Abstract
With the outbreak of COVID-19 (coronavirus disease 2019) as a global pandemic, various of its neurological manifestations have been reported. We report a case of a 54-year-old male with new-onset seizure who tested positive for severe acute respiratory syndrome coronavirus 2 from a nasopharyngeal swab sample. Investigative findings, which included contrast-enhancing right posterior temporal lobe T2-hyperintensity on brain magnetic resonance imaging, right-sided lateralized periodic discharges on the electroencephalogram, and elevated protein level on cerebrospinal fluid analysis, supported the diagnosis of possible encephalitis from COVID-19 infection. The findings in this case are placed in the context of the existing literature.
Keywords: COVID-19, SARS-CoV-2, encephalitis, seizure, review
Introduction
In December 2019, coronavirus disease 2019 (COVID-19) emerged from Wuhan as a global pandemic.1 It is a clinical syndrome caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a positive-sense RNA virus.2 Several neurological manifestations have been associated with COVID-19, among which hyposmia, headache, myalgia, hypogeusia, and altered sensorium are commonly identified.3 New-onset seizures or breakthrough seizure of previously known epilepsy patients are a less common manifestation of COVID-19.4,5 Likewise, there is sparse literature on new-onset seizure with encephalitis from COVID-19.6 We present a case of new-onset seizure likely secondary to possible limbic encephalitis in a patient with acute COVID-19 infection, along with an updated review.
Case Presentation
A 54-year-old African American male was evaluated in the emergency department (ED) after a 5-minute episode of loss of consciousness without convulsion preceded by sudden onset of a nonradiating pressure type holocranial headache along with diaphoresis, palpitation, and nausea. He was then working in his normal state of health at the hospital cafeteria and the event was witnessed by his coworkers. He regained consciousness without confusion. Fifteen minutes later, he had a generalized convulsion lasting 1 minute followed by 15 minutes of post-ictal confusion as described by the nurse. The patient could not recall any aura before seizure. He did not bite his tongue or become incontinent in either of the events. His past medical history included paroxysmal atrial fibrillation (PAF), hypertension, glucose-6-phosphate dehydrogenase deficiency, and hepatosteatosis. He did not report any past or family history of seizures and no risk factors for these. He was compliant on apixaban 5 mg 2 times daily as his home medication for PAF. He was afebrile, normotensive, and had an irregularly irregular pulse rate of 110 beats per minute. During the initial neurological examination, he was drowsy, oriented to time, place, and person with intact language, cranial nerves, motor, sensory, and cerebellar functions. He had no neck rigidity. He was treated with 2 mg of intravenous (IV) lorazepam and 1-g loading dose of IV levetiracetam in the ED.
The patient tested positive for SARS-CoV-2 infection in real-time reverse transcription polymerase chain reaction analysis taken from nasopharyngeal swab. Chest X-ray was unremarkable. Arterial blood gas analysis showed respiratory acidosis (pH 7.21, pCO2 [partial pressure of carbon dioxide] 69.8, pO2 [partial pressure of oxygen] 84.2, bicarb 28, and FIO2 [fraction of inspired oxygen] 32%). Blood work-up revealed sodium 137 mmol/L (ref = 135-144), potassium 3.6 mmol/L (ref = 3.7-5.0), glucose 115 mg/dL (ref = 70-110), calcium 9 mg/dL (ref = 8.9-10.3), magnesium 1.47 mEq/L (Ref = 1.4-1.9), hemoglobin 11.5 g/dL (ref = 13.7-17.5), white cell count 4.9 × 103/µL (ref = 4.1-10.8), total bilirubin 0.9 mg/dL (ref = 0.4-1.3), aspartate transaminase 30 U/L (ref = 12-38), alanine transaminase 22 U/L (ref = 8-60), ammonia 9 µmol/L (ref = 9-55), urea nitrogen 13 mg/dL (ref = 6-20), creatinine 1.2 mg/dL (ref = 0.64-1.27), vitamin B12 582 pg/mL (ref = 180-914), C-reactive protein 4.96 mg/L (ref = 0.0-4.0), and d-dimer <150 ng/mL (ref = 21-230). Thyroid function test, serum angiotensin-converting enzyme, cardiac troponins, urine toxicology, and analysis were unremarkable.
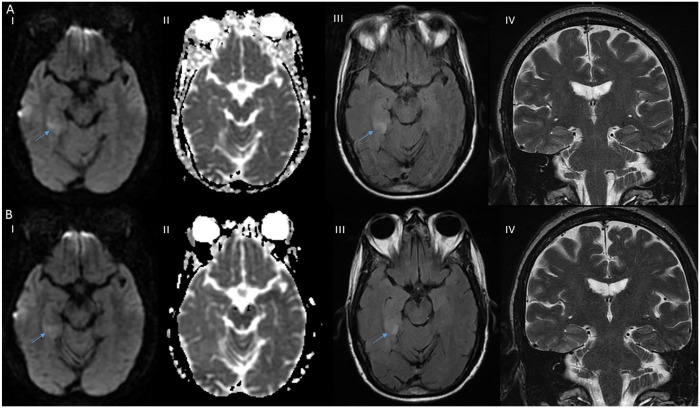
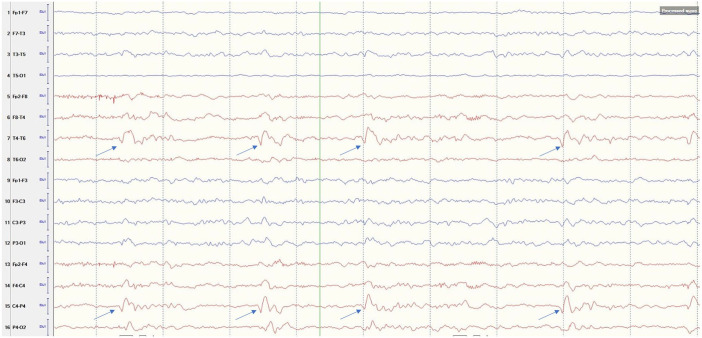
Magnetic resonance imaging (MRI) of the brain showed increased signal in diffusion-weighted imaging (DWI) without apparent-diffusion coefficient correlation in the posterior aspect of right medial temporal lobe and para-hippocampal gyrus with associated T2-weighted-Fluid-Attenuated Inversion Recovery (T2-FLAIR) signal hyperintensity. Repeat imaging 2 days later showed persistent findings with associated contrast enhancement (Figure 1). Computed tomography of the head and neck and transthoracic echocardiogram were unremarkable. Lumbar puncture was done after 24 hours before starting heparin drip without complications. Cerebrospinal fluid (CSF) analysis showed zero white blood cell and red blood cell count, protein 108 mg/dL (ref = 15-45 mg/dL), immunoglobulin G index 0.8 HI (ref = 0.0-0.7 HI), and glucose 56 mg/dL. CSF culture/stain, herpes simplex virus (HSV) 1 and 2, cryptococcal antigen, and cytology were negative. No definitive autoantibodies were detected in the CSF that included anti-amphiphysin, anti-ANNA-1/2/3, anti-AGNA-1, anti-CASPR2, anti-CRMP-5, anti-GAD-65, anti-GRAF1, anti-IgLON5, anti-MPR1, anti-LGI1, anti-mGluR1, anti-NIF, anti-NMDA-A, anti-PCA-Tr, anti-PCA-1, and anti-PCA-2. Electroencephalogram (EEG) showed right posterior quadrant lateralized periodic discharges with a frequency of 0.5 to 1 Hz, accompanied by focal delta slowing, but no electrographic seizures for the 1 hour duration of the study (Figure 2).
Figure 1.
Initial brain magnetic imaging resonance (MRI). (A) Axial section showed hyperintensity on right posterior medial temporal lobe on diffusion-weighted imaging (DWI) (A I) without apparent diffusion coefficient hypo intensity (A II), with associated T2-weighted-fluid-attenuated inversion recovery (T2-FLAIR) hyperintensity (A III), and coronal section T2-weighted imaging showed no hyperintensity on midbrain and thalamus (A IV). Two days later brain MRI (B) axial section showed persistent DWI hyperintensity on right posterior medial temporal lobe (B I), without ADC hypointensity (B II), persistent T2-FLAIR hyperintensity (B III), and unchanged coronal section T2 weighted imaging (B IV; lesion pointed by arrow).
Figure 2.
Electroencephalogram with longitudinal bipolar montage showing right posterior temporal-parietal quadrant lateralized periodic discharges (arrow).
During 4 days of hospitalization, the patient was initially treated with high-flow oxygen for hypoxia in the intensive care unit and was later transitioned to room air by maintaining his oxygen saturation above 93%. Atrial fibrillation with rapid ventricular rate was treated with an amiodarone drip and a heparin drip, which was later transitioned to oral diltiazem and apixaban. Levetiracetam was continued for seizure prophylaxis. He remained afebrile, normotensive, and had no further seizure. He had no cognitive or neurological deficits at the discharge.
Discussion
Common central nervous system (CNS) manifestations of COVID-19 incorporate ischemic stroke, CNS inflammation, encephalopathy, and myelitis.3 Encephalitis have been a pathological diagnosis usually caused by infection or autoimmune process. Viral encephalitis can be suspected with the clinical picture of fever, headache, altered sensorium or behavior, and seizure.7 CSF pleocytosis and elevated protein, T2/FLAIR hyperintensity of the temporal lobe, and focal abnormality on EEG support the diagnosis. However, CSF analysis might be normal in 5% to 10% cases or during the early stage of the disease.7
We lack the brain biopsy finding in our case to yield a definitive diagnosis. Our patient had a new-onset headache, seizure, contrast-enhancing T2 hyperintense lesion in the right medial temporal lobe, LPDs in right temporal leads on EEG, and elevated protein in the CSF with positive SARS-CoV-2 infection, which suggested the diagnosis of possible encephalitis. Though the lack of leukocytosis and mildly elevated protein in the CSF may be a nonspecific finding, however, similar CSF picture can be seen in early encephalitis. There were no metabolic abnormalities, cytokine storm, and cerebral hypoxic changes to attribute for the cause of seizure, which are theorized among the mechanisms of seizure with COVID-19.6
The lack of restricted diffusion in MRI brain ruled out an acute ischemic stroke. We cannot exclude the possibility of subacute stroke; however, other vascular territories of posterior cerebral artery that supplies medial temporal lobe like midbrain, thalamus, and occipital lobe were unaffected and patient compliance of apixaban for PAF made it less likely diagnosis. The lesion had DWI hyperintensity, which we think was likely secondary to the seizure. Seizure causes transient cytotoxic and vasogenic edema, which can reflect as a hyperintense DWI lesion on MRI of brain.8 The lack of heterogeneous lesion made brain tumor like low-grade glioma a lesser possibility. Demyelinating CNS diseases like multiple sclerosis usually affects periventricular and juxtacortical regions, which were not present in our case. Finally, CSF analysis showed no evidence of autoimmune antibody associated with encephalitis.
We did the systemic review of all cases published in English language with possible meningoencephalitis with COVID-19 to look for the clinical course and associated findings. A systematic review was done using PubMed and EMBASE database from January 2020 to September 2020 using the search terms “COVID-19,” “ SARS-CoV-2,” and “encephalitis,” modified as per the requirements for the search tool of each database. We found 14 such cases, which are elaborated in Table 1.9-21 The ages of the 14 cases ranged from 25 to 72 years (median = 45; interquartile range = 33.5-61). Of the 14, nine were males. Most of them initially had respiratory symptoms, such as cough and dyspnea (71.4%) and fever (42.8%). Four cases had no respiratory symptoms.15-17,19 Common neurological manifestations included altered sensorium (92.8%), headache (42.8%), new-onset seizure (35.7%), and dizziness (14.2%). Two cases initially had behavioral issues12; 1 case presented with mutism18; 1 case had left-sided paresis and paresthesia19; 1 case had ataxia, diplopia, oscillopsia, and bilateral facial weakness21; and 1 case reported hyposmia and ageusia.14
Table 1.
Summary of Published Cases of Encephalitis With COVID-19 Infection Until September 2020.
| Cases | Clinical presentation | Signs of meningism | CSF analysis | Imaging and EEG | Management and outcome |
|---|---|---|---|---|---|
| Moriguchi et al9 | 24-year-old male who had 9 days of fatigue, headache, fever, and sore throat; then multiple generalized seizure and decreased consciousness | Present | OP was elevated to 32 cm H2O with 10/mm3 mononuclear and 2/mm3 polymorphonuclear cells, HSV 1 and VZV negative. SARS-CoV-2 was positive. |
Brain MRI had findings of diffusion restriction in the right temporal lobe, hippocampal atrophy and ventriculitis. EEG-NA |
Intubated, treated with empiric antibiotics and antiviral, steroids, levetiracetam (at the time of report patient was 15 days in ICU) |
| Poyiadji et al10 | A female had 3 days of cough, fever, and altered sensorium | NA | Negative bacterial culture for 3 days, negative HSV 1 and 2, VZV, and WNV | CT head had bilateral symmetrical medial thalami hypoattenuation. Brain MRI had hemorrhagic ring-enhancing lesions within bilateral thalami, medial temporal lobes, and subinsular regions. EEG-NA |
Intravenous immunoglobulin; outcome not reported |
| Ye et al11 | A male had 12 days of fever, SOB, myalgia; and then altered sensorium | Present | Elevated OP 22 cm H2O, WBC 0.001 × 109/L, protein 0.27 g/L, glucose 3.14 mmol/L. SARS-CoV-2 was negative | CT head was normal EEG-NA |
Supportive, mannitol infusion; recovered nearly after a month of symptom onset |
| Bernard et al12 | A 64-year-old female had 5 days of cough, myalgia, behavior issues; and then GTCS | Absent | Cells 17/mm3; lymphocytes 97%, protein 466 mg/L, glucose CSF/serum 0.59, HSV 1 and 2, and anti-neuronal antibodies and SARS-CoV-2 was negative | Brain MRI was normal EEG showed nonconvulsive focal status epilepticus. Follow-up after 24 hours: moderate theta background slowing with no epileptiform activity. |
IV clobazam and valproate, acyclovir; improved after 96 hours of admission |
| Bernard et al12 | A 67-year-old female with 17 days of respiratory symptoms had headache, altered sensorium, behavior issues, left hemianopia, and sensory hemineglect | Absent | Cells 21/mm3; lymphocytes 89%, protein 461 mg/L, glucose CSF/serum 0.62, HSV 1 and 2, anti-neuronal antibody, and SARS-CoV-2 was negative | Normal imaging EEG-NA |
Empiric antibiotics, antiviral (acyclovir); recovered within 25 hours and discharged by 72 hours with no symptoms |
| Sohal and Mossammat13 | A 72-year-old male had weakness and light headedness following a hypoglycemic episode; then had SOB and altered sensorium; on day 3 of admission had multiple GTCS | NA | NA | CT head showed chronic microvascular changes with no acute findings EEG showed 6 temporal seizure |
Required intubation; oseltamivir, hydroxychloroquine, azithromycin, vancomycin, and piperacillin tazobactam. Seizure was treated with ativan, versed drip, leviteracetam, valporate; patient died on day 5. |
| Vandervorst et al14 | A 29-year-old male had 1 week of general weakness, cough, SOB, decreased appetite; 2 weeks later had altered sensorium, hyposmia, and hypogeusia | NA | CSF was within normal limits Repeat CSF was normal, HSV, enterovirus, antineuronal antibodies, and SARS-CoV-2 was negative |
CT head was normal MRI brain showed FLAIR signal hyperintensity of left medial temporal lobe with gyral expansion without diffusion restriction Repeat MRI brain 4 days after initial MRI, showed normalization of cortical hyperintensity and gyral expansion EEG showed excess beta activity with short-lasting left temporal delta activity not exceeding 10 seconds |
Acyclovir and hydroxychloroquine; discharged after 2 weeks with no neurological deficits |
| Efe et al15 | A 35-year-old female had headache, nausea, dizziness, and seizure | NA | NA | MRI brain showed T2 FLAIR hyperintensity in the left temporal lobe MR spectroscopy showed elevated choline peak, decreased N-acetylaspartate peak EEG-NA |
Multiple anti-epileptic (no details) and left anterior temporal lobectomy; symptoms improved completely |
| Huang et al16 and Duong et al17 | A 41-year-old female had headache, fever, and new-onset seizure | Present | WBC 70, 100% L, RBC 65, protein 100, glu 20/200 (units not mentioned). SARS-CoV-2 was present. | Imaging-NA EEG showed generalized slowing with no epileptic discharges |
Ceftriaxone, vancomycin, acyclovir, hydroxychloroquine, levetiracetam; recovered to baseline by the hospital day 12 |
| Pilotto et al18 | A 60-year-old male had 5 days of altered sensorium followed by fever, cough, and mutism | Present | At admission lymphocytes 18/µL, protein 69.6 mg/dL, HSV 1, 2, 6, and 8, EBV, VZV, adenovirus, and enterovirus was negative After day 1 of steroid lymphocytes 18/µL, protein 127.2 mg/dL, oligoclonal bands O, IL-6 2.36 pg/mL, IL-8 >1100 pg/mL, TNF-α 1.31 pg/mL, β-2 microglobulin 3.06 mg/L. Normal Tau, and NF light chain. SARS-CoV-2 was negative. CSF after 10 day of admission showed protein 91.4 mg/dL, lymphocytes 38/µL, IL-8 and TNF-α decreased to 97 pg/mL and 0.28 pg/mL, stable β2, and IL-6 levels. |
CT head was normal MRI brain was normal MRI after steroids was normal EEG showed generalized theta slowing EEG after steroids was normal |
Lopinavir/ritonavir, hydroxychloroquine, ampicillin, acyclovir, and steroids; discharged at day 11 with no neurological deficits |
| Bordo et al | 25-year-old male had a day of headache, left-sided paresthesia, and paresis followed by altered sensorium | NA | Nucleated cells 95 cells/mm3; lymphocytes 98%, protein 1055 mg/L, glucose 80 mg/L, RBC O/mm3, IL-1β 14.8 pg/mL, IL6 190 pg/mL, INF-α <0.58 pg/mL, INF-β <8.78 pg/Ml, ACE 15.5 U/L | CT head was normal MRI brain was normal EEG-NA |
Acyclovir, ampicillin, ceftriaxone; fully recovered after 2 days with amnesia of past 2 days |
| Bodro et al19 | A 49-year-old male had a week of fever, myalgias, cough, followed by altered sensorium | NA | Nucleated cells 90 cells/mm3; lymphocytes 99%, protein 1155 mg/L, glucose 54 mg/L, RBC 260/mm3, IL-1β <2.56 pg/mL, IL-6 25 pg/mL, INF-α <0.58 pg/mL, INF-β <8.78 pg/mL, ACE 10.9 U/L | CT head was normal MRI brain was normal EEG-NA |
Acyclovir, ampicillin, and ceftriaxone; recovered after 3 days with amnesia of previous days |
| El Aoud et al20 | A 60-year-oldmale had cough, headache, and brief loss of consciousness; 9 days later had persistent headache and intermittent altered sensorium | NA | Normal protein 0.49 g/L, glucose 0.55 g/L, WBC 1/mm3. Sterile culture. | CT head was normal Brain MRI showed focal hyperintense signal in DWI and T2-FLAIR in splenium MRI brain after a month was normal EEG showed slow oscillations without epileptiform features |
Amoxicillin/clavulanic acid; no residual symptoms after several weeks of follow-up |
| Wong et al21 | A 40-year-old male had 10 days of persistent fever, progressive dyspnea, followed 10 days later by productive cough and diarrhea presented with ataxia, diplopia, oscillopsia, and bilateral facial weakness | NA | Normal cell count and protein 0.042 g/L | Brain MRI showed increased signal lesion in right inferior cerebellar peduncle extending a small portion of the upper cord with associated swelling and microhemorrhage EEG-NA |
Amoxicillin with no other treatment; discharged after 11 days with persistent oscillopsia and ataxia |
Abbreviations: COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; EEG, electroencephalogram; OP, opening pressure; SOB, shortness of breath; HSV, herpes-simplex virus; VZV, varicella-zoster virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NA, not applicable; ICU, intensive care unit; WNV, West Nile virus; CT, computed tomography; WBC, white blood cell; GTCS, generalized tonic-clonic seizure; IV, intravenous; MRI, magnetic resonance imaging; FLAIR, Fluid-Attenuated Inversion Recovery; RBC, red blood cell; EBV, Epstein-Barr virus; IL, interleukin; TNF-α, tumor necrosis factor-α; NF, nuclear factor; INF, interferon; ACE, angiotensin-converting enzyme; DWI, diffusion-weighted imaging.
Six cases reported meningeal signs among which 71.4% had features of meningism, such as neck stiffness, Kernig’s and Brudzinski signs.9,11,12,17,18 Out of 12 cases who had CSF analysis, 4 (33.3%) had normal findings,10,14,20,21 while the others had elevated CSF opening pressure and/or lymphocytic pleocytosis with elevated protein. Three cases had elevated CSF interleukin levels.18,19 SARS-CoV-2 was detected in the CSF of 2 cases.9,16 Eleven cases had MRI of the brain imaging and 5 were reported to have normal findings (45.5%),12,18,19 3 cases had temporal lobe involvement suggesting limbic encephalitis (27.3%),9,14,15 1 case had necrotizing hemorrhagic encephalitis,10 1 case had rhombencephalitis,21 and 1 had type 1 mild encephalitis with reversible splenial lesion.20 One case with left temporal encephalitis had MR spectroscopy finding of elevated choline peak, and decreased N-acetylaspartate peak. Subsequent left temporal frozen biopsy showed concentric lymphocytic infiltration into perivascular spaces with neuronal damage and diffuse hypoxic changes.15
Most of the patients were managed with empirical antibiotics, acyclovir, hydroxychloroquine, and steroids. Out of 5 cases of new-onset seizures, MRI of the brain was done in 3 cases among which 2 had temporal lobe T2-hyperintense signal changes and 1 had normal findings. Seizures were managed by various anti-epileptics, including levetiracetam, lorazepam, versed, clobazam, and valproate.9,12,13,15,17 One case had a refractory focal seizure with concomitant use of hydroxychloroquine.13 Majority of the patients had good and early recoveries with median time to recovery of 11 days (interquartile range = 3-12.5).12,14,16,18,19,21
Our review suggests, frequent atypical presentation from possible encephalitis with COVID-19, like isolated behavior issues. Altered mental status was the foremost presentation while fever and new-onset seizure was reported in more than one third of the cases. Normal CSF analysis and MRI findings were seen in one third or more cases. Diagnosis of possible meningoencephalitis was made based on the clinical picture and investigations. As in this case, most of the reported cases of COVID associated encephalitis recover quickly with minimal residual deficits. Definitive diagnosis with evidence of SARS-CoV-2 in brain tissue were lacking.
Currently, there are numerous medications used for the treatment of COVID-19 infection. Care should be taken while using hydroxychloroquine, as it lowers the seizure threshold and interacts with several antiepileptics.22 For instance, in a patient with COVID-19 and refractory seizure, hydroxychloroquine may have lowered the seizure threshold.13 Levetiracetam is the favored antiepileptic medication for treating a seizure in COVID-19 patients, given its least cardiorespiratory adverse effects and drug-drug interactions.6
Our case adds to the literature on possible encephalitis with new-onset seizure associated with COVID-19. According to existing literature, more than one third of COVID-19 encephalitis cases had a new onset seizure and the majority quickly recovered with minimal residual deficits.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual case.
Informed Consent: Informed consent for patient information to be published in this article was obtained.
ORCID iD: Riwaj Bhagat  https://orcid.org/0000-0001-7730-3665
https://orcid.org/0000-0001-7730-3665
References
- 1. Thompson R. Pandemic potential of 2019-nCoV. Lancet Infect Dis. 2020;20:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nepal G, Rehrig JH, Shrestha GS, et al. Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020;24:421. doi: 10.1186/s13054-020-03121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anand P, Al-Faraj A, Sader E, et al. Seizure as the presenting symptom of COVID-19: a retrospective case series. Epilepsy Behav. 2020;112:107335. doi: 10.1016/j.yebeh.2020.107335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-660. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asadi-Pooya AA. Seizures associated with coronavirus infections. Seizure. 2020;79:49-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon T, Michael BD, Smith PE, et al. ; National Encephalitis Guidelines Development and Stakeholder Groups. Management of suspected viral encephalitis in adults—association of British Neurologists and British Infection Association National Guidelines. J Infect. 2012;64:347-373. [DOI] [PubMed] [Google Scholar]
- 8. Kim JA, Chung JI, Yoon PH, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. Am J Neuroradiol. 2001;22:1149-1160. [PMC free article] [PubMed] [Google Scholar]
- 9. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119-E120. doi: 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernard-Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;27:e43-e44. doi: 10.1111/ene.14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sohal S, Mossammat M. COVID-19 presenting with seizures. IDCases. 2020;20:e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vandervorst F, Guldolf K, Peeters I, et al. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip Neurosurg. 2020;22:100821. doi: 10.1016/j.inat.2020.100821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Efe IE, Aydin OU, Alabulut A, et al. COVID-19-associated encephalitis mimicking glial tumor. World Neurosurg. 2020;140:46-48. doi: 10.1016/j.wneu.2020.05.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pilotto A, Odolini S, Masciocchi S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88:423-427. doi: 10.1002/ana.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodro M, Compta Y, Llanso L, et al. Increased CSF levels of IL-1beta, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7:e821. doi: 10.1212/NXI.0000000000000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Aoud S, Sorial D, Selmaoui A, et al. A first case of mild encephalitis with reversible splenial lesion (MERS) as a presenting feature of SARS-CoV-2. Rev Neurol (Paris). Published online July 4, 2020. doi: 10.1016/j.neurol.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong PF, Craik S, Newman P, et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med (Lond). 2020;20:293-294. doi: 10.7861/clinmed.2020-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fish D, Espir M. Convulsions associated with prophylactic antimalarial drugs: implications for people with epilepsy. BMJ. 1988;297:526-527. [DOI] [PMC free article] [PubMed] [Google Scholar]