Abstract
Lessons Learned
Oral selective HDAC6 inhibitors could allow for decreased toxicity compared to pan‐class inhibitors, and increased ease of use.
ACY‐1215 is well tolerated and led to disease stabilization in 50% of patients treated on a twice‐daily dosing schedule.
Rational drug combinations with ACY‐1215 improve efficacy in patients with lymphoma.
Biomarkers such as XBP‐1 level or HDAC6‐score may improve patient selection.
Background
ACY‐1215, ricolinostat, is an oral, first‐in‐class isoform‐selective HDAC6 inhibitor. HDAC6 is a class IIb deacetylase and plays a critical role in protein homeostasis via the unfolded protein response (UPR). Lymphocytes generate a large repertoire of antibodies and depend on an activated UPR to maintain proteostasis. Lymphomas utilize this biology to evade programmed cell death. In preclinical models of lymphoma, ACY‐1215 disrupted proteostasis, triggering apoptosis.
Methods
We translated these findings into a multi‐institution, open‐label, dose‐escalation phase Ib/II study aimed to determine the safety and efficacy in patients with relapsed and refractory lymphoma.
Results
Twenty‐one patients with heavily pretreated lymphoma were accrued. Patients in the phase Ib portion were enrolled on one of two dose cohorts [Arm A: 160 mg daily (n = 3) or Arm B: 160 mg twice daily (n = 10)]. ACY‐1215 was well tolerated. There were no dose limiting toxicities. Most adverse events were grade 1–2, including diarrhea (57%), nausea (57%), and fatigue (43%). Grade 3–4 toxicities were rare and included anemia (9.5%) and hypercalcemia (9.5%). An additional 8 patients were enrolled on the phase II portion, at 160 mg twice daily. Sixteen patients were evaluable for response. ACY‐1215 did not result in any complete or partial responses in patients treated. Eight patients had stable disease (50%) lasting a median duration of 4.5 months, all of whom were treated twice daily. Disease progressed in eight patients (50%) at cycle 2. Five patients were not evaluable due to disease progression prior to cycle 2. The median PFS was 56 days.
Conclusion
ACY‐1215 is an oral selective HDAC6 inhibitor that was safe in patients with relapsed and refractory lymphoid malignancies and led to disease stabilization in half of the evaluable patients.
Keywords: Lymphoma, HDAC6 inhibitor, Ricolinostat, ACY‐1215
Figure 1.
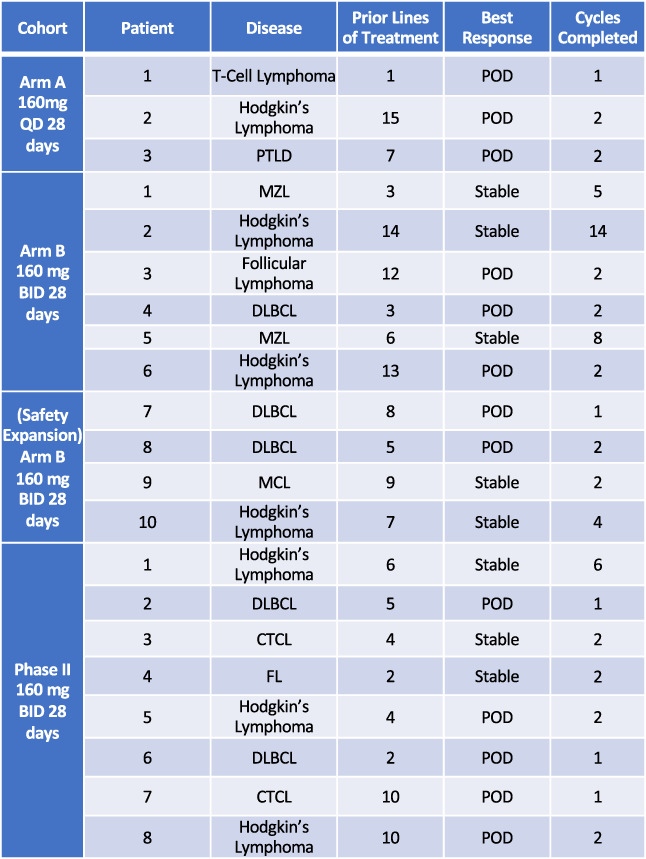
Patient response to therapy. Abbreviations: BID, twice daily; CTCL, cutaneous T‐cell lymphoma; DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; MZL, marginal zone B‐cell lymphoma; POD, progression of disease; PTLD, post‐transplant lymphoproliferative disorder; QD, once daily.
Discussion
This is the first reported experience of an isoform‐selective HDAC6 inhibitor to be studied for the treatment of patients with lymphoma. The rationale was based on the activity of drugs that disrupt protein degradation pathways, such as the proteasome inhibitor bortezomib. We have substantial preclinical evidence to support the notion that ACY‐1215 mediates its effects through the UPR and synergizes with complementary agents in the setting of lymphoma [1]. Rates of response to ACY‐1215, when used in combination in patients with multiple myeloma refractory to multiple agents, ranged from 29%–55% [2]. Several studies have evaluated ACY‐1215 in combination with paclitaxel based on the premise of dual targeting of microtubule dynamics [3, 4]. Given the pleiotropic effects of pan‐class HDAC inhibitors, efforts have been under way to develop isoform‐selective inhibitors. Based on the safety and efficacy of ACY‐1215 in other malignancies and our preclinical data, we sought to determine the effects of this first‐in‐class agent in the setting of lymphoma.
In this clinical study of ACY‐1215, the drug was well tolerated with no observed dose‐limiting toxicities. Toxicities consisted mainly of gastrointestinal symptoms, which were mild and easily controlled. Fifty percent of patients achieved prolonged stable disease, ranging from 2 to 14 months, on the twice‐daily dosing schedule. This is notable especially in the setting of heavily pretreated indolent lymphomas, as the goal of therapy for these patients is shifted from cure to maintaining quality of life and keeping disease in check. ACY‐1215 achieved both of those goals in patients with indolent lymphomas.
Pan‐class HDAC inhibitors are presently approved for use for patients with relapsed T‐cell lymphomas and in combination with bortezomib and dexamethasone for patients with myeloma but have had limited effect in other lymphoma subtypes as a single agent [5]. In this study, three patients with T‐cell lymphoma were enrolled. The first patient was treated on the single daily dosing schedule and achieved a clinical response, with resolution of B‐symptoms, prior to experiencing disease progression. The other two patients had cutaneous T‐cell lymphoma and were heavily pretreated with four and ten prior therapies. Of these patients, the first had achieved stable disease and the second had disease progression.
It is possible that certain subtypes of lymphoma may be more susceptible to HDAC6 inhibition. In our previous work, we discovered that marginal zone lymphomas have relatively lower expression of XBP‐1 compared with aggressive lymphomas when measured by immunohistochemistry [1]. Lower XBP‐1 may predict for a shift toward apoptosis and away from cellular homeostasis. Two patients with marginal zone lymphoma treated with ACY‐1215 achieved disease stabilization for 5 and 8 months.
ACY‐241, a second generation selective HDAC6 inhibitor, can achieve higher serum concentrations than ACY‐1215 and is in tablet formulation. The ACY‐1215 pharmacokinetic profile is such that its serum concentration plateaus at higher doses. ACY‐241 overcomes this phenomenon, leading to a greater degree of HDAC inhibition including HDAC1/2. ACY‐241 could be an alternative solution to providing a potent and well‐tolerated oral HDAC inhibitor to patient care.
Trial Information
| Disease | Lymphoma: Hodgkin and non‐Hodgkin |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase I/II, 3 + 3 |
| Primary Endpoints | Toxicity, tolerability, safety |
| Secondary Endpoint | Efficacy, pharmacokinetics |
| Additional Details of Endpoints or Study Design | |
| Phase Ib: The primary endpoint of the study was to establish the safety and tolerability of ACY1215 in two dosing schedules. | |
| Phase II: The primary endpoint of the study was the antitumor activity of ACY‐1215, as measured by objective response rate (complete response [CR] + partial response [PR]) assessed according to International Harmonization Project Revised Criteria (2007) [6]. | |
| Secondary endpoints of the study were as follows: (a) define the duration of response, (b) define the rate of progression‐free survival, (c) define safety and adverse events, and (d) describe the single‐dose and multiple‐dose ACY‐1215 pharmacokinetic profile. | |
| For the phase Ib portion of the study, patients with refractory lymphoma were treated with one of two dose schedules of ACY‐1215 (Acetylon Pharmaceuticals). Patients were accrued sequentially to two dose cohorts (160 mg once daily in Arm A and 160 mg twice daily in Arm B). After three patients enrolled in Arm A and ≤ 33% of patients (n ≤ 1) experienced a dose‐limiting toxicity, three additional patients were enrolled into Arm B for safety clearance, and then if ≤33% of patients (n ≤ 1) experienced a dose‐limiting toxicity, the cohort was to be further expanded to a total of 10 patients. | |
| The phase II study employed a minimax Simon two‐stage design. For stage 1, 2 of 22 patients must have achieved a response to allow for accrual to stage 2. For stage 2, there was a plan to enroll 18 patients, of whom 8 responses were needed to attain an efficacy of 25%. Accrual to the study was terminated early after the development of ACY‐1215 in lymphoma was halted following the acquisition of Acetylon by Celgene. | |
| Investigator's Analysis | Drug tolerable, hints of efficacy |
Drug Information
| Generic Name | ACY‐1215 |
| Trade Name | Ricolinistat |
| Company Name | Acetylon Pharmaceuticals |
| Drug Type | Small molecule |
| Drug Class | HDAC |
| Dose | 160 milligrams (mg) per flat dose |
| Route | Oral (p.o.) |
| Schedule of Administration | Days 1–28 |
Patient Characteristics
| Number of Patients, Male | 11 |
| Number of Patients, Female | 10 |
| Age | Median (range): 55 (24–86) years |
| Number of Prior Systemic Therapies | Median (range): 9 (1–5); 8 (6–10); 4 (>10) |
| Other |
Race: White, 16 (76%); Black or African American, 4 (19%); Asian 1 (4.8%) Ethnicity: Hispanic, 4 (19%); non‐Hispanic, 17 (81%) |
| Cancer Types or Histologic Subtypes |
Hodgkin lymphoma, 7 Diffuse large B‐cell lymphoma, 5 T‐cell lymphoma, 3 Follicular lymphoma, 2 Marginal zone B‐cell lymphoma, 2 Mantle cell lymphoma, 1 Post‐transplant lymphoproliferative disorder, 1 |
Primary Assessment Method
| Title | Dose Escalation |
| Number of Patients Screened | 23 |
| Number of Patients Enrolled | 21 |
| Number of Patients Evaluable for Toxicity | 21 |
| Number of Patients Evaluated for Efficacy | 16 |
| Evaluation Method | International Harmonization Project Revised Criteria (2007) |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 50 (8%) |
| Response Assessment PD | n = 50 (8%) |
| (Median) Duration Assessments PFS | 56 days |
| (Median) Duration Assessments Response Duration | 4.5 months |
| (Median) Duration Assessments Duration of Treatment | 2 months |
| Outcome Notes | |
| From April 2014 to October 2016, 23 patients who met the inclusion criteria were enrolled in the phase Ib and phase II portions of the study. Two patients were not accrued because of active infection and withdrawal of consent, respectively; therefore, of the 23 patients enrolled, 21 patients were accrued to the study. Fifteen patients were sequentially enrolled into cohorts of the phase Ib to determine maximum tolerated dose prior to the phase II expansion. Eight patients were enrolled to the phase II expansion. Most patients (57%) had received more than five prior lines of treatment. Sixteen patients were evaluable for response following cycle 2. No patients attained a complete or partial response. Of the 16 evaluable patients, 8 patients had stable disease (50%), all of whom were treated on twice‐daily schedule. Of the 16 evaluable patients, 8 patients experienced progression of disease (50%). There were five patients who were not evaluable for response at cycle 2. All five of these patients experienced progression of disease in cycle 1. Among patients with Hodgkin lymphoma treated on the twice‐daily dosing, 50% (3/6) had stable disease. All patients with marginal zone B‐cell lymphoma (n = 2) or mantle cell lymphoma (n = 1) had stable disease (100%). One patient with Hodgkin lymphoma who had 14 prior lines of therapy remained stable for 14 cycles and had symptomatic improvement corresponding to an increase of hemoglobin from 11.3 g/dL to 12.6 g/dL after nine cycles and resolution of B symptoms. Another patient with T‐cell lymphoma had immediate resolution of B symptoms including fevers and pruritus after starting once daily dosing of ACY‐1215. The median duration of disease stabilization was 4.5 months (range 2–14 months). The median progression‐free survival was 56 days (range 18–394 days), and the median 1‐year survival was not reached. | |
Adverse Events
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| Abdominal pain | 86 | 10 | 5 | 0 | 0 | 0 | 14 |
| Alanine aminotransferase increased | 90 | 0 | 5 | 0 | 5 | 0 | 10 |
| Anemia | 90 | 0 | 0 | 10 | 0 | 0 | 10 |
| Anorexia | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Aspartate aminotransferase increased | 90 | 0 | 5 | 0 | 5 | 0 | 10 |
| Cough | 71 | 19 | 10 | 0 | 0 | 0 | 29 |
| Confusion | 86 | 10 | 5 | 0 | 0 | 0 | 14 |
| Creatinine increased | 86 | 0 | 14 | 0 | 0 | 0 | 14 |
| Dehydration | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Diarrhea | 43 | 52 | 5 | 0 | 0 | 0 | 57 |
| Dyspnea | 81 | 10 | 5 | 5 | 0 | 0 | 19 |
| Fatigue | 57 | 33 | 10 | 0 | 0 | 0 | 43 |
| Fever | 86 | 10 | 5 | 0 | 0 | 0 | 14 |
| Gastrointestinal disorders ‐ Other, specify | 90 | 10 | 0 | 0 | 0 | 0 | 10 |
| Headache | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Hot flashes | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Hypercalcemia | 90 | 0 | 0 | 5 | 5 | 0 | 10 |
| Hyperkalemia | 90 | 10 | 0 | 0 | 0 | 0 | 10 |
| Nausea | 43 | 43 | 14 | 0 | 0 | 0 | 57 |
| Pain | 76 | 14 | 5 | 5 | 0 | 0 | 24 |
| Platelet count decreased | 90 | 5 | 0 | 0 | 5 | 0 | 10 |
| Rash maculo‐papular | 86 | 5 | 5 | 5 | 0 | 0 | 14 |
| Sinus tachycardia | 90 | 10 | 0 | 0 | 0 | 0 | 10 |
| Upper respiratory infection | 90 | 0 | 10 | 0 | 0 | 0 | 10 |
| Vomiting | 76 | 14 | 10 | 0 | 0 | 0 | 24 |
ACY‐1215 was well tolerated. There were no dose‐limiting toxicities. There were no dose reductions or delays. Most adverse events were grade 1 or 2. The table represents treatment‐emergent adverse events occurring in more than one patient. The most common grade 1–2 toxicities included diarrhea (57%), nausea (57%), fatigue (43%), cough (29%), vomiting (24%), and pain (19%). The most common grade 3–4 toxicities included anemia (9.5%) and hypercalcemia (9.5%).
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Appendicitis | 3 | Unrelated |
| Hypercalcemia | 4 | Unrelated |
| Hypotension | 4 | Unrelated |
There were three serious adverse events occurring in two patients, including one patient with appendicitis. Another patient with diffuse large B‐cell lymphoma who experienced both hypercalcemia and hypotension, which occurred during progression of disease, died on study because of progression of disease.
Pharmacokinetics/Pharmacodynamics
| Noncompartmental pharmacokinetic (PK) analysis was performed using Phoenix WinNonlin PK software. The observed maximum concentration (Cmax) of ACY‐1215 was 568 ± 232 at the time of 0.8 ± 0.3 hours (Fig. 2). The terminal half‐life was 1.17 ± 0.23 hours. The area under the curve (AUC) 0 to infinity was 1,281.4 ± 469.9 hours*ng/mL with a volume of distribution measured as 253.2 ± 220.5 L. The Cmax, time to reach Cmax, volume of distribution, and AUC 0 to infinity are similar to that reported by Yee and colleagues, as well as that demonstrated by the phase Ia ACY100 trial for once daily dosing of ACY‐1215 at 160 mg [15, 16]. However, the terminal half‐life was slightly lower in our study compared with that measured in the aforementioned trials (1.17 hours ±0.23 hours vs. 2.88 ± 0.33 hours vs. 2.78 ± 0.17 hours). Despite this slight variation, PK data in this study suggest little accumulation during repetitive dosing and no substantial differences in PK between daily and twice‐daily oral dosing of 160 mg ACY‐1215. |
Assessment, Analysis, and Discussion
| Completion | Study terminated before completion |
| Investigator's Assessment | Drug tolerable, hints of efficacy |
This study led to modest results. Prolonged durations of disease stabilization were achieved for many with indolent lymphomas. In these patients, symptoms and clinical parameters improved. In addition, disease shrank but remained greater than 50% its original size. Using biomarkers for response may lead to enriched responses in certain subtypes of lymphoma.
Recently, it was reported that calculating an HDAC6 score in breast cancer may predict for sensitivity to ACY‐1215 [2]; the HDAC6 score was based on mRNA expression in individuals’ tumors. In this study conducted by Kalinsky et al., patients with breast cancer were treated with ACY‐1215 in doses ranging from 120 mg to 240 mg daily for 21 days in combination with nab‐paclitaxel 100 mg/m2 given on days 1, 8, and 15 of a 28‐day cycle. The combination was well tolerated. Most patients achieved stable disease, and preliminary data suggest that the HDAC6 score may predict for activity. We look forward to updated data regarding the HDAC6 score. Perhaps as a means to enhance for biomarkers that predict for response, the HDAC6 score could be combined with immunohistochemistry staining of XBP‐1 to allow for even better selection of patients who may respond to HDAC6 inhibition. XBP‐1 is a component of the unfolded protein response and maintains homeostasis during endoplasmic reticular stress. Previously, we discovered that marginal zone lymphomas have relatively lower expression of XBP‐1 when measured by immunohistochemistry [6]. Lower XBP‐1 may predict for a shift toward apoptosis and away from cellular homeostasis and result in increased sensitivity to HDAC6 inhibition.
Given the pleotropic effects of pan‐class HDAC inhibitors [7, 8, 9, 10, 11, 12], isoform‐selective HDAC inhibitors have been of great interest; however, they have yet to gain clinical approval. Recently there has been piqued interest in selectively targeting HDAC3, as it cooperates with the master regulator of the germinal center, BCL6 [13, 14]. Taking lessons from ACY‐1215 and ACY‐241 [15, 16, 17, 18], perhaps isoform‐selective HDAC3 inhibitors may be developed [19]. This strategy may then allow a more precise epigenetic targeting with selective HDAC inhibition, rather than the broad stroke cytotoxic effects of pan‐class inhibitors.
ACY‐1215 demonstrated excellent tolerability. In general, HDAC inhibitors have had limited utility as single agents in most lymphoma subtypes; therefore, ACY‐1215 may be more effective when combined rationally with other targeted agents or immunotherapies [20, 21, 22, 23, 24].
Disclosures
Jennifer E. Amengual: Acetylon Pharmaceuticals, Appia Pharmaceuticals (RF), Karyopharm, Daiichi Sankyo, Janssen (H, SAB); Jennifer K. Lue: Daiichi Sankyo, AstraZeneca (C/A), Kymera Therapeutics (RF), Kymera Therapeutics, Astex Pharmaceuticals (SAB); Bijal Shah: Pfizer, Amgen, Kite/Gilead, Novartis, Celgene/Juno/BMS, Precision Biosciences, Adaptive, Spectrum/Acrotech, AstraZeneca, Beigene (C/A, H), Kite/Gilead, Jazz (RF); Simon Jones: Acetylon Pharmaceuticals (E); Ahmed Sawas: Seattle Genetics, Gilead, Daiichi Sanko (C/A), Affimed (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
Figure 2.
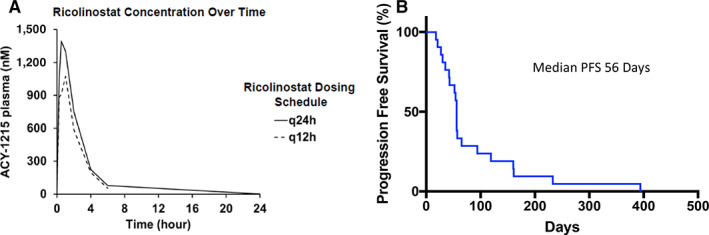
ACY‐1215 concentration over time (left) and progression‐free survival (right). Abbreviations: PFS, progression‐free survival; q12h, every 12 hours; q24h, every 24 hours.
Acknowledgments
This clinical trial was funding by Acetylon Pharmacueticals. The authors thank the patients for entrusting us with their care. This publication was supported in part through the National Cancer Institute Cancer Center Support Grant P30CA013696 and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001873.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02091063
- Sponsor: Acetylon Pharmaceuticals
- Principal Investigator: Jennifer E. Amengual
- IRB Approved: Yes
References
- 1. Amengual JE, Johannet P, Lombardo M et al. Dual targeting of protein degradation pathways with the selective HDAC6 inhibitor ACY‐1215 and bortezomib is synergistic in lymphoma. Clin Cancer Res 2015;21:4663–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raje NS, Bensinger W, Cole CE et al. Ricolinostat (ACY‐1215), the first selective HDAC6 inhibitor, combines safely with pomalidomide and dexamethasone and shows promising early results in relapsed‐and‐refractory myeloma (ACE‐MM‐102 Study). Blood 2015;126:4228a. [Google Scholar]
- 3. Kalinsky K, Onishi M, Yu J et al. Phase IB trial of ACY‐1215 (Ricolinostat) combined with nab‐paclitaxel in metastatic breast cancer. J Clin Oncol 2018;36(suppl 15):1058a. [Google Scholar]
- 4. Lee EK, Tan‐Wasielewski Z, Matulonis UA et al. Results of an abbreviated phase Ib study of the HDAC6 inhibitor ricolinostat and paclitaxel in recurrent ovarian, fallopian tube, or primary peritoneal cancer. Gynecol Oncol Rep 2019;29:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crump M, Coiffier B, Jacobsen ED et al. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large‐B‐cell lymphoma. Ann Oncol 2008;19:964–969. [DOI] [PubMed] [Google Scholar]
- 6. Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 7. Duvic M, Talpur R, Ni X et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T‐cell lymphoma (CTCL). Blood 2007;109:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piekarz RL, Frye R, Turner M et al. Phase II multi‐institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T‐cell lymphoma. J Clin Oncol 2009;27:5410–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piekarz RL, Frye R, Prince HM et al. Phase 2 trial of romidepsin in patients with peripheral T‐cell lymphoma. Blood 2011;117:5827–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ning ZQ, Li ZB, Newman MJ et al. Chidamide (CS055/HBI‐8000): A new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell‐mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol 2012;69:901–909. [DOI] [PubMed] [Google Scholar]
- 11. San‐Miguel JF, Hungria VT, Yoon SS et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double‐blind phase 3 trial. Lancet Oncol 2014;15:1195–1206. [DOI] [PubMed] [Google Scholar]
- 12. Amengual JE, Clark‐Garvey S, Kalac M et al. Sirtuin and pan‐class I/II deacetylase (DAC) inhibition is synergistic in preclinical models and clinical studies of lymphoma. Blood 2013;122:2104–2113. [DOI] [PubMed] [Google Scholar]
- 13. Jiang Y, Ortega‐Molina A, Geng H et al. CREBBP inactivation promotes the development of HDAC3‐dependent lymphomas. Cancer Discov 2017;7:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stengel K, Bhaskara S, Wang J et al. Therapeutic targeting of HDAC3 in FOXO1‐mutant DLBCL. Blood 2017;130(suppl 1):311a. [Google Scholar]
- 15. Gordon MS, Shapiro G, Sarantopoulos J et al. A phase 1b study of the safety, pharmacokinetics, and preliminary antitumor activity of citarinostat (ACY‐241) in combination with paclitaxel (Pac) in patients (pts) with advanced solid tumors (AST). J Clin Oncol 2018;36(suppl 15):2547a. [Google Scholar]
- 16. Niesvizky R, Richardson PG, Gabrail NY et al. ACY‐241, a novel, HDAC6 selective inhibitor: synergy with immunomodulatory (IMiD®) drugs in multiple myeloma (MM) cells and early clinical results (ACE‐MM‐200 Study). Blood 2015;126:3040a. [Google Scholar]
- 17. Awad MM, Le Bruchec Y, Lu B et al. Phase Ib study: Selective histone deacetylase (HDAC) inhibitor ACY‐241+ nivolumab (Nivo) in advanced non‐small cell lung cancer (NSCLC). J Clin Oncol 2019;37(suppl 15):9029a. [Google Scholar]
- 18. Hideshima T, Qi J, Paranal RM et al. Discovery of selective small‐molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma. Proc Natl Acad Sci USA 2016;113:13162–13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adhikari N, Amin SA, Trivedi P et al. HDAC3 is a potential validated target for cancer: An overview on the benzamide‐based selective HDAC3 inhibitors through comparative SAR/QSAR/QAAR approaches. Eur J Med Chem 2018;157:1127–1142. [DOI] [PubMed] [Google Scholar]
- 20. Santo L, Hideshima T, Kung AL et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY‐1215, in combination with bortezomib in multiple myeloma. Blood 2012;119:2579–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yee AJ, Bensinger WI, Supko JG et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: A multicentre phase 1b trial. Lancet Oncol 2016;17:1569–1578. [DOI] [PubMed] [Google Scholar]
- 22. Krukowski K, Ma J, Golonzhka O et al. HDAC6 inhibition effectively reverses chemotherapy‐induced peripheral neuropathy. Pain 2017;158:1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amengual JE, Prabhu SA, Lombardo M et al. Mechanisms of acquired drug resistance to the HDAC6 selective inhibitor ricolinostat reveals rational drug:drug combination with ibrutinib. Clin Cancer Res 2016:clincanres. 2022.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang P, Almeciga‐Pinto I, Jarpe M et al. Selective HDAC inhibition by ACY‐241 enhances the activity of paclitaxel in solid tumor models. Oncotarget 2017;8:2694. [DOI] [PMC free article] [PubMed] [Google Scholar]


