Abstract
AIM
To investigate the effect of leucine-rich-alpha-2-glycoprotein 1 (LRG1) on epithelial-mesenchymal transition (EMT) in retinal pigment epithelium (RPE) cells, and to explore the role of NADPH oxidase 4 (NOX4).
METHODS
RPE cells (ARPE-19 cell line) were treated with transforming growth factor-β1 (TGF-β1) to induce EMT. Changes of the mRNA and protein expression levels of LRG1 were tested in the TGF-β1 treated cells. The recombinant human LRG1 protein (rLRG1) and siRNA of LRG1 were used to establish accumulation of exogenous LRG1 model and the down-regulation of LRG1 model in ARPE-19 cells respectively, and to detect EMT-related markers including fibronectin, α-smooth muscle actin (α-SMA) and zonula occludens-1 (ZO-1). The mRNA and protein expression level of NOX4 were measured according to the above treatments. VAS2870 was used as a NOX4 inhibitor in rLRG1-treated cells. EMT-related markers were detected to verify the effect of NOX4 in the process of EMT.
RESULTS
TGF-β1 promoted the expression of LRG1 at both the mRNA and protein levels during the process of EMT which showed the up-regulation of fibronectin and α-SMA, as well as the down-regulation of ZO-1. Furthermore, the rLRG1 promoted EMT of ARPE-19 cells, which manifested high levels of fibronectin and α-SMA and low level of ZO-1, whereas knockdown of LRG1 prevented EMT by decreasing the expressions of fibronectin and α-SMA and increasing the expression of ZO-1 in ARPE-19 cells. Besides, the rLRG1 activated and LRG1 siRNA suppressed NOX4 expression. EMT was inhibited when VAS2870 was used in the rLRG1-treated cells.
CONCLUSION
These results for the first time demonstrate that LRG1 promotes EMT of RPE cells by activating NOX4, which may provide a novel direction to explore the mechanisms of subretinal fibrosis.
Keywords: leucine-rich-alpha-2-glycoprotein 1, epithelial-mesenchymal transition, NADPH oxidase 4, retinal pigment epithelium cells, subretinal fibrosis
INTRODUCTION
Currently, intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents is the first-line treatment of neovascular age-related macular degeneration (nAMD), which is characterized by the formation of choroidal neovascularization (CNV)[1]–[4]. However, clinical trials indicated that the emergence of subretinal fibrosis which is featured by the formation of fibrous membrane of CNV was deemed to be the prominent unfavorable effect of anti-VEGF therapy[5]–[7]. Although subretinal fibrosis is regarded as end-stage of the natural course of CNV due to the excessive wound healing response[8]–[11], anti-VEGF therapy was supposed to accelerate this process. The over-rapid progression of subretinal fibrosis not only give rise to the loss of previously gained visual acuity, but also lead to the retinal detachment on account of the traction of fibrous membrane[7]. Given the concrete mechanisms of subretinal fibrosis remain unclear at present, exploring possible mechanisms becomes particularly important.
In the fibrous membrane of CNV, myofibroblasts are the principal cellular constituents, which play significant roles in the process of subretinal fibrosis. Previous studies have shown that myofibroblasts can be derived from retinal pigment epithelium (RPE) cells[8]–[9],[12]. When RPE cells are stimulated under pathological conditions, they undergo the process of epithelial-mesenchymal transition (EMT) which leads to the trans-differentiation from epithelial cell phenotype to mesenchymal cell phenotype[9]. Thus, RPE cells participate in the forming process of subretinal fibrosis. A human retinal pigment epithelial cell line (ARPE-19) becomes an alternative to native RPE cells not only because they have the similar gene expression patterns[13], but also because ARPE-19 cells have the advantages of adequate sources and convenient use. In especial, in the aspect of researching EMT, ARPE-19 cells have become accepted widely as a reliable tool in numerous researches[14]–[17]. Given the above, ARPE-19 cells are used in this study to conduct EMT-related experiments and to explore the potential mechanisms of subretinal fibrosis.
Leucine-rich-alpha-2-glycoprotein1 (LRG1), a kind of secreted glycoprotein, was isolated and identified from human serum by Haupt and Baudner[18] in 1977 for the first time. It is a member of leucine-rich repeat family, which includes eight repeating consensus sequences[19]–[20]. Previous researches have indicated that LRG1 participates in various pathological process, such as promoting fibrosis, facilitating angiogenesis, accelerating cell proliferation, inhibiting cell apoptosis and so forth[19]–[23]. Although the pro-EMT function of LRG1 has been confirmed in some non-ocular cells[24]–[25], no study verifies whether LRG1 has an ability to promote EMT of ARPE-19 cells so far. In addition, many investigations have verified that NADPH oxidase 4 (NOX4) participate in the onset of EMT in diverse cells, especially in ocular cells[26]–[29]. For instance, NOX4 plays a vital role in transforming growth factor-β1 (TGF-β1)-induced lens EMT, and the inhibition of NOX4 may impede the process of EMT. Similarly, our preliminary experiment data (not yet published) also showed that NOX4 participated in the process of EMT when ARPE-19 cells were treated with bevacizumab, an anti-VEGF agent. These discoveries indicate that NOX4 as a valid target of promoting EMT is worth to be investigated as well. Therefore, in this research, we investigated the role of LRG1 in the process of EMT in ARPE-19 cells, and explored the function of NOX4 in the above condition in vitro.
MATERIALS AND METHODS
Cell Culture and Treatment
ARPE-19 cells, which were obtained from American Type Culture Collection (Manassas, VA, USA), were cultured in DMEM/F-12 medium (Invitrogen, Carlsbad, CA, USA) which contained 10% fetal bovine serum (FBS; Gibco, Life Technologies, Carlsbad, CA, USA), 100 U/mL penicillin and 100 µg/mL streptomycin (Sigma-Aldrich, St Louis, MO, USA) at 37°C with 5% CO2 humidified atmosphere. The original medium was replaced by DMEM/F-12 containing 2% FBS until cells reached 60%-70% confluence. In the meantime, the cells were treated differently on the basis of different purposes. The specific grouping situations were described as follows: 1) To detect the expression level of LRG1 in EMT model, ARPE-19 cells were classified into two groups. The first group was called blank control group, i.e. the cells were cultured in regular medium. In the second group, TGF-β1 (10 ng/mL; R&D Systems, Minneapolis, MN, USA) was added into the medium and kept for 72h to induce EMT of ARPE-19 cells. 2) To clarify the impact of over-accumulation of LRG1 on EMT in ARPE-19 cells, the recombinant human LRG1 protein (rLRG1; 100 ng/mL, R&D Systems) was used in this section. The cells were split into two groups according to whether or not adding rLRG1 in the medium and were cultured for 72h for both. The first group was regarded as the blank control group, and the second group was the rLRG1 treatment group. 3) To investigate the effect of knockdown of LRG1 on EMT in ARPE-19 cells, the siRNA of LRG1 was used to treat cells. The first group was the negative control group, i.e. the cells were transfected by siRNA sequence of negative control in regular medium. In the second group, the transfection of LRG1 siRNA was applied to cell treatment. The cells were transfected using siRNA transfection reagent (Santa Cruz Biotechnology, Dallas, Texas, USA) basing on the manufacturer's instruction. The sequence of siRNA for the negative control was depicted: sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense 5′-ACGUGACACGUUCGGAGAATT-3′. The sequence of siRNA for LRG1 was described as follows: sense 5′-GCUGGACCUCUCCAAUAACTT-3′ and antisense 5′-GUUAUUGGAGAGGUCCAGCTT-3′. All the sequences of siRNA were obtained from GenePharma (Shanghai, China). 4) To confirm the effect of NOX4 in rLRG1-treated ARPE-19 cells, VAS2870 (20 µmol/L; Absin Bioscience Inc, Shanghai, China), a NOX4 inhibitor, was added into the medium for 72h with or without rLRG1. The first group was blank control group, the second group was rLRG1 treatment group, and the third group was rLRG1 and VAS2870 double treatment group.
Reverse Transcription-Quantitative Polymerase Chain Reaction to Examine EMT-related Markers, LRG1 and NOX4
After the processes of cell treatment were completed in different groups, total RNA was isolated from ARPE-19 cells using TRIzol reagent (Invitrogen) and then was reversed transcribed into cDNA using reverse reagent (Vazyme, Nanjing, China). The amplifications of the target genes were conducted by a Rotor-Gene 6000 system (Qiagen, Hilden, Germany) using a SYBR qPCR Master Mix (Vazyme). The primer sequences were used as follows: fibronectin forward, 5′-GGGACCGTCAGGGAGAAAA-3′ and reverse, 5′-CGAGATATTCCTTCTGCCACTGTT-3′; α-smooth muscle actin (α-SMA) forward, 5′-GGTGACGAAGCACAGAGCAA-3′ and reverse, 5′-CAGGGTGGGATGCTCTTCAG-3′; zonula occludens-1 (ZO-1) forward, 5′-AGGATCCATATCCCGAGGAAA-3′ and reverse, 5′-CGAGGTCTCTGCTGGCTTGT-3′; LRG1, forward 5′-GGGAGAACCAGTTGGAGACCTT-3′ and reverse 5′-CCAGCTTGTTGCCGTTCAG-3′; NOX4, forward 5′-CAACTGTTCCTGGCCTGACA-3′ and reverse 5′-GCAACGTCAGCAGCATGTAGA-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′-CATGTTCGTCATGGGTGTGAA-3′ and reverse 5′-GGCATGGACTGTGGTCATGAG-3′. The expression levels of different genes were normalized by GAPDH mRNA, and then the results were presented using the fold change manner.
Western Blot Analysis to Detect EMT-related Markers, LRG1 and NOX4
After reaching the treatment time of diverse groups, ARPE-19 cells were harvested and the total protein lysates were obtained using RIPA lysis buffer which contained PMSF. The 4× SDS sample buffer was used in protein samples in order to cause protein denaturation at 95 degrees. The total proteins were separated by SDS-polyacrylamide gel electrophoresis and shifted onto polyvinylidene difluoride (PVDF) membranes. Next, immune responses were conducted after PVDF membranes were blocked with 5% skim milk for 1h at room temperature. According to different molecular weight, PVDF membranes were incubated with primary antibodies of fibronectin, α-SMA, ZO-1, LRG1, NOX4 (1:2000; Abcam, Cambridge, UK) and GAPDH (1:10 000, Abcam) overnight at 4°C. Subsequently, the HRP-conjugated secondary antibodies (1:4000; Absin Bioscience Inc) were used to combine with primary antibodies for 1h at ambient temperature in the following day. The immune complexes were expose using a ChemiDocTM Touch system (Bio-Rad, Shanghai, China) and analyzed by Image Lab software (Bio-Rad). The targeted protein expressions were normalized to GAPDH.
Statistical Analysis
The experimental results were repeated for three times. All the quantitative data were analyzed with SPSS 22.0 software. The data were indicated as means±standard deviations. Independent sample t-test was used to compare two groups. To make comparison between multiple groups, one-way analysis of variance was used to analyze data. A P-value<0.05 was deemed to be statistically significant.
RESULTS
LRG1 Expression was Increased in TGF-β1-induced EMT of ARPE-19 Cells
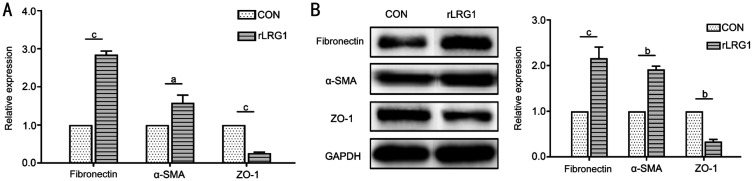
The establishment of model about EMT in ARPE-19 cells depended on the treatment of TGF-β1 for 72h. TGF-β1 up-regulated the expressions of fibronectin and α-SMA and down-regulated the expression of ZO-1 at the mRNA (Figure 1A) and protein levels (Figure 1B). In addition, the expression level of LRG1 was also increased. In the TGF-β1 treatment group, the mRNA expression levels of fibronectin and α-SMA were increased by 0.82- (P<0.001) and 2.48-folds (P<0.001) respectively when compared with untreated cells. Meanwhile, ZO-1 mRNA expression level was decreased by 85% (P<0.001). Western blot analysis showed that the protein expressions of fibronectin (P=0.005) and α-SMA (P=0.002) nearly doubled in the TGF-β1 treatment group, while ZO-1 decreased approximately 52% (P<0.001). In the meantime, the reverse transcription quantitative PCR and Western blot manifested the higher level of LRG1 during the process of EMT caused by TGF-β1. When the cells were treated with TGF-β1, the mRNA (Figure 1A) and protein (Figure 1B) expressions of LRG1 were increased by 1.03- (P=0.008) and 0.86-folds (P=0.005) respectively by comparison with the blank control group. Therefore, these results indicated that the expression level of LRG1 was increased in TGF-β1-induced EMT of ARPE-19 cells. This phenomenon implied that LRG1 might participate in the process of EMT in ARPE-19 cells.
Figure 1. The high expression level of LRG1 was detected in TGF-β1-induced EMT of ARPE-19 cells.
A: TGF-β1 promoted the expression of LRG1 at the mRNA level during the process of EMT which showed the up-regulation of fibronectin and α-SMA, as well as the down-regulation of ZO-1; B: TGF-β1 promoted the protein expression of LRG1 during the process of EMT in ARPE-19 cells. bP<0.01; cP<0.001.
The rLRG1 Promoted EMT of ARPE-19 Cells
Both reverse transcription quantitative PCR (Figure 2A) and Western blot analysis (Figure 2B) exhibited the similar variation tendency of EMT-related markers when using rLRG1 in ARPE-19 cells. In the rLRG1 treatment group, the mRNA expressions of fibronectin and α-SMA were increased by 1.84- (P<0.001) and 0.59-folds (P=0.036) respectively, meanwhile the ZO-1 mRNA expression level was reduced by about 74% (P<0.001) in comparison with the blank control group. Similarly, the protein expression levels of fibronectin and α-SMA were increased by 1.16- (P<0.001) and 0.92-folds (P=0.002) respectively, meanwhile the protein expression of ZO-1 was decreased by 66% (P=0.002) in rLRG1-treated cells.
Figure 2. The rLRG1 promoted EMT of ARPE-19 cells.
A: The mRNA expression levels of fibronectin and α-SMA were increased, and the expression of ZO-1 was decreased when ARPE-19 cells were treated with rLRG1; B: The protein expressions of fibronectin and α-SMA were up-regulated, and the expression of ZO-1 was down-regulated when using rLRG1 in ARPE-19 cells. aP<0.05; bP<0.01; cP<0.001.
Knockdown of LRG1 Prevented EMT of ARPE-19 Cells
Reverse transcription quantitative PCR (Figure 3A) and Western blot analysis (Figure 3B) indicated that the expressions of EMT-related markers showed obvious changes due to the knockdown of LRG1 in ARPE-19 cells. When compared with the negative control group, the process of EMT was suppressed by siRNA of LRG1. By reverse transcription quantitative PCR, the mRNA comparative expressions of fibronectin and α-SMA were decreased by 87% (P=0.003) and 80% (P<0.001) respectively. Meanwhile, the ZO-1 mRNA expression was increased by 0.49-fold (P=0.007). By Western blot analysis, the protein comparative expressions of fibronectin and α-SMA were reduced by 82% (P=0.002) and 64% (P<0.001) and the ZO-1 expression was increased by 0.93-fold (P=0.003) in the siRNA of LRG1 group.
Figure 3. Knockdown of LRG1 prevented EMT of ARPE-19 cells.
A: By reverse transcription quantitative PCR, the expressions of fibronectin and α-SMA were reduced, meanwhile the mRNA expression level of ZO-1 was increased when ARPE-19 cells were treated with siRNA of LRG1. B: By Western blot analysis, when using siRNA of LRG1 in ARPE-19 cells, the protein expressions of fibronectin and α-SMA were down-regulated, and the ZO-1 expression was up-regulated. bP<0.01; cP<0.001.
The rLRG1 Activated and LRG1 siRNA Inhibited the Expression of NOX4 in ARPE-19 Cells
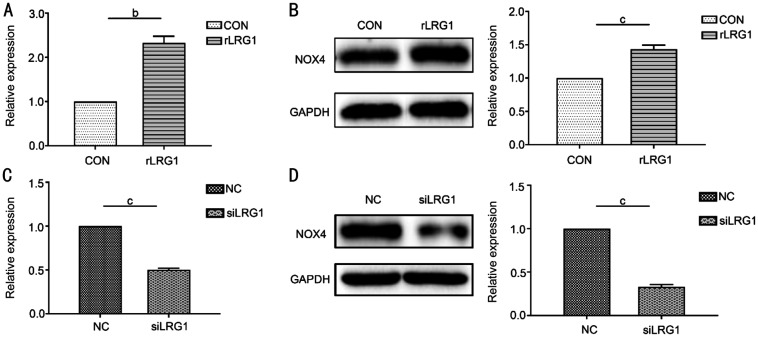
The comparative expression level of NOX4 depended on the use of rLRG1 and LRG1 siRNA in ARPE-19 cells. When the cells were treated with rLRG1, the mRNA (Figure 4A) and protein (Figure 4B) expressions of NOX4 were increased by 1.33- (P=0.004) and 0.43-folds (P<0.001) respectively when compared with the black control group. When using siRNA of LRG1, the mRNA (Figure 4C) and the protein expression (Figure 4D) of NOX4 were reduced by 50% (P<0.001) and 67% (P<0.001) respectively by comparison with the negative control group.
Figure 4. The rLRG1 activated and LRG1 siRNA suppressed the expression of NOX4 in ARPE-19 cells.
A: The mRNA expression of NOX4 was increased when ARPE-19 cells were treated with rLRG1; B: By western blot, the expression of NOX4 was increased when the cells received rLRG1 treatment; C: When using siRNA of LRG1, the expression of NOX4 was reduced at mRNA level; D: The protein expression of NOX4 was decreased when the cells were treated with siRNA of LRG1. bP<0.01; cP<0.001.
NOX4 Inhibition Suppressed rLRG1-induced EMT of ARPE-19 Cells
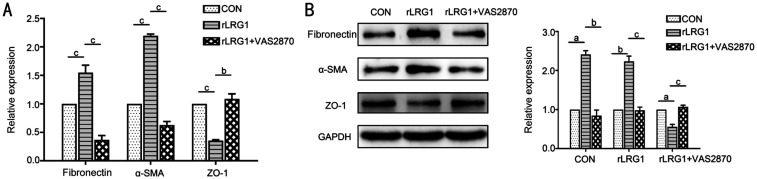
The phenomenon of rLRG1-induced EMT was reversed by VAS2870, a NOX4 inhibitor. In the rLRG1 and VAS2870 double treatment group, the mRNA expressions of fibronectin and α-SMA were decreased by 76% (P<0.001) and 71% (P<0.001) respectively, meanwhile the ZO-1 mRNA expression level was approximately 3.11-fold (P=0.008) compared with the rLRG1 treatment group (Figure 5A). Besides, Western blot analysis exhibited the similar variation tendencies of EMT-related markers (Figure 5B). The protein expressions of fibronectin and α-SMA were decreased by 65% (P=0.009) and 56% (P<0.001) respectively when the cells were treated with rLRG1 and VAS2870. Meanwhile, the protein expression of ZO-1 was about 1.91-fold (P<0.001) compared with the rLRG1 treatment group.
Figure 5. NOX4 inhibition attenuated rLRG1-induced EMT in ARPE-19 cells.
A: In the rLRG1 and VAS2870 treatment group, the expressions of fibronectin and α-SMA were decreased, and the expression of ZO-1 was increased at the mRNA level when compared with the rLRG1 treatment group; B: By Western blot, in the rLRG1 and VAS2870 treatment group, the protein expressions of fibronectin and α-SMA were lower than rLRG1 treatment group, whereas the ZO-1 expression was higher than rLRG1 treatment group. aP<0.05; bP<0.01; cP<0.001.
DISCUSSION
Intravitreal injection of anti-VEGF drugs has become the first-line therapeutic method of some neovascular fundus diseases including nAMD[1]–[4]. However, the enhanced subretinal fibrosis after anti-VEGF therapy affects the prognosis of patients seriously[5]–[7]. There is no consistent conclusion about the mechanism of subretinal fibrosis at present. In this research, we discussed the effect of LRG1 on TGF-β1-induced EMT of ARPE-19 cells in vitro and explored the possible relationship between LRG1 and NOX4. We found that LRG1 was capable of facilitating EMT in ARPE-19 cells, which might be accomplished by the activation of NOX4.
Previous studies have already confirmed that TGF-β1, as a classical pro-EMT factor, can induce EMT of RPE cells[30]–[31]. Moreover, TGF-β1-induced EMT model is generally used to investigate EMT-related mechanisms of ARPE-19 cells on account of the effectiveness of TGF-β1 in promoting EMT[32]–[33]. In this study, we also showed that the valid model of EMT in ARPE-19 cells was established when using TGF-β1 treatment, which exhibited high expressions of fibronectin and α-SMA as well as low expression of ZO-1 at both mRNA and protein level. Meanwhile, the mRNA and protein expression levels of LRG1 were increased during the process of EMT by reverse transcription quantitative PCR as well as Western blot analysis. Therefore, these results indicated that LRG1 might participate in the process of EMT of ARPE-19 cells.
To further elucidate the role of LRG1 in the course of EMT of ARPE-19 cells, rLRG1 and LRG1 siRNA were used to increase the exogenous LRG1 and to down-regulate the expression of LRG1, respectively. Our results showed that rLRG1 could obviously promote EMT by up-regulating fibronectin and α-SMA and down-regulating ZO-1, whereas the knockdown of LRG1 prevented EMT in ARPE-19 cells, which showed low expressions of above mesenchymal markers and high expression of ZO-1 at both mRNA and protein levels. The role of LRG1 in EMT of ocular cells is rarely reported at present and its EMT-related effects on other cells remains controversial. Zhang et al[24] reported that LRG1 could facilitate the progress of EMT in colorectal cancer cells via activating HIF-1α. Another study found that LRG1 could accelerate the migration ability of thyroid carcinoma cells via promoting EMT through promoting MAPK/p38 signaling[25]. On the contrary, some studies reported LRG1 might negatively modulate the process of EMT[34]–[35]. Therefore, the effect of LRG1 deserves to be further lucubrated in ocular cells, especially in RPE cells, which will be conducive to the investigation about subretinal fibrosis.
Although our study revealed the pro-EMT function of LRG1, its concrete mechanisms also should be revealed. In our preliminary experiments, the expression level of NOX4 increased obviously when ARPE-19 cells were treated with bevacizumab, an anti-VEGF agent, which led to EMT simultaneously, whereas the expressions of NOX1, NOX2 and NOX3 showed no obvious changes in comparison with black control group. Hence, NOX4 might exhibit a crucial role during the process of EMT after anti-VEGF treatment in ARPE-19 cells, and it is worthy of further investigation in EMT process. Although there are no relevant researches about the relationship between NOX4 and LRG1 at present, this study testified for the first time the upstream and downstream relationship between them. In our study, the results demonstrated that the expression level of NOX4 was increased due to the supplement of rLRG1, while the specific siRNA of LRG1 reduced the expression of NOX4 in ARPE-19 cells. Therefore, these results manifested that NOX4 might be in the downstream of LRG1 and be regulated by LRG1. Next, in order to verify the function of NOX4 during the process of EMT in ARPE-19 cells, VAS2870, a NOX4 inhibitor, was used with or without rLRG1. Our results indicated that the rLRG1-induced EMT of ARPE-19 cells was suppressed by VAS2870 treatment, which meant that NOX4 might facilitate the EMT of ARPE-19 cells. These results of our study were in accordance with Yang et al's[36] findings which also indicated VAS2870 reversed EMT of ARPE-19 cells through the suppression of NOX4. The role of NOX4 in promoting EMT in other ocular cells was reported too. For instance, NOX4 played a vital role in promoting TGF-β-induced EMT in lens epithelial cells[28]. GLX7013114 as a novel NOX4 inhibitor could suppress TGFβ-induced EMT of lens epithelial cells[29]. Therefore, in our study, it is reasonable to believe that LRG1 might induce EMT via activation of NOX4 in ARPE-19 cells.
In this study, the primary deficiency is the absence of animal models to further validate the function of LRG1 under the condition of subretinal fibrosis. Although the pro-EMT function of LRG1 has been demonstrated in ARPE-19 cells in vitro, the combination with animal experiments in vivo will make this study more rigorous.
In conclusion, our research indicated that LRG1 could promote EMT of ARPE-19 cells through activating NOX4. NOX4 inhibitor might attenuate the extent of EMT of ARPE-19 cells. Therefore, NOX4 inhibitor has the potential to reduce anti-VEGF agents-induced fibrosis. Further investigations, especially in CNV models, need to put into practice to elucidate more explicit functions of LRG1 as well as NOX4.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81670828); the Shandong Provincial Key Research and Development Program (No.2017GSF18141); the Innovation Project of Shandong Academy of Medical Sciences and the National Science and Technology Major Project of China (No.2017ZX09304-010). Yang LL is partially supported by the Taishan Scholar Youth Professional Program (No.tspd20150215; No.tsgn20161059).
Conflicts of Interest: Zhou L, None; Shi DP, None; Chu WJ, None; Yang LL, None; Xu HF, None.
REFERENCES
- 1.Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, MARINA Study Group Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(2):246–252. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 2.Talks JS, Lotery AJ, Ghanchi F, Sivaprasad S, Johnston RL, Patel N, McKibbin M, Bailey C, Mahmood S. First-year visual acuity outcomes of providing aflibercept according to the VIEW study protocol for age-related macular degeneration. Ophthalmology. 2016;123(2):337–343. doi: 10.1016/j.ophtha.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Rao P, Lum F, Wood K, Salman C, Burugapalli B, Hall R, Singh S, Parke DW, Williams GA. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1y in the IRIS registry. Ophthalmology. 2018;125(4):522–528. doi: 10.1016/j.ophtha.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Bakri SJ, Thorne JE, Ho AC, Ehlers JP, Schoenberger SD, Yeh S, Kim SJ. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American academy of ophthalmology. Ophthalmology. 2019;126(1):55–63. doi: 10.1016/j.ophtha.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Hwang JC, Del Priore LV, Freund KB, Chang S, Iranmanesh R. Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2011;42(1):6–11. doi: 10.3928/15428877-20100924-01. [DOI] [PubMed] [Google Scholar]
- 6.Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, Huang JY, Ying GS, Hagstrom SA, Winter K, Maguire MG, Comparison of Age-related Macular Degeneration Treatments Trials Research Group Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(3):656–666. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storey PP, Pancholy M, Wibbelsman TD, Obeid A, Su D, Borkar D, Garg S, Gupta O. Rhegmatogenous retinal detachment after intravitreal injection of anti-vascular endothelial growth factor. Ophthalmology. 2019;126(10):1424–1431. doi: 10.1016/j.ophtha.2019.04.037. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa K, Kannan R, Hinton DR. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp Eye Res. 2016;142:19–25. doi: 10.1016/j.exer.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa K, Sreekumar PG, Spee C, Nazari H, Zhu DH, Kannan R, Hinton DR. αB-crystallin regulates subretinal fibrosis by modulation of epithelial-mesenchymal transition. Am J Pathol. 2016;186(4):859–873. doi: 10.1016/j.ajpath.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117(3):524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinosa-Heidmann DG, Reinoso MA, Pina Y, Csaky KG, Caicedo A, Cousins SW. Quantitative enumeration of vascular smooth muscle cells and endothelial cells derived from bone marrow precursors in experimental choroidal neovascularization. Exp Eye Res. 2005;80(3):369–378. doi: 10.1016/j.exer.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Samuel W, Jaworski C, Postnikova OA, Kutty RK, Duncan T, Tan LX, Poliakov E, Lakkaraju A, Redmond TM. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol Vis. 2017;23:60–89. [PMC free article] [PubMed] [Google Scholar]
- 14.Choi K, Lee K, Ryu SW, Im M, Kook KH, Choi C. Pirfenidone inhibits transforming growth factor-β1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol Vis. 2012;18:1010–1020. [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Li M, Xu D, Zhao C, Liu G, Wang F. Overexpression of Snail in retinal pigment epithelial triggered epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014;446(1):347–351. doi: 10.1016/j.bbrc.2014.02.119. [DOI] [PubMed] [Google Scholar]
- 16.Wei Q, Liu Q, Ren C, Liu J, Cai W, Zhu M, Jin H, He M, Yu J. Effects of bradykinin on TGF-β1-induced epithelial-mesenchymal transition in ARPE-19 cells. Mol Med Rep. 2018;17(4):5878–5886. doi: 10.3892/mmr.2018.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji T, Inatani M, Tsuji C, Cheranov SM, Kadonosono K. Oxytocin induced epithelium-mesenchimal transition through Rho-ROCK pathway in ARPE-19 cells, a human retinal pigmental cell line. Tissue Cell. 2020;64:101328. doi: 10.1016/j.tice.2019.101328. [DOI] [PubMed] [Google Scholar]
- 18.Haupt H, Baudner S. Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author's transl) Hoppe Seylers Z Physiol Chem. 1977;358(6):639–646. [PubMed] [Google Scholar]
- 19.Wang X, Abraham S, McKenzie JAG, Jeffs N, Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai Z, Arthur HM, Bainbridge J, Moss SE, Greenwood J. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 2013;499(7458):306–311. doi: 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song WH, Wang XM. The role of TGFβ1 and LRG1 in cardiac remodelling and heart failure. Biophys Rev. 2015;7(1):91–104. doi: 10.1007/s12551-014-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda H, Fujimoto M, Serada S, Urushima H, Mishima T, Lee H, Ohkawara T, Kohno N, Hattori N, Yokoyama A, Naka T. Leucine-rich α-2 glycoprotein promotes lung fibrosis by modulating TGF-β signaling in fibroblasts. Physiol Rep. 2017;5(24):e13556. doi: 10.14814/phy2.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong D, Zhao SR, He GX, Li JK, Lang YB, Ye W, Li YL, Jiang CL, Li XF. Stable knockdown of LRG1 by RNA interference inhibits growth and promotes apoptosis of glioblastoma cells in vitro and in vivo. Tumor Biol. 2015;36(6):4271–4278. doi: 10.1007/s13277-015-3065-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Zhang X, Zhang J, Fang J, Ge Z, Li X. LRG1 promotes proliferation and inhibits apoptosis in colorectal cancer cells via RUNX1 activation. PLoS One. 2017;12(4):e0175122. doi: 10.1371/journal.pone.0175122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Zhu L, Fang J, Ge Z, Li X. LRG1 modulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res. 2016;35:29. doi: 10.1186/s13046-016-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ban Z, He J, Tang Z, Zhang L, Xu Z. LRG-1 enhances the migration of thyroid carcinoma cells through promotion of the epithelial-mesenchymal transition by activating MAPK/p38 signaling. Oncol Rep. 2019;41(6):3270–3280. doi: 10.3892/or.2019.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53(7):1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiraga R, Kato M, Miyagawa S, Kamata T. Nox4-derived ROS signaling contributes to TGF-β-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33(10):4431–4438. [PubMed] [Google Scholar]
- 28.Das SJ, Lovicu FJ, Collinson EJ. Nox4 plays a role in TGF-β-dependent lens epithelial to mesenchymal transition. Invest Ophthalmol Vis Sci. 2016;57(8):3665–3673. doi: 10.1167/iovs.16-19114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das S, Wikström P, Walum E, Lovicu FJ. A novel NADPH oxidase inhibitor targeting Nox4 in TGFβ-induced lens epithelial to mesenchymal transition. Exp Eye Res. 2019;185:107692. doi: 10.1016/j.exer.2019.107692. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Wang H, Wang F, Gu Q, Xu X. Snail involves in the transforming growth factor β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS One. 2011;6(8):e23322. doi: 10.1371/journal.pone.0023322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dvashi Z, Goldberg M, Adir O, Shapira M, Pollack A. TGF-β1 induced transdifferentiation of RPE cells is mediated by TAK1. PLoS One. 2015;10(4):e0122229. doi: 10.1371/journal.pone.0122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Li H, Liu XQ, Xu D, Wang F. MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells by targeting AKT2. Exp Cell Res. 2016;345(2):115–124. doi: 10.1016/j.yexcr.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Huang L, Zhang C, Su L, Song ZY. GSK3β attenuates TGF-β1 induced epithelial-mesenchymal transition and metabolic alterations in ARPE-19 cells. Biochem Biophys Res Commun. 2017;486(3):744–751. doi: 10.1016/j.bbrc.2017.03.113. [DOI] [PubMed] [Google Scholar]
- 34.Zhang NG, Ren YQ, Wang YS, Zhao L, Wang B, Ma NN, Gao ZX, Cao BW. LRG1 suppresses migration and invasion of esophageal squamous cell carcinoma by modulating epithelial to mesenchymal transition. J Cancer. 2020;11(6):1486–1494. doi: 10.7150/jca.36189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sng MK, Chan JSK, Teo Z, Phua T, Tan EHP, Wee JWK, Koh NJN, Tan CK, Chen JP, Pal M, Tong BMK, Tnay YL, Ng XR, Zhu PC, Chiba S, Wang XM, Wahli W, Tan NS. Selective deletion of PPARβ/δ in fibroblasts causes dermal fibrosis by attenuated LRG1 expression. Cell Discov. 2018;4:15. doi: 10.1038/s41421-018-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Li J, Wang Q, Xing Y, Tan Z, Kang Q. Novel NADPH oxidase inhibitor VAS2870 suppresses TGF-β-dependent epithelial-to-mesenchymal transition in retinal pigment epithelial cells. Int J Mol Med. 2018;42(1):123–130. doi: 10.3892/ijmm.2018.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]