In a very recent issue of Cell, Ghosh et al. describe how β-coronaviruses utilize deacidified lysosomes to egress from the infected cells and impair antigen presentation process1. This deacidified lysosome-mediated trafficking can be blocked by the Rab7 GTPase competitive inhibitor CID1067700 and alleviates β-coronavirus spread1.
SARS-Cov-2, one member of β-coronaviruses, brokes out all over the world in late 20192. Although remdesivir, hydroxychloroquine, and other targeted drugs were tested in SARS-Cov-2 treatment, until now, there is no fully effective cure and vaccine3–6. What gives us a glimmer of hope is: not all people exposed to SARS-CoV-2 were infected and not all infected patients developed severe respiratory diseases7,8. This shows that the way the virus enters, replicates, and exits vary from person to person. A series of studies on SARS-Cov-2 have been reported, and the details of β-coronavirus entry and replication in the host cells have been understood, however, how newly assembled viruses egress from the host cells is still unclear. Now, Ghosh et al. made new and important contributions to help understand this unconventional egress process by describing that β-coronaviruses traffic to lysosomes and egress by Arl8b-dependent lysosomal exocytosis1. This non-lytic release happens with the lysosome deacidification, limited lysosomal degradation enzyme activation, and impaired antigen presentation1. This deacidified lysosome-dependent exocytosis may be the reason why it is difficult for the host to generate an efficient antivirus immune response and antibody production.
The interface between β-coronaviruses and host cells largely determines the infection and spread of the viruses. The egress pathway of β-coronaviruses has been assumed as a biosynthetic secretory vesicle-dependent plasma membrane trafficking manner9. The newly synthesized genomic RNA of β-coronaviruses is coated by virus N proteins and subsequently budding into ER–Golgi for the next process of egress trafficking. Nevertheless, Ghosh et al. verified the above hypothesis and found that is not the case. Brefeldin A (BFA), a small molecule for shutting down all anterograde biosynthetic secretory traffic from the ER/ERGIC out to the plasma membrane, has no effect on the egress of β-coronaviruses1. In addition, β-coronaviruses are enriched in the late endosomes and lysosomes during egress, and Rab7 inhibitor significantly inhibits β-coronavirus egress through interrupting biogenesis and maintenance of lysosomes. Therefore, β-coronavirus egress is dependent on a lysosomal manner, but not the previously assumed biosynthetic secretory pathway.
When a large number of β-coronaviruses are generated in the cells, the new viruses actively invade the lysosomes, or the lysosomes swallow the new viruses. Ghosh et al. found that plasma membrane LAMP1 levels have ~2.5-fold increase in the infected cells, indicating obvious fusion of lysosomes with the plasma membrane1. In addition, about two-fold increased cathepsin D and three-fold increased pro-cathepsin D secreted to the extracellular media of the infected cells, and the further research confirmed that lysosomes are deacidified and lysosomal enzymes are inactive in β-coronavirus-infected cells1. Given that acidified lysosomes are important for protease processing and antigen presentation of antigen-presenting cells (APCs), the β-coronavirus infection must be bound to affect the host’s immune response. This coincides with the clinical phenomenon that susceptible COVID-19 patients cannot utilize the immune system to effectively eliminate the SARS-Cov-2. Instead, excessive activation of non-antiviral immune cells can even cause autoimmune diseases10.
Coronaviruses have been detected in lysosomes at the late stages of infection for decades11. Ghosh et al. pioneered the study of the central role of lysosomes in the egress of β-coronaviruses, and CID1067700 limits egress of β-coronaviruses by reducing lysosomal number and inhibiting the maturation of endolysosomes. Although β-coronavirus egress depending on a lysosomal manner has been verified, it is still uncertain whether the lysosomal deacidification of cells is caused by a β-coronavirus infection, or whether the infected cells actively deacidify lysosomes to release newly generated β-coronaviruses for relieving their own viral pressure (Fig. 1). Some researchers think that lysosomal deacidification may be a consequence of the action of specific coronavirus proteins, and some researchers believe that lysosomal deacidification indirectly caused by too much cargo loading and/or the disturbed proton pump or ion channel trafficking12,13.
Fig. 1. Actively or passively deacidified lysosomes push β-coronavirus egress.
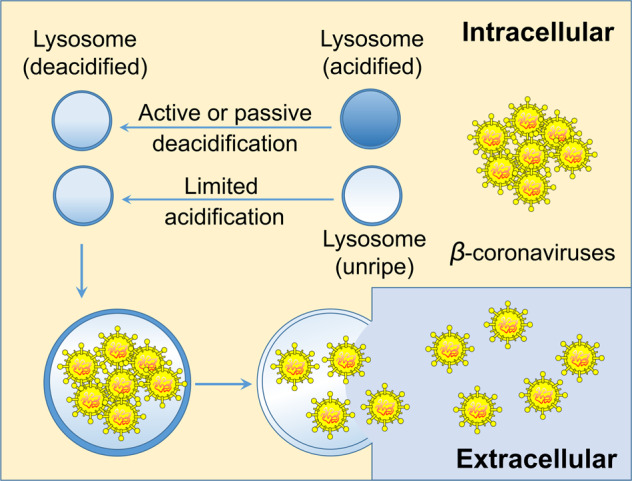
Lysosomal deacidification may be caused by β-coronavirus infection, or the infected cells actively deacidify lysosomes to release newly generated β-coronaviruses for relieving their own viral burden.
Recently, some studies have shown that lysosomal deacidification may be an active process of cells, for instance, insulin-like growth factor 2 receptor (IGF2R) activation promotes lysosomal deacidification-dependent proton rechanneling in maturing monocytes and dictated their oxidative phosphorylation metabolic inclination and anti-inflammatory potential14. Some interesting questions, provided that lysosomal deacidification is also an active process in cells infected by β-coronaviruses, what is the exact mechanism? Is it possible to artificially induce lysosome reacidification? Does re-acidifying of lysosomes limits β-coronavirus egress? As β-coronaviruses disrupted lysosome-dependent antigen cross-presentation pathways in the infected cells1. Simply inhibiting the lysosomal number and endolysosomal stability by Rab7-targeting inhibitors can only decrease β-coronavirus egress, however, the limited antigen presentation ability caused by impaired lysosomal acidification still cannot be restored. Therefore, it is necessary to explore the mechanism of lysosome deacidification and try to re-acidify lysosomes for inhibiting virus egress and restoring antigen presentation function of APCs. In addition, the identification of individual or clusters of predictive genetic alterations has been used for cancer predication15, therefore, examining the expression abundance of genes related to coronavirus emigration will be of great significance for distinguishing susceptible populations and formulating personalized prevention methods of COVID-19.
In summary, the β-coronavirus egress pathway involving lysosomal deacidification, impaired lysosomal degradation enzyme activation, and limited antigen presentation. This work highlight that targeting regulators of lysosomal trafficking and biogenesis, such as Arl8b and Rab7, may be the potential strategies to alleviate β-coronavirus infection and spread. More should be done in the future are to understand the mechanism of active and passive lysosome deacidification in β-coronavirus infected cells, to verify the effects of lysosomal reacidification on β-coronavirus egress and antigen presentation in APCs, and to extend the therapeutic potential of this discovery in the clinical treatment of β-coronavirus infection.
Acknowledgements
We thank Dr. Bin Lan for the initial discussions.
Author contributions
X.W., G.M., and Y.S. wrote the paper. X.W. designed and drawn the picture in Fig. 1.
Funding
This work was supported by grants from the National Key R&D Program of China (2018YFA0107500), National Natural Science Foundation of China (32000550, 31961133024, 81930085), Chinese Postdoctoral Science Foundation (2020 M671573), Open Project Funding from Key Laboratory of Tumor and Microenvironment of Chinese Academy of Sciences (no. 202004), Ministry of Health Italy-China cooperation grant and AIRC IG#20473 to G.M., a start-up fund from Soochow University, and Department of Science and Technology of Jiangsu Province research fund (BE2016671).
Conflict of interest
The authors declare no competing interests.
Ethics statement
No ethics approvals were required for this paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghosh, S. et al. Beta-coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell183, 1520–1535 (2020). [DOI] [PMC free article] [PubMed]
- 2.Chen J, et al. COVID-19 infection: the China and Italy perspectives. Cell Death Dis. 2020;11:438. doi: 10.1038/s41419-020-2603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11:512. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, et al. BCG vaccination policy and preventive chloroquine usage: do they have an impact on COVID-19 pandemic? Cell Death Dis. 2020;11:516. doi: 10.1038/s41419-020-2720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celardo I, Pace L, Cifaldi L, Gaudio C, Barnaba V. The immune system view of the coronavirus SARS-CoV-2. Biol. Direct. 2020;15:30. doi: 10.1186/s13062-020-00283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020;27:3209–3225. doi: 10.1038/s41418-020-00633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrati C, et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27:3196–3207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machamer CE. Accommodation of large cargo within Golgi cisternae. Histochemistry Cell Biol. 2013;140:261–269. doi: 10.1007/s00418-013-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verkhratsky A, Li Q, Melino S, Melino G, Shi Y. Can COVID-19 pandemic boost the epidemic of neurodegenerative diseases? Biol. Direct. 2020;15:28. doi: 10.1186/s13062-020-00282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducatelle R, Hoorens J. Significance of lysosomes in the morphogenesis of coronaviruses. Arch. Virol. 1984;79:1–12. doi: 10.1007/BF01314299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westerbeck JW, Machamer CE. The infectious bronchitis coronavirus envelope protein alters Golgi pH to protect the spike protein and promote the release of infectious virus. J. Virol. 2019;93:11. doi: 10.1128/JVI.00015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballabio A, Bonifacino JS. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020;21:101–118. doi: 10.1038/s41580-019-0185-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, et al. IGF2R-initiated proton rechanneling dictates an anti-inflammatory property in macrophages. Sci. Adv. 2020;6:48. doi: 10.1126/sciadv.abb7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amelio I, et al. Cancer predictive studies. Biol. Direct. 2020;15:18. doi: 10.1186/s13062-020-00274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


