9‐CM codes 070.2, 070.3, V02.61), hepatitis C (ICD‐9‐CM codes 070.41, 070.44, 070.51, 070.54, V02.62), inflammatory bowel disease (ICD‐9‐CM codes 555.*, 556.*), alcohol‐related illness (ICD‐9‐CM codes 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, A215, and V11.3), HIV infection (ICD‐9‐CM code 042–044, 795.8, V08), systemic lupus erythematosus (ICD‐9‐CM code 710.0), rheumatoid arthritis (ICD‐9‐CM code 714.0), Sjögren's syndrome (ICD‐9‐CM code 710.2), polymyositis (ICD‐9‐CM code 710.4), and dermatomyositis (ICD‐9‐CM code 710.3). Information on comorbid medical disorders was obtained by tracing all the inpatients records in the NHI database within 2 years before the index date.
Sensitivity Analysis
To validate the robustness of our finding, we also conducted a sensitivity analysis with alternative controls matched by propensity score. The propensity score matching was by a ratio of 1: 4 to balance the baseline characteristics as closely as possible between the two cohorts from the beginning of enrollment. The sensitivity analysis was conducted for the purpose of examining whether the finding was robust to different matching criteria.
Statistical Analysis
To describe the distribution of the study population, we presented the means and SDs for age and number as well as percentages of sex and cancer‐related comorbidity. The χ2 test was applied to examine the distribution of the categorical baseline characteristics between HPV cohort and HPV‐free cohort. We compared the age mean by the Student's t test. The incidence density for developing skin cancer was calculated for both groups. We also measured the cumulative incidence of skin cancer in the two cohorts by the Kaplan‐Meier method and tested the curve differences with the log‐rank test. To present the risk of cancer between both groups, the hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using single‐variable and multivariable Cox proportional hazard models. The data management and statistical analyses were implemented in SAS 9.4 software (SAS Institute, Cary, NC) The significance level was set at p < .05 for two‐side testing of the p value.
Results
We included 26,919 participants newly diagnosed with HPV infections from 2000–2012, and the comparison HPV‐free cohort matched 1 to 4 with the HPV cohort by sex, age, and index date in this study. Table 1 shows the baseline feature of patients with and without HPV infection. Significant differences in all covariates except for alcohol‐related illness were noted.
Table 1.
The baseline characteristics in HPV and non‐HPV cohorts
| Variable | Non‐HPV (n = 107,676), n (%) | HPV (n = 26,919), n (%) | p value |
|---|---|---|---|
| Gender | >.999 | ||
| Female | 55,988 (52) | 13,997 (52) | |
| Male | 51,688 (48) | 12,922 (48) | |
| Age, yr | >.999 | ||
| 14–30 | 45,072 (41) | 11,268 (41) | |
| 31–40 | 19,717 (18) | 4,928 (18) | |
| 41–50 | 18,043 (16) | 4,512 (16) | |
| >50 | 26,676 (24) | 6,669 (24) | |
| Mean (SD) | 38.0 ((17.5) | 37.9 (17.5) | .619 |
| Comorbidities | |||
| Hypertension | 17,332 (16) | 4,707 (17) | <.001 |
| Diabetes | 8,463 (7.7) | 2,259 (8.3) | .002 |
| Hyperlipidemia | 13,415 (12) | 4,292 (16) | <.001 |
| CKD | 1,133 (1.0) | 367 (1.3) | <.001 |
| Peptic ulcer disease | 20,750 (19) | 6,415 (23) | <.001 |
| Asthma | 9,063 (8) | 2,811 (10) | <.001 |
| COPD | 8,341 (8) | 2,638 (10) | <.001 |
| Helicobacter pylori | 307 (0.28) | 114 (0.42) | .030 |
| HBV | 3,356 (3) | 1,233 (5) | <.001 |
| HCV | 1,026 (0.9) | 285 (1.0) | .018 |
| IBD | 2,548 (2) | 746 (3) | <.001 |
| Alcohol‐related illness | 2,107 (2) | 555 (2) | .104 |
| HIV | 63 (0.06) | 31 (0.11) | .003 |
| Autoimmune disease | 2,933 (3) | 905 (3) | <.001 |
Follow‐up time: HPV, 5.08 (2.90); non‐HPV: 5.04 (2.91).
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease;
HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; IBD, inflammatory bowel disease.
Table 2 shows the Cox regression model, in which the incidence rate of skin cancers in HPV cohort was 1.61 per 10,000 person‐years and in control cohort was 0.66 per 10,000 person‐years. The adjusted HR of skin cancer for patients with HPV relative to controls was 2.45 (95% CI, 1.44–4.18; p < .01). Compared with patients under 30 years, patients aged 31–40 and 41–50 had an adjusted HR of 1.83 and 2.60, respectively, but the difference did not reach significance; however, the risk was significantly higher in patients older than 50 with an adjusted HR (aHR) of 22.5 (95% CI, 7.57–66.7). History of prior diagnosis of HPV infection have a higher risk of developing skin cancer in both melanoma and nonmelanoma (aHR, 17.1; 95% CI, 1.88–156; aHR, 2.06; 95% CI, 1.16–3.65, respectively) compared with the general population after adjusting for sex, comorbidities, and medication confounders. Figure 1 shows the cumulative incidence of skin cancers. The cumulative curve of skin cancers for the HPV cohort was higher than that of the comparison groups (log‐rank test, p < .001).
Table 2.
The association of explanatory variables and skin cancer
| Variable | Skin Cancer | cHR (95% CI) | aHR a (95% CI) | aSHR a (95% CI) | ||
|---|---|---|---|---|---|---|
| n | PY | IR | ||||
| All | ||||||
| Non‐HPV | 36 | 542,394 | 0.66 | 1.00 | 1.00 | 1.00 |
| HPV | 22 | 136,674 | 1.61 | 2.43 (1.43–4.12) b | 2.45 (1.44–4.18) b | 2.51 (1.48–4.26) b |
| Melanoma | ||||||
| Non‐HPV | 1 | 542,394 | 0.02 | 1.00 | 1.00 | 1.00 |
| HPV | 4 | 136,674 | 0.29 | 16.6 (1.85–149) c | 17.1 (1.88–156) c | 15.7 (1.59–153) c |
| Nonmelanoma | ||||||
| Non‐HPV | 35 | 542,394 | 0.65 | 1.00 | 1.00‐ | 1.00 |
| HPV | 18 | 136,674 | 1.32 | 2.03 (1.15–3.59) c | 2.06 (1.16–3.65) c | 2.12 (1.20–3.73) b |
| Gender | ||||||
| Female | 27 | 355,858 | 0.76 | 1.00 | ||
| Male | 31 | 323,210 | 0.96 | 1.27 (0.76–2.13) | ||
| Age, yr | ||||||
| 14–30 | 4 | 299,047 | 0.13 | 1.00 | 1.00 | 1.00 |
| 31–40 | 3 | 124,313 | 0.24 | 1.82 (0.41–8.14) | 1.83(0.41–8.17) | 1.79 (0.40–8.01) |
| 41–50 | 4 | 112,324 | 0.36 | 2.70 (0.68–10.8) | 2.60(0.65–10.5) | 2.57 (0.65–10.2) |
| >50 | 47 | 143,385 | 3.28 | 26.1 (9.40–72.5) b | 22.5(7.57–66.7) b | 20.5 (6.99–60.1) b |
| Comorbidities | ||||||
| Hypertension | ||||||
| No | 27 | 582,828 | 0.46 | 1.00 | 1.00 | 1.00 |
| Yes | 31 | 96,241 | 3.22 | 7.23 (4.32–12.1) b | 2.15(1.18–3.91) c | 2.20 (1.21–3.99) b |
| Diabetes | ||||||
| No | 50 | 634,109 | 0.79 | 1.00 | 1.00‐ | 1.00 |
| Yes | 8 | 44,959 | 1.78 | 2.37 (1.12–4.99) c | 0.63(0.28–1.40) | 0.61 (0.27–1.41) |
| Hyperlipidemia | ||||||
| No | 44 | 603,698 | 0.73 | 1.00 | 1.00 | 1.00 |
| Yes | 14 | 75,370 | 1.86 | 2.68 (1.47–4.9) b | 0.68(0.35–1.32) | 0.63 (0.32–1.25) |
| CKD | ||||||
| No | 58 | 674,072 | 0.86 | 1.00 | ||
| Yes | 0 | 4,997 | 0.00 | 0.00 (0–Inf) | ||
| Peptic ulcer disease | ||||||
| No | 41 | 557,729 | 0.74 | 1.00 | 1.00 | 1.00 |
| Yes | 17 | 121,340 | 1.40 | 1.97 (1.12–3.46) c | 0.77 (0.43–1.39) | 0.73 (0.40–1.34) |
| Asthma | ||||||
| No | 53 | 629,962 | 0.84 | 1.00 | ||
| Yes | 5 | 49,106 | 1.02 | 1.29 (0.52–3.23) | ||
| COPD | ||||||
| No | 51 | 630,748 | 0.81 | 1.00 | ||
| Yes | 7 | 48,321 | 1.45 | 1.86 (0.84–4.09) | ||
| Helicobacter pylori | ||||||
| No | 58 | 677,878 | 0.86 | 1.00 | ||
| Yes | 0 | 1,191 | 0.00 | 0.00 (0–Inf) | ||
| HBV | ||||||
| No | 55 | 659,002 | 0.83 | 1.00 | ||
| Yes | 3 | 20,066 | 1.50 | 1.86 (0.58–5.96) | ||
| HCV | ||||||
| No | 58 | 674,184 | 0.86 | 1.00 | ||
| Yes | 0 | 4,884 | 0.00 | 0.00 (0–Inf) | ||
| IBD | ||||||
| No | 55 | 665,248 | 0.83 | 1.00 | ||
| Yes | 3 | 13,821 | 2.17 | 2.76 (0.86–8.83) | ||
| Alcohol‐related illness | ||||||
| No | 57 | 668,653 | 0.85 | 1.00 | ||
| Yes | 1 | 10,416 | 0.96 | 1.21 (0.17–8.74) | ||
| HIV | ||||||
| No | 58 | 678,702 | 0.85 | 1.00 | ||
| Yes | 0 | 367 | 0.00 | 0.00 (0–Inf) | ||
| Autoimmune disease | ||||||
| No | 55 | 662,046 | 0.83 | 1.00 | ||
| Yes | 3 | 17,023 | 1.76 | 2.19 (0.69–7.01) | ||
Adjusted by age, hypertension, diabetes, hyperlipidemia and peptic ulcer disease.
p value < .05.
p value < .01.
p value < .001.
Abbreviations: aHR, adjusted hazard ratio; aHSR, adjusted subhazard ratio; cHR, crude hazard ratio; CI, confident interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease;; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; IBD, inflammatory bowel disease; Inf, infinity; IR, incidence rate (per 10,000 person‐years); PY, person‐years.
Figure 1.
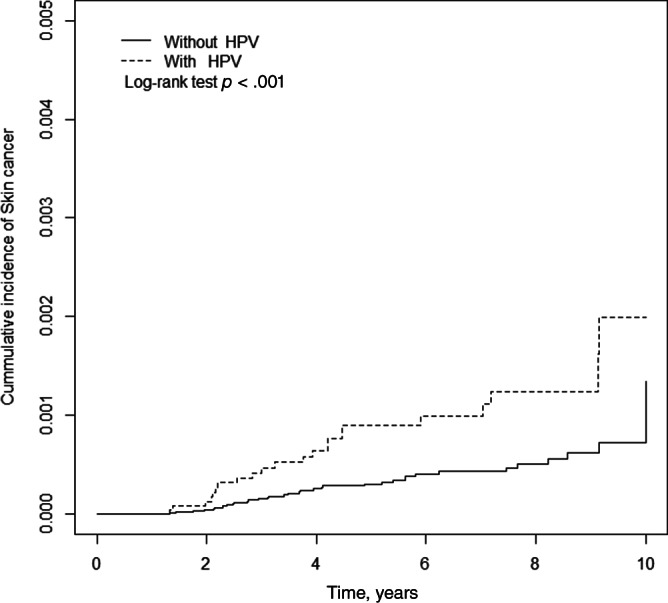
Cumulative incidence of skin cancers for the HPV cohort and the comparison groups (log‐rank test, p < .001). Abbreviation: HPV, human papillomavirus.
Table 3 shows the association of risks of skin cancers among different subgroups. In sex‐subgroup analysis, both genders with HPV showed a higher risk of developing skin cancers. In the age‐subgroup analysis, compared with matched non‐HPV age‐subgroups, those aged 41–50 and older than 50 had higher risk of developing skin cancers (adjusted HR, 10.09; 95% CI, 1.04–97.4; adjusted HR, 2.31; 95% CI, 1.27–4.21, respectively). Among participants with peptic ulcer diseases,there was a significant positive association of HPV infection with skin cancers (adjusted HR,4.52; 95% CI, 1.72–11.9), compared with matched non‐HPV subgroups.
Table 3.
The association of HPV and skin cancers in difference stratification levels
| Variable | non‐HPV | HPV | cHR (95% CI) | aHR a (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n | PY | IR | n | PY | IR | |||
| Gender | ||||||||
| Female | 16 | 284,345 | 0.56 | 11 | 71,513 | 1.54 | 2.75 (1.28–5.93) b | 2.74 (1.26–5.94) c |
| Male | 20 | 258,049 | 0.78 | 11 | 65,161 | 1.69 | 2.17 (1.04–4.53) c | 2.24 (1.07–4.71) c |
| Age, yr | ||||||||
| 14–30 | 3 | 239,166 | 0.13 | 1 | 59,881 | 0.17 | 1.33 (0.14–12.8) | 1.36 (0.14–13.1) |
| 31–40 | 2 | 99,385 | 0.20 | 1 | 24,928 | 0.40 | 1.98 (0.18–21.8) | 2.16 (0.2–23.82) |
| 41–50 | 1 | 89,701 | 0.11 | 3 | 22,622 | 1.33 | 11.68 (1.21–112) c | 10.09 (1.04–97.4) c |
| >50 | 30 | 114,142 | 2.63 | 17 | 29,243 | 5.81 | 2.23 (1.23–4.04) b | 2.31 (1.27–4.21) b |
| Comorbidities | ||||||||
| Hypertension | ||||||||
| No | 17 | 467,105 | 0.36 | 10 | 115,723 | 0.86 | 2.36 (1.08–5.16) c | 2.52 (1.15–5.52) c |
| Yes | 19 | 75,289 | 2.52 | 12 | 20,952 | 5.73 | 2.26 (1.10–4.65) c | 2.34 (1.13–4.83) c |
| Diabetes | ||||||||
| No | 31 | 507,351 | 0.61 | 19 | 126,758 | 1.50 | 2.46 (1.39–4.35) b | 2.46 (1.38–4.38) b |
| Yes | 5 | 35,043 | 1.43 | 3 | 9,916 | 3.03 | 1.98 (0.47–8.31) | 2.11 (0.50,8.97) |
| Hyperlipidemia | ||||||||
| No | 27 | 485,794 | 0.56 | 17 | 117,905 | 1.44 | 2.58 (1.41–4.74) b | 2.74 (1.49–5.04) b |
| Yes | 9 | 56,601 | 1.59 | 5 | 18,770 | 2.66 | 1.73 (0.58–5.17) | 1.81 (0.60–5.43) |
| CKD | ||||||||
| No | 36 | 538,707 | 0.67 | 22 | 135,365 | 1.63 | 2.43 (1.43–4.14) b | 2.46 (1.44–4.20) d |
| Yes | 0 | 3,688 | 0.00 | 0 | 1,309 | 0.00 | ||
| Peptic ulcer disease | ||||||||
| No | 29 | 450,754 | 0.64 | 12 | 106,975 | 1.12 | 1.75 (0.89–3.43) | 1.81 (0.92–3.56) |
| Yes | 7 | 91,641 | 0.76 | 10 | 29,699 | 3.37 | 4.35 (1.66–11.4) b | 4.52 (1.72–11.9) b |
| Asthma | ||||||||
| No | 34 | 505,208 | 0.67 | 19 | 124,755 | 1.52 | 2.27 (1.29–4.00) b | 2.30 (1.30–4.04) b |
| Yes | 2 | 37,187 | 0.54 | 3 | 11,920 | 2.52 | 4.66 (0.78–27.9) | 4.83 (0.80–29.1) |
| COPD | ||||||||
| No | 31 | 506,016 | 0.61 | 20 | 124,732 | 1.60 | 2.62 (1.49–4.60) d | 2.73 (1.55–4.80) d |
| Yes | 5 | 36,379 | 1.37 | 2 | 11,942 | 1.67 | 1.20 (0.23–6.21) | 1.23 (0.24–6.44) |
| Helicobacter pylori | ||||||||
| No | 36 | 541,488 | 0.66 | 22 | 136,390 | 1.61 | 2.43 (1.43–4.13) b | 2.45 (1.44–4.18) b |
| Yes | 0 | 907 | 0.00 | 0 | 284 | 0.00 | ||
| HBV | ||||||||
| No | 35 | 527,995 | 0.66 | 20 | 131,008 | 1.53 | 2.31 (1.33–3.99) b | 2.31 (1.33–4.02) b |
| Yes | 1 | 14,400 | 0.69 | 2 | 5,667 | 3.53 | 5.01 (0.45–55.3) | 5.17 (0.47–57.2) |
| HCV | ||||||||
| No | 36 | 538,716 | 0.67 | 22 | 135,468 | 1.62 | 2.43 (1.43–4.13) b | 2.46 (1.44–4.19) d |
| Yes | 0 | 3,679 | 0.00 | 0 | 1,206 | 0.00 | ||
| IBD | ||||||||
| No | 35 | 531,811 | 0.66 | 20 | 133,437 | 1.50 | 2.28 (1.32–3.95) b | 2.30 (1.33–4.01) b |
| Yes | 1 | 10,584 | 0.94 | 2 | 3,237 | 6.18 | 6.54 (0.59–72.2) | 9.54 (0.81–112) |
| Alcohol‐related illness | ||||||||
| No | 36 | 534,196 | 0.67 | 21 | 134,457 | 1.56 | 2.32 (1.35–3.97) b | 2.34 (1.36–4.03) b |
| Yes | 0 | 8,198 | 0.00 | 1 | 2,218 | 4.51 | NA (0–Inf) | NA (0–Inf) |
| HIV | ||||||||
| No | 36 | 542,169 | 0.66 | 22 | 136,533 | 1.61 | 2.43 (1.43–4.13) b | 2.45 (1.44–4.18) b |
| Yes | 0 | 225 | 0.00 | 0 | 141 | 0.00 | ||
| Autoimmune disease | ||||||||
| No | 35 | 529,413 | 0.66 | 20 | 132,633 | 1.51 | 2.28 (1.32–3.95) b | 2.33 (1.34–4.05) b |
| Yes | 1 | 12,982 | 0.77 | 2 | 4,041 | 4.95 | 6.31 (0.57–69.6) | 6.62 (0.60–73.5) |
Adjusted by age, hypertension, diabetes, hyperlipidemia, and peptic ulcer disease.
p value < .05.
p value < .01.
p value < .001.
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confident interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; IBD, inflammatory bowel disease; Inf, infinity; IR, incidence rate (per 10,000 person‐years); PY, person‐years.
We further investigated the subgroups divided by follow‐up time in Table 4. A significant higher risk of skin cancers was found in group diagnosed with HPV within the first 5‐year after index date (adjusted HR, 3.12; 95% CI, 1.66–5.87), and the risk was lowered 5 years after the index date. The adjusted hazard ratio was 1.53 (95% CI, 0.54–4.32) in patients with HPV infections after more than 5 years of diagnosis.
Table 4.
The incidence and hazard ratio of skin cancer stratified by follow‐up year
| Follow‐up time | non‐HPV | HPV | cHR (95% CI) | aHR a (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n | PY | IR | n | PY | IR | |||
| 1–5 yr | 27 | 91,866 | 2.94 | 13 | 78,480 | 1.66 | 2.96 (1.58–5.54) b | 3.12 (1.66–5.87) b |
| >5 yr | 9 | 281,462 | 0.32 | 9 | 227,261 | 0.40 | 1.53 (0.55–4.29) | 1.53 (0.54–4.32) |
Adjusted by age, hypertension, diabetes, hyperlipidemia, and peptic ulcer disease.
p value < .001.
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confident interval; HPV, human papilomavirus; IR, incidence rate (per 10,000 person‐years); PY, person‐years.
Table 5 and supplemental online Table 1 show the result after propensity score matching. It was shown that the risk of skin cancers was still higher in the HPV cohort than in the non‐HPV cohort. The adjusted HR of skin cancer for patients with HPV relative to controls was 2.88 (95% CI, 1.66–5; p < .001). As for the subtype of skin cancers, the adjusted HR of melanoma for patients with HPV relative to controls was 5.42 (95% CI, 1.19–24.6) after adjusting for demographic characteristics and all mentioned comorbidities; the adjusted HR of NMSC for patients with HPV relative to controls was 2.59 (95% CI, 1.43–4.71)
Table 5.
Hazard ratio of skin cancers in primary and sensitivity analysis
| Variable | Primary analysis | Sensitivity analysis | ||
|---|---|---|---|---|
| cHR (95% CI) | aHR a (95% CI) | cHR (95% CI) | aHR a (95% CI) | |
| Skin Cancers | ||||
| Non‐HPV | 1.00 | 1.00 | 1.00 | 1.00 |
| HPV | 2.43 (1.43–4.12) c | 2.45 (1.44–4.18) c | 2.92 (1.68–5.06) d | 2.88 (1.66–5) d |
| Melanoma | ||||
| Non‐HPV | 1.00 | 1.00 | 1.00 | 1.00 |
| HPV | 16.6 (1.85–149) b | 17.1 (1.88–156) b | 5.53 (1.24–24.78) b | 5.42 (1.19–24.6) b |
| Nonmelanoma | ||||
| Non‐HPV | 1.00 | 1.00 | 1.00 | 1.00 |
| HPV | 2.03 (1.15–3.59) b | 2.06 (1.16–3.65) b | 2.64 (1.45–4.79) c | 2.59 (1.43–4.71) c |
Primary analysis: 1:4 propensity score matching by gender, age, and index year. Sensitivity analysis: 1:4 propensity score matching by gender, age, index year. and all comorbidities.
Adjusted by age, hypertension, diabetes, hyperlipidemia, and peptic ulcer disease.
p value < .05.
p value < .01.
p value < .001.
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confident interval; HPV, human papillomavirus.
Table 6 presents the association between skin cancers and different types of HPV infections separately. The adjusted HR for skin cancers was 2.66 (95% CI, 1.41–5.00; p value < .01) in cutaneous types, whereas the adjusted HR was 2.32 (95% CI, 1.06–5.05; p value < .05) in mucosal types. The risk for developing skin cancers was indeed higher in individuals who had previous cutaneous HPV types infection compared with the mucosal ones. The cutaneous type was found to be highly associated with melanoma, with an adjusted HR of 180 (95% CI, 1.30–24962; p value < .01). As for NMSC, the adjusted HR was 2.12 (95% CI, 1.06–4.22; p value < .05) in the population with cutaneous type infections.
Table 6.
The association between skin cancers and different types of HPV infections separately
| Variable | Skin Cancer | cHR (95% CI) | aHR a (95% CI) | ||
|---|---|---|---|---|---|
| n | PY | IR | |||
| All | |||||
| Non‐HPV | 36 | 542,394 | 0.66 | 1.00 | 1.00 |
| HPV | 22 | 136,674 | 1.61 | 2.43 (1.43–4.12) b | 2.45 (1.44–4.18) b |
| Cutaneous types | 14 | 87,076 | 1.61 | 2.73 (1.46–5.12) c | 2.66 (1.41–5.00) b |
| Mucosal types | 8 | 49,599 | 1.61 | 2.15 (0.99–4.67) | 2.32 (1.06–5.05) d |
| Melanoma | |||||
| Non‐HPV | 1 | 542,394 | 0.02 | 1.00 | 1.00 |
| HPV | 4 | 136,674 | 0.29 | 16.6 (1.85–149) d | 17.1 (1.88–156) d |
| Cutaneous types | 3 | 87,076 | 0.34 | 76.3 (2.81–2076) d | 180 (1.30–24962) b |
| Mucosal types | 1 | 49,599 | 0.20 | 7.93 (0.46–137) | 7.40 (0.43–127) |
| Nonmelanoma | |||||
| Non‐HPV | 35 | 542,394 | 0.65 | 1.00 | 1.00 |
| HPV | 18 | 136,674 | 1.32 | 2.03 (1.15–3.59) d | 2.06 (1.16–3.65) d |
| Cutaneous types | 11 | 87,076 | 1.26 | 2.17 (1.09–4.30) d | 2.12 (1.06–4.22) d |
| Mucosal types | 7 | 49,599 | 1.41 | 1.96 (0.86–4.44) | 2.20 (0.96–5.02) |
Adjusted by age, hypertension, diabetes, hyperlipidemia, and peptic ulcer disease.
p value < .05.
p value < .01.
p value < .001.
Abbreviations: aHR, adjusted hazard ratio; cHR, crude hazard ratio; CI, confident interval; HPV, human papillomavirus; IR, incidence rate (per 10,000 person‐years); PY, person‐years.
Discussion
In this 13‐year nationwide population‐based retrospective cohort study, we found that individuals with prior diagnosis of HPV infection have a higher risk of developing skin cancer, both melanoma and nonmelanoma (adjusted HR, 17.1; 95% CI, 1.88–156; adjusted HR, 2.06; 95% CI, 1.16–3.65, respectively) compared with the general population after adjusting for sex, comorbidities, and medication confounders. Patients with HPV infection demonstrated higher risk of developing skin cancers as their age increased. In Cox proportional hazard regression model, the comorbidities did not pose significant effect to the development of skin cancers except from hypertension (adjusted HR, 2.20; 95% CI, 1.21–3.99). However, our stratification analysis further revealed that the HPV infection still manifested a distinct risk of skin cancers in all age, sex, and comorbidities subgroups, especially in group aged 41–50 (adjusted HR, 10.09; 95% CI, 1.04–97.4). Time‐to‐event analysis also indicated a strong relationship between HPV infection and skin cancers within 1–5 years of follow‐up time (adjusted HR, 3.12; 95% CI, 1.66–5.87), which may in part be because of more frequent dermatology department visits. Patients with previous cutaneous types infections are more associated with skin cancers compared with patients with mucosal types of infections.
NMSC and its association of HPV infection was widely investigated in previous studies. In a previous skin biopsy investigation of different oncogenic epitheliotropic viruses, HPV detection was more frequent in NMSC compared to noncancerous biopsies, supporting the role of HPV infection in NMSC development [21]. Alphapapillomaviruses were generally recognized as high‐risk HPV types, especially HPV 16, provoking anogenital, head, and neck cancers [22, 23]. A case report of pigmented Bowen's disease also confirmed the presence of high‐risk HPV types [24]. The association between high‐risk HPVs and skin cancers is elusive. One possible interpretation for the positive result in our study is that individuals infected with mucosal types may have higher chances to be affected by cutaneous types due to dysregulated immune status. Another possible interpretation contributes to the association between high‐risk HPV types and NMSC at head and neck region. In our study, we include the malignant skin neoplasm of head and neck region as a part of our primary outcome. The association between high‐risk‐HPV and a subset of head and neck cancers (HNC) has been addressed in previous studies. A previous study on HPV integration in HNC reported that 25 out of 35 HNCs showed integration of high‐risk HPV (type 16, 33, or 35) into the human genome [25]. Another study reported high‐risk HPV (type 16) integration rates of 39% in oral SCC [26]. Thus, the role of high‐risk types in the etiology of NMSC at head and neck region indeed requires further studies to elucidate.
As for the low‐risk HPVs, cutaneous HPVs are ubiquitously disseminated throughout healthy skin and may be an intrinsic part of the commensal flora in both immunosuppressed and immunocompromised individuals. Recently, among cutaneous HPVs, growing evidence of an etiological role of beta HPVs in NMSC has been proposed [27]. Beta HPV types play a role in so called the hit‐and‐run mechanism, by exacerbating the accumulation of UV radiation‐induced somatic mutations and acting as the mediator in initiating the skin carcinogenesis in NMSC [28]. High prevalence of mucosal HPV in NMSC was highlighted in a recent pathology study, indicating active infections assessed by E6 expression are associated with loss of p53 function. Together, these findings surmised that various HPV types take part in NMSC carcinogenesis. Even though the exact type of HPV infection could not be easily distinguishable in our study, the positive result of our study serves as an epidemiological evidence to cohere with previous hypothesis of the causative role of HPV infection in the subsequent risk of NMSC.
In melanomagenesis, UV radiation is addressed as the main risk factor whereas the probability of viral etiology of melanoma has been relatively little discussed. The role of HPV in melanoma development was contradictory. Variability in HPV detection rate in melanoma was found in previous studies, whereas high‐risk HPV viruses were detected in some cases, indicating that high‐risk mucosal HPV16 plays a role in a subgroup of melanoma [29, 30, 31, 32]. As for beta HPV, melanoma was found present significantly more in type 22 but less in type 21 than in control normal skin [31]. An intriguing case of melanosis of the vagina in a young woman infected with low‐risk HPV types was reported [33]. In a previous study of the cellular mechanism of HPV 18 in uveal melanoma (UM) development, researchers demonstrated that downregulation of HPV 18 E6/E7 led to growth inhibition and cell cycle block by activating the p53 and Rb pathways. Even though some justifiable threat of specimen contamination with viral DNA in vivo is possible, evidence showed that HPV is highly likely to be involved in the development of UM. Still, we could not purport a corollary between HPV infection and melanoma in our study because of the small number of events and insignificant result. This study may merely support a putative role of HPV infection in melanoma development.
In contrast, because UV light is a well‐established risk factor melanoma, it is indeed very appealing to postulate UV exposure may lead to an immune compromised status that triggers melanoma and/or HPV infection. However, because HPV infection and UV exposure could happen simultaneously, their “causative association” might be even more complicated. Several studies revealed that the E6 protein from β‐genus HPV decreases the amount of two essential UV‐repair kinases (ATM and ATR) [34, 35, 36, 37, 38]. These studies hypothesized that the diminished ATM and ATR availability has an impact on the ability of cells to protect themselves from UV damage. In such a case, the cumulative effect of UV may result from a prior HPV infection. Furthermore, previous Taiwanese studies revealed that the lesions of acral lentiginous melanoma (ALM), composing about 60% of all melanomas in Taiwan, are generally not a result of exposure to UV [39]. The understanding of the etiology of ALM is rudimentary, unclear, and contentious. It may be multifactorial, including interaction between genetic variants of small effect and certain environmental triggers, such as trauma [40]. Last but not least, we do not have any information about UV or sunlight exposure in our database. Therefore, the possibility of UV‐induced melanoma and/or HPV infection may be hard to tell from our study.
Age distribution and the risk of skin cancers were riveting in this study. In our demographic data, individuals aged between 14 and 30 years had the highest proportion of both cohorts. Most of the population in our study was younger than 50 years, which could be a result of the nature of HPV infection, which generally affects the younger population. In a Cox regression model, the effect of HPV infection on developing skin cancers became more profound as age increased. In our stratification analysis, the risk of developing skin cancers in the HPV cohort was extremely prominent in the group aged 41–50 years (adjusted HR, 10.09; 95% CI, 1.04–97.4). Mounting evidence has shown an increase in older population in HPV‐positive NMSC [41, 42, 43]. Distribution of HPV16 among HPV‐positive oropharynx squamous cell cancer also differs by age group [44]. As for melanoma, a previous study revealed several differences exist in risk factors such as family history, UV exposure, and sunburn history between young adults and middle‐aged patients [45]. Aside from a previous hypothesis of immunosenescence [46, 47], which attributes to reactivation or later age of presentation with HPV‐positive skin cancer, the combination of a growing population of elderly individuals and an increased proportion harboring HPV infections may also be explainable to the phenomenon of increasing older patients [41, 42]. Moreover, elderly patients seem to have lower clearance rates of HPV than a younger population, so a higher rate of cancer progression among elderly patients could be foreseen [48]. Our study is consistent with a paradigm shift of demographic changes in the age spectrum of HPV‐positive skin cancers. Interplay of various risk factors and patients’ characteristics could be investigated and substantiated in future studies.
This is a systematic retrospective observation study composed of a sizeable patient group to investigate the epidemiological association of melanoma, NMSC, and HPV infection. The results of this study highlighted the importance of enhanced knowledge in HPV status and cancer progression events. Advantages of using the NHIRD have been described in a previous study, which includes long‐term comprehensive follow‐up and universal coverage scheme [49]. In this study, we excluded the patients with any cancer diagnosis prior to or 1 year after the index date so that we could reduce the bias of increased melanoma risk associated with malignancy conditions, including basal cell or squamous cell skin carcinomas, and prostate cancer [50, 51]. We also qualified the HPV exposure group by implementing HPV infection treatment procedure codes such as excision, electro cauterization, CO2 laser operation, cryotherapy, and chemotherapy to ensure the accuracy of HPV cohort. Our database size ensures similar distributions due to well‐balanced matching and reduces the heterogeneity and selection bias.
Some limitations need to be considered while interpreting our study. Despite the fact that the Bureau of NHI uses strict auditing mechanism to reimburse insurance claim from patient‐care units, the ICD‐9‐CM codes claimed from the NHIRD might be inaccurate because of diagnostic uncertainty and misclassification. Squamous cell carcinoma and basal cell carcinoma could not be separated from the result. As for common immunosuppression drugs, because there were only two patients who used oral or intravenous corticosteroids (usage of medication was defined as the prescription for at least 30 days of drug within 180 days before or after index date) in our study, we did not include them in this study. Even though we tried to match both cohorts by common risk factors and comorbidities, some individuals may have an elevated susceptibility to mucosal and cutaneous HPV infections in general and/or to skin cancer development because of some confounders could not be identified in this study, including individual's genetic background information, skin type, occupational exposure to chemicals, and the amount of UV radiation exposure received during observation period. Information about HPV vaccination is not available in our database because HPV vaccination payment is not covered in the NHI scheme. Non‐Asian ethnic groups may need further investigation to verify the implication of our study because of a possible epidemiological difference as a result of ethnic and geological factors. A small number of skin cancer diagnoses occurred in the study, thus limiting the conclusions somewhat. Increased surveillance following a diagnosis of HPV might occur, thus potentially biasing the results.
Conclusion
A prominent interaction between HPV infection and skin cancers was observed in this study. The results of our analysis may raise consensus on the effect of HPV infection on skin cancers. Clinicians are advised to implement prudently on the differential diagnosis of skin cancers and HPV prevention and treatment, especially in older patients.
Author Contributions
Study conception/design: Ming‐Li Chen, Shuo‐Hsuan Wang, James Cheng‐Chung Wei, Hei‐Tung Yip, Yao‐Min Hung, Renin Chang
Collection and/or assembly of data: Hei‐Tung Yip
Analysis and interpretation of data: Ming‐Li Chen, James Cheng‐Chung Wie, Hei‐Tung Yip, Yao‐Min Hung, Renin Chang
Writing (original draft preparation): Ming‐Li Chen, Shuo‐Hsuan Wang, Yao‐Min Hung, Renin ChangWriting (review and editing): Hung, Renin Chang
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplement Table 1. The baseline characteristics in sensitivity analysis.
Acknowledgments
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109‐TDU‐B‐212–114004), MOST Clinical Trial Consortium for Stroke (MOST 109–2321‐B‐039–002), China Medical University Hospital (DMR‐109‐231), Tseng‐Lien Lin Foundation, Taichung, Taiwan.
The authors express appreciation to the Department of Medical Education and Research and the Research Center of Medical Informatics in Kaohsiung Veterans General Hospital for their comments.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Contributor Information
Yao‐Min Hung, Email: ymhung1@gmail.com.
Renin Chang, Email: rhapsody1881@gmail.com.
References
- 1. Asgari MM, Kiviat NB, Critchlow CW et al. Detection of human papillomavirus DNA in cutaneous squamous cell carcinoma among immunocompetent individuals. J Invest Dermatol 2008;128:1409–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018;78:237–247. [DOI] [PubMed] [Google Scholar]
- 3. Omland SH, Ahlström MG, Gerstoft J et al. Risk of skin cancer in patients with HIV: A Danish nationwide cohort study. J Am Acad Dermatol 2018;79:689–695. [DOI] [PubMed] [Google Scholar]
- 4. Jellouli‐Elloumi A, Kochbati L, Dhraief S et al. Cancers arising from burn scars: 62 cases [in French]. Ann Dermatol Venereol 2003;130:413–416. [PubMed] [Google Scholar]
- 5. Kharazmi E, Fallah M, Sundquist K et al. Familial risk of early and late onset cancer: Nationwide prospective cohort study. BMJ 2012;345:e8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasche D, Stephan S, Braspenning‐Wesch I et al. The interplay of UV and cutaneous papillomavirus infection in skin cancer development. PLoS Pathog 2017;13:e1006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karagas MR, Nelson HH, Sehr P et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst 2006; 98:389–395. [DOI] [PubMed] [Google Scholar]
- 8. Patel AS, Karagas MR, Perry AE et al. Exposure profiles and human papillomavirus infection in skin cancer: An analysis of 25 genus beta‐types in a population‐based study. J Invest Dermatol 2008;128:2888–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farzan SF, Waterboer T, Gui J et al. Cutaneous alpha, beta and gamma human papillomaviruses in relation to squamous cell carcinoma of the skin: A population‐based study. Int J Cancer 2013;133:1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iannacone MR, Gheit T, Waterboer T et al. Case‐control study of cutaneous human papillomaviruses in squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev 2012;21:1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Aldabagh B, Yu J et al. Role of human papillomavirus in cutaneous squamous cell carcinoma: A meta‐analysis. J Am Acad Dermatol 2014;70:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forslund O, Ly H, Reid C et al. A broad spectrum of human papillomavirus types is present in the skin of Australian patients with non‐melanoma skin cancers and solar keratosis. Br J Dermatol 2003;149:64–73. [DOI] [PubMed] [Google Scholar]
- 13. Iftner A, Klug SJ, Garbe C et al. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high‐risk genital types as possible risk factors. Cancer Res 2003;63:7515–7519. [PubMed] [Google Scholar]
- 14. Ben Ayed I, Tounsi H, Jaballah A et al. Mucosal human papillomavirus detection and TP53 immunohistochemical expression in non‐melanoma skin cancer in Tunisian patients. J Cutan Pathol 2019;46:591–598. [DOI] [PubMed] [Google Scholar]
- 15. Moody CA, Laimins LA. Human papillomavirus oncoproteins: Pathways to transformation. Nat Rev Cancer 2010;10:550–560. [DOI] [PubMed] [Google Scholar]
- 16. Akgül B, Lemme W, García‐Escudero R et al. UV‐B irradiation stimulates the promoter activity of the high‐risk, cutaneous human papillomavirus 5 and 8 in primary keratinocytes. Arch Virol 2005;150:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roussaki‐Schulze AV, Kouskoukis C, Rammos C et al. Identification of human papillomavirus DNA in melanoma biopsy specimens of Greek population. Int J Clin Pharmacol Res 2005;25:145–150. [PubMed] [Google Scholar]
- 18. Merrill SJ, Subramanian M, Godar DE. Worldwide cutaneous malignant melanoma incidences analyzed by sex, age, and skin type over time (1955‐2007): Is HPV infection of androgenic hair follicular melanocytes a risk factor for developing melanoma exclusively in people of European‐ancestry? Dermatoendocrinol 2016;8:e1215391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cun B, Song X, Jia R et al. Cell growth inhibition in HPV 18 positive uveal melanoma cells by E6/E7 siRNA. Tumour Biol 2013;34:1801–1806. [DOI] [PubMed] [Google Scholar]
- 20. Apalla Z, Nashan D, Weller RB et al. Skin cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther (Heidelb) 2017;7(suppl 1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baez CF, Gonçalves MTV, da Rocha WM et al. Investigation of three oncogenic epitheliotropic viruses shows human papillomavirus in association with non‐melanoma skin cancer. Eur J Clin Microbiol Infect Dis 2019;38:1129–1133. [DOI] [PubMed] [Google Scholar]
- 22. Haedicke J, Iftner T. Human papillomaviruses and cancer. Radiother Oncol 2013;108:397–402. [DOI] [PubMed] [Google Scholar]
- 23. Martínez‐Bailón C, Mantilla‐Morales A, Méndez‐Matías G et al. Human papillomavirus genotypes and P16INK4A expression in squamous penile carcinoma in Mexican patients. BMC Infect Dis 2019;19:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lellis RF, Veasey JV, Goncalves RDJ. Pigmented Bowen's disease associated with high‐risk HPV simulating melanoma of the hand. An Bras Dermatol 2017; 92:686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parfenov M, Pedamallu CS, Gehlenborg N et al. Characterization of HPV and host genome interactions in primary head and neck cancers. Proc Natl Acad Sci USA. 2014;111:15544–15549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olthof NC, Speel EJ, Kolligs J et al. Comprehensive analysis of HPV16 integration in OSCC reveals no significant impact of physical status on viral oncogene and virally disrupted human gene expression. PLoS One 2014;9:e88718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gheit T. Mucosal and cutaneous human papillomavirus infections and cancer biology. Front Oncol 2019;9:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viarisio D, Müller‐Decker K, Accardi R et al. Beta HPV38 oncoproteins act with a hit‐and‐run mechanism in ultraviolet radiation‐induced skin carcinogenesis in mice. PLoS Pathog 2018;14:e1006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. La Placa M, Ambretti S, Bonvicini F et al. Presence of high‐risk mucosal human papillomavirus genotypes in primary melanoma and in acquired dysplastic melanocytic naevi. Br J Dermatol 2005;152:909–914. [DOI] [PubMed] [Google Scholar]
- 30. Dahlgren L, Schedvins K, Kanter‐Lewensohn L et al. Human papilloma virus (HPV) is rarely detected in malignant melanomas of sun sheltered mucosal membranes. Acta Oncol 2005;44:694–699. [DOI] [PubMed] [Google Scholar]
- 31. Ruer JB, Pépin L, Gheit T et al. Detection of alpha‐ and beta‐human papillomavirus (HPV) in cutaneous melanoma: A matched and controlled study using specific multiplex PCR combined with DNA microarray primer extension. Exp Dermatol 2009;18:857–862. [DOI] [PubMed] [Google Scholar]
- 32. Schmidt SA, Hamilton‐Dutoit SJ, Farkas DK et al. Human papillomavirus and the incidence of nonmelanoma and melanoma skin cancer using cervical conization as a surrogate marker: A nationwide population‐based Danish cohort study. Ann Epidemiol 2015;25:293–296.e2. [DOI] [PubMed] [Google Scholar]
- 33. Nunez‐Troconis J, Delgado M, Gonzalez G et al. Melanosis of the vagina and human papillomavirus infection, an uncommon pathology: Case report. Invest Clin 2011;52:268–273. [PubMed] [Google Scholar]
- 34. Snow JA, Murthy V, Dacus D et al. β‐HPV 8E6 attenuates ATM and ATR signaling in response to UV damage. Pathogens 2019;8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5:a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blackford AN, Jackson SP. ATM, ATR, and DNA‐PK: The trinity at the heart of the DNA damage response. Mol Cell 2017;66:801–817. [DOI] [PubMed] [Google Scholar]
- 37. O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell 2015;60:547–560. [DOI] [PubMed] [Google Scholar]
- 38. Hufbauer M, Cooke J, van der Horst GT et al. Human papillomavirus mediated inhibition of DNA damage sensing and repair drives skin carcinogenesis. Mol Cancer 2015;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang JW, Yeh KY, Wang CH et al. Malignant melanoma in Taiwan: A prognostic study of 181 cases. Melanoma Res 2004;14:537–541. [DOI] [PubMed] [Google Scholar]
- 40. Basurto‐Lozada P, Molina‐Aguilar C, Castaneda‐Garcia C et al. Acral lentiginous melanoma: Basic facts, biological characteristics and research perspectives of an understudied disease. Pigment Cell Melanoma Res 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zumsteg ZS, Cook‐Wiens G, Yoshida E et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol 2016;2:1617–1623. [DOI] [PubMed] [Google Scholar]
- 42. Windon MJ, D'Souza G, Rettig EM et al. Increasing prevalence of human papillomavirus‐positive oropharyngeal cancers among older adults. Cancer 2018;124:2993–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. D'Souza G, Westra WH, Wang SJ et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol 2017;3:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fakhry C, Waterboer T, Westra WH et al. Distinct biomarker and behavioral profiles of human papillomavirus‐related oropharynx cancer patients by age. Oral Oncol 2020;101:104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palve JS, Korhonen NJ, Luukkaala TH et al. Differences in risk factors for melanoma in young and middle‐aged higher‐risk patients. In Vivo 2020;34:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gravitt PE, Rositch AF, Silver MI et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis 2013;207:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown DR, Weaver B. Human papillomavirus in older women: New infection or reactivation? J Infect Dis 2013;207:211–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beachler DC, Sugar EA, Margolick JB et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV‐infected and HIV‐uninfected adults. Am J Epidemiol 2015;181:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hsing AW, Ioannidis JP. Nationwide population science: Lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med 2015;175:1527–1529. [DOI] [PubMed] [Google Scholar]
- 50. Kvaskoff M, Mesrine S, Fournier A et al. Personal history of endometriosis and risk of cutaneous melanoma in a large prospective cohort of French women. Arch Intern Med 2007;167:2061–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li WQ, Qureshi AA, Ma J et al. Personal history of prostate cancer and increased risk of incident melanoma in the United States. J Clin Oncol 2013;31:4394–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplement Table 1. The baseline characteristics in sensitivity analysis.


