Abstract
During the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), many critically ill patients died of severe pneumonia, acute respiratory distress syndrome (ARDS), or multiple organ dysfunction syndrome. To date, no specific treatments have been proven to be effective for coronavirus disease 2019 (COVID-19). In the animal models and clinical applications, mesenchymal stromal/stem cells (MSCs) have been shown safety and efficacy for the treatment of respiratory virus infection through their abilities of differentiation and immunomodulation. Besides, possessing several advantages of MSC-derived extracellular vesicles (EVs) over MSCs, EV-based therapy also holds potential therapeutic effects in respiratory virus infection. In this review, we summarized the basic characteristics and mechanisms of COVID-19 and MSCs, outlined some preclinical and clinical studies of MSCs or MSC-EVs for respiratory virus infection such as influenza virus and SARS-CoV-2, shed light on the common problems that we should overcome to translate MSC therapy into clinical application, and discussed some safe issues related to the use of MSCs.
Keywords: mesenchymal stromal cells, coronavirus disease 2019, clinical translation
Introduction
COVID-19
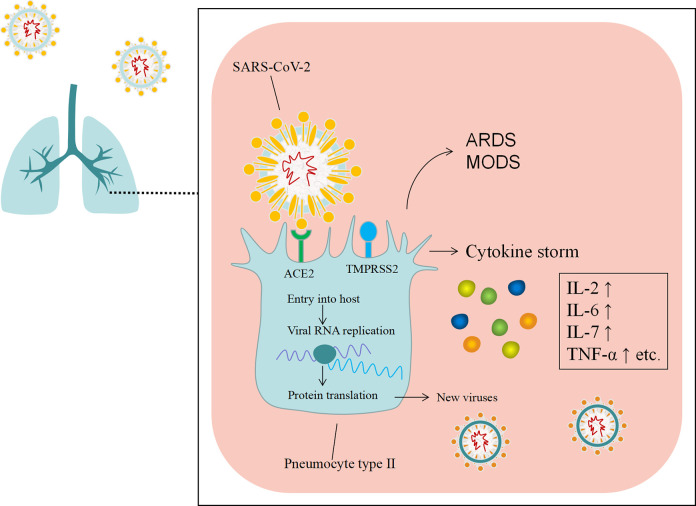
Sine the end of December 2019, a novel coronavirus, named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has emerged in Wuhan city, China, and has spread to other provinces of China and other counties quickly1,2. Human to human transmission was confirmed subsequently3. On 11 March 2020, the World Health Organization (WHO) officially declared the situation as a pandemic4. As of 21 Dec 2020, the novel virus has infected more than 75,700,000 people with more than 1,690,000 deaths worldwide, over 200 countries have been involved in this public health threat5. As with SARS-CoV and the Middle East respiratory syndrome (MERS)-CoV, SARS-CoV-2 is classified as a β CoV of group 2B6. It enters the host cell by its spike protein binding to the receptors identified as angiotensin-converting enzyme 2 (ACE2), which are primarily expressed in the lung epithelial cells7. Besides, study showed that the cellular serine protease TMPRSS2 for SASR-CoV-2 Spike protein priming is also important for entry into target cells8 (Fig. 1). The clinical symptoms of coronavirus disease 2019 (COVID-19) vary from asymptomatic to symptomatic, and the severe cases have been reported to be around 14% of all cases, and around 6% and 3% presented with critical and fatal outcomes, respectively9. Typical clinical symptoms of COVID-19 are fever, cough, dyspnea, and pneumonia. Complications during hospitalization include acute respiratory distress syndrome (ARDS), RNAaemia, acute cardiac injury, secondary infection, acute kidney injury, shock, and multiple organ dysfunction syndrome (MODS)10,11. So far, there are no effective treatments for COVID-19. Several potential drug candidates, including lopinavir/ritonavir, nucleoside analogues, neuraminidase inhibitors, remdesivir, DNA synthesis inhibitors (such as tenofovir disoproxil and lamivudine), chloroquine, and Chinese traditional medicines, have been proposed12,13. Besides, immunological therapy may have a role for COVID-19 as well, as SARS-CoV-2 infection can induce a cytokine storm in the lung, such as interleukine-2 (IL-2), IL-6, IL-7, GSCF, IP10, MCP1, MIP1A, and tumor necrosis factor-α (TNF-α), which leads to ARDS10. Therefore, avoiding the cytokine storm is a key step for the treatment of COVID-19. However, systemic corticosteroids may reduce the activity of immune system while inhibiting the cytokine storm14. In view of the rapid spread of SARS-CoV-2 and the increasing number of death worldwide, the potential of treatment lies in identifying novel therapeutics aimed at the patients with COVID-19 most likely to respond.
Figure 1.
The spike protein of SARS-CoV-2 mediates the entry of the virus into target cells via the ACE2 receptor, which leads to cytokine storm. ARDS: acute respiratory distress syndrome; ACE2: angiotensin-converting enzyme 2; MODS: multiple organ dysfunction syndrome; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Mesenchymal Stromal Cell-Based Therapy
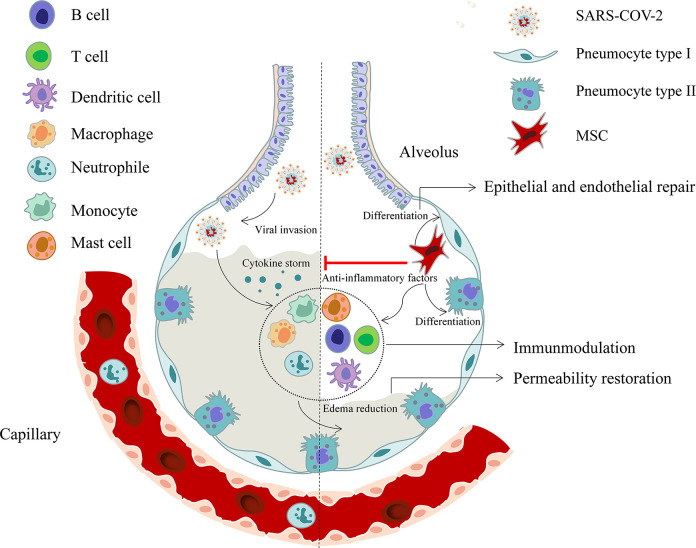
Mesenchymal stromal cells (MSCs) are non-hematopoietic, multipotent stromal precursor cells that can be derived from many tissues, including bone marrow (BM), adipose, dental pulp, placenta, cord blood, and matrix15. Possessing the ability of differentiating into osteoblasts, adipocytes, and chondroblasts in vitro, MSCs adhere to plastic under standard tissue conditions and express CD105, CD90, and CD73, yet not express other markers such as CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR surface molecules16. The minimal criteria for MSCs was firstly defined by the International Society for Cellular Therapy (ISCT) in 200616, followed by several updates mainly focusing on the refinement of standards for therapeutic efficacy17–19. It is known that MSCs are able to activate cell proliferation, prevent apoptosis, and improve regenerative responses by releasing growth factors and cytokines along with extracellular vesicles (EVs). In addition to the capability of differentiating into the particular phenotype of the damaged cells, such as lung epithelial and endothelial cells, which result in edema reduction and barrier repairation, MSCs also exert paracrine and immunoregulatory functions by cell to cell contact or secreting soluble factors. On one hand, MSCs are able to suppress CD4+ T helper cells, CD8+ cytotoxic T cells, and macrophages, and induce their differentiation into regulatory T cells (Treg) subsets and anti-inflammatory macrophages. Besides, they also inhibit the activity of dendritic cells (DCs) and natural killer (NK) cells to regulate immune response. On the other hand, MSCs can release soluble factors, such as indoleamine 2,3 dioxygenase (IDO), prostaglandin E2 (PGE-2), and nitric oxide (NO), to realize immunomodulation20. Importantly, MSCs reveal low immunogenicity and low tumorigenicity21,22, and can be isolated and expanded extensively without damage to their self-renewal or multipotency properties23. For decades, MSC therapy has been used for an array of ailments, especially in the immune-mediated inflammatory diseases, such as systemic lypus erythematosus (SLE) and graft versus-host disease (GVHD)24,25. Following intravenous infusion, MSCs are rapidly trapped in lungs, and a small amount of MSCs may recirculate to inflammatory or injured sites26. The migration of MSCs to the sites of injury or inflammation also plays an important role in its therapeutic effect. It is known that MSCs express a broad range of chemokine and growth factor receptors, such as CX chemokine receptors 1 and 2 (CXCR1 and CXCR2), CXCR4, CC chemokine receptor 1 (CCR1), CCR2, and platelet-derived growth factor (PDGF)27. With the damage of organ or tissue, the released cytokines cause migration of MSCs to sites of inflammation and injury, thus stimulate tissue repair. Therefore, in light of the pathogenesis of SARS-CoV-2, MSCs therapy holds great promise for the treatment of COVID-19 by inhibiting the cytokine storm and facilitating tissue repair and regeneration (Fig. 2).
Figure 2.
Schematic showing the potential role of MSCs in the treatment of COVID-19. MSCs play a role by releasing anti-inflammatory factors, regulating immune response, restoring endothelial barrier integrity, and other effects during SARS-CoV-2 infection. COVID-19: coronavirus disease 2019; MSCs: mesenchymal stromal cells; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
MSCs in Respiratory Virus Infection in Preclinical Studies
Multiple studies have shown beneficial effects of MSCs on preclinical models for ARDS and sepsis via paracrine section to modulate pathobiological pathways, including inflammation, endothelial and epithelial cell injury, alveolar fluid clearance (AFC), antimicrobial activity, and apoptosis28. However, little is known about MSCs therapy in the treatment of respiratory virus infection. In mice model, the effects of MSC therapy for influenza were inconsistent. In a preclinical study by Chan et al., mice infecting with influenza A/H5N1 were treated with BM-MSCs, the results showed a clinically significant reduction in lung pathology and increased survival rate among the aged mice, while no improved survival or histopathology in young mice. These reflect age-related differences in mechanisms of lung pathology and repair. In vitro, when H5N1 483/97-infected alveolar epithelial cells (AECs) were co-cultured with MSCs, a decrease in the impairment of AFC, reduction of the increased alveolar protein permeability (APP), and reduced proinflammatory cytokine responses were observed in AECs. Besides, angiopoietin-1 (Ang-1) and keratinocyte growth factor (KGF) were secreted by MSCs co-cultured with H5N1-infected AECs, which partly reduced MSC-mediated impairment of AFC and APP29. In Hong Kong, in vitro study of the lung injury model showed that umbilical cord (UC)-MSCs were more effectively in correcting impaired AFC, APP, and major epithelial ion transporter expression of H5N1-infected human AECs partly through Ang-1 and Hepatocyte Growth Factor (HGF) secretion compared with BM-MSCs or infected AEC alone. Besides, both UC-MSCs and BM-MSCs reduced H5N1 induction of several major pro-inflammatory cytokines and enhanced responses of anti-inflammatory cytokines in infected AECs compared with AECs alone. Particularly, these responses were weaker in infected AECs with BM-MSCs compared to AECs with UC-MSCs. Furthermore, the authors also found that UC-MSC secretome and UC-MSC exosomes restored H5N1-impaired AFC and APP of infected AECs. However, in vivo assay, only marginal therapeutic benefits of UC-MSCs in mice were observed, such as slightly improvement of body weight, lower concentration of Evans blue dye, and several reduced pro-inflammatory cytokines and chemokine protein levels of the bronchoalveolar lavage fluid (BALF) from H5N1 infected mice with UC-MSC treatment. And no beneficial effects of UC-MSC treatment on survival and anti-virus were shown30. In the study reporting on H9N2 avian influenza virus-induced acute lung injury in mice, infusion of BM-MSCs was associated with higher survival rate, lower dry weight ratios of lung, reduced airspace inflammation, improved lung hypoxemia and histopathology, reduced chemokines and proinflammatory cytokines levels, and decreased protein expression of CD14, TLR4, ERK, and JNK in the lung tissue as compared to the control group. These findings demonstrated that MSCs results in improvements in both pulmonary inflammation and lung tissue organization31. However, some studies have shown that administration of MSCs in a prophylactic or therapeutic regimen failed to improve outcomes in experimental severe influenza32,33. The conflicting observations of MSCs therapy for respiratory virus infection may result from different virus strains and experimental designs.
Clinical Application of MSCs in Respiratory Virus-Induced Lung Injury
Although compelling evidence of ARDS in patients have shown safety and efficacy of MSC administration, the clinical trial of the therapeutic effect of MSCs in respiratory virus induced lung injury is not currently extensive.
Chen et al. conducted a single center study of menstrual-blood-derived MSCs in patients with H7N9-induced ARDS, in which one million per kilogram of body weight for each time of MSCs were infused at the acute phase or late stage of ARDS in 17 voluntary patients with moderate to severe H7N9-induced ARDS. The results showed that death rate was significantly reduced in the MSCs treatment group compared with the control group (17.6% vs 54.5%, P = 0.006), with no MSC-infusion-related acute toxicities or seriously adverse events were observed in any of these patients. Besides, during the five years’ follow-up, significant improvement in lung function at each follow-up was found in four patients with MSC transplantation34. Currently, there is one published study investigating the therapeutic effect of MSCs in COVID-19, in which seven laboratory-confirmed COVID-19 patients, including one critically severe type, four severe types, and two common types were enrolled for MSCs transplantation intravenously, and three severe types were enrolled for placebo control. The results of the trial suggested that the pulmonary function and symptoms of these seven patients were significantly improved in two days after treatment. In 10 days after treatment, two common and one severe patient were recovered and discharged. Besides, increased peripheral lymphocytes, decreased C-reactive protein (CRP), and disappeared overactivated cytokine-secreting immune cell CXCR3+CD4+T cells, CXCR3+CD8+T cells, and CXCR3+NK cells were observed in 3-6 days after treatment. Furthermore, compared with the control group, the MSC treatment group had increased CD14+CD11c+CD11b mid-regulatory DC population, decreased TNF-α, and increased IL-10. No infusion-related or allergic reaction were observed after treatment. More importantly, the gene expression profile showed that MSCs were ACE2 and TMPRSS2 negative, indicating that MSCs were free from SARS-CoV-2 infection35.
In another randomized control trial (RCT) by Shu et al, 41 patients with severe COVID-19 were randomly divided into either the standard treatment group (29 patients) or the standard treatment group plus human UC-MSC (hUC-MSC) infusion group (12 patients). In the hUC-MSC treatment group, all patients improved and were discharged, while four patients in the control group deteriorated to critical illness and received invasive ventilation, and three of them died, with a 28-day mortality rate of 10.34%. In the hUC-MSC group, the time to clinical improvement was shorter, CRP and IL-6 levels were significantly lower from day 3 of infusion, the time for the lymphocyte count to return to the normal range was significantly faster, and lung inflammation absorption was significantly shorter on CT imaging as compared to the control group. No adverse reactions were observed in all patients who received hUC-MSC treatment36. These data suggest that MSC therapy was safe and effective for the treatment of COVID-19
Researches on MSC Derived Extracellular Vesicles (EVs) in Respiratory Virus Infection
Emerging studies have shown that MSC-derived EVs, which consist of the lipid bilayer with transmembrane proteins and contain cellular components such as lipids, cytosolic proteins, DNA, RNA, and microRNAs (miRNAs), can mitigate acute lung injury (ALI)37. In injured tissue, MSC-EVs interact between stem and injured cells, and realize tissue repair by transferring proteins, bioactive lipids, mRNA, and microRNA from MSCs to injured cells38. In addition to the similar therapeutic effects as MSCs, MSC-EVs offer several advantages over MSCs, including low immunogenicity and simple storage39.
Khatri et al. performed a study in a pig model of influenza virus, in which EVs from swine BM-MSCs were generated for in vitro and in vivo experiments. After incorporating into lung epithelial cells (LECs) in vitro, MSC-EVs had shown to reduce influenza virus replication and significantly inhibit the apoptosis of influenza-infected LEC. In vivo, MSC-EVs were administered intratracheally in influenza-infected pigs, the results revealed that minor infiltration of inflammatory cells and less inflammatory lesions were showed in lungs of pigs administered with MSC-EVs compared with the control group. Besides, virus titers and inflammatory cytokines production in the lung lysate and virus shedding in nasal swabs at day 3 post-EV administration were also lower in the EV-administrated group, while the levels of the anti-inflammatory cytokine IL-10 were slightly higher in the EV-administered group, as compared to the control group. These data suggested that, in addition to the anti-influenza and anti-inflammatory properties, MSC-EVs also attenuated influenza virus-induced ALI in a pig model40.
Role of MSC-Derived Exosomes in the Treatment of COVID-19
The term EVs comprises three types of vesicles namely exosomes, microvesicles (MVs), and apoptotic bodies (ABs) according to the international society for extracellular vesicles (ISEV)41. Of which, exosomes have size between 30 and 120 nm in diameter that participate in several pathological conditions. A prospective cohort study aimed at evaluating the safety and efficacy of exosomes (ExoFloTM) derived from allogeneic BM-MSCs for the treatment of severe COVID-19 and the results showed improvements in patients’ clinical status and oxygenation, significant reduction in levels of the acute phase reactants CRP, ferritin, D-Dimer, and absolute neutrophil count, and significant increase in absolute lymphocyte count, with no adverse events observed within 72 hours of ExoFlo administration42. Further studies are needed to testify the safety and efficacy of exosomes in the treatment of COVID-19. The preclinical and clinical studies investigating the effect of MSCs/MSC-derived EVs in respiratory virus infection are summarized in Table 1.
Table 1.
Preclinical and Clinical Studies Investigating the Effect of MSCs/MSC-Derived EVs in Respiratory Virus Infection.
| Study | Model/patient | Treatment | Mechanism | Reference |
|---|---|---|---|---|
| Chan et al (2016) | H5N1 influenza A virus-infected mice H5N1 influenza A viruses induced AECs |
Human BM-MSCs |
In vitro
↑ AFC and ↓ APP ↓ IL-1β, RANTES, IL6, IL8, and IP10 mRNA levels ↑ transporter proteins of CFTR, α1Na, and K-ATPase ↑ Ang-1 and KGF In vivo ↑ Survival and body weight in aged mice ↓Wet-to-dry lung weight, Evans blue dye in the lungs, and the concentration of total protein, most cytokine, and chemokine of the BALF in all mice. ↓ Peribronchial, perivascular, and overall histopathology scores. |
29 |
| Loy et al (2019) | Mouse ALI Influenza A/H5N1 virus induced AECs |
Human BM-MSCs and Human UC-MSCs |
In vitro
Restoration of AFC and APP (BM-MSCs, UC-MSCs, and UC-MSC secretome and exosomes) ↑ ENaC, Na+, K+-ATPase, and CFTR (both BM-MSCs and UC-MSCs) ↓ IFN-λ1, IL-6, IL-1β, IP-10, MCP-1, and RANTES (both BM-MSCs and UC-MSCs) and IFN-β (only UC-MSCs) ↑ IL-4, IL-10, IL-11, IL-13, and IL-1RA (both UC-MSCs and BM-MSCs) In vivo ↑ Body weight (UC-MSCs) ↓ Evans blue dye (UC-MSCs) ↓ IP-10, MCP-1, RANTES, TNF-α, IL1-β, IL-6 and IL-8 protein levels (UC-MSCs) |
30 |
| Li et al (2016) | Mice H9N2 avian influenza virus-induced ALI |
BM-MSCs of mice | ↑ Survival rate ↓ Lung weights and lung wet: dry weight ratios ↓ Airspace inflammation ↑ Lung histopathology ↑ PaO2, SaO2, and pH values ↓ PaCO2 ↓ GM-CSF, MCP-1, KC, MIP-1α, and MIG in both BALF and serum ↓ IL-1α, IL-6, IL-10, TNF-α, and IFN-γ in both BALF and serum ↓ CD14, TLR4, ERK and JNK protein in the lung tissue |
31 |
| Chen et al (2020) | Patients with H7N9-induced ARDS | Human menstrual-blood-derived MSCs | ↑ Survival rate ↓ PCT, ALT, sCr, CK, PT, D-Dimer In 4 patients with MSC transplantation during follow-up ↑ Hemoglobin levels ↓ PT Improvement in both lung function and lesions on CCT |
34 |
| Leng et al (2020) | Patients with COVID-19 | MSCs | ↓ Plasma CRP in one critically severe patient ↑ Oxygen saturation ↑ Lymphocyte ↓ ALT, CK activity and myoglobin Improvement on CCT ↑ Regulatory T cells and dendritic cell in the severe and critically severe patients ↑ CD14+CD11c+CD11bmid-regulatory dendritic cell and disappearance of overactivated T cells and NK cells in the critically severe patients. ↓ TNF-α ↑ IL-10 |
35 |
| Shu et al (2020) | Patients with severe COVID-19 | Human UC-MSCs | In the hUC-MSC group (compared with the control group) Reduced incidence of progression from severe to critical illness and reduced 28-day mortality rate Shorter time of clinical improvement Significantly lower levels of CRP and IL-6 from day 3 of infusion Significantly faster time of lymphocyte count to return to the normal range Significantly shorter time of lung inflammation absorption on CT imaging |
36 |
| Khatri et al (2018) | Pigs Influenza virus-induced ALI Influenza virus induced lung epithelial cells |
EVs from swine BM-MSCs |
In vitro
↓ Influenza replication ↑ Inhibition of apoptosis In vivo ↓ Infiltration of inflammatory cells and lung lesion score ↓ Total protein ↓ Virus shedding and virus titers ↓ TNF-α and CXCL10 ↑ IL-10 |
40 |
| Sengupta et al (2020) | Patients with severe COVID-19 | Exosomes (ExoFlo™) derived from allogeneic BM-MSCs | 83% of survival rate ↑ PaO2/FiO2 ↓ Absolute neutrophil count ↑ Average CD3+, CD4+, and CD8+ lymphocyte counts ↓ Mean CRP, ferritin, and D-dimer |
42 |
ALI: acute lung injury; AECs: alveolar epithelial cells; ARDS: acute respiratory distress syndrome; ALT: alanine aminotransferase; AFC: alveolar fluid clearance; APP: alveolar protein permeability; Ang-1: angiopoietin-1; BM: bone marrow; BALF: bronchoalveolar lavage fluid; COVID-19: coronavirus disease 2019; CRP: C-reactive protein; CK: creatine kinase; CCT: computed tomography of the chest; EVs: extracellular vesicles; FiO2: fraction of inspired oxygen; KGF: keratinocyte growth factor; MSCs: mesenchymal stem cells; PCT: procalcitonin; PT: prothrombin time; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide in artery; sCr: serum creatinine; SaO2: arterial oxygen saturation; UC: umbilical cord.
Clinical Translation of MSCs
MSCs Sources
The most common source of MSCs in respiratory disease has been BM43,44. But BM-MSCs are harvested by a highly invasive procedure. Besides, aging is associated with decreased differentiation potential, prolification capacity, cell number, and maximal life span of BM-MSCs, which limits their use in the clinical settings45,46. Gradually, more attention has been paid to adipose-derived (AD)-MSCs and UC-MSCs. A large amount of AD-MSCs can be easier acquired by liposuction. Furthermore, AD contains more than a thousand times of more MSCs47, which may present a higher proliferation rate. Likewise, UC-MSCs have higher proliferation rate, can be extracted noninvasively, and can grow more rapidly compared with BM-MSCs that allows the generation of high numbers of early passage MSCs. Unlike embryonic stem cells (ESCs), UC-MSC is an after birth tissue and is considered medical waste. Besides, the donor conditions of UC-MSCs are more uniform, thus reduce variability and enhance potency48,49. Nowadays, perinatal-derived cells (PDCs) including UC-MSCs have drawn public attention due to their early embryological origin and they possess unique plasticity and differentiation properties50. In vitro, PDCs from both maternal and fetal tissues do not induce immune response51–53, which makes them attractive for allogeneic transplantation. All these different types of stem cells have been shown efficacy in preclinical and clinical studies.
MSCs Dosage Regimens
MSCs can be administrated either intratracheally or intravenously. However, there is no evidence to prove which one is superior. And no study regarding the proper number of cells. In the STem cells for ARDS Treatment (START) phase 1 trial, three doses were administrated in three cohorts (1 × 106 cells/kg, 5 × 106 cells/kg, and 10 × 106 cells/kg predicted body weight, respectively), and the maximal dosage was well tolerated by patients. In the further START phase 2a trial, subjects were directly administered maximum tolerable dose of MSCs, and the results showed safety of this approach54,55. However, the optimum dose of MSC-based therapy remains to be determined.
Cell Manufacture and Storage
After cryopreservation, storage and transport, MSCs must retain viability and efficacy to be effective in clinical application. Administration immediately following thawing the cryopreserved MSCs and suspending them in plasmalyte. In this approach, MSCs are given with Dimethyl sulfoxide (DMSO), which has been most frequently used for years as a cryoprotectant agent. However, its use has been reported to induce some complications, including vomiting, abdominal pain or even fatal cardiopulmonary events, after infusion of cryopreserved bone marrow or cord blood. However, some data indicated that the thawing and washing to remove DMSO resulted in a substantial loss of cells56. In the START phase 1 and phase 2 trials, MSCs were thawed and washed to remove DMSO, and then resuspended in plasmalyte before intravenous administration. The results have shown that this approach can retain the efficacy of the MSCs in these studies54,55.
Administration Routes
Concerning the MSC-based therapy for COVID-19, whether intravenous route is the best choice is not yet clear. Local administration can help deliver paracrine mediators to the diseased sites directly, such as intratracheal or intramuscular injection. However, the therapeutic benefit may be hindered because of insufficient retention and survival of transplanted MSCs at the site of administration, as some of the administered cells remain at the site of injection in the hours following transplantation57. Alternatively, systematic administration of MSCs, such as intravenous injection, also brings some challenges, including the insufficient residence time and homing of MSCs to the target tissues limits the therapeutic effects, and the instant blood-mediated inflammatory reaction (IBMIR) following MSC contact with human serum57. Multiple strategies have been investigated to improve the administration of MSCs, including priming MSCs in vitro, using biomaterials to encapsulate MSCs to enhance MSC retention and survival, and some genetic engineering approaches to prevent MSC-mediated IBMIR and increase MSC residence time and sufficiently deliver MSCs to the target tissues57.
Safety Aspects Considering Infusion of MSC from Different Tissue Sources in COVID-19
Firstly, abnormal coagulation parameters have been reported in many severely and critically ill patients with COVID-19, and disseminated intravascular coagulation (DIC) was common in severe respiratory failure patients with COVID-19, which was usually associated with poor prognosis10,58–64. However, MSCs express variable degree of tissue factor (TF/CD142) on their surfaces and are procoagulant in the presence of blood or plasma, and infusion of MSCs systematically may adversely trigger the IBMIR in patients65,66, which was firstly reported by Moll et al. after infusion of BM- and PT-derived clinical MSC products67,68. Moll et al. also firstly described the coagulation-related issues of MSC therapies for COVID-199. TF expression and triggering of IBMIR by MSC products depend on the tissue source or starting material the MSC products were derived from. And AT- and PT-derived products express higher degree of TF and have reduced hemocompatibility than BM-MSCs, with substantial donor variability and culture passage-dependent TF induction65. Moreover, tissue factor expression changes over culture time, and handling conditions and growth media likely also affect procoagulant activity69. Therefore, before administrating MSCs intravenously, it is essential to testing the cells initially and selecting MSCs with low tissue factor/low procoagulant activity. Besides, cells with high tissue factor expression may also be subjected to preconditioning before administration to reduce tissue factor expression. Furthermore, it is necessary to develop administration protocols for delaying timing of administration to after resolution of coagulopathy or incorporating anticoagulants such as heparin to maximize therapeutic effect. Particularly, alternative routes of cell administration in patients with COVID-19 such as topical application of intramuscular injection are being increasingly explored70. Secondly, the source of MSCs should be comply with the national standards to ensure cell viability and quality and sterility of stem cells. MSC companies and hospitals treating patients should equip adequate facilities with qualified personnel to provide proper dose regimens, optimal route and method of administration, and MSC quality and counts. Thirdly, some studies have demonstrated that MSCs are susceptible to influenza virus. Thanunchai et al. reported that highly pathogenic avian influenza H5N1 virus could infect and replicate in MSCs, and virus infection resulted in apoptosis and losing of the immunoregulatory activity of MSCs28. Similarly, known influenza virus α-2,3 and α-2,6 sialic acid receptors were shown to express in BM-MSCs of pigs to support replication of swine and human influenza viruses, and viral infection of MSCs resulted in cell lysis and proinflammatory cytokine production71. However, no in vivo study has demonstrated the direct infection of influenza viruses to MSCs. The interaction between MSCs and virus can be characterized as double-edge sword, therefore, the safety of administration of MSCs should be ensured by screening the presence of viruses both in donors and recipients. Fourthly, precautions should be taken during the MSCs administration, medical health workers should monitor closely the occurrence of complications, such as embolism and allergic reaction, and take countermeasures actively. Fifthly, to ensure the safety of MSCs therapy, clinical studies should be carefully designed and the administration of MSCs should apply to the indications72.
Conclusions
By far, many clinical trials have been initiated worldwide, among them, bone marrow, umbilical cord (including umbilical cord blood and Wharton’s jelly), adipose tissue, menstrual blood, and some selected MSC therapy products of companies are the primary MSC sources. Based on the promising results of MSCs or MSC-derived EVs for respiratory virus infection in preclinical and clinical studies both in vitro and in vivo, and MSCs for COVID-19 treatment during pandemic, MSC-based therapy holds great promise and possibility for COVID-19. However, there were inconsistency of MSC-based therapy for influenza virus infection among some preclinical experiments, and problems and challenges for its clinical use still exist, including high cost, challenges associated with administration of MSCs, and the inevitable safe issues. Particularly, during the pandemic period, it is necessary to develop safe and accessible MSC-based therapy for low/middle income countries. Furthermore, to facilitate clinical translation, MSCs need to be manufactured with standard procedures in terms of cell delivery, cryopreservation/thawing, etc. Further research into the effect of MSC-based therapeutics for the treatment of moderate-severe phases of COVID-19 is essential, and novel approaches, such as MSC-derived exosomes, should be explored as well. What deserves expecting is that many trials of MSCs for COVID-19 are ongoing worldwide.
Footnotes
Author Contributions: JX drafted the initial manuscript. LC revised the manuscript. LZ designed and completed the table and the figures. LB and YS conceptualized the study and critically reviewed the manuscript for important intellectual content. All authors reviewed and approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yuan Shi  https://orcid.org/0000-0002-4571-4424
https://orcid.org/0000-0002-4571-4424
References
- 1. Na Z, Dingyu Z, Wenling W, Xingwang L, Bo Y, Jingdong S, Xiang Z, Baoying H, Weifeng S, Roujian L, Peihua N, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO Virtual press conference on COVID-19 [Internet]. Geneva: WHO; 2020 [cited 2020 Mar 16]. Available from: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final11mar2020.pdf?sfvrsn=cb432bb3_2
- 5. World Health Organization. (WHO. Novel Coronavirus (2019-nCoV). Weekly epidemiological update - 21 December 2020 [Internet]. Geneva: WHO; 2020 [cited 2020 Dec 21]. Available from: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---21-december-2020
- 6. Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann M, Kleine-Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. 2020. bioRxiv:2020 2001;2031.929042.
- 9. Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14(1):69–71. [DOI] [PubMed] [Google Scholar]
- 13. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019- nCoV) in vitro. Cell Res. 2020;30(3):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recentdevelopments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. [DOI] [PubMed] [Google Scholar]
- 16. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 17. Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L; MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells-the international society for cellular therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054–1061. [DOI] [PubMed] [Google Scholar]
- 18. Galipeau J, Krampera M. The challenge of defifining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17(2):125–127. [DOI] [PubMed] [Google Scholar]
- 19. Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, Debruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, Hematti P, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomez-Salazar M, Gonzalez-Galofre ZN, Casamitjana J, Crisan M, James AW, Péault B. Five decades later, are mesenchymal stem cells still relevant? Front Bioeng Biotechnol. 2020;8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14(3):252–265. [DOI] [PubMed] [Google Scholar]
- 22. Casiraghi F, Remuzzi G, Abbate M, Perico N. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev. 2013;9(1):65–79. [DOI] [PubMed] [Google Scholar]
- 23. Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;1068:352–366. [DOI] [PubMed] [Google Scholar]
- 24. Yamahara K, Hamada A, Soma T, Okamoto R, Okada M, Yoshihara S, Yoshihara K, Ikegame K, Tamaki H, Kaida K, Inoue T, et al. Safety and efficacy of amnion-derived mesenchymal stem cells (AM01) in patients with steroid-refractory acute graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: a study protocol for a phase I/II Japanese trial. BMJ Open. 2019;9(7):e026403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267–2277. [DOI] [PubMed] [Google Scholar]
- 26. Sensebé L, Fleury-Cappellesso S. Biodistribution of mesenchymal stem/stromal cells in a preclinical setting. Stem Cells Int. 2013;2013:678063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eseonu OI, De Bari C. Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology (Oxford). 2015;54(2):210–218. [DOI] [PubMed] [Google Scholar]
- 28. Thanunchai M, Kanrai P, Wiboon-Ut S, Puthavathana P, Hongeng S, Thitithanyanont A. Tropism of avian influenza A (H5N1) virus to mesenchymal stem cells and CD34+ hematopoietic stem cells. PLoS One. 2013;8(12):e81805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, Nicholls JM, Fang X, Guan Y, Lee JW, Chan RW, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A. 2016;113(13):3621–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, Peiris JSM, Chan MCW. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza a(h5n1) virus-associated acute lung injury. J Infect Dis. 2019;219(2):186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Xu J, Shi W, Chen C, Shao Y, Zhu L, Lu W, Han X. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res Ther. 2016;7(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darwish I, Banner D, Mubareka S, Kim H, Besla R, Kelvin DJ, Kain KC, Liles WC. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS One. 2013;8(8):e71761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol. 2014;307(5):L395–L406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Chen L, Gao H, Lu X, Yu L, Dai X, et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing). 2020;6(10):1153–1161. doi:10.1016/j.eng.2020.02.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, Ji N, Zheng Y, Chen X, Shi L, Wu M, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Huang R, Xu Q, Zheng G, Qiu G, Ge M, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles alleviate acute lung injury via transfer of miR-27a-3p. Crit Care Med. 2020;48(7):e599–e610. doi:10.1097/CCM.0000000000004315. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38. Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41(1):283–287. [DOI] [PubMed] [Google Scholar]
- 39. Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7(10):E355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lathrop MJ, Brooks EM, Bonenfant NR, Sokocevic D, Borg ZD, Goodwin M, Loi R, Cruz F, Dunaway CW, Steele C, Weiss DJ. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the Th17 signaling pathway. Stem Cells Transl Med. 2014;3(2):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, Antunes M, Robinson KL, Mitsialis SA, Kourembanas S, Thane K, et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4(11):1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919–926. [DOI] [PubMed] [Google Scholar]
- 46. Yu JM, Wu X, Gimble JM, Guan X, Freitas MA, Bunnell BA. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell. 2011;10(1):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McIntyre LA, Moher D, Fergusson DA, Sullivan KJ, Mei SH, Lalu M, Marshall J, Mcleod M, Griffin G, Grimshaw J, Turgeon A, et al. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: a systematic review. PLoS One. 2016;11(1):e0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Curley GF, Jerkic M, Dixon S, Hogan G, Masterson C, O’Toole D, Devaney J, Laffey JG. Cryopreserved, xeno-free human umbilical cord mesenchymal stromal cells reduce lung injury severity and bacterial burden in rodent escherichia coli–induced acute respiratory distress syndrome. Crit Care Med. 2017;45(2):e202–e212. [DOI] [PubMed] [Google Scholar]
- 49. Fong CY, Gauthaman K, Cheyyatraivendran S, Lin HD, Biswas A, Bongso A. Human umbilical cord Wharton’s jelly stem cells and its conditioned medium supporthematopoietic stem cell expansion ex vivo. J Cell Biochem. 2012;113(2):658–668. [DOI] [PubMed] [Google Scholar]
- 50. Parolini O, Soncini M. Human placenta: a source of progenitor/stem cells? J.Reproduktionsmed Endokrinol, 2006;3(2):117–126. [Google Scholar]
- 51. Papait A, Vertua E, Magatti M, Ceccariglia S, Munari SD, Silini AR, Sheleg M, Ofir R, Parolini O. Mesenchymal stromal cells from fetal and maternal placenta possess key similarities and differences: potential implications for their applications in regenerative medicine. Cells. 2020;9(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, Griensven MV, Stadler G, Redl H, Gabriel C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13(6):1173–1183. [DOI] [PubMed] [Google Scholar]
- 53. Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010;88(8):795–806. [DOI] [PubMed] [Google Scholar]
- 54. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, et al. Mesenchymal stem (stromal)cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ruiz-Delgado GJ, Mancías-Guerra C, Tamez-Gómez EL, Rodríguez-Romo LN, López-Otero A, Hernández-Arizpe A, Gómez-Almaguer D, Ruiz-Argüelles GJ. Dimethyl sulfoxide-induced toxicity in cord blood stem celltransplantation: report of three cases and review of the literature. Acta Haematol. 2009;122(1):1–5. [DOI] [PubMed] [Google Scholar]
- 57. Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, Biasio MD, Heinelt M, Reeve B, Abdi R, Alturki M, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study . Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost, 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, Paassen JV, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD, Geissler S, Reinke P. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–163. [DOI] [PubMed] [Google Scholar]
- 66. Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano-Furman NE, Triolo F, Kamhieh-Milz J, Moll G, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moll G, Rasmusson-Duprez I, Bahr LV, Connolly-Andersen A, Elgue G, Funke L, Hamad OA, Lönnies H, Magnusson PU, Sanchez J, Teramura Y, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cell Res Ther. 2019;10(1):322. [DOI] [PubMed] [Google Scholar]
- 68. Moll G, Ignatowicz L, Catar R, Luecht C, Sadeghi B, Hamad O, Jungebluth P, Dragun D, Schmidtchen A, Ringdén O. Different procoagulant activity of therapeutic mesenchymal stromal cells derived from bone marrow and placental decidua. Stem Cells Dev. 2015;24(19):2269–2279. [DOI] [PubMed] [Google Scholar]
- 69. Christy BA, Herzig MC, Montgomery RK, Delavan C, Bynum JA, Reddoch KM, Cap AP. Procoagulant activity of human mesenchymal stem cells. J Trauma Acute Care Surg. 2017;83: S164–S169. [DOI] [PubMed] [Google Scholar]
- 70. Barkama R, Mayo A, Paz A, Solopov A, Mann T, Vadasz Z, Appel T, Ofir R, Shani L, Sheleg M, Allenet H, et al. Placenta-derived cell therapy to treat patients with respiratory failure due to coronavirus disease 2019. Crit Care Explor. 2020;2(9):e0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Khatri M, Saif YM. Influenza virus infects bone marrow mesenchymal stromal cells in vitro: implications for bone marrow transplantation. Cell Transplant. 2013;22(3):461–468. [DOI] [PubMed] [Google Scholar]
- 72. Yim H, Jeong H, Cho Y, Jeong S. Safety of mesenchymal stem cell therapy: a systematic review and meta-analysis. Cytotherapy. 2016;18(6):S132. [Google Scholar]