Abstract
AIM
To evaluate the two-year outcomes of patients treated for diabetic macular edema (DME) with intravitreal Conbercept (IVC).
METHODS
The clinical data of 30 DME patients (36 eyes) were retrospectively reviewed. The patients were treated with IVC for 3mo. Additional IVC was given at subsequent monthly visits, if needed (3+PRN). The patients were followed up for 24mo.
RESULTS
The best-corrected visual acuity (BCVA) at 24mo significantly increased (66.7±15.3 letters) in comparison with the baseline (54.4±15.4 letters, P<0.0001). The mean improvement in BCVA was 11.0±2.9 letters. At 24mo, 44.1% of the eyes surveyed gained ≥15 letters, 52.9% of the eyes gained ≥10 letters, and 70.6% of the eyes gained ≥5 letters. No vision loss was noted in 96.8% of the eyes, and 5.9% of the eyes lost ≥5 letters, but ≤10 letters. The central retinal thickness (CRT) at 24mo was significantly reduced (277.1±122.9 µm) in comparison with the baseline (510.9±186.1 µm, P<0.0001). At 24mo, 43.3% of the patients had a CRT ≤250 µm. The mean number of injections over 24mo was 10.6±2.0. No severe eye or systemic adverse events related to either the drug or injection were noted.
CONCLUSION
IVC is safe and effective for the treatment of DME.
Keywords: diabetic macular edema, Conbercept, intravitreal, visual acuity
INTRODUCTION
The International Diabetes Federation reported that the 2013 global diabetic patient population was 382 million. China holds the record for most number of diabetic patients aged 20-79y worldwide and has 9.8 million diabetic patients in total[1]. Chronic hyperglycemia leads to systemic microvascular complications[2]. Diabetic retinopathy (DR) is the most common diabetic microvascular complication and the primary reason for vision loss in the working-aged population. Diabetic macular edema (DME) is the primary reason for vision loss in DR patients[3]–[5]. One mechanism of DME involves the breakdown of the inner/outer blood-retinal barrier, which results from the disruption of tight junctions between retinal capillary endothelial and the retinal pigment epithelial cells[5]–[7]. Laser is effective for the treatment of microaneurysm and focal DME. However, severe damage of the inner/outer blood-retinal barrier in diffuse DME renders patients prone to poorer vision and weaker responses to laser treatment[8]–[9]. The ETDRS study showed that macular laser photocoagulation (MPC) reduces the risk of immediate vision loss in 50% of the patients treated[10]. However, MPC shows very limited efficacy in treating diffuse DME, with improved vision in only 14.5% of the patients tested[9]. Modified MPC slightly increases the proportion of patients with visual acuity (VA) improvement of ≥5 letters by 51% after 1y, 47% after 2y, and 62% after 3y[11]–[13].Thus, effective therapy is still an unmet medical need.
Vascular endothelial growth factor (VEGF) can promote endothelial cell mitosis and increase vascular permeability, which contributes to the development of DME[14]–[16]. The READ-2 study indicated ranibizumab to be superior to MPC in terms of vision improvement after 2y and 4mo of treatment for DME[17]. Intravitreal injection of VEGFs, such as ranibizumab[18], bevacizumab[19], and aflibercept[20], has recently gained popularity for treating DME. Indeed, VEGF has become the first-line treatment for diffuse DME on account of its ability to reduce macular edema thickness and improve vision.
Conbercept is an anti-VEGF agent, whose core domain of is fused by human VEGFR1 immunoglobulin-like domain 2, human VEGFR2 immunoglobulin-like domains 3 and 4, and human immunoglobulin Fc fragment (molecular weight, 142 kd)[21]. Conbercept has higher affinity to VEGF than natural and monoclonal antibodies and can block all isoforms of VEGF-A, VEGF-B, and placental growth factor[22]. In comparison with aflibercept, Conbercept has a four-binding site of receptor 2, which increases the stability of the dimer and its affinity to VEGF[23]. The efficacy and safety of Conbercept for treating age-related macular degeneration and polypoidal choroidal vasculopathy have been confirmed in several clinical trials[24]–[25]. Our study aimed to investigate the efficacy and safety of intravitreal Conbercept (IVC) injection for DME treatment with 3+PRN.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the Ethical Committee and Institutional Review Board of Peking University People's Hospital (Beijing, China). Written informed consent was obtained from the guardian of each participant in accordance with the Declaration of Helsinki.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Patients
The clinical data of patients with diffuse DME treated with IVC injection at Peking University People's Hospital from May 2016 to January 2019 were retrospectively analyzed. Diffuse DME is defined as retinal thickening involving the central fovea and involves ≥2 optic discs. Most of the exudate in DME originates from diffuse leaky capillaries, and only 33% of this exudate is from leaky microaneurysms[26]–[28].
The diagnosis of diffuse DME was made based on clinical characteristics, fluorescein angiography, and optical coherence tomography (OCT). The inclusion criteria were: 1) age ≥18y; 2) type 1 or 2 diabetes; 3) HbA1c ≤10%; 4) vision loss resulted from diffuse DME involving the central fovea but not from other conditions; 5) best-corrected visual acuity (BCVA) was 24-78 letters (ETDRS letters); 6) central retinal thickness (CRT) ≥250 µm; and 7) no refractive media opacity that may affect the fundus examination. The exclusion criteria were: 1) active eye inflammation; 2) glaucoma; 3) active proliferative DR; 4) vision loss caused by other conditions, such as retinal vein occlusion, choroid neovascularization, macular hole, epiretinal membrane, vitreomacular traction, and macular degeneration and scarring; 5) intravitreal injection of corticosteroids, such as triamcinolone acetonide, within 3mo prior to the treatment; 6) intravitreal injection of other anti-VEGF drugs, such as ranibizumab or bevacizumab, within 2mo prior to the treatment; 7) uncontrolled hypertension (systolic pressure >160 mm Hg or diastolic pressure >100 mm Hg); and 8) stroke or myocardial infarction within 3mo prior to the treatment.
A total of 30 patients (36 eyes) were included in our study. All patients received their first IVC injection (0.5 mg/0.05 mL) during the period from May 2016 to January 2019. Follow-up was performed for at least 24mo with an interval of 1mo. A full set of ophthalmologic examinations, including BCVA, intraocular pressure, slit lamp, fundus examination, and OCT, were conducted at each follow-up visit. Fluorescein fundus angiography was performed at baseline and was ordered during the follow-up if deemed necessary by the investigators. BCVA was evaluated using the ETDRS chart. CRT was measured by Cirrus HD-OCT (Carl Zeiss, Meditec, Dublin, CA, USA).
The criteria for repeated injection were: 1) persistent or newly developed macular intraretinal/subretinal fluid and 2) CRT ≥250 µm or decreased BCVA by ≥5 letters and increased CRT.
Intravitreal injection was performed in an operating room under sterile conditions. Surface anesthesia was used. The periocular skin and conjunctival sac were disinfected with 5% povidone iodine, and the eye was opened with an eye speculum. Conbercept (0.5 mg/0.05 mL) was intravitreally injected with a 30 G syringe needle approximately 3.5-4 mm posterior to the corneal limbus. Patients were examined for signs of eye infection on the first day after the injection and followed up 1mo after the injection to determine the need for repeated treatment.
The primary outcome was changes in BCVA in comparison with the baseline at 24mo. The secondary outcomes were 1) the proportion of patients that had gained 15 letters and 2) changes in CRT.
Statistical Analysis
Categorical data were presented as percentages, and continuous data were reported as means±standard deviation. Paired t-test was used to compare the baseline BCVA and OCT with the follow-up results. All analyses were performed using SPSS v11.5 software (SPSS, Chicago, USA), and P<0.05 was considered statistically significant.
RESULTS
Our retrospective study included 30 patients (36 eyes), all of whom were followed up for at least 24mo. Fourteen patients were male (46.7%) and 16 patients were female (53.3%). The mean age was 52.2±13.2y. All patients had type 2 diabetes. The mean disease courses of diabetes and diffuse DME were 15.3±5.4y and 11.4±9.6mo, respectively. Panretinal photocoagulation (PRP) was previously used for 33 eyes (91.7%). All patients used insulin injection, and their mean HbA1c level was 8.1%±0.8%. The mean baseline BCVA was 54.4±15.4 letters, and the mean baseline CRT was 510.9±186.1 µm. The baseline patient and ophthalmologic information are listed in Table 1.
Table 1. Baseline patient and ophthalmologic information.
| Characteristics | Values |
| Mean age (y) | 52.2±13.2 |
| Sex | |
| Male | 14 (46.7) |
| Female | 16 (53.3) |
| Diabetes | |
| Type 1 | 0 |
| Type 2 | 100 (100) |
| HbA1c | 8.1%±0.8% |
| Diabetic disease course (y) | 15.3±5.4 |
| Diffuse DME disease course (mo) | 11.4±9.6 |
| DR types | |
| Non-proliferative DR | 3 (8.3) |
| Proliferative DR | 33 (91.7) |
| Previous laser treatment | |
| Yes | 33 (91.7) |
| No | 3 (8.3) |
| Hypoglycemic agents | |
| Oral hypoglycemic agents | 0 |
| Insulin injection | 30 (100) |
| VA (letter) | 54.4±15.4 |
| CRT (µm) | 510.9±186.1 |
DME: Diabetic macular edema; DR: Diabetic retinopathy; VA: Visual acuity; CRT: Central retinal thickness.
n (%)
Visual Acuity
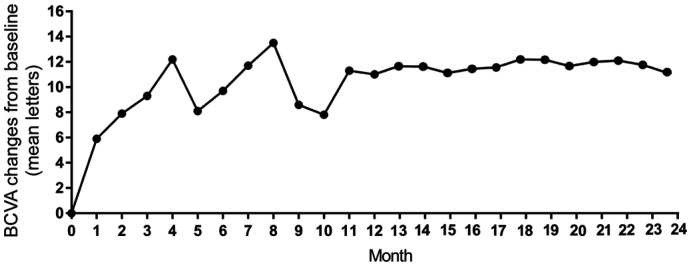
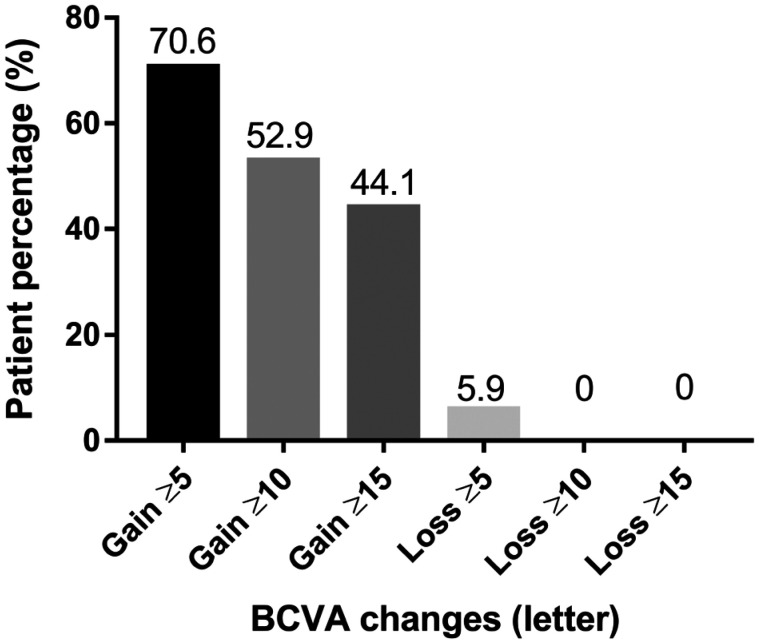
BCVA at 24mo (66.7±15.3 letters) was significantly improved in comparison with the baseline (54.4±15.4 letters, P<0.0001) by IVC injection. Improvements in vision in comparison with the baseline (Figure 1) were 9.3±2.6 letters at 3mo (P<0.0001), 9.5±1.8 letters at 6mo (P<0.0001), 8.7±2.5 letters at 9mo (P<0.0001), 10.8±2.0 letters at 12mo (P<0.0001), 11.3±3.3 letters at 15mo (P<0.0001), 11.7±2.7 letters at 18mo (P<0.0001), and 11.0±2.9 letters at 24mo (P<0.001). At 24mo, 44.1% of the eyes gained ≥15 letters, 52.9% of the eyes gained ≥10 letters, and 70.6% of the eyes gained ≥5 letters. No vision loss was noted in 96.8% of the eyes. No eyes lost ≥10 or ≥15 letters, and only 5.9% of the eyes lost ≥5 letters (Figure 2).
Figure 1. The mean change of BCVA in comparison with baseline during 24mo.
Figure 2. The percentage of gained or lost letters in comparison with baseline during 24mo.
Anatomical Outcomes
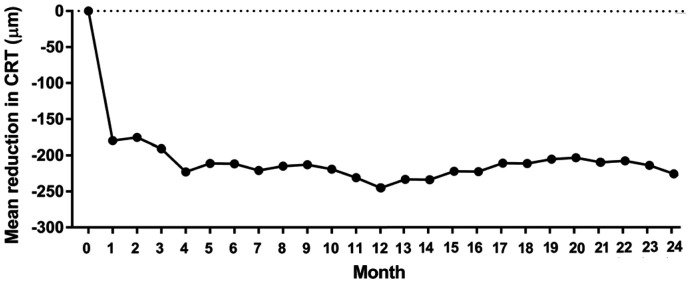
CRT at 1mo was significantly reduced (348.6±151.8 µm) in comparison with the baseline (510.9±186.1 µm, P<0.0001). The mean reduction in CRT at 1mo was 179.8±182.9 µm (P<0.0001). The mean CRT at 3mo was 325.8±147.5 µm, and the mean reduction in CRT at 3mo was 190.8±146.3 µm (P<0.0001). At 24mo, the mean CRT was 277.1±122.9 µm, and the mean reduction in CRT was 245.0±225.7 µm (P<0.0001). The percentage of patients with CRT ≤250 µm at 24mo was 43.3% (Figure 3).
Figure 3. The mean reduction in CRT in comparison with baseline during 24mo.
During the period of 24mo, a mean of 10.6±2.0 injections were performed for each eye. Three eyes (8.3%) received 14 injections, four eyes (11.1%) received 13 injections, nine eyes (25.0%) received 12 injections, six eyes (16.7%) received 10 injections, seven eyes (19.4%) received 9 injections, and seven eyes (19.4%) received 8 injections.
Safety Profile
A total of 381 injections were performed over 24mo of follow-up. No severe injection-related ophthalmologic adverse events, such as intraocular inflammation and rhegmatogenous retinal detachment, were observed. While subconjunctival hemorrhage was noted in five patients after the injections, this issue was not specifically treated and resolved spontaneously within 1wk. Moreover, no severe drug-related ophthalmologic adverse events, such as retinal artery occlusion, retinal ischemia, and vitreous hemorrhage, were recorded. On the first day after the injection, six patients had mild uveal inflammation, which was well-managed with non-steroid eye drops for 3d. Finally, no other drug-related non-ophthalmologic adverse events, such as death, myocardial infarction, stroke, and severe hypertension, were noted.
Two Cases Report
Case 1, female, 67 years old with proliferative DR. The patient suffered DME on the right eye. PRP has been completed. After 12 injections of IVC, macular edema subsided (Figure 4).
Figure 4. Fundus status of a patient with proliferative diabetic retinopathy before and after Conbercept treatment.
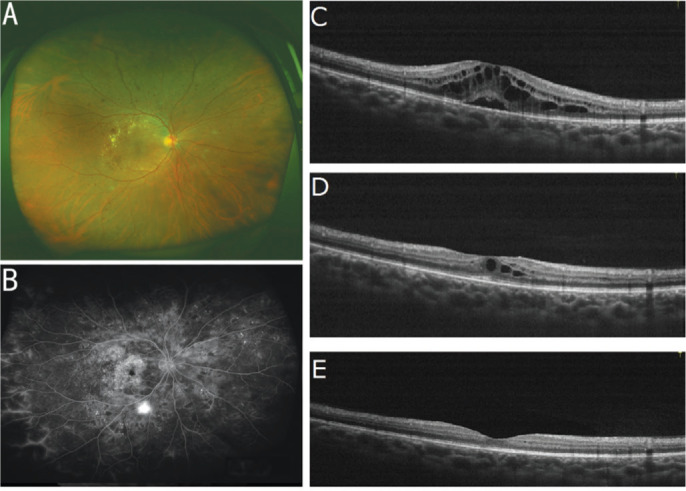
A: Fundus status before treatment; B: Fundus fluorescein angiography images before treatment; C: OCT examination images before treatment; D: OCT images after three successive Conbercept treatments; E: OCT images after 12 consecutive Conbercept treatments.
Case 2, male, 53 years old with nonproliferative DR. The patient suffered DME on the right eye. No laser treatment was performed. After 8 injections of IVC, macular edema subsided (Figure 5).
Figure 5. Fundus status of a patient with nonproliferative diabetic retinopathy before and after Conbercept treatment.
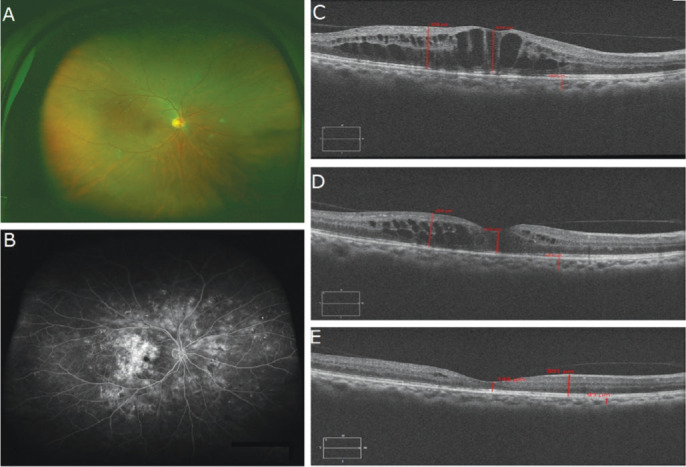
A: Fundus status before treatment; B: Fundus fluorescein angiography images before treatment; C: OCT examination images before treatment; D: OCT images after three successive Conbercept treatments; E; OCT images after eight successive Conbercept treatments.
DISCUSSION
Our single-center retrospective study aimed to evaluate the anatomical and functional outcomes of treatment of diffuse DME with IVC injection for 24mo. The results showed that Conbercept significantly improves vision, prevents severe vision loss, and rapidly reduces macular edema. More importantly, the treatment efficacy was maintained for 24mo.
The VISTA study showed that 41.6% and 31.1% of the patients in the one injection every 4wk (IAI2q4) and one injection per month for the first 5mo and then one injection every 8wk (IAI2q8) groups respectively gained ≥15 ETDRS letters; by comparison, in the VIVID study, 32.4% and 33.3% of the patients in the IAI2q4 and IAI2q8 groups respectively gained ≥15 ETDRS letters[20]. In another study, 0.5 mg ranibizumab injection once a month helped 45% patients in the RIDE group and 39.2% patients in the RISE group gain ≥15 ETDRS letters at 24mo; by the 36th month, 40.2% and 41.6% of the patients in the RIDE and RISE groups respectively gained ≥15 ETDRS letters. In our study, 44.1% of the eyes gained ≥15 ETDRS letters at 24mo, which is similar to previous studies. In the Bolt study, DME was treated with 1.25 mg of bevacizumab once every 6wk, and 11.9% of the patients gained ≥15 ETDRS letters at 12mo[29].
We also found a BCVA improvement of 11.0 letters at 24mo. In the RIDE and RISE studies, ranibizumab treatment achieved vision improvements of 12.0 and 11.9 letters, respectively, at 24mo and 11.4 and 11.0 letters, respectively, at 36mo[28]. The BCVA improvements of patients in the IAI2q4 and IAI2q8 groups at 52wk were 12.5 and 10.7 letters, respectively, in the VISTA study and 10.5 and 10.7 letters, respectively, in the VIVID study[20]. Our results are similar with these aforementioned clinical trials[20],[28]. In the Bolt study[29], treatment with bevacizumab for 12mo improved BCVA by eight letters. However, neither the proportion of patients gaining ≥15 ETDRS letters nor the vision improvement letter number was lower than our study. This finding may be explained by the injection of bevacizumab every 6wk, which may result in relapsed macular edema, in the Bolt study. In the VISTA and VIVID studies, both injection groups received laser treatment for 6mo as needed[20]. However, our patients did not receive laser therapy. Injection was performed every month for 2y in the RIDE and RISE studies [28]; by comparison, in our study, the treatment regimen was 3+PRN. These results suggest that Conbercept is effective in improving vision in DME patients and that the 3+PRN regimen can effectively improve vision. The RESTORE study showed that 0.5 mg of ranibizumab administered under the 3+PRN regimen can achieve a vision gain of ≥15 ETDRS letters in 22.6% of the patients at 12mo and a vision gain of 7 letters in the diffuse DME subgroup (n=45)[27]. The vision improvement of the RESTORE study was inferior to that of our study, as well as those of the VISTA, VIVID, RIDE, and RISE studies. This finding might be associated with the once-per-month injection of ranibizumab in the RIDE and RISE studies and the pharmacological characteristics of fusion protein. Fusion protein has higher affinity to VEGF-A and longer binding time. Fusion protein can also inhibit placental growth factor, which plays an important role in the development of DME[30].
In the RESTORE study, 1% of the patients treated with ranibizumab lost ≥15 ETDRS letters at 52wk[27]. In the Bolt study, 2.4% of the patients treated with bevacizumab lost ≥15 ETDRS letters at 12mo[29]. In the aflibercept clinical trial, the proportions of patients that lost ≥15 ETDRS letters at 52wk were 0.6% and 0.7% in the 2q4 and 2q8 groups of VISTA, respectively, and 0.7% and 0% in the 2q4 and 2q8 groups of VIVID, respectively[20]. In our study, none of the patients lost ≥15 ETDRS letters and 96.8% of the patients had no vision loss at 24mo. Our results are similar to previous treatment outcomes of VEGF administered every month. Thus, treatment of DME with IVC injection may prevent severe vision loss and the 3+PRN regimen is effective.
In the Bolt study, treatment with bevacizumab for 12mo reduced the CRT by a mean of 130±122 µm. In our study, IVC injection for 24mo reduced the CRT by a mean of 245.0±225.7 µm. CRT at 24mo (277.1±122.9 µm) was significantly reduced compared with the baseline (245.0±225.7 µm, P<0.0001). Our results suggest that Conbercept administered under the 3+PRN regimen can effectively improve DME for at least 24mo, similar to previous results[20]. In comparison with bevacizumab treatment administered once every 6wk, IVC injection administered over 24mo can more extensively improve macular edema.
Anti-VEGF treatment was scheduled monthly in most previous clinical trials. However, this treatment regimen is difficult to adhere to in real clinical practice. In the DRCR.net study, ranibizumab was administered under the 6+PRN regimen (including the immediate and delayed laser groups), and the mean number of injections was 8-9 for the first year, 2-3 for the second year, and 1-2 for the third year[18],[31]–[33]. The RESTORE study showed that ranibizumab administered under the 3+PRN regimen is injected a mean number of 7.0±2.8 times over 12mo[27]. In the VISTA and VIVID studies, aflibercept was injected 11.8 and 12.2 times, respectively, in the IAI2q4 group and 8.4 and 8.7 times, respectively, in the IAI2q8 group[20]. While the IAI2q8 group received fewer injections than the IAI2q4 group, CRT changes in the former group showed unmaintained anatomical outcomes. However, this group gained vision improvements similar to those of IAI2q4 group, and over 90% of the patients did not lose vision. Aflibercept has higher affinity to VEGF-A and a longer binding time, which enables longer administration intervals. In comparison with Aflibercept, Conbercept has a four-binding site of receptor 2, which increases the stability of the dimer and its affinity to VEGF[23]. Li et al[24] reported one-year outcome of conbercept for DME. At month 12, the mean improvement of BCVA was significantly higher with conbercept treatment than that of corresponding untreated controls: 18 (15) letters vs -4 (6) letters, P<0.001; 7 (1) letters vs -5 (5) letters, P<0.001; respectively. At month 12 the mean CRT from baseline was significantly declined with conbercept treatment than that of respective untreated controls (-212.8±11.9 vs -44.3±35.3 µm, P<0.001; -116.1±88.9 vs -33.7±49.8 µm, P=0.001; respectively)[1]. And Xu et al[9] also described the mean BCVA letter score improved by 9.3±5.2 with conbercept and the mean CRT reduction was 145.2±72.5 µm at month 12. In our study, the mean number of injections for each patient over 24mo was 10.6. Although the number of injections was low, the same vision and anatomical improvements were achieved. Multiple repeated injections may block neural factors and result in retinal atrophy[34]. Fewer injections reduce the risk of ophthalmologic and systemic adverse events. Prolonged administration intervals can reduce the numbers of injections and visits, relieve the patient's financial burden, and ease the burden of physicians. Earlier research suggested that prolonging the treatment interval in patients with DME may be feasible owing to the self-limitation of the disease. Patients with DME are at higher risk of experiencing adverse events than non-diabetic patients because diabetic patients often have systemic complications, such as cardiovascular and renal conditions, and are prone to infections. Thus, long-time and frequent intravitreal injection may increase the risk of infection in these patients[35].
In our study, 91.7% of the patients had previous PRP, and 47% of the eyes had been previously treated with other anti-VEGF agents (3mo prior to our study). Our results indicate that previous laser and anti-VEGF therapies do not affect the outcomes of IVC injection for DME, which is consistent with previous findings[20],[27]. The RESTORE study showed that ranibizumab treatment is superior to laser therapy alone in improving vision and that this result is not affected by DME type, diabetes type, and previous laser therapy (panretinal or macular laser therapy). Similarly, the RESOLVE study found that previous laser therapy has no effect on the BCVA letter number after ranibizumab treatment for 24mo[36].
In the VISTA and VIVID studies, less than 10% of the eyes in the aflibercept group received salvage laser therapy within half a year[20]. The RESOLVE study showed no significant differences in vision, anatomy, and injection times at 12mo between the drug-alone and drug-laser combination groups[27]. In fact, in our study, no patient received salvage macular laser therapy but similarly significant improvements in anatomy and function were achieved and maintained. Therefore, anti-VEGF treatment for DME significantly reduces the risk of macular laser therapy and, in turn, the risk of vision loss.
Early treatment of DME is desirable to achieve good efficacy. In the RIDE and RISE studies, the vision improvement in the laser group was 2.5 letters at 24mo[28]. Interestingly, even after the laser group was switched to 0.5 mg of ranibizumab monthly, the vision improvement was only 4.5 letters at 36mo. The READ study found that intravitreal ranibizumab injection can improve vision and significantly reduce CRT, and these treatment results were noted as early as a mean of 7d[37]. Some potential factors, such as neural cell loss resulting from chronic DR and retinal structural changes associated with the natural course of DR, may influence the ultimate vision recovery of chronic DME[28]. Our study is limited by its single-center retrospective design, short follow-up period, and small sample size. The long-term safety and efficacy of Conbercept in treating diffuse DME requires further investigation using large-sample multicenter prospective randomized studies.
Acknowledgments
Conflicts of Interest: Cheng Y, None; Yuan L, None; Zhao MW, None; Qian T, None.
REFERENCES
- 1.Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. doi: 10.1016/j.diabres.2020.108072. [DOI] [PubMed] [Google Scholar]
- 2.Mori K, Yoshida S, Kobayashi Y, Ishikawa K, Nakao S, Hisatomi T, Haruta M, Isihibashi T, Sonoda KH. Decrease in the number of microaneurysms in diabetic macular edema after anti-vascular endothelial growth factor therapy: implications for indocyanine green angiography-guided detection of refractory microaneurysms. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):735–741. doi: 10.1007/s00417-020-04608-9. [DOI] [PubMed] [Google Scholar]
- 3.Rasti R, Allingham MJ, Mettu PS, Kavusi S, Govind K, Cousins SW, Farsiu S. Deep learning-based single-shot prediction of differential effects of anti-VEGF treatment in patients with diabetic macular edema. Biomed Opt Express. 2020;11(2):1139–1152. doi: 10.1364/BOE.379150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehead M, Wickremasinghe S, Osborne A, Van Wijngaarden P, Martin KR. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expert Opin Biol Ther. 2018;18(12):1257–1270. doi: 10.1080/14712598.2018.1545836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy S, Kim D, Sankaramoorthy A. Mitochondrial structural changes in the pathogenesis of diabetic retinopathy. J Clin Med. 2019;8(9):1363. doi: 10.3390/jcm8091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezzola S, Nawaz IM, Cancarini A, Ravelli C, Calza S, Semeraro F, Presta M. 3D endothelial cell spheroid/human vitreous humor assay for the characterization of anti-angiogenic inhibitors for the treatment of proliferative diabetic retinopathy. Angiogenesis. 2017;20(4):629–640. doi: 10.1007/s10456-017-9575-4. [DOI] [PubMed] [Google Scholar]
- 7.Browning DJ, Altaweel MM, Bressler NM, Bressler SB, Scott IU, Diabetic Retinopathy Clinical Research Network Diabetic macular edema: what is focal and what is diffuse? Am J Ophthalmol. 2008;146(5):649–655. 655.e1–6. doi: 10.1016/j.ajo.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson TL, Nicod E, Angelis A, Grimaccia F, Pringle E, Kanavos P. Pars plana vitrectomy for diabetic macular edema: a systematic review, meta-analysis, and synthesis of safety literature. Retina. 2017;37(5):886–895. doi: 10.1097/IAE.0000000000001280. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Rong A, Bi Y, Xu W. Intravitreal conbercept injection with and without grid laser photocoagulation in the treatment of diffuse diabetic macular edema in real-life clinical practice. J Ophthalmol. 2016;2016:2143082. doi: 10.1155/2016/2143082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutton DW, Stein JD, Glassman AR, Bressler NM, Jampol LM, Sun JK, DRCR Retina Network Five-year cost-effectiveness of intravitreous ranibizumab therapy vs panretinal photocoagulation for treating proliferative diabetic retinopathy: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2019;137(12):1–9. doi: 10.1001/jamaophthalmol.2019.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Y, Takamura Y, Matsumura T, Morioka M, Gozawa M, Inatani M. Posterior subtenon infusion of triamcinolone acetonide as adjunctive treatment to panretinal photocoagulation using pattern scan laser for diabetic retinopathy. Jpn J Ophthalmol. 2018;62(6):686–692. doi: 10.1007/s10384-018-0628-z. [DOI] [PubMed] [Google Scholar]
- 13.Diabetic Retinopathy Clinical Research Network (DRCR net) Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol Chic Ill. 2009;127(3):245–251. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bressler NM, Beaulieu WT, Bressler SB, Glassman AR, Melia BM, Jampol LM, Jhaveri CD, Salehi-Had H, Velez G, Sun JK, DRCR Retina Network Anti-vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: pooled analysis of five DRCR retina network randomized clinical trials. Retina. 2020;40(6):1021–1028. doi: 10.1097/IAE.0000000000002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarbin MA. Extending our knowledge on systemic adverse events associated with intravitreal anti-vascular endothelial growth factor therapy. Ophthalmology. 2019;126(7):1016–1017. doi: 10.1016/j.ophtha.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Grant MB, Afzal A, Spoerri P, Pan H, Shaw LC, Mames RN. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs. 2004;13(10):1275–1293. doi: 10.1517/13543784.13.10.1275. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Diabetic Retinopathy Clinical Research Network. Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, Ferris FL, 3rd, Glassman AR, Maturi RK, Melia M. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312–2318. doi: 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nepomuceno AB, Takaki E, Paes de Almeida FP, Peroni R, Cardillo JA, Siqueira RC, Scott IU, Messias A, Jorge R. A prospective randomized trial of intravitreal bevacizumab versus ranibizumab for the management of diabetic macular edema. Am J Ophthalmol. 2013;156(3):502–510.e2. doi: 10.1016/j.ajo.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Bhandari S, Nguyen V, Fraser-Bell S, Mehta H, Viola F, Baudin F, Gabrielle PH, Creuzot-Garcher C, Gillies M, Barthelmes D. Ranibizumab or aflibercept for diabetic macular edema: comparison of 1-year outcomes from the fight retinal blindness! Registry. Ophthalmology. 2020;127(5):608–615. doi: 10.1016/j.ophtha.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B, Li H. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm Res. 2009;26(1):204–210. doi: 10.1007/s11095-008-9718-9. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi EG, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Zhang J, Yan M, Luo D, Zhu W, Kaiser PK, Yu DC, KH902 Phase 1 Study Group A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2011;118(4):672–678. doi: 10.1016/j.ophtha.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, Zhang F, Zhang J, Tang L, Wu Q, Luo D, Ke X, AURORA Study Group Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121(9):1740–1747. doi: 10.1016/j.ophtha.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Song Y, Xu G. Conbercept for treatment of neovascular age-related macular degeneration: results of the randomized phase 3 PHOENIX study. Am J Ophthalmol. 2019;197:156–167. doi: 10.1016/j.ajo.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88(9):1173–1179. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A, RESTORE study group The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang JM, Rubio RG, Adamis AP, Ehrlich JS, Hopkins JJ, RIDE and RISE Research Group Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086.e2. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Li J, Li M, Zeng M, Li T, Xiao W, Li J, Wu Q, Ke X, Luo D, Tang S, Luo Y. KH902 suppresses high glucose-induced migration and sprouting of human retinal endothelial cells by blocking VEGF and PIGF. Diabetes Obes Metab. 2013;15(3):224–233. doi: 10.1111/dom.12008. [DOI] [PubMed] [Google Scholar]
- 31.Bressler SB, Glassman AR, Almukhtar T, Bressler NM, Ferris FL, Googe JM, Jr, Gupta SK, Jampol LM, Melia M, Wells JA, 3rd, Diabetic Retinopathy Clinical Research Network Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57–68. doi: 10.1016/j.ajo.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998;273(47):31283–31288. doi: 10.1074/jbc.273.47.31283. [DOI] [PubMed] [Google Scholar]
- 34.Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, Pieramici DJ, Shami M, Singerman LJ, Stockdale CR. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130(9):1145–1152. doi: 10.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 36.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J, Zimmer-Galler I, Do DV, Campochiaro PA. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142(6):961–969.e4. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.