Abstract
Objective
The primary objective of this study was to evaluate the safety of niraparib 300 mg/day in Japanese patients with platinum-sensitive, relapsed ovarian cancer in a maintenance setting.
Methods
Phase 2, multicenter, open-label, single-arm study enrolled Japanese patients with platinum-sensitive, relapsed ovarian cancer who had received ≥2 platinum-based regimens. The primary endpoint (incidence of grade 3 or 4 thrombocytopenia-related events within 30 days after initial niraparib administration) was justified by the incidences of a global pivotal phase 3 study and its post-hoc safety analysis on thrombocytopenia, the major hematological adverse event of niraparib. The overall safety analysis examined other treatment-emergent adverse events (TEAEs).
Results
Enrolled patients (n=19) had a median (min, max) body weight of 53.9 (40.8–79.1) kg; all but one patient weighed <77 kg. Most (94.7%) patients initially received niraparib 300 mg/day but this decreased in subsequent cycles (mean±standard deviation dose intensity, 191.6±65.7 mg/day). In total, 6/19 (31.6%) patients experienced grade 3 or 4 thrombocytopenia-related events within 30 days of initial niraparib administration. Other common TEAEs included nausea, and decreased platelet or neutrophil counts. No progression-free or overall survival events occurred; only 1 of 4 response-evaluable patients had a post-baseline tumor assessment (stable disease).
Conclusion
The incidence of grade 3 or 4 thrombocytopenia-related events in Japanese ovarian cancer patients was similar to that in the corresponding non-Japanese study. Overall, the safety profile was acceptable and consistent with the known safety profile and previous experience with niraparib.
Trial Registration
ClinicalTrials.gov Identifier: NCT03759587
Keywords: Ovarian Cancer, Niraparib, Phase 2, Maintenance Therapy, Japanese
INTRODUCTION
Ovarian cancer is the leading cause of death from gynecological malignancies with a high fatality rate associated with late detection [1]. In Japan, ovarian cancer has been steadily increasing in incidence and many patients are initially diagnosed with more advanced stage cancers associated with low 5-year survival rates (46.0% for stage III and 25.1% for stage IV, 2009 figures) [2].
Most ovarian cancers are serous carcinomas, for which the standard treatment consists of a combination of debulking surgery and platinum-based chemotherapy. Although initial response to standard treatment is generally good, relapse is common. Poly(ADP-ribose) polymerase (PARP) inhibitors have emerged as an effective option for such cases of relapse, especially in a maintenance setting [3,4]. PARP inhibitors interfere with the ability of cells to repair DNA via PARP-mediated recruitment of DNA repair proteins such as topoisomerases and DNA ligase III [3,4]. In cancer cells with impaired DNA repair mechanisms, associated with homologous recombination deficiency (HRd; e.g., BRCA1 or BRCA2 mutations), PARP inhibitors lead to an accumulation of irreparable double-strand DNA breaks. Such vulnerable tumor cells are forced to rely on an error-prone non-homologous end-joining (NHEJ) mechanism for damage repair, which results in genetic instability and, eventually, apoptosis. This synergistic effect of HRd and drug-induced cytotoxicity is known as synthetic lethality.
Niraparib, a potent, selective PARP-1/2 inhibitor, was approved in the United States and Europe as a maintenance therapy for adult patients with platinum-sensitive relapsed epithelial ovarian, fallopian tube, or primary peritoneal cancer, including those without positive germline BRCA (gBRCA) mutation status [5]. This approval was based on positive results from ENGOT-OV16/NOVA, the first prospective phase 3 trial to analyze patients both with and without a deleterious gBRCA mutation [6]. Data from the NOVA trial also demonstrated that patients maintained quality of life during niraparib maintenance treatment [7]. A post-hoc analysis of the ENGOT-OV16/NOVA trial identified baseline platelet count and body weight as risk factors for grade ≥3 thrombocytopenia, which has been identified as the major dose-limiting toxicity for niraparib [8]. Specifically, patients with a body weight <77 kg or platelet count <150,000/µL had an approximately three-fold greater incidence of grade ≥3 thrombocytopenia during the first 30 days after the initial dose of niraparib 300 mg daily compared with patients with body weight of ≥77 kg and platelet count of ≥150,000/µL.
Based on the results of an open-label, single-arm, phase 2 study (QUADRA) in heavily pretreated ovarian cancer patients [9], the indication for niraparib in the US was extended to include patients who had 3 or more lines of therapy and either had a BRCA mutation regardless of platinum-sensitivity or were HRd-positive and platinum-sensitive [10]. Niraparib has been shown to be effective in heavily treated Japanese patients similar to the non-Japanese population enrolled in the QUADRA study [11]. Finally, positive results from a randomized, double-blind, placebo-controlled phase 3 trial (PRIMA) in patients with newly diagnosed advanced ovarian cancer was noteworthy in that it incorporated an individualized starting dose of 200 mg once daily for patients with a baseline body weight <77 kg, a platelet count <150,000/mm3, or both [12].
This phase 2 study (NCT03759587) was primarily designed to evaluate the safety of niraparib at a starting dose of 300 mg once daily in Japanese patients with platinum-sensitive, relapsed ovarian cancer. Considering the concerns regarding early onset of thrombocytopenia noted in patients receiving niraparib, this study was conducted under agreement with the Japanese regulatory agency (Pharmaceuticals and Medical Devices Agency [PMDA]) to evaluate the short-term safety of niraparib. Efficacy assessment was also requested by the PMDA as a secondary objective despite the short period of follow-up available.
MATERIALS AND METHODS
1. Study design
This phase 2, multicenter, open-label, single-arm study (NCT03759587) evaluated the safety and efficacy of niraparib in Japanese patients with platinum-sensitive, relapsed ovarian cancer who had achieved a complete response (CR) or partial response (PR) in the last chemotherapy containing platinum-based anticancer agents. Patients were enrolled at 15 sites in Japan from the date of the first subject's informed consent (28 December 2018) to data cutoff (17 March 2019).
Eligible patients were Japanese patients (20 years or older at consent) with histologically diagnosed ovarian cancer, fallopian tube cancer, or primary peritoneal cancer involving high-grade (or grade 3) serous or high-grade predominantly serous histology, regardless of biomarker status, or known to have gBRCA mutation. For inclusion, patients must have received ≥2 platinum-based regimens, including the last regimen (within 8 weeks after completion), to which they had a physician-assessed CR or PR response. Further, eligible patients must not have any measurable lesion >2 cm and cancer antigen (CA)-125 less than or equal to the upper limit of the normal range, or a >90% decrease following their last platinum regimen which had been stable for ≥7 days. Finally, patients must have had performance status of ≤1 on the Eastern Cooperative Oncology Group (ECOG) performance status as well as adequate organ function as assessed by laboratory testing.
Patients were mainly excluded if they had persisting grade ≥3 toxicity from previous chemotherapy, uncontrolled medical comorbidities and a history of treatment with PARP inhibitor(s). The study was conducted in accordance with ethical principles of the Declaration of Helsinki, the International Council for Harmonisation (ICH) Harmonised Tripartite Guideline for Good Clinical Practice (GCP), and the Institutional Review Board (IRB) regulations. The clinical study protocol, investigator's brochure, a sample informed consent form, and other study-related documents were reviewed and approved by the local or central IRBs of all study sites. Each investigator conducted the study according to applicable local or regional regulatory requirements and in accordance with the responsibilities listed in the protocol. All patients provided written informed consent to participate in the study.
2. Treatment
Niraparib 300 mg (3×100 mg hard capsules) once daily was administered orally in continuous 28-day cycles until subjects experienced objective progressive disease (PD), unacceptable toxicity, withdrawal of consent or until study discontinuation for any other prespecified reasons. Dose interruption and/or reduction were implemented and recorded if any grade toxicity was deemed intolerable to the patient. For non-hematologic toxicities, treatment was interrupted for any National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 4.03) grade 3 or 4 non-hematologic toxicity considered to be related to niraparib. Niraparib was restarted at 300 mg if the toxicity resolved to grade ≤1 within 28 days with intervention, or at 200 mg daily or 100 mg daily for first and second reductions, respectively, when adverse event intervention and prophylaxis was not considered feasible. If toxicity did not resolve to grade ≤1 within the 28-day dose interruption period and/or the patient had already undergone the maximum dose reductions, the patient permanently discontinued study treatment. For hematologic toxicities, dose interruption or modification criteria were based on predefined blood counts monitored by weekly blood draws until resolution or permanent discontinuation, if deemed necessary. With regard to platelet count, study treatment interruption was required if the platelet count decreased to 75,000–99,000/µL until platelet counts were ≥100,000/µL, with weekly monitoring until recovery. For the first occurrence of platelet decrease, treatment could then be resumed at the same dose or a reduced dose based on clinical judgement whereas the second occurrence after recovery required resumption at a reduced dose. If the platelet count decreased to <75,000/µL treatment interruption was required until platelet counts were ≥100,000/µL, with weekly monitoring until recovery, after which treatment could be resumed at a reduced dose.
3. Outcomes
The primary objective of this study was to evaluate the incidence of grade 3 or 4 thrombocytopenia-related events occurring within 30 days after initial administration of niraparib. In the analysis for the primary endpoint, the incidence of thrombocytopenia-related events was defined as the overall incidence of the MedDRA Preferred Term (PT) “thrombocytopenia” and the PT “platelet count decreased”.
Secondary objectives of this study related to the efficacy of niraparib. Efficacy was measured by investigator assessment of progression-free survival (PFS), overall response rate (ORR, CR + PR), and overall survival (OS) with disease progression. CR, and PR were assessed primarily via CT with contrast according to RECIST criteria (version 1.1) [13].
Safety of niraparib assessed as part of the primary objectives included the incidence of treatment-emergent adverse events (TEAEs), serious TEAEs, and TEAEs that led to drug reduction, interruption or discontinuation. TEAEs were defined as AEs that occurred after administration of the first dose of study drug and were coded using MedDRA and tabulated by PT and System Organ Class (SOC). Adverse events, including laboratory abnormalities, were considered according to severity (according to NCI CTCAE, version 4.03), seriousness, and causality in terms of relation to study drug administration. A comprehensive set of serum chemistry, hematology and urinalysis tests were performed on samples collected before study drug administration at the start of each cycle. Physical examinations and assessment of vital signs (blood pressure, pulse rate, body temperature) were also conducted at specified times during the study period.
4. Statistics
The threshold incidence of thrombocytopenia in non-Japanese patients was estimated using data from the NOVA study conducted outside of Japan, in which the incidence of grade 3 or 4 thrombocytopenia during the first 30 days after the initial niraparib dose was 34.6% in patients with baseline body weight of <77 kg or baseline platelet count of <150,000/µL. Further, the incidence of platelet count decrease less than 50,000/µL, which is almost equal to or worse than Grade 3 thrombocytopenia, was 46% in patients with baseline body weight of <58 kg. According to prescription data, ≥77% of ovarian cancer patients in Japan had a body weight of ≤59 kg. Therefore, the expected incidence of thrombocytopenia in this study population was considered as 46%. Based on these assumptions, 15 patients would provide 76% probability that the point estimate of the incidence of thrombocytopenia would be ≥35%, with which a certain level of evaluation is expected to be achievable.
RESULTS
1. Subject disposition and baseline characteristics
A total of 19 patients were enrolled and included in the full and safety analysis sets, defined as patients who received ≥1 dose of study drug. No patients discontinued the study drug. Only 4 patients were included in the response-evaluable analysis set, defined as patients who received ≥1 dose of study drug and had at least one measurable disease at baseline.
Baseline demographic and clinical characteristics are shown in Table 1. Patients had a median (min–max) age of 62.0 (44–79) years. The median (min–max) body weight of patients was 53.9 (40.8–79.1) kg and approximately 60% of patients weighed less than 58 kg, with all but one patient weighing less than 77 kg. The most common previous chemotherapy agents (≥20% of patients) were carboplatin (n=19, 100.0%), paclitaxel (n=19, 100.0%), bevacizumab (n=12, 63.2%), docetaxel (n=4, 21.1%), and doxorubicin hydrochloride (n=4, 21.1%).
Table 1. Demographic and baseline clinical characteristics.
| Characteristics | Safety population (n=19) | |
|---|---|---|
| Age (yr) | 62 (44–79) | |
| Age category (yr) | ||
| 18–64 | 11 (57.9) | |
| 65–74 | 6 (31.6) | |
| ≥75 | 2 (10.5) | |
| Weight (kg) | 53.9 (40.8–79.1) | |
| <58 | 11 (57.9) | |
| ≥58 and <77 | 7 (36.8) | |
| ≥77 | 1 (5.3) | |
| Mean±SD | 57.5±10.45 | |
| Time from initial diagnosis (yr) | 3.35 (1.2–19.6) | |
| Primary tumor site | ||
| Ovarian | 10 (52.6) | |
| Primary peritoneal | 5 (26.3) | |
| Fallopian tube | 4 (21.1) | |
| ECOG status | ||
| 0 | 17 (89.5) | |
| 1 | 2 (10.5) | |
| Cancer stage at initial diagnosis | ||
| IC | 1 (5.3) | |
| IIC | 1 (5.3) | |
| IIIB | 2 (10.5) | |
| IIIC | 10 (52.6) | |
| IV | 4 (21.1) | |
| Unknown | 1 (5.3) | |
| Time to progression after penultimate platinum therapy | ||
| 6–12 months | 5 (26.3) | |
| >12 months | 14 (73.7) | |
| Best response to most recent platinum therapy | ||
| CR | 9 (47.4) | |
| PR | 10 (52.6) | |
| Time from last platinum therapy to first dose of niraparib (days) | 42.0 (14–65) | |
| Histological subtype | ||
| Serous | 19 (100.0) | |
| Tumor grade | ||
| High-grade | 19 (100.0) | |
Values are presented as median (min–max) or number (%).
CR, complete response; ECOG, Eastern Cooperative Oncology Group; PR, partial response; SD, standard deviation.
Most (94.7%) patients received 300 mg of niraparib as the initial dose but this decreased in cycles 2 and 3. At the beginning of cycle 2, 57.9% (11/19 patients) received 200 mg, 26.3% (5/19 patients) received 300 mg, and 15.8% (3/19 patients) received 100 mg. Finally, in cycle 3, 2 of 5 (40.0%) patients received 300 mg and 200 mg (40.0%), and 1 subject received 100 mg. At data cutoff, 14 (73.7%) patients had started 2 cycles and 5 (26.3%) patients had started 3 cycles of niraparib (median [min–max] number of cycles, 2.0 [2–3] cycles). The median (min–max) total study duration and overall treatment exposure of the study drug were 51.0 (31–61) days and 49.0 (32–67) days, respectively, and the mean±standard deviation dose intensity was 191.6±65.74 mg/day.
2. Primary endpoint
Of the 19 enrolled patients, 6 (31.6%) patients experienced thrombocytopenia-related events of Grade 3 (n=4, 21.1%) or Grade 4 (n=2, 10.5%) severity within 30 days after initial administration of niraparib. In addition, grade 1 or 2 thrombocytopenia occurred in 7 (36.8%) patients.
3. Secondary endpoints
In terms of overall safety, at least one TEAE occurred in all 19 enrolled patients, including 6 patients who experienced grade 3 events and 3 patients who experienced grade 4 events. The most common TEAEs (≥30% of patients) were nausea (n=13, 68.4%), platelet count decreased (n=12, 63.2%), neutrophil count decreased (n=9, 47.4%), vomiting and decreased appetite (n=7, 36.8% each), and white blood cell count decreased and headache (n=6, 31.6% each) (Table 2). One serious TEAE was recorded in 1 patient who experienced grade 4 thrombocytopenia on day 15 and niraparib was interrupted although the patient resumed treatment following resolution. A total of 9 (47.4%) patients experienced grade ≥3 TEAEs and the most common events (≥10% patients) were decreased platelet count (n=5, 26.3%), decreased neutrophil count (n=4, 21.1%), and decreased white blood cell count (n=2, 10.5%). No deaths or study discontinuations due to TEAEs were recorded by the date of data cutoff.
Table 2. Overview of treatment-emergent adverse events leading to study drug discontinuation, reduction, or interruption.
| Status | No. (%) | |
|---|---|---|
| Leading to study drug discontinuation | 0 (0.0) | |
| Leading to study drug reduction | 15 (78.9) | |
| Leading to study drug interruption | 15 (78.9) | |
| Serious TEAEs | 1 (5.3) | |
| Leading to study discontinuation | 0 (0.0) | |
| Deaths | 0 (0.0) | |
TEAE, treatment-emergent adverse event.
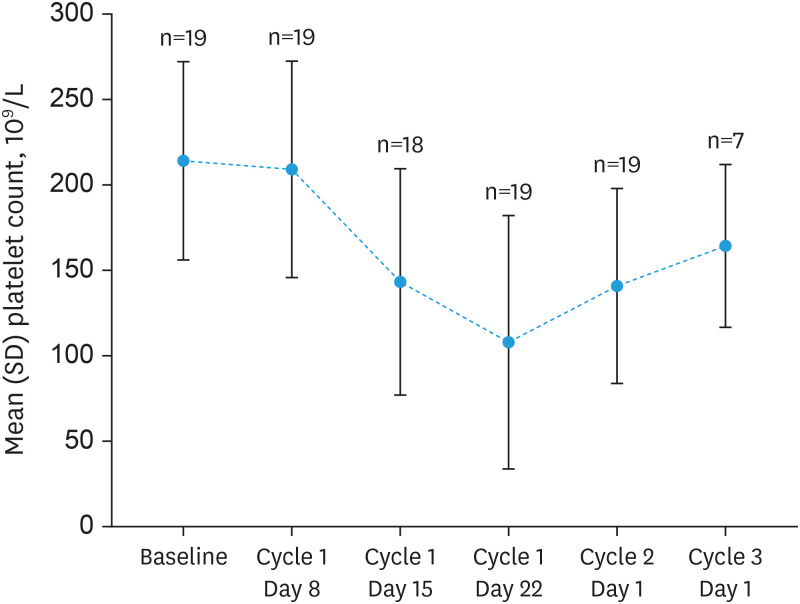
Reductions or interruptions in dosing of niraparib were required in 15 (78.9%) patients (Table 3). The most common TEAEs (≥15% of patients) leading to reduction or interruption of dosing were decreased platelet count (reduction: n=9, 47.4%; interruption: n=10, 52.6%), decreased neutrophil count (reduction or interruption: n=4, 21.1%) and nausea (reduction or interruption: n=3, 15.8%). In terms of laboratory examination and other findings, changes in hematology values were expected over the course of treatment given the known safety profile of niraparib from previous clinical studies and of other PARP inhibitors. In particular, mean platelet counts decreased as shown in Fig. 1, deepened or progressed to grade 3 toxicity in 4 patients and to Grade 4 toxicity in 2 patients. Similar progression to grade ≥3 toxicity was noted in neutrophil and leukocyte count and hemoglobin. No progression to higher grade toxicity was seen in relation to changes in serum chemistry, vital signs, or ECOG performance status, as well as in CA-125 status, which is suggestive of suppression of disease progression.
Table 3. Overall and grade 3 or higher treatment-emergent adverse events.
| Adverse events | TEAEs | Grade 3 or higher TEAEs | |
|---|---|---|---|
| Any TEAEs | 19 (100.0) | 9 (47.4) | |
| Blood and lymphatic system disorders | 5 (26.3) | 1 (5.3) | |
| Anemia | 3 (15.8) | 1 (5.3) | |
| Neutropenia | 3 (15.8) | 1 (5.3) | |
| Leukopenia | 2 (10.5) | - | |
| Thrombocytopenia | 1 (5.3) | 1 (5.3) | |
| Cardiac disorders | 2 (10.5) | - | |
| Palpitations | 2 (10.5) | - | |
| Gastrointestinal disorders | 18 (94.7) | - | |
| Nausea | 13 (68.4) | - | |
| Vomiting | 7 (36.8) | - | |
| Abdominal pain upper | 3 (15.8) | - | |
| Constipation | 2 (10.5) | - | |
| Abdominal distension | 1 (5.3) | - | |
| Abdominal pain | 1 (5.3) | - | |
| Diarrhea | 1 (5.3) | - | |
| Dyspepsia | 1 (5.3) | - | |
| Periodontal disease | 1 (5.3) | - | |
| Stomatitis | 1 (5.3) | - | |
| General disorders and administration site conditions | 5 (26.3) | - | |
| Fatigue | 2 (10.5) | - | |
| Malaise | 2 (10.5) | - | |
| Catheter site thrombosis | 1 (5.3) | - | |
| Infections and infestations | 4 (21.1) | - | |
| Influenza | 1 (5.3) | - | |
| Nasopharyngitis | 1 (5.3) | - | |
| Oral herpes | 1 (5.3) | - | |
| Upper respiratory tract infection | 1 (5.3) | - | |
| Investigations | 15 (78.9) | 8 (42.1) | |
| Platelet count decreased | 12 (63.2) | 5 (26.3) | |
| Neutrophil count decreased | 9 (47.4) | 4 (21.1) | |
| White blood cell count decreased | 6 (31.6) | 2 (10.5) | |
| Blood creatinine increased | 1 (5.3) | - | |
| Weight decreased | 1 (5.3) | - | |
| Metabolism and nutrition disorders | 7 (36.8) | - | |
| Decreased appetite | 7 (36.8) | - | |
| Dehydration | 1 (5.3) | - | |
| Nervous system disorders | 6 (31.6) | - | |
| Headache | 6 (31.6) | - | |
| Dizziness postural | 1 (5.3) | - | |
| Psychiatric disorders | 2 (10.5) | - | |
| Insomnia | 2 (10.5) | - | |
| Renal and urinary disorders | 1 (5.3) | - | |
| Renal impairment | 1 (5.3) | - | |
| Respiratory, thoracic and mediastinal disorders | 3 (15.8) | - | |
| Dyspnea | 1 (5.3) | - | |
| Epistaxis | 1 (5.3) | - | |
| Oropharyngeal discomfort | 1 (5.3) | - | |
| Rhinitis allergic | 1 (5.3) | - | |
| Skin and subcutaneous tissue disorders | 4 (21.1) | - | |
| Dermal cyst | 1 (5.3) | - | |
| Dermatitis exfoliative generalized | 1 (5.3) | - | |
| Hemorrhage, subcutaneous | 1 (5.3) | - | |
| Pruritus | 1 (5.3) | - | |
| Rash | 1 (5.3) | - | |
TEAE, treatment-emergent adverse event.
Fig. 1. Chronological change of platelet count.
SD, standard deviation.
In terms of efficacy, no PFS or OS events occurred during the study period and, consequently, all enrolled patients were alive at the data cutoff time point. The median (95% confidence interval) PFS and OS follow-up were 0 (NA–NA) and 1.7 (1.2–1.9) months, respectively. In the response-evaluable population, only 1 of 4 response-evaluable patients had a post-baseline tumor assessment at the data cutoff and was assessed as having stable disease.
DISCUSSION
This phase 2 study evaluated the safety of niraparib in Japanese patients with platinum-sensitive, relapsed ovarian cancer who had achieved CR or PR in the last chemotherapy containing platinum-based anticancer agents. The primary endpoint, grade 3 or 4 thrombocytopenia-related events within 30 days after the initial administration of niraparib, occurred in 6 of 19 (31.6%) patients overall with Grade 3 and Grade 4 thrombocytopenia-related events reported in 4 patients (21.1%), and 2 patients (10.5%), respectively. Although this incidence is lower than that associated with the subgroup of patients enrolled in the NOVA trial with body weight <58 kg (i.e., 45%) and initially anticipated for this population, it is similar to the incidences noted in patients from the NOVA trial with body weight <77 kg (34.6%) and in the overall population (28.9%).
The mean body weight of Japanese patients in this study (57.5 kg) was lower than the corresponding mean value from NOVA study (69.7 kg) with only one patient in the present study weighing more than 77 kg. The mean baseline platelet count was similar between this study and NOVA study. At the beginning of cycle 2, 14 of the 19 Japanese patients enrolled in the present study received a reduced dose (100 or 200 mg) of niraparib. Considering the result of study drug exposure and compliance, this early dose modification may have contributed to the lower incidence of Grade 3 or 4 thrombocytopenia-related events within 30 days after the initial administration of niraparib in Japanese patients. The mean dose intensity in the present study was approximately 192 mg daily, which is very similar to the dose intensity suggested for patients with baseline body weight <77 kg in a post hoc analysis of the NOVA trial.
One of 2 patients with Grade 4 thrombocytopenia-related events required transfusion although platelet counts in both patients recovered after dose interruption and the patients were able to continue the study with dose reduction at the data cutoff. This finding supports the notion that thrombocytopenia related to niraparib treatment was manageable in Japanese patients with ovarian cancer. The overall safety profile of niraparib in the present study was acceptable and consistent with the known safety profile of niraparib seen in non-Japanese patients enrolled in the NOVA trial and more recent QUADRA trial [6,9]. Although the incidence of TEAEs leading to reduction or interruption of niraparib dosing was higher in this phase 2 study than in the NOVA trial despite the short-term follow up in this study, no TEAEs leading to study drug discontinuation were observed.
The limitations of this study include small sample size and lack of a comparator arm. The sample size of this study was justified by the threshold or estimated incidences of grade 3 or 4 thrombocytopenia set by post-hoc safety analysis of NOVA study. Efficacy results in Japanese ovarian cancer patients in this maintenance setting will be complemented by another Japanese phase 2 study in a late-line treatment setting similar to that of the QUADRA study. In a post-approval setting, post-marketing surveillance will provide real-world safety and efficacy data in a larger population for Japanese patients with ovarian cancer.
In conclusion, the overall safety profile of niraparib in Japanese patients was acceptable and generally consistent with the known safety and previous experience with niraparib in non-Japanese patients. Although this study was not designed to demonstrate a statistically significant difference, the incidence of grade 3 or 4 thrombocytopenia-related events within 30 days (31.6%) was numerically lower than the expected incidence (46%) from the overseas phase 3 NOVA study subpopulation with baseline body weight of <58 kg, and similar to the incidence (34.6%) from NOVA study subpopulation with body weight <77 kg or baseline platelet count <150,000/µL, or the incidence (28.9%) from the overall population of NOVA study. The incidence of TEAEs leading to study drug dose reduction and study drug dose interruption was relatively higher in Japanese patients in this study than patients from the NOVA study in spite of short-term follow-up in this study. However, no TEAEs leading to study drug discontinuation were observed. Further, no new safety signals were identified in this study. Efficacy data in this study were too immature to provide conclusions, although no significant concerns were raised in relation to efficacy. Post-marketing surveillance will ultimately provide real-world safety and efficacy data in Japanese populations in the post-approval setting.
ACKNOWLEDGEMENTS
The authors thank the patients, their families, caregivers and all clinicians for their involvement and contribution to the study. The authors also thank: the Japanese Gynecologic Oncology Group (JGOG) providing valuable advice regarding study design and support for subject enrollment; TESARO, a GSK company, for providing valuable advice during manuscript development; Yuka Yamamoto, medical expert for Takeda Pharmaceutical Company Ltd., for providing valuable advice regarding study design and data interpretation during manuscript development; and Mark Snape, MBBS, CMPP of inScience Communications, Springer Healthcare, for writing the outline and the first draft of the manuscript. This medical writing assistance was funded by Takeda Pharmaceutical Company Ltd.
Footnotes
Presentation: Content included in this manuscript has been previously presented online at the Annual Congress of the Japan Society of Obstetrics and Gynecology, 23-28 April 2020, Abstract P-32-1.
Funding: This study was funded by Takeda Pharmaceutical Company Ltd.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.Y., S.S.
- Data curation: K.Y., S.S.
- Formal analysis: S.S.
- Funding acquisition: K.Y.
- Investigation: T.K., M.T., H.J., H.K., M.M., M.K., N.S., N.H., T.N.1, T.H., U.K., W.H., Y.Y., K.Y., S.S., S.A., I.H., T.N.2
- Methodology: K.Y., S.S., S.A.
- Project administration: K.Y.
- Resources: K.Y.
- Supervision: K.Y.
- Validation: K.Y., S.S.
- Writing - original draft: T.K., K.Y., S.S.
- Writing - review & editing: T.K., M.T., H.J., H.K., M.M., M.K., N.S., N.H., T.N.1, T.H., U.K., W.H., Y.Y., K.Y., S.S., S.A., I.H., T.N.2
1T.N., Naotake Tanaka; 2T.N., Nobuhiro Takeshima
References
- 1.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol. 2017;28:e32. doi: 10.3802/jgo.2017.28.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdev E, Tabatabai R, Roy V, Rimel BJ, Mita MM. PARP inhibition in cancer: an update on clinical development. Target Oncol. 2019;14:657–679. doi: 10.1007/s11523-019-00680-2. [DOI] [PubMed] [Google Scholar]
- 4.Yi M, Dong B, Qin S, Chu Q, Wu K, Luo S. Advances and perspectives of PARP inhibitors. Exp Hematol Oncol. 2019;8:29. doi: 10.1186/s40164-019-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ison G, Howie LJ, Amiri-Kordestani L, Zhang L, Tang S, Sridhara R, et al. FDA approval summary: niraparib for the maintenance treatment of patients with recurrent ovarian cancer in response to platinum-based chemotherapy. Clin Cancer Res. 2018;24:4066–4071. doi: 10.1158/1078-0432.CCR-18-0042. [DOI] [PubMed] [Google Scholar]
- 6.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 7.Oza AM, Matulonis UA, Malander S, Hudgens S, Sehouli J, Del Campo JM, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018;19:1117–1125. doi: 10.1016/S1470-2045(18)30333-4. [DOI] [PubMed] [Google Scholar]
- 8.Berek JS, Matulonis UA, Peen U, Ghatage P, Mahner S, Redondo A, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29:1784–1792. doi: 10.1093/annonc/mdy181. [DOI] [PubMed] [Google Scholar]
- 9.Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–648. doi: 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Zejula (niraparib) approved prescribing information [Internet] Research Triangle Park, NC: GlaxoSmithKline; c2020. [cited 2011 Feb 20]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208447s015s017lbledt.pdf. [Google Scholar]
- 11.Tabata T, Nakamura T, Yanagida S, Hamanishi J, Harano K, Hasegawa K, et al. A Japan study of niraparib monotherapy in patients with advanced, relapsed ovarian cancer who have received multiple prior lines of therapies; 72nd Annual Congress of the Japan Society of Obstetrics and Gynecology; 2020 April 23-28; p. Abstract P-32-2. [Google Scholar]
- 12.González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]