Abstract
Background
Cardiotoxicity induced by 5‐fluorouracil (5‐FU) is well known but poorly understood. In this study, we undertook ECG recording (Holter) and analyses of the biomarkers troponin and copeptin in patients receiving 5‐FU to increase our understanding of the cardiotoxicity.
Subjects, Materials, and Methods
Patients with colorectal or anal cancer that received first‐time treatment with 5‐FU‐based chemotherapy were prospectively included. Holter recording, clinical evaluation, 12‐lead electrocardiogram, and assessment of plasma concentrations of troponin I and copeptin were performed before (control) and during 5‐FU treatment (intervention).
Results
A total of 108 patients were included, 82 with colorectal and 26 with anal cancer. The proportion of patients with myocardial ischemia on Holter recording was significantly higher during the first 5‐FU infusion (14.1%) than before (3.7%; p = .001). The ischemic burden per day (p = .001), the number of ST depression episodes per day (p = .003), and the total duration of ischemic episodes per day (p = .003) were higher during the first 5‐FU infusion than before, as was plasma copeptin (p < .001), whereas plasma troponin I was similar (p > 0.999). Six patients (5.6%) developed acute coronary syndromes and two (1.8%) developed symptomatic arrhythmias during 5‐FU treatment.
Conclusion
5‐FU infusion is associated with an increase in the number of patients with myocardial ischemia on Holter recording. According to biomarker analyses, 5‐FU is associated with an increase in copeptin, but rarely with increases in cardiac troponin I. However, 5%–6% of the patients developed acute coronary syndromes during treatment with 5‐FU.
Implications for Practice
Symptomatic 5‐fluorouracil (5‐FU) cardiotoxicity occurs in 0.6%–19% of patients treated with this drug, but a small electrocardiographic (Holter) study has revealed silent myocardial ischemia in asymptomatic patients, suggesting a more prevalent subclinical cardiac influence. This study demonstrated a significant increase in the number of patients with myocardial ischemia on Holter recording during 5‐FU treatment and an increase in ischemic burden. Cardiac biomarker analyses suggested that 5‐FU infusion results in endogenous stress (increased copeptin) but rarely induces myocyte injury (no change in troponin). These findings suggest a more prevalent cardiac influence from 5‐FU and that Holter recording is an important tool in the evaluation of patients with suspected cardiotoxicity from 5‐FU.
Keywords: 5‐Flourouracil, Cardiotoxicity, Myocardial ischemia, Colorectal cancer, Anal cancer
Short abstract
Cardiotoxicity related to 5‐fluorouracil (5‐FU) treatment is poorly understood. This article reports results of a study using electrocardiogram results to analyze the biomarkers troponin and copeptin in patients receiving 5‐FU.
Introduction
The chemotherapeutic agent 5‐fluorouracil (5‐FU) is one of the most widely used drugs in oncology [1], and intravenous 5‐FU remains an essential part of the treatment of various solid tumors such as colorectal and anal cancer [2, 3, 4]. However, a severe side effect is cardiotoxicity [5, 6]. The incidence of symptomatic cardiotoxicity induced by 5‐FU varies between 0% and 20% in published studies, with larger studies reporting incidences around 1%–5% [6]. Most studies report symptomatic cardiotoxicity, but two small studies with continuous electrocardiographic (Holter) recording reveal silent myocardial ischemia and ventricular arrhythmias in asymptomatic patients, indicating a more prevalent subclinical cardiac influence [7, 8]. Furthermore, a study of exercise‐induced myocardial ischemia during 5‐FU infusion showed that silent myocardial ischemia was more prevalent than angina during a treadmill stress test, substantiating the suspicion of subclinical cardiotoxicity [9]. In addition, several case reports of patients incurring cardiac arrest or sudden death during 5‐FU have been published [10, 11, 12, 13], which underlines the need for an understanding of the cardiotoxic effects of 5‐FU.
Cardiac troponins are established biomarkers to detect and rule out myocardial infarction [14]. They are released from the cardiomyocytes during myocardial damage and are the most sensitive markers of myocardial injury [15, 16]. Yet, previous studies of cardiac troponins during 5‐FU treatment have not found a statically significant increase compared with baseline, but all studies included a small number of patients [17–21].
Copeptin is the C‐terminal part of the vasopressin prohormone [22]. It is secreted stoichiometrically together with arginine‐vasopressin from the neurohypophysis as part of the stress response [22]. The physiological role of copeptin itself is unknown [23], but the antidiuretic and vasoconstrictive effects of the arginine vasopressin hormone results in increased cardiac output and reduced vascular resistance [24, 25]. In contrast to arginine‐vasopressin, copeptin is stable in plasma and serum and easy to measure [23, 26]. Hence, copeptin is regarded as a marker of endogenous stress [27, 28]. In the diagnostics of acute myocardial infarction, the additional use of copeptin improves the diagnostic accuracy compared with troponin alone [29]. Furthermore, copeptin is a predictor of death in patients with coronary artery disease [30] and in patients with heart failure after acute myocardial infarction [23]. We measured the incidence of 5‐FU‐induced myocardial ischemia (ST elevation and/or ST depression) using Holter recording and evaluated plasma troponin I and plasma copeptin during 5‐FU treatment. In addition, we evaluated the incidence of clinical events and ventricular tachyarrhythmias and monitored the corrected QT (QTc) interval before and during 5‐FU administration.
Subjects, Materials, and Methods
Patients
Patients with colorectal cancer treated with 5‐FU‐based regimens in the adjuvant or metastatic setting (February 2013 to September 2016) and patients with local or locally advanced anal cancer treated with concomitant chemoradiation including 5‐FU (December 2014 to November 2016) were eligible. All patients were consecutively screened for inclusion. Exclusion criteria were age <18 years, pacemaker or implantable cardioverter defibrillator, prior treatment with fluoropyrimidines, and concurrent treatment with bevacizumab. Informed consent was obtained from all participants, and the study was approved by the Regional Committee on Health Research Ethics (H‐1‐2012‐054) and the Danish Data Protection Agency (HEH.750.24‐59).
Treatment Regimens
In the adjuvant setting, patients with colorectal cancer received 12 cycles of 5‐FU (modified de Gramont schedule) or 5‐FU + oxaliplatin (FOLFOX). In the metastatic setting, chemotherapy regimens included FOLFOX and 5‐FU + irinotecan (FOLFIRI) ± cetuximab or panitumumab. 5‐FU was administered as a bolus infusion (400 mg/m2) followed by a 46‐hour infusion (2,400 mg/m2). Patients with anal cancer received two cycles of 5‐FU and cisplatin concomitant with radiotherapy. 5‐FU was administered as a 96‐hour infusion (3,200 mg/m2; supplemental online Table 1).
Study Examinations
Information on cardiovascular health and risk factors for cardiovascular disease was obtained from medical records, and all participants filled in a questionnaire about cardiovascular comorbidities, symptoms, and risk factors (supplemental online Table 2).
Holter recording, 12‐lead ECG, clinical evaluation, and assessment of cardiac biomarkers were performed before and during the first cycle and again before and during the third/fourth cycle of chemotherapy in patients with colorectal cancer and during the second cycle in patients with anal cancer. Holter recording was scheduled to start 1–3 days before 5‐FU initiation and continue for 2 days during infusion in patients receiving bolus plus 46‐hour continuous infusion and for 4 days during infusion in patients receiving 96‐hour continuous infusion. 12‐lead ECG, clinical evaluation, and assessment of cardiac biomarkers were performed on the day the Holter recording was initiated (day 1–3 before 5‐FU initiation) and at the end of 5‐FU infusion (day 2 of treatment in patients receiving bolus plus 46‐hour continuous infusion and day 4 of treatment during infusion in patients receiving 96‐hour continuous infusion). Additionally, clinical evaluation, biomarker assessment, and 12‐lead ECG were performed in patients with cardiac symptoms. Patients were instructed to record cardiac symptoms in a diary and to contact the department if symptoms of possible cardiac origin occurred.
Two‐channel Lifecard CF Holter recorders (Delmar Reynolds, Spacelabs Healthcare, Snoqualmie, WA) with a capacity of up to 7 days of recording were used. Electrodes were placed to obtain the bipolar leads CM5 and CC5 (supplemental online Fig. 1).
Analyses of Holter recordings were performed semimanually in Pathfinder SL v1.7 (Spacelabs Healthcare) by a trained physician (A.D.‐P.). All episodes of myocardial ischemia or ventricular tachyarrhythmia were confirmed by an experienced cardiologist (M.V.‐N.). Intraobserver variability showed good to excellent agreement (intercorrelation coefficient or κ > 0.80; supplemental online Table 3).
All ECGs were performed with the same ECG device (MAC 5500 HD, GE Healthcare, Chicago, IL). Myocardial ischemia on 12‐lead ECG was defined as significant ST elevation or significant ST depression in at least two adjacent leads or negative T‐waves of ≥0.1 mV in two adjacent leads with prominent R or R/S >1 (supplemental online Table 4) [31, 32]. The QTc interval was estimated from 12‐lead ECGs by use of Bazett's formula (supplemental online Table 4) [33]. Troponin I was measured on Li‐heparin plasma using the ADVIA Centaur TnI‐Ultra assay (Siemens Healthcare Diagnostics Inc, Erlangen, Germany; upper 99th percentile cutoff: 40 ng/L, coefficient of variation at the 99th percentile: 10%, calibration traceable to NIST standard reference material 2921) [15]. Copeptin was measured on EDTA plasma by use of Thermo Scientific BRAMS Copeptin provasopressin immunofluorescent assay (Thermo Fisher Diagnostics, Allerød, DK; lower detection limit: 0.69 pmol/L, intra‐assay coefficient of variation <15%, interassay coefficient of variation <18%, reference ranges [2.5 and 97.5 percentiles] 1.7 and 11.25 pmol/L) [34].
Endpoints
The primary endpoint was myocardial ischemia on Holter recording. Secondary endpoints were elevations and fluctuations in troponin I, clinical events, QTc interval prolongation, and ventricular tachyarrhythmias (for definitions, see supplemental online Table 4). Copeptin was explored as a continuous variable. Second, the number of patients with copeptin levels above the suggested cutoff for myocardial infarction (>10 pmol/L) was calculated.On the Holter recordings, an episode of myocardial ischemia was defined as ST elevation of ≥1 mV measured in the J‐point lasting ≥1 minute or downsloped or horizontal ST depression of ≥1 mV measured 60 ms after the J‐point lasting ≥1 minute. An interval of ≥1 minute with no ST deviations should be present before a new episode was counted (supplemental online Table 4) [35]. The PR segment corrected for baseline ST abnormalities was used as a reference point. The extent of myocardial ischemia was expressed as the ischemic burden calculated by multiplication of the amplitude and the duration of ST deviations in the channel with most pronounced ischemia. We also recorded the duration of ischemic episodes per day and number of ST‐elevation and ST‐depression episodes per day. Day‐to‐day variation in ischemic episodes was calculated by subtracting the “best day” from the “worst day.” New‐onset myocardial ischemia was defined as myocardial ischemia during 5‐FU treatment in patients without any ischemia before 5‐FU start. Patients with left bundle branch block at baseline 12‐lead ECG were considered unsuitable for ischemia analyses at both 12‐lead ECG and Holter recordings and were only evaluated for arrhythmias.
Clinical events were defined as acute coronary syndromes, symptomatic tachyarrhythmias, and cardiac arrest. Acute coronary syndromes and myocardial infarction were defined according to current guidelines from the European Society of Cardiology [14, 36]. For definition of endpoints, see supplemental online Table 4.
After inclusion of one third of the patients, one patient developed cardiac arrest after cessation of 5‐FU, and Holter recording revealed ST elevation. After this event, we reviewed all recordings for myocardial ischemia and ventricular tachyarrhythmia on the day the Holter recorder was removed.
Statistical Analysis
Continuous variables and counts on Holter recording were adjusted for technically acceptable recording time per day. To compare variables before and during 5‐FU infusion, the McNemar test was used for proportions, a paired t test for continuous variables with a Gaussian distribution, and the Wilcoxon signed rank test for continuous outcomes with a non‐Gaussian distribution. For analyses of repeated measures on continuous outcomes with a non‐Gaussian distribution, the Friedman's test was used. The Wilcoxon pairwise test with Bonferroni correction was applied post hoc if the Friedman's test was significant. The distribution of plasma troponin I measurements was non‐Gaussian, whereas the log‐transformed copeptin values and log‐transformed QTc values followed a Gaussian distribution. All tests were two‐sided and p < .05 was considered statistically significant. Statistical analyses were performed in IBM SPSS Statistics 22 (IBM, Armonk, NY) and SAS version 9.4M2 (SAS Institute, Cary, NC).
Results
Of 270 patients asked to participate, 108 accepted (Fig. 1). Table 1 summarizes baseline characteristics. Eighty‐two patients received bolus 5‐FU followed by 46‐hour infusion, whereas 26 patients (24.1%) received 5‐FU for 96 hours.
Figure 1.
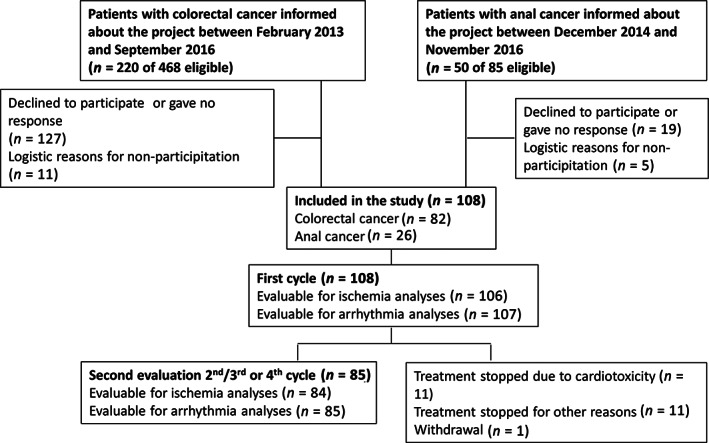
Flow diagram of patient inclusion and study examinations. Reasons for stopping treatment due to cardiotoxicity before second evaluation were as follows: acute coronary syndromes (five patients), silent myocardial ischemia (three patients), nonsustained ventricular tachycardia (two patients), and asymptomatic but excessive supraventricular ectopy and atrial fibrillation (one patient).
Table 1.
Patient characteristics
| All patients in the study (n = 108) | Patients with myocardial ischemia on Holter recording (n = 20) | Patients with acute coronary syndromes a (n = 6) | Patients with silent myocardial ischemia on Holter recording (n = 16) | Patients without symptoms or myocardial ischemia b (n = 84) | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Sex, male/female | 59/49 (54.6/45.4) | 13/7 (65.0/35.0) | 2/4 (33.3/66.7) | 4/12 (25.0/75.0) | 41/45 (47.7/52.3) |
| Age, median (range), years | 66 (35–81) | 66 (47–76) | 72 (47–78) | 66 (47–76) | 66 (35–81) |
| Localization of cancer | |||||
| Anal | 26 (24.1) | 6 (30.0) | 1 (16.7) | 5 (31.3) | 20 (23.3) |
| Colon | 63 (58.3) | 10 (50.0) | 3 (50.0) | 8 (50.0) | 52 (60.5) |
| Rectum | 19 (17.6) | 4 (20.0) | 2 (33.3) | 3 (18.8) | 14 (16.3) |
| Treatment setting | |||||
| Chemoradiation (curative intent) | 26 (24.1) | 6 (30.0) | 1 (16.7) | 5 (31.3) | 20 (23.3) |
| Adjuvant (curative intent) | 76 (70.4) | 11 (55.0) | 3 (50.0) | 9 (56.3) | 64 (74.4) |
| Metastatic (all palliative intent) | 6 (5.6) | 3 (15.0) | 2 (33.3) | 2 (12.5) | 2 (2.3) |
| Schedule of chemotherapy | |||||
| 5‐FU and cisplatin c | 26 (24.1) | 6 (30.0) | 1 (16.7) | 5 (31.3) | 20 (23.3) |
| FOLFOX d | 76 (70.4) | 12 (60.0) | 4 (66.7) | 10 (62.5) | 62 (72.1) |
| de Gramont | 2 (1.9) | 0 | 0 | 0 | 2 (2.3) |
| FOLFIRI e | 4 (3.7) | 2 (10.0) | 1 (16.7) | 1 (6.3) | 2 (2.3) |
| Dose intensity, start | |||||
| 100% | 95 (88.0) | 19 (86.3) | 4 (66.7) | 15 (93.7) | 76 (88.4) |
| 75%–85% | 13 (12.0) | 3 (13.6) | 2 (33.3) | 1 (6.3) | 10 (11.6) |
| Prior chest irradiation | 3 (2.8) | 1 (5.0) | 0 | 1 (6.3) | 2 (2.3) |
| Ischemic heart disease | 1 (0.9) | 1 (5.0) | 0 | 1 (6.3) | 0 |
| Previous stroke or transient cerebral ischemia | 8 (7.4) | 1 (5.0) | 0 | 1 (6.3) | 7 (8.1) |
| Heart failure | 1 (0.9) | 0 | 0 | 0 | 1 (1.2) |
| Atrial fibrillation or fluttering | 5 (4.6) | 0 | 0 | 0 | 5 (5.8) |
| Other heart diseases f | 3 (2.7) | 1 (5.0) | 0 | 1 (6.3) | 2 (2.3) |
| Hypertension | 35 (32.4) | 9 (45.0) | 3 (50.0) | 7 (43.8) | 25 (29.1) |
| Hypercholesterolemia | 73 (67.6) | 13 (65.0) | 4 (66.7) | 11 (68.8) | 58 (67.4) |
| Diabetes mellitus | 6 (5.6) | 0 | 0 | 0 | 6 (7.1) |
| Smoking status | |||||
| Current smoker | 16 (14.8) | 3 (15.0) | 0 | 3 (18.8) | 13 (15.1) |
| Former smoker | 58 (53.7) | 10 (50.0) | 2 (33.3) | 8 (50.0) | 48 (55.8) |
| Never smoked | 34 (31.5) | 7 (35.0) | 4 (66.7) | 5 (31.3) | 25 (29.1) |
| BMI | |||||
| Underweight (BMI <18.5) | 2 (1.9) | 0 | 0 | 0 | 2 (2.3) |
| Normal (BMI 18.5–24.9) | 45 (41.7) | 8 (40.0) | 3 (50.0) | 6 (37.5) | 36 (41.9) |
| Overweight (BMI 25.0–29.9) | 45 (41.7) | 10 (50.0) | 3 (50.0) | 8 (50.0) | 34 (39.5) |
| Obese (BMI >29.9) | 16 (14.8) | 2 (10.0) | 0 | 2 (12.5) | 14 (16.3) |
| Cardiac medications | |||||
| Beta blocker | 8 (7.4) | 2 (10.0) | 0 | 2 (12.5) | 6 (7.0) |
| Calcium channel blocker | 7 (6.5) | 1 (5.0) | 1 (16.7) | 1 (6.3) | 5 (5.8) |
| Digoxin | 0 | 0 | 0 | 0 | 0 |
| NYHA class at baseline | |||||
| 1–2 | 101 (93.5) | 18 (90.0) | 6 (100.0) | 14 (87.5) | 81 (94.2) |
| 3 | 7 (6.5) | 2 (10.0) | 0 | 2 (12.5) | 5 (5.8) |
| Baseline troponin I | |||||
| >40 ng/L (%) | 2 (1.9) | 1 (4.5) | 1 (16.7) | 0 | 1 (1.2) |
| Low eGFR (<60 mL/min/1.73 m2) | 1 (6.3) | 1 (5.0) | 0 | 1 (6.3) | 0 |
| Anemia g | 40 (37.0) | 11 (55.0) | 4 (66.7) | 8 (50.0) | 28 (32.6) |
One of six patients had chest pain without objective evidence of myocardial ischemia on Holter recording or 12‐lead ECG.
Patients with acute coronary syndromes and myocardial ischemia and the two patients with symptomatic arrhythmias are not included in this column.
One patient received only 5‐FU and no cisplatin.
One patient received treatment with FOLFOX + epidermal growth factor receptor inhibitor.
One patient received treatment with FOLFIRI + epidermal growth factor receptor inhibitor.
Aortic and mitral valve diseases.
Defined according to World Health Organization: hemoglobin <7.4 mmol/L for nonpregnant women and <8.1 mmol/L for men >15 years.
Abbreviations: 5‐FU, 5‐fluorouracil; BMI, body mass index; eGFR, estimated glomerular filtration rate; FOLFIRI, 5‐FU + irinotecan; FOLFOX, 5‐FU + oxaliplatin; NYHA, New York Heart Association.
A Holter recording during the first cycle of treatment was obtained in all patients but one (due to incorrect start of the recorder). One patient had left bundle branch block and was excluded from the ischemia analyses (Fig. 1). A Holter recording was obtained during the second/third or fourth cycle in 85 patients, and 84 were evaluable for ischemia analyses (one left bundle branch block; Fig. 1). Median recording time before and during 5‐FU administration is shown in supplemental online Table 7. Twenty‐eight patients reported possible cardiac symptoms (22 in the first cycle, 3 in the second cycle, 2 in the third cycle, and 1 in the fourth cycle) and these were interpreted as clinical events in 8 (acute coronary syndromes in 6 and arrhythmias in 2; supplemental online Fig. 2).
Myocardial Ischemia
Day‐to‐Day Variation in Ischemic Episodes Before and During 5‐FU
The day‐to‐day variation in number of ischemic episodes at baseline before the first cycle ranged from 0 to 19 (0%–60%), whereas the day‐to‐day variation in duration of ischemic episodes and total ischemic burden ranged from 0 to 65.3 minutes (0%–56%) and from 0 to 188.4 mm*min (58%), respectively (for detailed description, see supplemental online data). Before the second/third or fourth cycle, the day‐to‐day variation in number of episodes was 0–3 (0%–100%), the day‐to‐day variation in total duration of episodes was 0–42.0 minutes (0%–100%), and the day‐to‐day variation in ischemic burden was 0–73.5 (0%–100%).
The day‐to‐day variability in ischemic burden during 5‐FU treatment ranged from 0 to 3,049.6 mm*min. The ischemic burden for each day of recording in patients with myocardial ischemia during the first cycle is shown in supplemental online Figure 3 and during the second/third or fourth cycle in supplemental online Figure 4.
Myocardial Ischemia During 5‐FU Infusion Compared with Before Infusion
Myocardial ischemia was seen in 20 patients (18.7%) during Holter recording during either the first cycle or the second/third/fourth cycle (supplemental online Tables 5, 6). Of these, 16 patients (15.0% of all patients) had silent myocardial ischemia. The proportion of patients with myocardial ischemia was significantly higher during the first 5‐FU infusion (15 patients, 14.1%) than before 5‐FU initiation (four patients, 3.7%; p = .001; Fig. 2), as were the ischemic burden per day (p = .001), the number of ST depression episodes per day (p = .003), and the total duration of ischemic episodes per day (p = .003). The number of ST‐elevation episodes before and during the first infusion was similar (p = .12; supplemental online Table 8).
Figure 2.
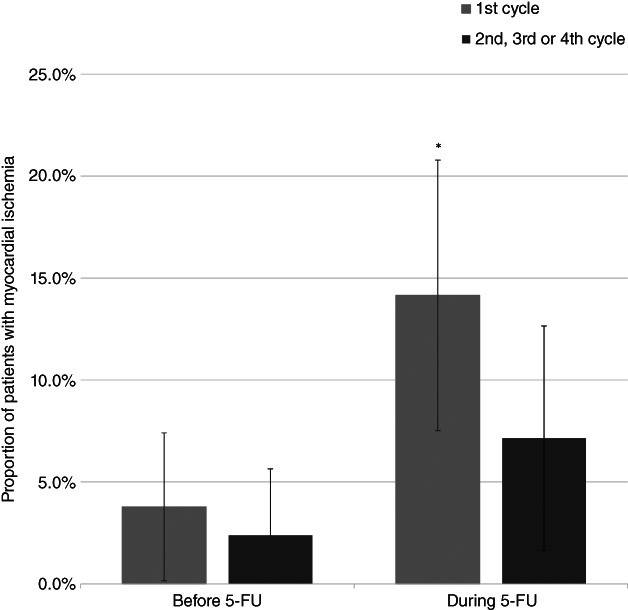
The proportion of patients with myocardial ischemia on Holter recording before and during 5‐FU infusion for the first cycle and the second/third/fourth cycle, respectively. The error bars illustrate the 95% confidence interval and * indicates a statistically significant difference between the proportion of patients with myocardial ischemia before 5‐FU infusion and proportion of patients with myocardial ischemia during 5‐FU infusion.Abbreviation: 5‐FU, 5‐fluorouracil.
During the second Holter recording, two patients (2.4%) had myocardial ischemia before the start of 5‐FU infusion and six (7.1%) had myocardial ischemia during infusion (p = .13). Again, the ischemic burden per day was significantly higher during 5‐FU infusion than before (p = .046), but the number of ST‐elevation episodes (p = .66), the number of ST‐depression episodes (p = .068), and the total duration of ischemia per day (p = .075) were similar (supplemental online Table 8).
The incidences of new‐onset myocardial ischemia according to the two 5‐FU schedules were similar, 11.1% for 46‐hour infusion and 15.3% for 96‐hour infusion (difference: 4.2%, 95% confidence interval [CI]: −11.2% to 19.7%, p = .51). Treatment with 5‐FU was stopped because of myocardial ischemia in eight patients (seven during/after the first cycle and one during the second cycle), while the dose was reduced in two (after first cycle).
Myocardial Ischemia According to Day in Cycle 1
The occurrence of myocardial ischemia according to the day in the cycle was examined separately in patients receiving bolus plus 46‐hour or 96‐hour infusion. For patients receiving bolus plus 46‐hour infusion, the ischemic burden (p = .027), the total duration of ischemia (p = .023), and the number of ST‐depression episodes (p = .042) were significantly higher on day 2 of treatment compared with before 5‐FU initiation (supplemental online Tables 9, 10). In contrast, there were no differences in ischemic burden (p = .93), total duration of ischemic episodes (p = .91), and number of ST‐elevation (p = .56) or ST‐depression episodes (p = .89) with regard to day of 5‐FU treatment in patients receiving 96‐hour infusion (supplemental online Table 10).
Biomarkers
Elevations in troponin I above the cutoff level were only observed in the 2 patients with myocardial infarction but increases in troponin I plasma concentrations larger than the assay variation but below the cutoff were observed in 7 patients (6.5%) during the first cycle and in 10 patients (11.8%) during the second/third/fourth cycle. However, compared with levels before 5‐FU infusion, plasma troponin I levels remained similar during the first cycle (p > .999) and during the second/third/fourth cycle (p = .37; Fig. 3). In contrast, the geometric mean of copeptin increased from 5.6 pmol/L (95% CI: 5.0–6.3 pmol/L) before to 7.7 pmol/l (6.8–8.7 pmol/L) during the first infusion (P < .001) and from 7.0 (95% CI: 6.2–7.9 pmol/L) before to 9.5 pmol/L (95% CI: 8.3–11.0 pmol/L) during the second/third/fourth cycle (p < .001; Fig. 4). Copeptin values above the suggested cutoff for myocardial infarction (>10 pmol/L) were observed in 17 patients (16.0%) before the first infusion, in 30 patients (29.4%) during the first infusion (p = 0), and in 23 patients (27.7%) before and 36 patients (42.4%) during the second/third/fourth cycle (p = .013). Among the patients with myocardial ischemia on Holter recording during the first infusion, 4 of 15 (27%) had copeptin levels >10 pmol/L, and during the second/third/fourth cycle, 3 of 6 (50%) patients with myocardial ischemia had copeptin >10 pmol/L. Only one patient with copeptin >10 pmol/L had elevated troponin above the cutoff for myocardial infarction (during the first cycle).
Figure 3.
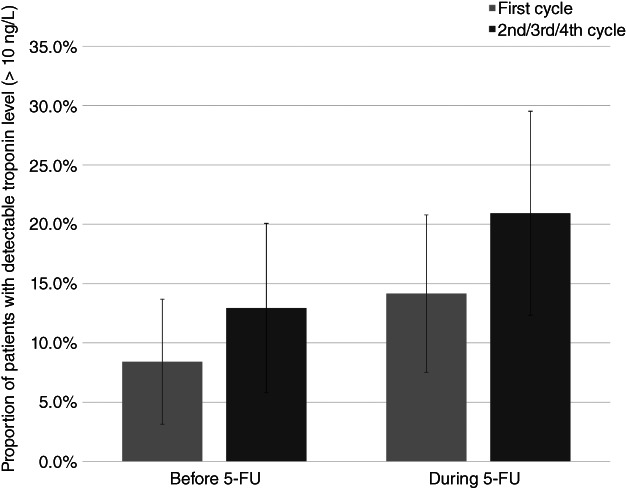
The proportion of patients with detectable troponin I (lower limit of detection: 10 ng/L) before and during 5‐FU infusion for the first cycle and the second/third/fourth cycle, respectively. The error bars illustrate the 95% confidence interval. No significant differences in troponin I levels before and during 5‐FU infusion were found (first cycle: p > .999; second/third/fourth cycle, p = .37).Abbreviation: 5‐FU, 5‐fluorouracil.
Figure 4.
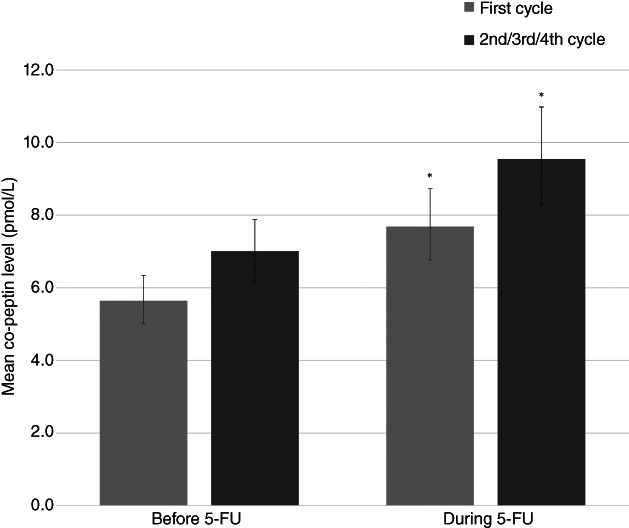
The geometric mean of plasma copeptin before and during 5‐FU infusion for the first cycle and for the second/third/fourth, respectively. The error bars illustrate the 95% confidence interval for the geometric mean and * indicate a statistically significant difference between the geometric mean before 5‐FU infusion and the geometric mean during 5‐FU infusion. Normal range for copeptin in healthy volunteers is 1.70–11.25 pmol/L (2.5 and 97.5 percentile, respectively).Abbreviation: 5‐FU, 5‐fluorouracil.
Nonsustained Ventricular Tachyarrhythmias
Nonsustained ventricular tachycardia (NSVT) was observed in 14 patients (13.0%) before and in 12 patients (11.2%) during the first 5‐FU infusion (p = .36). On the second Holter recording, NSVT was observed in nine patients (10.7%) before and in nine patients (10.7%) during 5‐FU infusion (p > .999). Treatment was stopped owing to NSVT in two patients after the first cycle. There was no statistically significant difference in the number of NSVT episodes according to days of treatment (46‐hour infusion: p = .62; 96‐hour infusion: p = .31).
QTc on 12‐Lead ECG
The geometric mean of the QTc interval decreased from 420 ms (95% CI: 415–425 ms) before to 410 ms (95% CI: 404–415 ms) during the first 5‐FU infusion (p = .001). There was no significant difference in the geometric mean of the QTc interval before (414 ms, 95% CI: 408–419 ms) and during (410 ms, 95% CI: 404–415 ms) the second/third/fourth cycle (p = .13).
Clinical Events
Six patients (5.6%) developed acute coronary syndromes: five during the first and one during the second cycle (supplemental online Table 5). One had unstable angina without ischemia (0.9%), two had unstable angina with ischemia on Holter recordings (1.9%), two had myocardial infarction confirmed by elevated troponin I (one ST‐elevation myocardial infarction and one non‐ST elevation myocardial infarction) (1.9%), and one patient sustained cardiac arrest (0.9%). None of these patients had symptoms or silent ischemia before the start of 5‐FU treatment. During 5‐FU treatment, three of six patients (50%) presented with silent ischemia on Holter recording before debut of clinical symptoms.
Two patients receiving 96‐hour infusion had symptomatic arrhythmias with palpitations and supraventricular tachycardia in one and dizziness and NSVT in one.
Discussion
The main finding in the present study is that myocardial ischemia measured on Holter recordings is increased during 5‐FU administration compared with before treatment. Also, our cardiac biomarker analyses showed that myocyte injury rarely occurs, while increases in copeptin suggest that endogenous stress is present. Furthermore, treatment with 5‐FU did not result in an increased number of NSVT episodes, and the QTc interval remained within normal limits (<460 ms). However, 5%–6% of the patients developed acute coronary syndromes, and 1%–2% developed symptomatic tachyarrhythmia during treatment.
The increase in myocardial ischemia during 5‐FU infusion is in line with findings in a previous Holter study of 25 patients [7]. Yet, the previous study reported a markedly higher prevalence (68%) of myocardial ischemia during 5‐FU. The difference can partly be explained by differences in the prevalence of coronary artery disease and myocardial ischemia at baseline [7]. In contrast, a study of exercise‐induced myocardial ischemia during 5‐FU reported an incidence of myocardial ischemia of 10.3% [9], which is in concordance with our findings of myocardial ischemia in 14.1% of patients during the first infusion. Additionally, the pattern of a higher ischemic burden on day 2 of the 46‐hour infusion is in keeping with the observation that major events often appear toward the end of infusion or during the first days after infusion [37, 38, 39].
Approximately three fourths of patients with myocardial ischemia on Holter recording had silent myocardial ischemia. Silent myocardial ischemia may carry an adverse prognosis, but the clinical significance is unclarified. In coronary heart disease and among middle‐aged and elderly individuals with no apparent heart disease, silent myocardial ischemia has been reported to be associated with increased risk of myocardial infarction and sudden death [40, 41, 42, 43, 44], although results are inconsistent [45]. Because ST elevation was the major finding on Holter recording in the patient sustaining cardiac arrest and ST elevations on Holter recording presented before symptoms in the two patients with myocardial infarction, myocardial ischemia may be a forerunner of severe cardiac events. Then again, only some patients with silent myocardial ischemia progressed to severe cardiac events, and some patients with silent myocardial ischemia during the first cycle did not have myocardial ischemia during the second recording and completed planned treatment without cardiac symptoms. Therefore, discontinuation of treatment because of silent myocardial ischemia may deprive patients of a beneficial treatment.
Furthermore, studies of patients with chronic ischemic heart disease have demonstrated a marked day‐to‐day variation in number and duration of ischemic episodes on Holter recording [46–49]. In line with these findings, the patients with myocardial ischemia on Holter recording before 5‐FU start showed considerable variability in number and duration of episodes and in total ischemic burden per day. Thus, it is possible that some of the increments in myocardial ischemia during 5‐FU treatment may not be due to 5‐FU treatment but may reflect the day‐to‐day variability of ischemic episodes, particularly in patients with minor increases in ischemic episodes.
The incidence of new‐onset myocardial ischemia was numerically higher in patients receiving the 96‐hour continuous 5‐FU infusion compared with patients receiving 46‐hour infusion (15.3% vs. 11.1%); however, the difference was not statistically significant. The lack of statistical significance may be due to the small number of patients receiving 96‐hour continuous infusion in our study. Based on our study, it cannot be concluded whether there are differences in the incidence or risk of myocardial ischemia according to the different 5‐FU infusion protocols. However, a prior study of symptomatic cardiotoxicity has suggested that the incidence of cardiotoxicity is lower for short 5‐FU infusion schedules (<3 hours) [5], and in a case‐series of 10 patients with 5‐FU or capecitabine cardiotoxicity, retreatment with bolus 5‐FU infusion resulted in no further cardiac symptoms [50]. For patients with metastatic colorectal cancer, the overall survival is comparable for bolus 5‐FU and 5‐FU administered according to the De Gramont schedule, but the De Gramont schedule is associated with higher response rates and reduced noncardiac toxicity (reduced incidence of grade 3–4 toxicities, granulocytopenia, diarrhea, and mucositis) [51].
Another alternative is the fluoropyrimidine S‐1, that is, a combination of tegafur, oteracil, and the DPD inhibitor gimeracil. Retreatment with S‐1 in patients with prior cardiotoxicity on 5‐FU or capecitabine is associated with a low rate of recurrent cardiotoxicity [52, 53].
Overall, we found no difference in troponin I levels before and during 5‐FU infusion. This might be due to lack of power but may also suggest that myocyte injury is uncommon and only occurs in severe cases of cardiotoxicity. Prior studies of troponin levels during 5‐FU treatment have shown comparable findings, with no change in troponin during treatment [17–21]. However, the number of patients included in the individual studies of troponin levels during 5‐FU treatment was small (n = 22–59). We found fluctuations in plasma troponin I concentrations below the upper 99th percentile in some patients during 5‐FU infusion. The clinical significance of such fluctuations in relation to 5‐FU cardiotoxicity is unknown. However, in the general population and in apparently healthy older adults, low levels of detectable cardiac troponin I have been associated with all‐cause mortality and cardiovascular death [54, 55], suggesting that even small amounts of detectable troponin are associated with adverse outcomes. Larger studies are needed to clarify the role of fluctuations in troponin during 5‐FU treatment.
Copeptin, the C‐terminal part of the vasopressin prohormone, increased during 5‐FU infusion, indicating a stress response [22]. However, copeptin is not specific for cardiac diseases, and copeptin elevations are seen during critical illness, for example, sepsis, diabetes, and respiratory infections [23]. Thus, the implications of the observed increase in the geometric mean of copeptin in our study are unknown. Importantly, our study was not designed to test copeptin as a diagnostic marker. We measured copeptin before infusion and at the end of the 5‐FU infusion simultaneously with troponin. In patients with myocardial infarction, copeptin is released early after symptom onset and decreases to values below the upper 99th percentile after approximately 10 hours [56]. Thus, the timing of copeptin measurements according to symptom onset is important, and because of the fixed time points for copeptin measurements in our study, the timing may not have been optimal. Furthermore, before the start of 5‐FU, 16% of the patients in our study had copeptin levels above the cutoff for myocardial infarction, indicating that the arginine‐vasopressin system was activated in some patients with colorectal or anal cancer even before start of chemotherapy. Future studies of the role of copeptin in 5‐FU cardiotoxicity should evaluate the biomarker together with cardiac troponins at symptom presentation.
We observed no increase in the number of NSVT episodes and changes in QTc interval during 5‐FU. These findings suggest that ventricular tachyarrhythmias are not the primary cause of cardiac arrest during 5‐FU but may occur secondary to myocardial ischemia. Comparable findings were reported in one prior Holter study of 5‐FU [7], whereas another reported an increased number and complexity of ventricular premature complexes [8]. Prior studies of QTc interval during 5‐FU treatment have revealed diverging findings, with significant prolongation of QTc interval in one study but no prolongation of the QTc interval in another study [57]. Most patients treated with 5‐FU receive antiemetics such as 5HT‐3 antagonists that can cause QTc interval prolongation, and this may have influenced the findings [58].
The incidence and patterns of clinical events in our study are consistent with findings in previous studies [59], and five of six patients undergoing coronary arteriography in our study had no significant stenosis (only the patient with symptomatic NSVT had stenoses on coronary angiography). Furthermore, troponin I levels rarely increased, and symptoms and ECG findings were transient and ceased a few days after cessation of 5‐FU, indicating that sustained occlusion was unlikely. These findings agree with the theory of coronary vasospasm. Coronary vasospasm is one of the leading theories regarding the cause of 5‐FU cardiotoxicity because of the clinical findings suggestive of coronary occlusion but the absence of coronary artery stenosis on coronary arteriography [38, 60]. This theory is further supported by visualization of coronary artery vasospasm during coronary angiography [61, 62] and brachial artery vasoconstriction demonstrated immediately after 5‐FU infusion [20, 63]. However, vasospasm is not always observed in the epicardial vessels in patients with 5‐FU cardiotoxicity [60, 64]. Therefore, Sara et al. [65] recently suggested that microvascular vasospasm may be involved in the pathophysiology of 5‐FU cardiotoxicity.
Strengths and Limitations
We did not quantify the degree of coronary atherosclerosis at baseline but relied on information from medical records and patient reporting regarding a history of ischemic heart disease. Hence, some patients may have had unrecognized coronary atherosclerosis, and this may have influenced the incidence of myocardial ischemia. We chose Holter recording to assess myocardial ischemia, because Holter recording can quantify myocardial ischemia during daily life, is inexpensive and noninvasive, and can detect both cardiac arrhythmias and episodes of myocardial ischemia [44]. A disadvantage is that only a few leads are used. Only myocardial ischemia in the areas covered by these leads is detected. Yet, lead CM5 has been reported to detect myocardial ischemia with a sensitivity of 89% compared with 12‐lead ECG [66]. Furthermore, interpretation of the ST segment on Holter recording can be challenging because left ventricular strain patterns may be difficult to differentiate from myocardial ischemia, and elevations in blood pressure can result in ST depression [44]. However, we observed a prevalence of myocardial ischemia of 3.7% before 5‐FU, which is lower than the incidences reported in population‐based studies of middle‐aged to elderly subjects (6%–35%) [42, 43, 67], indicating that we did not overestimate the incidence of myocardial ischemia.
Assays for troponin assessment are continuously improving, and more sensitive assays are now available on the market [15]. Thus, we may not have detected the smallest fluctuations in troponin.
Finally, we reviewed two thirds of Holter recordings for ischemic episodes and ventricular arrhythmias on the day the Holter recorder was removed. In five patients, we stopped 5‐FU owing to silent ischemia or nonsustained VT, which might have prevented clinical events.
Conclusion
5‐FU infusion is associated with a significant increase in the number of patients with myocardial ischemia on Holter recording and a significant increase in ischemic burden. Analyses of biomarkers suggest that 5‐FU infusion results in increases in copeptin levels but rarely induces myocyte injury. Acute coronary syndromes occurred in 5.6% of the patients during treatment. The presence of silent myocardial ischemia should alert the clinician about the risk of life‐threatening events.
Author Contributions
Conception/design: Anne Dyhl‐Polk, Morten Schou, Kirsten K. Vistisen, Merete Vaage‐Nilsen, Dorte L. Nielsen
Provision of study material or patients: Anne Dyhl‐Polk, Kirsten K. Vistisen, Eva Serup‐Hansen
Collection and/or assembly of data: Anne Dyhl‐Polk, Anne‐Sophie Sillesen
Data analysis and interpretation: Anne Dyhl‐Polk, Morten Schou, Kirsten K. Vistisen, Jens Faber, Tobias W. Klausen, Stig E. Bojesen, Merete Vaage‐Nilsen, Dorte L. Nielsen
Manuscript writing: Anne Dyhl‐Polk
Final approval of manuscript: Anne Dyhl‐Polk, Morten Schou, Kirsten K. Vistisen, Anne‐Sophie Sillesen, Eva Serup‐Hansen, Jens Faber, Tobias W. Klausen, Stig E. Bojesen, Merete Vaage‐Nilsen, Dorte L. Nielsen
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.
Acknowledgments
We thank Annie Therkelsen for help with patient visits and study examinations. This work was supported by grants from the University of Copenhagen, the Danish Heart Foundation, the Einar Willumsen Foundation, the Aase and Ejnar Danielsen Foundation, the August Frederik Wedell Erichsen Foundation, the Foundation of 17‐12‐1981, the Foundation for the Promotion of Clinical Cancer Research, and the Højmosegaard Grant/the Danish Medical Research Grant.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Grem JL. 5‐Fluorouracil: forty‐plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs 2000;18:299–313. [DOI] [PubMed] [Google Scholar]
- 2. Glynne‐Jones R, Wyrwicz L, Tiret E et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Cervantes A, Adam R et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–1422. [DOI] [PubMed] [Google Scholar]
- 4. Glynne‐Jones R, Nilsson PJ, Aschele C et al. Anal cancer: ESMO‐ESSO‐ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(suppl 3):iii10–iii20. [DOI] [PubMed] [Google Scholar]
- 5. Kosmas C, Kallistratos MS, Kopterides P et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol 2008;134:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polk A, Vaage‐Nilsen M, Vistisen K et al. Cardiotoxicity in cancer patients treated with 5‐fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013;39:974–984. [DOI] [PubMed] [Google Scholar]
- 7. Rezkalla S, Kloner RA, Ensley J et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: A prospective study. J Clin Oncol 1989;7:509–514. [DOI] [PubMed] [Google Scholar]
- 8. Yilmaz U, Oztop I, Ciloglu A et al. 5‐fluorouracil increases the number and complexity of premature complexes in the heart: A prospective study using ambulatory ECG monitoring. Int J Clin Pract 2007;61:795–801. [DOI] [PubMed] [Google Scholar]
- 9. Lestuzzi C, Vaccher E, Talamini R et al. Effort myocardial ischemia during chemotherapy with 5‐fluorouracil: An underestimated risk. Ann Oncol 2014;25:1059–1064. [DOI] [PubMed] [Google Scholar]
- 10. Fradley MG, Barrett CD, Clark JR et al. Ventricular fibrillation cardiac arrest due to 5‐fluorouracil cardiotoxicity. Tex Heart Inst J 2013;40:472–476. [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen K, Polk A, Nielsen DL et al. Cardiotoxicity in asymptomatic patients receiving adjuvant 5‐fluorouracil. J Clin Toxicol 2014;4:211. [Google Scholar]
- 12. Hannaford R. Sudden death associated with 5‐fluorouracil. Med J Aust 1994;161:225. [DOI] [PubMed] [Google Scholar]
- 13. Wijesinghe N. A case of late‐onset severe cardiotoxicity from 5‐fluorouracil therapy resulting in death. N Z Med J 2007;120:U2836. [PubMed] [Google Scholar]
- 14. Thygesen K, Alpert JS, Jaffe AS et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2018;40:237–269. [DOI] [PubMed] [Google Scholar]
- 15. Apple FS, Sandoval Y, Jaffe AS et al. Cardiac troponin assays: Guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 2017;63:73–81. [DOI] [PubMed] [Google Scholar]
- 16. Body R, Carlton E. Understanding cardiac troponin part 1: Avoiding troponinitis. Emerg Med J 2018;35:120–125. [DOI] [PubMed] [Google Scholar]
- 17. Ceyhan C, Meydan N, Barutca S et al. Ultrasound tissue characterization by integrated backscatter for analyzing Fluorouracil induced myocardial damage. Echocardiography 2005;22:233–238. [DOI] [PubMed] [Google Scholar]
- 18. Holubec L Jr, Topolcan O, Finek J et al. Dynamic monitoring of cardio‐specific markers and markers of thyroid gland function in cancer patients–a pilot study. Anticancer Res 2007;27:1883–1886. [PubMed] [Google Scholar]
- 19. Oztop I, Gencer M, Okan T et al. Evaluation of cardiotoxicity of a combined bolus plus infusional 5‐fluorouracil/folinic acid treatment by echocardiography, plasma troponin I level, QT interval and dispersion in patients with gastrointestinal system cancers. Jpn J Clin Oncol 2004;34:262–268. [DOI] [PubMed] [Google Scholar]
- 20. Salepci T, Seker M, Uyarel H et al. 5‐Fluorouracil induces arterial vasoconstrictions but does not increase angiotensin II levels. Med Oncol 2010;27:416–420. [DOI] [PubMed] [Google Scholar]
- 21. Turan T, Agac MT, Aykan AC et al. Usefulness of heart‐type fatty acid‐binding protein and myocardial performance index for early detection of 5‐fluorouracil cardiotoxicity. Angiology 2017;68:52–58. [DOI] [PubMed] [Google Scholar]
- 22. Morgenthaler NG, Struck J, Alonso C et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112–119. [DOI] [PubMed] [Google Scholar]
- 23. Dobsa L, Edozien KC. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem Med (Zagreb) 2013;23:172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parizadeh SM, Ghandehari M, Parizadeh MR et al. The diagnostic and prognostic value of copeptin in cardiovascular disease, current status, and prospective. J Cell Biochem 2018;119:7913–7923. [DOI] [PubMed] [Google Scholar]
- 25. Rotondo F, Butz H, Syro LV et al. Arginine vasopressin (AVP): A review of its historical perspectives, current research and multifunctional role in the hypothalamo‐hypophysial system. Pituitary 2016;19:345–355. [DOI] [PubMed] [Google Scholar]
- 26. Morgenthaler NG. Copeptin: A biomarker of cardiovascular and renal function. Congest Heart Fail 2010;16(suppl 1):S37–S44. [DOI] [PubMed] [Google Scholar]
- 27. Yalta K, Yalta T, Sivri N et al. Copeptin and cardiovascular disease: A review of a novel neurohormone. Int J Cardiol 2013;167:1750–1759. [DOI] [PubMed] [Google Scholar]
- 28. Morgenthaler NG, Struck J, Jochberger S et al. Copeptin: Clinical use of a new biomarker. Trends Endocrinol Metab 2008;19:43–49. [DOI] [PubMed] [Google Scholar]
- 29. Lipinski MJ, Escarcega RO, D'Ascenzo F et al. A systematic review and collaborative meta‐analysis to determine the incremental value of copeptin for rapid rule‐out of acute myocardial infarction. Am J Cardiol 2014;113:1581–1591. [DOI] [PubMed] [Google Scholar]
- 30. Khan SQ, Dhillon OS, O'Brien RJ et al. C‐terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation 2007;115:2103–2110. [DOI] [PubMed] [Google Scholar]
- 31. Ibanez B, James S, Agewall S et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 32. Cardiology TDSo . Guidelines from The Danish Society of Cardiology: Acute coronary syndromes. In. 2019. https://nbv.cardio.dk/aks. Accessed February 20, 2018.
- 33. QT interval and drug therapy. BMJ 2016;353:i2732. [DOI] [PubMed] [Google Scholar]
- 34. Scientific TF. Product Specifications: Thermo Scientific BRAMS Copeptin proAVP. Available at https://www.copeptin.com/images/downloads/pdf/data‐sheet‐Copeptin‐proAVP‐en.pdf. Accessed February 20, 2018.
- 35. Bjerregaard P, El‐Shafei A, Kotar SL et al. ST segment analysis by Holter Monitoring: Methodological considerations. Ann Noninvasive Electrocardiol 2003;8:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roffi M, Patrono C, Collet JP et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 37. Basselin C, Fontanges T, Descotes J et al. 5‐Fluorouracil‐induced Tako‐Tsubo‐like syndrome. Pharmacotherapy 2011;31:226. [DOI] [PubMed] [Google Scholar]
- 38. Kim SM, Kwak CH, Lee B et al. A case of severe coronary spasm associated with 5‐fluorouracil chemotherapy. Korean J Intern Med 2012;27:342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saif MW, Shah MM, Shah AR. Fluoropyrimidine‐associated cardiotoxicity: Revisited. Expert Opin Drug Saf 2009;8:191–202. [DOI] [PubMed] [Google Scholar]
- 40. Forslund L, Hjemdahl P, Held C et al. Prognostic implications of ambulatory myocardial ischemia and arrhythmias and relations to ischemia on exercise in chronic stable angina pectoris (the Angina Prognosis Study in Stockholm [APSIS]). Am J Cardiol 1999;84:1151–1157. [DOI] [PubMed] [Google Scholar]
- 41. Harkness JR, Morrow DA, Braunwald E et al. Myocardial ischemia and ventricular tachycardia on continuous electrocardiographic monitoring and risk of cardiovascular outcomes after non‐ST‐segment elevation acute coronary syndrome (from the MERLIN‐TIMI 36 Trial). Am J Cardiol 2011;108:1373–1381. [DOI] [PubMed] [Google Scholar]
- 42. Sajadieh A, Nielsen OW, Rasmussen V et al. Prevalence and prognostic significance of daily‐life silent myocardial ischaemia in middle‐aged and elderly subjects with no apparent heart disease. Eur Heart J 2005;26:1402–1409. [DOI] [PubMed] [Google Scholar]
- 43. Stagmo M, Juul‐Moller S, Israelsson B. Fifteen‐year risk of major coronary events predicted by Holter ST‐monitoring in asymptomatic middle‐aged men. Eur J Cardiovasc Prev Rehabil 2005;12:478–483. [DOI] [PubMed] [Google Scholar]
- 44. Wimmer NJ, Scirica BM, Stone PH. The clinical significance of continuous ECG (ambulatory ECG or Holter) monitoring of the ST‐segment to evaluate ischemia: A review. Prog Cardiovasc Dis 2013;56:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cohn PF, Fox KM, Daly C. Silent myocardial ischemia. Circulation 2003;108:1263–1277. [DOI] [PubMed] [Google Scholar]
- 46. Nademanee K, Christenson PD, Intarachot V et al. Variability of indexes for myocardial ischemia: A comparison of exercise treadmill test, ambulatory electrocardiographic monitoring and symptoms of myocardial ischemia. J Am Coll Cardiol 1989;13:574–579. [DOI] [PubMed] [Google Scholar]
- 47. Nabel EG, Barry J, Rocco MB et al. Variability of transient myocardial ischemia in ambulatory patients with coronary artery disease. Circulation 1988;78:60–67. [DOI] [PubMed] [Google Scholar]
- 48. Celermajer DS, Spiegelhalter DJ, Deanfield M et al. Variability of episodic ST segment depression in chronic stable angina: Implications for individual and group trials of therapeutic efficacy. J Am Coll Cardiol 1994;23:66–73. [DOI] [PubMed] [Google Scholar]
- 49. Patel DJ, Mulcahy D, Norrie J et al. Natural variability of transient myocardial ischaemia during daily life: An obstacle when assessing efficacy of anti‐ischaemic agents? Heart 1996;76:477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chakrabarti S, Sara J, Lobo R et al. Bolus 5‐fluorouracil (5‐FU) in combination with oxaliplatin is safe and well tolerated in patients who experienced coronary vasospasm with infusional 5‐FU or capecitabine. Clin Colorectal Cancer 2019;18:52–57. [DOI] [PubMed] [Google Scholar]
- 51. de Gramont A, Bosset JF, Milan C et al. Randomized trial comparing monthly low‐dose leucovorin and fluorouracil bolus with bimonthly high‐dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 1997;15:808–815. [DOI] [PubMed] [Google Scholar]
- 52. Kwakman JJM, Baars A, van Zweeden AA et al. Case series of patients treated with the oral fluoropyrimidine S‐1 after capecitabine‐induced coronary artery vasospasm. Eur J Cancer 2017;81:130–134. [DOI] [PubMed] [Google Scholar]
- 53. Osterlund PJ, Kinos S, Halonen P et al. Feasibility of switching to S‐1 after other fluoropyrimidine‐related cardiotoxicity during chemotherapy for solid tumors. J Clin Oncol 2020;38(suppl 15):7037a. [Google Scholar]
- 54. de Lemos JA, Drazner MH, Omland T et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Daniels LB, Laughlin GA, Clopton P et al. Minimally elevated cardiac troponin T and elevated N‐terminal pro‐B‐type natriuretic peptide predict mortality in older adults: Results from the Rancho Bernardo Study. J Am Coll Cardiol 2008;52:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gu YL, Voors AA, Zijlstra F et al. Comparison of the temporal release pattern of copeptin with conventional biomarkers in acute myocardial infarction. Clin Res Cardiol 2011;100:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Orditura M, De VF, Sarubbi B et al. Analysis of recovery time indexes in 5‐fluorouracil‐treated cancer patients. Oncol. Rep 1998;5:645–647. [DOI] [PubMed] [Google Scholar]
- 58. Brygger L, Herrstedt J. 5‐Hydroxytryptamine3 receptor antagonists and cardiac side effects. Expert Opin Drug Saf 2014;13:1407–1422. [DOI] [PubMed] [Google Scholar]
- 59. Polk A, Vistisen K, Vaage‐Nilsen M et al. A systematic review of the pathophysiology of 5‐fluorouracil‐induced cardiotoxicity. BMC Pharmacol Toxicol 2014;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tajik R, Saadat H, Taherkhani M et al. Angina induced by 5‐fluorouracil infusion in a patient with normal coronaries. Am Heart Hosp J 2010;8:E111–E112. [DOI] [PubMed] [Google Scholar]
- 61. Luwaert RJ, Descamps O, Majois F et al. Coronary artery spasm induced by 5‐fluorouracil. Eur Heart J 1991;12:468–470. [DOI] [PubMed] [Google Scholar]
- 62. Shoemaker LK, Arora U, Rocha Lima CM. 5‐fluorouracil‐induced coronary vasospasm. Cancer Control 2004;11:46–49. [DOI] [PubMed] [Google Scholar]
- 63. Sudhoff T, Enderle MD, Pahlke M et al. 5‐Fluorouracil induces arterial vasocontractions. Ann Oncol 2004;15:661–664. [DOI] [PubMed] [Google Scholar]
- 64. Mafrici A, Alberti A, Corrada E et al. Management of patients with persistent chest pain and ST‐segment elevation during 5‐fluorouracil treatment: report about two cases. Ital Heart J 2003;4:895–899. [PubMed] [Google Scholar]
- 65. Sara JD, Kaur J, Khodadadi R et al. 5‐fluorouracil and cardiotoxicity: A review. Ther Adv Med Oncol 2018;10:1758835918780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lanza GA, Mascellanti M, Placentino M et al. Usefulness of a third Holter lead for detection of myocardial ischemia. Am J Cardiol 1994;74:1216–1219. [DOI] [PubMed] [Google Scholar]
- 67. Aronow WS, Ahn C, Mercando AD et al. Prevalence and association of ventricular tachycardia and complex ventricular arrhythmias with new coronary events in older men and women with and without cardiovascular disease. J Gerontol A Biol Sci Med Sci 2002;57:M178–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.


