Graphical abstract
Keywords: Acute toxicity, Biomarker, Erythrocytic abnormalities, Fenitrothion, Genotoxicity, Organophosphate, Recuperation, Zebrafish
Highlights
-
•
Genotoxic effects of fenitrothion in the erythrocytes of zebrafish was measured.
-
•
Abnormality of erythrocyte were found to be raised after the exposure of fenitrothion.
-
•
Abnormality of erythrocyte recovered with a concentration-and duration-dependent way.
-
•
Fish erythrocyte is an effective biomarker for toxicity test of an organophosphate.
Abstract
The experiment was explicated to investigate the fenitrothion persuaded genotoxicity in the peripheral erythrocytes of zebrafish (Danio rerio) through in vivo exposures (10 %, 20 % and 40 % of LC50 of fenitrothion, i.e., 0.8, 1.6, and 3.2 mg/L, respectively) for variable periods (1, 3, and 7 days) and its subsequent post-exposure recuperation array in pesticide-free water for similar intervals was also evaluated. With the exception of the control group (0% of fenitrothion), the obtained results pointed out that with the promotion of time and concentrations, fenitrothion induced significantly (p < 0.05) higher prevalence and severity of erythrocytic nuclear abnormalities (ENA) such as- notched, micronucleus, nuclear bridges, blebbed, binucleated, nuclear bud and also erythrocytic cellular abnormalities (ECA) such as - echinocytic, elongated, tear-drop, crescentic, twin, fusion, and spindle-shaped cells. Recuperation data stated that zebrafish cured spontaneously and aberrated erythrocytic anomalies in all treatments were renormalized according to the concentration and duration dependence. Hence, we concluded that fenitrothion has a dangerous effect on the zebrafish, and this technology can be used to anticipate the sensitivity of aquatic animals to environmental pollution.
1. Introduction
The use of pesticides in agricultural areas to protects crops from harmful organisms, which may expand the aquatic environment and adversely affect aquatic organisms. Increased urbanization, industrial enterprise and technological expansion have prompted the heavy use of synthetic chemicals as pesticides, inflicting pollution and wastage in many ecosystems [1,2]. The aquatic habitat is the final meeting point of various chemical substances and water can be used as a medium for contact with various toxic substances [3]. When most rural farming areas are located near water bodies, these synthetic compounds penetrate the soil into lakes, waterways and streams and other aquatic systems, causing catastrophic effects on aquatic organisms. In addition to target pests, chemicals also significantly affect the presence of non-target organisms in aquatic environments such as invertebrates and fish. Even if direct contacts with chemicals in contaminated water are short, it will produce abnormalities in the fish blood [4,5].
Blood parameters have promoted toxicology research assessment tools as biomarkers, because fish blood components shrink due to any type of environmental change, and because they are directly connected through the gill surface, they respond quickly to any changes in water quality [6]. Water contaminated with toxic metals and pesticides can affect fish by changing normal behavior [7,8], histo-morphological alterations in intestine, kidney, liver, etc. and physiological function (modifications in blood parameters) ([[9], [10], [11], [12], [13], [14]]) and depletion of RNA, DNA and protein contents [15].
Fenitrothion (O, O Dimethyl O-3- methyl-4-nitrophenyl; a 94.2 % solution of fenitrothion is known as sumithion) is an insecticide, which is mainly used for crop protection and repelling of aquatic insects (especially tiger worms) before release of larvae in aquaculture ponds [16]. Fenitrothion is known to be somewhat harmful to fish [17], moderately toxic to warm and cold-water fish. Indiscriminate application of such a kind of pesticide may significantly distress the normal physiology, biology and early development of aquatic organisms [18,19].
For monitoring environment quality blood parameter analysis serves as an early warning tool and red blood cell irregularity test (nuclear and structural changes of red blood cells) is one of the relevant diagnostic tools to assess oxidative stress and genotoxicity caused by toxic substances present in aquatic ecosystems [15]. The red blood cells (erythrocytes) of fish are different from the red blood cells of mammals because they have cell nuclei, and they are interpreted in the form of morphological changes as important biological indicators of pollution. Red blood cell micronucleus analysis has made outstanding achievements in assessing the stress syndrome of pollutants and the genotoxicity and mutagenic effects of various environmental contaminants (Cavas and Konen [20]; Anbumani and Mohankumar [6,15,18],). In genotoxicity, the examination of the nuclear abnormalities of erythrocytic (ENA), an alternative of the common micronuclei analysis, a suitable indicator for detecting the health status of fish and environment, and is also extensively used in the toxicity test [15,21].
The recuperation of erythrocytes of aquatic animals especially in fish relies on the periods of duration [6,18] and understandings on the duration of full recovery after exposure to pesticide helps to ensure the compatible welfare of fish [22], henceforth the transition and recovery data may be a prognostic tool for environmental risk assessment [23,24]. So, this study aims to evaluate the possibility and pattern of red blood cell abnormalities (nuclear and cellular abnormalities) and the recovery of these biomarkers after exposure to fenitrothion in zebrafish (Danio rerio).
2. Materials and methods
2.1. Collection and rearing of experimental fish
Three hundred and sixty wild type adult zebrafish (Danio rerio) (body weight 0.9 ± 0.2 g; standard length 4 ± 0.7 cm) of both sexes were collected from the field laboratory complex of Bangladesh Agricultural University in a plastic bag filled with 1/3 water and 2/3 oxygen. Then they were acclimatized in aerated tap water for 15 days in a cemented tank prior to the experimentation under 12 h light and 14 h dark condition and tank water was renewed every alternative two day. These fish were given 33 % protein containing commercial fish feed (Spectra Feeds Ltd.) twice a day. Suctioning was performed daily for the removal of fecal and feed residues. Studied fish were managed and guided as reported by the ACP (Animal Care panel), Bangladesh Agricultural University, Bangladesh. Physico-chemical parameters of the water such as temperature, pH, and dissolved oxygen were observed. Fish were accepted as well adapted to laboratory conditions when less than 1% mortality was recorded during acclimatization period of 15 days period. The feeding supplement was abolished 24 h prior to the initiation of the experiment. The commercial-grade fenitrothion was procured from the authorized pesticide dealer from Dhaka, Bangladesh. The end date of pesticide has been verified before the start of the experiment.
2.2. Exposure of fish to fenitrothion
For the acute toxicity tests of fenitrothion on zebrafish, each experimental tank (36 × 10 × 12 inch3) was filled with 20 L of de-chlorinated tap water after cleaning with KMnO4 and sun-drying properly. The 96 h LC50 value of fenitrothion for zebrafish is 7.89 mg/L (Ahmed et al., 2011). Thirty healthy fish were introduced into each tank with 10 % (0.8 mg/L), 20 % (1.6 mg/L) and 40 % (3.2 mg/L) of the LC50 of fenitrothion for 7 days while the fourth group treated as control (0% of fenitrothion). During the experiment, the fish were fed twice a day, and a new dose of fenitrothion was used after changing the water on another day. Five fish were sampled from each group at the definite gaps (e.g., 1, 3, and 7 days) until the termination of the experiment.
2.3. Recuperation assessment
The pesticide exposure experiment was conducted for 1, 3 and 7 days and the recovery rate was evaluated at similar intervals in pesticide-free water. After 7 days of exposure to 10 %, 20 %, and 40 % of LC50 of fenitrothion, the fish were shifted to fenitrothion-free water tank for recuperation assessment. Renew the water every day and feed the fish every day. At the end of days 1, 3, and 7 after exposures, five individuals were collected from each tank and evaluated for their recuperation.
2.4. Estimation of erythrocytic abnormalities
Fish were caught gently from the tank on specific sampling day and anesthetized instantly with clove oil (5 mg/L). Blotting paper was used to clean the water and slime of the fish body. Peripheral blood samples of 5 fish from each group were obtained from the tail region using a surgical syringe. Immediate after sampling, blood sample was sprayed on pre-cleaned glass slides and then the slides were dried at room temperature for 15 min. After fixing the slides in 70 % methanol, place them at room temperature for 10 min, then stain the slides with 4% Giemsa for 7 min, and finally clean with tap water After air-drying overnight, the glass slides were mounted with DPX, and ENA including micronuclei (MN) and cellular abnormalities of erythrocytic (ECA) cells were scored under the Micros microscope (CX100, Austria) using a 40X objective lens. Scoring 2000 cells from each slide, 5 slides were prepared for each fish, and blindly scoring MN, ENA and ECA was performed on the randomly coded slides to reduce technical differences. For the score of MN, the following standard of Fenech et al. [25] was used: as long as the nuclear boundary can be clearly identified, the MN should be separated from the main nucleus or slightly overlapped with the main nucleus; the staining of the MN should be similar to the main nucleus. Abnormalities of nucleus other than MN in red blood cells were classified in the opinion of Sadiqul et al. [15]. In short, a cell with two nuclei is considered to be binuclear. A small tubular outgrowth of the nuclear membrane and contained euchromatin is known as blebbed. A nucleus with a tiny cavity and a considerable depth into a nucleus that does not contain nuclear material is called a notched. Nuclei with tube like out-growth are labeled as nuclear buds and nuclear bridges, which are thin lines that connect individual nuclei.
The identification standard for ECA should be different from structured red blood cells, which are elliptical structures with concentrated nuclei. Red blood cell abnormalities are categorized as echinocytic cells, which have jagged edges on the entire cell surface and have a more uniform shape; an elongated cell whose length is significantly greater than its width; fusion is the connection of two or more cells to form a larger cell volume; the main axis widens in the middle and then tapers at the ends termed as spindle; two cells are connected by the cell surface called as twin and a erythrocyte that is pulled to the nipple at one end, called tear-drop.
2.5. Statistical analysis
Before performing statistical analysis on all data sets (ENA and ECA), first complete the normality and homogeneity of the variance test. The data from ECA and ENA analyses were executed using ANOVA (one-way analysis of variance) followed by the post-hoc test. Statistical analysis was done with the SPSS Version 20.0 for Windows. All data are expressed as mean ± SD. The level of statistical significance was set at p < 0.05.
3. Results
3.1. Effects on ENA at different exposure days
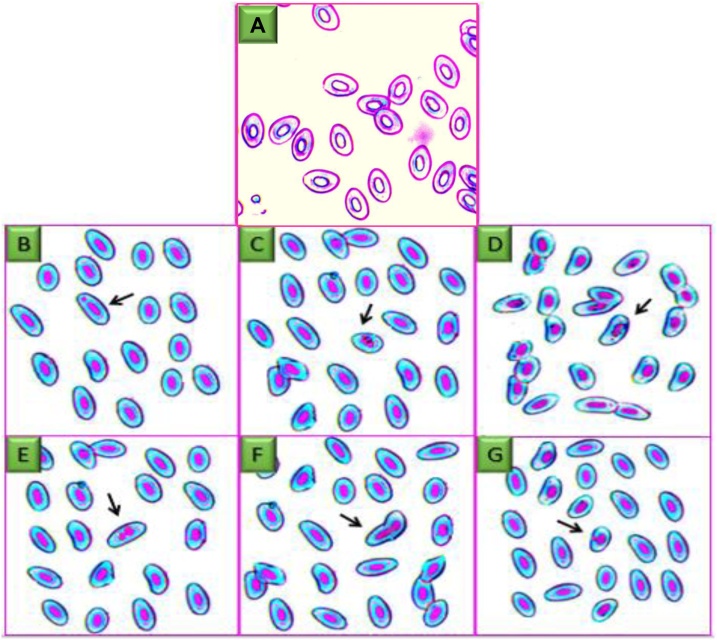
Mean ENA at individual exposure lengths including control are shown in Table 1. In the control group, the erythrocytes were oval and the nuclei arranged neatly, while in the fenitrothion treatment group, a number of nuclear irregularities were observed, such as bi-nuclei, blebbed nuclei, nuclear bud, nuclear bridge and notched nuclei (Fig. 1). After two consecutive days of exposure, the average erythrocyte nucleus abnormality increased significantly, and the change in ENA frequency (Table 1) was significantly different (p < 0.05).
Table 1.
Mean number of erythrocytic nuclear abnormalities (ENA) in zebrafish exposed to three sub-lethal concentrations (0.8, 1.6 and, 3.2 mg/L, i.e., 10 %, 20 %, and 40 % of LC50, respectively) of fenitrothion and the respective controls at each period of exposure (1, 3 and 7 days) and recovery for similar intervals.
| Abnormalities | Dose (% of LC50) | Exposure period |
Recovery period | Recovery rate (%) | Exposure period | Recovery period | Recovery rate (%) | Exposure period | Recovery period | Recovery rate (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 day | 1 day | 3 day | 3 day | 7 day | 7 day | |||||
| Micronuclei | 10 | 0.33 ± 0.10 ax | 0.48 ± 0.02a | 0.40 ± 0.01x | 16.66 | 1.50 ± 0.11b | 1.12 ± 0.30y | 25.33 | 4.18 ± 0.22c | 1.68 ± 0.13z | 59.80 |
| 20 | 0.68 ± 0.03 a | 0.60 ± 0.01 x | 11.76 | 1.71 ± 0.13 b | 1.40 ± 0.18 y | 18.12 | 4.84 ± 0.13 c | 2.12 ± 0.05z | 56.19 | ||
| 40 | 0.87 ± 0.12 a | 0.74 ± 0.11 x | 14.94 | 1.80 ± 0.20 b | 1.50 ± 0.17 y | 16.66 | 5.79 ± 0.21 c | 3.21 ± 0.20 z | 44.55 | ||
| Binucleated | 10 | 0.29 ± 0.03 ax | 0.51 ± 0.14ab | 0.45 ± 0.11 x | 11.76 | 0.33 ± 0.13 a | 0.25 ± 0.09y | 24..24 | 0.84 ± 0.02 b | 0.55 ± 0.11 x | 34.52 |
| 20 | 0.73 ± 0.05 a | 0.64 ± 0.19 x | 12.32 | 0.69 ± 0.21 a | 0.57 ± 0.15 x | 17.39 | 1.15 ± 0.13 b | 0.78 ± 0.09 x | 32.17 | ||
| 40 | 0.48 ± 0.14 a | 0.44 ± 0.05 x | 8.33 | 0.62 ± 0.16 b | 0.50 ± 0.21 x | 19.35 | 1.18 ± 0.14 c | 0.82 ± 0.05 y | 30.50 | ||
| Blebbed nuclei | 10 | 0.35 ± 0.11 ax | 0.77 ± 0.05 a | 0.65 ± 0.14 x | 15.58 | 1.20 ± 0.05 b | 0.86 ± 0.07 y | 28.33 | 1.95 ± 0.12 c | 1.12 ± 0.09 z | 42.56 |
| 20 | 0.96 ± 0.16 a | 0.84 ± 0.13 x | 12.50 | 1.57 ± 0.05 b | 1.00 ± 0.06 y | 26.75 | 2.10 ± 0.23 c | 1.30 ± 0.11 y | 38.09 | ||
| 40 | 1.13 ± 0.04 b | 1.00 ± 0.11 x | 11.50 | 1.55 ± 0.06 b | 1.25 ± 0.10 x | 19.35 | 2.55 ± 0.25 c | 1.60 ± 0.20 y | 37.25 | ||
| Notched nuclei | 10 | 0.15 ± 0.05 ax | 0.29 ± 0.04 a | 0.25 ± 0.08 x | 13.79 | 0.93 ± 0.12 b | 0.78 ± 0.20 y | 16.12 | 1.76 ± 0.3 c | 0.89 ± 0.18 y | 49.43 |
| 20 | 0.56 ± 0.06 a | 0.49 ± 0.09 x | 12.50 | 1.03 ± 0.12 b | 0.85 ± 0.13 y | 17.47 | 2.69 ± 0.42 c | 1.59 ± 0.10 z | 40.08 | ||
| 40 | 0.70 ± 0.03 a | 0.62 ± 0.16 x | 11.42 | 1.40 ± 0.12 b | 1.18 ± 0.36 y | 15.71 | 3.96 ± 1.09 c | 2.60 ± 0.10 z | 34.34 | ||
| Nuclear bridge | 10 | 0.18 ± 0.02 ax | 0.27 ± 0.08 a | 0.22 ± 0.08 x | 18.51 | 1.57 ± 0.08 b | 0.98 ± 0.27 y | 37.57 | 1.90 ± 0.07 c | 0.90 ± 0.02 y | 52.63 |
| 20 | 0.25 ± 0.02 a | 0.21 ± 0.08 x | 16.00 | 1.25 ± 0.12 b | 1.00 ± 0.08 y | 20.00 | 2.46 ± 0.27 c | 1.50 ± 0.17 z | 39.02 | ||
| 40 | 0.37 ± 0.11 a | 0.33 ± 0.06 x | 10.81 | 1.40 ± 0.14 b | 1.06 ± 0.11 y | 24.28 | 3.33 ± 0.19 c | 2.21 ± 0.15 z | 33.63 | ||
| Nuclear bud | 10 | 0.10 ± 0. ± 01ax | 0.13 ± 0.07 a | 0.11 ± 0.06 x | 15.38 | 0.40 ± 0.04ab | 0.29 ± 0.06 x | 27.50 | 0.75 ± 0.04 b | 0.44 ± 0.05 y | 41.33 |
| 20 | 0.20 ± 0.05 a | 0.18 ± 0.06 x | 10.00 | 0.50 ± 0.09ab | 0.37 ± 0.14 x | 26.00 | 0.94 ± 0.05 b | 0.68 ± 0.08 y | 27.65 | ||
| 40 | 0.30 ± 0.11 a | 0.26 ± 0.01 x | 13.33 | 0.68 ± 0.25 b | 0.52 ± 0.13x | 23.52 | 1.00 ± 0.04 c | 0.74 ± 0.05 y | 26.00 | ||
Values with different alphabet superscripts in the same row differ significantly (p < 0.05) between exposure and recovery periods, respectively in ENA cells. All values are expressed as mean±SD.
Fig. 1.
Blood smears of zebrafish exposed to sub-lethal concentrations of fenitrothion showing several ENA such as (A) control (an ovoid-shaped erythrocyte with a regular oval-shaped nucleus at the middle of the cell), (B) micronucleus (MN), (C) notched nucleus (D) blebbed, (E) bi-nucleus, (F) nuclear bridge and (G) nuclear bud (Giemsa stain: 40X).
3.2. Effects on ECA at different exposure days
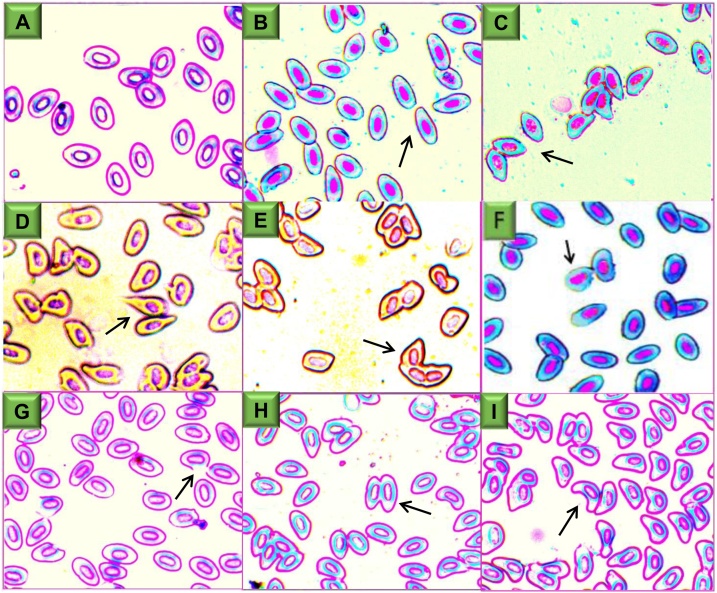
Significant changes in mean ECA frequency were observed within days of exposure (p < 0.05) (Table 2). The fish of the control treatment exhibited a normal red blood cell structure with regular cell walls. Compared with the control group, the frequency of ECA gradually increased after the treatment with fenitrothion (Table 2). The various patterns of ECA detected in this experiment, like - spindle-shaped, echinocytic cells, crescentic-shaped, elongated-shaped, fusion, twin-shaped, tear-drop shaped, and demembranated cells (Fig. 2).
Table 2.
Mean number of erythrocytic cellular abnormalities (ECA) in zebrafish exposed to three sub-lethal concentrations (0.8, 1.6 and 3.2 mg/L, i.e., 10 %, 20 %, and 40 % of LC50, respectively) of fenitrothion and the respective controls at each period of exposure (1, 3 and, 7 days) and recovery for similar intervals.
| Abnormalities | Dose (% of LC50) | Exposure period |
Recovery period | Recovery Rate (%) | Exposure period | Recovery period | Recovery Rate (%) | Exposure period | Recovery period | Recovery Rate (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 day | 1 day | 3 day | 3 day | 7 day | 7 day | |||||
| Tear-drop shaped | 10 | 0.30 ± 0.10ax | 2.30 ± 1.90a | 1.89 ± 0.41x | 17.82 | 3.97 ± 1.60b | 3.50 ± 0.38y | 11.83 | 2.29 ± 0.73a | 0.95 ± 0.18z | 58.51 |
| 20 | 5.29 ± 3.11a | 3.80 ± 0.60x | 28.16 | 1.52 ± 0.68b | 1.29 ± 0.23y | 15.13 | 2.38 ± 0.70c | 0.80 ± 0.16z | 60.08 | ||
| 40 | 7.45 ± 5.91a | 3.96 ± 1.02x | 46.84 | 5.71 ± 2.96b | 3.70 ± 0.75x | 35.20 | 2.77 ± 1.0 c | 1.52 ± 0.10y | 45.12 | ||
| Echinocytic | 10 | 0.30 ± 0.10ax | 0.80 ± 0.29a | 0.73 ± 0.39x | 8.75 | 2.78 ± 0.95b | 2.40 ± 0.34y | 13.66 | 2.31±.33c | 1.11 ± 0.09x | 51.94 |
| 20 | 1.20 ± 0.35a | 1.06 ± 0.44x | 11.66 | 2.09 ± 0.96b | 1.74 ± 0.75y | 16.74 | 1.63 ± 0.03a | 0.72 ± 0.07x | 55.82 | ||
| 40 | 1.44 ± 0.71a | 1.34 ± 0.70x | 6.94 | 2.51 ± 0.97b | 1.87 ± 0.30y | 25.49 | 2.60 ± 0.66b | 1.49 ± 0.04x | 42.69 | ||
| Elongated shaped | 10 | 0.20 ± 0.09ax | 1.03 ± 0.66a | 0.87 ± 0.16x | 15.53 | 1.34 ± 0.77a | 0.95 ± 0.37x | 29.10 | 1.53 ± 0.07b | 0.73 ± 0.49x | 52.28 |
| 20 | 3.03 ± 0.78a | 2.80 ± 0.11x | 7.59 | 1.20 ± 0.37b | 0.92 ± 0.25y | 23.33 | 0.87 ± 0.22c | 0.44 ± 0.03z | 49.42 | ||
| 40 | 0.47 ± 0.14a | 0.40 ± 0.15x | 14.89 | 1.96 ± 0.24b | 1.57 ± 0.24y | 19.89 | 0.48 ± 0.04a | 0.27 ± 0.11x | 43.75 | ||
| Fusion | 10 | 0.18 ± 0.07ax | 1.35 ± 0.37a | 1.16 ± 0.58x | 14.07 | 2.20 ± 0.93b | 1.75 ± 0.19y | 20.44 | 3.86 ± 1.46c | 1.55 ± 0.14xy | 59.84 |
| 20 | 3.50 ± 1.47a | 3.30 ± 1.07x | 5.71 | 1.92 ± 0.55b | 1.45 ± 0.96y | 24.47 | 2.62 ± 0.56c | 1.30 ± 0.12y | 50.38 | ||
| 40 | 2.90 ± 0.13a | 2.80 ± .87x | 3.44 | 1.62 ± 0.41b | 1.39 ± 0.63y | 14.19 | 1.90 ± 0.20b | 0.80 ± 0.12z | 57.89 | ||
| Demembranated | 10 | 0.23 ± 0.12ax | 2.02 ± 0.56a | 1.79 ± 0.12x | 11.38 | 1.57 ± 0.82b | 1.07 ± 0.10y | 31.84 | 1.78 ± 0.50ab | 0.77 ± 0.02z | 56.74 |
| 20 | 0.81 ± 0.14a | 0.75 ± 0.14x | 12.34 | 3.01 ± 1.37b | 2.43 ± 0.15y | 19.26 | 0.82 ± 0.02a | 0.42 ± 0.08x | 48.78 | ||
| 40 | 2.47 ± 0.83a | 2.30 ± 0.20x | 6.88 | 2.70 ± 0.76b | 1.97 ± 1.24y | 27.03 | 1.89 ± 0.79c | 0.95 ± 0.16z | 49.73 | ||
| Spindle shaped | 10 | 0.30 ± 0.14ax | 2.53 ± 0.56a | 2.24 ± 0.86x | 11.46 | 3.39 ± 0.78b | 2.39 ± 1.20x | 29.49 | 3.48 ± 0.14b | 1.46 ± 1.27y | 58.04 |
| 20 | 2.92 ± 1.54a | 2.41 ± 0.43x | 17.46 | 1.56 ± 0.63b | 1.10 ± 0.18y | 29.48 | 2.40 ± 0.71c | 0.99 ± 0.88y | 58.75 | ||
| 40 | 2.68 ± 2.06a | 2.32 ± 0.97x | 13.43 | 2.88 ± 0.66a | 1.95 ± 0.69y | 32.29 | 1.79 ± 0.69c | 0.90 ± 0.51z | 49.72 | ||
| Twin | 10 | 0.15 ± 0.06ax | 1.20 ± 0.24a | 1.00 ± 0.56x | 16.66 | 2.77 ± 0.41b | 2.07 ± 1.05y | 25.27 | 4.33 ± 1.84c | 1.99 ± 0.16z | 54.04 |
| 20 | 2.70 ± 0.81a | 2.35 ± 0.65x | 12.96 | 4.57 ± 2.83b | 3.51 ± 0.86y | 23.17 | 3.71 ± 0.72c | 1.82 ± 0.17z | 50.94 | ||
| 40 | 2.89 ± 1.25a | 2.50 ± 0.63x | 13.49 | 4.40 ± 1.57b | 3.20 ± 0.90y | 27.27 | 2.56 ± 0.50c | 1.60 ± .08z | 37.56 | ||
| Crescentic shaped | 10 | 0.03 ± 0.01ax | 1.30 ± 0.38a | 1.21 ± 0.64x | 6.92 | 2.82 ± 0.39b | 2.32 ± 0.89y | 17.73 | 1.23 ± 0.14a | 0.53 ± 0.43x | 56.91 |
| 20 | 1.10 ± 0.31a | 0.96 ± 0.54x | 12.72 | 2.20 ± 0.25b | 1.82 ± 0.36y | 17.27 | 0.95 ± 0.16a | 0.55 ± 0.02x | 42.10 | ||
| 40 | 1.50 ± 0.35a | 1.35 ± 0.67x | 10.00 | 1.80 ± 0.2b | 1.57 ± 0.91x | 12.77 | 0.23 ± 0.03a | 0.10 ± 0.01y | 56.52 | ||
Values with different alphabet superscripts in the same row differ significantly (p < 0.05) between exposure and recovery periods, respectively in ENA cells. All values are expressed as mean±SD.
Fig. 2.
Blood smears of zebrafish exposed to sub-lethal concentrations of fenitrothion showing several ECA like- (A) control (regular cells), (B) elongated (C) spindle-shaped, (D) tear-drop shaped, (E) fusion, (F) echinocytic cell, (G) demembranated, (H) twin-shaped and (I) crescentic shaped (Giemsa stain: 40X).
3.3. Recuperation responses of ENA induced by fenitrothion
The data of the recovery responses of individual ENA and all ENA are presented in Table 1, Table 3, respectively. The ENA content of the fish in the recovery period is low, and its value has a statistically significant change (p < 0.05). Almost all anomalous nuclei are normalized, and the recovery rate of the 10 % concentration of the fenitrothion shows a higher recovery rate than that of the fenitrothion concentration of 20 % and 40 %. The experimental results show that the recovery rate of micronucleus formation is the highest at 7 days at a concentration of 10 % (Table 1).
Table 3.
Frequency of all ENA in the blood of zebrafish after exposure and after recovery of fenitrothion.
| Dose (% of LC50) | Control | Exposure time (days) |
Recovery time (days) |
|||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 1 | 3 | 7 | |||
| Abnorm alities (%) | 10 | 0.57 ± 0.05ax | 0.70 ± 0.02a | 1.10 ± 0.09ab | 1.52 ± 0.10b | 0.62 ± 0.06x | 0.80 ± 0.07x | 0.88 ± 0.0x |
| 20 | 0.90 ± 0.03a | 1.40 ± 0.03b | 2.02 ± 0.07bc | 0.79 ± 0.09x | 1.12 ± 0.06xy | 1.37 ± 0.1y | ||
| 40 | 1.12 ± 0.06ab | 1.80 ± 0.10b | 2.92 ± 0.14c | 1.00 ± 0.08x | 1.52 ± 0.12y | 2.12 ± 0.2z | ||
Different superscripts of letters showed significant differences among exposure and recovered days (p < 0.05). Values are means±SD.
3.4. Recovery responses of ECA induced by fenitrothion
Table 2 shows the statistically significant differences in the recovery response of each ECA relative to the control group, and also shows the recovery responses of all ECAs between recovery days in Table 4. Recovery responses of ECA for the blood of zebrafish were duration and concentration-dependent. Increasing recovery time led to decreasing the frequency of ECA. All abnormal cells at a concentration of 10 % showed a higher recovery than fenitrothion at 20 % and 40 % (Table 2, Table 4).
Table 4.
Frequency of all ECA in the blood of zebrafish after exposure and after recovery of fenitrothion.
| Dose (% of LC50) | Control | Exposure time (days) |
Recovery time (days) |
|||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 1 | 3 | 7 | |||
| Abnorm alities (%) | 10 | 0.95 ± 0.09ax | 1.23 ± 0.02a | 1.82 ± 0.16a | 2.34 ± 0.13b | 1.04 ± 0.04x | 1.35 ± 0.09x | 1.30 ± 0.07x |
| 20 | 1.42 ± 0.06a | 2.19 ± 0.21b | 2.75 ± 0.13c | 1.22 ± 0.09x | 1.75 ± 0.08y | 1.70 ± 0.05y | ||
| 40 | 1.93 ± 0.12ab | 2.89 ± 0.20c | 4.35 ± 0.25d | 1.70 ± 0.13y | 2.42 ± 0.23z | 2.99 ± 0.12z | ||
Different superscripts of letters showed significant differences among exposure and recovered days (p < 0.05). Values are means±SD.
4. Discussion
The genotoxicity of fenitrothion on erythrocyte and its recuperation patterns in zebrafish was explicated in this study using nuclear abnormalities of erythrocyte (ENA) including micronuclei (MN) and cellular abnormalities of erythrocyte (ECA). The purpose of this study was to use zebrafish as a model organism to evaluate the applicability of these procedures and learn more about the prospective use of this fish as a biological monitor. The results showed that fenitrothion increased the frequency of ENA and ECA in zebrafish blood in a concentration and time-dependent manner. Blood is a reflection of pathophysiology and the frequency of erythrocyte aberrations (cellular and nuclear) is an indicator of the presence of pesticides in water, because erythrocytes have the ability to respond to certain environmental problems [26]. Different abnormalities of red blood cells are considered to be the leading biomarkers to evaluate the harmful effects associated with pollution and exhibit the relationship between chronic health effects and mutagenicity [20].
In this study, several erythrocyte nuclear abnormalities were perceived in zebrafish blood smears, including notched nuclei, binuclear, blebbed, nuclear bridges, nuclear buds, and micronuclei. The pattern of ENA frequency enhancement depends on the time and dose-dependent manner. Indistinguishable inspections were made by some other researchers [15,21]. In this study, the frequency of ENA reached its maximum after 7 days of exposure, and the rate of occurrence of each irregularity in all treatments were in the order: micronuclei > notched > nuclear bridge > blebbed > bi-nucleus > nuclear bud. The rate of occurrence of erythrocyte irregularities in a cell is depending on the kinetics of cell proliferation [27]. A sort of toxic substances, which influence chromosomal irregularities, can regulate to these nuclear malformations [28]. In addition, due to toxic effects, notched, concentration, fragmentation and erythrocyte with blebbed nucleus may also be linked to the collapse of tubulin polymerization [29,30]. The increased frequency of ENA indicates that there are genotoxic and mutagenic effects in this experiment, which may be owing to the expanded production of caspase-activated DNase, leading to the cleavage of nuclear and cytoskeletal proteins and aneuploidy [1].
Fish's MN proved to be a convenient technique for assessing genotoxic pollutants present in the aquatic environment and reported originating owing to either clastogenicity or aneugenicity (mitotic spindle dysfunction) during anaphase from lagging acentric chromosome or symmetrical and asymmetrical chromatid fragments caused by unrepaired DNA breaks or miss-repair of DNA breaks from exposure to a genotoxic contaminant [6,15,18,20,31]. In this study, authors speculated that, MN prevalence and severity increased after 7 days of fenitrothion exposure. Micronucleated red blood cells found in fish 1–5 days after exposure to pollutants [32]. Therefore, the maximum number of MNs observed after 7 days. It is envisaged that micronucleus provides indirect evidence for chromosomal instability, DNA damage and genotoxic events, thereby increasing the risk of fish development and degenerative diseases.
The study results of ENA in zebrafish treated to fenitrothion exhibited that binucleated cells and notched nuclei increased significantly at different exposure days, where the exposure concentration was high, and the highest value was observed at 7 days of exposure. Ventura et al. [29] suggested that mitotic fusions caused by the aneugenic effects of poisons can lead to the formation of binucleated cells and notched nuclei. On the first day of fenitrothion exposure, the appearance of nuclear buds can be almost controlled but with the increase of exposure days, the appearance of nuclear buds gradually increased. Perhaps, fenitrothion generated reactive free radicals in the fish’s body and Hussain et al. [1] said that the generated free radicals can react with DNA molecules in the interphase stage and form nuclear buds.
Moreover, in the current study, it can be seen that the highest recuperation array is exhibited during the 7-day recovery period, and the reduced MN formation may indicate the reconstruction of damaged cells, DNA, or both, as suggested by Banu et al. [33]. This reverse correlation between exposure time and DNA damage may be owing to the toxicity of xenobiotics. This biotin may distract the enzymatic process in the generation of DNA destruction [34]. An additional speculated mechanism may be the gene activation of metabolic enzymes (such as cytochrome P450) in diverse tissues, thus providing a defense mechanism against hazardous chemicals [35]. In isolated human lymphocytes and fish, similar repair mechanisms have been observed for pesticides such as malathion and monocrotophos [33,36]. In addition, other factors, including DNA repair efficiency and cell removal kinetics, can eliminate those damaged cells, which may be related to spontaneous MN and nuclear abnormalities frequency interspecies changes [20]. Moreover, increased erythropoiesis may dilute or obscure the amount of MN, and cell cycle and maturation time should be considered [32].
The assay also specifically evaluates the deformities of erythrocytes such as twin cells, tear-drop shaped cells, echinocytic cells, de-membranated cells, elongated cells, crescentic shaped cells and fusion cells that might be owing to concentration-dependent increase of lipid per-oxidation products in erythrocytes of fish treated to pesticides. It is generally accepted that many poisons with oxidative stress potential can attack DNA, leading to molecular, clastogenic and morphological injury [37]. Many toxic substances can affect the order of cell modification and cause hypoxia, which leads to the isolation of ATP, and various deformations of red blood cells proposed by Ateeq et al. [38]. Because the toxic substances destroy the membrane structure of red blood cells, cell metabolism, and iron permeability resulting in the atypical configuration of red blood cells [39]. Most of the RBCs transformed either elongated shaped, fusion shaped, spindle shaped, tear-drop shaped or twin shaped was studied by Arutjunov et al. [40] in mammals caused by exposures to occupational toxic agents.
In this study, the maximum commonness of elongated and tear-drop shaped cells was detected instantly after the first day of exposure, and then slowed down, and the highest recuperation array was seen on the 7th day of recovery. Das and Nanda [41] also reported a time-dependent decrease in the ECA in peripheral blood of stinging catfish. In contrast, spindle-shaped cells and twin-shaped cells were found to be the largest at 7 days of exposure, and the largest recovery response was found at the 7 day recovery period. The literature reveals that spindle-shaped and twin-shaped cells peaked between the 1st and 5th days of treatment [27,42].
In the results of this study, the number of echinocytic cells on day 7 was more than that on day 1 and day 3. Brecher and Bessis [43] reported that the appearance of echinocytic cells is due to the distortion of the membrane lipid microenvironment, which expands the lipid peroxidation, thereby improving the membrane porosity and fluidity. It takes some time to form holes in the plasma membrane. As the number of holes increased, abnormalities also increased [44].
Exposure to fenitrothion-free freshwater causes a concentration and duration-dependent decrease in almost all ENA and ECA of zebrafish. After exposure to pesticides, the nucleus and cell abnormalities of red blood cells gradually returned to normal over time and the measurement was recovered [6]. The recovery of erythrocyte after exposure indicates that fenitrothion entering the system cannot be concentrated in the body and is steadily abolished, leading to recuperation. Nearly all relevant observations have been described in previous studies conducted by [22], which involved the toxicity of carbofuran and cypermethrin on blood parameters and recuperation of a bony fish.
5. Conclusion
The results indicated that sub-lethal concentrations of fenitrothion induced genotoxicity with the especial reference to ECA as well as ENA, MN induction in the erythrocytes of zebrafish portraying a direct relationship with an increase in the pesticide concentration and exposure period. The ENA and ECA of the fish blood can be used as an early biomarker of toxicity of fenitrothion as well as ecological disruption. So far, little is known about the recuperation array or duration of time needed for the recuperation of erythrocytes in fish. The findings of this research indicate that the recovery rates of ENA and ECA are time and dose-dependent, as the parameters are gradually standardized after exposure to pesticide-free water. The information on decontamination helps to suggest remedial measures to deal with fish affected by poisons that are farmed near the area exposed to pesticides. However, more investigations are needed to better understand the mechanisms involved in recovery. The results of the study will help control the indiscriminate use of pesticides and suggest the development of sustainable technology to solve environmental problems.
Declaration of Competing Interest
The authors declare of no competing interests. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
This work was partially supported by a grant of Impact of Aquaculture Drugs and Chemicals on Aquatic Ecology and Productivity Project (IADCAEPP) provided by Bangladesh Fisheries Research Institute (BFRI), Mymensingh-2201, Bangladesh.
Edited by Dr. A.M Tsatsaka
References
- 1.Hussain R., Mahmood F., Khan A., Javed M.T., Rehan S., Mehdi T. Cellular and biochemical effects induced by atrazine on blood of male Japanese quail (Coturnix japonica) Pestic. Biochem. Physiol. 2014;103(1):38–42. doi: 10.1016/j.pestbp.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Witeska M., Sarnowski P., Ługowska K., Kowal E. The effects of cadmium and copper on embryonic and larval development of Ide Leuciscus idus L. Fish Physiol. Biochem. 2014;40(1):151–163. doi: 10.1007/s10695-013-9832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amoatey P., Baawain M.S. Effects of pollution on freshwater aquatic organisms. Water Environ. Res. 2019;9:1272–1287. doi: 10.1002/wer.1221. [DOI] [PubMed] [Google Scholar]
- 4.Saravanan M., Kumar K.P., Ramesh M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: cypriniformes) during acute and chronic sub-lethal exposure to lindane. Pestic. Biochem. Physiol. 2011;100(3):206–211. [Google Scholar]
- 5.Peter V.S., Babitha G.S., Bonga S.W., Peter M.S. Carbaryl exposure and recovery modify the interrenal and thyroidal activities and the mitochondria-rich cell function in the climbing perch Anabas testudineus Bloch. Aquat. Toxicol. 2013;126:306–313. doi: 10.1016/j.aquatox.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Islam M.S., Khan M.M., Moniruzzaman M., Mostakim G.M., Rahman M.K. Recuperation patterns in fish with reference to recovery of erythrocytes in Barbonymus gonionotus disordered by an organophosphate. Int. J. Environ. Sci. Technol. 2019;16(11):7535–7544. [Google Scholar]
- 7.Satyavardhan K. A comparative toxicity evaluation and behavioral observations of freshwater fishes to FenvalerateTM. Middle East J. Sci. Res. 2013;13(2):133–136. [Google Scholar]
- 8.Rani G.I., Kumaraguru A.K. Behavioural responses and acute toxicity of Clarias batrachus to synthetic pyrethroid insecticide, ƛ-cyhalothrin. J. Environ. Appl. Biores. 2014;2(1):19–24. [Google Scholar]
- 9.Mostakim G.M., Zahangir M.M., Mishu M.M., Rahman M.K., Sadiqul M.I. Alteration of blood parameters and histoarchitecture of liver and kidney of silver barb after chronic exposure to quinalphos. J. Toxicol. 2015 doi: 10.1155/2015/415984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sricharani R.B., Uthra S., Vadivel V.K., Savarimuthu P.A., Aristides M.T., Kirill S.G., Thiagarajan R. Effect of surfactant in mitigating cadmium oxide nanoparticle toxicity: implications for mitigating cadmium toxicity in environment. Environ. Res. 2017;152:141–149. doi: 10.1016/j.envres.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Elisavet A.R., Dimitris G.S., Athanasios A.A., Irina V.S., Aleksandra B., Vesna M., Maria T., Boris B.D., Pascal D., Maroudio K., Aristidis M.T. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study. Environ. Res. 2017;157:173–181. doi: 10.1016/j.envres.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Moniruzzaman M., Khan M.M., Rahman M.K., Sadiqul M.I. Effects of profenofos induced histopathology and recovery patterns in silver barb (Barbonymus gonionotus) Progress. Agric. 2017;28(3):240–248. [Google Scholar]
- 13.Bakshi A., Panigrahi A.K. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol. Rep. 2018;5:440–447. doi: 10.1016/j.toxrep.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naz S., Hussain R., Ullah Q., Chatha A.M.M., Shaheen A., Khan R.U. Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). 2020. Environ. Sci. Pollut. Res. Int. 2020 doi: 10.1007/s11356-020-10980-0. [DOI] [PubMed] [Google Scholar]
- 15.Sadiqul M.I., Ferdous Z., Nannu M.T.A., Mostakim G.M., Rahman M.K. Acute exposure to a quinalphos containing insecticide (convoy) causes genetic damage and nuclear changes in peripheral erythrocytes of silver barb, Barbonymus gonionotus. Environ. Pollut. 2016;219:949–956. doi: 10.1016/j.envpol.2016.09.066. [DOI] [PubMed] [Google Scholar]
- 16.Ray B.P., Baten M.A., Saha M.K. Effects of some selected pesticides on the mortality of tilapia fish (Oreochromis niloticus) J. Biological Chem. Res. 2012;29:189–205. [Google Scholar]
- 17.Thomson W.T. Thomson Publications; Fresno, California: 1989. Agricultural Chemicals. Book I: Insecticides; p. 120. [Google Scholar]
- 18.Khan M.M., Moniruzzaman M., Mostakim G.M., Sadequr M.R.K., Rahman M.K., Sadiqul M.I. Aberrations of the peripheral erythrocytes and its recovery patterns in a freshwater teleost, silver barb exposed to profenofos. Environ. Pollut. 2018;234:830–837. doi: 10.1016/j.envpol.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Zakia S., Mansura M.K., Golam M.M., Moniruzzaman M., Rahman M.K., Shahjahan M., Sadiqul M.I. Studying the effects of profenofos, an endocrine disruptor, on organogenesis of zebrafish. Environ. Sci. Pollut. Res. - Int. 2020 doi: 10.1007/s11356-020-11944-0. [DOI] [PubMed] [Google Scholar]
- 20.Bolognesi C., Hayashi M. Micronucleus assay in aquatic animals. Mutagenesis. 2011;26(1):205–213. doi: 10.1093/mutage/geq073. [DOI] [PubMed] [Google Scholar]
- 21.Ergene S., Çavaş T., Çelik A., Köleli N., Aymak C. Evaluation of river water genotoxicity using the piscine micronucleus test. Environ. Mol. Mutagen. 2007;48(6):421–429. doi: 10.1002/em.20291. [DOI] [PubMed] [Google Scholar]
- 22.Adhikari S., Sarkar B., Chatterjee A., Mahapatra C.T., Ayyappan S. Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton) Ecotoxicol. Environ. Saf. 2004;58(2):220–226. doi: 10.1016/j.ecoenv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Wu R.S., Siu W.H., Shin P.K. Induction, adaptation and recovery of biological responses: implications for environmental monitoring. Mar. Pollut. Bull. 2005;51(8–12):623–634. doi: 10.1016/j.marpolbul.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Du Y., Shi X., Liu C., Yu K., Zhou B. Chronic effects of water-borne PFOS exposure on growth, survival and hepatotoxicity in zebrafish: a partial life-cycle test. Chemosphere. 2009;74(5):723–729. doi: 10.1016/j.chemosphere.2008.09.075. [DOI] [PubMed] [Google Scholar]
- 25.Fenech M., Chang W.P., Kirsch-Volders M., Holland N., Bonassi S., Zeiger E. HUMAN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. Toxicol. Environ. Mutagen. 2003;534(1–2):65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 26.Sawhney A.K., Johal M.S. Erythrocyte alterations induced by malathion in Channa punctatus (Bloch) Bull. Environ. Contam. Toxicol. 2000;64(3):398–405. doi: 10.1007/s001280000014. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sabti K., Metcalfe C.D. Fish micronuclei for assessing genotoxicity in water. Mutat. Res. Toxicol. 1995;343(2–3):121–135. doi: 10.1016/0165-1218(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 28.Lim S., Ahn S.Y., Song I.C., Chung M.H., Jang H.C., Park K.S., Lee K.U., Pak Y.K., Lee H.K. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One. 2009;4(4) doi: 10.1371/journal.pone.0005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura B.D.C., de Angelis D.D.F., Marin-Morales M.A. Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pestic. Biochem. Physiol. 2008;90(1):42–51. [Google Scholar]
- 30.Campos-Pereira F.D., Oliveira C.A., Pigoso A.A., Silva-Zacarin E.C., Barbieri R., Spatti E.F., Marin-Morales M.A., Severi-Aguiar G.D. Early cytotoxic and genotoxic effects of atrazine on Wistar rat liver: a morphological, immunohistochemical, biochemical, and molecular study. Ecotoxicol. Environ. Saf. 2012;78:170–177. doi: 10.1016/j.ecoenv.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Fenech M., Kirsch-Volders M., Natarajan A.T., Surralles J., Crott J.W., Parry J., Norppa H., Eastmond D.A., Tucker J.D., Thomas P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26(1):125–132. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- 32.Udroiu I. The micronucleus test in piscine erythrocytes. Aquat. Toxicol. 2006;79(2):201–204. doi: 10.1016/j.aquatox.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Banu B.S., Danadevi K., Rahman M.F., Ahuja Y.R., Kaiser J. Genotoxic effect of monocrotophos to sentinel species using comet assay. Food Chem. Toxicol. 2001;39(4):361–366. doi: 10.1016/s0278-6915(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 34.Rank J., Jensen K. Comet assay on gill cells and hemocytes from the blue mussel. Mytilusedulis. Ecotoxicol. Environ. Saf. 2003;54(3):323–329. doi: 10.1016/s0147-6513(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 35.Wong C.K.C., Yeung H.Y., Woo M.H. Specific expression of cytochrome P450 (A) gene in gill, intestine and liver of tilapia exposed to coastal sediments. Aquat. Toxicol. 2001;54:69–80. doi: 10.1016/s0166-445x(00)00173-9. [DOI] [PubMed] [Google Scholar]
- 36.Blasiak J., Jaloszynski P., Trzeciak A., Szyfte K. In vitro studies on the genotoxicity of the organophosphorous insecticide malathion and its two analogues. Mutat. Res. 1999;445:275–283. doi: 10.1016/s1383-5718(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 37.Jha A.N. Ecotoxicological applications and significance of the comet assay. Mutagenesis. 2008;23(3):207–221. doi: 10.1093/mutage/gen014. [DOI] [PubMed] [Google Scholar]
- 38.Ateeq B., Ali M.N., Ahmad W. Induction of micronuclei and erythrocyte alterations in the catfish Clarias batrachus by 2, 4-dichlorophenoxyacetic acid and butachlor. Mutat. Res. Toxicol. Environ. Mutagen. 2002;518(2):135–144. doi: 10.1016/s1383-5718(02)00075-x. [DOI] [PubMed] [Google Scholar]
- 39.Nikinmaa M. How does environmental pollution affect red cell function in fish? Aquat. Toxicol. 1992;22(4):227–238. [Google Scholar]
- 40.Arutjunov V.D., Ju D.B., Gribova I.A., Kruglikov G.G. Scanning electron-microscopic and light-optic investigations of erythrocytes in toxic anaemia. Occup. Environ. Med. 1981;38(1):72–75. doi: 10.1136/oem.38.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das R.K., Nanda N.K. Induction of micronuclei in peripheral erythrocytes of fish Heteropneustes fossilis by mitomycin C and paper mill effluent. MutationResearch Letters. 1986;175(2):67–71. [Google Scholar]
- 42.Palhares D., Grisolia C.K. Comparison between the micronucleus frequencies of kidney and gill erythrocytes in tilapia fish, following mitomycin C treatment. Genet. Mol. Biol. 2002;25(3):281–284. [Google Scholar]
- 43.Brecher G., Bessis M. Present status of spiculed red cells and their relationship to the discocyte-echinocyte transformation: a critical review. Blood. 1972;40(3):333–344. [PubMed] [Google Scholar]
- 44.Nepomuceno J.C., Ferrari Í, Spanó M.A., Centeno A.J. Detection of micronuclei in peripheral erythrocytes of Cyprinus carpio exposed to metallic mercury. Environ. Mol. Mutagen. 1997;30(3):293–297. doi: 10.1002/(sici)1098-2280(1997)30:3<293::aid-em7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]