Highlights
-
•
AMD patients show weaker connection in the splenium and left optic radiation.
-
•
Verbal fluency scores correlate with connection in the splenium and left IFOF/ILF.
-
•
AMD is linked with weaker correlations of cognition and white matter connection.
Keywords: AMD, DTI, White matter connectivity, Quantitative anisotropy (QA), Verbal fluency
Abstract
Age-related macular degeneration (AMD) is a common retina disease associated with cognitive impairment in older adults. The mechanism(s) that account for the link between AMD and cognitive decline remain unclear. Here we aim to shed light on this issue by investigating whether relationships between cognition and white matter in the brain differ by AMD status. In a direct group comparison of brain connectometry maps from diffusion weighted images, AMD patients showed significantly weaker quantitative anisotropy (QA) than healthy controls, predominantly in the splenium and left optic radiation. The QA of these tracts, however, did not correlate with the visual acuity measure, indicating that this group effect is not directly driven by visual loss. The AMD and control groups did not differ significantly in cognitive performance. Across all participants, better cognitive performance (e.g. verbal fluency) is associated with stronger connectivity strength in white matter tracts including the splenium and the left inferior fronto-occipital fasciculus/inferior longitudinal fasciculus. However, there were significant interactions between group and cognitive performance (verbal fluency, memory), suggesting that the relation between QA and cognitive performance was weaker in AMD patients than in controls. This may be explained by unmeasured determinants of performance that are more common or impactful in AMD or by a recruitment bias whereby the AMD group had higher cognitive reserve. In general, our findings suggest that neural degeneration in the brain might occur in parallel to AMD in the eyes, although the participants studied here do not (yet) exhibit overt cognitive declines per standard assessments.
1. Introduction
Age-related macular degeneration (AMD) is a common eye disease in the macula, which is the portion of retina responsible for sharp, central vision (Bressler et al., 1988, National Eye Institute, 2011). AMD affects people over 50 years of age, and the annual incidence of AMD increases with age, accounting for approximately 30% of Americans over 75 years of age (National Eye Institute, 2011). AMD not only causes visual recognition difficulties (Stelmack et al., 2004, Szlyk et al., 2004), but also is associated with higher rates of cognitive decline in late-life (Pham et al., 2006, Woo et al., 2012), higher risk of incident dementia (Klaver et al., 1999), and worse performance on non-visually displayed cognitive tests, particularly on tasks of phonemic verbal fluency (Baker et al., 2009, Clemons et al., 2006, Whitson et al., 2010, Wong et al., 2002). However, the relation between vision loss and cognitive impairment, and the role of brain structure and function in this relation, remain unknown.
The relationship between sensory impairments and impaired cognitive functioning could be either causal or correlational (Whitson et al., 2018). Sensory and cognitive impairments might co-occur more frequently than expected by chance because they share common risk factors, such as aging, smoking, or genetics. Sensory impairments may cause cognitive declines directly or through mediating factors, such as differences in cognitive load, depression, social isolation, activity, or brain structure. Previous studies revealed substantial association between mild cognitive impairment and visual system deficits such as vascular fractal dimension in the retina, drusen-like regions in the peripheral retina, and pigment dispersion (DeBuc et al., 2018). Furthermore, AMD has been associated with differences in intrinsic neural connectivity patterns in the primary visual cortex and high-level language processing network (Zhuang et al., 2018). Szlyk and Little (2009) found that AMD patients exhibited increased task-related activation in the left prefrontal and superior and inferior parietal regions, compared to controls, in recognizing single words. More severe visual impairment, such as blindness that occurs in early life, has been shown to alter patterns of functional brain connectivity, specifically in decreased functional connectivity between the visual cortices and the parietal somatosensory, frontal motor and temporal regions (Liu et al., 2007). Although these studies have provided clear evidence of a link between AMD and cognition, and between AMD and the brain, it remains unknown whether brain-cognition relationships may be different in the context of AMD.
To better understand the relationship between AMD and cognitive function, here we investigated whether AMD alters cognitive systems at both behavioral and neural levels. To this end, AMD patients and age-matched healthy controls performed a battery of cognitive tests and underwent magnetic resonance imaging (MRI), including both structural and diffusion weighted images. A diffusion MRI connectometry approach (Yeh et al., 2013b, Yeh et al., 2016) was adopted to explore potential associations between white matter pathways and cognitive performance in both groups. This whole-brain, voxel-wise approach measures the density of diffusing water along major fiber bundles from diffusion MRI using Q-space diffeomorphic reconstruction. To avoid the low reliability problem of conventional tractography methods, this approach adopts a new local connectome method of measuring the density of the diffusion spins as the degree of connectivity between two adjacent voxels within a white matter fascicle. The mapping and analysis of the local connectome, termed as connectometry, tracks the difference along specific segment of fiber bundles, and can be combined with cognitive measures to reveal brain-behavior relations via permutation tests.
In this study we compared AMD with controls in their cognitive performance (e.g. verbal fluency, memory) and white matter connectivity, and also correlated their performance scores with corresponding diffusion weighted images (DWI) to explore group differences in the relation between white matter connectivity and cognitive performance.
2. Methods
2.1. Participants
Thirty-nine AMD patients (26 females, ages 62–94, mean age = 75.8) and 33 healthy controls (20 females, ages 56–85, mean age = 74.1) participated in this study. All participants were community-dwelling, right-handed (Edinburgh Handedness Inventory; Oldfield, 1971), fluent speakers of American English (English was the primary language used throughout adulthood by all participants and was the first language of all but one). Individuals with a diagnosis of dementia or who lacked capacity to provide consent were excluded. AMD patients were referred from local ophthalmology clinics; all had central visual impairment attributable to AMD in one or both eyes for at least one year, with visual acuity ranging from 0.1 (mild impairment) to 2 (severe impairment) per the Logarithm of the Minimal Angle of Resolution (LogMAR) (Kaiser, 2009), and no other significant eye disease. Thirty-six of the 39 AMD patients had at least some vision impairment in both eyes, and 22 patients had moderate to severe impairment in both eyes (the better eye LogMAR ≥ 0.3). All healthy controls underwent examination by an ophthalmologist to confirm normal vision without any significant eye diseases. All participants provided informed consent and were compensated for their time. All experimental procedures were approved by the Duke University Medical Center Institutional Review Board.
3. Cognitive tests
All participants performed a battery of cognitive tests chosen to be suitable for individuals with vision impairment, in that no tasks involved visual cueing or relied on visual ability (i.e., no reading, drawing, image recognition, etc.). Additionally, all of the tests were validated for use with auditory cues. These tests included the Controlled Oral Word Association Test with letters F, A, S (COWAT-FAS, Spreen and Benton, 1977), Animal Naming (Monsch et al., 1992, Sager et al., 2006), the Fuld Object Memory Evaluation (FOME) (Fuld et al., 1990), the Brief Test of Adult Cognition by Telephone (BTACT, administered in person) (Tun and Lachman, 2006), the Digit Span subtest from the Wechsler Adult Intelligence Scale Version III (WAIS-III) and the Logical Memory Test from the Wechsler Memory Scale Version III (WMS-III) (Iverson, 2001). To obtain separate, composite measures of verbal fluency and memory, we performed factor analyses of the individual tests. The verbal fluency factor was a composite of a summative measure of phonemic verbal fluency from the COWAT (summed score for letters F, A, and S) and four categorical verbal fluency measures, drawn from the FOME and the Animal Naming tests, that included the semantic categories, foods, things that make people happy, boys’ or girls’ names, and animals. We extracted a single factor with the highest eigenvalue of 2.94 as a representative measure of verbal fluency processes. The eigenvalues of the remaining factors were all lower than 1.0. The verbal fluency factor correlated highly with each of the five input measures, with Pearson r correlation coefficients of 0.86, 0.79, 0.79, 0.87, and 0.74, respectively. We also extracted a factor of memory with the highest eigenvalue of 2.18, based on the input measures of BTACT 15-word item recall, WMS-III Logical Memory immediate and delayed recall, and total item recall from the FOME, which is based on a selective reminding procedure. The correlations between the memory factor and the four input measures were 0.43, 0.95, 0.96, and 0.58, respectively. The verbal fluency factor correlated with the memory factor at a moderate level, r = 0.48. Each participant also performed tests of visual and auditory acuity. All the cognitive tests were performed after the MRI scans on the same day or a few days later. The duration of these cognitive tests was approximately 3 h for each participant.
4. MRI acquisition and imaging analysis
Scanning was performed on a 3.0 Tesla GE MR 750 whole-body 60 cm bore human scanner equipped with 40 mT/m gradients and a 150 T/m/s slew rate. An eight-channel head coil was used for radio frequency (RF) transmission and reception (General Electric, Milwaukee Wisconsin, USA). Sagittal T-1 weighted localizer images were acquired and used to define a volume for data collection and high order shimming. A semi-automated high-order shimming program was used to ensure global field homogeneity. High-resolution structural images were acquired using a 3D fSPGR pulse sequence (Repetition time [TR] = 8.156 s; echo time [TE] = 3.18 ms; TI = 450 ms; field of view [FOV] = 25.6 cm2; flip angle = 12°; voxel size = 1 × 1 × 1 mm; 166 contiguous slices, sense factor = 2). Diffusion MR scans were acquired for each participant (TR = 9,000 ms; TE = 85.6 ms; FOV = 25.6 cm2; flip angle = 90°; voxel size = 1 × 1 × 2 mm; 68 contiguous oblique-axial slices parallel to the AC-PC plane; 34 diffusion-weighted directions; b = 1000 s/mm2; 1 non-diffusion-weighted reference image).
The diffusion weighted images (DWI) were visually inspected for sufficient quality and orientation, then were preprocessed with DTIPrep (Liu et al., 2010), including slice-wise, interlace-wise, and gradient-wise intensity artifact correction, eddy-current distortion and motion correction. We reoriented the diffusion directions with these corrections. We performed diffusion MRI connectometry analyses using DSI Studio (Yeh et al., 2016) (http://dsi-studio.labsolver.org/) to investigate the relation between cerebral white matter connectivity and cognitive performance. We loaded the b-table extracted from the DWI and created an SRC image for each participant, which was reconstructed to generate an FIB file using the DSI Studio reconstruction function. Here we used the Q-Space diffeomorphic reconstruction (QSDR) approach which estimated the density of diffusing water at different orientations, i.e. the spin distribution function (SDF). Whereas diffusion-tensor based measures, such as fractional anisotropy (FA), reflect the rate of water diffusion along vectors principle eigenvectors of the tensor model, the quantitative anisotropy (QA) reflects the volume of water diffusing in a q-space distribution. Studies using MR phantoms have demonstrated that QA is more robust to the free water effect and is more adept at resolving partial volume effects due to crossing fibers (Yeh et al., 2013b), and is especially relevant for the current analysis, which relies on both scalar and tractography-based measures of white matter health. The QA map was calculated using QSDR in each participant’s native space, then normalized to the MNI QA map. The reconstruction quality was evaluated by the r-squared value of each image, and all but one participant’s images in this study were > 0.6 which suggests good registration results (http://dsi-studio.labsolver.org/Manual/Reconstruction). The exceptional participant’s r2 = 0.58, and his image passed manual inspection without any registration problems as well, so no participants were removed from this analysis. A connectometry database was further created by including the FIB images of all participants in DSI Studio with the Human Connectome Project (HCP) 1021 atlas (http://brain.labsolver.org/diffusion-mri-templates/hcp-842-hcp-1021) as the standard template.
We first performed a direct group comparison between AMD patients and controls in terms of participants’ SDF 3D maps, then correlated each participant’s SDF map in the connectometry database with corresponding cognitive performance (verbal fluency and memory) for all AMD and control participants combined together. Positive correlations between SDF maps and continuous variables (e.g. verbal fluency, memory) indicate stronger white matter connection strength associates with better cognitive performance. Two further interaction variables were calculated by multiplying the mean-corrected group variable by the mean-corrected verbal fluency/memory variables. Positive correlations between these two interaction variables and SDF maps indicates that the verbal fluency/memory effects are significantly stronger in controls than in AMD patients. In these analyses, connection strength was represented by the measure of QA which is similar to FA (fractional anisotropy), a widely used index in the DTI research field. Yeh et al. (2013a) has demonstrated the advantages of QA over FA in the performance of deterministic fiber tracking.
In each group-level connectometry analysis, the local connectomes were tracked using a deterministic fiber tracking algorithm (Yeh et al., 2013b). All tracts generated from bootstrap resampling were included. Tract trimming was performed with one iteration. The SDF was normalized. Significant tracts were reported at a t score threshold of 3, with a length threshold of 25 voxels and a tract density of 20 per voxel. To estimate the false discovery rate, we applied a total of 10,000 randomized permutations to each analysis to obtain the null distribution of the tract length. As age and year of education tend to affect behavioral performance and corresponding neural representations, we partialled these two variables out by taking them as extraneous variables in each of the analysis models reported in this study. We also performed test models by additionally partialling out gender in each analysis, and found that all effects of interest remain the same as in the original analysis, indicating that gender does not affect the effects in this study.
5. Results
Participant demographics and behavioral performance are reported in Table 1. There was no significant group difference between AMD patients and healthy controls in age, gender, or years of education, all p > 0.05. AMD patients showed significantly worse scores in visual acuity measures compared to controls, t = 6.15, for OD, t = 6.89, for OS, t = 7.82, for mean, all p < 0.001. Although hearing and cognitive scores tended to be worse in the AMD group, no significant group differences were detected in any cognitive measures or in the hearing test, all p > 0.05.
Table 1.
Participant demographics.
| AMD | control | |
|---|---|---|
| N | 39 | 33 |
| Age | 75.75 (8.84) | 74.14 (7.35) |
| Gender (female proportion) | 66.67% | 60.61% |
| Education | 14.74 (2.61) | 15.48 (2.09) |
| Pure tone average threshold, decibels (right ear) | 35.28 (17.83) | 33.52 (16.68) |
| Pure tone average threshold, decibels (left ear) | 39.51 (19.76) | 38.81 (16.55) |
| Visual acuity (OS)* | 0.64 (0.48) | 0.06 (0.07) |
| Visual acuity (OD)* | 0.64 (0.52) | 0.08 (0.09) |
| Visual acuity (mean)* | 0.64 (0.42) | 0.07 (0.07) |
| Verbal fluency (phonemic) | 33.87 (13.22) | 38.58 (16.70) |
| Verbal fluency (categorical) | 16.90 (4.77) | 19.06 (6.09) |
| Wechsler (initial) | 23.74 (6.30) | 24.94 (6.42) |
| Wechsler (delayed) | 19.51 (7.12) | 20.73 (7.91) |
| BTACT | 5.87 (2.70) | 5.85 (1.94) |
| Digit span | 3.72 (1.47) | 4.03 (1.51) |
| Fuld Cued Item Recall | 24.62 (4.30) | 25.12 (4.37) |
| Fuld Fluency - Names | 20.59 (6.86) | 20.88 (6.22) |
| Fuld Fluency - Food | 21.95 (5.74) | 23.55 (6.04) |
| Fuld Fluency – Happy | 14.08 (4.53) | 16.24 (5.10) |
Values provided are means, with standard deviation in parentheses.
Visual acuity is provided in the Logarithm of the Minimum Angle of Resolution (LogMAR).
OD = right eye; OS = left eye; BTACT = brief test of adult cognition by telephone.
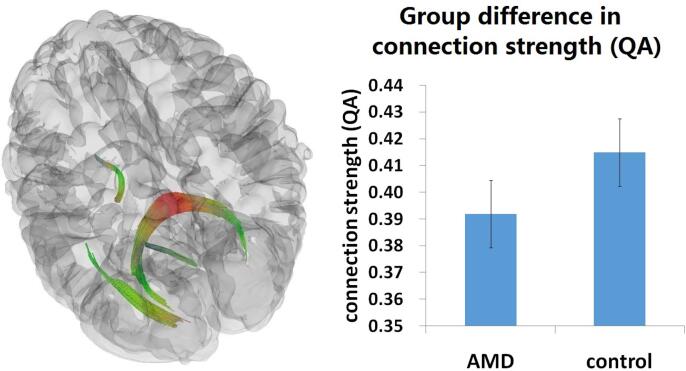
Turning to our connectometry results, it is important to clarify the multidimensional quality of these data. Connectometry delivers quantitative information on the volume, length, and fiber count of regions, as well as a quantitative measure of the volume of water diffusing along the fiber orientation. The quantitative measure, quantitative anisotropy (QA), is a normalized value which can be compared across subjects. In a direct group comparison on participants’3D SDF connectometry maps in DSI Studio, we found a significant group effect between AMD patients and controls in the splenium and left optic radiation (Fig. 1), p < 0.001, uncorrected, at the voxel level, and p < 0.05, cluster level corrected for multiple comparisons (this threshold was applied to all the following contrasts). The fiber region demonstrating this significant effect consisted of 6736 tract streamlines per million, and the mean length of all tract streamlines was 34.82 mm (standard deviation = 9.84 mm) with the tract volume of 8507 mm3. A comparison of connection strength (QA) in this contrast demonstrated substantially weaker connection strength (QA) in these tracts in the AMD patients compared to healthy controls (controls = 0.42, AMD = 0.39), as shown in the plot in Fig. 1. As this effect is a direct comparison between AMD patients and controls across the whole brain, we did not perform further t-tests on the plot data between these two groups to avoid duplicative comparisons. To further test whether these two significant tracts were related to the degree of visual loss in AMD, we correlated the visual acuity measure (the mean scores of both eyes) of AMD patients with their corresponding SDF connectometry maps, but did not find any significant effect in these two tracts. There were no white matter tracts that had significantly weaker connection strength (QA) in controls, compared to AMD patients, across the whole brain.
Fig. 1.
Group differences in Connection Strength (QA). A significant effect of group with weaker connectivity in AMD patients than controls rendered at p < 0.001, voxel level uncorrected, p < 0.05, cluster level corrected. Plots showing mean connection strength across these significant tracts within each group. A standard RGB code was used to label the spatial locations of tracts: red for left–right, green for anterior-posterior, and blue for dorsal–ventral. QA = quantitative anisotropy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
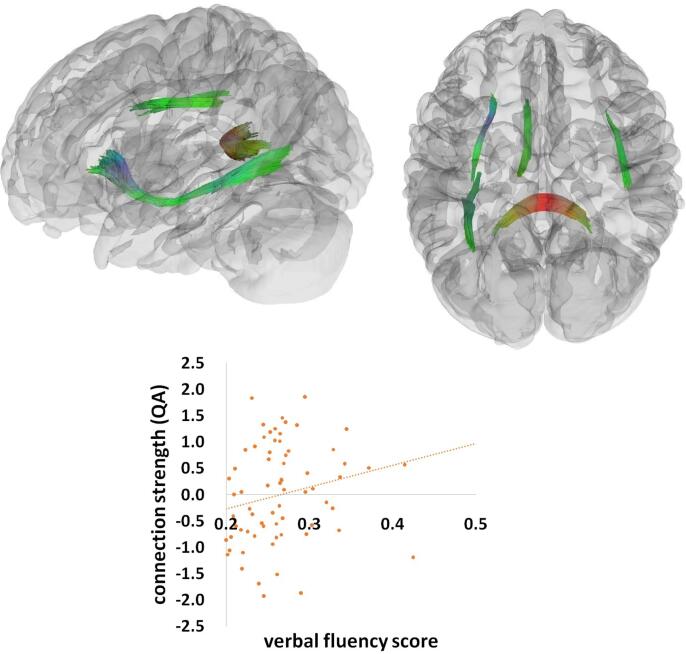
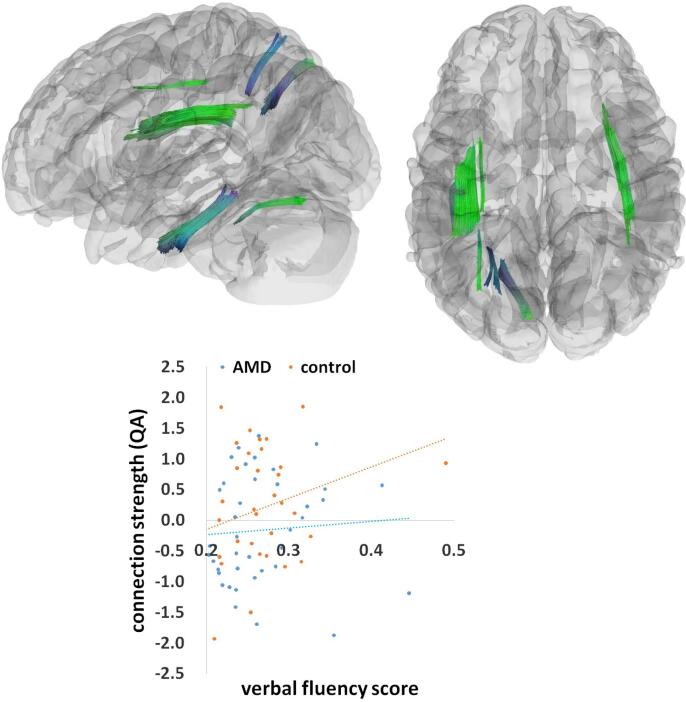
For all participants combined, there was a significant relation between verbal fluency factor and connection strength (QA), predominantly in the splenium and the left inferior fronto-occipital fasciculus (IFOF)/inferior longitudinal fasciculus (ILF), as well as in the left internal capsule and right arcuate fasciculus (Fig. 2). This significant effect consisted of 15,448 tract streamlines per million, and the mean length of all tract streamlines was 30.51 mm (sd = 5.07 mm) with the tract volume of 13942 mm3. Stronger connection strength (QA) in these tracts was significantly correlated with higher verbal fluency scores across all patients and controls (r = 0.25). We also found a significant interaction between verbal fluency and group, mainly in the superior and inferior parts of the left arcuate fasciculus, and additionally in the superior part of the right arcuate fasciculus, left superior longitudinal fasciculus (SLF), and left cingulum (Fig. 3). This effect contained 22,449 tract streamlines per million, and the mean length of all tract streamlines was 29.39 mm (sd = 4.33 mm) with the tract volume of 19700 mm3. This interaction effect indicated that in these tracts, the relation between white matter connection strength (QA) and cognitive performance was weaker in AMD patients (r = 0.07) than in healthy controls (r = 0.27).
Fig. 2.
The verbal fluency effect. A significant effect of verbal fluency across all participants rendered at p < 0.001, voxel level uncorrected, p < 0.05, cluster level corrected. Plot showing a linear correlation between tract connection strength and behavioral performance (verbal fluency scores) across all AMD and control participants. A standard RGB code was used to label the spatial locations of tracts: red for left–right, green for anterior-posterior, and blue for dorsal–ventral. QA = quantitative anisotropy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
The interaction between verbal fluency and group. A significant interaction effect between verbal fluency and group rendered at p < 0.001, voxel level uncorrected, p < 0.05, cluster level corrected. Plot showing linear correlations between tract connection strength and behavioral performance (verbal fluency scores) in the AMD and control groups separately. A standard RGB code was used to label the spatial locations of tracts: red for left–right, green for anterior-posterior, and blue for dorsal–ventral. QA = quantitative anisotropy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
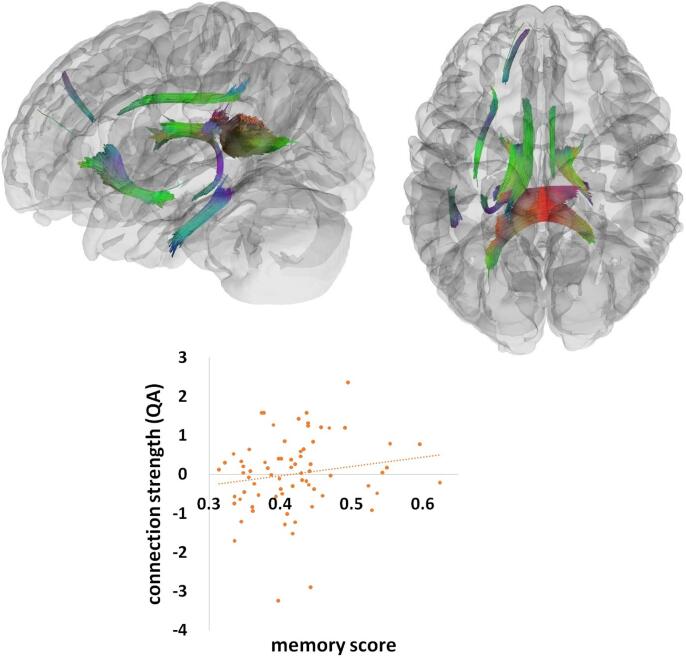
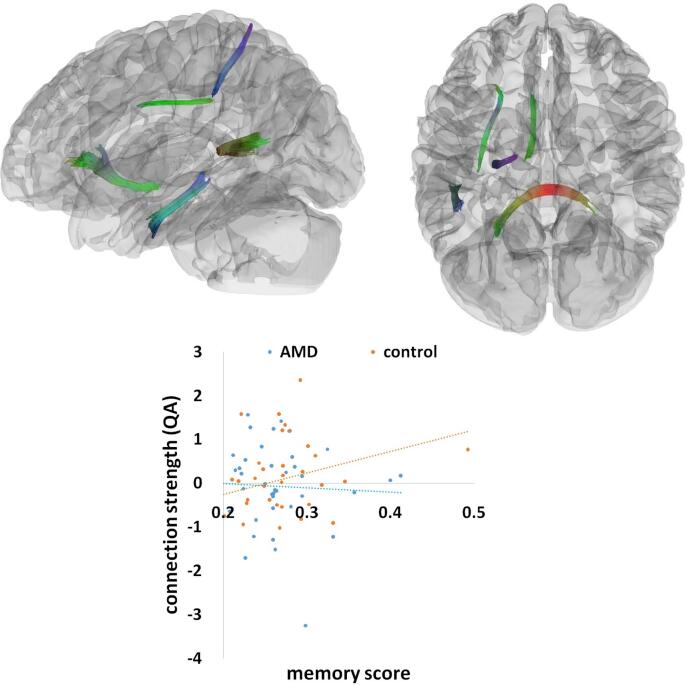
A similar analysis indicated that the memory factor significantly correlated with white matter connection strength (QA) in the splenium, the anterior part of the left IFOF, the inferior part of the left arcuate fasciculus, and the bilateral cingulum (Fig. 4). This effect was composed of 33,368 tract streamlines per million, and the mean length of all tract streamlines was 34.29 mm (sd = 8.87 mm) with the tract volume of 15423 mm3. Increasing memory scores were associated with stronger connection strength (QA) in these white matter tracts across all AMD patients and controls (r = 0.16). There was also a significant interaction effect between the memory factor and group in the splenium, the anterior part of the left IFOF, the inferior part of the left arcuate fasciculus, left cingulum, and the SLF (Fig. 5). This significant effect consisted of 5275 tract streamlines per million, and the mean length of all tract streamlines was 31.84 mm (sd = 6.87 mm) with the tract volume of 6055 mm3. The neural-behavioral correlations in these tracts were significantly weaker in AMD patients (r = -0.05) than healthy controls (r = 0.27).
Fig. 4.
The memory effect. A significant effect of memory rendered at p < 0.001, voxel level uncorrected, p < 0.05, cluster level corrected. Plot showing a linear correlation between tract connection strength and behavioral performance (memory scores) across all AMD and control participants. A standard RGB code was used to label the spatial locations of tracts: red for left–right, green for anterior-posterior, and blue for dorsal-ventral. QA = quantitative anisotropy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
The interaction between memory and group. A significant interaction effect between memory and group rendered at p < 0.001, voxel level uncorrected, p < 0.05, cluster level corrected. Plot showing linear correlations between tract connection strength and behavioral performance (memory scores) in the AMD and control groups separately. A standard RGB code was used to label the spatial locations of tracts: red for left–right, green for anterior-posterior, and blue for dorsal–ventral. QA = quantitative anisotropy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
6. Discussion
In this study we investigated the relation between cerebral white matter connection strength (QA) and cognitive performance in AMD patients and controls. Compared to controls, AMD patients showed weaker relationships between cognitive performance and white matter connection strength (QA) as well as lower white matter connection strength (QA), primarily in the splenium of the corpus callosum and left optic radiation. The optic radiation is the posterior part of the visual pathway from the lateral geniculate nucleus (LGN) to the primary visual cortex (Dayan et al., 2015). Macular degeneration in the eyes might trigger white matter deterioration in part of the visual pathway such as the left optic radiation. The function of the splenium is more complex since it is part of the visual system linking primary and secondary visual regions (Knyazeva, 2013), and also heavily engaged in higher level cognitive processes, such as language production and acquisition (Swanson et al., 2017). The white matter density measure in the splenium did not correlate with the measured visual acuity in AMD patients. Instead, decreasing density in the splenium significantly correlated with cognitive declines in verbal fluency and memory tests across all participants. From this cross-sectional analysis, we cannot determine whether neural deficits in the splenium were driven by macular degeneration in the eyes, although the lack of association with visual acuity suggests that it is not.
Several major white matter tracts were found to be associated with verbal fluency, memory and an interaction with group, including IFOF/ILF, AF, SLF, mainly in the left hemisphere. These findings are in agreement with previous research that these tracts play essential roles in processing language and memory information (Catani et al., 2005, Dick and Tremblay, 2012, Glasser and Rilling, 2008, Parker et al., 2005, Rauschecker and Scott, 2009). For example, IFOF or ILF is the white matter bundle that connects occipital cortex to the anterior temporal and inferior frontal cortex. IFOF/ILF has been implicated in language comprehension (Saur et al., 2008, Saur et al., 2010, Wong et al., 2011), particularly semantic working memory processing (Turken and Dronkers, 2011). AF is the major bundle connecting the posterior superior temporal regions to the inferior frontal regions or premotor regions, which has long been taken as the critical pathway engaged in speech processing and production (Catani et al., 2005, Rolheiser et al., 2011), semantic processing (Glasser and Rilling, 2008), reading (Yeatman et al., 2011), syntactic representation (Friederici, 2009, Papoutsi et al., 2011), verbal working memory (Meyer et al., 2014), and even non-linguistic sound localization and auditory-spatial awareness (Makris et al., 2005, Petrides and Pandya, 2002, Schmahmann and Pandya, 2009). As the verbal fluency factor correlated with the memory factor at a moderate level, it is not surprising to observe some overlapping white matter tracts across these verbal fluency and memory effects, such as we observed in the anterior part of IFOF. The distinct overall white matter tracking patterns across the verbal fluency and memory effects indicate that the participants with these deficits exhibited widespread neural disruption which was associated with not only verbal fluency, but general cognitive functions including memory as well.
The AMD patients and controls were comparable in their performance across 10 cognitive tests, although there was a tendency for worse performance in AMD patients (the mean absolute values of nine tests were numerically lower in AMD patients compared to controls). Prior epidemiological literature has reported significantly lower cognitive scores in AMD patients, compared to their peers with intact vision (Baker et al., 2009, Clemons et al., 2006, Whitson et al., 2010, Wong et al., 2002). The absence of a significant difference in cognitive performance in this study may be attributable to a small sample size. Another possibility is that the AMD patients recruited into this study may have had higher than average cognitive reserve, enabling this group to maintain cognitive performance despite structural brain changes.
In terms of the relations among AMD, the brain, and cognition, we found AMD patients and controls differed from each other in two significant ways. First, when the brain MRIs from AMD patients and controls were directly compared, AMD patients had weaker white matter connections in certain tracts, including the splenium. Second, while stronger connection strength (QA) within the splenium was associated with better verbal fluency and memory performance in all patients, we found a significant moderation effect by group. The neural-behavioral relationships were weaker in AMD patients, compared to controls, such that splenium connection strength (QA) was not as tightly linked to cognitive performance in AMD patients. The finding may suggest that some variable, other than white matter tract connectivity, was a more important driver of cognitive performance in this AMD group. One might presume that cognitive performance in AMD patients is a function of vision loss. However, previous population-based studies have shown that people with very early AMD, even in the absence of vision loss, under-perform on verbal fluency tests (Wong et al., 2002). Additionally, white matter connectivity in our AMD patients was not related to visual acuity. Taken together, these findings point toward a mechanism for cognitive change in AMD that is not dependent on vision loss and is not entirely mediated by white matter.
It has been speculated that the link between AMD and cognitive impairment noted in previous studies may arise because AMD is a comorbidity of other chronic neurodegenerative diseases such as Alzheimer’s disease (AD). AD is associated with amyloid beta (Aβ) plaques in the brain (Hardy and Selkoe, 2002), while AMD is associated with amyloid deposits in the macula, particularly within the drusen (Rozzini et al., 2014). Additionally, AMD and neurodegenerative dementias share several risk factors such as hypertension, cigarette smoking, and obesity (Rozzini et al., 2014), therefore, both disease processes might be caused or affected by similar environmental mechanisms. Future studies are needed to further investigate the degree to which brain amyloid or other dementia biomarkers might explain cognitive and neural patterns seen in AMD patients.
Our study has several important limitations that affect the interpretation of results. First, it is difficult for functionally impaired people to travel to a study site and undergo brain imaging. Therefore, our AMD group is likely biased towards individuals with high cognitive reserve and resources. To mitigate the bias, we offered transportation services to study visits and we matched groups on education, which is often used as a proxy indicator of cognitive reserve. Nonetheless, one possible explanation for the observation of a weaker brain-behavior relationship in the AMD group is higher cognitive reserve in that group that rendered them better able to cope with white matter damage, which may reflect a recruitment bias. Second, our analyses rely on cross-sectional data, and causal inferences should be interpreted with caution. Third, we lack detailed data on patients’ AMD course (e.g., time since onset, treatment courses, changes in visual acuity over time) or comorbidities (e.g., history of traumatic brain injury, post-traumatic stress disorder) which could impact brain effects. Additionally, AMD is a heterogeneous disease and especially in the era of more effective medications for neovascular disease, many AMD patients experience dynamic and unequal vision loss in their two eyes. To enhance feasibility of recruitment and generalizability of our findings, we did not require our participants have binocular vision loss, although over 90% of our AMD sample did have vision impairment in both eyes. More substantial differences between the patient and AMD groups may have been observed if the AMD group were restricted to individuals with severe, bilateral vision impairment. Finally, these analyses do not account for brain function or other metrics of brain structure (e.g., volume) or damage that could confound the relationships interrogated here, which focused on white matter connectivity.
These limitations notwithstanding, the analyses presented here provide new insights about differences in brain structure associated with AMD, which is the most common cause of significant vision loss in Americans. The observation of lower white matter connectivity in AMD patients’ optic radiation, corpus callosum, and splenium is not surprising given the role that these structures play in relaying visual information. Although it is plausible that damage to these same structures could have a downstream causal effect that contributes to any cognitive performance differences in people with AMD, we did not find evidence of group differences in cognition here, although it is possible that cognitive deficits may arise later. Taken together, our findings point to the alternate possibility that at least some of the cognitive changes associated with AMD arise from neurodegenerative processes that develop in parallel with AMD. If AMD patients are at heightened risk of neuropathological changes affecting gray matter or specific brain structures, it could explain the observation of weaker relationships between white matter connectivity and cognitive scores in the AMD group. To further understand the brain and cognitive consequences of AMD, we need longitudinal studies that include representative samples of AMD patients and controls with functional and structural brain data as well as markers (e.g., amyloid) of common dementing illnesses.
CRediT authorship contribution statement
Jie Zhuang: Conceptualization, Methodology, Formal analysis, Data curation, Writing - review editing. David J. Madden: Conceptualization, Funding acquisition, Writing - review editing. Priscila Cunha: Data curation, Writing - review editing. Alexandra Badea: Data curation, Writing - review editing. Simon W. Davis: Data curation, Writing - review editing. Guy G. Potter: Data curation, Writing - review editing. Eleonora M. Lad: Data curation, Writing - review editing. Scott W. Cousins: Data curation, Writing - review editing. Nan-Kuei Chen: Data curation, Writing - review editing. Kala Allen: Data curation, Writing - review editing. Abigail J. Maciejewski: Data curation, Writing - review editing. Xuan Duong Fernandez: . Michele T. Diaz: Funding acquisition, Writing - review editing. Heather E. Whitson: Conceptualization, Methodology, Funding acquisition, Data curation, Writing - review editing.
Acknowledgements
This research was funded by NIH grant R01 AG043438 to HEW, R01 AG039684 to DJM, and R01 AG034138 to MTD. This manuscript has not been published elsewhere, nor is it currently under consideration for publication elsewhere. This research has been approved by the Duke University Medical Center Institutional Review Board. All authors have reviewed the contents of the manuscript, approved its contents, and validated the accuracy of the data. The authors declare no conflicts of interest.
Contributor Information
Jie Zhuang, Email: jie.zhuang@duke.edu.
Heather E. Whitson, Email: heather.whitson@duke.edu.
References
- Baker M.L., Wang J.J., Rogers S., Klein R., Kuller L.H., Larsen E.K., Wong T.Y. Early age-related macular degeneration, cognitive function, and dementia: the cardiovascular health study. Arch. Ophthalmol. 2009;127(5):667–673. doi: 10.1001/archophthalmol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler N.M., Bressler S.B., Fine S.L. Age-related macular degeneration. Surv. Ophthalmol. 1988;32(6):375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Clemons, T.E., Rankin, M.W., McBee, W.L., 2006. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report No. 16. Archives of ophthalmology (Chicago, Ill.: 1960). Arch. Ophthalmol., 124(4), 537–543. https://doi.org/10.1001/archopht.124.4.537. [DOI] [PMC free article] [PubMed]
- Dayan M., Munoz M., Jentschke S., Chadwick M.J., Cooper J.M., Riney K., Vargha-Khadem F., Clark C.A. Optic radiation structure and anatomy in the normally developing brain determined using diffusion MRI and tractography. Brain Struct. Funct. 2015;220(1):291–306. doi: 10.1007/s00429-013-0655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBuc D.C., Somfai G.M., Arthur E., Kostic M., Oropesa S., Santiesteban C.M. Investigating multimodal diagnostic eye biomarkers of cognitive impairment by measuring vascular and neurogenic changes in the retina. Front. Physiol. 2018;9:1721. doi: 10.3389/fphys.2018.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Tremblay P. Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain. 2012;135(12):3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Pathways to language: fiber tracts in the human brain. Trends Cognitive Sci. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Fuld P.A., Masur D.M., Blau A.D., Crystal H., Aronson M.K. Object-memory evaluation for prospective detection of dementia in normal functioning elderly: predictive and normative data. J. Clin. Exp. Neuropsychol. 1990;12(4):520–528. doi: 10.1080/01688639008400998. [DOI] [PubMed] [Google Scholar]
- Glasser, M.F., Rilling, J.K., 2008. DTI tractography of the human brain’s language pathways. Cerebral Cortex (New York, NY). https://doi.org/10.1093/cercor/bhn011. [DOI] [PubMed]
- Hardy J., Selkoe D.J. The amyloid hypothesis of alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Iverson G.L. Interpreting change on the WAIS-III/WMS-III in clinical samples. Arch. Clin. Neuropsychol. 2001;16(2):183–191. doi: 10.1016/S0887-6177(00)00060-3. [DOI] [PubMed] [Google Scholar]
- Kaiser, P., 2009. Prospective evaluation of visual acuity assessment: A comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans. Am. Ophthalmol. Soc., https://www.scienceopen.com/document?vid=d87bbef1-aa2d-48bf-9180-b8667a83feb6. [PMC free article] [PubMed]
- Klaver C.C.W., Ott A., Hofman A., Assink J.J.M., Breteler M.M.B., de Jong P.T.V.M. Is age-related maculopathy associated with Alzheimer’s Disease?: the Rotterdam study. Am. J. Epidemiol. 1999;150(9):963–968. doi: 10.1093/oxfordjournals.aje.a010105. [DOI] [PubMed] [Google Scholar]
- Knyazeva M.G. Splenium of corpus callosum: patterns of interhemispheric interaction in children and adults. Neural Plasticity. 2013;2013:1–12. doi: 10.1155/2013/639430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yu C., Liang M., Li J., Tian L., Zhou Y., Qin W., Li K., Jiang T. Whole brain functional connectivity in the early blind. Brain. 2007;130(8):2085–2096. doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- Liu, Z., Wang, Y., Gerig, G., Gouttard, S., Tao, R., Fletcher, T., Styner, M., 2010. Quality control of diffusion weighted images. Medical Imaging 2010: advanced PACS-based imaging informatics and therapeutic applications, 7628, undefined-undefined. https://doi.org/10.1117/12.844748.
- Makris N., Kennedy D.N., McInerney S., Sorensen A.G., Wang R., Caviness V.S., Pandya D.N. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Meyer L., Cunitz K., Obleser J., Friederici A.D. Sentence processing and verbal working memory in a white-matter-disconnection patient. Neuropsychologia. 2014;61:190–196. doi: 10.1016/j.neuropsychologia.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Monsch A.U., Bondi M.W., Butters N., Thal L.J., Salmon D.P., Katzman R. Comparisons of verbal fluency tasks in the detection of dementia of the alzheimer type. Arch. Neurol. 1992;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- National Eye Institute. (2011). Facts about age-related macular degeneration. National Institutes of Health. www.nei.nih.gov/health/maculardegen/armd_facts.
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papoutsi M., Stamatakis E.A., Griffiths J., Marslen-Wilson W.D., Tyler L.K. Is left fronto-temporal connectivity essential for syntax? effective connectivity, tractography and performance in left-hemisphere damaged patients. NeuroImage. 2011;58(2):656–664. doi: 10.1016/j.neuroimage.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Parker G.J.M., Luzzi S., Alexander D.C., Wheeler-Kingshott C.A.M., Ciccarelli O., Ralph M.A.L. Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage. 2005;24(3):656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Pham T.Q., Kifley A., Mitchell P., Wang J.J. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology. 2006;52(6):353–358. doi: 10.1159/000094984. [DOI] [PubMed] [Google Scholar]
- Rauschecker J.P., Scott S.K. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat. Neurosci. 2009;12(6):718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolheiser T., Stamatakis E.A., Tyler L.K. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J. Neurosci. 2011;31(47):16949–16957. doi: 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini L., Riva M., Ghilardi N., Facchinetti P., Forbice E., Semeraro F., Padovani A. Cognitive dysfunction and age-related macular degeneration. Am. J. Alzheimer’s Dis. Dementias. 2014;29(3):256–262. doi: 10.1177/1533317513517032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager M.A., Hermann B.P., Rue A.L., Woodard J.L. Screening for dementia in community-based memory clinics. Wis. Med. J. 2006;105(7):25–29. [PubMed] [Google Scholar]
- Saur D., Kreher B.W., Schnell S., Kümmerera D., Kellmeyera P., Vrya M.S., Umarova R., Musso M., Glauche V., Abel S., Huber W., Rijntjes M., Hennig J., Weiller C. Ventral and dorsal pathways for language. PNAS. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Schelter B., Schnell S., Kratochvil D., Küpper H., Kellmeyer P., Kümmerer D., Klöppel S., Glauche V., Lange R., Mader W., Feess D., Timmer J., Weiller C. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. NeuroImage. 2010;49(4):3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Fiber pathways of the brain. 2009. pp. 1–654. [Google Scholar]
- Spreen O., Benton A.L. University of Victoria; Neuropsychology Laboratory: 1977. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA), 1977 revision: Manual of Instructions. [Google Scholar]
- Stelmack J.A., Szlyk J.P., Stelmack T.R., Demers-Turco P., Williams R.T., Moran D., Massof R.W. Psychometric properties of the veterans affairs low-vision visual functioning questionnaire. Invest. Ophthalmol. Vis. Sci. 2004;45(11):3919–3928. doi: 10.1167/iovs.04-0208. [DOI] [PubMed] [Google Scholar]
- Swanson M.R., Wolff J.J., Elison J.T., Gu H., Hazlett H.C., Botteron K., Styner M., Paterson S., Gerig G., Constantino J., Dager S., Estes A., Vachet C., Piven J., Piven J., Hazlett H.C., Chappell C., Dager S., Estes A., Gu H. Splenium development and early spoken language in human infants. Developmental Sci. 2017;20(2) doi: 10.1111/desc.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlyk J.P., Little D.M. An fMRI study of word-level recognition and processing in patients with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2009;50(9):4487–4495. doi: 10.1167/iovs.08-2258. [DOI] [PubMed] [Google Scholar]
- Szlyk J.P., Stelmack J., Massof R.W., Stelmack T.R., Demers-Turco P., Williams R.T., Wright B.D. Performance of the veterans affairs low vision visual functioning questionnaire. J. Visual Impairm. Blind. 2004;98(5):261–275. [Google Scholar]
- Tun P.A., Lachman M.E. Telephone assessment of cognitive function in adulthood: the brief test of adult cognition by telephone [3] Age Ageing. 2006;35(6):629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Turken, A.U., Dronkers, N.F., 2011. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci., FEBRUARY 2011, undefined-undefined. https://doi.org/10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed]
- Whitson H.E., Ansah D., Whitaker D., Potter G., Cousins S.W., MacDonald H., Pieper C.F., Landerman L., Steffens D.C., Cohen H.J. Prevalence and patterns of comorbid cognitive impairment in low vision rehabilitation for macular disease. Arch. Gerontol. Geriatr. 2010;50(2):209–212. doi: 10.1016/j.archger.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson H.E., Cronin-Golomb A., Cruickshanks K.J., Gilmore G.C., Owsley C., Peelle J.E., Recanzone G., Sharma A., Swenor B., Yaffe K., Lin F.R. American geriatrics society and national institute on aging bench-to-bedside conference: sensory impairment and cognitive decline in older adults. J. Am. Geriatr. Soc. 2018;66(11):2052–2058. doi: 10.1111/jgs.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F.C.K., Chandrasekaran B., Garibaldi K., Wong P.C.M. White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. J. Neurosci. 2011;31(24):8780–8785. doi: 10.1523/JNEUROSCI.0999-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.Y., Klein R., Nieto F.J., Moraes S.A.D., Mosley T.H., Couper D.J., Klein B.E.K., Boland L.L., Hubbard L.D., Sharrett A.R. Is early age-related maculopathy related to cognitive function? The atherosclerosis risk in communities study. Am. J. Ophthalmol. 2002;134(6):828–835. doi: 10.1016/S0002-9394(02)01672-0. [DOI] [PubMed] [Google Scholar]
- Woo S.J., Park K.H., Ahn J., Choe J.Y., Jeong H., Han J.W., Kim T.H., Kim K.W. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012;119(10):2094–2101. doi: 10.1016/j.ophtha.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Rykhlevskaia E., Sherbondy A.J., Deutsch G.K., Wandell B.A., Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J. Cognit. Neurosci. 2011;23(11):3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F.C., Badre D., Verstynen T. Connectometry: A statistical approach harnessing the analytical potential of the local connectome. NeuroImage. 2016;125:162–171. doi: 10.1016/j.neuroimage.2015.10.053. [DOI] [PubMed] [Google Scholar]
- Yeh F.C., Tang P.F., Tseng W.Y.I. Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. NeuroImage: Clinical. 2013;2(1):912–921. doi: 10.1016/j.nicl.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F.C., Verstynen T.D., Wang Y., Fernandez-Miranda J.C., Tseng W.I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE. 2013 doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J., Madden D.J., Duong-Fernandez X., Chen N., Cousins S.W., Potter G.G., Diaz M.T., Whitson H.E. Language processing in age-related macular degeneration associated with unique functional connectivity signatures in the right hemisphere. Neurobiol. Aging. 2018;63:65–74. doi: 10.1016/j.neurobiolaging.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]