Abstract
Background
The optimal treatment of BRCA wild-type patients with platinum-sensitive recurrent ovarian cancer remains unknown. Recently, there is an increase in the evidence to support the role of the combination of a poly(adenosine diphosphate-ribose) polymerase inhibitor, anti-angiogenic agents, and immunotherapy as maintenance therapy in BRCA wild-type patients with platinum-sensitive recurrence. We hypothesized that adding pembrolizumab and bevacizumab to olaparib maintenance can increase progression-free survival (PFS) in BRCA wild-type patients with platinum-sensitive recurrent ovarian cancer.
Methods
BRCA wild-type patients who received two previous courses of platinum-containing therapy, achieved complete or partial response to last treatment, and the treatment-free interval is >6 months after the penultimate platinum-based chemotherapy offered olaparib maintenance with pembrolizumab and bevacizumab. Forty-four patients will be included from 4 sites across Singapore and Korea. The primary endpoint of the study is 6-month PFS rate.
Trial Registration
ClinicalTrials.gov Identifier: NCT04361370, Clinical Research Information Service Identifier: KCT0005144
Keywords: Ovarian Neoplasms, Bevacizumab, Poly(ADP-ribose) Polymerase Inhibitors, Immunotherapy Recurrence
INTRODUCTION
Ovarian cancer remains one of the most lethal gynecological malignancies in women. Most women present with advanced-stage cancer and develop recurrence after first-line therapy. Platinum-based chemotherapy is the standard of care for patients with platinum-sensitive recurrent ovarian cancer, defined as development of recurrence ≥6 months after completing first-line chemotherapy. However, repeated exposure to platinum agents can cause toxicity and resistance to chemotherapy, leading to death. New therapeutic options for recurrent cancer would, therefore, be of clinical interest.
Poly(ADP-ribose) polymerase inhibitors (PARPis) are approved for the treatment of patients with ovarian cancer harboring BRCA1/2 mutations, as well as for BRCA1/2-wildtype patients with platinum-sensitive recurrent ovarian cancer. The rationale supporting the development of PARPis for BRCA1/2-mutant cancers was based on the concept of synthetic lethality, which predicated anti-tumor efficacy of PARPis in tumors with defects in homologous recombination repair. As maintenance therapy after second-line chemotherapy in a platinum-sensitive relapsed setting, three PARPis have demonstrated efficacy in patients without BRCA1/2 mutations, although the magnitude of benefit has been consistently much smaller compared to that in BRCA1/2-mutant patients. For example, the median progression-free survival (PFS) improvement in patients with platinum-sensitive relapsed BRCA1/2-mutant ovarian cancer treated with olaparib compared to that in patients treated with placebo was 19.1 months versus 5.5 months (hazard ratio [HR]=0.30; p<0.001), conferring an overall survival (OS) benefit of 1 year [1], but in patients with platinum-sensitive relapsed BRCA1/2-wildtype epithelial ovarian cancer, this was only 8.4 versus 4.8 months (HR=0.35; p<0.001) [2], and no OS benefit was seen. Similar results showing the disparity in benefit between BRCA1/2-mutated and -wildtype populations were seen in two phase III trials of maintenance PARPis therapy, using niraparib [3] and rucaparib [4], separately.
Bevacizumab is another maintenance therapy option for BRCA wild-type patients with recurrent ovarian cancer, and its use is supported by the growing clinical experience with antiangiogenics in ovarian cancer. In Korea, the Food and Drug Administration has permitted bevacizumab therapy for concurrent use and maintenance in all cases of platinum-sensitive recurrence [5,6]. This is supported by the OCEANS trial [5] and Gynecologic Oncology Group (GOG) 213 [6] that evaluated the addition of bevacizumab to chemotherapy concurrently and then as maintenance post-chemotherapy until disease progression. In these trials, only a 4–6-month improvement was observed in the median PFS after second-line chemotherapy even with bevacizumab maintenance. Thus, there is an unmet medical need for therapies that, when used in combination with the current standard of care, substantially increases the proportion of patients with complete remission and prevents disease recurrence in patients with advanced-stage ovarian cancer.
To date, combinations of PARPis + anti-angiogenic therapy, PARPis + programmed cell death protein 1 and programmed cell death ligand 1 (PD-1/PD-L1) blockade, and anti-angiogenic therapy + PD-1/PD-L1 blockade, have been investigated and shown to be synergistic and safe, even in BRCA1/2-wildtype patients. Although clinical trials are ongoing, no published studies have described the effect of combination therapy of all three classes of drugs, namely, PARPis, anti-angiogenic therapy, and immunotherapy, in recurrent ovarian cancer patients. The safety profile of the MEDIOLA combining olaparib, durvalumab and bevacizumab was well tolerated in patients with BRCA wild-type platinum-sensitive recurrent ovarian cancer, consistent with the known safety profiles of the single agents. The combination of PARPis, PD-L1 and anti-angiogenic therapy is now being tested as part of first-line maintenance treatment in the clinical trials, including DUO-O (olaparib, durvalumab, bevacizumab), ENGOT-ov43 (olaparib, pembrolizumab, bevacizumab), it is unknown yet whether combination of three drugs has synergistic and safe for BRCA wild-type patients. Therefore, the OPEB-01 study endeavors to study the combination of olaparib, bevacizumab, and pembrolizumab as maintenance therapy in BRCA1/2-wildtype patients with platinum-sensitive recurrent ovarian cancer, by leveraging its potential synergy.
METHODS
1. Trial design
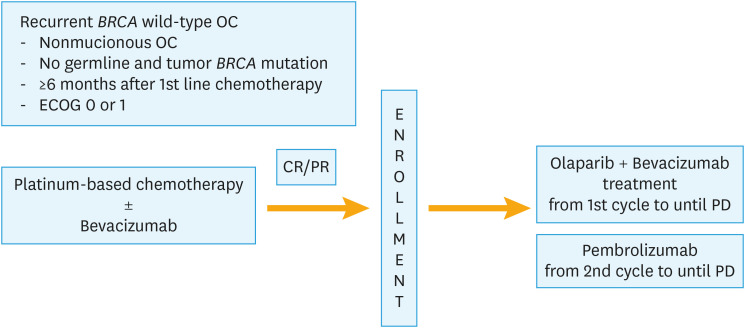
This phase II study of olaparib maintenance with pembrolizumab and bevacizumab in BRCA non-mutated patients with platinum-sensitive recurrent ovarian cancer (OPEB-01) is a multi-center, investigator-initiated, single-arm study which recruited from four sites across Singapore and Korea, with institutional review board approval obtained from each site. Patients with recurrent BRCA wild-type ovarian cancer who have responded to their last platinum-based chemotherapy (complete or partial response) are offered olaparib, pembrolizumab, and bevacizumab as maintenance treatment (Fig. 1). The treatment to be used in this trial is outlined in Table 1. Trial treatment will be administered on day 1 of each cycle on an outpatient basis. Pembrolizumab (200 mg) will be administered as an intravenous infusion for 30 minutes every 3 weeks from cycle 2 onwards.
Fig. 1. Study schema.
CR, complete remission; ECOG, Eastern Cooperative Oncology Group; OC, ovarian cancer; PD, progressive disease; PR, partial remission.
Table 1. Trial treatment.
| Drug | Dose/potency | Dose frequency | Route of administration | Regimen/treatment period | Use |
|---|---|---|---|---|---|
| Pembrolizumab | 200 mg | Q3W | IV infusion | Q3W; day 1 of each 3-week cycle from cycle 2 | Experimental |
| Olaparib | 300 mg | BID | Oral | During each treatment cycle | Experimental |
| Bevacizumab | 15 or 7.5 mg/kg | Q3W | IV infusion | Q3W; day 1 of each 3-week cycle | Background therapy |
BID, twice daily; IV, intravenous; Q3W, every 3 weeks
2. Participants
Patients with the following inclusion criteria will be enrolled in this study and all patients must provide informed consent to participate in this study: 1) Histologically confirmed diagnosis of high-grade predominantly serous, endometrioid, carcinosarcoma with high-grade serous component, clear cell, or low-grade serous ovarian cancers, primary peritoneal cancer, or fallopian tube cancer (only up to 8 patients with clear cell carcinoma will be included); 2) Participant has received two previous courses of platinum-containing therapy and has disease that was considered platinum-sensitive following the penultimate (next to last) platinum course (more than 6 months of interval between penultimate platinum regimen and progression of disease); 3) Participant has responded to the last platinum regimen (complete or partial response), remains in response, and is enrolled on study within 8 weeks after completing the last platinum regimen; 4) Participant is able to provide a newly obtained core or excisional biopsy of a tumor lesion for prospective testing of BRCA 1/2 and PD-L1 status prior to enrollment; 5) Females aged 20 years or older at the time of acquisition of informed consent; 6) Eastern Cooperative Oncology Group performance status of 0–1; 7) Participant has adequate organ function; 8) Participant is not pregnant or breastfeeding.
We will exclude patients based on the following criteria: 1) Participant has mucinous, germ cell, or borderline tumor of the ovary; 2) Participant has a known or suspected deleterious mutation (germline or somatic) in either BRCA1 or BRCA2; 3) Participant has a history of non-infectious pneumonitis that required treatment with steroids or currently has pneumonitis; 4) Participant either has myelodysplastic syndrome/acute myeloid leukemia or features suggestive of myelodysplastic syndrome/acute myeloid leukemia; 5) Participant has a known additional malignancy that is progressing or has required active treatment within the past 3 years; 6) Participant has known active central nervous system metastases and/or carcinomatous meningitis; 7) Participant has a known history of active tuberculosis; 8) Participant has a known history of Hepatitis B (defined as hepatitis B surface antigen reactive) or known active hepatitis C virus (defined as presence of HCV RNA [qualitative]) infection; 9) Participant has uncontrolled hypertension, defined as systolic >140 mmHg or diastolic >90 mmHg documented by two blood pressure readings taken at least 1 hour apart; 10) Participant has a history of hemorrhage, hemoptysis, or active gastrointestinal bleeding within 6 months.
3. Primary endpoint
The primary endpoint of the study is a PFS rate of 6 months. PFS is defined as the time from the start of treatment until the first documented sign of disease progression or death from any cause.
4. Secondary endpoints
The secondary endpoints include OS (time frame: up to 1 year), time to progression (time frame: up to 1 year), time to first subsequent treatment (or death) (time frame: date of first documented subsequent treatment or date of death, assessed up to 72 months), time to second subsequent treatment (time frame: date of first documented second subsequent treatment assessed up to 72 months), and PFS 2. OS is defined as the time from the first treatment until death from any cause.
5. Exploratory endpoints
The exploratory endpoint of this study is to identify molecular biomarkers of clinical response/resistance, safety, and/or the mechanism of action of pembrolizumab, olaparib, and bevacizumab in platinum-sensitive recurrent ovarian cancer. Comprehensive genomic profiling will be performed for all samples to find the predictive biomarkers. Tumors and blood samples from this study will be examined to identify BRCA reversion mutation and homologous recombination deficiency status changes. This research will include exome sequencing, RNA sequencing, whole genome sequencing, and circulating tumor DNA.
6. Sample size
The sample size was calculated based on Simon's 2-stage optimal design with assumptions concerning the estimated PFS rate in ovarian cancer. The rate of patients with disease-free state at 6 months is expected to be 50% with bevacizumab maintenance (current standard of care and results from GOG 213), and the HR of adding maintenance therapy of 3 combinations (PARPis, antiangiogenic therapy, and immune checkpoint inhibitor) was assumed to be 0.5, which was equivalent to 70.7% of PFS rate. When applying the same expected efficacy (HR=0.5) with DUO-O study (NCT04361370), the null hypothesis for this study will be 50% of the 6-month PFS rate, and alternative hypothesis of interest will be 70% of 6-month PFS rate. Using Simon's two-stage optimal design at a one-sided 5% level of significance and 80% power, a total of 39 patients are required in this study. In the 1st stage, 22 patients will be enrolled; if 10 or more progressive disease (PD) are observed, the trial will be terminated. If not, the trial will continue to the second stage, and a total of 39 patients will be studied. If the total number of PD is less than 15 or equal to, the null hypothesis will be rejected. Considering 10% follow-up loss, the sample size will be 44 patients in each dose.
7. Randomization and blinding
Not applicable.
8. Statistical methods
Efficacy analyses are based on the modified intent-to-treat approach (patients should receive at least one treatment dose). Safety analyses are based on the safety population (treated with at least one dose of study drug). Adverse events are graded according to common terminology criteria for adverse events version 5.0. After recruiting 22 patients, interim analysis will be conducted to determine futility of the treatment.
DISCUSSION
Recently, several randomized clinical trials on maintenance treatment with PARPis showed significant improvement in PFS and OS in patients with platinum-sensitive recurrent ovarian cancer and a BRCA mutation (somatic or germline) [1,3,4]. Therefore, such patients are most likely to benefit from treatment with PARPis. For BRCA-wildtype patients with platinum-sensitive recurrent ovarian cancer, few options for maintenance therapy are suggested; olaparib, niraparib, and bevacizumab as monotherapies are possible maintenance treatment options. Randomized studies comparing maintenance PARPis and placebo have consistently shown a smaller degree of benefit for BRCA-wildtype patients compared to BRCA-mutant patients. Study19 was a phase II trial of patients with platinum-sensitive recurrent ovarian cancer who were treated with at least two prior courses of platinum-based chemotherapy and achieved complete or partial response to the last chemotherapy. BRCA-wildtype patients treated with olaparib showed greater improvement in PFS compared to those treated with placebo; however, the absolute difference between the groups was small (7.4 vs. 5.5 months; HR=0.54; p=0.0075). The NOVA trial [3], evaluating niraparib maintenance, included two independent cohorts, BRCA-mutation and BRCA-wildtype. BRCA-wildtype group showed longer PFS compared to the placebo group; however, the absolute difference between the groups was similarly small (9.3 vs. 3.9 months; HR=0.45; p<0.001). Based on the results from GOG 213, median PFS after second-line chemotherapy is 6 months even with bevacizumab maintenance. Thus, there is an unmet need for novel therapy for patients with platinum-sensitive recurrent ovarian cancer without a BRCA mutation.
Novel treatments such as PD-1/PD-L1 immune checkpoint blockade have been investigated in recurrent ovarian cancer. The importance of the immune system in ovarian cancer has been demonstrated [7], whereby increased presence of tumor-infiltrating lymphocytes correlated with improved prognosis. However, immune checkpoint blockade monotherapy has had disappointing results, showing low objective response rates of <15% in several trials [8,9,10]. In patients with recurrent ovarian cancer treated with pembrolizumab monotherapy, PFS was only 2.1 months [10]. Certain populations may benefit more from immune checkpoint inhibitor monotherapy (e.g., PD-L1 positive and/or deficient mismatch repair patients). In order to expand current indications for PARPis, immune checkpoint blockade, and anti-angiogenics, including their application to biomarker negative patients, combination strategies are suggested. In a study of PARPis combined with anti-angiogenic agents, Liu et al. showed synergistic effects of olaparib and cediranib in BRCA-wildtype patients [11]. The AVANOVA randomized phase II study showed that the combination of niraparib and bevacizumab significantly improved PFS better than niraparib alone [12]. This survival benefit was more pronounced in non-BRCA mutation and/or homologous recombination deficiency negative patients as well as BRCA and/or homologous recombination deficiency positive group. The PAOLA randomized phase III study is currently evaluating the synergistic effects of olaparib and bevacizumab [13]. In PARPis with immune checkpoint inhibitor, PARPis treatment led to an accumulation of cytosolic dsDNA, which activates the cGAS-STING-TBK1-IRF3 innate immune axis, thus promoting antitumor immunity. This can be further enhanced by the introduction of immune checkpoint blockade. The MEDIOLA study shows synergistic effects of olaparib and durvalumab. TOPACIO study shows promising effects of combination between niraparib and pembrolizumab regardless of BRCA/homologous recombination deficiency status in platinum-resistant setting [14].
The combination of bevacizumab and a PARPis, together with immune checkpoint blockade is a developing area of interest in ovarian cancer. At present, several randomized trials on the combination of agents from these three classes are ongoing in the front-line therapy of advanced epithelial ovarian cancer, including ENGOT-ov44/FIRST (niraparib, TSR042, and bevacizumab), DUO-O (olaparib, durvalumab, and bevacizumab), and ENGOT-ov43/KEYLYNK-001 (olaparib, pembrolizumab, and bevacizumab). In our study, we focus on the combination of olaparib, bevacizumab, and pembrolizumab as maintenance therapy after second-line chemotherapy in a platinum-sensitive recurrent setting, specifically in BRCA-wildtype patients. It is unknown whether BRCA-wildtype patients could derive clinical benefits from olaparib maintenance when combined with other novel agents. We hypothesize that olaparib maintenance with bevacizumab and pembrolizumab is synergistic in this population. In this study, we aim to evaluate the efficacy and safety of olaparib maintenance with bevacizumab and pembrolizumab in BRCA-wildtype patients with platinum-sensitive recurrent ovarian cancer.
ACKNOWLEDGMENTS
We appreciate the contribution of the investigators and study teams at the participating sites.
Footnotes
Conflict of Interest: L.Y.J., K.B.G., N.N.Y.L., C.C.H., P.S.Y., and G.Y. have no competing interests to declare.
L.J.Y. has received honoraria and research funding from AstraZeneca and MSD.
L.M.C. has received honoraria and research funding from AstraZeneca, Clovis, MSD, Roche, and Takeda.
T.D.S.P. has received honoraria from AstraZeneca, Bayer, Foundation Medicine, Janssen, Merck, Roche, and Tessa Therapeutics as well as research funding from AstraZeneca, Bayer, and Karyopharm Therapeutics.
- Conceptualization: L.Y.J., L.M.C., K.B.G., N.N.Y.L., C.C.H., P.S.Y., T.D.S.P., G.Y., L.J.Y.
- Data curation: L.J.Y.
- Project administration: L.J.Y.
- Supervision: K.B.G.
- Visualization: L.Y.J., L.M.C., K.B.G., N.N.Y.L., C.C.H., P.S.Y., T.D.S.P., G.Y., L.J.Y.
- Writing - original draft: L.Y.J., L.J.Y.
- Writing - review & editing: L.Y.J., L.M.C., K.B.G., N.N.Y.L., C.C.H., P.S.Y., T.D.S.P., G.Y., L.J.Y.
References
- 1.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 2.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 3.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman R, Brady M, Herzog T, Sabbatini P, Armstrong D, Walker J, et al. A phase III randomized controlled clinical trial of carboplatin and paclitaxel alone or in combination with bevacizumab followed by bevacizumab and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian, peritoneal primary and fallopian tube cancer (Gynecologic Oncology Group 0213) Gynecol Oncol. 2015;137:3–4. [Google Scholar]
- 7.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 8.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 9.Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019;5:393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30:1080–1087. doi: 10.1093/annonc/mdz135. [DOI] [PubMed] [Google Scholar]
- 11.Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang GB, Malander S, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409–1419. doi: 10.1016/S1470-2045(19)30515-7. [DOI] [PubMed] [Google Scholar]
- 13.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 14.Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5:1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]