Abstract
AIM
To evaluate intraocular pressure (IOP) measurements and fluctuations using the iCare ONE rebound tonometer (RT-ONE), during home monitoring, in diagnosed and suspected glaucoma patients.
METHODS
A retrospective case series of consecutive patients with known glaucoma or glaucoma suspects who were followed-up and treated between January 2016 and January 2017. The study included 80 eyes of 40 patients with a mean age of 59.1±14.6y (range, 24-78). All patients have undergone 4-5d of IOP home monitoring with RT-ONE at morning, noon, afternoon, and night time.
RESULTS
Baseline mean IOP, as measured in the clinic (8 a.m.-12 p.m.), was 17.4±5.1 mm Hg, compared to RT-ONE home monitoring mean IOP of 15.6±4.1 mm Hg (P=0.002). Mean IOP was significantly lower at noon, afternoon and night times compared to clinic measured IOP and morning measurements (P=0.005). IOP peak measured during home monitoring was significantly higher compared to the clinic measured IOP (21.3±5.6 mm Hg and 17.4±5.1 mm Hg, P<0.001). IOP peaks during home monitoring demonstrated a majority of 47 peaks during morning measurements, compared to 23 at noon, 19 at afternoon and only 12 at night (P<0.001). The home monitoring results led to treatment modification of 44 eyes (55%), treatment regime was insufficient for 40 (50%) eyes.
CONCLUSION
Home monitoring IOP with RT-ONE can provide good assessment of mean IOP, IOP fluctuations and peaks throughout the hours of the day, which lead to an accurate treatment for glaucoma patients.
Keywords: glaucoma, intraocular pressure, home monitoring, iCare ONE, peak
INTRODUCTION
Glaucoma is the second leading cause of blindness worldwide. Although it is understood to have a multifactorial pathogenesis, intraocular pressure (IOP) is currently the only modifiable risk factor for development and progression of visual field defect and optic nerve damage characteristic of this disease[1]–[3].
IOP is a continuous and dynamic parameter, with a circadian rhythm and spontaneous changes[4]. As IOP peaks and IOP fluctuations are considered significant independent risk factor for the progression of glaucoma[5], the accurate assessment of a patient's IOP profile is critical in the management of glaucoma[6]–[7]. Monitoring patients' IOP during different hours of the day provides valuable clinical information in the management of a glaucoma patient.
The iCare ONE rebound tonometer (RT-ONE; Icare Finland, Helsinki, Finland) is a portable handheld tonometer for patient self-use which can be performed at home and does not require topical anesthesia. The mechanism of the RT-ONE has been described in previous studies[8]–[10]. The apparatus measures IOP by detecting the deceleration of a plastic rod probe as it is bounced off the cornea detected by a solenoid inside the instrument. The higher the IOP, the faster the probe bounces off the cornea. Several studies have shown reliability, ease of use, accuracy and correlation of the RT-ONE with the Goldmann applanation tonometer (GAT) readings[11]–[19].
The aim of this study was to measure IOP and IOP peaks during home monitoring using the RT-ONE on diagnosed glaucoma patients and glaucoma suspect patients and assess the disease progress accordingly to those measures and clinical evaluation.
SUBJECTS AND METHODS
Ethical Approval
This is a retrospective case series of consecutive patients with known primary open angle glaucoma (POAG), pseudoexfoliation glaucoma (PXFG), normal tension glaucoma (NTG), uveitic glaucoma, ocular hypertension (OHT) or glaucoma suspects who were followed-up and treated by a single glaucoma specialist (Kurtz S) between January 2016 and January 2017. The study adhered to the tenets of the declaration of Helsinki and was approved by the Institutional Review Board (IRB) of the Tel Aviv Medical Center, Tel Aviv, Israel. Informed consent was waived due to the retrospective nature of the study.
Follow-up
All patients underwent complete ocular examination, including GAT measurement, at the glaucoma clinic by a single glaucoma specialist (Kurtz S), between 8 a.m.-12 p.m.; followed by RT-ONE IOP home monitoring in both eyes.
All participants were trained for home measurements by a professional technician. First, the iCare ONE and its characteristics were explained, then patients were instructed on its use with their dominant hand in front of a mirror. The iCare ONE was placed 4 to 8 mm from the cornea according to the manufacturer's instructions. Two adjustable support elements and an eye cup were used to facilitate obtaining good-quality measurements. When the patients became familiar with the use of the iCare ONE, three valid measurements for each eye were recorded. After the training, all patients were referred to 4-5d of home monitoring. Patients were instructed to IOP measuring at 4 specific times during the day: morning (5-7 a.m.), noon (12 a.m.-2 p.m.), afternoon (5-7 p.m.) and night (10 p.m.-12 p.m.). Each measurement value was the average of five IOP readings, with a difference of less than 2 mm Hg between each reading.
IOP readings of the RT-ONE measurements were saved on the instrument, downloaded to a readable file form, and sent to the referring physician. All patients underwent a complete ophthalmological examination and the data collected included patients' demographics, best-corrected visual acuity (BCVA), slit-lamp examination, type of glaucoma, number of topical IOP lowering drops, clinic measured IOP, four daily readings of IOP, IOP peak (defined as the highest measurement during entire follow-up) and change in treatment. Glaucoma progression was defined as worsening in visual field exams and optic disc cupping and treatment was adjusted accordingly.
Patients under the age of 18, patients with corneal or retinal disease, ocular trauma or optic neuropathy and patients with less than 4d of home monitoring were excluded from this study.
Statistical Analysis
Data were recorded in Microsoft Excel (2010)™ and analyzed using SPSS version 23 (SPSS Inc., Chicago, IL, USA). Continuous variables such as age were compared between subjects using Mann-Whitney, non-parametric test was used. Comparison of IOP measurements over time compared to baseline clinical IOP was performed used repeated measures analysis of variance (ANOVA). Binary variables were compared between subjects using the Fisher's exact test. All tests were 2-tailed, and the threshold for statistical significance was defined as a P-value <0.05. BCVA was recorded using a Snellen chart and was converted to logarithm of minimal angle of resolution (logMAR) value for statistical purposes.
RESULTS
The study included 80 eyes of 40 patients (23 males and 17 females) with a mean age of 59.1±14.6y (range, 24-78). The most common diagnosis was POAG and NTG (50% and 25%, respectively) and baseline-number of topical IOP lowering medications were 2±1.6. Table 1 presents the baseline characteristics of the patients.
Table 1. Patients' baseline characteristics.
| Baseline characteristics | Eyes (n=80) |
| Diagnosis | |
| POAG | 40 (50) |
| NTG | 20 (25) |
| PXFG | 8 (10) |
| OHT | 6 (7.5) |
| Glaucoma suspect | 4 (5) |
| Secondary glaucoma | 2 (2.5) |
| Number of topical medications | |
| 0 | 25 (31.3) |
| 1 | 7 (8.7) |
| 2 | 11 (13.8) |
| 3 | 20 (25) |
| 4 | 17 (21.2) |
POAG: Primary open angle glaucoma; NTG: Normal tension glaucoma; PXFG: Pseudoexfoliation glaucoma; OHT: Ocular hypertension.
n (%)
Intraocular Pressure Measurements
Baseline mean IOP, as measured in the clinic with GAT, was 17.4±5.1 mm Hg in comparison to 15.6±4.1 mm Hg (P=0.002) with RT-ONE home monitoring.
When analyzing right and left eyes independently, there was no difference in baseline IOP and no difference when comparing the baseline mean IOP to mean IOP during home monitoring (P=0.009 and P=0.002 for right and left eyes, respectively). Also, the mean IOP of each day's measurements by the RT-ONE were lower than the clinic measured IOP, statistically significant on day one through four (Table 2). When examining the changes throughout the day, mean IOP was significantly lower at noon (P=0.01), afternoon (P=0.005) and night times (P<0.001) compared to clinic measured IOP (P=0.005). Morning measurements were found to be the highest (17.1±5.5 mm Hg), statistically significant compared to noon (15.9±4.5 mm Hg, P=0.045), afternoon (15.8±4.8 mm Hg, P=0.03) and night (13.9±3.9 mm Hg, P<0.001) and were not statistically significant compared to the clinic measured IOP (P=1.00). IOP measurements during the follow up are presented in Figure 1.
Table 2. Comparison of mean IOP measurements throughout the follow-up period.
| Follow-up period | IOP (mean±SD) | Pa |
| Clinic measuring | 17.4±5.1 | |
| Day 1 | 15.3±4.6 | >0.001 |
| Day 2 | 15.7±4.5 | 0.004 |
| Day 3 | 15.5±4.0 | 0.001 |
| Day 4 | 15.5±4.8 | 0.001 |
| Day 5 | 16.1±4.7 | 0.76 |
IOP: Intraocular pressure. aAll statistical analysis compared to clinic measured IOP.
Figure 1. IOP measurements during follow up.
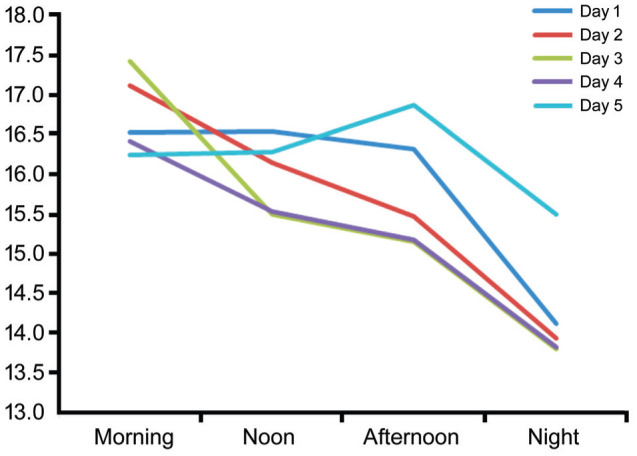
Intraocular Pressure Peaks and Fluctuations
IOP peaks measured during home monitoring was significantly higher compared to the clinic measured IOP (21.3±5.6 mm Hg and 17.4±5.1 mm Hg, P<0.001). It was also significantly higher in the treatment modified group (18.5±5.3 vs 23.6±5.2 mm Hg, P=0.001) and in the non-modified group (15.9±4.5 vs 18.4±4.8 mm Hg, P=0.01). The fluctuation index, calculated as mean IOP peaks–clinic mean IOP, was 3.9 for all eyes. When comparing the treatment-modified to the non-modified groups, the fluctuation index was significantly higher for the treatment modified group (5.02 vs 2.5, P=0.03). There was a total of 101 IOP peaks during home monitoring, as some patients had multiple measurements of peak IOP during their follow-up. A majority of 47 peaks were measured in the morning in comparison to 23 at noon, 19 at the afternoon and only 12 at night (P<0.001 for all values). When examining the total number of peaks, there was a range of 17-23 peaks for each day. We did not find a statistical difference between any of the days of the home monitoring.
Treatment Modification
The home monitoring results led to treatment modification in 44 eyes (55%). The treatment regime was insufficient for 40 eyes (50%): large fluctuations of IOP were noticed in 16 eyes, IOP measurements above target pressure in one eye and both reasons in 23 eyes. Of these 40 eyes, nine eyes (22%) were treated with the maximum tolerated medical therapy (MTMT), and therefore referred to trabeculectomy. Four (10%) eyes (all with NTG) had a reduction of the number of medications secondary to the RT-ONE measurements.
DISCUSSION
Previous studies demonstrated that RT-ONE measurements were within limits of agreement compared to the GAT[16],[18]–[19], and the apparatus is relatively easy for personal use after a short training session. Thus, it is reasonable to use it for the purpose of glaucoma patients home monitoring.
In this current study, although RT-ONE home monitoring measurements were significantly lower than the IOP clinic measurements (15.6 vs 17.4), the treatment regime was insufficient for 50% of the study eyes. Those patients suffered from glaucoma progression (in terms of worsening in visual field exams and optic disc cupping) despite no change in office hours IOP. This progression can be explained by IOP fluctuations, with a difference of 3.8 mm Hg between average IOP peaks and average IOP at clinic for all patients, and a 5.1 mm Hg difference in the treatment-modified group. Previous smaller studies showed similar results. Sood and Ramanathan[17] demonstrated a change in the management of 10 out of 18 (56%) patients with NTG following RT-ONE home IOP phasing, mostly due to IOP peaks. Barkana et al[7] demonstrated that mean peak of 24-hour IOP measurements (16.8 mm Hg) was significantly higher than peak office IOP (14.7 mm Hg). In this study, 24-hour IOP monitoring led to treatment modification in one-third of the patients. Hughes et al[20], in their review, concluded that 24-hour monitoring of IOP can lead to change in treatment due to IOP fluctuations and spikes which are underestimated in clinic follow-up evaluations. These results demonstrate the importance of 24h IOP monitoring on patient' treatment. Having said that, glaucoma patients' monitoring through isolated, in-office measurements, is of common practice. This approach may lead patients with apparently normal or controlled IOP measured during office hours, to disease progression[6]–[7].
In this study, we also found that mean morning IOP was significantly higher than noon, afternoon and night measurements. Also, most of the IOP peaks (47%) occurred in the morning as opposed to only 12% of IOP peaks at night. Our results correlate with previous studies who found IOP peaks in the morning and decline over the course of the day. The exact timing of the peaks is unknown[14],[21]–[25]. Because morning IOP measurements were significantly higher and most IOP peaks occurred in the morning, if it is possible, we suggest clinic visit should be performed at this time of day. However, our results show that more than 50% of IOP peaks occurred at noon, afternoon or night, and a single office measurement is not sufficient for adequate follow up and monitoring of glaucoma patients.
The limitations of this study are the retrospective nature of the study and lack of complete data for all patients, including rate of progression. A large prospective study is needed in order to know the exact impact of IOP home monitoring with RT-ONE.
In conclusion, home monitoring IOP with RT-ONE can provide good assessment of glaucoma patients mean IOP, IOP fluctuations and peaks throughout the hours of the day, which may lead to accurate treatment for glaucoma patients.
Acknowledgments
Conflicts of Interest: Rosenfeld E, None; Rabina G, None; Barequet D, None; Mimouni M, None; Fischer N, None; Kurtz S, None.
REFERENCES
- 1.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. discussion 829–830. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 2.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 3.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 4.Liu JHK, Zhang XY, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 5.Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, Caprioli J. Predictive factors for glaucomatous visual field progression in the Advanced glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–1635. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Mosaed S, Liu JH, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol. 2005;139(2):320–324. doi: 10.1016/j.ajo.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 7.Barkana Y, Anis S, Liebmann J, Tello C, Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124(6):793–797. doi: 10.1001/archopht.124.6.793. [DOI] [PubMed] [Google Scholar]
- 8.Kontiola AI. A new induction-based impact method for measuring intraocular pressure. Acta Ophthalmol Scand. 2000;78(2):142–145. doi: 10.1034/j.1600-0420.2000.078002142.x. [DOI] [PubMed] [Google Scholar]
- 9.Cervino A. Rebound tonometry: new opportunities and limitations of non-invasive determination of intraocular pressure. Br J Ophthalmol. 2006;90(12):1444–1446. doi: 10.1136/bjo.2006.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danias J, Kontiola AI, Filippopoulos T, Mittag T. Method for the noninvasive measurement of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2003;44(3):1138–1141. doi: 10.1167/iovs.02-0553. [DOI] [PubMed] [Google Scholar]
- 11.Quérat L, Chen E. Monitoring daily intraocular pressure fluctuations with self-tonometry in healthy subjects. Acta Ophthalmol. 2017;95(5):525–529. doi: 10.1111/aos.13389. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi A, Nakakura S, Fujio Y, Fukuma Y, Mori E, Tabuchi H, Kiuchi Y. A pilot evaluation assessing the ease of use and accuracy of the new self/home-tonometer IcareHOME in healthy young subjects. J Glaucoma. 2016;25(10):835–841. doi: 10.1097/IJG.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 13.Takagi D, Sawada A, Yamamoto T. Evaluation of a new rebound self-tonometer, icare HOME. J Glaucoma. 2017;26(7):613–618. doi: 10.1097/IJG.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Katalinic P, Kalloniatis M, Hennessy MP, Zangerl B. Diurnal intraocular pressure fluctuations with self-tonometry in glaucoma patients and suspects: a clinical trial. Optom Vis Sci. 2018;95(2):88–95. doi: 10.1097/OPX.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valero B, Fénolland JR, Rosenberg R, Sendon D, Mesnard C, Sigaux M, Giraud JM, Renard JP. Reliability and reproducibility of introcular pressure (IOP) measurement with the Icare® Home rebound tonometer (model TA022) and comparison with Goldmann applanation tonometer in glaucoma patients. J Fr Ophtalmol. 2017;40(10):865–875. doi: 10.1016/j.jfo.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Montañés J, Martínez-De-la-casa JM, Sabater AL, Morales-Fernandez L, Sáenz C, Garcia-Feijoo J. Clinical evaluation of the new rebound tonometers icare PRO and icare ONE compared with the goldmann tonometer. J Glaucoma. 2015;24(7):527–532. doi: 10.1097/IJG.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 17.Sood V, Ramanathan US. Self-monitoring of intraocular pressure outside of normal office hours using rebound tonometry. J Glaucoma. 2016;25(10):807–811. doi: 10.1097/IJG.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 18.Termühlen J, Mihailovic N, Alnawaiseh M, Dietlein TS, Rosentreter A. Accuracy of measurements with the iCare HOME rebound tonometer. J Glaucoma. 2016;25(6):533–538. doi: 10.1097/IJG.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 19.Mudie LI, LaBarre S, Varadaraj V, Karakus S, Onnela J, Munoz B, Friedman DS. The icare HOME (TA022) study. Ophthalmology. 2016;123(8):1675–1684. doi: 10.1016/j.ophtha.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma. 2003;12(3):232–236. doi: 10.1097/00061198-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kitazawa Y, Horie T. Diurnal variation of intraocular pressure in primary open-angle glaucoma. Am J Ophthalmol. 1975;79(4):557–566. doi: 10.1016/0002-9394(75)90792-8. [DOI] [PubMed] [Google Scholar]
- 22.Mottet B, Chiquet C, Aptel F, Noel C, Gronfier C, Buguet A, Romanet JP. 24-hour intraocular pressure of young healthy humans in supine position: rhythm and reproducibility. Invest Ophthalmol Vis Sci. 2012;53(13):8186. doi: 10.1167/iovs.12-10877. [DOI] [PubMed] [Google Scholar]
- 23.Bhorade AM, Gordon MO, Wilson B, Weinrab RN, Kass MA, Ocular Hypertension Treatment Study Group Variability of intraocular pressure measurements in observation participants in the ocular hypertension treatment study. Ophthalmology. 2009;116(4):717–724. doi: 10.1016/j.ophtha.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David R, Zangwill L, Briscoe D, Dagan M, Yagev R, Yassur Y. Diurnal intraocular pressure variations: an analysis of 690 diurnal curves. Br J Ophthalmol. 1992;76(5):280–283. doi: 10.1136/bjo.76.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MS, Kim JM, Park KH, Choi CY. Asymmetry of diurnal intraocular pressure fluctuation between right and left eyes. Acta Ophthalmol. 2011;89(4):352–357. doi: 10.1111/j.1755-3768.2009.01672.x. [DOI] [PubMed] [Google Scholar]


