Abstract
AIM
To compare the clinical outcomes of a variety of multifocal intraocular lenses (MIOLs) in patients diagnosed with presbyopia or cataracts.
METHODS
This clinical trial study included 141 patients (282 eyes) with different MIOLs implantation. The Symfony (60 eyes), the ReSTOR (100 eyes), the AT LISAtri (60 eyes), and the PanOptix (62 eyes) intraocular lenses were evaluated in this prospective interventional study. The near, intermediate, and distant visual acuities, contrast sensitivity, and defocus curve were measured as valid criteria. To statistically analyze the results, we used the Statistical Package for Social Science software, the non-parametric Wilcoxon signed-rank t, the one-way analysis of variance and the Tukey's post-hoc test in our analysis. Moreover, we conducted a detailed literature search on the PubMed database in English about MIOLs, in total 59 studies were included in this review article.
RESULTS
The four approaches did not show any significant difference in the best-corrected distance visual acuity (P>0.05). The defocus curves at the contrast of 100% showed that trifocal IOLs had better intermediate performance than the bifocal IOL (P<0.05). There were no statistically significant differences between AT LISAtri and PanOptix lenses for visual acuity at all distances. The eyes with PanOptix, Symfony, and AT LISAtri IOL showed better contrast sensitivity than those ReSTOR at spatial frequencies of 1, 3, and 6 cpd in photopic and mesopic conditions (P<0.001).
CONCLUSION
All four groups of the multifocal lenses were satisfying in terms of distance and near vision. Also, the group of trifocal lenses led to satisfactory outcomes in intermediate vision, without degradation in quality of vision.
Keywords: multifocal lens, visual acuity, cataract, presbyopia
INTRODUCTION
Multifocal intraocular lenses (MIOLs) have been reported as a substitute for improving near vision and quality of life for many patients[1]. A large portion of the intraocular lenses (IOLs) that are industrially accessible has two principal foci comparing to near and far vision. Notwithstanding, subjects with these IOLs encounter poor vision at intermediate vision because of its trademark V-design, containing two crests relating to near and far vision and a hole in the middle of it for intermediate vision[2]–[5]. Today, intermediate vision plays a significant role, such as working with computers. Trifocal IOLs have designed by join two diffractive profiles to enhance the intermediate vision. The in vitro and also in vivo researches have demonstrated the ability of these IOLs for the restoration of far, intermediate, and near visual capacity[5]–[7], reduction of spectacle dependency[8]–[12], and patient satisfaction[6]. The trifocal IOLs illustrated excellent optical quality at the intermediate distance rather than bifocal IOLs[4]–[5],[13]–[14]. However, photopic phenomena such as halo and glare and loss of contrast sensitivity may occur unintentionally. Furthermore, the most sensitive division of the eye that includes the dominant of the optical principle of the eye is green light while the life is polychromatic. Besides the conceivable compensation, both refraction and diffraction make longitudinal chromatic aberration (LCA)[15]. In order to correct chromatic aberration, multifocal IOLs with an extended range of vision and the proprietary of achromatic technology were designed[15]. Regarding these detections, the authors realized that a comparison of the visual outcome by trifocal (with three different types of performance) and bifocal IOLs were needed.
To date, there is no simultaneous comparison of visual performance after implantation of a quadrifocal IOL with trifocal function (AcrySof IQ PanOptix, Alcon Laboratories Inc., USA), a trifocal IOLs (AT LISAtri 839MP Carl Zeiss Meditec AG, Jena, Germany), extended depth of focus (EDOF) IOL (TECNIS Symfony; Abbott Medical Optics, Inc., Abbott Park, IL, USA) and a bifocal IOL (ReSTOR, Alcon Laboratories Inc., USA).
The current prospective clinical trial study investigates far, intermediate, and near visual acuities (VA) of a quadrifocal IOL with trifocal function (AcrySof IQ PanOptix, Alcon Laboratories Inc., USA), a trifocal IOLs (AT LISAtri 839MP Carl Zeiss Meditec AG, Jena, Germany), EDOF IOL (TECNIS Symfony; Abbott Medical Optics, Inc., Abbott Park, IL, USA) and a bifocal IOL (ReSTOR, Alcon Laboratories, Inc, USA). Furthermore, the difference affectability of various multifocal IOLs implantation on the contrast sensitivity function (CSF) under photopic and mesopic conditions and defocus curves in presbyopic patients or cataract after implantation of the four intended IOLs were assessed.
SUBJECTS AND METHODS
Ethical Approval
All procedures performed in studies involving human participants followed the ethical standards of the Institutional Review Board of the Shahid Beheshti University of Medical Sciences, Tehran, Iran, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
In this prospective clinical trial study (No.NCT03454334), 141 patients (282 eyes) with different IOLs included AMO Symfony in 60 eyes, AcrySof ReSTOR (Alcon) in 100 eyes, Zeiss AT LISATri in 60 eyes, PanOptix (Alcon) in 62 eyes and were at least 40y of age and had bilateral cataracts or presbyopia. Enrollment criteria included uncomplicated bilateral IOL implantation and good ocular health with no pathology that could compromise best VA (outside of residual refractive error). Cases meeting these criteria had a best corrected distance visual acuity (BDVA) less than 20/40, glare VA test worse than 20/40 preoperatively. Individuals also had a potential need for VA of 20/30 or better after surgery, and were completely willing to conform to the investigation requirements. Excluded from entry were subjects with irregular astigmatism or regular astigmatism; ≥1.25 D with the rule (WTR), and ≥0.75 D against the rule (ATR) to consider; probable increment of ATR astigmatism with age and posterior corneal astigmatism[16], large mesopic pupil[17] using the millimeter scale with the Colvard pupillometer in different light conditions and angle kappa and alpha[18]–[19] by the iTrace aberrometer (Tracey Technologies), corneal transplant surgery or previous glaucoma-filtering, a history of retinal detachments. Also excluded were patients with other clinically non-cataract ocular abnormality (e.g., microphthalmos, chronic drug-induced miosis, any pathology in the cornea to the retina, ocular surface problems and amblyopia). IOL implantation accomplished by one surgeon (Doroodgar F) at a single site. Surgery had to consist of bilateral implantation of either the AMO Symfony, AcrySof ReSTOR (Alcon), Zeiss AT LISATri, PanOptix (Alcon; Table 1). Trifocal implantation was accomplished based on the patient's interest in a higher degree of spectacle freedom at near, intermediate, and far distances after cataract surgery. The axial length and anterior segment size were assessed with a swept-source optical coherence tomography-based biometer (IOLMaster 700, Carl Zeiss Meditec AG). The A-constant was 119.1 for the AcrySof IQ PanOptix IOL, 118.6 for the AT LISAtri 839MP IOL, and 119.3 for the Symfony IOL. The target refraction was emmetropia in all groups, except the Symfony group that ordered to be -0.5 in the non-dominant eye.
Table 1. Optical features of the bifocal (ReSTORE), trifocals (PanOptix, AT LISA tri 839MP), and EDOF (Symphony) IOLs.
| IOL characteristics | AcrySof IQ PanOptix | ReSTORE | AT LISA tri 839MP | TECNIS Symfony |
| Optical design | Diffractive-refractive hybrid | Diffractive | Diffractive | Diffractive |
| Optic type | Non-apodized | Apodized | Non-apodized | Non-apodized |
| Addition (near/intermediate) | +3.25 D/+2.17 D | +3.00 D | +3.33 D/+1.66 D | -/+1.75 D |
| IOL size, mm | 13.0 | 13.0 | 11.0 | 13.0 |
| Optic size, mm | 6.0 | 6.0 | 6.0 | 6.0 |
| Diffractive zone, mm | 4.5 | 6.0 | 6.0 | ∼4.9 |
| Optic material | Hydrophobic acrylate/methacrylate copolymer | Hydrophilic acrylate | 25% hydrophilic acrylate with hydrophobic surface properties | Hydrophobic acrylate |
| Spherical aberration, µm | -0.10 | -0.1 | -0.18 | -0.27 |
| Refractive index | 1.55 | 1.4 | 1.46 | 1.47 |
| Range | +6.0 to +34.0 D | +10.0 to +35.0 D | 0 to +32.0 D | +5.0 to +34.0 D |
| Pupil dependence | Less dependent diffractive zone (4.5 mm) and lower energy utilization (up to 88%). The IOL incorporates 3 focal points at 40, 60, and 120 cm plus a distance focus from the base curve. | Dependent | Independent diffractive zone (6.0 mm) energy utilization (85%-86%) | Independent diffractive zone (6.0 mm) energy utilization (85%-56%) |
D: Diopter; IOL: Intraocular lens.
Patient Follow-up and Study Outcomes
All patients underwent full ophthalmologic examinations two weeks after surgery to confirm the healing process and another after second eye surgery. The binocular and monocular uncorrected distance visual acuity (UDVA), uncorrected intermediate visual acuity (UIVA; 60 cm), and corrected intermediate visual acuity (CIVA; 60 cm, 40 cm), uncorrected near visual acuity (UNVA), corrected near visual acuity (CNVA; 40 cm) were measured with Early Treatment of Diabetic Retinopathy Study (ETDRS) charts. The Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany) was used to assess posterior cornea.
Postsurgical evaluations were directed at a regular follow-up program (baseline and at 6mo and 1, 2y for the PanOptix group and 6mo, 1 and 4y for another group (AT LISAtri, Symfony, ReSTOR) between November 2013 and July 2019.
The postsurgical convention also contained measuring of VA in far, intermediate and near distances, contrast sensitivity test was accomplished under photopic (85 cd/m2) and mesopic (3 cd/m2) conditions using the CVS1000 contrast sensitivity test (VectorVision, Greenville, SC) for each group separately.
Binocular distance corrected defocus curves were measured in each subject with the Test Chart 2000, positioned at 6 m, to measure the VA with each defocus lens. These were sequenced in a random order over the range of +2.0 to -3.00 D in 0.50 D steps with the letters on the Test Chart 2000 randomized between measures. An Oculus Universal Trial Frame (Keeler Ltd., Windsor, UK), adjusted to ensure a 12-mm back vertex distance, was used to house the manifest refraction and each additional defocus lens. For each measurement of VA, subjects were prompted once with the phrase, “can you read any more letters on the line below”? According to the methodology described by Gupta and colleagues[20]. Each subject also subjectively rated his or her intermediate and near vision on a scale of 0 (completely unsatisfied) to 5 (completely satisfied).
Intraocular Lens Power Calculation
The IOL Master 700 (Carl Zeiss Meditec AG, Jena, Germany) biometry outcomes were optimized, and the SRK-T between 21 and 26, the Haggis above, the Hoffer Q formula below and the Barret universal two formulas[17],[21] calculated the IOL power for all patients. Target refraction was emmetropia for PanOptix, AT LISAtri, and ReSTOR while in the Symfony IOLs group, micro monovision was considered; target refraction for dominant eye was emmetropia and for the fellow eye was low myopia of -0.75 D.
Surgical Technique
Surgery was accomplished by one surgeon (Doroodgar F) using a standardized procedure. Cataracts were extracted by phacoemulsification through a 2.2 mm clear corneal incision. Multifocal IOLs were implanted in the capsular bag with utilizing the injector produced for the every particular IOL[18]–[19],[22]–[23]. The time between two surgical procedures was about 14d based on the patient's condition and other factors. Postoperatively, ciprofloxacin eye drops (Ciplex; Sina Darou, Tehran, Iran) were prescribed of times per day for ten days. Betamethasone eye drops (Betasonate, 0.1% betamethasone disodium phosphate; Sina Darou, Tehran, Iran) were given every 6h for two weeks then tapered, and artificial tears (ArtelacTM, Hypromellose; Bausch and Lomb, Montpellier, France) were administered every six hours for two weeks.
Statistical Analysis
To statistically analyze the results, we used the SPSS software (SPSS Statistics for Windows, V.23.0, 2013; IBM). The non-parametric Wilcoxon signed-rank test was applied to determine the significant differences between the objective results before and after the implantation of the trifocal lenses, such as contrast sensitivity and the logMAR VA. Given that these factors had a normal distribution, we report the mean and SD for them. Also, we used the one-way analysis of variance (ANOVA) to determine any statistically significant differences between the means of the effectiveness of four studied independent groups. We applied Tukey's post-hoc test to make pairwise comparisons of means and considered 5% level to find the statistically significant differences in our analysis.
RESULTS
There were 141 enrolled patients. This study consisted of 30 patients (60 eyes) for the Zeiss AT LISAtri IOL also ReSTOR (n=100), PanOptix (n=62), Symfony (n=60) with range age 40 to 70y in the four groups that underwent cataract surgery. Table 2 shows the preoperative demographics of each group of 80 women and 61 men in the study. There were no discontinuations throughout the study. The cases in the four groups had similar demographic features.
Table 2. Descriptive measures for the number of implanted IOL in each group, age, pupil size, spherical equivalent, astigmatism, and visual acuities preoperatively.
| Measurement | AT LISA (n=60) | PanOptix (n=62) | ReSTOR (n=100) | Symfony (n=60) |
| Age, y | 51.73±6.08 | 53.62±6.97 | 51.46±6.12 | 52.7±6.48 |
| Mesopic pupil size | 4.02±0.35 | 4.30±0.35 | 4.51±0.37 | 4.28±0.35 |
| Photopic pupil size | 3.48±0.33 | 3.48±0.33 | 3.43±0.36 | 3.45±0.35 |
| Spher | 0.91±2.75 | +0.72±2.94 | +0.81±2.96 | +0.95±2.82 |
| Astigmatism | -0.73±0.39 | -0.80±0.32 | -0.69±0.42 | -0.70±0.41 |
| UCVA (logMAR) | 0.50±0.17 | 0.51±0.19 | 0.58±0.15 | 0.61±0.10 |
| BCVA (logMAR) | 0.30±0.17 | 0.29±0.15 | 0.30±0.17 | 0.34±0.16 |
UCVA: Uncorrected visual acuity, BCVA: Best corrected visual acuity.
mean±SD
In the trifocal group (AT LISA tri 839 MP, PanOptix and Symfony), the mean binocular UNVA; 40 cm and UIVA; 60 cm were significantly better than in the bifocal group (ReSTOR) respectively (UNVA: 0.037±0.07, 0.033±0.05, 0.017±0.04 vs 0.086±0.07; P<0.001 and UIVA: 0.037±0.07, 0.040±0.06, 0.052±0.07 vs 0.105±0.07; P<0.001).
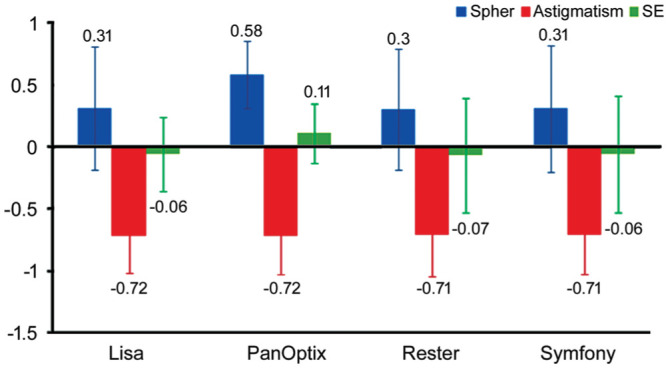
The considerable improvement was observed postoperatively in logMAR UDVA, CDVA, UNVA (40 cm), UIVA (60 cm) in four groups at 0.05 significance level (Table 3). Likewise, as expected, a significant decrease in the refractive error was observed postoperatively (P=0.02; Figure 1). According to Table 3, ReSTOR lens has less vision than the other groups at near and intermediate distances.
Table 3. Postoperative UCVA and BCVA.
| VA | AT LISA (n=60) | PanOptix (n=62) | ReSTOR (n=100) | Symfony (n=60) | P |
| UCVA | |||||
| Bilateral near vision | |||||
| 6mo | 0.025 (0.05) | 0.020 (0.04) | 0.089 (0.07)a | 0.017 (0.04) | <0.001 |
| 1y | 0.022 (0.05) | 0.027 (0.04) | 0.087 (0.07)a | 0.12 (0.03) | <0.001 |
| 2 or 4y | 0.037 (0.07) | 0.033 (0.05) | 0.086 (0.07)a | 0.017 (0.04) | <0.001 |
| Bilateral intermediate vision | |||||
| 6mo | 0.020 (0.05) | 0.020 (0.04) | 0.17 (0.07)a | 0.045 (0.07) | <0.001 |
| 1y | 0.015 (0.05) | 0.027 (0.04) | 0.096 (0.07)a | 0.05 (0.13) | <0.001 |
| 2 or 4y | 0.037 (0.07) | 0.040 (0.06) | 0.105 (0.07)a | 0.052 (0.07) | <0.001 |
| Far vision | |||||
| 6mo | 0.032 (0.06) | 0.020 (0.04) | 0.040 (0.06) | 0.092 (0.08)a | <0.001 |
| 1y | 0.035 (0.06) | 0.047 (0.06) | 0.036 (0.06) | 0.062 (0.08)a | 0.06 |
| 2 or 4y | 0.032 (0.06) | 0.020 (0.04) | 0.033 (0.06) | 0.090 (0.08)a | <0.001 |
| BCVA | |||||
| Bilateral near vision | |||||
| 6mo | 0.025 (0.05) | 0.019 (0.04) | 0.059 (0.07)a | 0.025 (0.05) | <0.001 |
| 1y | 0.021 (0.05) | 0.025 (0.04) | 0.057 (0.07)a | 0.022 (0.05) | <0.001 |
| 2 or 4y | 0.037 (0.07) | 0.038 (0.6) | 0.055 (0.07)a | 0.015 (0.05) | <0.001 |
| Bilateral intermediate vision | |||||
| 6mo | 0.058 (0.14) | 0.019 (0.04) | 0.077 (0.07)a | 0.058 (0.14) | <0.001 |
| 1y | 0.040 (0.06) | 0.025 (0.04) | 0.088 (0.07)a | 0.040 (0.06) | <0.001 |
| 2 or 4y | 0.037 (0.07) | 0.031 (0.05) | 0.097 (0.07)a | 0.027 (0.06) | <0.001 |
| Far vision | |||||
| 6mo | 0.025 (0.05) | 0.019 (0.04) | 0.023 (0.04) | 0.025 (0.05) | 0.96 |
| 1y | 0.022 (0.05) | 0.025 (0.04) | 0.036 (0.06) | 0.022 (0.05) | 0.28 |
| 2 or 4y | 0.037 (0.07) | 0.038 (0.04) | 0.023 (0.04) | 0.027 (0.06) | 0.38 |
UCVA: Uncorrected visual acuity; BCVA: Best corrected visual acuity; SD: Standard deviation. aStatistically significant.
logMAR
Figure 1. Post-operative refraction.
Contrast Sensitivity
Figures 2 and 3 demonstrate the mean postoperative contrast sensitivity in the logarithmic scale under binocular mesopic and photopic conditions. There was no significant difference in the values obtained between AT LISAtri and PanOptix at spatial frequencies of 1 cpd and 3, 6, 12, 18 cpd. Eyes with the PanOptix, Symfony, and AT LISAtri IOL illustrated preferred contrast sensitivity than ReSTOR at spatial frequencies of 1, 3, 6 cpd photopic and mesopic conditions (P<0.001). The curves achieved with monocular vision were equivalent to binocular vision.
Figure 2. Binocular photopic contrast sensitivity for four IOL types.
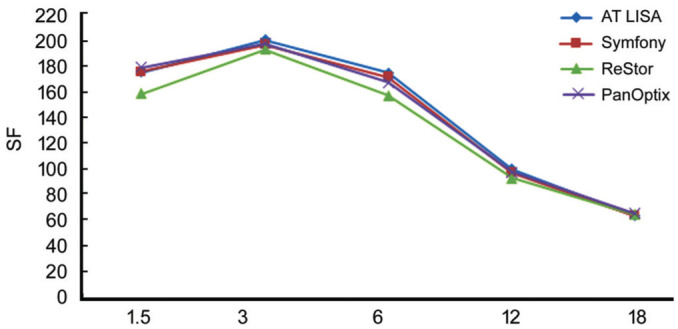
Figure 3. Binocular mesopic contrast sensitivity for four IOL types.
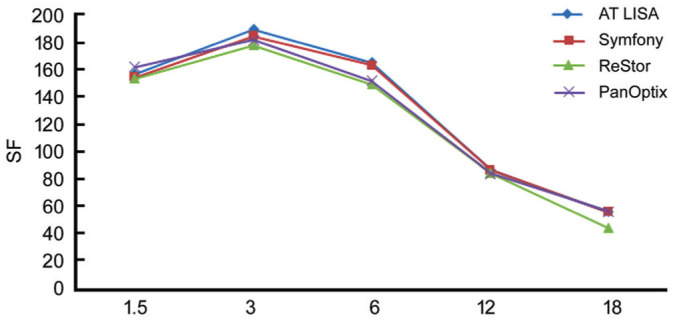
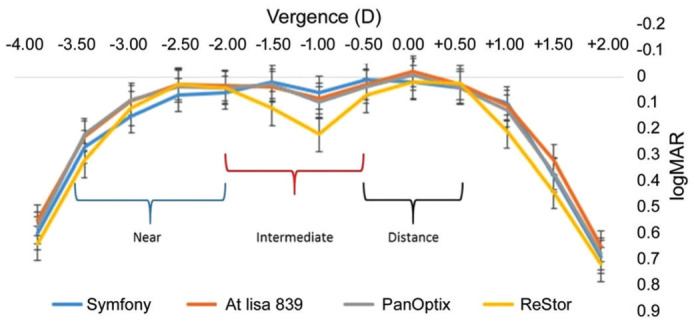
Defocus Curves
Figure 4 demonstrates the defocus curves under 100% of contrast. No statistically significant differences were observed between four groups in -2.00 D defocus (P=0.167). There was no significant difference for three groups trifocal IOLs in defocus of -1.00 D (P=0.07), all of which would do well to results than the bifocal group (AcrySof ReSTOR; P=0.04). At this level of defocus, VA results were comparable between the other two groups of trifocal IOLs (P=0.09), the two of which had essentially preferred VA over the symfony group with 0.0 D defocus (P<0.001 and P=0.008 for AT LISA and Pan Optix, respectively). In any case, there were no contrasts between the three groups with -2.00 D defocus (P=0.1). At defocus level -2 and from -2.5 D to -4.0 D the PanOptix and AT LISA group showed better acuity than the Symfony group. With -2.00 D defocus, the AcrySof IQ PanOptix group and AT LISAtri 839MP group acquired practically identical outcomes.
Figure 4. Mean monocular 100% contrast logarithm of the minimum angle of resolution visual acuity (logMAR) with correction for distance as a function of lens defocus in the four groups.
Spectacle Independence
All subjects were spectacle independent in four groups.
DISCUSSION
The current clinical trial study provides a comparative analysis of the clinical performance of the PanOptix IOL, the AT LISAtri 839MP IOL, the TECNIS Symfony IOL, and ReSTOR IOL. The clinical results demonstrated satisfactory outcomes for four groups. However, the group of ReSTOR achieved less intermediate vision in comparison to the trifocal IOLs.
We conducted a detailed literature search on the PubMed database in the English language, without date limitation. Search terms included “Multifocal IOL”, “cataract surgery”, “Monofocal IOL”, “Angle κ and α”, “IOL formulas in Multifocal IOL implantation”, “Pupil size” and commercially available trifocal IOLs such as “FineVision Micro F (PhysIOL, Liege, Belgium)”, “the AT LISAtri 839MP (Carl Zeiss Meditec AG, Jena, Germany)”, “the AcrySof IQ PanOptix, Alcon Laboratories, Inc.”, “the EDOF IOL, TECNIS Symfony (Abbott Medical Optics, Santa Ana, CA)”, and “Aspheric IOLs”. In cases of non-English articles, abstract information was used whenever possible. The review of related articles including six randomized clinical trials and two cohort studies illustrated that the trifocal IOLs analyzed remarkably prefer to give intermediate VA than bifocal IOLs without dealing distance or near visual acuity (Table 4)[1],[3],[7],[9],[24]–[55].
Table 4. Summary of outcomes included in the review.
| Study author | Others | Bifocal |
Trifocal |
Bifocals better | Trifocals better | ||||||
| ReSTOR | Lentis Mplus | TECNIS | AT LISA | LISA 839 | PanOptix | Symfony | FineVision | ||||
| Cochener et al (2012)[7] | Accommodative | a | a | R ↑UNVA, ↑UDVA, and ↑SI | |||||||
| Alfonso et al (2012)[3] | a | a | IV<N, F similarly, Photopic CS; LM>R at high SF | ||||||||
| Alió et al (2011) [1] | a | a | IV, CS LM>R whereas, NVR>LM | ||||||||
| Alió et al (2013)[9] | a | a | IV and CS L.M ↑>Lisa, while Lisa ↑ in D, NV | ||||||||
| van der Linden et al (2012)[55] | a | a | R↑had higher patient satisfaction | ||||||||
| Marques et al (2015)[42] | a | a | Excellent predictability and optical performance in both lenses | ||||||||
| Domínguez-Vicent et al (2016)[43] | a | a | a | MTF (sagittal tangential Strehl ratio); defocus: trifocal showed 3 (F, I, N); MW: 2 (F, N) mean peaks respectively | |||||||
| Jonker et al (2015)[30] | a | a | DC: Id in -2, -3.5, -4 D | DC: Sd in -1 D; Id in 2, 1.5, 1, 0.5, 0, -0.5, -1.5, -2.5, -3 D; CS: Id in 3, 6, 12 c/d | |||||||
| Gundersen et al (2016)[41] | a | a | DC: Id in 0.5 D | DC: Sd in 2, -1.5 D; Id in 1.5, 1, 0, -0.5, -1, -2, -2.5, -3, -3.5, -4 D | |||||||
| Cochener et al (2016)[34] | a | a | DC: Id in 2, 1.5, 1, 0.5 D CS: Id in 1.5, 3 c/d |
DC: Sd in -1, -1.5, -2, -2.5 D; Id in 0, -0.5, -3, -3.5 D CS: Id in 6, 12 c/d | |||||||
| Bilbao-Calabuig et al (2016)[44] | a | a | DC: Sd in -1.5, -2, -2.5, -3, -3.5 D; Id in 2, 1.5, 1, 0.5, 0, -0.5, -1 D; CS: Sd in 3 c/d; Id in 1.5, 6, 12, 18 c/d | ||||||||
| Plaza-Puche et al (2016)[38] | a | a | a | a | a | DC: Sd in -1, -1.5, -2, -2.5, -3, -3.5, -4 D; Id in 1, 0.5, 0, -0.5 D | |||||
| Mojzis et al (2014)[39] | a | a | DC: Sd in -3.5, -4 D; Id in 1, 0.5, -3 D, CS: Id in 3 c/d | DC: Sd in -1, -1.5 D; Id in 0, -0.5, -2, -2.5 D, CS: Id in 6, 12, 18 c/d | |||||||
| Lubiński et al (2020)[31] | a | a | DC: Sd in 1.5, 0.5, -0.5, -1.0, -2.0, -2.5, -3.0, -3.5 and -4.0 D; Id in VFQ-25 in NV | ||||||||
| Monaco et al (2017)[40] | a | a | DC: Sd in 1.5 D, and from -2.5 to -4.0 D | ||||||||
| Mencucci et al (2018)[45] | a | a | a | CS: Id in Photopic, Sd in Mesopic (S>L at the SF of 18 cpd); the best outcomes in 60 cm: P, in 80 cm: S | |||||||
| Cochener et al (2018)[35] | a | a | a | Id in UDVA, Sd in UNVA (F and P>S), micromonovision gave; UIVA, UNVA>non-micromonovision | |||||||
| Esteve-Taboada et al (2015)[46] | a | a | a | F: ↑far vision; S: ↑intermediate vision; L839: ↑near vision | |||||||
| Ruiz-Mesa et al (2018)[48] | a | a | a | F and P>S in NVA | |||||||
| Ruiz-Mesa et al (2017)[47] | a | a | Best corrected | ||||||||
| Sudhir et al (2019)[49] | a | a | P>S in intermediate focal point of 60 cm (arms-length), a more natural and comfortable | ||||||||
| de Medeiros et al (2017)[33] | a | a | a | S, T>P for I, FVA and photopic CS in low sf, P>S, T for UIVA at 60 cm and for UNVA at 40 cm | |||||||
| Pedrotti et al (2016)[50] | a | a | The mesopic CS of S>L, the S compensates Ch, SA in large pupil | ||||||||
| Chang et al (2016)[51] | a | clinical utility aberrations for EDOF and presbyopia | |||||||||
| Eppig et al (2015)[52] | Accommodative M | mesopic CS aspheric IOL>photopic, due to the correction of SA at large pupil diameters | |||||||||
| Crnej et al (2014)[53] | Accommodative M | CS and SA at 12 cycles/degree: significantly lower and better, capsulorhexis size effects | |||||||||
| Kretz et al (2015)[54] | a | Functional vision and stereopsis outcome binocular>monocular for all distances | |||||||||
| Kohnen et al (2017)[32] | a | P is quadrifocal, good VA at all distances; (logMAR>0.1), best VA at 60 cm | |||||||||
SI: Spectacle independence; UCNVA: Uncorrected near visual acuity; UCIVA: Uncorrected intermediate visual acuity; ↑: Higher; VP: Visual performance; DC: Defocus curve in 100%; CS: Contrast sensitivity; I: Intermediate; F: Far; N: Near; SF: Spatial frequency; SA: Spherical aberrations; IOHOA: Internal and ocular higher-order aberration; Sd: Statistically significant differences; Id: Insignificant differences; L: Zeiss AT LISA Tri; P: AcrySof IQ PanOptix (Alcon Laboratories, Inc); R: ReSTOR multifocal IOLs (Alcon Laboratories, Inc., Fort Worth, TX) with +2.50 and +3.00 diopters, EDOF IOLs; S: TECNIS Symfony (Abbott Medical Optics, Inc., Abbott Park, IL); MW: Mini well (SIFI, Catania, Italy); LMp: Oculentis GmbH-Lentis Mplus LS-312; F: FineVision Micro F (PhysIOL SA, Liège, Belgium). aImplanted lens.
The findings of a prospective randomized multicenter study of Alió et al[1],[9],[36] studies of Cochener et al[7]–[8],[34]–[35], and review of the previous studies (Table 4) suggested incorporation of a third focus does not harm reading performance when compared with the performance of bifocal IOLs. In agreement, the authors found no differences between the IOLs, even when the measurements were performed. The findings of defocus vergences in the current study are comparable with the previous studies that described the defocus curve between different trifocal IOLs (Table 4)[31],[35],[45],[49].
The PanOptix IOL provided better VA results at the distance range from 50 to 60 cm than Symfony, although acuity was slightly worse at 80 cm, Kohnen et al[32] also reported a statistically significantly better VA, 1 line better VA, at defocus level -1.5 D, and from -2.5 D to -4.0 D of PanOptix than the Symfony IOL[32].
Sudhir et al[49] illustrated the better optical quality of Trifocals (Symfony and PanOptix) at a distance and near vision rather than bifocal lenses. Furthermore, they showed comparable modulation transfer function (MTFs) at -1.50 D and -3.00 D for Symfony and PanOptix but better performance through Symfony at the intermediate range (highest MTF at -2.00 D and -2.50 D)[49]. Ruiz-Mesa et al[48] reported a significantly better near and better reading distance (the range of 37 to 39 cm for both IOL) with PanOptix than Symfony, the distance corrected intermediate vision between PanOptix (60 cm) and Symfony (80 cm) was similar. The contrast sensitivity under photopic and mesopic conditions and the high order aberrations were identical between two groups at all spatial frequencies[47]–[48]. Mencucci et al[45] PanOptix provided better VA at 60 cm than the Symfony and at; similarly, 80 cm, Symfony was significantly better than the PanOptix and AT LISA. The near vision was relatively better with PanOptix than AT LISA; both IOLs showed significantly better near vision than Symfony[45].
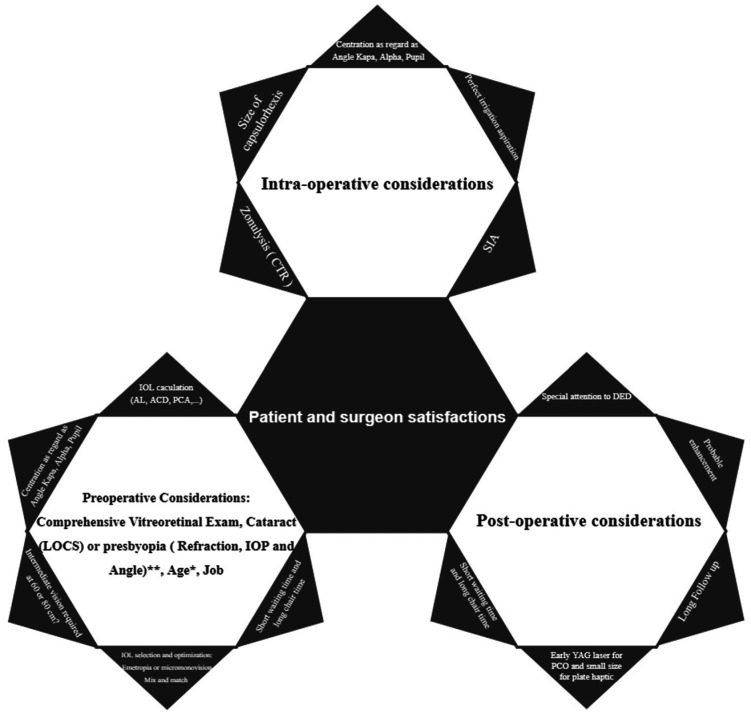
Our findings are similar, whereas Trifocals (AT LISA, Symfony, and PanOptix) had not priority rather bifocal lenses (ReSTOR) at a distance. Furthermore, the AT LISA and PanOptix lenses had no preference in near vision. Fortunately, all subjects in the current study are spectacle independent, except ReSTOR group that somehow complain about blurred vision when working with computers. Previous studies reported 100% and 90% spectacle independence for AT LISA and Symfony respectively to see small letters thus needing prescription about 1.0 D add[31]. The target refraction -0.5 to -0.75 D in the non-dominant eye in the Symfony group and emmetropia for the other three lenses in our study might have supported near vision tasks in the Symfony group. This fantastic development (particularly in hyperopic patients with individual adaptability and flexibility) can be troublesome for myopic patients who have to drive a long time. The patient should not be losing any visual capacity. Thereby age, intraocular pressure (IOP), and angle (Figure 5) are important preoperative factors[25],[56]–[59]. Post-operative residual astigmatism ought to be under 1.00 diopter. The difference in surgically induced astigmatism is negligible in eyes with a corneal incision below 2.2 mm, and in surgical incisions <3.5 mm, astigmatism decreased with the longer follow-up. According to the previous studies, combining two subgroups (clear corneal incision 1.8 mm and 2.2 mm) did not significantly affect postoperative visual outcome[29]–[30].
Figure 5. Factors requirement for best outcomes in MIOL implantation.
It should be noted that every MIOL has its advantages and de-escalations, which along with the patient's personality and necessity expectation and clinical conditions, are essential factors to consider while selecting an IOL for the best result. An excellent near vision for reading is necessary for daily activities. Writing and reading is a complicated procedure that involves rather than just distinction optotypes. Furthermore, to achieve optimal postoperative outcomes, the decision about post-operative emmetropia or micro monovision and mix and match IOLs are some optional management[44].
Although all the patients were satisfied with near vision as well in the ReSTOR group, we observed some back falling off the ReSTOR group in near vision. It was not established whether it is due to patient cooperation or other confounding factors for better near VA by preoperative planning micro monovision. Even though the defocus curves illustrate thoughtful information, however, regarding evaluation at 100% contrast can lead to overestimation and decline in low contrast[29],[34].
By applying of the swept-source optical coherence tomography technology as the IOL Master 700 (Carl Zeiss Meditec AG, Jena, Germany) and OA-2000 (Tomey, Nagoya, Japan), advanced tomography and formula such as Barret universal two and ray trace technology, we tried to overcome the carelessness to some extent[21]. However, one of the limitations of studies was lack of complete measurement of higher-order aberrations before and after IOL implantation. Furthermore, because of the small number of the patients who received the other MIOLs, all the different kinds of MIOLs were not included in one prospective study. The authors suggest an attractive idea to customized lens for each patient based on bag volume, aberrometry, and necessity expectations for additional comparisons of the visual performance.
In conclusion, if all the criteria, as discussed above, are met, the results of the current study indicate good visual outcomes following implantation of these four group MIOLs. The visual outcome at a distance and near was comparable between four groups, although the trifocal lenses performed better at intermediate vision.
Acknowledgments
We acknowledge the professional manuscript services of American Journal Experts.
Conflicts of Interest: Doroodgar F, None; Niazi F, None; Sanginabadi A, None; Karimian F, None; Niazi S, None; Alinia C, None; Javadi MA, None.
REFERENCES
- 1.Alió JL, Plaza-Puche AB, Piñero DP, Amparo F, Rodríguez-Prats JL, Ayala MJ. Quality of life evaluation after implantation of 2 multifocal intraocular lens models and a monofocal model. J Cataract Refract Surg. 2011;37(4):638–648. doi: 10.1016/j.jcrs.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 2.Madrid-Costa D, Cerviño A, Ferrer-Blasco T, García-Lázaro S, Montés-Micó R. Visual and optical performance with hybrid multifocal intraocular lenses. Clin Exp Optom. 2010;93(6):426–440. doi: 10.1111/j.1444-0938.2010.00518.x. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso JF, Fernández-Vega L, Blázquez JI, Montés-Micó R. Visual function comparison of 2 aspheric multifocal intraocular lenses. J Cataract Refract Surg. 2012;38(2):242–248. doi: 10.1016/j.jcrs.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Madrid-Costa D, Ruiz-Alcocer J, Ferrer-Blasco T, García-Lázaro S, Montés-Micó R. Optical quality differences between three multifocal intraocular lenses: bifocal low add, bifocal moderate add, and trifocal. J Refract Surg. 2013;29(11):749–754. doi: 10.3928/1081597X-20131021-04. [DOI] [PubMed] [Google Scholar]
- 5.Montés-Micó R, Madrid-Costa D, Ruiz-Alcocer J, Ferrer-Blasco T, Pons ÁM. In vitro optical quality differences between multifocal apodized diffractive intraocular lenses. J Cataract Refract Surg. 2013;39(6):928–936. doi: 10.1016/j.jcrs.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Voskresenskaya A, Pozdeyeva N, Pashtaev N, Batkov Y, Treushnicov V, Cherednik V. Initial results of trifocal diffractive IOL implantation. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1299–1306. doi: 10.1007/s00417-010-1424-8. [DOI] [PubMed] [Google Scholar]
- 7.Cochener B, Vryghem, Rozot Visual and refractive outcomes after implantation of a fully diffractive trifocal lens. Clin Ophthalmol. 2012:1421. doi: 10.2147/OPTH.S32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cochener B, Vryghem J, Rozot P, Lesieur G, Chevalier JP, Henry JM, David T, Lesueur L, Gatinel D, Ganem C, Blanckaert J, van Acker E, Heireman S, Ghekiere S. Clinical outcomes with a trifocal intraocular lens: a multicenter study. J Refract Surg. 2014;30(11):762–768. doi: 10.3928/1081597X-20141021-08. [DOI] [PubMed] [Google Scholar]
- 9.Alió JL, Montalbán R, Peña-García P, Soria FA, Vega-Estrada A. Visual outcomes of a trifocal aspheric diffractive intraocular lens with microincision cataract surgery. J Refract Surg. 2013;29(11):756–761. doi: 10.3928/1081597X-20131021-05. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard AL, Shah S, Bhatt U, Bhogal G, Wolffsohn JS. Visual outcomes and subjective experience after bilateral implantation of a new diffractive trifocal intraocular lens. J Cataract Refract Surg. 2013;39(3):343–349. doi: 10.1016/j.jcrs.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Vryghem JC, Heireman S. Visual performance after the implantation of a new trifocal intraocular lens. Clin Ophthalmol. 2013;7:1957–1965. doi: 10.2147/OPTH.S44415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law EM, Aggarwal RK, Kasaby H. Clinical outcomes with a new trifocal intraocular lens. Eur J Ophthalmol. 2014;24(4):501–508. doi: 10.5301/ejo.5000407. [DOI] [PubMed] [Google Scholar]
- 13.Piovella M, Colonval S, Kapp A, Reiter J, Cauwenberge F, Alfonso J. Patient outcomes following implantation with a trifocal toric IOL: twelve-month prospective multicentre study. Eye (Lond) 2019;33(1):144–153. doi: 10.1038/s41433-018-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M, Kim JH, Lim TH, Cho BJ. Comparison of reading speed after bilateral bifocal and trifocal intraocular lens implantation. Korean J Ophthalmol. 2018;32(2):77. doi: 10.3341/kjo.2017.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loicq J, Willet N, Gatinel D. Topography and longitudinal chromatic aberration characterizations of refractive-diffractive multifocal intraocular lenses. J Cataract Refract Surg. 2019;45(11):1650–1659. doi: 10.1016/j.jcrs.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Shajari M, Sonntag R, Ramsauer M, Kreutzer T, Vounotrypidis E, Kohnen T, Priglinger S, Mayer WJ. Evaluation of total corneal power measurements with a new optical biometer. J Cataract Refract Surg. 2020;46(5):675–681. doi: 10.1097/j.jcrs.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 17.Teshigawara T, Meguro A, Mizuki N. Influence of pupil dilation on the Barrett Universal II (new generation), Haigis (4th generation), and SRK/T (3rd generation) intraocular lens calculation formulas: a retrospective study. BMC Ophthalmol. 2020;20(1):299. doi: 10.1186/s12886-020-01571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu YN, Kou JJ, Chen DJ, Wang DD, Zhao YY, Hu M, Lin XL, Dai Q, Li JH, Zhao YE. Influence of angle kappa and angle alpha on visual quality after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2019;45(9):1258–1264. doi: 10.1016/j.jcrs.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Vallejo M, Piñero DP, Fernández J. Avoiding misinterpretations of Kappa angle for clinical research studies with Pentacam. J Optom. 2019;12(2):71–73. doi: 10.1016/j.optom.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, Wolffsohn JSW, Naroo SA. Optimizing measurement of subjective amplitude of accommodation with defocus curves. J Cataract Refract Surg. 2008;34(8):1329–1338. doi: 10.1016/j.jcrs.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Kuthirummal N, Vanathi M, Mukhija R, Gupta N, Meel R, Saxena R, Tandon R. Evaluation of Barrett universal II formula for intraocular lens power calculation in Asian Indian population. Indian J Ophthalmol. 2020;68(1):59–64. doi: 10.4103/ijo.IJO_600_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Zheng T, Lu Y. Effect of decentration on the optical quality of monofocal, extended depth of focus, and bifocal intraocular lenses. J Refract Surg. 2019;35(8):484–492. doi: 10.3928/1081597X-20190708-02. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Long T, Gu X, Ma T. Changes in angle kappa and angle alpha before and after cataract surgery. J Cataract Refract Surg. 2020;46(3):365–371. doi: 10.1097/j.jcrs.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 24.McNeely RN, Pazo E, Spence A, Richoz O, Nesbit AM, Moore TCB, Moore JE. Visual quality and performance comparison between 2 refractive rotationally asymmetric multifocal intraocular lenses. J Cataract Refract Surg. 2017;43(8):1020–1026. doi: 10.1016/j.jcrs.2017.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, Eom Y, Chung HW, Song JS, Jeong JW, Park SK, Kim HM. Factors for good near and distance visual outcomes of multifocal intraocular lens with inferior segmental near add. Graefes Arch Clin Exp Ophthalmol. 2020;258(8):1735–1743. doi: 10.1007/s00417-020-04761-1. [DOI] [PubMed] [Google Scholar]
- 26.Petzold A, Wilke C, Renner K, Kunert K. Retrospective study of long-term patient satisfaction after bilateral implantation of multifocal intraocular lenses of different generations-a 10 year follow-up. Klin Monbl Augenheilkd. 2019;236(8):969–975. doi: 10.1055/a-0842-6735. [DOI] [PubMed] [Google Scholar]
- 27.Venter JA, Pelouskova M, Collins BM, Schallhorn SC, Hannan SJ. Visual outcomes and patient satisfaction in 9366 eyes using a refractive segmented multifocal intraocular lens. J Cataract Refract Surg. 2013;39(10):1477–1484. doi: 10.1016/j.jcrs.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Cao K, Friedman DS, Jin S, Yusufu M, Zhang J, Wang J, Hou S, Zhu G, Wang B, Xiong Y, Li J, Li X, He H, Chai L, Wan XH. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv Ophthalmol. 2019;64(5):647–658. doi: 10.1016/j.survophthal.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Kaymak H, Breyer D, Alió JL, Cochener B. Visual performance with bifocal and trifocal diffractive intraocular lenses: a prospective three-armed randomized multicenter clinical trial. J Refract Surg. 2017;33(10):655–662. doi: 10.3928/1081597X-20170504-04. [DOI] [PubMed] [Google Scholar]
- 30.Jonker SMR, Bauer NJC, Makhotkina NY, Berendschot TTJM, van den Biggelaar FJHM, Nuijts RMMA. Comparison of a trifocal intraocular lens with a +3.0 D bifocal IOL: results of a prospective randomized clinical trial. J Cataract Refract Surg. 2015;41(8):1631–1640. doi: 10.1016/j.jcrs.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Lubiński W, Podborączyńska-Jodko K, Kirkiewicz M, Mularczyk M, Post M. Comparison of visual outcomes after implantation of AtLisa tri 839 MP and Symfony intraocular lenses. Int Ophthalmol. 2020;40(10):2553–2562. doi: 10.1007/s10792-020-01435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohnen T, Herzog M, Hemkeppler E, Schönbrunn S, De Lorenzo N, Petermann K, Böhm M. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52–62. doi: 10.1016/j.ajo.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 33.de Medeiros AL, de Araujo Rolim AG, Motta AFP, Ventura BV, Vilar C, Chaves MAPD, Carricondo PC, Hida WT. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin Ophthalmol. 2017;11:1911–1916. doi: 10.2147/OPTH.S145945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochener B. Prospective clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses. J Refract Surg Thorofare N J. 2016;32(3):146–151. doi: 10.3928/1081597X-20160114-01. [DOI] [PubMed] [Google Scholar]
- 35.Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507–514. doi: 10.3928/1081597X-20180530-02. [DOI] [PubMed] [Google Scholar]
- 36.Alió JL, Kaymak H, Breyer D, Cochener B, Plaza-Puche AB. Quality of life related variables measured for three multifocal diffractive intraocular lenses: a prospective randomised clinical trial. Clin Exp Ophthalmol. 2018;46(4):380–388. doi: 10.1111/ceo.13084. [DOI] [PubMed] [Google Scholar]
- 37.Alió JL, Pikkel J, editors. Multifocal intraocular lenses: the art and the practice. Springer Nature; 2019. [Google Scholar]
- 38.Plaza-Puche AB, Alio JL, Sala E, Mojzis P. Impact of low mesopic contrast sensitivity outcomes in different types of modern multifocal intraocular lenses. Eur J Ophthalmol. 2016;26(6):612–617. doi: 10.5301/ejo.5000777. [DOI] [PubMed] [Google Scholar]
- 39.Mojzis P, Kukuckova L, Majerova K, Liehneova K, Piñero DP. Comparative analysis of the visual performance after cataract surgery with implantation of a bifocal or trifocal diffractive IOL. J Refract Surg. 2014;30(10):666–672. doi: 10.3928/1081597X-20140903-06. [DOI] [PubMed] [Google Scholar]
- 40.Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737–747. doi: 10.1016/j.jcrs.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 41.Gundersen KG, Potvin R. Comparison of visual outcomes and subjective visual quality after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of apodized diffractive bifocal intraocular lenses. Clin Ophthalmol. 2016;10:805–811. doi: 10.2147/OPTH.S107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marques EF, Ferreira TB. Comparison of visual outcomes of 2 diffractive trifocal intraocular lenses. J Cataract Refract Surg. 2015;41(2):354–363. doi: 10.1016/j.jcrs.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 43.Domínguez-Vicent A, Esteve-Taboada JJ, Del Águila-Carrasco AJ, Monsálvez-Romin D, Montés-Micó R. In vitro optical quality comparison of 2 trifocal intraocular lenses and 1 progressive multifocal intraocular lens. J Cataract Refract Surg. 2016;42(1):138–147. doi: 10.1016/j.jcrs.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 44.Bilbao-Calabuig R, González-López F, Amparo F, Alvarez G, Patel SR, Llovet-Osuna F. Comparison between mix-and-match implantation of bifocal intraocular lenses and bilateral implantation of trifocal intraocular lenses. J Refract Surg. 2016;32(10):659–663. doi: 10.3928/1081597X-20160630-01. [DOI] [PubMed] [Google Scholar]
- 45.Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi: 10.1007/s00417-018-4052-3. [DOI] [PubMed] [Google Scholar]
- 46.Esteve-Taboada JJ, Domínguez-Vicent A, Del Águila-Carrasco AJ, Ferrer-Blasco T, Montés-Micó R. Effect of large apertures on the optical quality of three multifocal lenses. J Refract Surg. 2015;31(10):666–676. doi: 10.3928/1081597X-20150928-01. [DOI] [PubMed] [Google Scholar]
- 47.Ruiz-Mesa R, Abengózar-Vela A, Aramburu A, Ruiz-Santos M. Comparison of visual outcomes after bilateral implantation of extended range of vision and trifocal intraocular lenses. Eur J Ophthalmol. 2017;27(4):460–465. doi: 10.5301/ejo.5000935. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Mesa R, Abengózar-Vela A, Ruiz-Santos M. A comparative study of the visual outcomes between a new trifocal and an extended depth of focus intraocular lens. Eur J Ophthalmol. 2018;28(2):182–187. doi: 10.5301/ejo.5001029. [DOI] [PubMed] [Google Scholar]
- 49.Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. AcrySof IQ PanOptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens. Asia - Pac J Ophthalmol. 2019;8(4):335–349. doi: 10.1097/APO.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedrotti E, Bruni E, Bonacci E, Badalamenti R, Mastropasqua R, Marchini G. Comparative analysis of the clinical outcomes with a monofocal and an extended range of vision intraocular lens. J Refract Surg. 2016;32(7):436–442. doi: 10.3928/1081597X-20160428-06. [DOI] [PubMed] [Google Scholar]
- 51.Chang DH, Rocha KM. Intraocular lens optics and aberrations. Curr Opin Ophthalmol. 2016;27(4):298–303. doi: 10.1097/ICU.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 52.Eppig T, Filser E, Goeppert H, Schroeder AC, Seitz B, Langenbucher A. Index of contrast sensitivity (ICS) in pseudophakic eyes with different intraocular lens designs. Acta Ophthalmol. 2015;93(3):e181–e187. doi: 10.1111/aos.12538. [DOI] [PubMed] [Google Scholar]
- 53.Crnej A, Buehl W, Greslechner R, Hirnschall N, Findl O. Effect of an aspheric intraocular lens on the ocular wave-front adjusted for pupil size and capsulorhexis size. Acta Ophthalmol. 2014;92(5):e353–e357. doi: 10.1111/aos.12344. [DOI] [PubMed] [Google Scholar]
- 54.Kretz FTA, Muller M, Gerl M, Gerl RH, Auffarth GU. Binocular function to increase visual outcome in patients implanted with a diffractive trifocal intraocular lens. BMC Ophthalmol. 2015;15(1):110. doi: 10.1186/s12886-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Linden JW, van Velthoven M, van der Meulen I, Nieuwendaal C, Mourits M, Lapid-Gortzak R. Comparison of a new-generation sectorial addition multifocal intraocular lens and a diffractive apodized multifocal intraocular lens. J Cataract Refract Surg. 2012;38(1):68–73. doi: 10.1016/j.jcrs.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 56.Chang PY, Wang JK, Weng HY, Chang SW. Cataract surgery reduces intraocular pressure but not posture-induced intraocular pressure changes in patients with angle-closure glaucoma. Sci Rep. 2019;9(1):1–7. doi: 10.1038/s41598-019-50598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez CI, Chansangpetch S, Nguyen A, Feinstein M, Mora M, Badr M, Masis M, Porco T, Lin SC. How to predict intraocular pressure reduction after cataract surgery? A prospective study. Curr Eye Res. 2019;44(6):623–631. doi: 10.1080/02713683.2019.1580375. [DOI] [PubMed] [Google Scholar]
- 58.Xu BY, Chiang M, Chaudhary S, Kulkarni S, Pardeshi AA, Varma R. Deep learning classifiers for automated detection of gonioscopic angle closure based on anterior segment OCT images. Am J Ophthalmol. 2019;208:273–280. doi: 10.1016/j.ajo.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahbaz I. Importance of multifocal intraocular lenses in the elderly population. J Ophthalmol Res. 2020;3(1):8–15. [Google Scholar]