Highlights
-
•
DIBH results in cardiac sparing in left sided breast cancer radiotherapy.
-
•
Heart volume in field and maximum heart distance are predictors of cardiac sparing.
-
•
Thresholds of these anatomical predictors can help to select patients for DIBH.
Keywords: Breast cancer, Radiotherapy, Deep inspiratory breath-hold, Dosimetric predictors
Abbreviations: DIBH, Deep Inspiratory Breath-hold; FB, Free Breathing; ABC™, Active Breathing Coordinator™; ROC, Receiver Operating Characteristic; LAD, Left Anterior Descending; HVIF, Heart Volume in Field; MHD, Maximum Heart Depth; RPM, Real-time Position Management; EORTC, European Organization for Research and Treatment of Cancer; BCS, Breast Conservation Surgery; MRM, Modified Radical Mastectomy; RTOG, Radiation Therapy Oncology Group; 3DCRT, Three-Dimensional Conformal Radiation Therapy; SCF, Supraclavicular Fossa; PTV, Planning target volume; HV, Heart Volume; LV, Lung Volume; HH, Heart Height; HCWL, Heart Chest Wall Length; CS, Chest Separation; CD, Chest Depth; HCWD, Heart Chest Wall Distance; LOD, Lung Orthogonal Distance; CLD, Central Lung Distance; DVH, Dose Volume Histograms; CT, Computer Tomography; RNI, Regional Nodal Irradiation; OAR, Organs-at-risk; IMC, Internal Mammary Chain; AUC, Area under the curve; CCD, Cardiac Contact Distance; NTCP, Normal Tissue Complications Probability; BMI, Body Mass Index
Abstract
Introduction
The risk of radiotherapy-associated cardiovascular disease has been a concern for decades in breast cancer survivors. The objective of our study is to evaluate the dosimetric benefit of Deep Inspiratory Breath-hold technique (DIBH) on organs-at-risk (OAR) sparing in left-sided breast cancer radiotherapy and to find out pre-treatment predictors of cardiac doses for guiding patient selection for DIBH.
Material and methods
Pre-radiotherapy planning CT scans were done in Free Breathing (FB) and in DIBH [using Active Breathing Coordinator system (ABC™)] in 31 left sided breast cancer patients. 3DCRT plans were generated for both scans. Comparison of anatomical and dosimetric variables were done using paired t test and correlation was evaluated using Pearson correlation. Linear regression was used to get independent predictors of cardiac sparing and Receiver Operating Characteristic (ROC) curve analysis was done to find out the specific threshold of the predictors.
Results
There was a 39.15% reduction in mean heart dose in DIBH compared to FB (2.4 Gy vs 4.01 Gy) (p < 0.001), 19% reduction in maximum Left Anterior Descending (LAD) dose and a 9.9% reduction in ipsilateral lung mean dose (p = 0.036) with DIBH. A significant correlation was observed between reduction in Heart Volume in Field (HVIF) and Maximum Heart Depth (MHD) with reduction in mean heart dose. Reduction in HVIF (ΔHVIF) independently predicted cardiac sparing.
Conclusion
DIBH leads to significant reduction in OAR doses and is suggested for all patients of left-sided breast cancer undergoing radiotherapy. However, HVIF and MHD predicted for cardiac sparing and threshold criteria of ΔHVIF and ΔMHD may be used by centres with high workload to select patients for DIBH.
Introduction
Adjuvant radiotherapy improves locoregional control and survival in breast cancer patients both after breast-conservation surgery [1] and mastectomy [2]. With increase in survival, long term radiation toxicity becomes a major concern. The heart is the most important organ at risk in breast cancer radiotherapy and cardiac irradiation is associated with long term cardiac co-morbidities particularly coronary artery disease. A study by Darby et al [3] has shown that rates of major coronary events increase linearly by 7.4% per Gray mean dose to the heart, with no apparent threshold. Apart from the mean dose to the heart, there are also several other factors contributing to the cardiotoxicity. These are: dose of radiation, fraction size, Left Anterior Descending (LAD) coronary artery dose and left ventricular doses [4], [5]. Studies have also shown that left-sided breast cancer patients have an increased risk of radiation-related cardiotoxicity compared to right-sided cancers [6], [7].
Dose to the LAD is an important factor in the development of RT-related cardiotoxicity [8]. Therefore, for the past few years, the focus has been on reducing the mean heart dose and LAD dose with the use of modern radiotherapy techniques. Different strategies like cardiac shielding [9], partial breast irradiation [10], breath-hold technique [11] are used for reducing heart doses without compromising target coverage or other organ-at-risk. Deep Inspiratory Breath-hold (DIBH) technique is one of the most well studied techniques. The separation between the heart and the chest wall/breast increases with deep inspiration and if radiation is delivered in this phase, it decreases the heart and LAD doses. DIBH can be achieved by using Elekta Active Breathing Coordinator™ (ABC) or by Varian RPM system. The UK HeartSpare [12] randomized study compared the two techniques and reported that they are comparable in terms of positional reproducibility and normal tissue sparing.
Though several studies [13], [14], [15], [16] have shown dosimetric benefit of the DIBH technique on cardiac sparing, it requires intensive patient coaching and tends to increase departmental workload. The routine use of this technique in a center with high patient load may lead to an increase in patient waiting time. A survey conducted by van der Laan et al [17] reported that breath-hold radiotherapy technique was used only in 19% of the European Organization for Research and Treatment of Cancer (EORTC) Radiation oncology group-affiliated institutes for treating breast cancer. Another recent study by Desai et al [18] has reported the nationwide trends in heart-sparing techniques utilized in breast cancer radiotherapy in United Kingdom. Results of this survey, based on 530 responses from radiation oncologists, have shown that though DIBH is the most common cardiac sparing technique used, only 43% of the physicians have used it frequently for cardiac sparing (in more than three-fourth of their breast cancer patients). When asked about the reasons for not offering DIBH, 15% of the respondents mentioned increased planning and treatment time. This reflects that this technique can be more labor intensive, which may deter some physicians from using it routinely [18]. With this background our study was conducted not only to assess the dosimetric benefit of the DIBH technique in our patient population but also to quantify the dosimetric benefit and correlate the changes in patients’ anatomical parameters with the changes in dosimetric parameters, to enable selection of patients who would benefit more from this technique.
Materials and methods
From October 2017 to May 2019, 31 consecutive patients of left-sided breast cancer who received adjuvant radiotherapy to the breast or chest wall with DIBH technique using ABC were prospectively analysed. The inclusion criteria were, left sided breast cancer patients who have undergone Breast Conservation Surgery (BCS) and those who had high-risk factors after Modified Radical Mastectomy (MRM) [T3-T4 and/ or lymph node positive disease or high risk as per Cambridge score]. Pregnant women, patients treated previously with breast or chest wall radiotherapy and those who had difficulty holding their breath for more than 10 s were excluded. Informed consent was obtained from all the patients before the study. This study was approved by the institutional ethical committee before initiation.
Simulation and treatment planning procedure
Before simulation patients were extensively trained for breath-hold technique. Depending on the performance, the threshold of breath-hold volume and duration was determined for individual patient. All patients were scanned in the supine position, lying on a breast board with both arms abducted at 90 degrees. Patients who required supraclavicular nodal irradiation had their faces turned to the side opposite to the affected side. The breast tissue and tumor bed scar or chest wall scar were marked with radiopaque wire. A simulation CT scan was performed for each patient in Siemens Somatom Emotion 16 slice CT scanner and 2 sets of images were taken, one in free breathing (FB) and another in DIBH. The Active Breath Coordinator (ABC™) system from Elekta was used for monitoring respiratory breath-hold where the predetermined threshold of breath-hold volume and duration was set for every individual patient and CT data was acquired without contrast using 3 mm slices (this slice thickness was chosen in accordance with the cardiac contouring atlas developed by Duane et al) [19]. The FB and DIBH CT scan images were then transferred to our treatment planning system.
The treatment planning was done using Monaco TPS v.5.11 software of Elekta Versa HD machine. The target volume and organ at risk structures were delineated on both CT scans sets by the same physician as per Radiation Therapy Oncology Group (RTOG) contouring atlas [20] to reduce inter-observer variation. The heart and the LAD were contoured according to the validated University of Michigan cardiac contouring atlas [21]. In the University of Michigan cardiac atlas development and validation study, use of intravenous contrast did not make any difference to inter-observer variation in dose reporting, while thecardiac contouring atlas developed by Duane et al is also based on the non-contrast CT planning scan [19]. We, therefore, did not use contrast for our planning CT scans. 3DCRT plans were generated on both the image sets using tangential photon beams. with a field- in- field technique. Cardiac shielding with multileaf collimator was used to keep the cardiac doses as low as possible. For the Supraclavicular Fossa (SCF), a single direct anterior field angled 5–10 degrees was used and asymmetric jaws were used for matching tangential and SCF portals. Bolus was used in chest wall cases in half of the fractions (i.e. first seven fractions) and in all the fractions wherever there was skin involvement as per institutional protocol. The patients were prescribed 40 Gy in 15 fractions over 3 weeks for whole breast/ chest wall irradiation and a tumour bed boost dose of 12.5 Gy in 5 fractions over 1 week for patients undergoing breast conserving surgery. Tumour bed boost was delivered using electron beam of specific energy depending on the depth of the tumour bed. The plan acceptability criteria were kept as the follows: 95% of the breast planning target volume (PTV-breast) or 90% of PTV-chest wall should be covered by at least 90% isodose and volume covered by 107% isodose, should be less than 2 cc. Both the plans should have a comparable target coverage.
Anatomical and dosimetric parameters
Eleven anatomical parameters were calculated and recorded for FB and DIBH CT scans for each patient and the change in these parameters between the two scans were also documented. Definition of all these anatomical parameters are given in Table 1 and is based on a study by Register et al. [22].
Table 1.
Definition of the anatomical parameters [22].
| Anatomical parameters | Definition |
|---|---|
| Heart Volume (HV) | Volume of contoured heart in cc |
| Lung Volume (LV) | Volume of contoured bilateral lungs in cc |
| Heart Chest Wall Length (HCWL) | The maximum length of contact between the heart and chest wall |
| Heart Height (HH) | The distance from the superior to inferior extent of the contoured heart |
| Chest Separation (CS) | Maximum separation between medial edge and lateral edge of 50% isodose line along the central axis of beam |
| Chest Depth (CD) | The anterior-posterior thickness of the chest at the level of the maximum chest separation |
| Heart Chest Wall Distance (HCWD) | The distance from the maximal heart point to the chest wall |
| Maximum Heart Depth (MHD) | The maximum distance from the field edge to the heart border |
| Heart Volume in Field (HVIF) | The heart volume encompassed by the 50% isodose line |
| Lung Orthogonal Distance (LOD) | The maximum distance from the field edge to the lung-chest wall interface at the level of maximum cs |
| Central Lung Distance (CLD) | The distance between the midpoint of the posterior field and the edge of chest wall |
Dose Volume Histograms (DVHs) were generated for both the plans (FB and DIBH) and the following dosimetric parameters were noted – percentage of volume of planning target volume (PTV) covered by 90% and 95% of prescribed (PTV_V90, V95), heart and LAD mean dose (Dmean), maximum dose (Dmax), ipsilateral and bilateral lung mean dose, percentage volumes of heart receiving doses ≥ 5 Gy (V5), 10 Gy (V10), 30 Gy (V30) , percentage volumes of LAD receiving doses ≥ 5 Gy (V5), 40 Gy (V40) of LAD, percentage volume of ipsilateral and bilateral lung receiving doses ≥ 5 Gy (V5), 12 Gy (V12), 20 Gy (V20) were recorded.
Each approved plan was subjected to Quality Assurance using the iMatrixx phantom (an ion chamber array), Octavius 4D phantom and Qasar phantom, keeping the gamma value = 3% for acceptance. Treatment was delivered in all patients using ABC-DIBH technique in Elekta Versa HD Linear accelerator. For image-verification, daily kilovoltage cone beam CT was used, and online correction was also carried out. Whenever there was a systemic error, appropriate action was taken.
Statistical methods
The data was analysed using the IBM SPSS® software version 22. The descriptive data was represented as mean, median, standard deviation or percentage. The paired t test was used for continuous numerical variables, to test whether the DIBH technique differed from FB technique with respect to particular anatomical or dosimetric parameters. For each anatomical and dosimetric parameter, the mean difference between FB and DIBH scans was recorded and correlations of differences in between relevant dose metrics with patient related parameters were analysed using Pearson's correlation. Unpaired t-test was used to compare between BCS and MRM groups. Linear regression analysis was done to find out independent predictors of cardiac sparing. Receiver Operating Characteristic (ROC) analysis was performed to find out the threshold value of the predictor of cardiac sparing which may help in selecting the patient for DIBH. A p value ≤ 0.05 was considered statistically significant.
Results
Patient and treatment characteristics
Sixty-two CT scan data of 31 left sided breast cancer patients, who met the selection criteria, were analyzed. Patient and treatment characteristics are presented in Table 2. Majority of the patients underwent BCS (61.3%). 54.8% of the patients had node positive disease and 64.5% received Regional Nodal Irradiation (RNI) to SCF. 77.4% of the patients received chemotherapy of whom, 21 patients (67.7%) received anthracycline based chemotherapy. The mean breath-hold volume was 1.1 L and mean duration of breath-hold was 15 s.
Table 2.
Patient and treatment characteristics (N = 31).
| Patient/Treatment Parameters | Number | Percentage | |
|---|---|---|---|
| Median Age | 49 yrs (32-75yrs) | ||
| Median BMI | 28 (19.8–39.9) | ||
| Tumour stage | |||
| Stage I | 3 | 9.7% | |
| Stage II | 19 | 61.3% | |
| Stage III | 9 | 29% | |
| Location of the tumour | |||
| Upper outer quadrant | 14 | 45.1% | |
| Upper inner quadrant | 6 | 19.4% | |
| Lower outer quadrant | 4 | 12.9% | |
| Lower inner quadrant | 3 | 9.7% | |
| Central tumours | 4 | 12.9% | |
| Node positive disease present | 17 | 54.8% | |
| Type of surgery done | |||
| MRM | 12 | 38.7% | |
| BCS | 19 | 61.3% | |
| Regional nodal irradiation (RNI) | |||
| Yes | 20 | 64.5% | |
| No | 11 | 35.5% | |
| Chemotherapy received | |||
| Yes | 24 | 77.4% | |
| No | 7 | 22.6% | |
| Received anthracycline based chemotherapy | 21 | 67.7% | |
| Mean breath-hold volume | 1.1 L | ||
| Mean duration of breath-hold | 15sec |
MRM-Modified Radical Mastectomy, BCS- Breast Conservation Surgery
Comparison of anatomical parameters between FB and DIBH scans
The changes of the anatomical parameters with DIBH is summarised in Table 3. With DIBH, as the lung expanded and the heart moved away from the chest wall, we observed a significant reduction in mean Heart Volume (HV) (p = 0.01), mean Heart Chest wall Length (HCWL) (p < 0.001) and an increase in mean Chest Depth (CD) (p < 0.001), mean Heart Chest Wall Distance (HCWD) (p < 0.001), mean Lung Volume (LV) (p < 0.001), mean Lung Orthogonal Distance (LOD) and mean Central Lung Distance (CLD) (p < 0.001).
Table 3.
Anatomic and planning characteristics compared between Free Breathing (FB) and Deep Inspiratory Breath-hold (DIBH) scans (N = 31).
| PARAMETERS | FB | DIBH | Δ (Difference) | Δ% (percentage of difference) | p value |
|---|---|---|---|---|---|
| Measured on Treatment Planning Scans | |||||
| Mean HV (cc) | 496.90 | 478.27 | −18 | −3.6 | 0.010 |
| Mean HCWL (cm) | 7.7 | 6.8 | −0.9 | −11.6 | <0.001 |
| Mean HH (cm) | 8.71 | 8.87 | 0.16 | 1.83 | 0.139 |
| Mean LV (cc) | 2320.62 | 3451.17 | 1130.55 | 48.71 | <0.001 |
| Measured after tangent fields set | |||||
| Mean CD (cm) | 20.21 | 21.04 | 0.8 | 3.96 | <0.001 |
| Mean CS (cm) | 22.66 | 22.64 | 0 | 0 | 0.0978 |
| Mean HCWD (cm) | 1.18 | 1.84 | 0.66 | 55.9 | <0.001 |
| Mean MHD (cm) | 2.01 | 1.07 | −0.94 | −46.7 | <0.001 |
| Mean HVIF (cc) | 26.58 | 7.02 | −19.56 | −73.8 | <0.001 |
| Mean LOD (cm) | 2.57 | 3.11 | 0.54 | 21 | <0.001 |
| Mean CLD (cm) | 2.37 | 2.95 | 0.53 | 22.3 | <0.001 |
HV-Heart Volume; HCWL- Heart Chest Wall Length; HH– Heart Height; LV- Lung Volume; CD- Chest Depth; CS- maximum Chest Separation; HCWD- Heart Chest Wall Distance; MHD- Maximum Heart Depth; HVIF- Heart Volume in Field; LOD- Lung Orthogonal Distance; CLD- Central Lung Distance
The DIBH technique resulted in 46.7% reduction in Maximum Heart Depth (MHD) (2.01 cm in FB scans vs 1.07 cm in DIBH scans) (p < 0.001), and 73.8% reduction in Heart Volume In Field (HVIF) (26.58 cc in FB scans vs 7.02 cc in DIBH scans) (p < 0.001).
Dosimetric comparison
The dose-volume parameters are summarized in Table 4. Target coverage was comparable in both the scans. In MRM cases, PTV V90% was 90.8% in FB scan and 90.01% in DIBH scan. In case of BCS, PTV V90% was 94.0% in FB Scan and 94.9% in DIBH Scan. DIBH significantly decreased the mean heart dose (39.15%) from 4.01 Gy in FB to 2.4 Gy with DIBH. 64% patients had > 20% decrease, 29% patients > 50% decrease in mean heart dose. Maximum LAD dose was reduced to 25.8 Gy in DIBH scans compared to 31.9 Gy in FB scans (p < 0.001). V30, V10, V5 of the heart was also significantly reduced. DIBH also resulted in significant improvement of lung doses. V20 and V12 of total and ipsilateral lung reduced significantly (p values are 0.006 and 0.02 respectively) and there was also a reduction in ipsilateral lung mean doses (p = 0.03).
Table 4.
Dosimetric comparisons between Free Breathing (FB) and Deep Inspiratory Breath-hold (DIBH) scans (N = 31).
| PARAMETER | FB | DIBH | Δ (Difference) | Δ% (percentage of difference) | p value |
|---|---|---|---|---|---|
| Mean Heart dose (Gy) | 4.0 | 2.4 | −1.5 | −39.1 | 0.00 |
| Mean LAD dose (Gy) | 12.6 | 8.7 | −3.8 | −30.1 | 0.00 |
| Max Heart dose (Gy) | 39.4 | 31.5 | −7.8 | −19.8 | 0.00 |
| Max LAD dose (Gy) | 31.9 | 25.8 | −6.0 | −18.9 | 0.00 |
| Heart V5 (%) | 14.2 | 7.6 | −6.6 | −46.5 | 0.00 |
| Heart V10 (%) | 8.9 | 3.4 | −5.5 | −61.8 | 0.00 |
| Heart V30 (%) | 2.9 | 0.4 | −2.4 | −82.7 | 0.00 |
| LAD V5 (%) | 52.5 | 52.6 | 0.1 | 0 | 0.97 |
| LAD V40 (%) | 0.6 | 0.4 | −0.1 | −21.7 | 0.83 |
| Mean total lung dose (Gy) | 7.1 | 4.7 | −2.4 | –33.8 | 0.17 |
| Total Lung V5 (%) | 19.6 | 17.4 | −2.1 | −10.9 | 0.24 |
| Total Lung V12 (%) | 13.9 | 11.7 | −2.1 | −15.3 | 0.02 |
| Total lung V20 (%) | 11.1 | 9.1 | −1.9 | −17.4 | 0.00 |
| Mean Left Lung dose (Gy) | 10.2 | 9.2 | −1.0 | −9.9 | 0.03 |
| Left lung V5 (%) | 38 | 37 | −0.8 | −2.2 | 0.67 |
| Left lung V12 (%) | 27.6 | 24.9 | −2.7 | −9.7 | 0.05 |
| Left lung V20 (%) | 22.3 | 19.4 | −2.9 | −13.2 | 0.01 |
LAD- Left Anterior Descending Artery.
Though MRM patients had a larger reduction in mean heart dose (Δmean heart dose) than BCS patients (difference of FB mean heart dose and DIBH mean heart dose was 2.1 in PMRT patients and 1.2 in BCS patients), it was not statistically significant (p = 0.22). Similarly, there was no significant difference in improvement of other dosimetric parameters between the MRM and BCS group (supplementary table 1). There was also no significant difference of cardiac sparing in patients receiving regional nodal irradiation to SCF versus those who did not (p = 0.25).
Correlation and predictors of cardiac sparing
The correlations between the changes in anatomical and dosimetric parameters are shown in Table 5. The change in three anatomical parameters with DIBH significantly correlated with improvement in cardiac doses. They were Δmean CD, ΔMHD and ΔHVIF. Δmean CD and ΔHVIF correlated significantly with some of the dosimetric parameters of the heart (mean heart dose, V5, V10 and V20), but did not show any correlation with LAD doses except for ΔHVIF which showed significant correlation with mean LAD dose. ΔMHD significantly correlated with the reduction in V5, V10 and mean heart dose but not show any correlation with other heart and LAD dose parameters. No other correlation was observed in between the anatomical and dosimetric parameters of cardiac sparing.
Table 5.
Correlations between anatomic and planning characteristics and doses to OAR (N = 31).
| Dosimetric parameter | Anatomical parameters |
|||||
|---|---|---|---|---|---|---|
| ΔHVIF | p value | Δmean CD | p value | Δ MHD | p value | |
| HEART | ||||||
| Max Heart Dose(Gy) | −0.18 | 0.31 | 0.023 | 0.901 | 0.03 | 0.83 |
| Mean Heart Dose(Gy) | 0.89 | 0.000 | 0.72 | 0.000 | 0.35 | 0.05 |
| V5 (%) | 0.78 | 0.000 | 0.68 | 0.000 | 0.45 | 0.01 |
| V10 (%) | 0.84 | 0.000 | 0.69 | 0.000 | 0.39 | 0.02 |
| V30 (%) | 0.87 | 0.000 | 0.59 | 0.000 | 0.17 | 0.36 |
| LAD | ||||||
| Max LAD Dose(Gy) | 0.27 | 0.13 | 0.15 | 0.39 | 0.20 | 0.26 |
| Mean LAD Dose(Gy) | 0.65 | 0.000 | 0.31 | 0.08 | 0.20 | 0.28 |
| V5 (%) | 0.09 | 0.60 | 0.26 | 0.15 | 0.07 | 0.69 |
| V40 (%) | 0.22 | 0.23 | 0.21 | 0.25 | 0.29 | 0.11 |
LAD- Left Anterior Descending Artery; Δ- Difference of the dosimetric parameters between FB and DIBH scans; HVIF- Heart Volume in Field; CD- Chest Depth; MHD- Maximum Heart Depth.
The linear regression analysis showed that ΔHVIF and Δmean CD were independent predictors of reduction of mean heart dose (p < 0.001, ANOVA). ΔMHD did not predict for Δmean heart dose in our study (p = 0.53). None of the parameters predicted a reduction in LAD max dose. In the regression model, the dependent variables analyzed were Δmean heart dose and ΔLAD max dose and the independent variables were Δmean CD, ΔMHD and ΔHVIF, based on the correlation seen.
Discussion
Our study is a prospective one which showed the dosimetric benefit of the DIBH technique over free breathing technique in reducing OAR doses (heart, LAD and lung). Analysis from our study showed a significant reduction in mean heart dose, maximum heart dose, V5, V10 and V30 of the heart Also, significant reduction in mean and maximum LAD doses were observed. These results are in concordance with other published studies. A systematic review by Smyth et al [23] on cardiac dose sparing benefit of DIBH, which included 10 studies, showed a statistically significant reduction in mean heart and LAD dose with the DIBH technique in all studies. The mean heart doses ranged from 2.3 Gy to 6.9 Gy in FB scans to 1.3 Gy to 3.9 Gy in DIBH scans. DIBH reduced the mean heart dose by up to 3.4 Gy and LAD max dose by up to 14.1 Gy [23].
Most of the studies published highlight the reduction in heart and LAD doses but there are few studies that comment on lung doses. Our study showed not only a reduction in heart and LAD doses but also a reduction in V20, V12 of total lung and ipsilateral lung mean dose, V20 and V12. Zurl et al [24] reported that the mean dose to the ipsilateral lung was significantly reduced by 15% with DIBH. In our study, there was significant reduction of both the mean dose to ipsilateral lung (10%, p = 0.03) and V20 (13%, p = 0.01). A study by Yeung et al. [25] showed that a greater reduction in mean heart and LAD dose was possible with DIBH, in patients receiving RNI, including Internal Mammary Chain (IMC) nodal irradiation, compared to the patients not receiving RNI. As per our institutional protocol, we irradiated the IMC chain only if it was radiologically or pathologically positive, which was not the case in any of the patients analysed.. Therefore, our cohort of patients receiving RNI (only to the SCF group) did not show any cardiac sparing benefit with DIBH over patients not receiving RNI.
Though there are many studies reporting dosimetric benefit of DIBH in breast cancer radiotherapy, few studies have analysed predictors of cardiac sparing with DIBH. We tried to assess around 11 anatomical parameters in both the CT simulatory scans, before and after tangent fields are set, in order to assess the patients who will benefit more with DIBH. There are several predictors reported in the literature. A strong correlation between the mean heart dose and MHD was observed by Taylor et al [26] who reported that for every 1 cm increase in MHD, the mean heart dose increased by 2.9% on average. Therefore, MHD on free breathing scan of >1 cm has been used as a criterion for selecting patients in other studies [27]. In our study we have also observed that ΔMHD significantly correlated with the reduction in V5, V10 and mean heart dose.
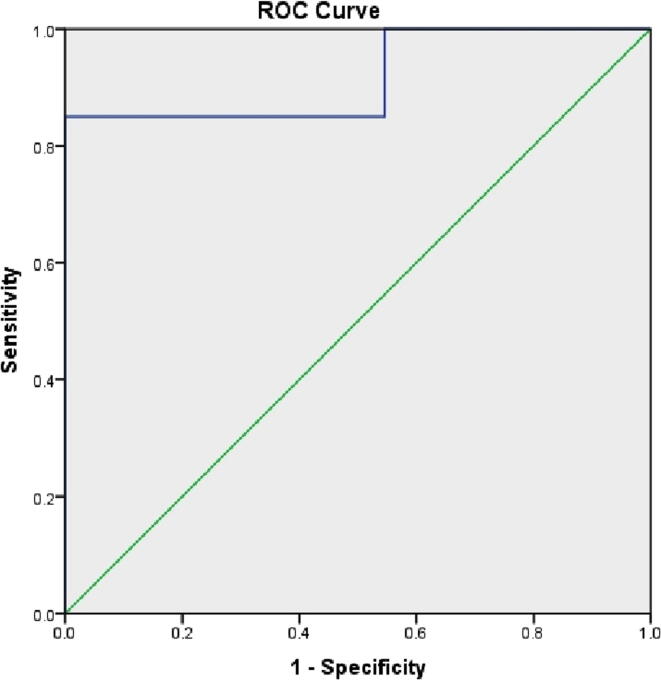
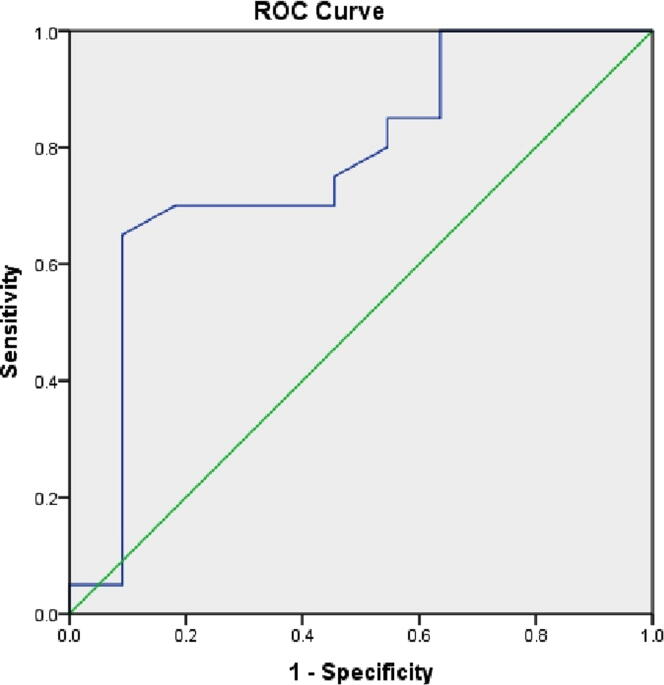
We performed ROC curve analysis which showed that estimated mean area under the curve (AUC) for ΔHVIF predicting for > 20% reduction in mean heart dose was 0.91 (p = 0.001; 95% CI-0.81–0.98) with a cut-off value of 6 cc (illustrated in Fig. 1) and ΔHVIF predicting for > 50% reduction in mean heart dose was 0.84 (p = 0.002; 95% CI-0.69–0.98) with a cut-off value of 13 cc. The AUC for ΔMHD predicting for > 20% reduction in mean heart dose was 0.768 (p = 0.15; 95%CI 0.58–0.95) with cut-off value of 7 mm (illustrated in Fig. 2) and AUC for ΔMHD predicting for > 50% reduction in mean heart dose was 0.69 (p = 0.23; 95%CI 0.42–0.85) with cut-off value of 1 cm. Out of 10 patients who had < 20% reduction in mean heart dose, 9 had ΔHVIF less than 6 cc and ΔMHD < 7 mm. Whereas, 3 patients whose ΔHVIF was below the threshold, achieved dosimetric benefit. Therefore, from the ROC curve analysis we can conclude that patients with ΔMHD of >1 cm are likely to have > 50% reduction in mean heart dose with DIBH.
Fig. 1.
ROC curve for ΔHVIF predicting for >20% reduction in mean heart dose: AUC was 0.91 (p = 0.001; 95% CI-0.81–0.98) with a cut-off value of 6 cc.
Fig. 2.
ROC curve for ΔMHD predicting for >20% reduction in mean heart dose: AUC was 0.768 (p = 0.15; 95%CI 0.58–0.95) with cut-off value of 7 mm.
Another dosimetric predictor reported in the literature is the heart volume in the field (HVIF). A study by Wang et al. [28] reported that mean heart dose increases by 0.67 Gy per 1-cc increase in HVIF. Another retrospective study similar to our study, looking at predictors of cardiac sparing by Register et al [22], also found a strong correlation of ΔHVIF and ΔMHD with Δmean heart dose, though they did not suggest any threshold value. In our study, we have found that ΔHVIF is a strong and independent predictor of cardiac sparing and have also reported a cut-off of ΔHVIF, which means that a patient with a reduction of HVIF > 6 cc is likely to have a reduction in mean heart dose by > 20%. These are the candidates who are going to benefit more with DIBH. We cannot recommend a routine use of these threshold values as absolute criteria for selection of patients for DIBH, as, in our study, there were patients who benefitted from DIBH despite having a reduction in HVIF, by less than 6 cc. This cut-off can be used along with ΔMHD as a relative criterion for selecting patients for DIBH in centres with high patient load. A study by Czeremszyńska et al. [29] tried to find out the threshold of dosimetric predictors but concluded that the anatomical characteristics’ thresholds could not be used to select patients for whom DIBH-RT will not be considered. They have not included HVIF in their study. The disadvantage of this method is that it requires two scans for a particular patient, and also coaching of the patient for DIBH but selecting patients with this method can lead to a reduction in the longer treatment time slots required for DIBH and therefore, less waiting time for other patients. While performing our study, we have seen that treatment planning does not take extra time for DIBH scans compared to FB scans, but as ABC-DIBH requires the patient to actively breathe-in to a predefined threshold volume before every treatment field delivery, their coaching and respiratory co-ordination during treatment requires some extra time. In our study, average time taken for breath-hold training before CT simulation was 20 min. Despite of this training, during the initial fractions of radiotherapy, treatment time was longer compared to later fractions of radiotherapy, because the patients required some time to get used to the breath-hold technique.
Few studies have reported Cardiac Contact Distance (CCD) as a predictor of cardiac sparing with DIBH. [29], [30] In a study by Rochet N et al. [30], CCD was measured in both axial (CCDax) and parasagittal plane (CCDps) of planning CT and concluded that FB-CCDps is potentially a very good predictor for cardiac exposure. In our study, HCWL is the CCDax and we have not found any correlation between this predictor and heart doses. We have not analysed for CCDps.
Study by Tanna et al [27] suggested an upfront selection criterion for selecting patients for treatment with DIBH so that two radiotherapy planning scans can be avoided. These upfront selection criteria were tumours in the lower part of the breast or tumours extending across more than one quadrant, should be selected for DIBH. By using this criterion, they reported that almost 2/3rd patients would have been over selected for DIBH.
In our study we had 10 patients who had < 20% reduction in mean heart dose and out of them 9 had ΔHVIF < 6 cc and ΔMHD < 7 mm. Therefore, out of the total 31 patients in our study, we could select 9 patients i.e. 29% of the study cohort who will be less benefitted from DIBH.
There are several limitations to our study. First, our study cohort size is small but is comparable to most of the published DIBH case series. Second, we have not reported any clinical outcome or Normal Tissue Complications Probability (NTCP) in our study. Third, we have not analysed for clinical predictors like Body Mass Index (BMI), age, preexisting heart disease etc. Fourth, two sets of CT scan images were taken for each patient, one in free breathing and other in DIBH, so a concern may arise regarding the additional dose of radiation exposure from one extra CT scan. McCollough et al. [31] stated that the effective doses of radiation from diagnostic CT scan ranges from less than 1 mSv to around 10 mSv (CT chest around 7 mSv). Even if one extra scan is taken, the doses would still be 10 to 100 times lower than the dose levels that have been reported to increase the risk of secondary cancer.
Conclusion
From the dosimetric comparisons depicted in our study, we conclude that when compared with free breathing scans, there is a significant reduction in heart, LAD and lung doses with DIBH, which may lead to reduction in late cardiac toxicity. We found a significant correlation of reduction in Heart Volume in Field and Maximum Heart Depth with a reduction in mean heart dose. ΔHVIF is an independent predictor of cardiac sparing. Most of the left-sided breast cancer patients are likely to benefit and should be treated with DIBH. However, HVIF and MHD predicted for cardiac sparing and threshold criteria of ΔHVIF and ΔMHD may be used by centres with high workload to select patients who will benefit more with DIBH. Though this would require two simulation scans and coaching time, it will decrease treatment duration in some patients and waiting time in the radiotherapy department.
Funding source
None.
Ethical committee approval
The study is approved by institutional ethical committee.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2021.02.006.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Early Breast Cancer Trialists' Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. The Lancet. 2011 Nov 12;378(9804):1707-16. https://doi.org/10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed]
- 2.McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (London, England). 2014 Jun 5;383(9935) http://doi.org/10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed]
- 3.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005 Dec 17;366(9503):2087-106. https://doi.org/10.1016/S0140-6736(05)67887-7. [DOI] [PubMed]
- 5.Nielsen H.M., Overgaard M., Grau C., Jensen A.R., Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24(15):2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 6.Giordano S.H., Kuo Y.F., Freeman J.L., Buchholz T.A., Hortobagyi G.N., Goodwin J.S. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97(6):419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 8.Lu H.M., Cash E., Chen M.H., Chin L., Manning W.J., Harris J. Reduction of cardiac volume in left-breast treatment fields by respiratory maneuvers: a CT study. Int J Radiat Oncol Biol Phys. 2000;47(4):895–904. doi: 10.1016/S0360-3016(00)00512-5. [DOI] [PubMed] [Google Scholar]
- 9.Raj KA, Evans ES, Prosnitz RG, Quaranta BP, Hardenbergh PH, Hollis DR et al. Is there an increased risk of local recurrence under the heart block in patients with left-sided breast cancer? The Cancer Journal. 2006 Jul 1;12(4):309-http://doi.org/10.1097/00130404-200607000-00010. [DOI] [PubMed]
- 10.Hiatt J.R., Evans S.B., Price L.L., Cardarelli G.A., DiPetrillo T.A., Wazer D.E. Dose-modeling study to compare external beam techniques from protocol NSABP B-39/RTOG 0413 for patients with highly unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys. 2006;65(5):1368–1374. doi: 10.1016/j.ijrobp.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 11.Korreman S.S., Pedersen A.N., Nøttrup T.J., Specht L., Nyström H. Breathing adapted radiotherapy for breast cancer: comparison of free breathing gating with the breath-hold technique. Radiother Oncol. 2005;76(3):311–318. doi: 10.1016/j.radonc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett F.R., Colgan R.M., Carr K., Donovan E.M., McNair H.A., Locke I. The UK HeartSpare Study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiotherapy Oncol. 2013;108(2):242–247. doi: 10.1016/j.radonc.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Swanson T., Grills I., Ye H., Entwistle A., Teahan M., Letts N. Six-year experience routinely utilizing moderate deep inspiration breath-hold (mDIBH) for the reduction of cardiac dose in left-sided breast irradiation for patients with early stage or locally advanced breast cancer. Am J Clin Oncol. 2013;36(1):24. doi: 10.1016/j.ijrobp.2009.07.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden A.J., Rains M., Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol. 2012;56(4):464–472. doi: 10.1111/j.1754-9485.2012.02405.x. [DOI] [PubMed] [Google Scholar]
- 15.Shim J.G., Kim J.K., Park W., Seo J.M., Hong C.S., Song K.W. Dose-volume analysis of lung and heart according to respiration in breast cancer patients treated with breast conserving surgery. J Breast Can. 2012;15(1):105–110. doi: 10.4048/jbc.2012.15.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vikström J., Hjelstuen M.H., Mjaaland I., Dybvik K.I. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncologica. 2011;50(1):42–50. doi: 10.3109/0284186X.2010.512923. [DOI] [PubMed] [Google Scholar]
- 17.van der Laan H.P., Hurkmans C.W., Kuten A., Westenberg H.A. Current technological clinical practice in breast radiotherapy; results of a survey in EORTC-Radiation Oncology Group affiliated institutions. Radiother Oncol. 2010;94(3):280–285. doi: 10.1016/j.radonc.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Desai N., Currey A., Kelly T., Bergom C. Nationwide trends in heart-sparing techniques utilized in radiation therapy for breast cancer. Adv Radiat Oncol. 2019;4(2):246–252. doi: 10.1016/j.adro.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duane F., Aznar M.C., Bartlett F., Cutter D.J., Darby S.C., Jagsi R. A cardiac contouring atlas for radiotherapy. Radiotherapy Oncol. 2017;122(3):416–422. doi: 10.1016/j.radonc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx [accessed 17nth August 2019].
- 21.Feng M., Moran J.M., Koelling T., Chughtai A., Chan J.L., Freedman L. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Register S., Takita C., Reis I., Zhao W., Amestoy W., Wright J. Deep inspiration breath-hold technique for left-sided breast cancer: an analysis of predictors for organ-at-risk sparing. Medical Dosimetry. 2015;40(1):89–95. doi: 10.1016/j.meddos.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Smyth L.M., Knight K.A., Aarons Y.K., Wasiak J. The cardiac dose-sparing benefits of deep inspiration breath-hold in left breast irradiation: a systematic review. J Med Radiat Sci. 2015;62(1):66–73. doi: 10.1002/jmrs.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zurl B., Stranzl H., Winkler P., Kapp K.S. Quantitative assessment of irradiated lung volume and lung mass in breast cancer patients treated with tangential fields in combination with deep inspiration breath hold (DIBH) Strahlentherapie und Onkologie. 2010;186(3):157–162. doi: 10.1007/s00066-010-2064-y. [DOI] [PubMed] [Google Scholar]
- 25.Yeung R., Conroy L., Long K., Walrath D., Li H., Smith W. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiation Oncology. 2015;10(1):200. doi: 10.1186/s13014-015-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor C.W., McGale P., Povall J.M., Thomas E., Kumar S., Dodwell D. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys. 2009;73(4):1061–1068. doi: 10.1016/j.ijrobp.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 27.Tanna N., McLauchlan R., Karis S., Welgemoed C., Gujral D.M., Cleator S.J. Assessment of upfront selection criteria to prioritise patients for breath-hold left-sided breast radiotherapy. Clin Oncol. 2017;29(6):356–361. doi: 10.1016/j.clon.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Purdie TG, Rahman M, Marshall A, Liu FF, Fyles A. Rapid automated treatment planning process to select breast cancer patients for active breathing control to achieve cardiac dose reduction. Int J Radiat Oncol Biol Phys 2012 Jan 1;82(1):386-93. https://doi.org/10.1016/j.ijrobp.2010.09.026. [DOI] [PubMed]
- 29.Czeremszyńska B., Drozda S., Górzyński M., Kępka L. Selection of patients with left breast cancer for deep-inspiration breath-hold radiotherapy technique: results of a prospective study. Rep Pract Oncol Radiotherap. 2017;22(5):341–348. doi: 10.1016/j.rpor.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochet N., Drake J.I., Harrington K., Wolfgang J.A., Napolitano B., Sadek B.T. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Practical radiation oncology. 2015 May 1;5(3):e127–e134. doi: 10.1016/j.prro.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 31.McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. InMayo Clinic Proceedings 2015 Oct 1 (Vol. 90, No. 10, pp. 1380-1392). Elsevier. https://doi.org/10.1016/j.mayocp.2015.07.011. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.