Abstract
We describe a case of recurrent glioblastoma treated with anlotinib in this report. The patient was administered anlotinib 12 mg p.o. once every day (days 1–14, with a 21‐day cycle) (anlotinib clinical study NCT04004975) and oral temozolomide chemotherapy 100 mg/m2 (days 1–7, days 15–21, 28‐day cycle; 12 cycles). After 2 months of therapy, the patient achieved a partial response that has been maintained for >17 months of follow‐up. Molecular characterization confirmed the presence of a TERT promoter mutation, wild‐type IDH1/2, an FGFR3‐TACC3 fusion, and FGFR3 amplification in the patient. Anlotinib is a multitarget tyrosine kinase inhibitor that was originally designed to inhibit VEGFR2/3, FGFR1–4, PDGFRα/β, and c‐Kit. Patients with TERT promoter mutations and high‐grade IDH‐wild‐type glioma have shorter overall survival than patients with IDH‐wild‐type glioma without TERT promoter mutations. However, this patient had a favorable clinic outcome, and FGFR3‐TACC3 fusion may be a new marker for treatment of glioma with anlotinib.
Key Points
This case study is believed to be the first report that FGFR3‐TACC3 fusion could be a novel indication to treat recurrent glioblastoma with the drug anlotinib.
This case exhibited an exceptional response (maintained partial response >17 months) after 2‐month combined therapy of anlotinib and oral temozolomide chemotherapy.
This case also underscores the importance of molecular diagnosis for clinically complex cases. Tumor tissue‐based assessment of molecular biomarkers in brain tumors has been successfully translated into clinical application.
Short abstract
Currently, effective target therapy that can prolong the overall survival of patients with recurrent glioblastoma is limited. This case report describes the promising treatment of a patient with recurrent GBM with an FGFR3‐TACC3 fusion treated with anlotinib.
Introduction
Glioblastoma (GBM) is the most common and fatal primary brain tumor. Despite aggressive treatment, the median overall survival (OS) is less than 15 months [1]. The preferred treatment for GBM is surgery, and subsequent treatment is a combination of radiotherapy and chemotherapy. For patients with recurrent GBM, targeted therapies are limited [2, 3, 4]. Molecular markers of GBM are needed to improve personalized diagnosis and treatment and to better understand the biology of the disease to facilitate the development of new therapies [1].
Carcinogenic fusion because of chromosomal translocation is recognized as a molecular driver in gliomas [5]. A fusion involving fibroblast growth factor receptors (FGFR1/FGFR3) and TACC1/TACC3 occurs in approximately 3% of patients with GBM. FGFR3‐TACC3 fusion gliomas exhibit FGFR3 expression [6]. Gliomas carrying the FGFR3‐TACC3 fusion are also IDH1/2 wild type and almost always (∼75%) have TERT promoter mutations or CDKN2A loss [7]. IDH1/2‐wild‐type gliomas with TERT promoter mutations are associated with reduced OS compared with gliomas without TERT mutations [8, 9, 10].
Anlotinib is a multitarget tyrosine kinase inhibitor that was originally designed to inhibit VEGFR2/3, FGFR1–4, PDGFRα/β, c‐Kit, and Ret. Anlotinib can inhibit not only tumor angiogenesis but also tumor cell growth. It has been used to treat advanced lung cancer [11]. Anlotinib is approved by the National Medical Products Administration for use in non‐small cell lung cancer. Preclinical results showed that anlotinib significantly inhibited FGFR1–4, especially FGFR2 [12].
Patient Story
A 44‐year‐old woman was admitted to the hospital as a new patient on October 1, 2017; she experienced three episodes of sudden grand mal epilepsy prior to being admitted to the hospital. Magnetic resonance imaging (MRI) revealed space‐occupying lesion in the left thalamus and left parietal lobe (Fig. 1). The patient underwent tumor resection in the left parietal lobe under general anesthesia on October 10, 2017. The immunohistochemical results showed that glial fibrillary acidic protein, Olig‐2, and p53 were expressed; epithelial membrane antigen and IDH‐1 were negative; and the Ki67 proliferation index was ∼3% (Fig. 1). The final diagnosis was anaplastic astrocytoma, IDH wild type, World Health Organization (WHO) grade III. The patient was treated with intensity modulated radiation therapy to a total dose of 60 Gy in 30 fractions, together with temozolomide (TMZ) chemotherapy 75 mg/m2 taken orally on November 29, 2017. Two adjuvant TMZ cycles (200 mg/m2 per day, every day, days 1–5, every 28 days for one cycle) were given to this patient. MRI revealed relapse of the tumor on April 5, 2018, and the patient received bevacizumab 300 mg (5 mg/kg) targeted therapy and temozolomide (200 mg/m2, days 1–5, every 28 days). Brain MRI monitoring revealed a partial response on June 7, 2018. Then, the patient received oral temozolomide chemotherapy at home (100 mg/m2, days 1–7, days 15–21, every 28 days). Subsequent brain MRI monitoring showed progressive disease on July 7, 2018.
Figure 1.
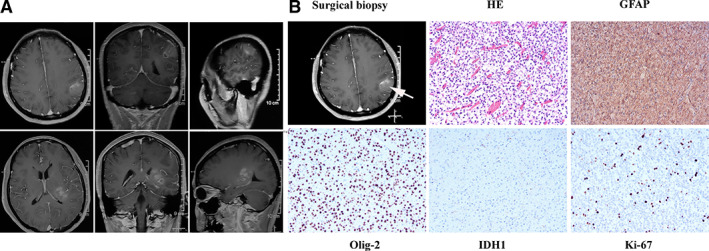
Image of pathological diagnosis. (A): Magnetic resonance imaging revealed space‐occupying lesion in left thalamus and left parietal lobe. (B): Hematoxylin and eosin stain (200×) shows that tumor cells are dense and unevenly distributed, with obvious nuclear heterogeneity, and that small blood vessels proliferate in the tumor tissue. The immunohistochemical results showed that GFAP and Olig‐2 were expressed, IDH‐1 was negative, and the Ki67 proliferation index was ∼3%. The final diagnosis was anaplastic astrocytoma, IDH wild type, World Health Organization grade III. Abbreviations: GFAP, glial fibrillary acidic protein; HE, hematoxylin and eosin.
Next‐generation sequencing analysis (NGS) of DNA was performed for the primary surgical tissue. The results showed the TERT p.C228T mutation (mutation allele frequency [MAF] = 40%, formalin‐fixed, paraffin‐embedded [FFPE] sample), FGFR3 amplification (copy number [N] = 3.13, FFPE sample), and an FGFR3‐TACC3 fusion (Catalogue of Somatic Mutations in Cancer [COSMIC] mutation identifier, COSF1353; FFPE sample) (Fig. 2). Then, the FGFR3‐TACC3 fusion was verified with an RNA fusion panel. The FGFR3‐TACC3 fusion can result in diffuse FGFR3 expression in most tumor cells. FGFR‐TACC fusions are potent oncogenic events that, when present in brain tumor cells, confer sensitivity to FGFR inhibitors [11]. Anlotinib is a novel multitarget tyrosine kinase inhibitor that is designed to primarily inhibit VEGFR2/3, FGFR1–4, PDGFRα/β, and c‐Kit. The patient was administered anlotinib 12 mg p.o. every day (days 1–14, 21‐day cycle) (anlotinib clinical study NCT04004975) and oral TMZ chemotherapy 100 mg/m2 (days 1–7, days 15–21, 28‐day cycle; 12 cycles). After 2 months of therapy, the patient achieved a partial response that has been maintained for >17 months of follow‐up.
Figure 2.
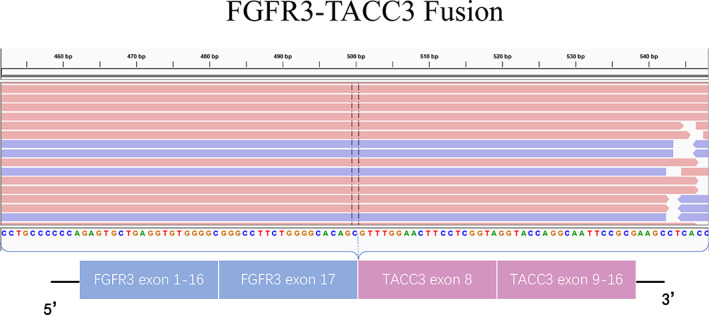
Structure of FGFR‐TACC gene fusions identified by next‐generation sequencing. The FGFR3‐TACC3 fusion (COSF1353) was a classic fusion in the Catalogue of Somatic Mutations in Cancer (COSMIC) and was confirmed by RNA sequencing. Abbreviation: bp, base pairs.
No serious adverse reactions occurred during anlotinib treatment in this case. The adverse reactions were grade 1 hypertension (well controlled by antihypertensive drugs), grade 2 hand and foot syndrome, and grade 1 white blood cell decline, with no bleeding and no proteinuria. In an ongoing clinical trial, the most common grade ≥ 3 adverse events during anlotinib treatment in advanced non‐small cell lung cancer were hypertension, triglyceride elevation, hand and foot skin reaction, and lipase elevation [13]. The timeline of treatment and radiographic responses is shown in Figure 3.
Figure 3.
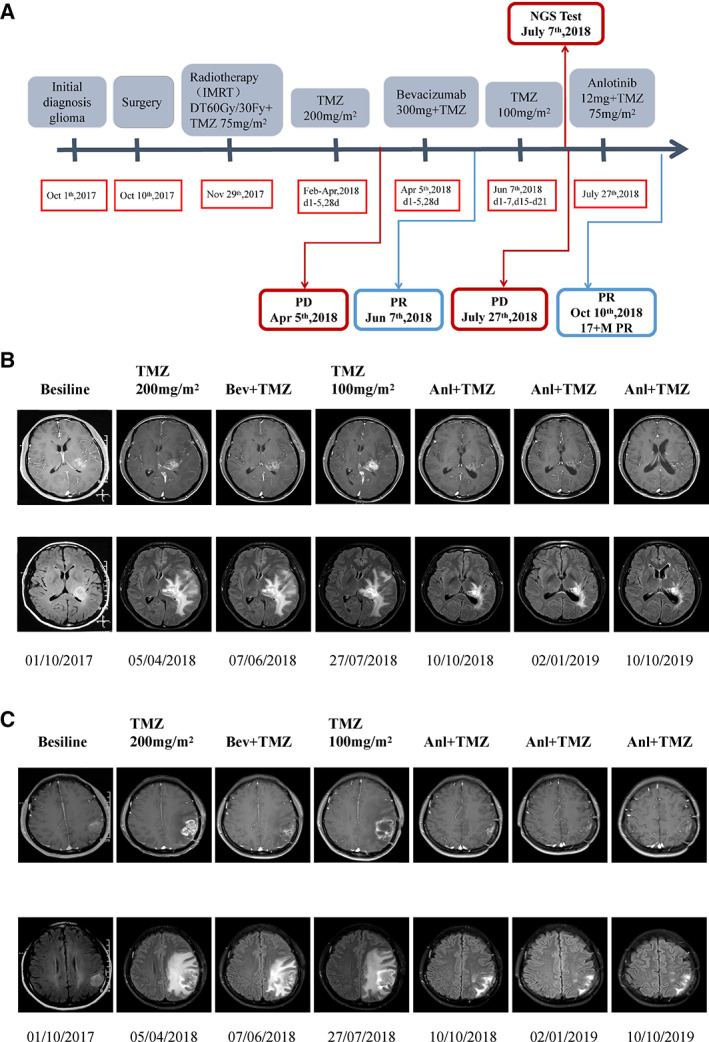
Timeline of treatment and radiographic responses. (A): Timeline of treatment. (B): Radiographic responses to anlotinib on left thalamus: the image of the previous row at each time is an enhanced image of the lesion; the image of the lower row of each time is the fluid attenuated inversion recovery (FLAIR) image of the lesion. FLAIR image mainly shows that the edema subsided after antivascular treatment. (C): Radiographic responses to anlotinib on left parietal: the image of the previous row at each time is an enhanced image of the lesion; the image of the lower row of each time is the FLAIR image of the lesion. FLAIR image mainly shows that the edema subsided after antivascular treatment. Abbreviations: 17 + M, more than 17 months; Anl, anlotinib; Bev, bevacizumab; IMRT, intensity modulated radiation therapy; NGS, next‐generation sequencing; PD, progressive disease; PR, partial response; TMZ, temozolomide.
Discussion
Patients with TERT promoter mutations and high‐grade IDH‐wild‐type glioma have shorter OS than patients with IDH‐wild‐type glioma without TERT promoter mutations [14, 15, 16]. The prognosis of patients with recurrent GBM is poor. For recurrent gliomas, bevacizumab is the preferred drug in the National Comprehensive Cancer Network (NCCN) guidelines. Bevacizumab, a monoclonal antibody primarily inhibiting VEGF, has been used in patients with GBM as a later‐line treatment since its approval in the U.S. for recurrent GBM in 2009 and has become the standard treatment for patients with recurrent GBM in the NCCN guidelines [17]. Another recommended regimen is bevacizumab plus chemotherapy (carmustine or lomustine, TMZ, or carboplatin). A meta‐analysis showed that the combination of bevacizumab and chemotherapy in the treatment of recurrent GBM significantly improved the progression‐free survival and objective response rate but did not extend the OS compared with single‐agent bevacizumab [18]. In cases in which standard regimens fail for patients with relapsed GBM, targeted therapies are limited [2]. Molecular markers of GBM are needed to improve personalized diagnosis and treatment and to better understand the biology to facilitate the development of new therapies [1].
The significant difference in efficacy between bevacizumab and anlotinib in our case suggests that anlotinib is a suitable and promising treatment for GBM with FGFR3‐TACC3 fusion. It may provide a new option for patients with recurrent GBM after TMZ or bevacizumab treatment.
Anlotinib is a multitarget tyrosine kinase inhibitor and an oral small molecule inhibitor. The drug was originally designed to inhibit VEGFR2/3, FGFR1–4, PDGFRα/β, and c‐Kit; thus, it has broad inhibitory effects on tumor angiogenesis and growth [12]. In our case report, the patient carried an FGFR3‐TACC3 fusion (COSF1353) and FGFR3 amplification according to the NGS analysis. The FGFR3‐TACC3 fusion (COSF1353) is a classic fusion (an oncogenic driver mutually exclusive with IDH mutation and EGFR amplification that mainly occurs in GBM) [6] and was once reported in the recurrent tumor of two patients who were diagnosed with glioblastoma, WHO grade IV [7]. The FGFR3‐TACC3 fusion (COSF1353) was also reported in a 64‐year‐old woman who was affected by left parietal GBM, whose tumor showed diffuse FGFR3 expression in most tumor cells [11]. FGFR‐TACC fusions are clear oncogenic events in vitro, and brain tumor cells carrying these fusions have sensitivity to FGFR inhibitors [5, 18]. In glioblastoma, a relationship between FGFR3 amplification and FGFR3 protein expression has not been reported. However, the two are correlated up to approximately 90% of the time in bladder cancer [19]. Therefore, the reason for the significant effect of anlotinib treatment in this patient may be that the patient carried two alterations at the same time and was thus particularly sensitive to FGFR3 inhibitors. Patients with FGFR3‐TACC3 rearrangements and/or FGFR3 amplification should be considered for clinical trials featuring FGFR inhibitors.
Gliomas carrying FGFR3‐TACC3 fusions are also IDH1/2 wild type and almost always (∼75%) have TERT promoter mutations or CDKN2A loss, which can provide an important clue for the molecular characterization of this glioma subtype [7].
Currently, it is very difficult to treat recurrent glioblastoma. There is no effective targeted drug that can prolong the overall survival of patients with recurrent glioblastoma. Our case is the first report in the world in which a patient with recurrent GBM with an FGFR3‐TACC3 fusion was treated with anlotinib, and the patient achieved a partial response that has been maintained for >17 months. In recent years, a variety of FGFR inhibitors are also undergoing clinical trials (Table 1). In view of the particularly effective treatment of a patient with recurrent GBM with FGFR3‐TACC3 fusion with anlotinib, it is worth conducting further clinical trials. Perhaps anlotinib can change the current treatment status of patients with GBM with FGFR3‐TACC3 fusion. Such a finding will have important clinical significance.
Table 1.
Clinical trials of FGFR inhibitors
| Tumor type | Drug | Company | Target | Clinical development stage |
|---|---|---|---|---|
| Glioblastoma | BGJ398 | Novartis | FGFR1–3 | Phase II (NCT01975701) |
| AZD4547 | AstraZeneca | FGFR1–3 | Phase I/II (NCT02824133) | |
| Solid tumors/ lymphoma | E‐7090 | Eisai | FGFR1–3 | Phase I (NCT02275910) |
| LY2874455 | Eli Lilly & Co. | Pan‐FGFR | Phase I (NCT03125239) | |
| TAS‐120 | Taiho Pharm | FGFR1–4 | Phase I/II (NCT02052778) | |
| Erdafitinib (JNJ42756493) | Janssen | FGFR1–4, VEGFR2 | Phase II (NCT02421185) | |
| Rogaratinib (BAY1163877) | Bayer | FGFR1–4 | Phase II (NCT03410693) | |
| Debio‐1347 | Debio Pharm | FGFR1–3 | Phase I (NCT01948297) |
Limitation
There are some interesting points to be addressed. First, the patient has multiple tumors located in important functional areas (left thalamus and left parietal lobe). Therefore, the first surgery only removed the tumor node from left parietal lobe. Second, because the surgical biopsy tissue did not penetrate the central area of the tumor, we inferred that the pathological result may not comprehensively represent the true clinical situation of the patient. We invited a pathologist to perform another pathological assessment. The final pathological diagnosis was anaplastic astrocytoma, IDH wild type, WHO grade III. Third, the patient did not take a molecular diagnosis after the first surgery, and she only took the traditional treatment (because of economic reasons). Then the patient had a short clinical relapse, and the disease could not be controlled after traditional treatment. In order to find a better treatment, NGS of DNA was performed for the primary surgical tissue. The results showed IDH wild type, codeletion of 1q/19p absent, TERT p.C228T mutation. Based on the above data and the initial pathological results, we concluded that this patient had a glioblastoma [20, 21].
After relapse, MRI of tumor lesions showed a cystic change and enhanced aggravation, accompanied by tumor cell necrosis. The characteristics of MRI were also consistent with the recurrent glioblastoma.
Combining the pathological result, molecular features, and postrelapse MRI characteristics, the patient had a recurrent glioblastoma. This also reflects the importance of molecular diagnosis for clinically complex cases.
Author Contributions
Conception/design: Yong Wang, Dandan Liang
Provision of study material or patients: Yong Wang, Jimin Chen
Collection and/or assembly of data: Jimin Chen, Huan Chen
Data analysis and interpretation: Yong Wang, Rui Fan
Manuscript writing: Rui Fan, Rongjie Tao
Final approval of manuscript: Yong Wang, Dandan Liang, Jimin Chen, Huan Chen, Rui Fan, Ye Gao, Yongsheng Gao, Rongjie Tao, Henghui Zhang
Disclosures
The authors indicated no financial relationships.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Rongjie Tao, Email: rongjietao@163.com.
Henghui Zhang, Email: zhhbao@ccmu.edu.cn.
References
- 1. Diplas BH, He X, Brosnan‐Cashman JA et al. The genomic landscape of TERT promoter wildtype‐IDH wildtype glioblastoma. Nat Commun 2018;9:2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lv Y, Zhang J, Liu F et al. Targeted therapy with anlotinib for patient with recurrent glioblastoma: A case report and literature review. Medicine (Baltimore) 2019;98:e15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lau D, Magill ST, Aghi MK. Molecularly targeted therapies for recurrent glioblastoma: Current and future targets. Neurosurg Focus 2014;37:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Touat M, Idbaih A, Sanson M et al. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann Oncol 2017;28:1457–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh D, Chan JM, Zoppoli P et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012;337:1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bielle F, Di Stefano AL, Meyronet D et al. Diffuse gliomas with FGFR3‐TACC3 fusion have characteristic histopathological and molecular features. Brain Pathol 2018;28:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballester LY, Moghadamtousi SZ, Leeds NE et al. Coexisting FGFR3 p.K650T mutation in two FGFR3‐TACC3 fusion glioma cases. Acta Neuropathol Commun 2019;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camille P, Anne G, Emilie B et al. Increasing incidence of central nervous system (CNS) tumors (2000–2012): Findings from a population based registry in Gironde (France). BMC Cancer 2018;18:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol 2013;125:621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiao Y, Killela PJ, Reitman ZJ et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 2012;3:709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Stefano AL, Fucci A, Frattini V et al. Detection, characterization, and inhibition of FGFR‐TACC fusions in IDH wild‐type glioma. Clin Cancer Res 2015;21:3307–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun Y, Niu W, Du F et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi‐target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Syed YY. Anlotinib: First global approval. Drugs 2018;78:1057–1062. [DOI] [PubMed] [Google Scholar]
- 14. Eckel‐Passow JE, Lachance DH, Molinaro AM et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 2015;372:2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Killela PJ, Reitman ZJ, Jiao Y et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self‐renewal. Proc Natl Acad Sci USA 2013;110:6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arita H, Yamasaki K, Matsushita Y et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun 2016;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Capelletti M, Dodge ME, Ercan D et al. Identification of recurrent FGFR3‐TACC3 fusion oncogenes from lung adenocarcinoma. Clin Cancer Res 2014;20:6551–6558. [DOI] [PubMed] [Google Scholar]
- 18. Chen Z, Xu N, Zhao C et al. Bevacizumab combined with chemotherapy vs single‐agent therapy in recurrent glioblastoma: Evidence from randomized controlled trials. Cancer Manag Res 2018;10:2193–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tomlinson DC, Baldo O, Harnden P et al. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol 2007;213:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foote MB, Papadopoulos N, Diaz LA Jr. Genetic classification of gliomas: Refining histopathology. Cancer Cell 2015;28:9–11. [DOI] [PubMed] [Google Scholar]
- 21. Masui K, Mischel PS, Reifenberger G. Molecular classification of gliomas. Handb Clin Neurol 2016;134:97–120. [DOI] [PubMed] [Google Scholar]


