Abstract
Cancer has become the second leading cause of death worldwide; however, its complex pathogenesis remains largely unclear. Previous research has shown that cancer development and progression are closely associated with various non-coding RNAs, including long non-coding RNAs and microRNAs, which regulate gene expression. Target gene abnormalities are regulated and engaged in the complex mechanism underlying tumor formation, thereby controlling apoptosis, invasion, and migration of tumor cells and providing potentially effective targets for the treatment of malignant tumors. Chemotherapy is a commonly used therapeutic strategy for cancer; however, its effectiveness is limited by general toxicity and tumor cell drug resistance. Therefore, increasing attention has been paid to developing new cancer treatment modalities using traditional Chinese medicines, which exert regulatory effects on multiple components, targets, and pathways. Several active ingredients in Chinese medicine, including ginsenoside, baicalin, and matrine have been found to regulate ncRNA expression levels, thus, exerting anti-tumor effects. This review summarizes the scientific progress made regarding the anti-tumor mechanisms elicited by various active ingredients of Chinese medicine in regulating non-coding RNAs, to provide a theoretical foundation for treating tumors using traditional Chinese medicine.
Keywords: microRNA, lncRNA, non-coding RNA, active ingredients of Chinese medicine, anti-cancer
Introduction
The incidence of cancer has tripled over the past three decades and is expected to increase five-fold by 2030 (1). Nevertheless, the precise mechanism associated with cancer pathogenesis remains largely unclear as it is highly complex and diverse. Since the 1970s, gene therapy has become an increasingly attractive strategy for the treatment of various diseases. Accordingly, more recently, the focus has shifted toward identifying specific genetic etiologies of cancer to design effective gene therapies to overcome the challenges associated with traditional cancer treatment modalities. As the human genome is gradually deciphered, RNA has been shown to play an auxiliary role as an intermediate vector of genetic information, and an increasing number of regulatory functions have been ascribed to this class of molecules. In particular, non-coding sequences, which account for 99% of the total human genome, have received greater attention (2). Most of the identified non-coding RNAs (ncRNAs), including long non-coding (lnc)RNAs, micro (mi)RNAs, small interfering (si)RNAs, Piwi-interacting (pi)RNAs, ribosomal (r)RNAs, transfer (t)RNAs, and small nuclear (sn)RNAs, participate in the translation, modification, and other cellular functions. Among these molecules, lncRNAs and miRNAs have been researched more intensively to decipher their fundamental roles in many diseases, including cancer (3). In fact, lncRNAs were initially believed to be the “noise” of genome transcription, a by-product of RNA polymerase II transcription, and were considered biologically non-functional (4). However, recent studies have shown that lncRNAs play an essential role in cancer signal transduction pathways by interacting with proteins and RNA (5) and are reportedly associated with various cancers, including those of the stomach (6–8), lung (9, 10), breast (11, 12), and prostate (13, 14). Specifically, inhibition of lncRNA H19 and lncRNA PVT1 expression can effectively inhibit the cancerization, metastasis (15), and angiogenesis (7) of gastric cancer.
miRNA is a class of small (18–22 nucleotides) ncRNA molecules; it is present in all eukaryotic cells, with over 2,000 of these RNAs being identified in humans (3). However, ncRNAs have also been described in insects, plants, fungi, bacteria, and viruses (16, 17). Compared with lncRNA, the regulatory mechanism of miRNA is relatively simple and clear. It regulates gene expression by either suppressing mRNA translation or degrading mRNA molecules (18). Previous studies have found that the abnormal expression of miRNAs can disrupt many signaling pathways resulting in reduced proliferation, migration, and invasion of cancer cells and the promotion of apoptosis (19–21). Numerous carcinogenic miRNAs, including miR-638, miR-155, miR-31, miR-21, miR-221, miR-222, and miR-294 among others, are overexpressed in various malignant tumors (22–24), whereas others, including miR-429, miR-211, miR-1271, and miR-34a, exert anti-cancer effects and are under-expressed in cancer cells (25, 26).
The emergence of ncRNA provides new alternatives for cancer treatment, but its effective application in clinical cancer treatment remains challenging. At present, chemotherapy is one of the most important means of treating malignant tumors. However, its efficacy is limited by systemic toxicity and tumor cell resistance (27). Traditional Chinese medicine has been practiced for thousands of years because of its safety and minimal side effects. To date, many cancer patients have used traditional Chinese medicine as an alternative therapy for cancer treatment (28). Moreover, some of the active ingredients of Chinese medicine have been shown to exert anti-cancer effects by regulating ncRNAs and acting on various signaling pathways and cancer-related molecular targets, thus, inhibiting tumor proliferation, metastasis, and invasion and inducing cancer cell apoptosis (29). For instance, the isoflavone calycosin inhibits nasopharyngeal carcinoma cell growth by regulating the lncRNA EWSAT1 and its downstream TRAF6 pathways (30). Additionally, curcumin upregulates miR-145 expression to inhibit cell proliferation and invasion in vitro, while inducing cell cycle arrest (31). These reports suggested that ncRNA have anti-tumor abilities that regulate cancer cell apoptosis, proliferation, resistance, metastasis, and invasion.
Active Ingredients of Traditional Chinese Medicine Regulate the Anti-Tumor Mechanism of miRNA
Over the past decades, miRNA has been shown to play a regulatory role in many cancer signaling pathways (32). The active ingredients in traditional Chinese medicine act through different mechanisms to upregulate the expression of tumor-suppressing miRNAs and downregulate the expression of oncogenic miRNAs (33). They also inhibit tumor occurrence and development by inducing cell apoptosis to inhibit tumor metastasis, enhancing cell cycle arrest to reduce drug resistance, and by downregulating other pathways (34) ( Table 1 ).
Table 1.
Detailed information on Chinese medicine active ingredients targeting miRNAs.
| Active Compound | Types of cancer | miRNA | Target Genes | Related Hallmark | Reference |
|---|---|---|---|---|---|
| Ginsenoside Rh2 | Acute myeloid leukemia | miR-21 | Bcl-2 | Induce apoptosis | (35) |
| Ginsenoside Rh2 | Human liver cancer | miR-21 | MCL1/Nrf2/Bcl-2 | Induce apoptosis | (36) |
| Ginsenoside Rh2 | Prostate cancer | miR-4295 | CDKN1A | Anti-proliferation | (37) |
| Ginsenoside Rh2 | Lung adenocarcinoma | miR-491 | MMP-9 | Anti-metastasis | (38) |
| Matrine | Human thyroid cancer | miR-21 | PTEN/p-Akt | Induce apoptosis | (39, 40) |
| Honokiol | Osteosarcoma | miR-21 | PTEN/PI3K/AKT | Induce apoptosis | (41) |
| Berberine | Non-small cell lung cancer | miR-19a | TF/MAPK | Induce apoptosis | (42) |
| Baicalin | Colon cancer | miR-217 | DKK1 | Induce apoptosis | (33) |
| Baicalin | Hepatoma | miR-3127-5p | PI3K/Akt | Cell cycle arrest Anti-proliferation |
(43) |
| Resveratrol | Breast cancer | miR-122-5p/miR-542-3p | XIAP/Bcl-2 | Induce apoptosis | (44) |
| Resveratrol | Acute lymphoblastic leukemia | miR-196b/miR-1290 | Caspase-3 | Induce apoptosis/anti-migration/cycle arrest | (45) |
| Resveratrol | Cancer | miR-326 | PKM2 | Induce apoptosis | (46) |
| Resveratrol | Breast cancer | miR-34a/miR-424/miR-503 | Bcl2/p53 | Anti-proliferation | (47) |
| Triacetyl resveratrol | Pancreatic cancer | miR-200 | Shh | Anti-MET process/Anti-metastasis | (48) |
| Resveratrol | Low invasive breast cancer |
miR-122-5p | Bcl2/CDKs | Enhance chemosensitivity | (49) |
| Resveratrol | Colorectal cancer | miR-200c | Vimentin/ZEB1 | Anti-MET process/anti-metastasis | (50) |
| Ginsenoside Rg3 | Oral squamous cell carcinoma | miR-221 | PI3K/AKT, MAPK/ERK | Anti-EMT process | (51) |
| Ginsenoside Rg3 | Ovarian cancer | miR-145 | DNMT3A/FSCN1 | Anti-EMT process | (52) |
| Camptothecin | Cancer | miR-125b | Bak1/Mcl1/p53 | Induce apoptosis | (53) |
| Camptothecin | Cervical cancer | miR-15a/16 | Rictor | Enhance chemosensitivity | (54) |
| Paeoniflorin | Gastric carcinoma | miR-124 | PI3K/Akt/STAT3 | Anti-proliferation | (55) |
| Paeoniflorin | Human glioma cells | miR-16 | MMP-9 | Anti-proliferation/induce apoptosis | (56) |
| Paeoniflorin | Multiple myeloma | miR-29b | MMP-2 | Anti-proliferation/induce apoptosis | (57) |
| Shikonin | Glioblastoma | miR-143 | BAG3 | Induce apoptosis | (58) |
| Shikonin | Endometrioid endometrial cancer | miR-106b | AKT/mTOR | Anti-proliferation/Induce apoptosis | (59) |
| Shikonin | Gastric carcinoma | miR-195 | PI3K/AKT | Anti-migration/anti-invasion | (60) |
| Shikonin | Cervical Cancer | miR-183-5p | E-cadherin | Anti-migration/anti-invasion | (61) |
| Shikonin | Retinoblastoma | miR-34a/miR-202 | MYCN | Anti-proliferation | (62) |
| Celastrol | Ovarian carcinoma | miR-21 | PI3K/p-Akt/NF-κB | Induce apoptosis | (63) |
| Celastrol | Colon cancer | miR-21 | PI3K/AKT/GSK-3β | Anti-proliferation | (64) |
| Celastrol | Gastric cancer | miR-21 | MMP9/Vimentin Cyclin D1/CDK6 |
Anti-migration/induces cell cycle arrest | (65) |
| Celastrol | Gastric cancer | miR-21 | P27/mTOR | Induces cell cycle arrest | (66) |
| Celastrol | Prostate cancer | miR-101 | NA | Induces autophagy | (67) |
| Celastrol | Prostate cancer | miR-17-92a | NA | Induces autophagy | (68) |
| Celastrol | Lung adenocarcinoma | miR-24/miR-181b | STAT3 | Anti-proliferation | (69) |
| Celastrol | Lung adenocarcinoma | miR-33a-5p | mTOR/p-p70S6K/p-4EBP1 | Enhance chemosensitivity | (70) |
Active Ingredients of Chinese Medicine Targeting miRNA Induce Tumor Apoptosis
Apoptosis is a basic biological phenomenon, that involves the activation, expression, and regulation of a series of genes. The active ingredients of traditional Chinese medicine regulate target genes by influencing an abnormal expression of miRNAs and inducing tumor cell apoptosis ( Figure 1 ). miR-21 inhibits apoptosis in various tumor cells (71–73). Ginsenoside Rh2 is a natural monosome of ginseng total saponin, and its anti-cancer effects have been demonstrated in various tumors. A former report showed that ginsenoside Rh2 inhibited Bcl-2 by increasing miR-21 levels, which induced apoptosis and significantly decreased leukemia cell viability (35). PTEN is a classical anti-oncogene, the inhibition of PTEN is key for cell apoptosis, mainly relying on the phosphorylation and dephosphorylation of Akt. In FTC-133 human follicular thyroid cells, the alkaloid Chinese medicine active ingredient matrine is induced by upregulating the PTEN/Akt signaling pathway via the downregulating miR-21 (39) it induces apoptosis of TPC-1 human thyroid cancer cells (40). Similarly, the reverse quantitative polymerase chain reaction of an extract of Magnolia officinalis revealed that magnolol could induce abnormal expression of miRNA in human osteosarcoma cells, with miR-21 showing a very strong ability to downregulate mRNAs. Recent evidence has suggested that Honokiol is able to suppress the PI3K/AKT signaling pathway; however, it was reactivated by miR-21 overexpression. Honokiol inhibits proliferation and induces apoptosis by regulating the miR-21/PTEN/PI3K/AKT signaling pathway in human osteosarcoma cells (41).
Figure 1.
Active ingredients of Chinese medicine targeting miRNAs induce tumor apoptosis and cell cycle blocking.
A previous report described that Rh2 reduces the expression levels of MCL1 and Nrf2, suppresses colony formation, and induces HepG2 cell apoptosis by inhibiting miR-146a-5p in HepG2 cells (36). Berberine is a quaternary ammonium alkaloid isolated from Coptis chinensis. It inhibits the growth of non-small-cell lung cancer via the miR-19a/TF/MAPK axis and promotes apoptosis. A previous study of the mechanism of anti-tumor baicalin in human colon cancer showed that baicalin induced colon cancer cell apoptosis via the Wnt signaling pathway mediated by miR-217/DDK1, in which DDK1 was identified as a direct downstream target gene of miR-217 (33). A previous study showed that resveratrol regulated the apoptosis and cell cycle of breast cancer cells by regulating miRNAs such as miR-125b-5p, miR-200C-3p, miR-409-3p, miR-122-5p, and miR-542-3p. Resveratrol-mediated miRNA modulation regulates key anti-apoptotic and cell cycle proteins, including Bcl-2, X-linked inhibitor of apoptosis protein, and CDKs, which are critical for its activity. Among these, miR-542-3p and miR-122-5P play key roles in resveratrol-mediated apoptosis of MCF-7 and MDA-MB-231 breast cancer cells, respectively (44). Resveratrol significantly reduced miR-196b/miR-1290 expression in the T-ALL (T-cell acute lymphoblastic leukemia) TTL-104 and B-ALL (B-cell acute lymphoblastic leukemia) SUP-B15 cell lines and upregulated the expression of IGFBP3. As a miR-196b/miR-1290 inhibitor, resveratrol was further demonstrated to exert antitumor effects on ALL cells including antiproliferation, cell cycle arrest, apoptosis and inhibition of migration (45). Pyruvate kinase PKM2 is highly expressed in various tumors. The expression of miR-326 was increased after resveratrol treatment, and miR-326/PKM2-mediated stress and mitochondrial dysfunction were involved in apoptosis induced by resveratrol (46). Camptothecin, a cytotoxic quinoline alkaloid, is an anti-cancer compound found in plants. Deep sequencing analysis of miRNA expression profiles during the camptothecin-induced apoptosis showed that 79 miRNAs were downregulated post-treatment (74). A study of camptothecin verified that miR-125b was down-regulated in camptothecin induced apoptosis in cancer cells. Camptothecin induced apoptosis in cancer cells through miR-125b-mediated mitochondrial pathways by targeting the 3’-untranslated (UTR) regions of Bak1, Mcl1, and p53 (53). In glioblastoma stem cells, miR-143 expression was downregulated after shikonin administration, whereas the regulation factor BAG3 was upregulated. Notably, miR-143 overexpression reversed this phenomenon and enhanced the anti-tumor activity of shikonin in glioblastoma stem cells (58). Celastrol is found in the root of Celastrus orbiculatus, belonging to the family Celastraceae. It can regulate apoptosis-promoting signaling pathways (75). Celastrol suppresses cellular proliferation and induces apoptosis of ovarian cancer OVCAR3 cells by downregulating miR-21 to inhibit the PI3K/P-Akt-NF-κB signaling pathway, thereby revealing a potential therapeutic approach (63).
Active Ingredients of Chinese Medicine Target miRNAs to Block Tumor Cell Cycle
The occurrence of most tumors is related to the disruption of cell cycle regulation, leading to uncontrolled cell growth. Many active ingredients of Chinese medicine play an anti-tumor role by blocking the tumor cell cycle and inhibiting cell proliferation ( Figure 1 ). For instance, in patients with acute lymphoblastic leukemia (ALL), a study demonstrated that the expression of IGFBP3 was decreased in ALL patients. The authors further identified that miR-196b and miR-1290 were overexpressed in T-ALL TALL-104 and B-ALL SUP-B15 cell lines, respectively. As an miR-196b/miR-1290 inhibitor, resveratrol was further demonstrated to exert antitumor effects on ALL cells including cell cycle arrest. Resveratrol blocks T-ALL T-ALL-104 cells during the G1 phase and the B-ALL SUP-B15 cells in the S phase by inhibiting miR-196b/miR-1290 (45). Baicalin upregulates miR-3127-5p, which increases p21/CDKN1A and P27/CDKN1B expression to inhibit cell proliferation arresting the cell cycle in S and G2/M phases in Bel-7402 cells (43). Celastrol caused G2/M cell cycle arrest that was accompanied by the down-regulation of miR-21 expression. Further study showed that celastrol inhibited p27 protein degradation by inhibiting the miR-21 and mTOR signaling pathways in BGC-823 and MGC-803 cells. The effect of celastrol on cell cycle arrest of gastric cancer cells was due to an increase in the p27 protein level via inhibition of the miR-21-mTOR signaling pathway (66).
Active Ingredients of Chinese Medicine Targeting miRNA Inhibit Tumor Cell Proliferation
Many traditional Chinese medicines regulate cell proliferation through miRNAs ( Figure 2 ). Paeoniflorin is a monoterpene glycoside with various anti-cancer activities and is derived from Paeonia lactiflora. Studies have shown that paeoniflorin has broad-spectrum anti-tumor activities against various cancers (76), including those of liver (77), lungs (78), breast (79), and pancreas (80). miR-124 levels are significantly increased in paeoniflorin-treated MGC-803 cells, which inhibits PI3K/Akt and p-STAT3 expression. A PI3K agonist or STAT3 overexpression can reverse effects of paeoniflorin on MGC-803 cell proliferation (55).
Figure 2.
Active ingredients of Chinese medicine targeting miRNAs inhibit tumor cell proliferation.
Shikonin, a natural naphthoquinone isolated from traditional Chinese herbs. miR-106b is one of the most significantly downregulated miRNAs among the several miRNAs dysregulated by shikonin. miR-106b targets the tumor suppressor gene phosphatase and tensin homolog (PTEN), thereby modulating AKT/mTOR signaling pathway and ultimately inhibiting the proliferation of endometrial cancer cells (59). An earlier report investigated changes in the proliferation of the retinoblastoma cell lines Y-79 and Weri-RB-1 after shikonin administration. The results revealed that shikonin upregulated miR-34a and miR-202 expression and directly targeted the oncogene MYCN to degrade its mRNA while inhibiting the proliferation of retinoblastoma cells (62).
Several studies have shown that celastrol can inhibit tumor cell proliferation in several types of cancers. For example, in colon cancer cells, the overexpression of miR-21 enhanced cell viability, inhibited apoptosis, increase Bcl-2 expression, and decreased Bax levels; these effects were reversed by celastrol. As enzymes are involved in cell survival, the PI3K/AKT/GSK-3β pathway provides important signals for tumor cell proliferation Clastrol may inhibit colon cancer cell proliferation by negatively regulating the PI3K/AKT/GSK-3 pathways via miR-21 (64). Similar results were reported in lung adenocarcinoma, in which celastrol inhibited cell proliferation and induced apoptosis by regulating the expression levels of miR-24 and miR-181b (69).
Another study showed that ginsenoside Rh2 inhibited the proliferation of prostate cancer cells in a dose-dependent manner, and no CDKN1A cell cycle inhibitor was observed in the increased protein of ginsenoside Rh2. Screening all candidate miRNAs for binding to the 3′-untranslated region of CDKN1A showed that miR-4295 was dose-dependently inhibited by ginsenoside Rh2. Therefore, comprehensive experimental investigations revealed that ginsenoside Rh2 inhibits prostate cancer cell growth by inhibiting miRNA-4295, which activates CDKN1A (37).
Active Ingredients of Chinese Medicine Targeting miRNA Inhibit Tumor Cell Metastasis and Invasion
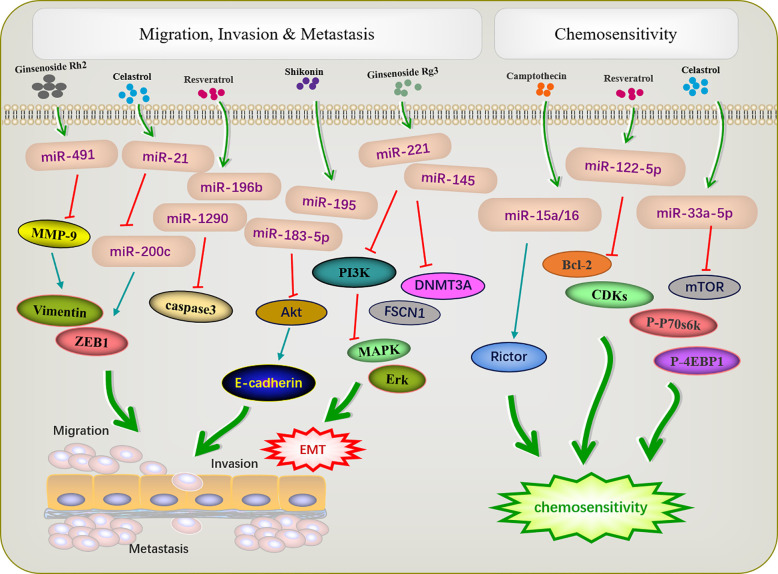
The metastasis and invasion are essential feature characterizing the biological behavior of malignant tumors. The active ingredients of traditional Chinese medicine can inhibit the invasion and metastasis of tumors by regulating miRNA ( Figure 3 ). EMT is an important prerequisite for tumor cell metastasis. The miR-200 family inhibits EMT, thereby inhibiting tumor metastasis. A previous study reported that triacetyl resveratrol, a derivative of resveratrol, inhibited pancreatic cancer growth and EMT by upregulating the expression of the members of the miR-200 family and targeting the Shh pathway (48). Moreover, resveratrol increased the expression of miR-200c to inhibit cell proliferation and invasion in HCT116 cells (50). Ginseng saponin Rg3 suppressed EMT in oral squamous cell carcinoma and ovarian cancer via miR-221 (51) and miR-145 (52). E-cadherin, the most common EMT protein, plays an important role in tumor invasion, and the loss of E-cadherin expression promotes tumor and EMT. In the cervical cancer cell lines HeLa and C33a, Shikonin inhibits EMT by inducing miR-183-5p expression via E-cadherin (61). Similarly, miR-17-5p expression was upregulated in triple-negative breast cancer. The PTEN is a direct target of miR-17-5p. Studies have shown that increased expression of PTEN can inhibit miR-17-5p and reduce the expression of Akt and P-Akt, thereby inhibiting EMT and the migration and invasion of triple-negative breast cancer cells (81).
Figure 3.
Active ingredients of Chinese medicine targeting miRNAs inhibit tumor cell metastasis and invasion, and reverse tumor cell resistance.
Shikonin can inhibit tumor migration and invasion via modulating miRNA-mediated regulation of multiple pathways. miR-195 is an important member of the micro-15/16/195/424/497 family and can be used as a diagnostic biomarker in breast cancer (82). In NCI-N87 cells, shikonin inhibited the proliferation, migration, and invasion by regulating miR-195 to inhibit the PI3K/AKT signaling pathway (60). Matrix metalloproteinases (MMPs) play important roles in mediating angiogenesis, metastasis, and invasion. MMP-2/9 expression is related to the progression of many tumors, such as colon cancer (83), neuroblastoma (84), and bladder cancer (85). Paeoniflorin regulated miR-16/29b, which targeted MMP-9/2 (56) to inhibit the growth and invasion of multiple myeloma cells (57). Celastrol can downregulate miR-21 and MMP9 and regulate the expression of the cell migration protein vimentin; this reduces the migration and invasion ability of MKN45 gastric cancer cells (65). Resveratrol significantly inhibited cell migration in T-cell ALL T-ALL-104 and B-cell ALL SUP-B15 cells by inhibiting miR-196b/miR-1290 (45).
Active Ingredients of Chinese Medicine Targeting miRNA Reverse Tumor Cell Resistance
Increasing evidence has revealed that dysfunctional miRNAs significantly affect chemotherapy resistance. Active ingredients of Chinese medicine play an important role in reducing the toxic and side effects of chemotherapy and improving resistance to chemotherapy ( Figure 3 ). A previous study showed that low-invasive breast cancer cells were resistant to amycin and this resistance was reversed by resveratrol, which also targeted the regulatory inhibitor miR-122-5p to influence the cell cycle and apoptosis (49). Increased autophagy during chemotherapy can promote tumor apoptosis or mediate autophagy-related apoptosis. miR-15a and miR-16 effectively induce autophagy, enhancing the therapeutic effect of camptothecin (54). Celastrol reduced mTOR, P-P70S6K, and p-4EBP1 expression by increased miR33a-5p to inhibited tumor growth (70).
The Active Ingredients of Traditional Chinese Medicine Regulate the Anti-Tumor Mechanism of lncRNAs
Generally, lncRNAs are defined as molecules comprising more than 200 nucleotides lacking protein-coding capacity. However, more recently, lncRNAs have been reported to regulate gene expression (86). Meanwhile, various active ingredients in Chinese medicines, such as curcumin and resveratrol, modulate tumor development via lncRNA expression regulation in vitro and in vivo ( Table 2 ). In gemcitabine-resistant pancreatic ductal adenocarcinoma cell, a phenolic compound extracted from turmeric, curcumin, desensitizes chemotherapy-resistant pancreatic ductal adenocarcinoma via inhibiting the PRC2-PVT1-c-Myc axis. Hence, emerging evidence suggested that curcumin may be an effective sensitizing agent for chemotherapeutic drugs (96). Moreover, curcumin induces the expression of the lncRNA PINT to inhibit acute lymphoblastic leukemia cell growth (88).
Table 2.
Detailed information on Chinese medicine active ingredients targeting lncRNAs.
| Active Compound | LncRNA | Cancer | Related Hallmark | Reference |
|---|---|---|---|---|
| Curcumin | H19 | Gastric cancer | Proliferation | (87) |
| Curcumin | GAS5 | Breast cancer | Apoptosis | (12) |
| Curcumin | PINT | Acute lymphoblastic leukemia | Proliferation | (88) |
| Curcumin | ROR | Prostate cancer | Proliferation | (31) |
| Curcumin | PANDAR | Colorectal cancer | Apoptosis | (89) |
| Curcumin | MEG3 | Ovarian cancer | Drug resistance | (90) |
| Resveratrol | AK001796 | Lung cancer | Proliferation/cycle arrest | (91) |
| Resveratrol | NEAT1 | Multiple myeloma | Proliferation/migration | (92) |
| Matrine | LINC00472 | Bladder carcinoma | Growth/metastasis | (93) |
| Artesunate | UCA1 | Prostate cancer | Apoptosis/migration | (94) |
| Triptolide | THOR | Nasopharyngeal carcinoma | Growth | (95) |
| Calycosin | EWSAT1 | Nasopharyngeal carcinoma | Growth | (30) |
Resveratrol is a non-flavonoid polyphenol compound with a wide pharmacological spectrum of anti-cancer, anti-inflammatory, anti-microbial, and antioxidant activity (97). One study on lung cancer reported the upregulation of 21 lncRNAs and downregulation of 19 lncRNAs resveratrol treated A549 cells. Among these, decreased levels of the lncRNA AK001796 weakened the inhibitory effect of resveratrol on cell proliferation (91). Resveratrol inhibits cell proliferation, migration, and invasion by downregulating AK001796 and NEAT1 in lung cancer and multiple myeloma (91, 92). Similarly, triptolide and isoflavone calycosin inhibit tumor cell growth by inhibiting specific lncRNAs in nasopharyngeal cancer. Triptonide inhibits human nasopharyngeal carcinoma cell growth via disrupting lncRNA THOR-IGF2BP1 signaling. Conversely, ectopic lncRNA THOR overexpression inhibits Triptonide-induced cytotoxicity in NPC cells (95).
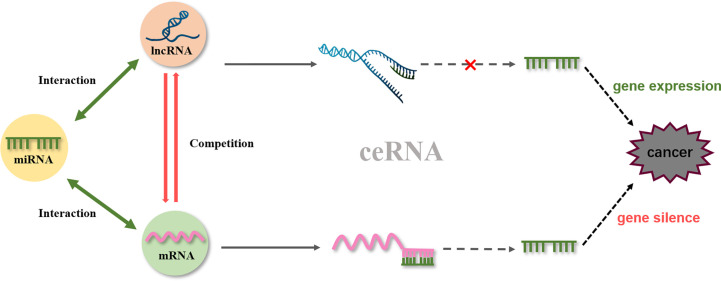
One of the representative lncRNAs, H19, is recognized as a cancer biomarker and is associated with the occurrence of esophageal cancer (98), colorectal cancer (99), liver cancer (100), breast cancer (101), bladder cancer (102), and stomach cancer. Furthermore, reduced expression of H19 can inhibit cancer development (103, 104). Specifically, curcumin inhibited cell proliferation via c-Myc/H19 pathway, which reduced the expression of H19 in stomach cancer cells. This indicated that curcumin is a potential drug for gastric cancer (87). Moreover, microarray data identified H19 as a potential target of Huaier (a fungal parasite on locust trees), the extract from which reduced the expression of H19, while also reducing the viability of breast cancer cells by inducing apoptosis via regulation of the H19-miR-675-5p-CBL axis (105). Besides, certain lncRNAs and miRNAs mutually restrict and regulate target genes to achieve tumor inhibition. For example, in bladder cancer, H19 can directly bind miR-29b-3p to derepress the target DNMT3B. Further, upregulating H19 antagonizes miR-29b-3p-mediated proliferation, migration, and epithelial-mesenchymal transition (EMT) suppression in bladder cells. This evidence demonstrated, for the first time, that H19 may function as a competing endogenous RNA (ceRNA) for miR-29b-3p and relieve the suppression of DNMT3B, leading to EMT and metastasis of bladder cancer (106). In 2011, a new theory was proposed that ceRNAs and miRNA response elements could mediate the interactions between mRNA pseudogenes and some ncRNAs to form a large-scale regulatory network in the transcriptome and serve as a “new language” for “mutual conversation” (107) ( Figure 4 ). These networks are characterized by sponge activity, in which ncRNA interacts with the target gene to competitively bind or inhibit. In addition, ceRNAs have been identified as key regulatory factors in cancer (108, 109). PVT1, located downstream of the proto-oncogene Myc in chromosome 8q24, was used as a ceRNA of miR-216b and miR-152 in non-small-cell lung cancer and osteosarcoma to promote the tumor resistance to anti-cancer drugs (110, 111). In prostate cancer cells, lncRNA-ROR and the stem cell marker Oct4 mRNA contain binding regions for miR-145 and directly compete with this microRNA. Curcumin reduced the expression of endogenous lncRNA-ROR and effectively increased the available concentration of miR-145 in human prostate cancer stem cells, where miR-145 prevented cell proliferation by decreasing Oct4 expression (31). The ceRNA hypothesis has revealed new mechanisms of RNA interactions, which incentivized the analysis of ncRNA role in cancer development.
Figure 4.
ceRNA network.
Regulation of the Anti-Tumor Mechanism by circRNA and Other ncRNAs
As potential targets for the active ingredients of Chinese medicine, circRNA, siRNA, rRNA, and other non-coding RNAs are involved in tumor development. Some circRNAs affect cancer biogenesis in diverse manners, such as by functioning as miRNA sponges, combining with RNA-binding proteins, acting as transcription factors, and affecting protein translation (112). Matrine decreased circRNA-104075 and Bcl-9 expression significantly via inhibition of PI3K/AKT and Wnt-β-catenin pathways,it suppressed cell viability while induceing apoptosis and autophagy in glioma cell line U251 (113). Another study shows that matrine down-regulated the levels of circ_0027345 and HOXD3, and up-regulated miR-345-5p expression. Meanwhile, matrine restrained tumor growth, invasion and promoted autophagy of HCC by regulating the circ_0027345/miR-345-5p/HOXD3 axis in vivo (114). Curcumin has antioxidant and anti-cancer properties, and it has also been used as a radiosensitizer. A study compared the differences in circRNA levels in NPC cell lines after radiotherapy and after treatment with curcumin, using a high-throughput microarray. Finally, it was demonstrated by reverse transcription-quantitative polymerase chain reaction assay and wound healing assay that curcumin could enhance radiosensitization of NPC cell lines via mediating regulation of tumor stem-like cells by the “hsa_circRNA_102115”-“hsa-miR-335-3p”-“MAPK1” interaction network (115). At present, although circRNA has shown significant activity in the treatment of cancer, there are few reports on the regulation of circRNA by Active Ingredients of Chinese Medicine, which is also an important direction for future researchers to concern and research. circRNA has been expected to become a new molecular biomarker for the clinical diagnosis, treatment and prognosis, and the potential target for targeted therapy. siRNAs are double-stranded RNAs of 20-25 nucleotides and are involved in RNA interference; they regulate gene expression in a specific manner. Multiple synthetic siRNAs can achieve long-term silencing of target genes without interfering with endogenous microRNA pathways. Ginsenoside Rh2 downregulated P-STAT3/STAT3 and intracellular oxidative stress by upregulating PPAR. In response to siRNA-mediated knockdown of PPAR, STAT3 and intracellular oxidative stress were increased (116). rRNA is the most abundant type of RNA in cells. In lung cancer cells, triptolide interrupts rRNA synthesis by inhibiting transcriptional activation of RNA Pol I and UBF, thereby activating the apoptosis regulators caspase 9 and caspase 3 to inhibit BCL2 and induce apoptosis and cell cycle arrest (117).
Discussion
During cancer development, abnormal ncRNAs modulate cell proliferation, migration, and invasion by regulating the expression of proto-oncogenes and tumor suppressor genes. Many active ingredients of traditional Chinese medicine, such as resveratrol, matrine, and berberine, have been evaluated in vivo and in vitro to target specific ncRNA and shown to play anti-cancer roles. This review summarized the finding regarding 16 active ingredients of traditional Chinese medicine that can target miRNAs, lncRNAs, and other ncRNAs, thereby playing an effective role in suppressing cancer growth. Among the ncRNAs regulated by Chinese medicine active ingredients, miR-21 is the most reported ncRNA and is extensively studied in various cancers. It is involved in most of the cancer-related processes, such as cell apoptosis, proliferation, migration, and cell cycle. These findings indicate that miR21 is one of the promising ncRNAs to develop targeted therapeutic agents for many types of cancer. Some active ingredients of traditional Chinese medicine, such as Ginsenoside Rh2 and Resveratrol, promote apoptosis by regulating ncRNAs to target common apoptosis-related target genes, such as BCL2 and Caspase3.
In conclusion, traditional Chinese medicine’s active ingredients significantly ameliorate malignant neoplasms via ncRNA regulation, suggesting that active ingredients of traditional Chinese medicine may become alternative therapeutic agents for cancer in the future. At present, most studies have reported that the active ingredients of traditional Chinese medicine mainly target one kind of ncRNA for cancer treatment. However, ceRNA mechanism suggests that several kinds of ncRNA have complex interactions in cancer treatment. Therefore, we need to further explore the detailed anti-cancer mechanism and clinical safety of each of the active ingredients of traditional Chinese medicine. We hope that this review on the regulation of ncRNA by active ingredients of traditional Chinese medicine on tumor will be helpful for future research studies on anti-cancer of traditional Chinese medicine and provide a reference for their clinical application.
Author Contributions
TL and SH participated in writing, editing, and making figures. ZQ and DL read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant 81973712,81803680,82003985), China Postdoctoral Science Foundation (grant 2020M670825, 2020T130568), Jilin Province Science and Technology Development Project in China (grant 20170309005YY, 20200504005YY), Jilin Province TCM science and technology project (grant 2020041).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ncRNA, non-coding RNA; lncRNA, long non-coding RNA; miRNA, microRNA; siRNA, small interfering RNA; piRNA, Piwi-interacting RNA; rRNA, ribosomal RNA; tRNA, transfer RNA; snRNA, small nuclear RNA; EMT, epithelial-mesenchymal transition; ceRNA, competing endogenous RNA; T-ALL, T-cell acute lymphoblastic leukemia; B-ALL, B-cell acute lymphoblastic leukemia; PKM2, pyruvate kinase M2; MMPs, Matrix metalloproteinases; circRNA, circular RNA.
References
- 1. Sohyla R, Shahram S, Alireza Z, Arash Z, Fariba S. Cancer CMJJoG. a comparative study of spatial distribution of gastrointestinal cancers in poverty and affluent strata (kermanshah metropolis, iran). J Gastrointest Cancer (2018) 50(4):838–47. 10.1007/s12029-018-0163-7 [DOI] [PubMed] [Google Scholar]
- 2. Hong M, Wang N, Tan HY, Tsao SW, Feng Y. MicroRNAs and chinese medicinal herbs: new possibilities in cancer therapy. Cancers (2015) 7(3):1643–57. 10.3390/cancers7030855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romano G, Veneziano D, Acunzo M, Croce CM. Small non-coding RNA and cancer. Carcinogenesis (2017) 38(5):485–91. 10.1093/carcin/bgx026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long J, Xiong J, Bai Y, Mao J, Lin J, Xu W, et al. Construction and investigation of a lncrna-associated cerna regulatory network in cholangiocarcinoma. Front Oncol (2019) 9:649. 10.3389/fonc.2019.00649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol (2018) 28(4):287–301. 10.1016/j.tcb.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hao NB, He YF, Li XQ, Wang K, Wang RL. The role of miRNA and lncRNA in gastric cancer. Oncotarget (2017) 8(46):81572–82. 10.18632/oncotarget.19197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao J, Du P, Cui P, Qin Y, Hu C, Wu J, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene (2018) 37(30):4094–109. 10.1038/s41388-018-0250-z [DOI] [PubMed] [Google Scholar]
- 8. Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci (2017) 21(17):3850–6. [PubMed] [Google Scholar]
- 9. Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao X, et al. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett (2016) 371(1):99–106. 10.1016/j.canlet.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 10. Fang Z, Chen W, Yuan Z, Liu X, Jiang H. LncRNA-MALAT1 contributes to the cisplatin-resistance of lung cancer by upregulating MRP1 and MDR1 via STAT3 activation. Biomed Pharmacother (2018) 101:536–42. 10.1016/j.biopha.2018.02.130 [DOI] [PubMed] [Google Scholar]
- 11. Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol (2019) 21(4):498–510. 10.1038/s41556-019-0299-0 [DOI] [PubMed] [Google Scholar]
- 12. Gu J, Wang Y, Wang X, Zhou D, Shao C, Zhou M, et al. Downregulation of lncRNA GAS5 confers tamoxifen resistance by activating miR-222 in breast cancer. Cancer Lett (2018) 434:1–10. 10.1016/j.canlet.2018.06.039 [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Pitchiaya S, Cieślik M, Niknafs YS, Tien JC, Hosono Y, et al. Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat Genet (2018) 50(6):814–24. 10.1038/s41588-018-0120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitobe Y, Takayama KI, Horie-Inoue K, Inoue S. Prostate cancer-associated lncRNAs. Cancer Lett (2018) 418:159–66. 10.1016/j.canlet.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 15. Li H, Yu B, Li J, Su L, Yan M, Zhu Z, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget (2014) 5(8):2318–29. 10.18632/oncotarget.1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammond SM. An overview of microRNAs. Adv Drug Deliver Rev (2015) 87:3–14. 10.1016/j.addr.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashrafizadeh M, Ahmadi Z, Mohammadinejad R, Farkhondeh T, Samarghandian S. MicroRNAs mediate the anti-tumor and protective effects of ginsenosides. Nutr Cancer (2020) 72(8):1264–75. 10.1080/01635581.2019.1675722 [DOI] [PubMed] [Google Scholar]
- 18. Solomon MC, Radhakrishnan RA. MicroRNA’s - The vibrant performers in the oral cancer scenario. Japanese Dental Sci Rev (2020) 56(1):85–9. 10.1016/j.jdsr.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan S, Wang H, Chen X, Liang C, Shang W, Wang L, et al. MiR-182-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-C. Cancer Lett (2020) 488:18–26. 10.1016/j.canlet.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Zhang L, Chen M, Liu D. miR-324-5p inhibits gallbladder carcinoma cell metastatic behaviours by downregulation of transforming growth factor beta 2 expression. Artif cells Nanomed Biotechnol (2020) 48(1):315–24. 10.1080/21691401.2019.1703724 [DOI] [PubMed] [Google Scholar]
- 21. Xia H, Zhao Y. miR-155 is high-expressed in polycystic ovarian syndrome and promotes cell proliferation and migration through targeting PDCD4 in KGN cells. Artif cells Nanomed Biotechnol (2020) 48(1):197–205. 10.1080/21691401.2019.1699826 [DOI] [PubMed] [Google Scholar]
- 22. Mackenzie NC, Staines KA, Zhu D, Genever P, Macrae VE. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem Funct (2014) 32(2):209–16. 10.1002/cbf.3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li M, Zhang D, Cheng J, Liang J, Yu F. Ginsenoside Rh2 inhibits proliferation but promotes apoptosis and autophagy by down-regulating microRNA-638 in human retinoblastoma cells. Exp Mol Pathol (2019) 108:17–23. 10.1016/j.yexmp.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Shang H, Zhang S, Zhang X. Ginsenoside Rh2 inhibits proliferation and migration of medulloblastoma Daoy by down-regulation of microRNA-31. J Cell Biochem (2018) 119(8):6527–34. 10.1002/jcb.26716 [DOI] [PubMed] [Google Scholar]
- 25. Li N, Wang C, Zhang P, You S. Emodin inhibits pancreatic cancer EMT and invasion by up−regulating microRNA−1271. Mol Med Rep (2018) 18(3):3366–74. 10.3892/mmr.2018.9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng F, Li J, Ma C, Tang X, Tang Q, Wu J, et al. Novel regulation of miR-34a-5p and HOTAIR by the combination of berberine and gefitinib leading to inhibition of EMT in human lung cancer. J Cell Mol Med (2020) 24(10):5578–92. 10.1111/jcmm.15214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ripani P, Delp J, Bode K, Delgado ME, Dietrich L, Betzler VM, et al. Thiazolides promote G1 cell cycle arrest in colorectal cancer cells by targeting the mitochondrial respiratory chain. Oncogene (2020) 39(11):2345–57. 10.1038/s41388-019-1142-6 [DOI] [PubMed] [Google Scholar]
- 28. Xiang Y, Guo Z, Zhu P, Chen J, Huang YJCM. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med (2019) 8(5):1958–75. 10.1002/cam4.2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan Z, Lai Z, Lin J. Anticancer properties of traditional chinese medicine. Combinatorial Chem High throughput screening (2017) 20(5):423–9. 10.2174/1386207320666170116141818 [DOI] [PubMed] [Google Scholar]
- 30. Kong L, Li X, Wang H, He G, Tang A. Calycosin inhibits nasopharyngeal carcinoma cells by influencing EWSAT1 expression to regulate the TRAF6-related pathways. Biomed Pharmacother (2018) 106:342–8. 10.1016/j.biopha.2018.06.143 [DOI] [PubMed] [Google Scholar]
- 31. Liu T, Chi H, Chen J, Chen C, Huang Y, Xi H, et al. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene (2017) 631:29–38. 10.1016/j.gene.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 32. Tang J, Ahmad A, Sarkar FH. MicroRNAs in breast cancer therapy. Curr Pharm design (2014) 20(33):5268–74. 10.2174/1381612820666140128205239 [DOI] [PubMed] [Google Scholar]
- 33. Jia Y, Chen L, Guo S, Li Y. Baicalin induced colon cancer cells apoptosis through miR-217/DKK1-mediated inhibition of Wnt signaling pathway. Mol Biol Rep (2019) 46(2):1693–700. 10.1007/s11033-019-04618-9 [DOI] [PubMed] [Google Scholar]
- 34. Farooqi AA, Khalid S, Ahmad A. Regulation of cell signaling pathways and miRNAs by resveratrol in different cancers. Int J Mol Sci (2018) 19(3):652. 10.3390/ijms19030652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Wang Y. Ginsenoside Rh2 mitigates pediatric leukemia through suppression of Bcl-2 in leukemia cells. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol (2015) 37(2):641–50. 10.1159/000430383 [DOI] [PubMed] [Google Scholar]
- 36. Chen W, Chu S, Li H, Qiu Y. MicroRNA-146a-5p enhances ginsenoside Rh2-induced anti-proliferation and the apoptosis of the human liver cancer cell line HepG2. Oncol Lett (2018) 16(4):5367–74. 10.3892/ol.2018.9235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao Q, Zheng J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A. Cell Proliferation (2018) 51(3):e12438. 10.1111/cpr.12438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, Zhang Y, Song W, Zhang Y, Dong X, Tan M. Ginsenoside Rh2 Inhibits migration of lung cancer cells under hypoxia via mir-491. Anti-cancer Agents Medicinal Chem (2019) 19(13):1633–41. 10.2174/1871520619666190704165205 [DOI] [PubMed] [Google Scholar]
- 39. Li Q, Zhang S, Wang M, Dong S, Wang Y, Liu S, et al. Downregulated miR-21 mediates matrine-induced apoptosis via the PTEN/Akt signaling pathway in FTC-133 human follicular thyroid cancer cells. Oncol Lett (2019) 18(4):3553–60. 10.3892/ol.2019.10693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao L, Zhang X, Cui S. Matrine inhibits TPC-1 human thyroid cancer cells via the miR-21/PTEN/Akt pathway. Oncol Lett (2018) 16(3):2965–70. 10.3892/ol.2018.9006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang J, Zou Y, Jiang D. Honokiol suppresses proliferation and induces apoptosis via regulation of the miR−21/PTEN/PI3K/AKT signaling pathway in human osteosarcoma cells. Int J Mol Med (2018) 41(4):1845–54. 10.3892/ijmm.2018.3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen QQ, Shi JM, Ding Z, Xia Q, Zheng TS, Ren YB, et al. Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manage Res (2019) 11:9005–15. 10.2147/cmar.S207677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bie B, Sun J, Li J, Guo Y, Jiang W, Huang C, et al. Baicalein, a Natural Anti-Cancer Compound, Alters MicroRNA Expression Profiles in Bel-7402 Human Hepatocellular Carcinoma Cells. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol (2017) 41(4):1519–31. 10.1159/000470815 [DOI] [PubMed] [Google Scholar]
- 44. Venkatadri R, Muni T, Iyer AK, Yakisich JS, Azad N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis (2016) 7(2):e2104. 10.1038/cddis.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou W, Wang S, Ying Y, Zhou R, Mao P. miR-196b/miR-1290 participate in the antitumor effect of resveratrol via regulation of IGFBP3 expression in acute lymphoblastic leukemia. Oncol Rep (2017) 37(2):1075–83. 10.3892/or.2016.5321 [DOI] [PubMed] [Google Scholar]
- 46. Wu H, Wang Y, Wu C, Yang P, Li H, Li Z. Resveratrol induces cancer cell apoptosis through MiR-326/PKM2-Mediated ER stress and mitochondrial fission. J Agric Food Chem (2016) 64(49):9356–67. 10.1021/acs.jafc.6b04549 [DOI] [PubMed] [Google Scholar]
- 47. Otsuka K, Yamamoto Y, Ochiya T. Regulatory role of resveratrol, a microRNA-controlling compound, in HNRNPA1 expression, which is associated with poor prognosis in breast cancer. Oncotarget (2018) 9(37):24718–30. 10.18632/oncotarget.25339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fu J, Shrivastava A, Shrivastava SK, Srivastava RK, Shankar S. Triacetyl resveratrol upregulates miRNA−200 and suppresses the Shh pathway in pancreatic cancer: A potential therapeutic agent. Int J Oncol (2019) 54(4):1306–16. 10.3892/ijo.2019.4700 [DOI] [PubMed] [Google Scholar]
- 49. Zhang W, Jiang H, Chen Y, Ren F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J Cell Biochem (2019) 120(9):16283–92. 10.1002/jcb.28910 [DOI] [PubMed] [Google Scholar]
- 50. Karimi Dermani F, Saidijam M, Amini R, Mahdavinezhad A, Heydari K, Najafi R. Resveratrol inhibits proliferation, invasion, and epithelial-mesenchymal transition by increasing miR-200c expression in HCT-116 colorectal cancer cells. J Cell Biochem (2017) 118(6):1547–55. 10.1002/jcb.25816 [DOI] [PubMed] [Google Scholar]
- 51. Cheng Z, Xing D. Ginsenoside Rg3 inhibits growth and epithelial-mesenchymal transition of human oral squamous carcinoma cells by down-regulating miR-221. Eur J Pharmacol (2019) 853:353–63. 10.1016/j.ejphar.2019.03.040 [DOI] [PubMed] [Google Scholar]
- 52. Li J, Lu J, Ye Z, Han X, Zheng X, Hou H, et al. 20(S)-Rg3 blocked epithelial-mesenchymal transition through DNMT3A/miR-145/FSCN1 in ovarian cancer. Oncotarget (2017) 8(32):53375–86. 10.18632/oncotarget.18482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeng CW, Zhang XJ, Lin KY, Ye H, Feng SY, Zhang H, et al. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Mol Pharmacol (2012) 81(4):578–86. 10.1124/mol.111.076794 [DOI] [PubMed] [Google Scholar]
- 54. Huang N, Wu J, Qiu W, Lyu Q, He J, Xie W, et al. MiR-15a and miR-16 induce autophagy and enhance chemosensitivity of Camptothecin. Cancer Biol Ther (2015) 16(6):941–8. 10.1080/15384047.2015.1040963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zheng YB, Xiao GC, Tong SL, Ding Y, Wang QS, Li SB, et al. Paeoniflorin inhibits human gastric carcinoma cell proliferation through up-regulation of microRNA-124 and suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol (2015) 21(23):7197–207. 10.3748/wjg.v21.i23.7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li W, Qi Z, Wei Z, Liu S, Wang P, Chen Y, et al. Paeoniflorin inhibits proliferation and induces apoptosis of human glioma cells via microRNA-16 upregulation and matrix metalloproteinase-9 downregulation. Mol Med Rep (2015) 12(2):2735–40. 10.3892/mmr.2015.3718 [DOI] [PubMed] [Google Scholar]
- 57. Wang S, Liu W. Paeoniflorin inhibits proliferation and promotes apoptosis of multiple myeloma cells via its effects on microRNA−29b and matrix metalloproteinase−2. Mol Med Rep (2016) 14(3):2143–9. 10.3892/mmr.2016.5498 [DOI] [PubMed] [Google Scholar]
- 58. Liu J, Qu CB, Xue YX, Li Z, Wang P, Liu YH. MiR-143 enhances the antitumor activity of shikonin by targeting BAG3 expression in human glioblastoma stem cells. Biochem Biophys Res Commun (2015) 468(1-2):105–12. 10.1016/j.bbrc.2015.10.153 [DOI] [PubMed] [Google Scholar]
- 59. Huang C, Hu G. Shikonin suppresses proliferation and induces apoptosis in endometrioid endometrial cancer cells via modulating miR-106b/PTEN/AKT/mTOR signaling pathway. Biosci Rep (2018) 38(2):BSR20171546. 10.1042/bsr20171546 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Jia L, Zhu Z, Li H, Li Y. Shikonin inhibits proliferation, migration, invasion and promotes apoptosis in NCI-N87 cells via inhibition of PI3K/AKT signal pathway. Artif cells Nanomed Biotechnol (2019) 47(1):2662–9. 10.1080/21691401.2019.1632870 [DOI] [PubMed] [Google Scholar]
- 61. Tang Q, Liu L, Zhang H, Xiao J, Hann SS. Regulations of miR-183-5p and snail-mediated shikonin-reduced epithelial-mesenchymal transition in cervical cancer cells. Drug design Dev Ther (2020) 14:577–89. 10.2147/dddt.S236216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Su Y, Lu S, Li J, Deng L. Shikonin-mediated up-regulation of miR-34a and miR-202 inhibits retinoblastoma proliferation. Toxicol Res (2018) 7(5):907–12. 10.1039/c8tx00079d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang H, Li J, Li G, Wang S. Effects of celastrol on enhancing apoptosis of ovarian cancer cells via the downregulation of microRNA−21 and the suppression of the PI3K/Akt−NF−κB signaling pathway in an in vitro model of ovarian carcinoma. Mol Med Rep (2016) 14(6):5363–8. 10.3892/mmr.2016.5894 [DOI] [PubMed] [Google Scholar]
- 64. Ni H, Han Y, Jin X. Celastrol inhibits colon cancer cell proliferation by downregulating miR-21 and PI3K/AKT/GSK-3β pathway. Int J Clin Exp Pathol (2019) 12(3):808–16. [PMC free article] [PubMed] [Google Scholar]
- 65. Yao SS, Han L, Tian ZB, Yu YN, Zhang Q, Li XY, et al. Celastrol inhibits growth and metastasis of human gastric cancer cell MKN45 by down-regulating microRNA-21. Phytother Res PTR (2019) 33(6):1706–16. 10.1002/ptr.6359 [DOI] [PubMed] [Google Scholar]
- 66. Sha M, Ye J, Luan ZY, Guo T, Wang B, Huang JX. Celastrol induces cell cycle arrest by MicroRNA-21-mTOR-mediated inhibition p27 protein degradation in gastric cancer. Cancer Cell Int (2015) 15:101. 10.1186/s12935-015-0256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guo J, Huang X, Wang H, Yang H. Celastrol induces autophagy by targeting AR/miR-101 in prostate cancer cells. PLoS One (2015) 10(10):e0140745. 10.1371/journal.pone.0140745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Guo J, Mei Y, Li K, Huang X, Yang H. Downregulation of miR-17-92a cluster promotes autophagy induction in response to celastrol treatment in prostate cancer cells. Biochem Biophys Res Commun (2016) 478(2):804–10. 10.1016/j.bbrc.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 69. Yan YF, Zhang HH, Lv Q, Liu YM, Li YJ, Li BS, et al. Celastrol suppresses the proliferation of lung adenocarcinoma cells by regulating microRNA-24 and microRNA-181b. Oncol Lett (2018) 15(2):2515–21. 10.3892/ol.2017.7593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li YJ, Sun YX, Hao RM, Wu P, Zhang LJ, Ma X, et al. miR-33a-5p enhances the sensitivity of lung adenocarcinoma cells to celastrol by regulating mTOR signaling. Int J Oncol (2018) 52(4):1328–38. 10.3892/ijo.2018.4276 [DOI] [PubMed] [Google Scholar]
- 71. Canfrán-Duque A, Rotllan N, Zhang X, Fernández-Fuertes M, Ramírez-Hidalgo C, Araldi E, et al. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Mol Med (2017) 9(9):1244–62. 10.15252/emmm.201607492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis (2016) 7(6):e2277. 10.1038/cddis.2016.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med (2018) 22(1):261–76. 10.1111/jcmm.13316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Erdoğan İ, Coşacak M, Nalbant A, Akgül B. Deep sequencing reveals two Jurkat subpopulations with distinct miRNA profiles during camptothecin-induced apoptosis. Turkish J Biol = Turk biyoloji dergisi (2018) 42(2):113–22. 10.3906/biy-1710-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kashyap D, Sharma A, Tuli HS, Sak K, Mukherjee T, Bishayee A. Molecular targets of celastrol in cancer: recent trends and advancements. Crit Rev Oncol/Hematol (2018) 128:70–81. 10.1016/j.critrevonc.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 76. Xiang Y, Zhang Q, Wei S, Huang C, Li Z, Gao Y. Paeoniflorin: a monoterpene glycoside from plants of Paeoniaceae family with diverse anticancer activities. J Pharm Pharmacol (2020) 72(4):483–95. 10.1111/jphp.13204 [DOI] [PubMed] [Google Scholar]
- 77. Liu H, Zang L, Zhao J, Wang Z, Li L. Paeoniflorin inhibits cell viability and invasion of liver cancer cells via inhibition of Skp2. Oncol Lett (2020) 19(4):3165–72. 10.3892/ol.2020.11424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu Q, Chen GL, Li YJ, Chen Y, Lin FZ. Paeoniflorin inhibits macrophage-mediated lung cancer metastasis. Chin J Natural Medicines (2015) 13(12):925–32. 10.1016/s1875-5364(15)30098-4 [DOI] [PubMed] [Google Scholar]
- 79. Zhang J, Yu K, Han X, Zhen L, Liu M, Zhang X, et al. Paeoniflorin influences breast cancer cell proliferation and invasion via inhibition of the Notch−1 signaling pathway. Mol Med Rep (2018) 17(1):1321–5. 10.3892/mmr.2017.8002 [DOI] [PubMed] [Google Scholar]
- 80. Li Y, Gong L, Qi R, Sun Q, Xia X, He H, et al. Paeoniflorin suppresses pancreatic cancer cell growth by upregulating HTRA3 expression. Drug Design Dev Ther (2017) 11:2481–91. 10.2147/dddt.S134518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li J, Lai Y, Ma J, Liu Y, Bi J, Zhang L, et al. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer (2017) 17(1):745. 10.1186/s12885-017-3674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu Y, Tang D, Zheng S, Su R, Tang Y. Serum microRNA-195 as a potential diagnostic biomarker for breast cancer: a systematic review and meta-analysis. Int J Clin Exp Pathol (2019) 12(11):3982–91. [PMC free article] [PubMed] [Google Scholar]
- 83. Hsu HH, Kuo WW, Day CH, Shibu MA, Li SY, Chang SH, et al. Taiwanin E inhibits cell migration in human LoVo colon cancer cells by suppressing MMP-2/9 expression via p38 MAPK pathway. Environ Toxicol (2017) 32(8):2021–31. 10.1002/tox.22379 [DOI] [PubMed] [Google Scholar]
- 84. Li XY, Huang GH, Liu QK, Yang XT, Wang K, Luo WZ, et al. Porf-2 Inhibits Tumor Cell Migration Through the MMP-2/9 Signaling Pathway in Neuroblastoma and Glioma. Front Oncol (2020) 10:975. 10.3389/fonc.2020.00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen Z, Zhang F, Zhang S, Ma L. The down-regulation of SNCG inhibits the proliferation and invasiveness of human bladder cancer cell line 5637 and suppresses the expression of MMP-2/9. Int J Clin Exp Pathol (2020) 13(7):1873–9. [PMC free article] [PubMed] [Google Scholar]
- 86. Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene (2017) 36(41):5661–7. 10.1038/onc.2017.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu G, Xiang T, Wu QF, Wang WX. Curcumin suppresses the proliferation of gastric cancer cells by downregulating H19. Oncol Lett (2016) 12(6):5156–62. 10.3892/ol.2016.5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Garitano-Trojaola A, José-Enériz ES, Ezponda T, Unfried JP, Carrasco-León A, Razquin N, et al. Deregulation of linc-PINT in acute lymphoblastic leukemia is implicated in abnormal proliferation of leukemic cells. Oncotarget (2018) 9(16):12842–52. 10.18632/oncotarget.24401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen T, Yang P, Wang H, He ZY. Silence of long noncoding RNA PANDAR switches low-dose curcumin-induced senescence to apoptosis in colorectal cancer cells. OncoTargets Ther (2017) 10:483–91. 10.2147/ott.S127547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang J, Liu J, Xu X, Li L. Curcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer. Cancer Chemother Pharmacol (2017) 79(3):479–87. 10.1007/s00280-017-3238-4 [DOI] [PubMed] [Google Scholar]
- 91. Yang Q, Xu E, Dai J, Liu B, Han Z, Wu J, et al. A novel long noncoding RNA AK001796 acts as an oncogene and is involved in cell growth inhibition by resveratrol in lung cancer. Toxicol Appl Pharmacol (2015) 285(2):79–88. 10.1016/j.taap.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 92. Geng W, Guo X, Zhang L, Ma Y, Wang L, Liu Z, et al. Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/β-catenin signaling pathway. Biomed Pharmacother = Biomedecine Pharmacotherapie (2018) 107:484–94. 10.1016/j.biopha.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 93. Li L, Qi F, Wang K. Matrine restrains cell growth and metastasis by up-regulating LINC00472 in bladder carcinoma. Cancer Manage Res (2020) 12:1241–51. 10.2147/cmar.S224701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhou Y, Wang X, Zhang J, He A, Wang YL, Han K, et al. Artesunate suppresses the viability and mobility of prostate cancer cells through UCA1, the sponge of miR-184. Oncotarget (2017) 8(11):18260–70. 10.18632/oncotarget.15353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang SS, Lv Y, Xu XC, Zuo Y, Song Y, Wu GP, et al. Triptonide inhibits human nasopharyngeal carcinoma cell growth via disrupting Lnc-RNA THOR-IGF2BP1 signaling. Cancer Lett (2019) 443:13–24. 10.1016/j.canlet.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 96. Yoshida K, Toden S, Ravindranathan P, Han H, Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis (2017) 38(10):1036–46. 10.1093/carcin/bgx065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Amiri A, Tehran MM, Asemi Z, Shafiee A, Hajighadimi S, Moradizarmehri S, et al. Role of resveratrol in modulating microRNAs in human diseases: from cancer to inflammatory disorder. Curr Medicinal Chem (2019) 2(28):360–76. 10.2174/0929867326666191212102407 [DOI] [PubMed] [Google Scholar]
- 98. Zhou YW, Zhang H, Duan CJ, Gao Y, Cheng YD, He D, et al. miR-675-5p enhances tumorigenesis and metastasis of esophageal squamous cell carcinoma by targeting REPS2. Oncotarget (2016) 7(21):30730–47. 10.18632/oncotarget.8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li S, Hua Y, Jin J, Wang H, Du M, Zhu L, et al. Association of genetic variants in lncRNA H19 with risk of colorectal cancer in a Chinese population. Oncotarget (2016) 7(18):25470–7. 10.18632/oncotarget.8330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li X, Liu R. Long non-coding RNA H19 in the liver-gut axis: a diagnostic marker and therapeutic target for liver diseases. Exp Mol Pathol (2020) 115:104472. 10.1016/j.yexmp.2020.104472 [DOI] [PubMed] [Google Scholar]
- 101. Collette J, Le Bourhis X, Adriaenssens E. Regulation of human breast cancer by the long non-coding RNA H19. Int J Mol Sci (2017) 18(11):2319. 10.3390/ijms18112319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Taheri M, Omrani MD, Ghafouri-Fard S. Long non-coding RNA expression in bladder cancer. Biophys Rev (2018) 10(4):1205–13. 10.1007/s12551-017-0379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang L, Zhou Y, Huang T, Cheng AS, Yu J, Kang W, et al. The interplay of LncRNA-H19 and its binding partners in physiological process and gastric carcinogenesis. Int J Mol Sci (2017) 18(2):450. 10.3390/ijms18020450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: Roles in tumorigenesis. Biomed Pharmacother (2020) 123:109774. 10.1016/j.biopha.2019.109774 [DOI] [PubMed] [Google Scholar]
- 105. Wang J, Wang X, Chen T, Jiang L, Yang Q. Huaier extract inhibits breast cancer progression through a LncRNA-H19/MiR-675-5p pathway. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol (2017) 44(2):581–93. 10.1159/000485093 [DOI] [PubMed] [Google Scholar]
- 106. Lv M, Zhong Z, Huang M, Tian Q, Jiang R, Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res (2017) 1864(10):1887–99. 10.1016/j.bbamcr.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 107. Salmena L, Poliseno L, Tay Y, Kats L. Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell (2011) 146(3):353–8. 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ogunwobi OO, Kumar A. Chemoresistance mediated by ceRNA networks associated with the PVT1 locus. Front Oncol (2019) 9:834. 10.3389/fonc.2019.00834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer (2018) 18(1):5–18. 10.1038/nrc.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chen L, Han X, Hu Z, Chen L. The PVT1/miR-216b/Beclin-1 regulates cisplatin sensitivity of NSCLC cells via modulating autophagy and apoptosis. Cancer Chemother Pharmacol (2019) 83(5):921–31. 10.1007/s00280-019-03808-3 [DOI] [PubMed] [Google Scholar]
- 111. Sun ZY, Jian YK, Zhu HY, Li B. lncRNAPVT1 targets miR-152 to enhance chemoresistance of osteosarcoma to gemcitabine through activating c-MET/PI3K/AKT pathway. Pathology Res Pract (2019) 215(3):555–63. 10.1016/j.prp.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 112. Shang Q, Yang Z, Jia R, Ge S. The novel roles of circRNAs in human cancer. Mol Cancer (2019) 18(1):6. 10.1186/s12943-018-0934-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chi G, Xu D, Zhang B, Yang F. Matrine induces apoptosis and autophagy of glioma cell line U251 by regulation of circRNA-104075/BCL-9. Chemico Biological Interact (2019) 308:198–205. 10.1016/j.cbi.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 114. Lin S, Zhuang J, Zhu L, Jiang Z. Matrine inhibits cell growth, migration, invasion and promotes autophagy in hepatocellular carcinoma by regulation of circ_0027345/miR-345-5p/HOXD3 axis. Cancer Cell Int (2020) 20:246. 10.1186/s12935-020-01293-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhu D, Shao M, Yang J, Fang M, Liu S, Lou D, et al. Curcumin enhances radiosensitization of nasopharyngeal carcinoma via mediating regulation of tumor stem-like cells by a CircRNA network. J Cancer (2020) 11(8):2360–70. 10.7150/jca.39511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tong-Lin Wu T, Tong YC, Chen IH, Niu HS, Li Y, Cheng JT. Induction of apoptosis in prostate cancer by ginsenoside Rh2. Oncotarget (2018) 9(13):11109–18. 10.18632/oncotarget.24326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang J, Zhang ZQ, Li FQ, Chen JN, Gong X, Cao BB, et al. Triptolide interrupts rRNA synthesis and induces the RPL23−MDM2−p53 pathway to repress lung cancer cells. Oncol Rep (2020) 43(6):1863–74. 10.3892/or.2020.7569 [DOI] [PMC free article] [PubMed] [Google Scholar]