Abstract
Background
In this phase II clinical trial, we evaluated the efficacy of the nonanthracycline combination of carboplatin and nab‐paclitaxel in early stage triple‐negative breast cancer (TNBC).
Patients and Methods
Patients with newly diagnosed stage II–III TNBC (n = 69) were treated with neoadjuvant carboplatin (area under the curve 6) every 28 days for four cycles plus nab‐paclitaxel (100 mg/m2) weekly for 16 weeks. Pathological complete response (pCR) and residual cancer burden (RCB) were analyzed with germline mutation status, tumor‐infiltrating lymphocytes (TILs), TNBC molecular subtype, and GeparSixto immune signature (GSIS).
Results
Sixty‐seven patients were evaluable for safety and response. Fifty‐three (79%) patients experienced grade 3/4 adverse events, including grade 3 anemia (43%), neutropenia (39%), leukopenia (15%), thrombocytopenia (12%), fatigue (7%), peripheral neuropathy (7%), neutropenia (16%), and leukopenia (1%). Twenty‐four patients (35%) had at least one dose delay, and 50 patients (72%) required dose reduction. Sixty‐three (94%) patients completed scheduled treatment. The responses were as follows: 32 of 67 patients (48%) had pCR (RCB 0), 10 of 67 (15%) had RCB I, 19 of 67 (28%) had RCB II, 5 of 67 (7%) had RCB III, and 1 of 67 (2%) progressed and had no surgery. Univariate analysis showed that immune‐hot GSIS and DNA repair defect (DRD) were associated with higher pCR with odds ratios of 4.62 (p = .005) and 4.76 (p = .03), respectively, and with RCB 0/I versus RCB II/III with odds ratio 4.80 (p = .01). Immune‐hot GSIS was highly correlated with DRD status (p = .03), TIL level (p < .001), and TNBC molecular subtype (p < .001). After adjusting for age, race, stage, and grade, GSIS remained associated with higher pCR and RCB class 0/I versus II/III with odds ratios 7.19 (95% confidence interval [CI], 2.01–25.68; p = .002) and 8.95 (95% CI, 2.09–38.23; p = .003), respectively.
Conclusion
The combination of carboplatin and nab‐paclitaxel for early stage high‐risk TNBC showed manageable toxicity and encouraging antitumor activity. Immune‐hot GSIS is associated with higher pCR rate and RCB class 0/1. This study provides an additional rationale for using nonanthracycline platinum‐based therapy for future neoadjuvant trials in early stage TNBCs. Clinical trial identification number: NCT01525966
Implications for Practice
Platinum is an important neoadjuvant chemotherapy agent for treatment of early stage triple‐negative breast cancer (TNBC). In this study, carboplatin and nab‐paclitaxel were well tolerated and highly effective in TNBC, resulting in pathological complete response of 48%. In univariate and multivariate analyses adjusting for age, race, tumor stage and grade, “immune‐hot” GeparSixto immune signature (GSIS) and DNA repair defect (DRD) were associated with higher pathological complete response (pCR) and residual cancer burden class 0/1. The association of immune‐hot GSIS with higher pCR holds promise for de‐escalating neoadjuvant chemotherapy for patients with early stage TNBC. Although GSIS is not routinely used in clinic, further development of this immune signature into a clinically applicable assay is indicated.
Keywords: Neoadjuvant, Carboplatin, Nab‐paclitaxel, Triple‐negative breast cancer
Short abstract
Metastatic triple‐negative breast cancer is associated with poor clinical outcomes, largely due to a lack of effective targeted therapy. This article reports the results of a phase II trial evaluating the efficacy of neoadjuvant carboplatin plus nab‐paclitaxel in patients with stage II–III triple‐negative breast cancer.
Introduction
Triple‐negative breast cancer (TNBC) is characterized by the lack of estrogen receptor, progesterone receptor, and overexpression of human epidermal growth factor receptor 2 (HER2) expression. It accounts for approximately 10% to 15% of all breast cancer [1, 2]. TNBC is a highly heterogeneous disease with four to six molecular subtypes based on mRNA expression [3, 4]. Despite treatment with anthracycline‐taxane–based standard chemotherapy, 30% to 40% of patients with early stage TNBC relapse [5, 6]. Furthermore, metastatic TNBC is associated with poor clinical outcome, largely because of a lack of effective targeted therapy [5, 7].
Neoadjuvant chemotherapy (NCT) with anthracycline‐taxane is the standard treatment for early stage (tumor size ≥2 cm) or locally advanced TNBC [8]. Pathological complete response (pCR) and low residual cancer burden (RCB) after NCT predict improved survival in TNBC [9, 10, 11]. However, with standard neoadjuvant anthracycline‐taxane regimen, pCR rate is approximately 30% to 40% in TNBC [6, 10, 12]. Platinum agents have received renewed interest in the treatment of TNBC because of a close association between TNBC and hereditary breast cancer [13]. Adding the DNA‐damaging agent carboplatin to a neoadjuvant regimen has shown improved pCR rates in TNBC without significant event‐free survival benefit in the Cancer and Leukemia Group B (CALGB 40603) Alliance trial [14, 15]. In the GeparSixto trial, addition of carboplatin to a neoadjuvant anthracycline and taxane–based chemotherapy regimen increased pCR from 43% to 57% in patients with TNBC [16, 17]. Interestingly, the benefit in a subset analysis of the trial was more pronounced in patients without germline BRCA mutations [16]. In an anthracycline‐free neoadjuvant trial combining carboplatin and docetaxel, a pCR rate of 55% was observed in TNBC [18]. Moreover, nab‐paclitaxel, an albumin‐bound particle form of paclitaxel, has shown preferential tumor uptake and a more favorable safety profile compared with paclitaxel, and nab‐paclitaxel has been evaluated in the neoadjuvant setting [19, 20, 21].
Previous studies have shown that the immune system is important for response to NCT in breast cancer. This is particularly relevant in TNBC, which is characterized by a higher immune response compared with other breast cancer subtypes. Indeed, there is strong evidence that tumor‐infiltrating lymphocytes (TILs) in TNBC have prognostic value and are associated with improved clinical outcome and survival [22, 23]. The GeparSixto immune signature (GSIS) is composed of 12 immune genes that differentiate “immune‐hot” tumors from “immune‐cold” tumors, which includes immune‐activating genes (CCL5, CXCL9, CXCL13, CD80, CD21, CD8A, IGKC) and immunosuppressive genes (PD‐1, PD‐L1, CTLA4, FOXP3, and IDO1), which were selected to include B‐cell and T‐cell markers, chemokines, and immune checkpoint inhibitors. This immune signature was previously reported to predict pCR in patients with TNBC who received neoadjuvant anthracycline‐plus‐taxane combination in addition to carboplatin [24]. DNA repair deficiency or “BRCAness” resulting from somatic mutations or epigenetic modification of DNA repair genes are associated with higher pCR in neoadjuvant trials; however, the precise definition of DNA repair deficiency is controversial [25]. In this study, DNA repair defect (DRD) is defined as germline mutations in genes that are known to be associated with DNA repair pathways, including BRCA1, BRCA2, CHEK2, and RAD51C gene mutations [26, 27].
Recent U.S. Food and Drug Administration approval of immune checkpoint inhibitor (ICI) in metastatic TNBC has elicited strong interest in the neoadjuvant regimen [28, 29, 30]. Several studies combining ICI and anthracycline‐taxane showed significantly promising pCR with additional toxicities [31, 32, 33]. Thus, assessing the status of immunological parameters in TNBC may provide clinical utility in informing treatment decisions that include immunotherapy approaches. In addition, non–anthracycline‐containing regimens with carboplatin‐taxane showed promising pCR in early stage TNBC [18] and may serve as a chemotherapy backbone for future ICI combinations.
Here we report the results of a phase II trial evaluating the efficacy of neoadjuvant carboplatin plus nab‐paclitaxel in patients with stage II–III TNBC. Additionally, we report the association of pCR with biomarkers including germline BRCA mutation, DRD, TILs, and microarray‐based immune signature.
Patients and Methods
Patient Population
This study was conducted between January 2012 and August 2018 with institutional review board approval of the City of Hope Comprehensive Cancer Center (COH) (protocol 11174). The study followed the guidelines of the Declaration of Helsinki and good clinical practice and was registered at the clinical trial web site ClinicalTrials.gov under number NCT01525966. Voluntary informed consent was signed by all patients prior to study entry. Key eligibility criteria included patients with newly diagnosed stage II–III TNBC, primary tumor size over 2 cm by imaging or clinical measurement, histologically confirmed TNBC (estrogen receptor <10%, progesterone receptor <10%, HER2‐neu negative defined by immunohistochemistry score 0 or 1, or fluorescence in situ hybridization negative), and no evidence of distant metastases.
Study Procedures
A total of 69 patients with stage II–III TNBC were accrued. Two patients were subsequently found to be HER2 positive and were removed from study. Neoadjuvant carboplatin (area under the curve [AUC] 6) on day 1 of every 28 days for four cycles (to minimize severe myelosuppression), plus nab‐paclitaxel (100 mg/m2) weekly for 16 weeks (four 28‐day cycles) was given intravenously. If necessary, treatment was delayed allowing recovery from toxicity; however, if treatment was delayed for more than 2 weeks because of toxicity, the patient stopped protocol treatment. Toxicity‐based dose adjustments were carried out according to drug‐specific standard guidelines. Patients did not receive prophylactic colony‐stimulating factors (e.g., granulocyte colony‐stimulating factor [G‐CSF], granulocyte‐macrophage colony‐stimulating factor) during cycle 1, but G‐CSF use was permitted from cycle 2 onwards according to American Society of Clinical Oncology guidelines [34].
Pathological Response Assessment
Pathological response was determined by COH pathologists. pCR was defined as the absence of residual invasive breast cancer with or without ductal carcinoma in situ in the breast and axilla (ypT0/TisN0). RCB was scored for all patients using the Symmans criteria [35]. Patients who had pCR (RCB 0) or near pCR (RCB I) were included in the group RCB 0/I.
Germline Genetic Testing
Of 67 patients, 56 underwent germline genetic testing per National Comprehensive Cancer Network (NCCN) guidelines. Eleven patients did not meet testing criteria. Most patients were tested through the myRisk Hereditary Cancer test or Comprehensive BRCAnalysis from Myriad Genetics (Salt Lake City, UT). DRD was defined by identification of germline mutations in genes that are known to be associated with DNA repair pathways, including BRCA1, BRCA2, CHEK2, and RAD51C gene mutations [26].
TNBC Molecular Subtyping
Formalin‐fixed, paraffin‐embedded (FFPE) baseline tumor biopsies were subjected to mRNA microarray testing via 70‐gene MammaPrint profile, the 80‐gene BluePrint subtypes, and full genome mRNA profiling (Agendia, Irvine, CA). Of 67 samples, 63 had RNA quality and quantity with at least 30% tumor cell percentage for sequencing. MammaPrint categorized patients as having a high risk or low risk of recurrence, whereas BluePrint stratified tumor samples into three molecular subgroups: luminal type, HER2 type, and basal type. MammaPrint further stratified BluePrint luminal type into luminal A (MammaPrint low risk) and luminal B (MammaPrint high risk) [36, 37]. The mRNA expression array was used for Vanderbilt TNBC molecular subtyping: basal‐like 1 (BL1), basal‐like 2 (BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem‐like (MSL), and luminal androgen receptor (LAR) subtypes [3, 38].
Immune Signatures
Using the above full genome mRNA expression array data (Agendia), the expression of the GSIS 12‐gene immune signature (CCL5, CXCL9, CXCL13, CD80, CD21, CD8A, IGKC, PD‐1, CD274 (PD‐L1), CTLA4, FOXP3, and IDO1) was analyzed. Hierarchical clustering of mRNA expression distinguished immune‐hot tumors (high immunologic gene expression) from immune‐cold tumors (low immunologic gene expression) [39].
Stromal TIL Analysis
H&E‐stained slides from pretreatment specimens (biopsies) were analyzed for stromal TILs by microscopic analysis of H&E‐stained slides of FFPE surgical specimens by pathologists at City of Hope. Stromal TILs were reported in percentages according to the International TILs Working Group 2014 and categorized as the following: low (0%–10%), intermediate (11%–59%), and high (≥60%) [40]. TILs in the tumor area with artifacts or necrosis were excluded, as were polymorphonuclear leukocytes [41].
Statistical Design
A two‐stage design was proposed based on detecting a promising pCR rate. In the first stage, accrual was continued until 22 patients were enrolled, with second stage accrual to an evaluable 45 and total of 49 patients. The design was selected to meet the objectives and permit the early termination of the trial in the event that the therapy was inferior to other neoadjuvant regimens. An expanded cohort of 20 patients (given the promising results in the first 45) was added to better evaluate the response rates in association with molecular and genomic features of TNBC. This resulted in a total of 69 patients. The primary objective of this study was to evaluate pCR and RCB after NCT based on the surgical specimen analyzed by COH pathologists after completion of study treatment. The secondary objectives were to evaluate disease‐free survival (DFS), measured from start of treatment to progression of disease or death from any cause and overall survival (OS) of the patients, and to assess the toxicities using the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE; version 4.0). All enrolled patients who received at least one dose of therapy were evaluated for toxicity. Survival times were measured from the start date of neoadjuvant treatment to the date of event or death. DFS and OS were estimated by the Kaplan‐Meier method (one patient progressed during neoadjuvant therapy and was assigned the date of progression for DFS). The corresponding median survival times (with 95% confidence limits) were determined. Odds ratios and 95% confidence intervals (CIs) with p values were used. A value of p < .05 (two‐sided) was considered statistically significant. The associations of pCR and RCB class with TNBC molecular subtype, DRD status (wild type vs. mutation), BRCA (wild type vs. mutation), stromal TIL level (low, medium, and high), and GSIS (immune‐hot vs. immune‐cold) were tested by univariate logistic regression with either pCR (yes vs. no) or RCB class (0/I vs. II/III) as the response variable. Demographic variables (age and race), clinical variables (tumor stage and grade), and biomarkers were included in the model. Because the GSIS was highly correlated with other biomarkers, a multivariate logistic regression was carried out for pCR with GSIS as the only biomarker, with patient's age, race, tumor stage, and tumor grade adjusted as covariates.
Results
Patient Characteristics
A total of 69 patients with stage II–III TNBC were enrolled between January 2012 and August 2018 at City of Hope. Two patients were found to be ineligible because of HER2‐positive status on repeat biopsy (supplemental online Fig. 1). Patient characteristics, disease status, and treatment variables are summarized in Table 1 (n = 67). Median age of the patients was 52 years (28–79). Thirty‐five of 67 patients (52%) were non‐Hispanic White, and 24 of 67 (36%) were Hispanic. Fifty‐five of 67 patients (82%) had clinical stage II, and 12 of 67 (18%) had stage III disease. Fifty‐one of 67 patients (76%) had grade 3 disease. Of the 67 patients, 56 had genetic testing per NCCN guideline: BRCA1, 8 of 56 patients (14%); BRCA2, 3 of 56 (5%); CHEK2, 2 of 56 (4%); and RAD51C, 1 of 56 (2%); and 42 of 56 (75%) were wild type. Eleven patients did not meet NCCN testing criteria. Surgery and adjuvant radiation therapy were performed according to NCCN guidelines. After NCT, 66 of 67 patients underwent breast surgery, and 1 of 67 progressed with new distant metastases; hence, this patient did not have surgery or radiation. Of the 66 patients who had surgery, 19 of 66 (29%) had lumpectomy, and 47 of 66 (71%) had mastectomy. Thirty‐seven of 66 patients (55%) received adjuvant radiation therapy. Seven patients received additional adjuvant chemotherapy: three had adriamycin/cyclophosphamide (AC), two had capecitabine, one had AC followed by capecitabine, and one had carboplatin/nab‐paclitaxel.
Table 1.
Patient and disease characteristics (n = 67 a )
| Characteristic | n (%) |
|---|---|
| Age, years, median (range) | 52 (28–79) |
| Race/ethnicity (n = 67) | |
| Non‐Hispanic White | 35 (52) |
| Hispanic | 24 (36) |
| Asian | 4 (6) |
| Black | 1 (1) |
| Other/unknown | 3 (5) |
| Menopausal status (n = 67) | |
| Premenopausal | 24 (36) |
| Perimenopausal | 6 (9) |
| Postmenopausal | 37 (55) |
| Clinical stage (n = 67) | |
| II | 55 (82) |
| III | 12 (18) |
| Tumor grade (n = 67) | |
| 1 | 2 (3) |
| 2 | 14 (21) |
| 3 | 51 (76) |
| Initial nodal status (n = 67) | |
| Positive | 34 (51) |
| Negative | 33 (49) |
| Germline mutation (n = 56) b | |
| BRCA1 | 8 (14) |
| BRCA2 | 3 (5) |
| CHEK2 | 2 (4) |
| RAD51C | 1 (2) |
| Wild type | 42 (75) |
| Surgery (n = 66) c | |
| Lumpectomy | 19 (29) |
| Mastectomy | 47 (71) |
| Adjuvant radiation (n = 66) c | |
| Yes | 37 (55) |
| No | 29 (45) |
Two patients were not eligible because of human epidermal growth factor receptor 2–positive status on repeat biopsy.
A total of 56 of 67 had genetic test results per National Comprehensive Cancer Network guidelines.
One patient progressed and had distant metastases; no surgery or radiation.
Pathological Response and Survival
Among the 67 patients, 32 of 67 (48%) achieved pCR (RCB 0), 10 of 67 (15%) had RCB I, 19 of 67 (28%) had RCB II, 5 of 67 (7%) had RCB III, and 1 of 67 (2%) progressed (supplemental online Fig. 2). A total of 42 of 67 patients (63%) achieved RCB 0/I.
Median follow‐up was 43.7 months. Nine of 67 patients (13%) experienced disease relapse. The Kaplan‐Meier curve for DFS at median follow‐up is shown in Figure 1A, with a 3‐year DFS of 87.3% (95% CI, 74.9–93.8; n = 67, events = 9). The Kaplan‐Meier curve for OS is shown in Figure 1B, with a 3‐year OS of 90.2% (95% CI, 77.8–95.8; n = 67, events = 6). A total of 35 patients had residual disease with a 3‐year DFS of 79.0% (95% CI, 58.5–90.2%; n = 35, events = 8; Fig. 1C).
Figure 1.
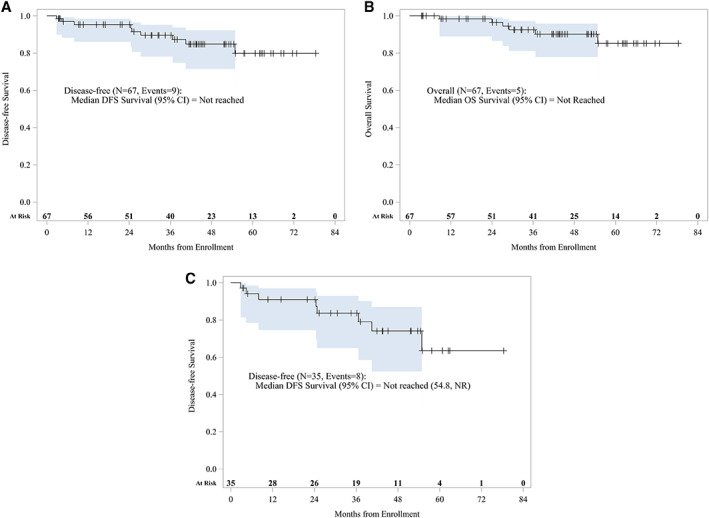
Kaplan‐Meier survival curves (n = 67): Survival analysis showed 3‐year DFS of 87.3% (95% CI, 74.9%–93.8%; n = 67, events = 9) (A), 3‐year OS of 90.2% (95% CI, 77.8%–95.8%; n = 67, events = 6) (B), and for the 35 patients with residual disease, 3‐year DFS of 79.0% (95% CI, 58.5%–90.2%; n = 35, events = 8) (C).Abbreviations: CI, confidence interval; DFS, disease‐free survival; NR, not reached; OS, overall survival.
Adverse Events
A total of 67 patients were evaluable for adverse events (Table 2). Overall, 53 of 67 patients (79%) experienced one or more CTCAE (version 4.0) grade 3/4 adverse events (AEs). Hematological AEs were the most common grade 3/4 toxicities. Grade 3 AEs were anemia (29/67, 43%), neutropenia (26/67, 39%), leukopenia (10/67, 15%), thrombocytopenia (8/67, 12%), lymphopenia (5/67, 7%), and febrile neutropenia (2/67, 3%). Forty‐three percent of patients had blood transfusion. Significant grade 3 nonhematological AEs were fatigue (5/67, 7%) and peripheral neuropathy (5/67, 7%). Grade 4 hematological AEs were neutropenia (11/67, 16%) and leukopenia (1/67, 1%). There were no grade 4 nonhematologic AEs. Twenty‐four patients (35%) had at least one dose delay (range, one to four), and 50 patients (72%) required dose reductions (3 patients had reduction because of carboplatin, 49 because of nab‐paclitaxel, and 2 because of both). Median duration of dose delay was 1 week. Thirty‐seven of 67 patients (55%) used G‐CSF support. Sixty‐three of 67 patients (94%) completed scheduled treatment. Two patients were taken off the study early because of hematological toxicities, one patient refused treatment prior to completing cycle 2, and one patient went off treatment for progression prior to completing cycle 3.
Table 2.
Treatment‐related adverse events (n = 67)
| Adverse event a | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|
| Hematological AEs | 14 (21) | 40 (60) | 11 (16) |
| Neutropenia | 19 (28) | 26 (39) | 11 (16) |
| Leukopenia | 35 (52) | 10 (15) | 1 (1) |
| Anemia | 29 (43) | 29 (43) | 0 |
| Thrombocytopenia | 8 (12) | 8 (12) | 0 |
| Lymphopenia | 10 (15) | 5 (7) | 0 |
| Febrile neutropenia | 0 | 2 (3) | 0 |
| Nonhematological AEs | 34 (51) | 12 (18) | 0 |
| Fatigue | 23 (34) | 5 (7) | 0 |
| Alopecia | 16 (24) | 0 | 0 |
| Hypertension | 8 (12) | 0 | 0 |
| Nausea/vomiting | 6 (9) | 0 | 0 |
| Peripheral sensory neuropathy | 3 (4) | 5 (7) | 0 |
| Upper respiratory infection | 3 (4) | 0 | 0 |
| Hypokalemia | 2 (3) | 1 (1) | 0 |
| Urinary tract infection | 1(1) | 1(1) | 0 |
| Dehydration | 0 | 1 (1) | 0 |
| Hyperglycemia | 0 | 1 (1) | 0 |
| Hyponatremia | 0 | 1 (1) | 0 |
| Premature menopause | 0 | 1 (1) | 0 |
| Hypophosphatemia | 4 (6) | 0 | 0 |
| Depression | 3 (4) | 0 | 0 |
| Oral mucositis | 3 (4) | 0 | 0 |
| Elevated alanine transferase | 2 (3) | 0 | 0 |
| Dyspnea | 2 (3) | 0 | 0 |
| Hypocalcemia | 2 (3) | 0 | 0 |
Includes events with at least two grade 2 occurrences or one grade 3 or higher. Only the highest grade per person for each category is counted.
Abbreviation: AE, adverse event.
Analysis of Biomarkers with pCR: DRD Mutation, TNBC Molecular Subtype, TILs, and GSIS
Of 67 patients, Agendia microarray was performed from 63 baseline tumor biopsies to obtain MammaPrint profiles and full genome mRNA profiling. MammaPrint classified all tumors as at high risk for recurrence. Of the 63 patients, 56 had germline genetic testing, and 61 had stromal TIL analysis (supplemental online Fig. 1). All subsequent biomarker analysis is based on the 63 patients with Agendia microarray result.
GSIS is composed of 12 immune genes (CCL5, CXCL9, CXCL13, CD80, CD21, CD8A, IGKC, PD‐1, CD274 (PD‐L1), CTLA4, FOXP3, and IDO1) that were selected based on immunological relevance and was previously reported to predict pCR in patients with TNBC who received neoadjuvant anthracycline‐plus‐taxane combination in addition to carboplatin [24]. Hierarchic clustering of GSIS across 63 tumors showed two different immune groups: immune‐cold tumors, which showed low expression of all immune genes, and immune‐hot tumors, which showed high expression of immunologic genes (Fig. 2).
Figure 2.
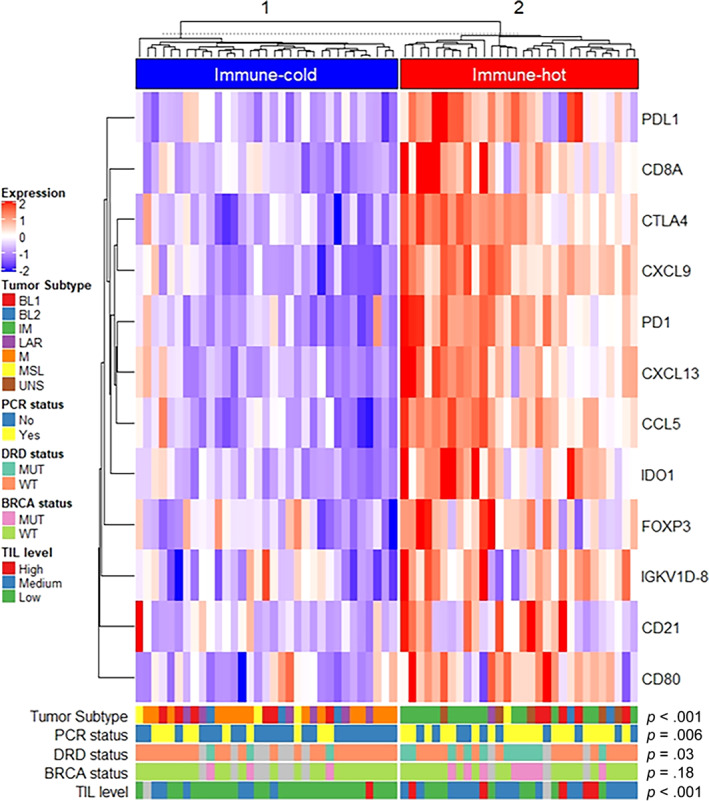
Association of GeparSixto immune signature with triple‐negative breast cancer molecular subtype, pCR status, and biomarkers (n = 63). Of 67 patients enrolled, 63 pretreatment tumor specimens were available for analysis. Hierarchical clustering of 12 immunologically relevant genes in 63 tumors showed two distinct immune groups with different expression levels, including immune‐cold tumors with low expression of immune genes, and immune‐hot tumors with high expression of immune genes. Gray blocks on DRD, BRCA, and TILs indicate no results (not analyzed because of limited specimen availability).Abbreviations: BL1, basal‐like 1; BL2, basal‐like 2; DRD, DNA repair defect; IM, immunomodulatory; LAR, luminal androgen receptor; M, mesenchymal; MSL, mesenchymal stem‐like; MUT, mutation; pCR, pathological complete response; TIL, tumor‐infiltrating lymphocyte; UNS, unspecified, WT, wild type.
In this study, DRD is defined by identification of germline mutations in genes that are known to be associated with DNA repair pathways, including BRCA1, BRCA2, CHEK2, and RAD51C. There were no PALB2 or ATM mutations in this patient population. Of 56 patients with germline genetic testing, 14 (25%) had DRD, including BRCA1 (n = 8), BRCA2 (n = 3), CHEK2 (n = 2), and RAD51C (n = 1), and 42 of 56 (75%) were wild type (Table 3). Univariate logistic regression showed that pCR was associated with DRD status and immune‐hot GSIS with odds ratios of 4.76 (p = .03) and 4.62 (p = .005), respectively. Similarly, RCB 0/I class was associated with immune‐hot GSIS with an odds ratio of 4.80 (p = .01; Table 3).
Table 3.
Univariate analysis of pCR and RCB class with demographics, clinical variables, and biomarkers (n = 63)
| pCR, n | pCR OR (95% CI), p value | RCB class OR (95% CI), p value | ||
|---|---|---|---|---|
| Yes (n = 30) | No (n = 33) | |||
| Median age, years | 51.8 | 52.08 | 1.00 (0.96–1.04), .09 | 0.99 (0.95–1.03), .65 |
| Race | ||||
| White | 25 | 27 | 1.00 | 1.00 |
| Other | 5 | 6 | 0.90 (0.24–3.32), .87 | 0.69 (0.19–2.57), .58 |
| Clinical stage | ||||
| 2 | 25 | 26 | 1.00 | 1.00 |
| 3 | 5 | 7 | 0.74 (0.21–2.64), .65 | 0.55 (0.16–1.82), .35 |
| Tumor grade | ||||
| Low | 1 | 1 | 1.00 | Inf (0.00–Inf), .99 |
| Intermediate | 4 | 9 | 0.45 (0.02–9.03), .60 | 1.00 a |
| High | 25 | 23 | 1.08 (0.06–18.36), .95 | 3.20 (0.90–11.37), .07 |
| TNBC molecular subtype | ||||
| BL1 | 6 | 5 | 1.00 | 1.00 |
| BL2 | 2 | 3 | 0.55 (0.07–4.76), .59 | 0.33 (0.03–3.52), .36 |
| IM | 10 | 6 | 1.39 (0.29–6.62), .68 | 0.67 (0.10–4.48), .68 |
| LAR | 2 | 5 | 3.00 (0.04–2.53), .29 | 0.17 (0.02–1.27), .10 |
| M | 5 | 10 | 0.41 (0.08–2.05), .28 | 0.19 (0.03–1.22), .08 |
| MSL | 2 | 2 | 0.84 (0.08–8.25), .88 | 0.22 (0.18–2.67), .24 |
| UNS | 3 | 2 | 1.25 (0.15–10.70), .84 | 0.33 (0.03–3.52), .36 |
| DRD status | ||||
| Wild type | 17 | 22 | 1.00 | 1.00 |
| Mutation | 11 | 3 | 4.76 (1.14–19.69), .03 | 3.75 (0.73–19.14), .11 |
| N/A | 2 | 8 | ||
| BRCA status | ||||
| Wild type | 20 | 22 | 1.00 | 1.00 |
| Mutation | 8 | 3 | 2.94 (0.68–12.55), .15 | 2.50 (0.48–13.11), .28 |
| N/A | 2 | 8 | ||
| Stromal TIL level | ||||
| Low (0%–10%) | 13 | 20 | 1.00 | 1.00 |
| Medium (11%–59%) | 13 | 9 | 2.23 (0.74–6.69), .16 | 2.22 (0.70–7.10), .18 |
| High (≥60%) | 4 | 2 | 3.06 (0.49–9.30), .23 | 1.67 (0.27–10.39), .58 |
| N/A | 0 | 2 | ||
| GSIS | ||||
| Immune‐hot | 20 | 10 | 1.00 | 1.00 |
| Immune‐cold | 10 | 23 | 4.62 (1.58–13.33), .005 | 4.80 (1.56–14.81), .01 |
Intermediate grade was chosen as the reference category because there was no RCB II/III with low grade.
Abbreviations: BL1, basal‐like 1; BL2, basal‐like 2; CI, confidence interval; DRD, DNA repair defect; GSIS, GeparSixto immune signature; IM, immunomodulatory; Inf, infinity; LAR, luminal androgen receptor; M, mesenchymal; MSL, mesenchymal stem‐like; N/A, not applicable; OR, odds ratio; pCR, pathological complete response; RCB, residual cancer burden; TIL, tumor‐infiltrating lymphocyte; TNBC, triple‐negative breast cancer; UNS, unspecified.
Fisher's exact test showed that GSIS (immune‐hot vs. immune‐cold) was significantly associated with DRD status with mutation in 11 of 27 patients (41%) with immune‐hot status and mutation in 3 of 26 patients (12%) with immune‐cold status (p = .03). Stromal TIL level (p < .001) and TNBC molecular subtypes (p < .001) were also associated with GSIS (Table 4). In addition, GSIS was associated with both pCR (20/30, 67% in immune‐hot vs. 10/33, 30% in immune‐cold) and RCB 0/I class (24/30, 80% in immune‐hot vs. 15/33, 45% in immune‐cold) (p = .005 and p = .009, respectively; Table 4).
Table 4.
Correlation between GSIS and biomarkers (n = 63)
| GSIS | p value | ||
|---|---|---|---|
| Immune‐hot | Immune‐cold | ||
| DRD status | |||
| Wild type | 16 | 23 | .03 |
| Mutation | 11 | 3 | |
| N/A | 3 | 7 | |
| BRCA status | |||
| Wild type | 19 | 23 | .18 |
| Mutation | 8 | 3 | |
| N/A | 3 | 7 | |
| Stromal TIL level | |||
| Low (0%–10%) | 8 | 25 | <.001 |
| Medium (11%–59%) | 16 | 6 | |
| High (≥60%) | 5 | 1 | |
| N/A | 1 | 1 | |
| TNBC molecular subtype | |||
| BL1 | 5 | 6 | <.001 |
| BL2 | 2 | 3 | |
| IM | 16 | 0 | |
| LAR | 1 | 6 | |
| M | 0 | 15 | |
| MSL | 1 | 3 | |
| UNS | 5 | 0 | |
| pCR | |||
| Yes | 20 | 10 | .005 |
| No a | 10 | 23 | |
| RCB class | |||
| 0/I | 24 | 15 | .009 |
| II/III a | 6 | 18 | |
| BluePrint | |||
| Luminal | 0 | 2 | .49 |
| Basal | 30 | 31 | |
| MammaPrint | |||
| H1 | 3 | 8 | .19 |
| H2 | 27 | 25 | |
One immune‐cold patient progressed prior to surgery and was included in the non‐pCR RCB II/III group for analysis purposes.
Abbreviations: BL1, basal‐like 1; BL2, basal‐like 2; DRD, DNA repair defect; GSIS, GeparSixto immune signature; IM, immunomodulatory; LAR, luminal androgen receptor; M, mesenchymal; MSL, mesenchymal stem‐like; N/A, not applicable; pCR, pathological complete response; RCB, residual cancer burden; TIL, tumor‐infiltrating lymphocyte; TNBC, triple‐negative breast cancer; UNS, unspecified.
DRD and BRCA status were available for 53 of 63 patients with substantial overlap. Only three patients showed different status (wide type vs. mutation) for DRD and BRCA. DRD status was significantly correlated with GSIS (p = .03), as shown in Table 4. Because of missing data in DRD and colinearity between GSIS and DRD, we only included one variable (GSIS or DRD) in the model. After adjusting for patient age, race, tumor stage, and tumor grade with a multivariate logistic model, immune‐hot GSIS was significantly associated with pCR and RCB 0/I class with notably higher odds ratios of 7.19 (95% CI, 2.01–25.68; p = .002) and 8.95 (95% CI, 2.09–38.32; p = .003), respectively (Table 5). Using DRD status in the multivariate model in place of GSIS showed that DRD was associated with pCR but not with RCB 0/I with odds ratios of 6.93 (95% CI, 1.32–36.47; p = .022) and 4.88 (95% CI, 0.74–32.10; p = .10), respectively (data not shown). These results show that DRD status, TIL level, and mRNA‐based immune gene signatures such as GSIS can detect patients who may achieve higher pCR rate from platinum‐based neoadjuvant chemotherapy.
Table 5.
Multivariate analysis of pCR and RCB class with demographics, clinical variables, and GSIS (n = 63)
| n | pCR (yes vs. no) OR (95% CI), p value | RCB class (0/I vs. II/III) OR (95% CI), p value | |
|---|---|---|---|
| Age as a continuous variable | 63 | 1.02 (0.98–1.08), .33 | 1.02 (0.97–1.07), .46 |
| Race | |||
| White | 52 | 1.00 | 1.00 |
| Other | 11 | 1.23 (0.28–5.37), .79 | 1.00 (0.22–4.47), .996 |
| Clinical stage | |||
| 2 | 51 | 1.00 | 1.00 |
| 3 | 12 | 0.37 (0.08–1.65), .19 | 0.24 (0.05–1.22), .08 |
| Tumor grade | |||
| Low | 2 | 1.00 | Inf (0.00–Inf), .99 |
| Intermediate | 13 | 0.43 (0.02–10.76), .61 | 1.00 a |
| High | 48 | 1.37 (0.07–28.08), .84 | 4.81 (1.07–21.69), .04 |
| GSIS | |||
| Immune‐hot | 30 | 1.00 | 1.00 |
| Immune‐cold | 33 | 7.19 (2.01–25.68), .002 | 8.95 (2.09–38.32), .003 |
Intermediate grade was chosen as the reference category because there were no RCB II/III with low grade.
Abbreviations: CI, confidence interval; GSIS, GeparSixto immune signature; Inf, infinity; OR, odds ratio; pCR, pathological complete response; RCB, residual cancer burden.
Discussion
Anthracycline and taxane–based neoadjuvant therapy has been the mainstay for HER2‐negative breast cancer regardless of hormone receptor status [8, 42]. Although pCR after NCT is associated with favorable clinical outcome, 30% to 40% of patients with early stage TNBC relapse despite receiving anthracycline‐taxane neoadjuvant regimen [9, 10]. Our study demonstrated excellent pCR rate of 48% and an RCB 0/I rate of 63% with a nonanthracycline regimen of carboplatin‐nab‐paclitaxel in patients with stage II–III TNBC. The most common grade 3 or 4 AEs were anemia and neutropenia. Notably, 35% had dose delay, and 72% had dose reduction; however, patients achieved encouraging pCR with four cycles of carboplatin plus weekly nab‐paclitaxel. Similarly, Sharma et al. reported a pCR of 55% and RCB 0/I of 68% with six cycles of carboplatin and docetaxel in early stage TNBC [18]. In the BrighTNess trial, Loibl et al. reported a pCR rate of 58% in patients who received paclitaxel (80 mg/m2 weekly × 12 doses) plus carboplatin (AUC 6 every 3 weeks for four cycles) followed by four cycles of doxorubicin and cyclophosphamide [43]. The GeparSixto trial demonstrated that the addition of carboplatin to paclitaxel and nonpegylated loposomal doxorubicine increased the pCR rate from 43% to 57% among 315 patients with TNBC [17]. In CALGB 40603, a randomized phase II trial evaluated for stage II–III TNBC, adding carboplatin to paclitaxel followed by dose‐dense doxorubicin plus cyclophosphamide increased the pCR rate from 39% to 49%, although adding carboplatin was associated with more frequent neutropenia and thrombocytopenia [15]. Addition of carboplatin was not associated with event‐free survival benefit in CALGB 40603 [14]. Our study adds to the body of literature supporting the addition of platinum to neoadjuvant therapy for TNBC.
The different pathological responses to NCT is attributed to the molecular heterogeneity of TNBC [3, 44]. Lehmann et al. first described that TNBC can be subclassified into six distinctive subtypes using molecular profiling [3]. The relation between subtypes and different treatment responses has been evaluated [45, 46, 47]. In the BrighTNess trial, TNBC subtypes were mostly BL1 or BL2 (23.3%), IM (22.4%), or M/MSL (31.7%), with only 6% LAR. pCR was higher for basal versus nonbasal tumors (52.3% vs. 35.4%, p = .003). IM had the highest pCR rate of 64.2% (95% CI, 59.9%–68.5%). Masuda et al. reported that IM had the highest pCR rate of 93%, LAR and M had the lowest pCR rates of 29% and 28%, respectively, and BL1 and BL2 had 54.5% and 50% pCR rates, respectively [48]. In our study, the pathological response based on the TNBC subtypes was similar with previous reports, but the results were not statistically significant, likely reflecting the small sample size. However, the higher response rate for IM and the lower response rate for LAR and M types were consistent with immune‐hot and immune‐cold tumor status by GSIS analysis. Although a larger number of primary tumors need to be assessed to confirm the current finding, our data highlight the need for valid immune biomarkers in the area of immune checkpoint therapies for early stage breast cancers.
Although the current study showed that the combination of carboplatin and nab‐paclitaxel is effective, there was increased hematological toxicity. Therefore, the identification of predictive biomarkers to better define subsets of patients who benefit the most from the regimen would be helpful. Several biomarkers have been reported that predict pCR to neoadjuvant therapy in TNBCs, including BRCA mutation [49, 50], homologous recombination deficiency (HRD) [51], and TILs [52]. HRD resulting from the loss of BRCA function is the main rationale of platinum efficacy in TNBC [53, 54, 55, 56]. Based on genomewide effects, BRCA‐like classifiers can identify the functional loss of BRCA and serve as predictors. HRD score identifies BRCAness and predicts the sensitivity of platinum and is increasingly being considered [51]. In the current study, 8 of 11 patients (73%) with BRCA mutation achieved pCR compared with 20 of 42 (48%) with wild‐type BRCA; however, it was not statistically significant (with either pCR or RCB 0/I). Recently, emerging evidence of high platinum sensitivity in BRCA‐related breast cancer has been reported. Byrski et al. reported a pCR rate of 61% from patients with BRCA1‐positive breast cancer when patients were treated with single agent cisplatin as NCT [57]. In another study, neoadjuvant carboplatin and docetaxel demonstrated a 59% pCR rate in BRCA‐associated TNBC [18]. However, in the randomized phase II trial TBCR C031 comparing neoadjuvant single agent cisplatin with AC, the pCR rates were 23% and 26%, respectively, which was statistically insignificant [58]. HRD status, but not BRCA mutation, was associated with improved DFS in SWOG S9313 [56]. We evaluated DRD by analyzing germline mutations of BRCA1, BRCA2, CHEK2, and RAD51C. In our univariate analysis, pCR was associated with DRD and GSIS immune‐hot with odds ratio of 4.76 and 4.62. Our data suggest that DRD and GSIS are potential tools for predicting pCR, with a stronger signal for GSIS based on multivariate logistic regression (see below).
Increased stromal TILs have been associated with improved pCR to NCT in TNBC and improved DFS and OS in TNBC [40]. In this study, we evaluated the association of stromal TILs, TNBC molecular subtypes, DRD status, and GeparSixto immune signature with pCR. Among the biomarkers evaluated, univariate logistic regression showed that pCR was associated with DRD status and immune‐hot GSIS, and RCB class was associated with immune‐hot GSIS. In the I‐SPY 2 study, a seven‐gene DNA repair deficiency expression signature (PARPi‐7) and BRCA1ness signatures [59] were associated with response in the neoadjuvant veliparib and carboplatin arm (p < .05) [60]. After adjusting for age as a continuous variable, race, stage, and grade with a multivariate logistic model, immune signature further supported the association of GSIS with pCR and RCB class with striking odds ratios of 7.19 and 8.95, respectively, whereas HRD was associated with higher pCR only. Based on these findings, GSIS outperforms all other biomarkers, such as TILs, TNBC molecular subtype, BRCA status, and DRD, in association with pCR.
There has been increasing interest in incorporating ICIs into the treatment of TNBC because of relatively high preexisting immunogenicity reflected by a higher percentage of stromal TILs, which are predictive and prognostic in TNBC [24, 61]. The IMpassion130 trial showed the benefit of incorporating the ICI atezolizumab, which moderately increased progression‐free survival in PD‐L1‐positive TNBC [28, 62]. In the I‐SPY 2 study, adding pembrolizumab to neoadjuvant adriamycin, cyclophosphamide, and paclitaxel increased pCR from 20% to 60% [30, 32]. In GeparNuevo, a randomized phase II NCT trial in TNBC, addition of durvalumab to nab‐paclitaxel and epirubicin plus cyclophosphamide increased pCR rate from 44% to 53% [63]. From these encouraging results, multiple ongoing studies have been testing the combination of ICI with chemotherapy in the neoadjuvant setting (NCT03742986) [64, 65]. In KEYNOTE‐522, adding pembrolizumab to carboplatin/paclitaxel followed by anthracycline/cyclophosphamide for treatment of early stage TNBCs showed a promising improved pCR rate of 64.8% versus 54.2% in the chemotherapy‐alone arm (p = .00055) [33]. Grade 3 or higher toxicities were seen in 76.8% and 72.2% of the patients, respectively. Of the patients treated with the pembrolizumab/chemotherapy combination in KEYNOTE‐522, 23.3% had discontinuation of treatment related to adverse events [33]. Furthermore, PD‐L1 positivity trended toward higher pCR rate in the subset analysis [33]. Despite these developments, one may question the chemotherapy backbone currently being tested: carboplatin and paclitaxel or paclitaxel followed by anthracycline and cyclophosphamide. These escalated approaches, in addition to 1 year of ICI treatment, could increase treatment‐associated toxicities and lower quality of life for patients who are potentially curable when given a less toxic regimen. Our study underscores, in addition to the importance of carboplatin‐based nonanthracycline neoadjuvant regimens [18], the urgent need to identify biomarkers that predict pCR or RCB 0/I in order to spare patients from unnecessary therapy. Carboplatin and taxane–based doublets may serve as an appropriate NCT backbone in the immunotherapy era for TNBC, especially BRCA wild‐type tumors, whereas BRCA germline mutated breast cancer may be most effectively treated with PARP inhibitor–based therapy, considering the promising efficacy of single talazoparib neoadjuvant therapy [66].
The current study was limited by small sample size, as well as availability of genomic data. A future neoadjuvant study incorporating GSIS for prospective patient selection is required to validate the current findings.
Conclusion
Carboplatin and nab‐paclitaxel showed manageable toxicity and had encouraging antitumor activity in patients with early stage TNBC resulting in a high pCR rate. This study adds to the existing data on the efficacy of platinum agents in early stage TNBC. Our results showing that immune‐hot GSIS is associated with significantly higher pCR and RCB 0/I hold promise for de‐escalating neoadjuvant therapy and provide further rationale for using a nonanthracycline platinum‐based therapy backbone for future neoadjuvant trials.
Author Contributions
Conception/design: Yuan Yuan, George Somlo, Joanne Mortimer
Provision of study material or patients: Daniel Schmolze, Daphne Stewart, James Waisman, Laura Kruper, Veronica Jones, John H. Yim, Christina Yeon
Collection and/or assembly of data: Jin Sun Lee, Susan E. Yost, Kim Robinson, Aileen Tang, Norma Martinez
Data analysis and interpretation: Sierra Min Li, Paul H. Frankel, Christopher Ruel, Andrea Menicucci, Sahra Uygun, Erin Yoder, Bastiaan van der Baan
Manuscript writing: Yuan Yuan, Jin Sun Lee, Susan E. Yost
Final approval of manuscript: Yuan Yuan, Jin Sun Lee, Susan E. Yost, Sierra Min Li, Paul H. Frankel, Christopher Ruel, Daniel Schmolze, Kim Robinson, Aileen Tang, Norma Martinez, Daphne Stewart, James Waisman, Laura Kruper, Veronica Jones, Andrea Menicucci, Sahra Uygun, Erin Yoder, Bastiaan van der Baan, John H. Yim, Christina Yeon, George Somlo, Joanne Mortimer
Disclosures
Yuan Yuan: Merck, Eisai, Novartis, Puma, Genentech, Pfizer (RF), Eisai, Genentech, AstraZeneca, Immunomedics, Novartis (C/A); Bastiaan van der Baan: Agendia (E, OI); Joanne Mortimer: Puma, Karyopharm (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 Diagram of patient distribution. Numbers of patient with pathology responses (black), mRNA array (green), and genetic or germline mutation testing (blue) are shown. pCR, pathological complete response; pts, patients
Supplemental Figure 2. Pathological response and DRD mutations (N = 66*). Size of bar graph depicts the pathological response among patients with the numbers of patients within each RCB class and DRD group. WT, wild type; pCR, pathological complete response; RCB, residual cancer burden. *One patient progressed and did not receive surgery (no RCB status represented in this graph). Unknown, patient not eligible for genetic testing per NCCN guideline.
Acknowledgments
This clinical trial was funded by Celgene. In addition, nab‐paclitaxel was provided by Celgene. mRNA array analysis was performed by Agendia Precision Oncology. This study was supported by the City of Hope Comprehensive Cancer Center Pathology Research Services Core and Biostatistics and Mathematical Modeling Core (National Cancer Institute of the National Institutes of Health under award P30CA033572). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Howlader N, Altekruse SF, Li CI et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plasilova ML, Hayse B, Killelea BK et al. Features of triple‐negative breast cancer: Analysis of 38,813 cases from the National Cancer Database. Medicine (Baltimore) 2016;95:e4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lehmann BD, Bauer JA, Chen X et al. Identification of human triple‐negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burstein MD, Tsimelzon A, Poage GM et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple‐negative breast cancer. Clin Cancer Res 2015;21:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dent R, Trudeau M, Pritchard KI et al. Triple‐negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–4434. [DOI] [PubMed] [Google Scholar]
- 6. Carey LA, Dees EC, Sawyer L et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007;13:2329–2334. [DOI] [PubMed] [Google Scholar]
- 7. Park S, Koo JS, Kim MS et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012;21:50–57. [DOI] [PubMed] [Google Scholar]
- 8. Senkus E, Kyriakides S, Ohno S et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2015;26(suppl 5):v8–v30. [DOI] [PubMed] [Google Scholar]
- 9. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 10. von Minckwitz G, Untch M, Blohmer JU et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–1804 [DOI] [PubMed] [Google Scholar]
- 11. Symmans WF, Wei C, Gould R et al. Long‐term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 2017;35:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liedtke C, Mazouni C, Hess KR et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol 2008;26:1275–1281. [DOI] [PubMed] [Google Scholar]
- 13. Stevens KN, Vachon CM, Couch FJ. Genetic susceptibility to triple‐negative breast cancer. Cancer Res 2013;73:2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sikov WM, Polley MY, Twohy E et al. CALGB (Alliance) 40603: Long‐term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) + /‐ carboplatin (Cb) and bevacizumab (Bev) in triple‐negative breast cancer (TNBC). J Clin Oncol 2019;37(suppl 15):591s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sikov WM, Berry DA, Perou CM et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once‐per‐week paclitaxel followed by dose‐dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple‐negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015;33:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hahnen E, Lederer B, Hauke J et al. Germline mutation status, pathological complete response, and disease‐free survival in triple‐negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol 2017;3:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Minckwitz G, Schneeweiss A, Loibl S et al. Neoadjuvant carboplatin in patients with triple‐negative and HER2‐positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol 2014;15:747–756. [DOI] [PubMed] [Google Scholar]
- 18. Sharma P, Lopez‐Tarruella S, Garcia‐Saenz JA et al. Efficacy of neoadjuvant carboplatin plus docetaxel in triple‐negative breast cancer: Combined analysis of two cohorts. Clin Cancer Res 2017;23:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gradishar WJ, Tjulandin S, Davidson N et al. Phase III trial of nanoparticle albumin‐bound paclitaxel compared with polyethylated castor oil‐based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794–7803. [DOI] [PubMed] [Google Scholar]
- 20. Desai N, Trieu V, Yao Z et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor‐free, albumin‐bound paclitaxel, ABI‐007, compared with cremophor‐based paclitaxel. Clin Cancer Res 2006;12:1317–1324. [DOI] [PubMed] [Google Scholar]
- 21. Untch M, Jackisch C, Schneeweiss A et al. Nab‐paclitaxel versus solvent‐based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto‐GBG 69): A randomised, phase 3 trial. Lancet Oncol 2016;17:345–356. [DOI] [PubMed] [Google Scholar]
- 22. Loi S, Drubay D, Adams S et al. Tumor‐infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early‐stage triple‐negative breast cancers. J Clin Oncol 2019;37:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adams S, Goldstein LJ, Sparano JA et al. Tumor infiltrating lymphocytes (TILs) improve prognosis in patients with triple negative breast cancer (TNBC). Oncoimmunology 2015;4:e985930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Denkert C, von Minckwitz G, Brase JC et al. Tumor‐infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2‐positive and triple‐negative primary breast cancers. J Clin Oncol 2015;33:983–991. [DOI] [PubMed] [Google Scholar]
- 25. Nicolas E, Bertucci F, Sabatier R et al. Targeting BRCA deficiency in breast cancer: What are the clinical evidences and the next perspectives? Cancers (Basel) 2018;10:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walsh T, Casadei S, Coats KH et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA 2006;295:1379–1388. [DOI] [PubMed] [Google Scholar]
- 27. Easton DF, Pharoah PD, Antoniou AC et al. Gene‐panel sequencing and the prediction of breast‐cancer risk. JAMA Oncol 2015;372:2243–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmid P, Adams S, Rugo HS et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 29. Loibl S, Untch M, Burchardi N et al. Randomized phase II neoadjuvant study (GeparNuevo) to investigate the addition of durvalumab to a taxane‐anthracycline containing chemotherapy in triple negative breast cancer (TNBC). J Clin Oncol 2018;36:2. [Google Scholar]
- 30. Nanda R, Liu MC, Yau C et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early‐stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I‐SPY2 trial. JAMA Oncol 2020;6:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmid P, Park YH, Munoz‐Couselo E et al. KEYNOTE‐173: Phase 1b multicohort study of pembrolizumab (Pembro) in combination with chemotherapy as neoadjuvant treatment for triple‐negative breast cancer (TNBC). Cancer Res 2019;79:2. [Google Scholar]
- 32. Nanda R, Liu MC, Yau C et al. Pembrolizumab plus standard neoadjuvant therapy for high‐risk breast cancer (BC): Results from I‐SPY 2. J Clin Oncol 2017;35(suppl 15):506a.28029304 [Google Scholar]
- 33. Schmid P, Cortes J, Pusztai L et al. Pembrolizumab for early triple‐negative breast cancer. N Engl J Med 2020;382:810–821. [DOI] [PubMed] [Google Scholar]
- 34. Smith TJ, Bohlke K, Lyman GH et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015;33:3199–3212. [DOI] [PubMed] [Google Scholar]
- 35. Symmans WF, Peintinger F, Hatzis C et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 36. Delahaye LJ, Wehkamp D, Floore AN et al. Performance characteristics of the MammaPrint® breast cancer diagnostic gene signature. Per Med 2013;10:801–811. [DOI] [PubMed] [Google Scholar]
- 37. Krijgsman O, Roepman P, Zwart W et al. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat 2012;133:37–47. [DOI] [PubMed] [Google Scholar]
- 38. Chen X, Li J, Gray WH et al. TNBCtype: A subtyping tool for triple‐negative breast cancer. Cancer Inform 2012;11:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Denkert C, Minckwitz Gv, Brase JC et al. Tumor‐infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2–positive and triple‐negative primary breast cancers. J Clin Oncol 2015;33:983–991. [DOI] [PubMed] [Google Scholar]
- 40. Denkert C, von Minckwitz G, Darb‐Esfahani S et al. Tumour‐infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40–50. [DOI] [PubMed] [Google Scholar]
- 41. Salgado R, Denkert C, Demaria S et al. The evaluation of tumor‐infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Early Breast Cancer Trialists’ Collaborative Group ; Peto R, Davies C, Godwin J et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loibl S, O'Shaughnessy J, Untch M et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple‐negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol 2018;19:497–509. [DOI] [PubMed] [Google Scholar]
- 44. Prat A, Adamo B, Cheang MC et al. Molecular characterization of basal‐like and non‐basal‐like triple‐negative breast cancer. The Oncologist 2013;18:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santonja A, Sanchez‐Munoz A, Lluch A et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 2018;9:26406–26416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masuda H, Masuda N, Kodama Y et al. Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple‐negative breast cancer patients. Cancer Chemother Pharmacol 2011;67:911–917. [DOI] [PubMed] [Google Scholar]
- 47. Filho OM, Stover DG, Asad S et al. Immunophenotype and proliferation to predict for response to neoadjuvant chemotherapy in TNBC: Results from BrighTNess phase III study. J Clin Oncol 2019;37(suppl 15):510a. [Google Scholar]
- 48. Masuda H, Baggerly KA, Wang Y et al. Differential response to neoadjuvant chemotherapy among 7 triple‐negative breast cancer molecular subtypes. Clin Cancer Res 2013;19:5533–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caramelo O, Silva C, Caramelo F et al. The effect of neoadjuvant platinum‐based chemotherapy in BRCA mutated triple negative breast cancers‐systematic review and meta‐analysis. Hered Cancer Clin Pract 2019;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fasching PA, Loibl S, Hu C et al. BRCA1/2 mutations and bevacizumab in the neoadjuvant treatment of breast cancer: Response and Prognosis results in patients with triple‐negative breast cancer from the GeparQuinto Study. J Clin Oncol 2018;36:2281–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Telli ML, Hellyer J, Audeh W et al. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple‐negative or BRCA1/2 mutation‐associated breast cancer. Breast Cancer Res Treat 2018;168:625–630. [DOI] [PubMed] [Google Scholar]
- 52. Loi S, Sirtaine N, Piette F et al. Prognostic and predictive value of tumor‐infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node‐positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin‐based chemotherapy: BIG 02‐98. J Clin Oncol 2013;31:860–867. [DOI] [PubMed] [Google Scholar]
- 53. Pellegrino B, Mateo J, Serra V et al. Controversies in oncology: Are genomic tests quantifying homologous recombination repair deficiency (HRD) useful for treatment decision making? ESMO Open 2019;4:e000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Telli ML, Timms KM, Reid J et al. Homologous recombination deficiency (HRD) score predicts response to platinum‐containing neoadjuvant chemotherapy in patients with triple‐negative breast cancer. Clin Cancer Res 2016;22:3764–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Loibl S, Weber KE, Timms KM et al. Survival analysis of carboplatin added to an anthracycline/taxane‐based neoadjuvant chemotherapy and HRD score as predictor of response‐final results from GeparSixto. Ann Oncol 2018;29:2341–2347. [DOI] [PubMed] [Google Scholar]
- 56. Sharma P, Barlow WE, Godwin AK et al. Impact of homologous recombination deficiency biomarkers on outcomes in patients with triple‐negative breast cancer treated with adjuvant doxorubicin and cyclophosphamide (SWOG S9313). Ann Oncol 2018;29:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Byrski T, Huzarski T, Dent R et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1‐positive breast cancer patients. Breast Cancer Res Treat 2014;147:401–405. [DOI] [PubMed] [Google Scholar]
- 58. Tung N, Arun B, Hacker MR et al. TBCRC 031: Randomized phase II study of neoadjuvant cisplatin versus doxorubicin‐cyclophosphamide in germline BRCA carriers with HER2‐negative breast cancer (the INFORM trial). J Clin Oncol 2020;38:1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Severson TM, Wolf DM, Yau C et al. The BRCA1 ness signature is associated significantly with response to PARP inhibitor treatment versus control in the I‐SPY 2 randomized neoadjuvant setting. Breast Cancer Res 2017;19:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolf DM, Yau C, Sanil A et al. DNA repair deficiency biomarkers and the 70‐gene ultra‐high risk signature as predictors of veliparib/carboplatin response in the I‐SPY 2 breast cancer trial. NPJ Breast Cancer 2017;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kwa MJ, Adams S. Checkpoint inhibitors in triple‐negative breast cancer (TNBC): Where to go from here. Cancer 2018;124:2086–2103. [DOI] [PubMed] [Google Scholar]
- 62. Schmid P, Cruz C, Braiteh FS et al. Abstract 2986: Atezolizumab in metastatic TNBC (mTNBC): Long‐term clinical outcomes and biomarker analyses. Cancer Res 2017;77(suppl 13):2986a. [Google Scholar]
- 63. Loibl S, Untch M, Burchardi N et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane‐based neoadjuvant therapy in early triple‐negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279–1288. [DOI] [PubMed] [Google Scholar]
- 64. Schmid P, Cortes J, Bergh JCS et al. KEYNOTE‐522: Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo + chemo as neoadjuvant therapy followed by pembro vs placebo as adjuvant therapy for triple‐negative breast cancer (TNBC). Ann Oncol 2017;28(suppl 5):V72a. [Google Scholar]
- 65. Litton JK, Moulder SL, Hess KR et al. Neoadjuvant trial of nab‐paclitaxel and atezolizumab (Atezo), a PD‐L1 inhibitor, in patients (pts) with chemo‐insensitive triple negative breast cancer (TNBC). Ann Oncol 2018;29(suppl 8):VIII72a. [Google Scholar]
- 66. Litton JK, Scoggins ME, Hess KR et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol 2020;38:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 Diagram of patient distribution. Numbers of patient with pathology responses (black), mRNA array (green), and genetic or germline mutation testing (blue) are shown. pCR, pathological complete response; pts, patients
Supplemental Figure 2. Pathological response and DRD mutations (N = 66*). Size of bar graph depicts the pathological response among patients with the numbers of patients within each RCB class and DRD group. WT, wild type; pCR, pathological complete response; RCB, residual cancer burden. *One patient progressed and did not receive surgery (no RCB status represented in this graph). Unknown, patient not eligible for genetic testing per NCCN guideline.


