Abstract
We investigated the association between serum adenosine deaminase and coronary artery calcification (CAC) in type 2 diabetes mellitus (T2DM) patients. The cross-sectional study included 459 patients with T2DM, the clinical and laboratory tests were performed, and all T2DM patients were separated into the 3 groups based on the tertile of serum adenosine deaminase levels. In the baseline data, the CAC score had statistically significant differences between the 3 groups (p < 0.001). Serum adenosine deaminase levels were positively correlated with CAC score in T2DM patients (r = 0.355, p < 0.001). The results of multiple linear regression analysis showed that serum adenosine deaminase was independent positively correlated with CAC score in T2DM patients (r = 0.255, p < 0.001). Receiver-operating characteristic curve analysis showed that area under curve was 0.750 to identify T2DM patients with CAC. Serum adenosine deaminase levels are correlated with CAC scores in T2DM patients, clinically, serum adenosine deaminase should be considered as an underlying marker to determine the severity of atherosclerosis in T2DM patients.
Keywords: serum adenosine deaminase, type 2 diabetes mellitus, coronary artery calcification, cardiovascular disease
Introduction
Cardiovascular disease is a major complication in patients with type 2 diabetes mellitus (T2DM), and T2DM patients increase the 2-3 folds risk of cardiovascular disease.1 Contemporary data have showed that diabetes mellitus is close related with the risk of cardiovascular mortality.2 Clinically, coronary artery calcification (CAC) has been acknowledged to the deposition of calcium in coronary arteries, CAC is highly correlated to the occurrence and progress of atherosclerosis,3 and the CAC score is an outstanding predictor of cardiovascular events in patients with T2DM.4
Adenosine deaminase is a widely catalytic enzyme that catalyzes adenosine deamination to inosine.5 Serum adenosine deaminase is a clinical biomarker in multiple diseases, such as hepatic injury, hemolytic anemia and tuberculous disease.6 Recent studies have suggested that serum adenosine deaminase levels are associated with patients with recent-onset schizophrenia, cutaneous anthrax and chronic tonsillitis.7–9 Recently, due to the fact increased serum adenosine deaminase levels in T2DM patients with adverse glycemic control,10 then, the poor glycemic control is a risk factor for atherosclerosis in T2DM patients.11 Thus, we investigated the association between serum adenosine deaminase and CAC in T2DM patients.
Methods and Materials
T2DM Patients
459 patients with type 2 diabetes who referred to WHO criteria12 were diagnosed, and all T2DM patients were separated into the 3 groups based on the tertile of serum adenosine deaminase levels, in these T2DM patients, patients were subjected to computed tomography scan. This study was approved by Ethics Committee of Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, and our study obtained the informed consent of the patients. Exclusion criteria: patients with acute or chronic infections, tuberculous disease, cancer, liver damage, rheumatic disease, renal insufficiency, pregnancy and hematologic disease were excluded.
Blood Analytes
Blood samples were drawn in the morning after fasted overnight in all patients. In total, serum was separated to test laboratory indexes by high-speed centrifugation with 3,000 rpm at room temperature for 10 minutes, and further biochemical tests were performed.
Clinical Materials
Physical examinations were carried out. Blood pressure was assessed by using a mercury sphygmomanometer. The clinical materials such as body mass index, medication use and diabetes duration et al were obtained in electronic medical records.
The Measurements for CAC Score
Image tests were implemented by computed tomography scan with a gantry rotation speed of 0.4 s/rotation, 120 kV and 100 mA, the CAC score was estimated by computed tomography scan, and CAC score was categorized on the basis of following grades: ≤10 (minimal or none); 11-100 (mild calcification); 101-400 (moderate calcification); 401-1000 (severe calcification); and greater than 1,000 (extensive calcification).13
Statistical Analysis
We used SPSS version 20.0 statistical software to assess the data. The data are shown as mean and standard deviation or percentage. Chi-square test and One-ANOVA were used to determine the differences between the 3 groups. The correlations were made by Pearson correlation test. We used multiple linear regression analysis to analyze the further correlation between serum adenosine deaminase and CAC score. Receiver-operating characteristic curve analysis was selected to assess the performance to identify T2DM patients with CAC form all patients. The p values <0.05 were considered statistically significant.
Results
Table 1 provided all T2DM patients’ physical demographic, clinical materials and metabolic parameters, these data were separated into 3 groups on the basis of the tertile of serum adenosine deaminase levels, the CAC score had statistically significant differences between the 3 groups (p<0.001), and the statistically significant differences were observed in body mass index, duration of diabetes, insulin use, high-sensitivity C-reactive protein, fasting blood glucose and glycosylated hemoglobin between the 3 groups.
Table 1.
Clinical Parameters Are Separated According to the Tertile of Serum Adenosine Deaminase.
| <9 | 9-13 | >13 | ||
|---|---|---|---|---|
| n = 150 | n = 174 | n = 135 | p-values | |
| Gender (Male, n %) | 94 (62.7) | 113 (64.9) | 94 (69.6) | 0.455 |
| Age(yr) | 46.8 ± 8.2 | 47.8 ± 9.7 | 48.4 ± 9.7 | 0.313 |
| Body mass index (kg/m2) | 21.7 ± 2.2 | 23.0 ± 2.5 | 23.0 ± 2.4 | <0.001 |
| Smoking, n (%) | 37 (24.7) | 39 (22.4) | 30 (22.2) | 0.856 |
| Alcohol use, n (%) | 15 (10.0) | 15 (8.6) | 10 (7.4) | 0.740 |
| Hypertension, n (%) | 40( 26.7) | 54 (31.0) | 46 (34.1) | 0.391 |
| Hyperlipidemia, n (%) | 75 (50%) | 75 (43.1%) | 61 (45.2%) | 0.452 |
| Duration of diabetes (years) | 7.7 ± 7.5 | 9.3 ± 8.6 | 10.1 ± 9.4 | 0.048 |
| Insulin, n (%) | 26 (17.3) | 44 (25.3) | 40 (29.6) | 0.046 |
| Aspirin, n (%) | 110 (73.3%) | 109 (62.6%) | 84 (62.6) | 0.070 |
| ACEI/ARBs, n (%) | 135 (90.0) | 166 (95.4) | 124 (91.9) | 0.167 |
| High-sensitivity C-reactive protein (mg/L) | 3.0 ± 2.1 | 3.2 ± 2.2 | 4.2 ± 2.2 | <0.001 |
| Fasting blood glucose (mmol/L) | 6.8 ± 3.1 | 7.9 ± 2.6 | 9.9 ± 3.7 | <0.001 |
| Glycosylated hemoglobin (%) | 7.8 ± 4.6 | 8.2 ± 4.3 | 10.5 ± 4.1 | <0.001 |
| Coronary artery calcification scores | 25.6 ± 56.1 | 32.0 ± 82.1 | 143.6 ± 136.4 | <0.001 |
ACEI = angiotensin-converting enzyme inhibitor, ARBs = angiotensin-2 receptor blockers.
In the correlation analyses, serum adenosine deaminase levels were positively correlated with body mass index, fasting blood glucose, glycosylated hemoglobin in patients with T2DM (r = 0.134, p = 0.004; r = 0.382, p < 0.001; and r = 0.237, p < 0.001), moreover, there was an additional positively correlation between serum adenosine deaminase and high-sensitivity C-reactive protein (r = 0.227, p<0.001), and serum adenosine deaminase was observed to be positively correlated with CAC score in T2DM patients (r = 0.355, p < 0.001).
Table 2 provided the results of multiple linear regression analysis, we adjusted the variables including gender, age, body mass index, smoking, alcohol use, hypertension, duration of diabetes, medication use, high-sensitivity C-reactive protein, fasting blood glucose, glycosylated hemoglobin, and hyperlipidemia in the multiple linear regression analysis, afterward, the serum adenosine deaminase was independently positively correlated with CAC score in patients with T2DM (r = 0.255, p < 0.001).
Table 2.
The Serum Adenosine Deaminase Is Independently Correlated With CAC Scores in T2DM Patients in Multiple Linear Regression Analysis.
| Unstandardized cofficients | Standardized cofficients | t | P-value | ||
|---|---|---|---|---|---|
| B | Std Error | Beta | |||
| Body mass index | 0.282 | 0.070 | 0.165 | 4.035 | <0.001 |
| Aspirin | -0.810 | 0.364 | -0.091 | -2.228 | 0.026 |
| Fasting blood glucose | 0.181 | 0.060 | 0.144 | 3.037 | 0.003 |
| Glycosylated hemoglobin | 0.087 | 0.043 | 0.092 | 2.029 | 0.043 |
| High-sensitivity C-reactive protein | 0.403 | 0.078 | 0.213 | 5.189 | <0.001 |
| Coronary artery calcification scores | 0.010 | 0.002 | 0.255 | 5.695 | <0.001 |
Patients who the CAC score (>10) were defined as CAC positive, so receiver-operating characteristic curve analysis was used to identify the T2DM patients with CAC from all patients, the results showed that area under curve was 0.750 with sensitivity of 0.699 and specificity of 0.657, (Figure 1) and the cutoff values of serum adenosine deaminase were 10.2 in identifying T2DM patients with CAC.
Figure 1.
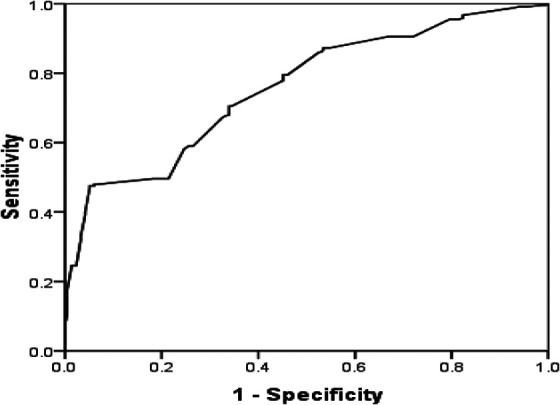
Receiver-operating characteristic curve analysis of serum adenosine deaminas in identifying T2DM patients with CAC.
Discussion
Subclinical atherosclerosis is a clinical disadvantage factor for cardiovascular disease in patients with T2DM, and the CAC is an independently predictor for the overall load of atherosclerosis in persons with and without obstructive coronary artery disease.14 In this study, we found the link between serum adenosine deaminase and fasting blood glucose and glycosylated hemoglobin in T2DM patients, the results are consistent with previous study,15 Interesting, we observed an additional correlation between serum adenosine deaminase and CAC score in T2DM patients.
Low-grade inflammation is active in T2DM patients,16 inflammatory components have been observed in the pathogenesis of atherosclerosis in T2DM, and population-based study has found that inflammation markers are correlated with intermediate cardiovascular endpoints, especially in intima-media thickness,17 and the inflammatory parameters are associated with coronary calcification.18,19 Furthermore, experimental study has suggested that inflammation mediated-molecular and cellular pathways can promote atherosclerosis, key inflammatory mechanism is important in atherogenesis as an active process.20 More direct evidence has been suggested that the increased adenosine deaminase activity is observed as a marker in early stage of atherosclerosis, which contributes to its progression and development of CAC.21 Thus, inflammation may be a major mechanism to elevate serum adenosine deaminase in T2DM patients with CAC. As expected, the association between adenosine deaminase and inflammation have been reported, inhibition of ADA activity can contribute to relieve inflammation and cell proliferation.22 There are several reports indicating that the increase of serum adenosine deaminase due to the stimulation of inflammation. An association of serum adenosine deaminase with systemic inflammation is observed in experimental colitis,23 and serum adenosine deaminase activity is elevated in inflammatory diseases such as acute lymphoblastic leukemia, autoimmune hepatitis and rheumatic disease.24–27 Serum adenosine deaminase has been reported to be an inflammatory marker in rheumatoid arthritis.28 In another study showed that serum adenosine deaminase, an endogenous anti-inflammatory metabolite, is elevated in response to inflammatory status resulted by adipose tissue in obesity.29 Serum adenosine deaminase levels have been found to be increased in patients with Inflammatory bowel disease, and serum adenosine deaminase is a useful biomarker for assessment of intestinal inflammation.30 It is well established in the literature that a significantly correlation between serum adenosine deaminase and C-reactive protein has been suggested in patients with chagas disease,31 supporting the view, our data also showed the link between serum adenosine deaminase and high-sensitivity C-reactive protein in T2DM patients.
In another aspect, poor glycemic control increases the risk of coronary atherosclerosis plaque in T2DM patients, in return, adenosine deaminase can increase insulin sensitivity for glucose transport, and elevate the accessibility about 25% of GLUT4 to cell envelope for glucose transportation.32 Therefore, long-term hyperglycemia also may increase the adaptation of adenosine in patients with T2DM, leading to an increased serum adenosine deaminase levels in T2DM patients with CAC.
We have to consider some limitations. First, our study is only a hospital-based cross-sectional design in general diabetic population, further study should be considered in community population. Second, the effects of anti-inflammatory treatment on serum adenosine deaminase levels were not evaluated in patients with T2DM. Third, use of hypoglycemic and lipid-lowering drugs may affect adenosine deaminase levels in patients with T2DM. Fourth, CAC is an estimated value, but a direct measure of coronary atherosclerosis,33 thus, some patients with coronary atherosclerotic plaques may not be detected. In addition, our study did not examine the relationship between serum adenosine deaminase and clinical outcomes in T2DM patients with CAC.
Conclusions
In the light of our results, serum adenosine deaminase levels are positively correlated with CAC score in T2DM patients, serum adenosine deaminase may be a useful marker to assess the severity of atherosclerosis in patients with T2DM.
Footnotes
Author Contribution: Ming Yu and Hanyun Zhou contributed to this study as co-first authors. Conceived and designed the study: Hongxia Shuai, Juan Ding and Ming Yu. Performed the experiments: Ming Yu, Hanyun Zhou and Qingan Li. Analyzed the data: Ming Yu, Hanyun Zhou. Contributed materials and analysis: Ming Yu, Ji Zhang. Wrote the manuscript: Ming Yu.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Xiangyang Science and Technology Projects (2012 No. 40).
ORCID iD: Juan Ding  https://orcid.org/0000-0002-5929-5830
https://orcid.org/0000-0002-5929-5830
References
- 1. Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21(7):1138–1145. [DOI] [PubMed] [Google Scholar]
- 2. Pan A, Wang Y, Talaei M, Hu FB. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132(19):1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elkeles RS, Godsland IF, Feher MD, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29(18):2244–2251. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal S, Morgan T, Herrington DM, et al. Coronary calcium score and prediction of all-cause mortality in diabetes: the Diabetes Heart study. Diabetes Care. 2011;34(5):1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. da Cunha JG. Adenosine deaminase. A pluridisciplinary enzyme. Acta Med Port. 1991;4(6):315–323. [PubMed] [Google Scholar]
- 6. Sorribas Vivas A, Blanco Vaca F, Gómez Gerique JA, González Sastre F. Adenosine deaminase: biochemical characteristics and clinical significance of an enzyme key to cellular immunity. Med Clin (Barc). 1988;90(13):548–552. [PubMed] [Google Scholar]
- 7. Sasidharan A, Kumar S, John JP, et al. Elevated serum adenosine deaminase levels in neuroleptic-naïve patients with recent-onset schizophrenia. Asian J Psychiatr. 2017;29:13–15. [DOI] [PubMed] [Google Scholar]
- 8. Sunnetcioglu M, Karadas S, Aslan M, et al. Serum adenosine deaminase activity in cutaneous anthrax. Med Sci Monit. 2014;20:1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garca MF, Demir H, Turan M, et al. Assessment of adenosine deaminase (ADA) activity and oxidative stress in patients with chronic tonsillitis. Eur Arch Otorhinolaryngol. 2014;271(6):1797–1802. [DOI] [PubMed] [Google Scholar]
- 10. Niraula A, Thapa S, Kunwar S, Lamsal M, Baral N, Maskey R. Adenosine deaminase activity in type 2 diabetes mellitus: does it have any role? BMC Endocr Disord. 2018;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keen H. Glucose intolerance, diabetes mellitus and atherosclerosis; prospects for prevention. Postgrad Med J. 1976;52(609):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alberti KG, Zimmet PG. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 13. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826–833 [DOI] [PubMed] [Google Scholar]
- 14. Janowitz WR, Agatston AS, Viamonte M, Jr. Comparison of serial quantitative evaluation of calcified coronary artery plaque by ultrafast computed tomography in persons with and without obstructive coronary artery disease. Am J Cardiol. 1991;68(1):1–6. [DOI] [PubMed] [Google Scholar]
- 15. Sapkota LB, Thapa S, Subedi N. Correlation study of adenosine deaminase and its isoenzymes in type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2017;5(1):e000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poreba M, Rostoff P, Siniarski A, et al. Relationship between polyunsaturated fatty acid composition in serum phospholipids, systemic low-grade inflammation, and glycemic control in patients with type 2 diabetes and atherosclerotic cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ray A, Huisman MV, Tamsma JT, et al. The role of inflammation on atherosclerosis, intermediate and clinical cardiovascular endpoints in type 2 diabetes mellitus. Eur J Intern Med. 2009;20(3):253–260. [DOI] [PubMed] [Google Scholar]
- 18. Hartmann G, Tsch PM, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246–252. [DOI] [PubMed] [Google Scholar]
- 19. Hirota T, Suzuki E, Ito I, et al. Coronary artery calcification, arterial stiffness and renal insufficiency associate with serum levels of tumor necrosis factor-alpha in Japanese type 2 diabetic patients. Diabetes Res Clin Pract. 2008;82(1):58–65. [DOI] [PubMed] [Google Scholar]
- 20. Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond). 2018;132(12):1243–1252. [DOI] [PubMed] [Google Scholar]
- 21. Kutryb-Zajac B, Mateuszuk L, Zukowska P, et al. Increased activity of vascular adenosine deaminase in atherosclerosis and therapeutic potential of its inhibition. Cardiovasc Res. 2016;112(2):590–605. [DOI] [PubMed] [Google Scholar]
- 22. Khemka VK, Bagchi D, Ghosh A, et al. Raised serum adenosine deaminase level in nonobese type 2 diabetes mellitus. ScientificWorld J. 2013;2013:404320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antonioli L, Fornai M, Colucci R, et al. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther. 2007;322(2):435–442. [DOI] [PubMed] [Google Scholar]
- 24. Moustafa YM, Elsaied MA, Abd-Elaaty EM, Elsayed RA. Evaluation of serum adenosine deaminase and inflammatory markers in psoriatic patients. Indian J Dermatol. 2019;64(3):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ebrahimi-Rad M, Khatami S, Ansari S, Jalylfar S, Valadbeigi S, Saghiri R. Adenosine deaminase 1 as a biomarker for diagnosis and monitoring of patients with acute lymphoblastic Leukemia. J Med Biochem. 2018;37(2):128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torgutalp M, Efe C, Babaoglu H, Kav T. Relationship between serum adenosine deaminase levels and liver histology in autoimmune hepatitis. World J Gastroenterol. 2017;23(21):3876–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zamani B, Jamali R, Jamali A. Serum adenosine deaminase may predict disease activity in rheumatoid arthritis. Rheumatol Int. 2012;32(7):1967–1975. [DOI] [PubMed] [Google Scholar]
- 28. Vinapamula KS, Pemmaraju SV, Bhattaram SK, Bitla AR, Manohar SM. Serum adenosine deaminase as inflammatory marker in rheumatoid arthritis. J Clin Diagn Res. 2015;9(9):BC08–BC10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jadhav AA, Jain A. Elevated adenosine deaminase activity in overweight and obese Indian subjects. Arch Physiol Biochem. 2012;118(1):1–5. [DOI] [PubMed] [Google Scholar]
- 30. Yordanova M, Gerova D, Atanassova A, Galunska B. Adenosine deaminase as a useful biomarker for diagnosis and monitoring of inflammatory bowel disease. Clin Lab. 2020;66(7). [DOI] [PubMed] [Google Scholar]
- 31. Bravo-Tobar ID, Nello-Pérez C, Fernández A, et al. Adenosine deaminase activity and serum c-reactive protein as prognostic markers of Chagas disease severity. Rev Inst Med Trop Sao Paulo. 2015;57(5):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vannucci SJ, Nishimura H, Satoh S, Cushman SW, Holman GD, Simpson IA. Cell surface accessibility of GLUT4 glucose transporters in insulin-stimulated rat adipose cells. Modulation by isoprenaline and adenosine. Biochemi J. 1992;288(1):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mautner GC, Mautner SL, Froehlich J, et al. Coronary artery calcification: assessment with electron beam CT and histomorphometric correlation. Radiology. 1994;192(3):619–623. [DOI] [PubMed] [Google Scholar]


