Abstract
The severity of osteoporosis in humans manifests in its high incidence and by its complications that diminish quality of life. A societal consequence of osteoporosis is the substantial burden that it inflicts upon patients and their families. Several bone-modifying drugs have been prescribed to patients with osteoporosis. However, evidence for their anti-fracture efficacy remains inconclusive. To the contrary, long-term use of anti-osteoporotic drugs such as bisphosphonates and Denosumab, an RANKL inhibitor, have resulted in adverse events. We now present an alternative and adjuvant approach for treatment of osteoporosis. The data derive from in vivo studies in an ovariectomized rat model and from a randomized double blind, placebo-controlled human clinical study. Both studies involved treatment with Panaceo Micro Activation (PMA)-zeolite-clinoptilolite, a defined cation exchange clinoptilolite, which clearly improved all bone histomorphometric parameters examined from ovariectomized animals, indicative for increased bone formation. Moreover, intervention with PMA-zeolite-clinoptilolite for one year proved safe in humans. Furthermore, patients treated with PMA-zeolite-clinoptilolite showed an increase in bone mineral density, an elevated level of markers indicative of bone formation, a significant reduction in pain, and significantly improved quality of life compared with patients in the control (placebo) group. These encouraging positive effects of PMA-zeolite-clinoptilolite on bone integrity and on osteoporosis warrant further evaluation of treatment with PMA-zeolite-clinoptilolite as a new alternative adjuvant therapy for osteoporosis.
Keywords: Zeolite, clinoptilolite tuff, clinoptilolite Panaceo Micro Activation, osteoporosis, ovariectomized rats, double-blinded clinical trial
Impact statement
Osteoporosis and osteoarthritis are common chronic diseases that reduce quality of life and carry a substantial financial burden. Despite pharmacological advances, the burden of osteoporosis remains problematic and current pharmacotherapies often come with adverse side effects. Here, we present an innovative and cost-effective approach for treating osteoporosis based on studies with an animal model of osteoporosis and a first in human clinical trial. The approach takes advantage of properties exhibited by zeolites, particularly Panaceo Micro Activation (PMA)-clinoptilolite, a surface modified zeolite with ion exchange properties. Intervention of osteoporotic animals and patients with PMA-zeolite-clinoptilolite yielded sufficient beneficial markers of bone formation and maintenance of bone integrity to warrant further evaluation of this compound, alone or in combination with a current therapy, for the treatment of osteoporosis.
Introduction
Osteoporosis is a major public health issue with consequential complications such as hip and vertebral fractures due to compromised bone integrity. The disease causes diminished quality of life and imposes a substantial burden on patients, their families, and society as a whole.1 A primary cause of osteoporosis in females is menopausal loss of estrogens.2 However, numerous other conditions increase the risk of debilitating fractures including compromised bone microarchitecture, quality of osteoid, and demineralization that decreases bone strength.2 Further evidence suggests that the gut microbiome may also play an essential role in determining bone integrity.3,4 Since 1994, the benchmark for the diagnosis of osteoporosis has been based on bone mineral density (BMD), alterations in which occur when the homeostatic balance between bone resorption and bone formation is disrupted. Increased bone resorption results in reduced bone mass and increased risk of fracture. Thus, the pharmaceutical industry has focused on therapeutics that suppress bone resorption or stimulate bone formation. Neither approach, however, has effectively resolved the problem of osteoporosis nor decreased its incidence. Both approaches have the potential to cause unanticipated and severe adverse events.5
As an alternative to treatment and management of osteoporosis with biotherapeutics, such as bisphosphonates and Denosumab, an receptor activator of nuclear factor kappa-Β ligand (RANKL) inhibitor,6,7 others have proposed the use of natural products, such as calcium-containing zeolite granules,8 or strong anti-oxidants such as quercetin, curcumin, and phytoestrogens or administration of anti-resorptive compounds such as flavonoids, terpenoids, or polyphenols in olive oil.9,10 The experimental basis upon which these proposals are founded is limited to in vitro or in vivo animal studies.
Here, we present preclinical results and clinical data that support the use of the activated zeolite, PMA-zeolite-clinoptilolite, as a new therapeutic alternative for treating osteoporosis. It should be noted that PMA-zeolite-clinoptilolite has been registered as a medical device for several years according to the European directive 93/42/EEC and tested in a clinical trial11 but not for treatment of osteoporosis. The impetus for pursuing the proposition that treatment with PMA-zeolite-clinoptilolite may provide benefit to patients with osteoporosis derives from several sources. In addition to anecdotal physician reports describing improved bone density in patients who received treatment with PMA-zeolite-clinoptilolite, there are previously documented cases describing positive effects of zeolites on bones.12–14 Soluble silica promotes biological and mechanical properties of bone by stimulating osteogenesis-mediated gene expression and expression of genes that suppress osteoclastogenesis14 and stimulating collagen type 1 synthesis and osteoblast differentiation.15
Clinoptilolite is a crystalline, hydrated aluminosilicate with a highly hydrophilic three-dimensional crystal structure that can retain or shed water reversibly. It also acts as a cation exchanger without changing its basic crystal structure. The large structural cavities and channels contain water molecules which form hydration spheres around exchangeable cations. Upon removal of water by heating to 100°C, clinoptilolite behaves like a molecular sieve allowing small molecules to pass through its channels but excluding larger molecules. Furthermore, clinoptilolite may adsorb polar molecules with high selectivity.16 In the last decade, the potential clinical utility of natural zeolite-clinoptilolite materials as medical devices or therapeutics has become intriguingly apparent.17–20 The potential use of clinoptilolite for clinical purposes remains elusive since well-designed animal studies and clinical trials are lacking. Furthermore, the absence of standardized preparations of the clinoptilolite and the necessary quality control raises justifiable concerns over their use for medical purposes. We now describe experiments in rats with ovariectomy-induced osteoporosis plus data from a double-blinded human clinical trial showing that treatment with a well-defined double-activated clinoptilolite, designated PMA-zeolite-clinoptilolite,21 has a beneficial effect on bone density in osteoporosis patients.
Materials and methods
Source and preparation of zeolite materials
Double-activated zeolite-clinoptilolite (PMA-zeolite-clinoptilolite) and clinoptilolite with added dolomite at 10% (Clino+dol) were provided by Panaceo International GmbH, Finkensteinerstr. 5, A-9585, Goedersdorf, Austria. Synthetic zeolite-A was obtained from A. E. Fischer-Chemie (Germany). Detailed physical–chemical properties and preparation of PMA-zeolite-clinoptilolite are described in Kraljević Pavelić et al.21
Animals
Female Wistar HsdBrlHan rats were obtained from the breeding colony at Institute for Medical Research and Occupational Health in Zagreb and maintained under pathogen-free conditions in steady state micro-environment cages. They were fed with a standard good laboratory practice (GLP) certified chow ad libitum (Dieta Standard 4RF21 from Mmucedola s.r.l., Italy; the certificate of ingredients and analysis is given in the Supplementary Material 1), with free access to tap water and alternating 12 h light and dark cycles. Sixty 5-month-old rats were split into six groups of 10 animals each. Experimental rats were ovariectomized (subjected to total hysterectomy) to induce an osteoporotic condition (loss of bone density) using a dorsal approach under general anesthesia with i.p. Narketan, (80 mg/kg) and Xylapan (12 mg/kg) purchased from Chassot AG, Bern Switzerland. Control rats were either sham-operated (operation but without ovariectomy) or ovariectomized without any treatment. One month after ovariectomy, experimental and control rats were treated daily with test substances. The six groups of 10 animals were treated daily post-surgery by gavage with an intra-gastric probe for 16 weeks. The group design was as follows:
Group #1 (Negative Control, sham-operated) treated with 3 mL redistilled H2O
Group #2 (Control, Ovariectomized − Ovx) treated with 3 mL redistilled H2O
Group #3 (Control 2, Ovariectomized − Ovx) treated with 3 mL suspension of alendronate in H2O (5 mg/kg)
Group #4 (Experimental, Ovariectomized − Ovx+Zeolite A) treated with 3 mL suspension of zeolite-A in H2O (8 g/kg)
Group #5 (Experimental, Ovariectomized − Ovx+Clino/dol) treated with 3 mL suspension of PMA-zeolite-clinoptilolite in H2O with addition of dolomite to 10% (Clino+dol at 8 g/kg)
Group #6 (Experimental, Ovariectomized – Ovx + PMA) treated with 3 mL suspension of PMA-zeolite-clinoptilolite in H2O (PMA at 8 g/kg)
Tissue sampling and analysis
Following final treatment, animals were euthanized by exsanguination under general anesthesia. The left femur and tibia were harvested and stored in 70% ethanol until further analysis. Femur and tibia BMD and bone mineral content (BMC) were measured using an Hologic QDR 4000 bone densitometer (dual-energy X-ray absorptiometry, dual x-ray absorptiometry (DXA), Hologic, Bedford, MA, USA), and changes in bone architecture and in cancellous bone of the proximal tibia and distal femoral metaphysis were assessed using microcomputerized tomography (Skyscan µCT, Bruker). The X-ray energy was set to 50 kV (strength 202 µA). For histomorphometric analysis, a pixel value of 18 µm was selected, and an aluminum filter (0.5 mm) was applied to improve bone visualization; 303 projections were obtained using rotational shift of 0.6° over a full rotation. The scan time for a single sample was approximately 12 min. The reconstructed µCT images were analyzed using NRecon software (Bruker) with standardized histomorphometric values. Histomorphometric values were obtained using the CT Analyzer software (Omicron) based on cross-sectional images of the proximal tibiae, where the cubical regions of interest (2 × 2 × 1 mm) were designated and used for calculation. The following histomorphometric values were analyzed: bone volume (BV) fraction (BV/total volume (TV) – %), bone surface (BS)/volume ratio (BS/BV – 1/mm), BS density (BS/TV – 1/mm), trabecular thickness (Tb.Th – mm), trabecular number (Tb.N – 1/mm), trabecular separation (Tb.Sp – mm), structure model index, total porosity (Po(tot) – %), and connectivity density (Conn.Dn – 1/mm³). The data were analyzed by multifactorial comparisons between ovariectomized rats (positive control), sham-operated rats (negative control), and rats subjected to various experimental treatments using Statistica, version 13.2 (Duncan test at P < 0.05).
Aluminum concentration in bones and liver
The distribution of aluminum in the liver was assessed by low-energy X-ray fluorescence (LEXRF) microscopy. The X-ray source was synchrotron radiation (Elettra Synchrotron Trieste, Italy, Beamline: TwinMic). Dried organ samples were cryo-sectioned at −23°C into 10 µm thick slices and flattened by pre-cooled filter paper. The flattened sections were freeze-dried and mounted onto gold folding grids for analysis with the TwinMic spectromicroscope.22 The brightfield and differential phase contrast images were acquired at 1.95 keV in scanning transmission mode23 with a spot size of 1.2 µm in diameter. Images were supplemented with LEXRF microscopy emission maps of C, O, O/C ratio, Al, Si, and other trace elements (e.g. Mg and Zn). The data showing no accumulation of aluminum in the rat liver during the intervention with PMA are presented in the Supplementary Material 2 (Supplementary Figure 2).
Clinical study
Inclusion and exclusion criteria
The study enrolled 100 osteoporotic patients (6 male and 94 female patients, Croatian Caucasians) ranging in age from 56 to 74 years. This included a subgroup of 20 patients with type 2 diabetes. To be eligible for the study, all patients had to have a BMD T-score of 2.5 or lower. They also had to be untreated or to have failed treatment. All female participants had postmenopausal osteoporosis. All subjects completed a medical history file and underwent an initial blood chemistry panel analysis. Subjects were excluded if they had other severe diseases such as cancer, autoimmune disease, chronic renal failure, and secondary osteoporosis. Female subjects who were pregnant were also disqualified.
Methodology
Subjects were randomized into two groups each of which received predefined medication or placebo. Subjects were instructed to maintain their daily diet and lifestyle throughout the study but to restrict intake of drugs and supplements except for Vitamin D3. All subjects received boxes containing either PMA-zeolite-clinoptilolite or placebo to be taken for a period of 12 months. The subjects in the treated group (n = 50) received 9 g/day PMA-zeolite-clinoptilolite powder (Panaceo International GmbH, Villach, Austria). The placebo-group (n = 50) received microcrystalline cellulose powder which was similar in appearance to the PMA-zeolite-clinoptilolite. All subjects were instructed to take 1 spoon of the powder dissolved in a glass of water three times daily: with breakfast, with lunch, and with dinner for the full 12 months. To ensure compliance, the subjects were contacted every three months for a complete check-up and health monitoring and to receive motivation to remain compliant with the protocol.
Markers of osteoporosis status
Subjects were monitored at the start of the study, at 6 months and at 12 months. The markers used were indicators of bone formation or bone maintenance and included: (1) assessment of BMD as a function of time; (2) changes in levels of osteocalcin, a marker of bone remodeling; and (3) changes in bone remodeling based on the Beta-crosslaps C-terminal telopeptide (CTx) assay, which measures collagen breakdown products in the serum including the CTx. The BMD was assessed by bone densitometry of lumbar vertebrae (L1–L4) and the femoral neck. Calcifications and deformities due to bone fractures were excluded to avoid potential skewing of data. In addition, subjects provided additional data including the frequency of falling and the occurrence of fractures. At the conclusion of the study, subjects were evaluated for general health status which was compared to that at the start of the study. In addition, subjects provided subjective assessment of the intensity of their musculoskeletal pain using a visual analog scale (VAS).
Statistical methods
Clinical trial data were analyzed using Dell Statistica, version 12 (2015). The variables are presented as frequencies or percentages and compared using the Pearson chi-square test or Fisher exact test, where appropriate. Normally distributed continuous variables (distribution tested with Kolmogorov–Smirnov test) are presented as means with standard deviation or as median with interquartile range. Comparisons of variables in two groups were done using the parametric t-test and non-parametric Mann Whitney U test. Pairwise comparisons were performed using the paired t-test or Wilcoxon matched pairs test. Multifactorial comparisons (between groups and subgroups or between study time points and groups) were carried out using factorial analysis of variance (ANOVA) or repeated measures ANOVA within factors, where appropriate. Overall health status at the end of the study was assessed by applying multiple logistic regression modeling. Statistical significance was determined at the level of 0.05.
Results
All markers of bone integrity assessed showed statistically significant differences between sham-operated control animals and those that were ovariectomized, confirming the validity of this rat osteoporotic model (Supplementary Table 1). The compromised bone quality of ovariectomized rats serves as a surrogate for osteopenia, which is a risk factor for bone fractures.24,25 Ovariectomized and control female rats were treated daily with each of the zeolite materials or with distilled water (vehicle) for 16 weeks. Upon termination of the study, the rats were assessed for several markers of bone integrity including BMD and BMC of the tibia and femur trabeculae. In addition, we quantified in each group the percent BV, BS density, trabecular thickness, the number of trabeculae and trabecular separation, as well as total porosity and connectivity density (Figures 1 and 2, Supplementary Table 2).
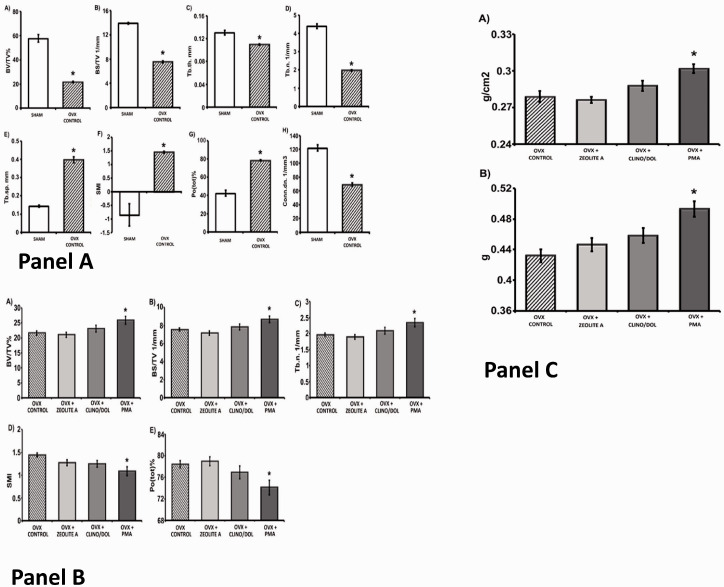
Figure 1.
Comparison of markers of tibia metaphysis bone integrity between control untreated ovariectomized rats and ovariectomized rats treated with zeolite A (Ovx + zeo A), PMA-zeolite-clinoptilolite + 10% dolomite (Ovx + clino/dol), and PMA-zeolite-clinoptilolite (Ovx + PMA) by ex vivo µCT. Panel A compares levels of markers of bone integrity at 16 weeks between sham-operated (group #1) and untreated ovariectomized rats (Ovx, group #2). Markers include (A) BV/TV%, (B) BS density based on TV presented as BS/TV 1/mm, (C) trabecular thickness (Tb.th mm), (D) trabecular number (Tb. N. 1/mm), (E) trabecular separation (Tb. Sp. mm), (F) SMI, (G) total porosity (Pot(tot)%), and (H) connectivity density (Conn. dn. 1/mm3). Data are presented as mean ± standard error of mean. *Significantly different from sham (analysis of variance (ANOVA) Duncan post hoc, P < 0.05, n = 9–10 in both groups). Panel B compares bone integrity between Ovx controls (group #2), animas treated with zeolite A (Ovx + zeo A, group #4), with PMA-zeolite-clinoptilolite + dolomite (Ovx + clino/dol, group #5), or with PMA-zeolite-clinoptilolite alone (Ovx + PMA, group #6). The markers include (A) percent BV BV/TV%, (B) BS density BS/TV 1/mm, (C) trabecular number (Tb. N. 1/mm), (D) SMI, (E) total porosity (Pot(tot)%). Panel C compares bone mineral density and bone mineral content after 16 weeks between untreated ovariectomized rats (Ovx control) with ovariectomized animals treated with zeolite A (Ovx + zeoA), with PMA-zeolite-clinoptilolite with addition of 10% dolomite (Ovx + clino/dol), or with PMA-zeolite-clinoptilolite alone (Ovx + PMA). In all cases, the data are presented as the mean ± standard error of the mean with an N = 9 or 10 in all groups. The asterisk denotes statistically significant difference from untreated ovariectomized controls (Ovx control) based on ANOVA Duncan post hoc, P < 0.05. BV: bone volume; BS: bone surface; TV: total volume; PMA: Panaceo Micro Activation; SMI: structure model index.
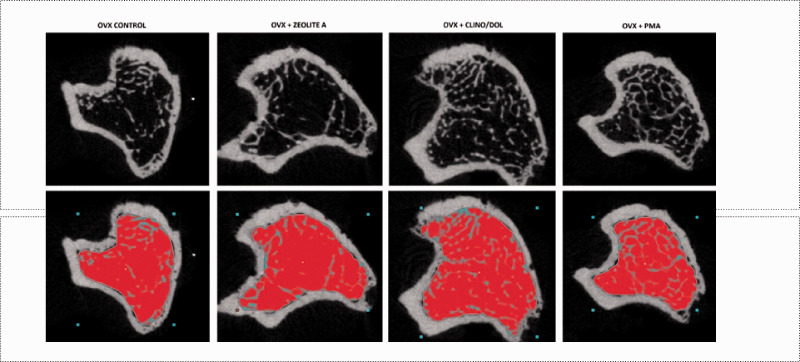
Figure 2.
Ex vivo µCT images of proximal tibia metaphysis in control, untreated ovariectomized rats and ovariectomized rats treated with zeolite A (Ovx + zeo A), PMA-zeolite-clinoptilolite + 10% dolomite (Ovx + clino/dol), and PMA-zeolite-clinoptilolite (Ovx + PMA). Images show horizontal sections of proximal tibia metaphysis in ovariectomized (Ovx control, group #2) and treated groups. Red area represents measured region of interest. From left to right: control (group #2), Ovx + Zeolite A (group #4), Ovx + Clino/dol (group #5), and Ovx + PMA-zeolite-clinoptilolite (group #6). PMA: Panaceo Micro Activation. (A color version of this figure is available in the online journal.)
There were significant differences in some, but not all, markers of bone integrity between control animals (groups #1, #2 and #3) and ovariectomized animals treated with PMA-zeolite-clinoptilolite (groups #5 and #6). These data are summarized in Supplementary Table 2. Bone mass (BV/TV) is represented by the ratio of BV to the TV of the bone region of interest (TV) and correlates directly with BMD in both regular and irregular trabecular distribution. The lowest BV/TV values were in the control ovariectomized-untreated (group #2) and the zeolite-A-treated group (group #4). The PMA-zeolite-clinoptilolite-treated animals (groups #5 and #6) had a significantly higher BV/TV value than the Ovx-untreated animals. Likewise, the BS density (BS/TV) was also significantly greater in PMA-zeolite-clinoptilolite-treated animals (groups #5 and #6) than in untreated ovariectomized animals (group #2). These data are consistent with the proposition that PMA-zeolite-clinoptilolite has a protective effect on osteoporotic bone in this rat model of osteoporosis. The PMA-zeolite-clinoptilolite promotes increased bone formation and reduced bone resorption after ovariectomy.
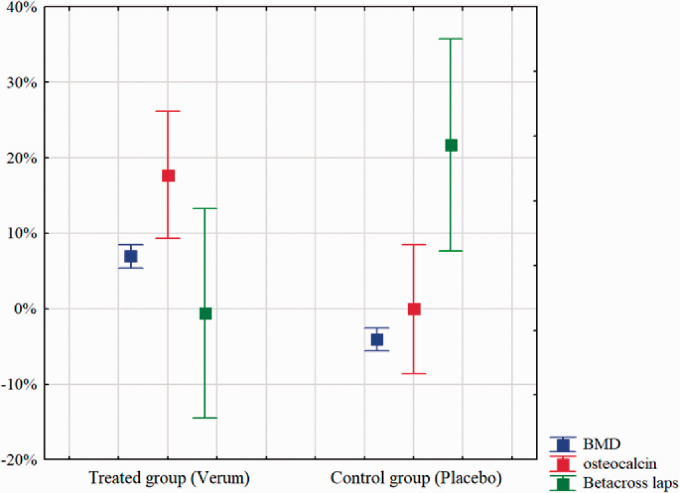
The encouraging response of PMA-zeolite-clinoptilolite treatment in the rat model of induced osteoporosis prompted us to initiate a double-blind clinical trial to test safety and efficacy of PMA-zeolite-clinoptilolite treatment of osteoporotic patients for one year. The study enrolled 100 participants. The average age of those receiving PMA-zeolite-clinoptilolite (experimental group) was 66 ± 8 years and that of the control group was 65 ± 10 years. Of the 100 enrollees, 81 completed a full year-long course. The study sample was well balanced with respect to participant age, gender, the diagnosis of diabetes, as well as to bone quality parameters (Supplementary Tables 5 and 6). Figures 3 and 4 and Supplementary Table 3 show that PMA-zeolite-clinoptilolite-treated subjects had elevated BMD values that were elevated at the end of the study compared to the start while the BMD values of the control group were decreased. The osteocalcin values showed a similar pattern except that osteocalcin levels in the control group did not increase (Supplementary Table 4, Figures 3 and 4). The beta crosslaps values were reduced in the treated subjects and increased in the control group, suggestive of reduced bone resorption in the former and increased resorption in the latter. The relative differences (Δ) between treated and control groups for all three parameters were expressed as the difference in value at the end and start of the study divided by the value at the start, expressed as a percent (Supplementary Table 4). The relative changes in values between two groups were statistically significant. The BMD in the PMA-zeolite-clinoptilolite-treated group increased by an average of 6.9% and decreased by 4.1% in the control group. Osteocalcin increased on average by 9% in the treated group compared with 0.7% in the control group. The betacross laps value, a measure of bone resorption, decreased by 0.6% in the treated group and increased by an average of 21.7% in the control group, indicative of continued bone resorption in the absence of treatment.
Figure 3.
Measures of bone integrity at the start and end of the study on osteoporosis patients within the presented clinical trial. A: BMD values at the beginning and the end of the study, measured in PMA-zeolite-clinoptilolite-treated group (Verum, Blue) and control (Placebo, Red) group. B: Osteocalcin values at the beginning and the end of the study, measured in PMA-zeolite-clinoptilolite-treated group (Verum, Blue) and control (Placebo, Red) group. C: Beta-cross laps values at the beginning and the end of the study, measured in PMA-zeolite-clinoptilolite-treated group (Verum, Blue) and control (Placebo, Red) group. Mean values with 95% confidence interval are presented. BMD: bone mineral density. (A color version of this figure is available in the online journal.)
Figure 4.
BMD, osteocalcin, and Betacross laps values at the beginning and the end of the clinical study on osteoporosis patients, measured in PMA-zeolite-clinoptilolite-treated group (Verum) and control (Placebo) group. Mean values with 95% confidence interval are presented. BMD: bone mineral density. (A color version of this figure is available in the online journal.)
As part of the quality of life assessment, subjects were queried about the incidence of accidental falls and of bone fractures prior to and during their one-year participation in the study. They were also queried about their pain level using the VAS as an assessment tool. In addition, we used a new subjective assessment of overall health status at the conclusion of the study. Subjects assessed their overall health condition on a scale from ranging range from 1 to 5 where 1 is “much worse than before entering the study”; 2 is “worse than before entering the study”; 3 is “unchanged”; 4 is “better than before entering the study”; and 5 “much better than before entering the study.” Based on this subjective assessment, there appears to have been a perceived but not statistically significant improvement in overall health. The treated group gave an average score of 4 compared with the control group with an average score of 3.
There was no apparent difference in frequency of new bone fractures prior to, during, and at the end of the study between control and PMA-zeolite-clinoptilolite-treated groups, although the numbers are too small to be objective. The same was true for the frequency of accidental falls recorded. Both groups reported a reduction in pain level over the course of the study, which may have been a placebo effect.
The change in overall health status between start and end of study was based on the VAS criteria described earlier and presented as ΔVAS. The overall health status at the end of the study was estimated to be significantly better in the treated group having an average score of 4 (better at end than at start of the study) compared with the control group which had an average score of 3 (registered as unchanged). A multiple regression analysis was performed to identify correlates with overall health estimates. The only such correlate identified was the change in pain level (ΔVAS) (Supplementary Table 7). The multiple regression analysis was collapsed into a single correlation with a coefficient of −0.74 and P < 0.001 (Supplementary Figure 1), indicative of a highly significant correlation.
Discussion
Zeolites are comprised of a large family of about 40 naturally occurring highly porous minerals of which clinoptilolite is the most abundant member. In addition, there are more than 150 artificial, synthetic zeolites. Zeolites generally consist of hydrated Si044− and Al045− aluminosilicate tetrahedra and act as effective ion exchangers with multiple industrial and potential medical applications. Many of the characteristics that render zeolites attractive for industrial and medical use have been summarized and reviewed with a particular emphasis on clinoptilolite.26,27 The PMA-zeolite-clinoptilolite used in this study is a registered clinoptilolite medical device. While there are limited data on the effects of clinoptilolite on human physiology, the available data indicate that its administration to healthy alcohol drinkers has no adverse effects.28 Similarly, it has been given orally to patients for the treatment of dyslipidemia with no effect on blood lipid levels nor other harmful consequences.29 Clinoptilolite has been used to successfully control severe diarrheal diseases in cattle and pigs,30 and its efficacy and tolerability led to the human anti-diarrheal drug Enterex, a drug developed in Cuba. Based on its immunomodulatory and biological ion-exchange capacities, the beneficial clinical properties of clinoptilolite have been accepted by the pharmaceutical communities in the European Union and the USA as safe for human use https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2013.3039.31 Until recently, clinical use of natural clinoptilolite material in humans has been hampered by the inconsistency of its chemical composition, which depends, in part, on its source of origin. To be accepted by the medical community, the clinical application of zeolites requires that they be chemically consistent and non-toxic. The PMA-zeolite-clinoptilolite material used in this study complies with the European directive 93/42/EEC and has been tested in a clinical trial for intestinal issues.11
This study supports PMA-zeolite-clinoptilolite as a safe and effective intervention for osteopenia. Administration of PMA-zeolite-clinoptilolite to osteoporotic rats over a 16-week period yielded statistically significant improvements in markers of bone density and bone architecture as well as in multiple morphometric characteristics. The likelihood of aluminum release following long-term treatment appears not to be consequential since our previous study showed that in animals treated with PMA-zeolite-clinoptilolite there is no such release.21 In addition, data from a clinical trial confirm the safety of PMA-zeolite-clinoptilolite with respect to aluminum release.11
Data from the rat osteoporosis model suggest that treatment with PMA-zeolite-clinoptilolite has beneficial effects and is encouraging for the use of PMA-zeolite-clinoptilolite treatment of osteoporotic patients. Our current on-going trial provides data for a one-year course of treatment and has shown no adverse effects. A subset of markers of bone integrity provides statistically significant improvement with treatment. However, the one-year timeframe is insufficient for establishing the overall positive effects of PMA-zeolite-clinoptilolite treatment of osteoporotic patients. Therefore, patients who concluded year 1 of the trial have been immediately enrolled into the second, third, and fourth year of the study, for which data are not yet available and will be presented separately upon trial completion.
The mechanisms by which PMA-zeolite-clinoptilolite exerts its beneficial effect on bone mineralization and bone integrity are still unclear. Potential molecular mechanisms are confounded by the observation that detectable amounts of PMA-zeolite-clinoptilolite do not directly enter the bloodstream but act in the digestive tract and are excreted through feces. As mentioned earlier, zeolites consist of a hydrated aluminosilicate substance from which soluble silica can be released in the digestive tract from where it can be absorbed into the bloodstream.20 It is noteworthy that minute levels of silica may be beneficial for bone integrity while a deficiency of silica may have deleterious effects.31–34 These and other observations have led us to hypothesize that the soluble Si released from PMA-zeolite-clinoptilolite may, at least partially, contribute to the observed effects on bone. The requirement for Si in bone formation and preservation is well established but is not well understood mechanistically. A silica-deficient diet in chickens, for example, induces skeletal deformations and causes joint abnormalities and changes in collagen synthesis in the chick and in rats.31,32 Conversely, an elevated level of silica induces a significant increase in femoral BMD in osteoporotic females,35 consistent with increased bone quality in animals fed a dietary Si supplement.36 As a cautionary note, high levels of silica may also lead to decreased intestinal absorption of calcium and magnesium.37
Administration of orthosilicic acid as a source of silica in animal models induces numerous activities conducive to bone development and maintenance15,38,39 including stimulation of collagen type1 synthesis and of alkaline phosphatase activity. Orthosilicic acid also elevates osteocalcin in human osteoblast-like cells and enhanced osteoblastic differentiation in vitro. It is plausible, therefore, to propose that silica has the capacity to promote bone integrity through modulation of signaling pathways in cartilage and extracellular matrix maintenance. Collagen maturation and deposition depends in part on silica due to its involvement in prolyl hydroxylase activity.15,34 In addition, silica participates in cross-linking proteoglycans and collagen within the bone matrix which is critical for bone stabilization and prevention of its enzymatic degradation. This observation is consistent with the reported benefit of a moderate silicon dietary supplement on bone integrity in postmenopausal females.40
An alternative mechanism underlying the activity of PMA-zeolite-clinoptilolite on bone integrity may entail the immune system since clinoptilolite administration increases the number of circulating CD4+ T cells and CD19+ B cells,41 both of which are decreased in osteoporotic females.42 Furthermore, administration of clinoptilolite increases the number of peritoneal macrophages, consistent with intestine being a primary site of action.43 Since the gut microbiota can modulate host immune responses44 and affect the status of bone health,4,45 it is plausible that under appropriate conditions PMA-zeolite-clinoptilolite functions cooperatively with the gut microbiota and the host immune system to promote good bone health. This hypothesis, however, needs further research. At last, we would also like to state some possible limitations of our work. The study may be considered a preliminary study on PMA effects in osteoporotic patients as it was done in only one clinical center and on a limited sample size. Conclusive data on the bone status may be therefore, obtained in additional, multi-centric study as well as upon completion of the five-year study period that is underway. The presented study has indeed been extended to a total of five years with the emphasis on monitoring of fractures occurrence, especially in cases of severe fallings. This portion of data will be published upon completion of the study as well.
In summary, our data are encouraging for the use of PMA-zeolite-clinoptilolite in a clinical setting, in particular for bone integrity in patients with osteoporosis. Pre-clinical studies in ovariectomy-induced osteoporotic rats provide “proof of concept” that oral administration of PMA-zeolite-clinoptilolite is well tolerated and that markers of bone integrity are elevated.
The human trial to date reflects results from the first year of the trial with continued enrollment to document further developments over several years. Nevertheless, despite the relatively short timeframe, some markers of bone integrity showed statistically significant improvements within that short timeframe. For osteoporosis, a long-term view is important. It is important to note that clinoptilolite has low-binding affinity to many existing anti-osteoporotic drugs that are used clinically but that have severe side effects. The bisphosphonates that impair bone resorption by inhibiting osteoclast activity are one such example. The independent action of the PMA-zeolite-clinoptilolite suggests that it may act in an additive way or synergistically with bisphosphonates if administered cooperatively, allowing administration of lower doses of the bisphosphonates and reducing risk of adverse events. Thus, PMA-zeolite-clinoptilolite presents as a potentially promising alternative or adjuvant therapy for osteoporosis.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220968752 for Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial by Sandra Kraljević Pavelić, Vedran Micek, Dragica Bobinac, Edo Bazdulj, Alessandra Gianoncelli, Dalibor Krpan, Marta Žuvić, Sandra Eisenwagen, Peter J Stambrook and Krešimir Pavelić in Experimental Biology and Medicine
Supplemental material, sj-pdf-2-ebm-10.1177_1535370220968752 for Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial by Sandra Kraljević Pavelić, Vedran Micek, Dragica Bobinac, Edo Bazdulj, Alessandra Gianoncelli, Dalibor Krpan, Marta Žuvić, Sandra Eisenwagen, Peter J Stambrook and Krešimir Pavelić in Experimental Biology and Medicine
Authors’ contributions
SKP, KP and DK – study design, conceptualization; SKP – project management and administration, laboratory logistics, experimental supervision and study oversight, literature search, writing – original draft preparation, literature search, discussion, analysis and interpretation of experimental and clinical data, visualization of statistical data from animal studies; SKP and AG – LEXRF analyses; VM – in vivo animal study, basic statistics of obtained animal data, visualization of statistical data from animal studies; DB and EB – measurements of the animal bones and interpretation of obtained parameters; DK – clinical trial supervision and logistic, participated in manuscript drafting; MZ – statistical analysis and visualization of statistical data from clinical trials; SE – technical support and technical logistics to the clinical trial registration and organization; PJS – writing – critical review of the manuscript and literature; and KP – principal project investigator, analyzed clinical data, writing – review and editing, funding acquisition.
Acknowledgements
Technical assistance of Mrs. Tanja Oberwinkler is greatly acknowledged in the preparation of animal studies documentation and at the outset of the human trial.
Declaration OF CONFLICTING INTERESTS: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SKP and KP are independent scientific advisors of Panaceo International Gmbh, Austria. SE is employed at Panaceo International Gmbh, Austria. Other authors do not have any competing interest to declare.
ETHICAL APPROVAL: The study was conducted according to the guidelines of the Declaration of Helsinki for Research on Human Subjects 1989 and was approved by the Ethical committee of the University of Rijeka, Department of Biotechnology on 3 April 2014 (reference number 001–2013). Clinical trial data are deposited at: https://register.clinicaltrials.gov/ under the registration number NCT03901989.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The studies were funded by the research grants from Panaceo International GmbH, Villach, Austria “Effects of the PMA-zeolite-clinoptilolite on bone metabolism. Experimental and clinical study” to the University of Rijeka (Rijeka, Croatia) to yield research data on different mineral materials performance in osteoporosis treatment in animals and humans.
SupplementAL MATERIAL: Supplemental material for this article is available online.
ORCID iDs
Sandra Kraljević Pavelić https://orcid.org/0000-0003-0491-673X
Alessandra Gianoncelli https://orcid.org/0000-0002-2457-3618
References
- 1.Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep 2010; 12:186–91 [DOI] [PubMed] [Google Scholar]
- 2.Ivanova S, Vasileva L, Ivanova S, Peikova L, Obreshkova D. Osteoporosis: therapeutic options. Folia Med (Plovdiv) 2015; 57:181–90 [DOI] [PubMed] [Google Scholar]
- 3.Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab 2015; 26:69–74 [DOI] [PubMed] [Google Scholar]
- 4.Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep 2015; 13:125–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabatabaei-Malazy O, Salari P, Khashayar P, Loijani B. New horizons in treatment of osteoporosis. Daru 2017; 25:2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorentzon M. Treating osteoporosis to prevent fractures: current concepts and future developments. J Intern Med 2019; 285:381–94 [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol 2017; 5:898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabiano A, Piras AM, Calderone V, Testai L, Flori L, Puppi D, Chiellini F, Zambito Y. A new calcium oral Controlled-Release system based on zeolite for prevention of osteoporosis. Nutrients 2019; 11:2467–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garzia-Martinez O, Rivas A, Ramos- Torrecilas J, DeLuna E, Ruiz C. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr 2014; 65:834–40 [DOI] [PubMed] [Google Scholar]
- 10.Banu J, Varela E, Guerra JM, Halade G, Williams PJ, Bahadur AN, Hanaoka K, Fernandes G. Dietary coral calcium and zeolite protects bone in a mouse model for postmenopausal bone loss. Nutr Res 2012; 32:965–75 [DOI] [PubMed] [Google Scholar]
- 11.Lamprecht M, Bogner S, Steinbauer K, Schuetz B, Greilberger JF, Leber B, Wagner B, Zinser E, Petek T, Wallner-Liebmann S, Oberwinkler T, Bachl N, Schippinger G. Effects of zeolite supplementation on parameters of intestinal barrier integrity, inflammation, redoxbiology and performance in aerobically trained subjects. J Int Soc Sports Nutr 2015; 12:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins KL, Vagnoni DB, Southern LL. Effect of dietarysodium zeolite a and excess calcium on growth and tibia calcium and phosphorus concentration in uninfected and Eimeria acervulina –infected chicks. Poult Sci 1989; 68:1236–40 [DOI] [PubMed] [Google Scholar]
- 13.Watkins KL, Southern LL. Effect of dietary sodium zeolite a and graded levels of calcium and phosphorus on growth, plasma, and tibia characteristics of chicks. Poult Sci 1992; 71:1048–58 [DOI] [PubMed] [Google Scholar]
- 14.Mladenović Ž, Johansson A, Willman B, Shahabi K, Björn E, Ransjö M. Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater 2014; 10:406–18 [DOI] [PubMed] [Google Scholar]
- 15.Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HF, Evans BA, Thompson RP, Powell JJ, Hampson GN. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003; 32:127–35 [DOI] [PubMed] [Google Scholar]
- 16.Mumpton FA. La roca magica: uses of natural zeolites in agriculture and industry. Proc Natl Acad Sci U S A 1999; 96:3463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collela C. A critical reconsideration of biomedical and veterinary applications of natural zeolites. Clay Miner 2011; 46:295–309. [Google Scholar]
- 18.Pavelic K, Hadžija M. Medical applications of zeolites. Handbook of zeolites science and technology. New York: Marcel Dekker Inc., 2003, pp. 1143–74 [Google Scholar]
- 19.Rodrigues Fuentes G, Rivera Denis A, Barrios Alvarez M, Iraizos Colarte A. Antacid drug based on purified natural clinoptilolite. Micropor Mesopor Mat 2006; 94:200–7 [Google Scholar]
- 20.Jurkić LM, Pavelić SK, Cepanec I, Pavelić K. Zeolites and orthosilicic acid: new perspectives for therapy. Nutr Metab (Lond) 2013; 10:2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraljević Pavelić S, Micek V, Filošević A, Gumbarević D, Žurga P, Bulog A, Orct T, Yamamoto Y, Preočanin T, Plavec J, Peter R, Petravić M, Vikić-Topić D, Pavelić K. Novel, oxygenated clinoptilolite material efficiently removes aluminium from aluminium chloride-intoxicated rats in vivo. Micropor Mesopor Mat 2017; 249:146–56 [Google Scholar]
- 22.Gianoncelli A, Kourousias G, Merolle L, Altissimo M, Bianco A. Current status of the TwinMic beamline at elettra: a soft X-ray transmission and emission microscopy station. J Synchrotron Radiat 2016; 23:1526–37 [DOI] [PubMed] [Google Scholar]
- 23.Gianoncelli A, Kourousias G, Altissimo M, Bedolla DE, Merolle L, Stolfa A, Shin H. Combining multiple imaging techniques at the TwinMic X-ray microscopy beamline. AIP Conf Proc 2016; 1764:030002–9 [Google Scholar]
- 24.Berry SD, McLean RR, Hannan MT, A, Cupples L, Kiel DP. Changes in bone mineral density (BMD) may predict the risk of fracture differently in older adults according to fall history. J Am Geriatr Soc 2014; 62:2345–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng HW, Xu FH, Davies KM, Heaney R, Recker RR. Differences in bone mineral density, bone mineral content, and bone areal size in fracturing and non-fracturing women, and their interrelationships at the spine and hip. J Bone Miner Metab 2002; 20:358–66 [DOI] [PubMed] [Google Scholar]
- 26.Kraljević Pavelić S, Simović Medica S, Gumbarević D, Filošević A, Pržulj N, Pavelić K. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front Pharmacol 2018; 9:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastinu A, Kumar A, Maccarinelli G, Anna Bonini S, Premoli M, Aria F, Gianoncelli A, Memo M. Zeolite clinoptilolite: therapeutic virtues of an ancient mineral. Molecules 2019; 24:1517–32[Mismatch [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federico A, Dallio M, Gravina AG, Iannotta C, Romano M, Rossetti G, Somalvico F, Tuccillo C, Loguercio C. A pilot study on the ability of clinoptilolite to absorb ethanol in vivo in healthy drinkers: effect of gender. J Physiol Pharmacol 2015; 66:441–7 [PubMed] [Google Scholar]
- 29.Cutovic M, Lazovic M, Vukovic-Dejanovic V, Nikolic D, Petronic-Markovic I, Cirovic D. Clinoptilolite for treatment of dyslipidemia: preliminary efficacy study. J Altern Complement Med 2017; 23:738–44 [DOI] [PubMed] [Google Scholar]
- 30.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific opinion on the safety and efficacy of clinoptilolite of sedimentary origin for all animal species. EFSA J 2013; 11:3039–52 [Google Scholar]
- 31.Schwarz K, Milne DB. Growth promoting effects of silicon in rats. Nature 1972; 239:333–4 [DOI] [PubMed] [Google Scholar]
- 32.Carlisle EM. Silicon: an essential element for the chick. Science 1972; 178:619–21 [DOI] [PubMed] [Google Scholar]
- 33.Jugdaohsingh R, Tucker KL, Qiao N, Cupples LA, Kiel DP, Powel JJ. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort. J Bone Miner Res 2004; 19:297–307 [DOI] [PubMed] [Google Scholar]
- 34.Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging 2007; 11:99–110 [PMC free article] [PubMed] [Google Scholar]
- 35.Spector TD, Calomme MR, Anderson SH, Clement G, Bevan L, Demeester N, Swaminathan R, Jugdaohsingh R, Berghe DA, Powell JJ. Choline-stabilized orthosilicic acid supplementation as an adjunct to calcium/vitamin D3 stimulates markers of bone formation in osteopenic females: a randomized, placebo-controlled trial. BMC Musculoskel Dis 2008; 9:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MH, Bae YJ, Choi MK, Chung YS. Silicon supplementation improves the bone mineral density of calcium-deficient ovariectomized rats by reducing bone resorption. Biol Trace Elem Res 2009; 128:239–47 [DOI] [PubMed] [Google Scholar]
- 37.Kayongo-Male H, Julson JL. Effects of high levels of dietary silicon on bone development of growing rats and turkeys fed semi-purified diets. Biol Trace Elem Res 2008; 123:191–201 [DOI] [PubMed] [Google Scholar]
- 38.Calomme MR, Vanden Berghe DA. Supplementation of calves with stabilised orthosilicic acid. Biol Trace Elem Res 1997; 56:153–64 [DOI] [PubMed] [Google Scholar]
- 39.Calomme R, Cos P, D’Haese PC, Vingerhoets R, Lamberts LV, De Broe ME, Van Hoorebeke C, Vanden Berghe DA. Absorption of silicon in healthy subjects. Metal ions in biology and medicine 5. Paris: John Libbey Eurotext, 1998, pp. 228–32 [Google Scholar]
- 40.Price TC, Koval KJ, Langford JR. Silicon: a review of its potential role in the prevention and treatment of postmenopausal osteoporosis. Int J Endocrinol 2013; 2013:316783–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivkovic S, Deutsch U, Silberbach A, Walraph E, Mannel M. Dietary supplementation with an activated zeolite clinoptilolite in immunodeficiency: effects on the immune system. Adv Ther 2004; 21:135–47 [DOI] [PubMed] [Google Scholar]
- 42.Breuil V, Ticchioni M, Testa J, Roux CH, Ferrari P, Breittmayer JP, Albert-Sabonnadière C, Durant J, De Perreti F, Bernard A, Euller-Ziegler L, Carle GF. Immune changes in post-menopausal osteoporosis: the immunos study. Osteoporos Int 2010; 21:805–14 [DOI] [PubMed] [Google Scholar]
- 43.Pavelic K, Katic M, Sverko V, Marotti T, Bosnjak B, Balog T, Stojkovic R, Radacic M, Colic M, Poljak-Blazi M. Immunostimulatory effect of natural clinoptilolite as a possible mechanism of its antimetastatic ability. J Cancer Res Clin Oncol 2002; 128:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazar V, Ditu L-M, Gradisteanu Pircalabioru G, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of gut microbiota and immune system interactions in infectious diseases. Front Immunol 2018; 9:1830–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep 2015; 13:363–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220968752 for Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial by Sandra Kraljević Pavelić, Vedran Micek, Dragica Bobinac, Edo Bazdulj, Alessandra Gianoncelli, Dalibor Krpan, Marta Žuvić, Sandra Eisenwagen, Peter J Stambrook and Krešimir Pavelić in Experimental Biology and Medicine
Supplemental material, sj-pdf-2-ebm-10.1177_1535370220968752 for Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial by Sandra Kraljević Pavelić, Vedran Micek, Dragica Bobinac, Edo Bazdulj, Alessandra Gianoncelli, Dalibor Krpan, Marta Žuvić, Sandra Eisenwagen, Peter J Stambrook and Krešimir Pavelić in Experimental Biology and Medicine