Abstract
Medulloblastoma (MB) is the most common malignant pediatric brain tumor, however, the mechanisms underlying tumorigenesis in different MB subgroups remain incompletely understood. Although previous studies of MB predisposition have been conducted in tertiary referral centers primarily in Caucasian cohorts, it is not unclear clear whether there exist population-specific genetic alterations in MBs. In this study, we investigated the contribution of genomic and transcriptomic alterations to the risk of malignant MB in the Chinese population (designated as the Asian cohort). We analyze the genomic and transcriptomic alterations of the Asian MB cohort by using a combination of whole-exome sequencing (WES) and RNA-deep-sequencing. In addition, we integrate publicly available data with the Asian MB cohort and identify a subset of potential MB-driving genes specifically enriched in each of the MB subgroups. We further characterize a newly identified group-3-enriched transcriptional regulator, ZNF124, and demonstrate that ZNF124 is critical for the growth of the most aggressive group-3 MB cells. Together, our analyses indicate conserved yet distinct genetic alterations and gene expression patterns of MBs between different ethnic groups. Our studies further provide an important resource for identifying potential tumor-driving factors in MBs, enhancing our understanding of the disease process for developing ethnically targeted therapies in patients with MB.
Keywords: medulloblastoma, Asian cohort, whole exome sequencing, RNA-seq, subgroup-specific regulator, ZNF124, racial and ethnic disparities
Introduction
Medulloblastoma (MB) is the most common pediatric malignant brain tumor, accounting for approximately 63% of pediatric intracranial embryonal tumors and is associated with a high mortality and morbidity rates (Northcott et al., 2019). Patients receiving conventional multimodal therapy suffer from chronic sequelae such as physical disability and neurodevelopmental disorders for years after treatment completion, indicating a need for identification of effective targets for therapy (Narayan et al., 2019; De Medeiros et al., 2020). MBs originate within the developing cerebellum or brainstem (Waszak et al., 2018) though the etiology of MB tumorigenesis is not fully understood.
Based on distinct transcriptomic and epigenomic profiling, MBs can be classified into four broad subgroups: WNT, sonic-hedgehog (SHH), group 3 (G3) and group 4 (G4). Although a cohort of MBs arises from patients with risk-enhancing familial cancer syndromes such as Gorlin syndrome, Li-Fraumeni syndrome, and Turcot syndrome (Northcott et al., 2011), the majority of MBs arise due to somatic alterations in the genome. The WNT and SHH subgroups are usually associated with genetic alterations in WNT and SHH signaling, respectively. The WNT subtype comprises 10% of all MB patients and tends to have a favorable prognosis. The majority (97%) of WNT MBs are characterized by somatic mutations in CTNNB1 or APC (Waszak et al., 2018), though somatic mutations in DDX3X, SMARCA4, and monosomy 6 are also detected in WNT MB (Northcott et al., 2012a). The SHH subgroup is prevalent in infants and adult patients, accounting for ∼25% of MBs (Srinivasan et al., 2016) and shows high heterogeneity in genetic changes and clinical presentation (Kumar et al., 2019). The typical genetic events that occur in the SHH signaling pathway are loss-of-function mutations or deletions including PTCH1 (Smith et al., 2014), GNAS (He et al., 2014), and GPR161 (Begemann et al., 2020), activating mutations in SMO (Twigg et al., 2016) and amplifications of GLI1, GLI2, and MYCN (Cavalli et al., 2017). Mutant TP53 is another key hallmark in SHH MB, especially in the SHHa-MB subgroup (Louis et al., 2016).
The criteria for G3 and G4 subgroup classification are still controversial due to the lack of validated molecular driver events (less than 10% have a clear driver mutation) (Hovestadt et al., 2020). A recent reclassification of MBs has proposed that G3 and 4 tumors exist on a continuum between the previously described G3 and G4 molecular states (Northcott et al., 2017; Sharma et al., 2019). G3 MB is mainly characterized by MYC and OTX2 amplifications or activation of GFI1 and GFI1B in some cases (Northcott et al., 2017). A set of G4 MB is associated with enhancer-hijacking-mediated PRDM6 overexpression (Northcott et al., 2014, 2017). Loss of 17p and gain of 17q (isochromosome 17q) is also prevalent in G3 and G4 MB (Northcott et al., 2017).
Most omics studies of MBs to date are from predominantly Caucasian cohorts with the genetic alterations and genomic features from Asian cohorts remaining poorly defined. It is not completely understood whether there are differences in genetic drivers of tumors between different racial ethnicities. Only two studies of MBs have been reported from Asian patients including a whole exome study of recurrent MB from only 17 pediatric patients in South Korea (Phi et al., 2018) and a small SHH-subtype MB cohort from Taiwan (Wu et al., 2020), however, a comprehensive analysis of Asian MB subgroups has not been conducted. In this study, we report the genomic landscape across a cohort of 89 MBs in the Han population from China. We further integrate molecular profiling of the new cohort of pediatric brain tumors with publicly available MB cohorts and found a set of novel potential driver genes in the individual MB subgroups, pointing to potential therapeutic targets. This dataset will serve as a foundation to gain greater fundamental insight into MB etiology and tumorigenic mechanisms for devising potential targeted therapies.
Materials and Methods
Study Subjects and Ethics Statement for Medulloblastoma Cohort
Human tumor and matched blood samples for WES or RNA-seq analysis were obtained with informed consent. Pediatric medulloblastoma tissues (89) and matched blood samples (57 used as controls) were collected from patients from the Children’s Hospital of Fudan University and the West China Hospital. All tumor tissues and blood were examined by whole exome sequencing (WES). Fifty-nine MB tissues were subject to RNA-sequencing (RNA-seq). Four normal apparent brain tissue samples from patients diagnosed with brain diseases other than brain tumor were used as a control for RNA-seq or WES. The study was approved by the Institutional Review Boards at the Children’s Hospital of Fudan University and West China Hospitals. The sequencing platform for WES and RNA-seq was an Illumina HiSeq X10, using pair end with 150bp for RNA-seq. The subtype for MB samples is identified by a set of subtype-specific marker gene expression: WIF1, TNC, GAD1, DKK2, and EMX2 for WNT; PDLIM3, EYA1, HHIP, ATOH1, and SFRP1 for SHH; IMPG2, GABRA5, EGFL11, NRL, MAB21L2, NPR3, and MYC for G3 and KCNA1, EOMES, KHDRBS2, RBM24, and UNC5D for G4.
Whole Exome Sequencing and Somatic Variant Calling
Somatic variant analyses was conducted using the Genome Analysis Tool Kit (GATK) with best practices guidelines for genetic data preprocessing and variant calling (Mckenna et al., 2010; Mclean et al., 2019). Briefly, the fastq data files from WES were mapped to human genome (hg38) by the Burrows-Wheeler Aligner (BWA) (Li and Durbin, 2009) in the GATK4 module (Mckenna et al., 2010). We used HaplotypeCaller to call the germline mutations of single nucleotide variants (SNV) and insertion deletions (Indel) and followed the suggested pipeline of Mutect2 (Benjamin et al., 2019) to call the somatic SNVs and Indels. All variants were then annotated by ANNOVAR (Wang et al., 2010). Somatic mutations were filtered by the following criteria: (1) remove variants with alternative count reads smaller than 3; (2) remove allele frequency smaller than 5%; (3) remove variants with minor allele frequency larger than 0.01% in any of the public databases (ExAC, 1000 genome, ESP, Kaviar, HRC); (4) only include variants with ExonicFunc type in “frameshift deletion,” “frameshift insertion,” “non-synonymous SNV,” “stopgain,” and “stoploss.”
Copy Number Variation (CNV) Analysis
The mapped bam files from WES were used for CNV analysis. We followed the somatic copy number variation pipeline from GATK4 CNV1. The final segment ratio files with CNV type annotation for all tumor samples were further annotated by AnnotSV (Geoffroy et al., 2018). Considering the limitation of WES in calling CNV, we only reported large CNVs. Somatic CNVs were filtered by the following criteria: (1) AnnotSV ranking smaller than 4; (2) segment log ratio smaller than 0.5; (3) CNV length smaller than 75% of its located chromosome p/q length.
RNA-Seq Expression and Gene Fusion Analysis
The fastq data files from RNA-seq were processed by eXpress, a software package for efficient probabilistic assignment of ambiguously mapping sequenced fragments (Roberts and Pachter, 2013) with reference gene annotation from Gencode v29 (Harrow et al., 2012). The datasets were imported into DESeq2 (Anders and Huber, 2010) to get normalized expression matrix and differentiated expression statistics. We followed the pipeline from Arriba2 for the detection of gene fusions from RNA-seq data. Fusion events were filtered as follows: (1) remove events with confidence level at “low”; (2) remove events with site at intergenic regions; (3) remove events of the fused genes that only exist once in all samples; (4) remove events with both side gene type in “protein_coding,” “antisense,” “lincRNA,” “transcribed_unprocessed_pseudogene,” “transcribed_processed_pseudogene,” and at least one side with gene type in “protein_coding” and “lincRNA”; (5) remove events with only one read at the split point of the fusion gene; (6) remove events with the breakpoint in the 5′UTR or 3′UTR.
Gene Annotation
The functional annotation of genes was collected from MSigDB3. The MB-related gene lists were collected from (1) marker genes; (2) DisGeNEt (Pinero et al., 2017); (3) 51 driver gene list from published datasets (Northcott et al., 2017); (4) literature search by easyPubMed (R package).
Lentivirus shRNA Production
shRNAs against ZNF124 were designed based on the algorithm4 and the shRNA target sequences (shZNF124-1: GCAAGGACATATAAAGGCTCA and shZNF124-2: GCCAGTTCCCTTCAGAAACAC) were inserted to clone into pGreen-puro vector (SBI System Biosciences, Inc.). To produce lentiviruses, HEK293T cells were co-transfected with shRNA or GFP vector packaging using Lipofectamine 3000 reagent (Life Sciences). Supernatants were collected and filtered at 48 and 72 h following transfection. Viral supernatant was concentrated by centrifugation at 25,000 × rpm for 2 h at 4°C and used to infect cells (MOI = 5–10) overnight in the presence of 10 μg/mL polybrene. Cells were selected and maintained with puromycin (2 μg/ml). Gene expression was verified by real time PCR.
Medulloblastoma Cell Culture and Proliferation Assays
Medulloblastoma cell line D458 was cultured in DMEM/F12 media with 10% FBS, 2 mM L-glutamine, and 1% Penicillin/Streptomycin at 37°C in an atmosphere of 5% CO2.
Cell proliferation was measured by WST-1 or BrdU assays. For WST-1 assay, we used the cell proliferation reagent WST-1 (Millipore Sigma) according to its protocol. For BrdU assay, procedures were performed according to the protocols as described5. Briefly, we added the BrdU (10 μM) into medium, cultured the cells for 3 h at 37°C in an atmosphere of 5% CO2, fixed cells with 4% paraformaldehyde and permeabilized with 0.3% Triton X-100. For BrdU staining, cells were treated with 1N HCl incubating 10 min on ice and neutralized by phosphate/citric acid buffer. BrdU antibody (Thermo Fisher Scientific, # MA3-071) was added into the cells and incubated overnight at 4°C. Mouse-anti BrdU (BD Bioscience, 1:500) antibody was used to label BrdU overnight at 4°C. DAPI counterstain was included in the final washes before the samples were mounted in Fluoromount G (SouthernBiotech) for microscopy. Cell images were quantified in a blinded manner. All immunofluorescence-labeled images were captured using a Nikon C2+ confocal microscope.
Cell Apoptosis Analysis by Flow Cytometry
Medulloblastoma cells were washed with PBS and stained by Ghost DyeTM UV450 (Tonbo Biosciences) for 30 min on ice to detect the viable and dying cells according to the protocol6. After staining, cells were washed with FACS buffer (ice-cold HBSS supplemented with 2% bovine serum) and washed twice with PBS before multiparameter flow cytometric detection on a BD LSRFortessa (Becton Dickinson, San Jose, CA, United States).
Western Blotting and Quantitative PCR
Medulloblastoma cells were lysed in RIPA buffer, the protein concentration quantified (Bio-Rad), and the lysates were separated by 4–12% SDS-PAGE. Antibodies to ZNF124 (Abcam, ab168627) and GAPDH (Thermo Fisher Scientific, Catalog # 39-8600) were used in an incubation overnight. Bands were visualized with secondary antibodies conjugated to horseradish peroxidase (Bio-Rad) and ECL western blotting detection reagents (Pierce) per the manufacturer’s instructions.
Total RNA was extracted using the TRIzol Reagent (Invitrogen) and subsequently, 1 μg RNA was reverse-transcribed using the Advantages of a High-Capacity cDNA Reverse Transcription Kit (Bio-Rad). The cDNAs were then subject to real-time PCR analysis using Fast SYBR Green Master Mix in StepOnePlus Real-Time PCR system (Thermo Fisher Scientific). Primer sequences used were as follows:
ZNF124 forward 5′- AGCCTTCCGTTACTCCAA; ZNF124 reverse 5′- AGGGTTCTTCACCAGCAT; SOX2 forward 5′- TGGGTTCGGTGGTCAAGT; SOX2 reverse 5′- TCTGGTAGTGCTGGGACA; P21 forward 5′- TCACTGTCT TGTACCCTTGTGC; P21 reverse 5′- CTTCCTGTGGGC GGATTAG; BAX forward 5′- TTGCTTCAGGGTTTCATCCA; BAX reverse 5′- AGACACTCGCTCAGCTTCTTG; PUMA forward 5′- GTCCCCTGCCAGATTTGTGGC; PUMA reverse 5′- GACACTGCCGAGGGCACCAGG.
Statistical Analysis
All analyses in this research were performed using Microsoft Excel or GraphPad Prism 6.00 (San Diego, CA, United States)7 or RStudio (R v.3.3.0, https://www.R-project.org/)8. Data are presented as means ± standard deviation (SD). Comparisons in gene expression among patients were evaluated by the Friedman non-parametric analysis of variance (GraphPad PRISM 8, GraphPad Software, United States). Differences were considered significant at p < 0.05. We used a Fisher’s exact test to assess the differences in mutation rates in each subgroup MB between tumor cohorts.
Results
Pediatric Brain Tumors in Asian Patients Have Similar Genetic Alterations to Their Caucasian Counterparts
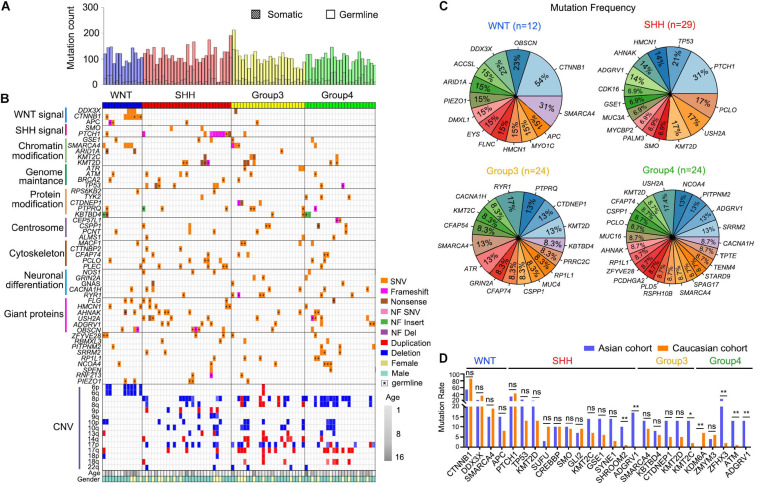
We have analyzed a total of 89 MB patient tumor samples and 59 matched blood samples through whole exome sequencing (WES) and performed an analysis to determine the somatic and germline mutations via the Genome Analysis Tool Kit (GATK; Supplementary Table 1). We identified an average of 23.8 somatic mutations and 90.9 germline mutated genes on average for each Chinese pediatric brain tumor (Figure 1A and Supplementary Table 1).
FIGURE 1.
Genetic alteration landscape of Asian pediatric Medulloblastoma cohort. (A) Number of germline and somatic mutations in 89 pediatric medulloblastomas tested by WES. (B) OncoPrint summarizing recurrently altered genes and copy number variations of the 89 pediatric Medulloblastomas in the Asian cohort. (C) Recurrently mutated genes within the four subgroups of MBs. (D) Comparison between recurrently mutated genes in the Caucasian cohort (Northcott et al., 2017) based on WGS or WES data and the Asian cohort detected using WES data (this work). *P < 0.05, **P < 0.01, Fisher’s Exact test was used to assess the significance of the mutation rate.
Similar to the previously reported studies (Brennan et al., 2013; Hara et al., 2018), in WNT MBs we found frequent mutations in CTNNB1 (54%, 7/13), DDX3X (23%, 3/13), SMARCA4 (31%, 4/13), ARID1A (15%, 2/13), and chromosome 6p deletion (54%, 7/13) (Northcott et al., 2017). In SHH MBs, we found common mutations in PTCH1 (31%, 9/29) and TP53 (21%, 6/29). G3 MBs had recurrent mutations such as KMT2D (13%, 3/24), PTPRQ (13%, 3/24), and CTDNEP1 (13%, 3/24) (Northcott et al., 2012b, 2017). G4 MBs exhibited recurrent mutations in USH2A (17.4%, 4/23), ADGRV1 (13%, 3/23), NCOA4 (13%, 3/23), and PITPNM2 (13%, 3/23) (Figures 1B,C). We have further identified new sets of mutated genes associated with the centrosome (16%) and neural differentiation pathways (25%), especially in G3 (42%) and G4 (61%) MBs (Figure 1B). Interestingly, the genetic mutations in MBs did not exhibit apparent differences between genders except TP53, for which seven out of eight affected patients were female (Figure 1B and Supplementary Table 1).
When comparing the recurrent somatic mutations in the Asian cohort with the publicly available cohort (Northcott et al., 2017) with Caucasian populations, we found the mutation rates of most recurrently mutated genes are consistent between the two ethnic cohorts within MB subgroups (Figure 1D). However, there are some subtle differences. For instance, a recurrent KDM6A mutation, which is most frequently mutated in G4 MB in the Caucasian cohort (Northcott et al., 2017), but was not detected in G4 MBs in the Asian cohort (Figure 1D). The H3K27me3 demethylase KDM6A was reported to have a tumor suppressor function in medulloblastoma (Yi et al., 2020). Conversely, several recurrent mutations in the Asian cohort occurred rarely in the Caucasian cohort, including SHROOM2, encoding a key mediator of RhoA–ROCK pathway to inhibit tumor metastasis (Yuan et al., 2019), and ADGRV1, encoding an adhesion G protein-coupled receptor V1 associated with tumor invasion and metastasis (Aust et al., 2016), in SHH-MB, KMT2C, encoding a lysine methyltransferase 2C that regulates MB tumorigenesis (Roussel and Stripay, 2018) in G3-MB, ZFHX3 (zinc finger homeobox 3), ATM, encoding ataxia telangiectasia mutated protein kinase, a DNA damage checkpoint-regulator, and ADGRV1 in G4-MB (Figure 1D). These data suggest that heterogeneity exists in the prevalence of genetic mutations in MBs across distinct ethnic groups.
Pediatric Asian MB Cohort Shows Transcriptomic Pathway Alterations Similar to Caucasian Patients
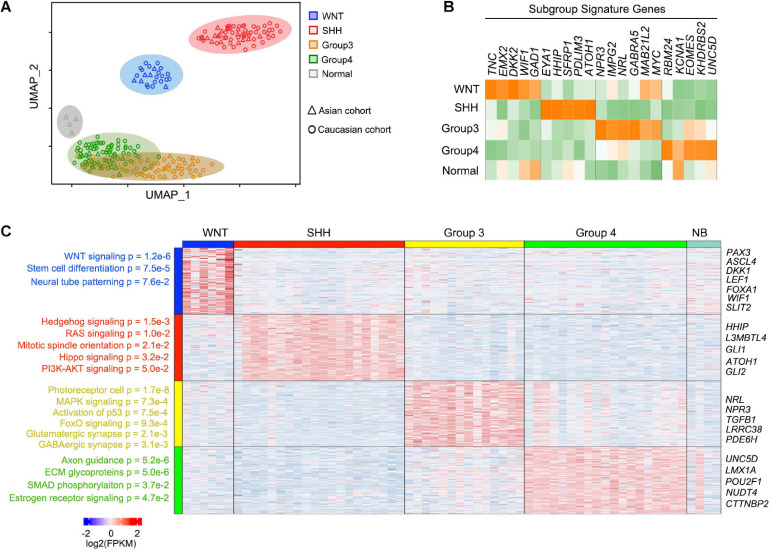
To characterize the gene expression pattern of the Asian MB cohorts, we performed RNA-seq for 63 MB tumors (Supplementary Table 2), including WNT (n = 6), SHH (n = 20), G3 (n = 14), G4 (n = 19) MBs and apparently normal brain controls (n = 4, marked as NB). Clustering analyses indicated that SHH and WNT formed distinct clusters, as opposed to G3 and G4 which are less well defined, consistent with previous subgrouping studies (Cavalli et al., 2017; Figure 2A). Previously established subgroup-specific marker genes clearly differentiate between each of these MB subgroups (Figure 2B). Signature genes of each MB subgroup from the Asian cohort were consistent with previously defined Caucasian MB subgroups (Figure 2C and Supplementary Table 3).
FIGURE 2.
Signature genes for each MB subgroup and function enrichment. (A) UMAP plots for the 59 newly reported MBs and four normal cerebellar samples with similarity measured by the expression level of highly variable genes. The Caucasian MB cohort was used as the control of the known subgroup MB. (B) Heatmap of known marker genes for four subgroups of newly reported MBs showing significant differential expression across subgroups. (C) Heatmap and enriched pathway for the differential feature genes across MBs.
In the WNT MBs, highly enriched genes were related to WNT signaling pathways (p = 1.2e-6) including DKK1, LEF1, and WIF1, as well as stem cell differentiation regulators (p = 7.5e-5) such as PAX3 and FOXA1. In SHH MBs, the enriched genes were associated with Hedgehog signaling (p = 1.5e-3), RAS (p = 1.0e-2), mitotic spindle orientation (p = 2.1e-2), as well as HIPPO (p = 3.2e-2), and PI3K-AKT (p = 5.0e-2) signaling. Although a cohort of patients in the G3 subgroup shared similar gene expression patterns with G4 MB patients overall, G3 MBs were distinguished by signatures of photoreceptor cells (p = 1.7e-8), or of MAPK (p = 7.3e-4) and FOXO (p = 9.4e-4) signaling, while signatures for axon guidance (p = 5.2e-6), ECM glycoproteins (p = 5.0e-6), SMAD phosphorylation (p = 3.7e-2), and estrogen receptor signaling (p = 4.7e-2) were enriched in G4 MBs (Figure 2C). The signature genes or pathways of each MB subgroup from the Asian cohort were consistent with previously defined MB subgroups (Figure 2C), suggesting that there is no major difference in MB subgroups among different racial populations based on gene expression patterns.
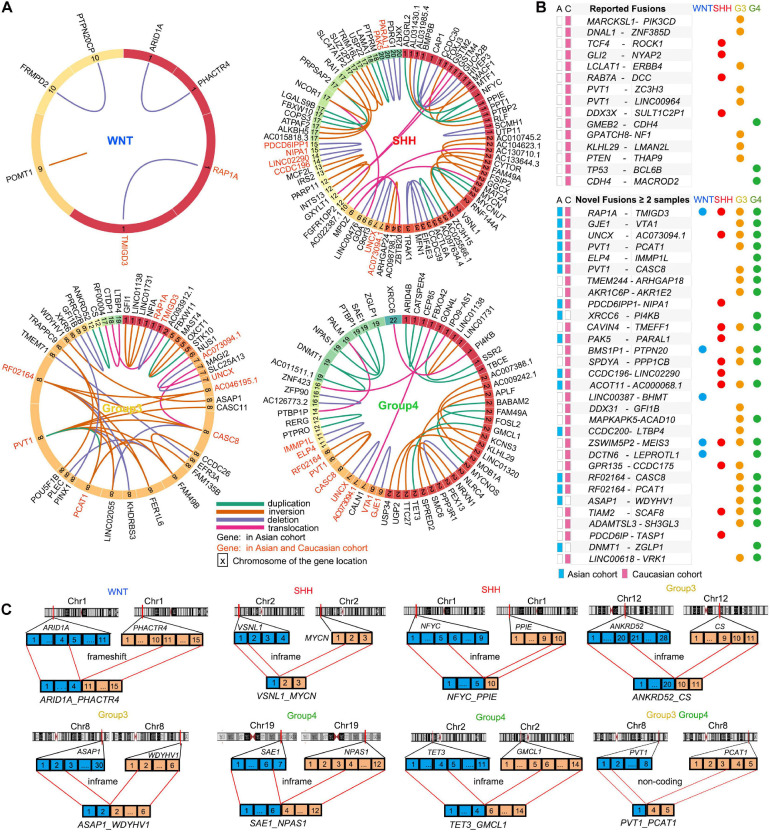
Identification of Novel Gene Fusions in Asian Medulloblastomas
To identify the gene fusions within MB subgroups, we used the Arriba algorithm (Haas et al., 2019) to identify approximately 149 gene fusion events from 52 tumor samples including six WNT-MBs, 16 SHH-MB, 14 G3-MB, and 16 G4-MB in the Asian MB cohort (Figure 3A and Supplementary Table 4). Among the MB subgroups in the Asian cohort, we observed a greater number of fusion events in the SHH and G4 subgroups when compared to G3 and WNT subgroups. The most frequent fusion genes were RAP1A-TMIGD3 in two WNT-MBs and one G3-MB, and PVT1-CASC8 in two G3-MBs and one G4-MB, which is consistent with the Caucasian cohort (Northcott et al., 2017; Figure 3A). Notably, three fusion genes (XRCC6-PI4KB, LINC01138-LINC01731, and DNMT1-ZGLP1) were detected in at least two MB patients in the Asian cohort but not in the Caucasian cohort (Figure 3A). Conversely, a set of the reported MB fusion genes such as MARCKSL1-PIK3CD, DNAL1-ZNF385D, TCF4-ROCK1, GLI2-NYAP2 in the Caucasian cohort were not detected in the Asian cohort (Figures 3A,B).
FIGURE 3.
Gene fusion events in MB subtypes. (A) Gene fusions in WNT, SHH, Group3, and Group4 patients in the Asian cohort. The genes in red indicate the fusion genes also detected in the Caucasian cohort. The colors of the lines shows different types of fusions: duplication (green), inversion (brown), deletion (purple), and translocation (pink). The numbers in the block with different color indicate the numbered chromosomes where the gene located. (B) The reported fusion genes in the Caucasian cohort (up) and the novel fusion genes found in the Asian and Caucasian cohorts (down). (C) Schematic summaries of high-confidence fusion transcripts targeting known or putative MB driver genes in the Asian cohort. Selected gene fusion events were shown: ARID1A-PHACTR4 in WNT MB, VSNL1-MYCN and NFYC-PPIE in SHH MBs, ANKRD52-CS, ASAP1-WDYHV1, and PVT1-PCAT1 in G3 MBs, SAE1-NPAS1, TET3-GMCL1, and PVT1-PCAT1 in G4 MBs.
Our analysis also identified 31 novel fusion genes from at least two samples in the Asian and Caucasian MB cohorts (Figure 3B). In the Asian cohort, we identified MB subgroup-specific fusion genes such as a frameshift fusion gene ARID1A-PHACTR4 in WNT-MB (Figures 3A,C), and in-frame fusion genes (e.g., VSNL1-MYCN and NFYC-PPIE) in SHH-MB (Figure 3C), in-frame fusion genes (e.g., ANKRD52-CS and ASAP1-WDYHV1) and frameshift gene fusions such as (e.g., NF1A-GFI1 and NUS1-SLC25A13) in G3-MB (Figure 3C), as well as in-frame fusion genes (e.g., DNMT1-ZGLP1, SAE1-NPAS1, and TET3-GMCL1) and non-coding fusion genes (e.g., DNMT1-AC011511.1) in G4-MBs (Figure 3C and Supplementary Table 4). In addition, the fusion genes shared among different MB subgroups were also identified such as GJE1-VTA1 (G3- and G4-MB), PVT1-PCAT1 (G3- and G4-MB), and UNCX-AC073094.1 (SHH-, G3-, and G4- MB; Figures 3B,C) in both Asian and Caucasian cohorts. The oncogenic effect of mutations in the fusion genes such as ARID1A, PVT1, GFI1, MYCN, DNMT1, and TET3 has been identified in various tumors including medulloblastomas to regulate tumorigenesis (Bell et al., 2010; Northcott et al., 2014; Mathur, 2018; Mo et al., 2020; Onagoruwa et al., 2020; Wong, 2020), suggesting that the predicted inframe fusion proteins may possess oncogenic potential. Although the precise functions of the fusion genes in MB remain to be defined, our data suggest that novel gene fusion events might contribute to tumorigenesis in the MB cohorts.
Identification of ZNF124 as a G3-MB-Enriched Gene
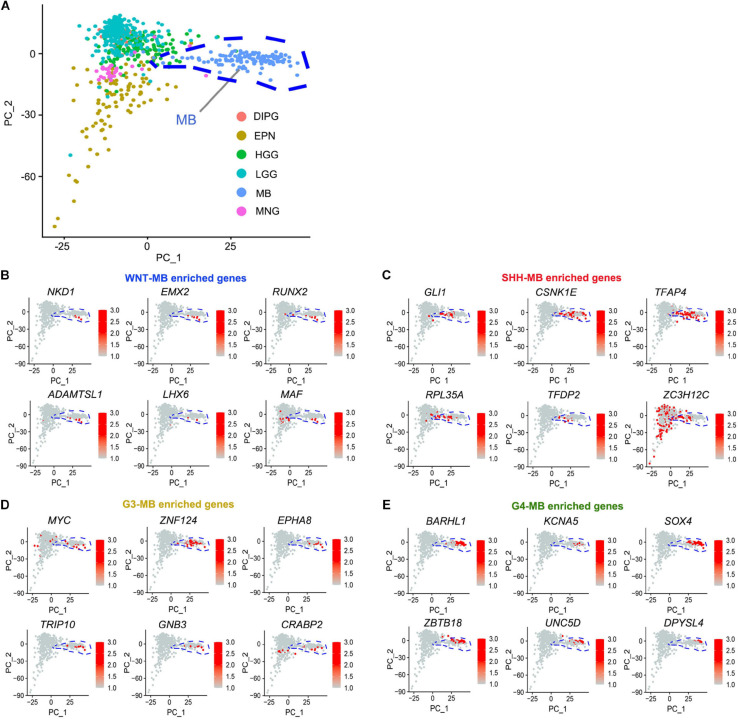
To identify the candidate drivers specific to MBs, but not in other types of tumors, we integrated transcriptomic data from other pediatric brain tumors including ependymoma (EPN), high-grade glioma (HGG), low-grade glioma, (LGG), meningiomas (MNG), diffuse intrinsic pontine glioma (DIPG), and medulloblastomas (MB) from the CBTTC9 database and performed principal component analysis (PCA) to identify the MB-specific genes (Figure 4A). When integrating them with subgroup-enriched genes (Supplementary Table 4), we identified WNT signaling regulator NKD1, transcriptional regulators such as a RUNX family transcription factor (RUNX2), a homeobox-containing transcription factor (EMX2), and a LIM homeobox gene (LHX6) as potential WNT-MB subgroup-specific drivers (Figure 4B). In the SHH subgroup, we uncovered an effector of SHH signaling (GLI1), activating enhancer binding protein (TFAP4), and transcriptional regulator TFDP2 as candidate SHH-MB subgroup-specific drivers (Figure 4C). In G3 MB, besides the previously known G3 driver MYC, we also identified candidate drivers including ZNF124, also known as ZK7, a transcriptional regulator linked to cell survival and tumorigenesis in other cellular contexts (Miyake et al., 2002), TRIP10, a member of the thyroid hormone receptor interactor family, and CRABP2, a member of the retinoic acid binding protein family (Figure 4D). In G4 MB, we identified several transcriptional regulators of neural development including ZBTB18, BARHL1, and SOX4 as candidate driver genes in this subgroup (Figure 4E).
FIGURE 4.
Identification of MB-subgroup-specific candidate driver genes. (A) Principal component analysis (PCA) of MB (133), EPN (98), DIPG (32), HGG (139), LGG (261), and MNG (34) based on the publicly available transcriptomics dataset (CBTTC, https://cbttc.org/). (B–E) The exclusive potential driver genes of WNT (B), SHH (C), G3 (D), and G4 (E) MBs expression in brain tumors using CBTTC cohort.
Loss of Group 3 Specific Driver ZNF124 Attenuates Growth and Promotes Apoptosis of G3-MB Cells
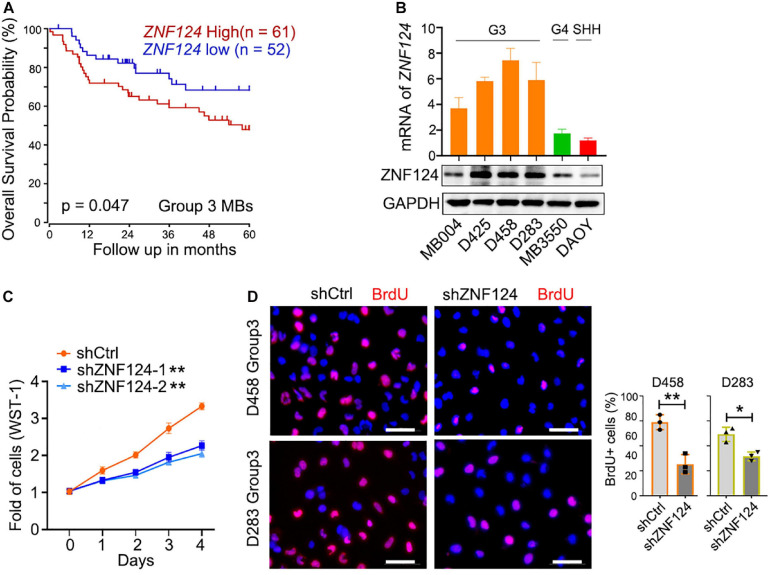
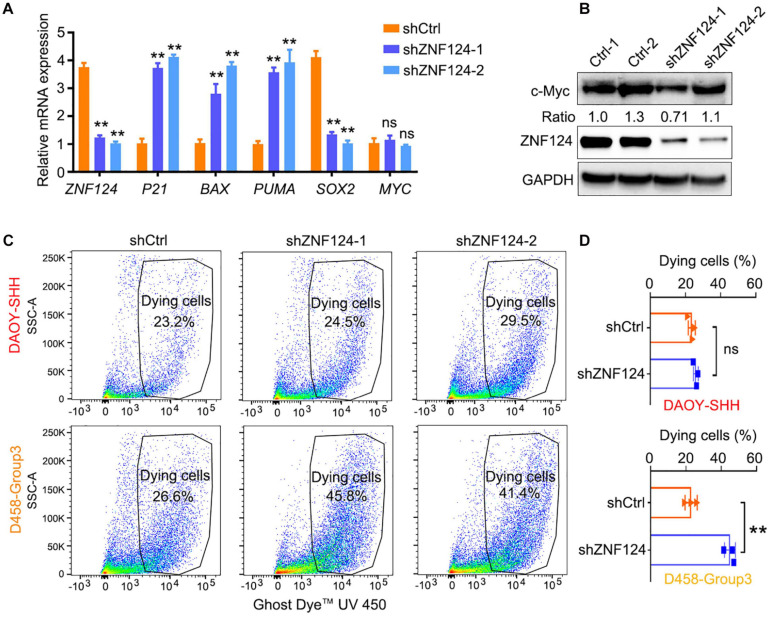
We hypothesize that these MB-subgroup specific genes may play a role in MB tumorigenesis. To define and validate the function of the potential drivers in MB cell growth, we focused on ZNF124, a transcription factor whose loss has been linked to defects in cerebellar development (Poot et al., 2007), as a potential driver for G3-MB, the most aggressive G3 MB subgroup. High expression of ZNF124 is associated with poor prognosis in G3-MBs (Figure 5A), suggesting that ZNF124 may have potential functions in G3 MB tumorigenesis. qPCR and western blotting analyses indicated that ZNF124 expression is higher in G3 MB cell lines (e.g., D425, D458, and D283) (Ivanov et al., 2016) compared with SHH-MB cells (DAOY) and G4 MB cells (MB3550; Figure 5B). Knockdown of ZNF124 using lentiviral shRNA substantially reduced the proliferation of D458 cells, a G3 MB cell line (Ivanov et al., 2016), as measured by the cell proliferation assay (WST-1) and BrdU incorporation (Figures 5C,D). In addition, we found that lentiviral shRNA targeting ZNF124 effectively inhibited expression of a stemness gene SOX2 but significantly increased expression of a cell cycle inhibitor P21/CDKN1A and apoptosis-related genes such as PUMA/BBC3 and BAX in G3 MB D458 cells (Figure 6A). However, ZNF124 knockdown in the MYC-amplified D458 G3-MB cells (Ivanov et al., 2016) did not significantly change the MYC expression at both mRNA and protein levels (Figures 6A,B), suggesting that ZNF124 does not directly regulate MYC expression.
FIGURE 5.
ZNF124 is specifically required for the growth of the aggressive G3MB cell. (A) Survival (5 years) analysis of the driver gene based on the ZNF124 expression using the publicly available datasets (GEO: gse85217). (B) RT-PCR experiment to detect ZNF124 mRNA (upper) and Western blotting to exam ZNF124 protein (lower) expression in human MB cell lines. Data represent means ± SD, n = 3 independent experiments. (C) Fold change in proliferation (as measured by WST-1) of control and shZNF124-expressing D458 cells. Values are means ± SD of five independent measurements across three replicate experiments. **p < 0.01, two-tailed unpaired t-test. (D) BrdU staining images for the control and shZNF124 D425 cells (left) and the quantification (right). Data represent means ± SD, n = 3 independent experiments. *p < 0.05; **p < 0.01, two-tailed unpaired t-test.
FIGURE 6.
Knockdown ZNF124 promotes apoptosis of G3-MB cells. (A) RT-PCR data showed the efficiency of shZNF124 in MYC amplificated G3-MB D458 cells and the cell cycle inhibitor gene CDKN1A/P21, apoptosis genes BAX and PUMA and tumor stemness gene MYC and SOX2 mRNA expression. Data represent means ± SD, n = 3 independent experiments. **p < 0.01, two-tailed unpaired t-test. (B) Western blotting analysis of MYC and ZNF124 protein expression in human MB D458 cell after ZNF124 knockdown with Lenti-shRNA for 72 h. Ratio of MYC expression over GAPDH control was shown. (C,D) Dying cells were detected by flow cytometry in G3-D458 and SHH-DAOY cells after knockdown ZNF124 (C) and the comparison of the dead cells in control and shZNF124 treatment cells (D). Data represent means ± SD, n = 3 independent experiments. **p < 0.01, two-tailed unpaired t-test.
Given that ZNF124 inhibits apoptotic death in other cell contexts (Kuramoto et al., 2000; Miyake et al., 2002), we then examined the proportion of apoptotic cells after ZNF124 knockdown in different MB cell lines. ZNF124 knockdown increased cellular apoptosis in G3-MB D458 cells, but not SHH-DAOY cells (Figures 6C,D), suggesting that targeting ZNF124 increases cell death specifically in G3-MB cells.
Discussion
The characteristics of distinct MBs differ by their genetic alterations and gene expression profiles. An important aspect of MB tumorigenesis is its somatic mutations. However, the genetic characteristics of MBs in Asian populations are poorly understood. Our present analyses of the landscape of somatic mutations and transcriptomic profiles of MBs reveal similar genetic alterations and gene expression profiles between Caucasian and Asian cohorts, two large racial groups, suggesting that the major tumor driving events are conserved among different races.
In this study, most of the recurrent mutations in the Asian cohort are similar to Caucasian cohorts, however, there are novel gene fusion events unique to Asian MBs. The differences among racial or ethnic groups may lie in genetic, environmental, or behavioral factors that act alone or in combination. At present, whether and how the novel gene fusion events contribute to MB tumorigenesis or progression in each MB subgroup remain to be defined in Asian cohort. To our knowledge, our study provides the first comprehensive view of somatic mutations and gene expression profiles of MBs in Han Chinese population patients. Our study provides evidence of distinct genetic alteration events or heterogeneity among different racial and ethnic groups. Given that rare mutations can be responsible for severe effects in complex human disease and cancer (Mcclellan and King, 2010; Turajlic et al., 2019), it suggests the importance of tailoring an ethnicity-based genetic testing panel and treatment strategies for Asian patients. This Asian cohort of pediatric brain tumors presents an important resource for future studies that better represent this previously underrepresented population in pediatric brain tumor research.
Our genomic analysis of MBs identifies potential genetic alterations across different MB subtypes. In the Asian cohort and the publicly available MB cohorts, CNV is more prevalent in aggressive MBs (G3 and G4) when compared with WNT and SHH MBs and other pediatric brain tumors (Northcott et al., 2017). In addition, we identified unique inframe fusion proteins that may possess oncogenic potential in the Asian MBs, suggesting that the novel gene fusion events might contribute to tumorigenesis in the Asian MB cohort.
By performing a comprehensive analysis of this new Asian cohort in combination with publicly available cohorts, we identify a set of subgroup-specific potential driver genes across different MBs (Figure 4A). Particularly, we identified ZNF124, which is highly enriched in G3 MB, the most aggressive MB subgroup. High expression of ZNF124 is associated with poor prognosis in MBs, suggesting that ZNF124 may serve as a prognostic marker in G3 MB. ZNF124 has been shown to inhibit cell death in other cellular contexts (Kuramoto et al., 2000; Miyake et al., 2002). Interestingly, we show that ZNF124 knockdown inhibits tumor cell growth and increases cell death specifically in G3-MB cells but not in SHH-MB cells, suggesting a unique role of ZNF124 in regulating the growth of the most aggressive G3 MB cells. Thus, our integrative transcriptomic profiling analysis for MB specific genes allows the identification of novel candidate driver genes that are critical for the growth of subgroup-specific MBs. Together, our present studies identify the genetic mutation landscape of the Asian ethnic population and provide an important reference for future genetic testing of cancer risk and potential targeted treatment in the patients with MBs.
Data Availability Statement
The datasets presented in this study can be found in the NCBI Gene Expression Omnibus. The accession code is GSE164677.
Ethics Statement
The study was approved by the Ethics Committee of Children’s Hospital of Fudan University, Shanghai, China, and conducted in accordance with the Declaration of Helsinki. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
QRL, ZL, and XD conceptualized, designed, and wrote the manuscript with input from all authors. ZL, LX, YL, JaY, and HL performed the experiments. XD and JyY performed data analysis. YX, PX, LX, GH, TC, HL, and WZ collected the samples. KB and RR revised the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This project was supported by grants from the CancerFree KIDS and Pray Hope Believe Foundations.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.634056/full#supplementary-material
References
- Anders S., Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust G., Zhu D., Van Meir E. G., Xu L. (2016). Adhesion GPCRs in tumorigenesis. Handb. Exp. Pharmacol. 234 369–396. 10.1007/978-3-319-41523-9_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann M., Waszak S. M., Robinson G. W., Jager N., Sharma T., Knopp C., et al. (2020). Germline GPR161 mutations predispose to pediatric medulloblastoma. J. Clin. Oncol. 38 43–50. 10.1200/jco.19.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E., Chen L., Liu T., Marshall G. M., Lunec J., Tweddle D. A., et al. (2010). MYCN oncoprotein targets and their therapeutic potential. Cancer Lett. 293 144–157. 10.1016/j.canlet.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Benjamin D., Sato T., Cibulskis K., Getz G., Stewart C., Lichtenstein L., et al. (2019). Calling somatic SNVs and indels with mutect2. Biorxiv 2019:861054. 10.1101/861054 [DOI] [Google Scholar]
- Brennan C. W., Verhaak R. G., Mckenna A., Campos B., Noushmehr H., Salama S. R., et al. (2013). The somatic genomic landscape of glioblastoma. Cell 155 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli F. M. G., Remke M., Rampasek L., Peacock J., Shih D. J. H., Luu B., et al. (2017). Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 31:e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Medeiros C. B., Moxon-Emre I., Scantlebury N., Malkin D., Ramaswamy V., Decker A., et al. (2020). Medulloblastoma has a global impact on health related quality of life: findings from an international cohort. Cancer Med. 9 447–459. 10.1002/cam4.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy V., Herenger Y., Kress A., Stoetzel C., Piton A., Dollfus H., et al. (2018). AnnotSV: an integrated tool for structural variations annotation. Bioinformatics 34 3572–3574. 10.1093/bioinformatics/bty304 [DOI] [PubMed] [Google Scholar]
- Haas B. J., Dobin A., Li B., Stransky N., Pochet N., Regev A., et al. (2019). Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 20:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Akutsu H., Takano S., Kino H., Ishikawa E., Tanaka S., et al. (2018). Clinical and biological significance of adamantinomatous craniopharyngioma with CTNNB1 mutation. J. Neurosurg. 131 217–226. 10.3171/2018.3.jns172528 [DOI] [PubMed] [Google Scholar]
- Harrow J., Frankish A., Gonzalez J. M., Tapanari E., Diekhans M., Kokocinski F., et al. (2012). GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 22 1760–1774. 10.1101/gr.135350.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Zhang L., Chen Y., Remke M., Shih D., Lu F., et al. (2014). The G protein alpha subunit Galphas is a tumor suppressor in sonic hedgehog-driven medulloblastoma. Nat. Med. 20 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovestadt V., Ayrault O., Swartling F. J., Robinson G. W., Pfister S. M., Northcott P. A., et al. (2020). Medulloblastomics revisited: biological and clinical insights from thousands of patients. Nat. Rev. Cancer 20 42–56. 10.1038/s41568-019-0223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov D. P., Coyle B., Walker D. A., Grabowska A. M. (2016). In vitro models of medulloblastoma: choosing the right tool for the job. J. Biotechnol. 236 10–25. 10.1016/j.jbiotec.2016.07.028 [DOI] [PubMed] [Google Scholar]
- Kumar R., Liu A. P. Y., Northcott P. A. (2019). Medulloblastoma genomics in the modern molecular era. Brain Pathol. 30 679–690. 10.1111/bpa.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto K., Uesaka T., Kimura A., Kobayashi M., Watanabe H., Katoh O., et al. (2000). ZK7, a novel zinc finger gene, is induced by vascular endothelial growth factor and inhibits apoptotic death in hematopoietic cells. Cancer Res. 60 425–430. [PubMed] [Google Scholar]
- Li H., Durbin R. (2009). Fast and accurate short read alignment with burrows–wheeler transform. Bioinformatics 25 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis D. N., Perry A., Reifenberger G., Von Deimling A., Figarella-Branger D., Cavenee W. K., et al. (2016). The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131 803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Mathur R. (2018). ARID1A loss in cancer: towards a mechanistic understanding. Pharmacol. Ther. 190 15–23. 10.1016/j.pharmthera.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Mcclellan J., King M. C. (2010). Genetic heterogeneity in human disease. Cell 141 210–217. 10.1016/j.cell.2010.03.032 [DOI] [PubMed] [Google Scholar]
- Mckenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A. M., et al. (2010). The genome analysis toolkit: a mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20 1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclean C. Y., Hwang Y., Poplin R., Depristo M. A. (2019). Genomewarp: an alignment-based variant coordinate transformation. Bioinformatics 35 4389–4391. 10.1093/bioinformatics/btz218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N., Katoh O., Hirata S., Kimura S., Watanabe H., Yajin K. (2002). Expression of the Kruppel-type zinc finger gene, ZK7, in head and neck squamous cell carcinoma and normal mucosa. Cancer Lett. 185 111–118. 10.1016/s0304-3835(02)00303-8 [DOI] [PubMed] [Google Scholar]
- Mo H. Y., An C. H., Choi E. J., Yoo N. J., Lee S. H. (2020). Somatic mutation and loss of expression of a candidate tumor suppressor gene TET3 in gastric and colorectal cancers. Pathol. Res. Pract. 216:152759. 10.1016/j.prp.2019.152759 [DOI] [PubMed] [Google Scholar]
- Narayan V., Sugur H., Jaiswal J., Arvinda H. R., Arivazhagan A., Somanna S., et al. (2019). Medulloblastoma: distinctive histo-molecular correlation with clinical profile, radiologic characteristics, and surgical outcome. Pediatr. Neurosurg. 54 329–340. 10.1159/000501913 [DOI] [PubMed] [Google Scholar]
- Northcott P. A., Buchhalter I., Morrissy A. S., Hovestadt V., Weischenfeldt J., Ehrenberger T., et al. (2017). The whole-genome landscape of medulloblastoma subtypes. Nature 547 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott P. A., Korshunov A., Pfister S. M., Taylor M. D. (2012a). The clinical implications of medulloblastoma subgroups. Nat. Rev. Neurol. 8 340–351. 10.1038/nrneurol.2012.78 [DOI] [PubMed] [Google Scholar]
- Northcott P. A., Shih D. J., Remke M., Cho Y. J., Kool M., Hawkins C., et al. (2012b). Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 123 615–626. 10.1007/s00401-011-0899-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott P. A., Korshunov A., Witt H., Hielscher T., Eberhart C. G., Mack S., et al. (2011). Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 29 1408–1414. 10.1200/jco.2009.27.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott P. A., Lee C., Zichner T., Stutz A. M., Erkek S., Kawauchi D., et al. (2014). Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma. Nature 511 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott P. A., Robinson G. W., Kratz C. P., Mabbott D. J., Pomeroy S. L., Clifford S. C., et al. (2019). Medulloblastoma. Nat. Rev. Dis. Primers 5:11. [DOI] [PubMed] [Google Scholar]
- Onagoruwa O. T., Pal G., Ochu C., Ogunwobi O. O. (2020). Oncogenic Role of PVT1 and therapeutic implications. Front. Oncol. 10:17. 10.3389/fonc.2020.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phi J. H., Park A. K., Lee S., Choi S. A., Baek I. P., Kim P., et al. (2018). Genomic analysis reveals secondary glioblastoma after radiotherapy in a subset of recurrent medulloblastomas. Acta Neuropathol. 135 939–953. 10.1007/s00401-018-1845-8 [DOI] [PubMed] [Google Scholar]
- Pinero J., Bravo A., Queraltrosinach N., Gutierrezsacristan A., Deupons J., Centeno E., et al. (2017). DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 45 D833–D839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M., Kroes H. Y., Se V. D. W., Eleveld M. J., Rooms L., Nievelstein R. A., et al. (2007). Dandy-Walker complex in a boy with a 5 Mb deletion of region 1q44 due to a paternal t(1;20)(q44;q13.33). Am. J. Med. Genet. A 143A 1038–1044. 10.1002/ajmg.a.31690 [DOI] [PubMed] [Google Scholar]
- Roberts A., Pachter L. (2013). Streaming fragment assignment for real-time analysis of sequencing experiments. Nat. Method 10 71–73. 10.1038/nmeth.2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Stripay J. L. (2018). Epigenetic drivers in pediatric medulloblastoma. Cerebellum 17 28–36. 10.1007/s12311-017-0899-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T., Schwalbe E. C., Williamson D., Sill M., Hovestadt V., Mynarek M., et al. (2019). Second-generation molecular subgrouping of medulloblastoma: an international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 138 309–326. 10.1007/s00401-019-02020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Beetz C., Williams S. G., Bhaskar S. S., O’sullivan J., Anderson B., et al. (2014). Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J. Clin. Oncol. 32 4155–4161. 10.1200/jco.2014.58.2569 [DOI] [PubMed] [Google Scholar]
- Srinivasan V. M., Ghali M. G., North R. Y., Boghani Z., Hansen D., Lam S. (2016). Modern management of medulloblastoma: molecular classification, outcomes, and the role of surgery. Surg. Neurol. Int. 7 S1135–S1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turajlic S., Sottoriva A., Graham T., Swanton C. (2019). Resolving genetic heterogeneity in cancer. Nat. Rev. Genet. 20 404–416. 10.1007/3-540-30683-8_749 [DOI] [PubMed] [Google Scholar]
- Twigg S. R. F., Hufnagel R. B., Miller K. A., Zhou Y., Mcgowan S. J., Taylor J., et al. (2016). A recurrent mosaic mutation in SMO, encoding the hedgehog signal transducer smoothened, is the major cause of curry-jones syndrome. Am. J. Hum. Genet. 98 1256–1265. 10.1016/j.ajhg.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Li M., Hakonarson H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38:e164. 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszak S. M., Northcott P. A., Buchhalter I., Robinson G. W., Sutter C., Groebner S., et al. (2018). Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 19 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. K. (2020). DNMT1: A key drug target in triple-negative breast cancer. Semin. Cancer Biol. 2020:10. 10.1016/j.semcancer.2020.05.010 [DOI] [PubMed] [Google Scholar]
- Wu K. S., Ho D. M., Jou S. T., Yu A. L., Tran H. M., Liang M. L., et al. (2020). Molecular-clinical correlation in pediatric medulloblastoma: a cohort series study of 52 cases in taiwan. Cancer Lett. 12:653. 10.3390/cancers12030653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J., Shi X., Xuan Z., Wu J. (2020). Histone demethylase UTX/KDM6A enhances tumor immune cell recruitment, promotes differentiation and suppresses medulloblastoma. Cancer Lett. 499 188–200. 10.1016/j.canlet.2020.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Chen L., Xiao J., Qi X. K., Zhang J., Li X., et al. (2019). SHROOM2 inhibits tumor metastasis through RhoA-ROCK pathway-dependent and -independent mechanisms in nasopharyngeal carcinoma. Cell Death Dis. 10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in the NCBI Gene Expression Omnibus. The accession code is GSE164677.