Abstract
A 62-year-old man presented with a history of atypical meningioma (World Health Organization grade II) and recurrent as anaplastic meningioma (World Health Organization grade III). His previous treatments included multiple surgical resections, fractionated radiation therapy, stereotactic radiosurgery, everolimus/octreotide long-acting release, bevacizumab, and hydroxyurea. Magnetic resonance imaging revealed rapid volumetric progression over the prior 9 months, with a near tripling in size from 29.9 cm3 to 80.4 cm3. Indium In 111 octreotide scanning confirmed the presence of somatostatin receptors within the tumor. Lutetium Lu 177 dotatate was administered intravenously at a dose of 200 mCi per dose every 8 weeks for 4 cycles. Treatment was tolerated very well, with no notable adverse events. Tumor volume initially increased to 98.3 cm3 after cycle 1 of treatment and subsequently decreased to 91.2 cm3 after cycle 2. Eight months after treatment onset, the tumor volume remained stable (93.4 cm3).
Abbreviations and Acronyms: FDA, Food and Drug Administration; MRI, magnetic resonance imaging; SSTR, somatostatin receptor; WHO, World Health Organization
Atypical (World Health Organization [WHO] grade II) and anaplastic (WHO grade III) meningiomas are relatively uncommon subtypes, collectively accounting for approximately 20% of all meningiomas.1,2 Both are associated with poorer prognosis than the more common WHO grade I meningiomas. Initial treatments typically involve surgical resection and radiation therapy but recurrence is common. Systemic therapy is often pursued thereafter, with limited known efficacy. Somatostatin receptor (SSTR) types 2 through 5 are commonly overexpressed in meningiomas and have been found to be present in the vast majority of meningiomas, regardless of tumor grade.3, 4, 5 Lutetium Lu 177 (177Lu) dotatate, which is a beta particle–emitting somatostatin analogue, has been approved recently by the US Food and Drug Administration (FDA) for the treatment of SSTR-positive gastroenteropancreatic neuroendocrine tumors. Given the near-universal presence of SSTRs in meningiomas, 177Lu-dotatate may be a promising strategy for treatment of an anaplastic meningioma.6
For the case reported in this article, informed consent was obtained for treatment with the described therapy. All data are documented in the electronic medical record. Mayo Clinic Institutional Review Board approval was not required to report findings of routine clinical patient care.
Report of Case
Presentation and Treatment
A 62-year-old man presented with recurrent meningioma. A left parietal meningioma had been diagnosed in 2000 after the patient had presented with a seizure. He underwent his first surgical resection in 2001, with pathologic examination documenting WHO grade I meningioma.
Two years after the initial diagnosis, he presented with recurrent generalized tonic-clonic seizures. Subsequent magnet resonance imaging (MRI) revealed multiple sites of new meningiomas along with tumor regrowth at the primary tumor site. Between 2003 and 2017, the patient experienced several episodes of tumor recurrence and underwent multimodality therapy, including a total of 8 surgical interventions and fractionated external beam radiotherapy with a total of 59.4 Gy in 33 fractions in 2002 and 54 Gy in 30 fractions in 2013. Additionally, stereotactic radiosurgery with gamma knife was performed in 2007 and 2016 with maximum radiation doses of 24 Gy and 28 Gy. Findings on pathologic examination of specimens from surgical resection of tumor in 2016 were suspicious for anaplastic progression (WHO grade III), which was confirmed at the next resection in 2017 when pathologic examination revealed over 40 mitotic cells per 10 high-power fields within an area of the tumor. Eventually, continued progression of the treatment-refractory anaplastic meningioma led to referral of the patient for systemic therapeutic measures because standard surgical and radiation options had been exhausted.
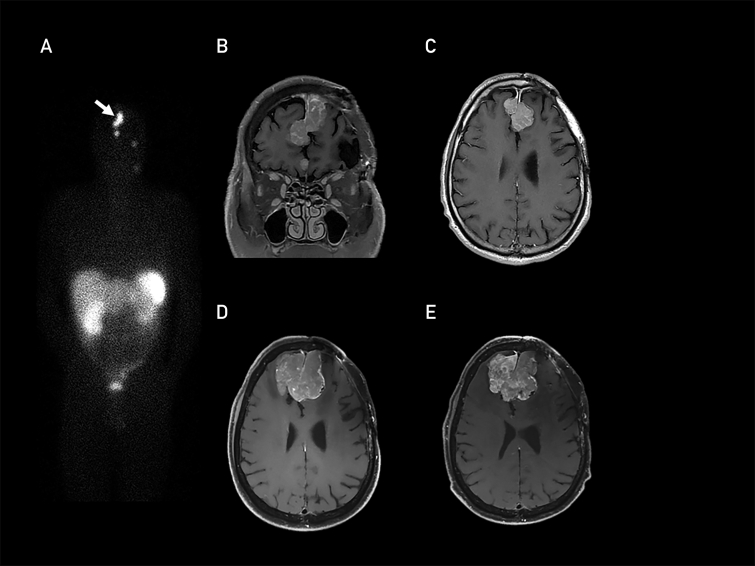
An indium In 111 (111In)-octreotide scan performed in 2017 revealed radiotracer uptake within the meningioma, documenting SSTR expression (Figure A). The patient was initially treated with everolimus and octreotide long-acting release based on results of the CEVOREM (Combination of Everolimus and Octreotide in Resistant Meningiomas) trial.7 At progression, he was treated with bevacizumab and hydroxyurea.8,9 However, these systemic therapies were not effective, and the meningioma exhibited rapid volumetric progression, with a near tripling in size from 29.9 cm3 in November 2017 to 80.4 cm3 in August 2018 (Figure B-D).
Figure.
Indium In 111-octreotide scan performed in November 2017 showing somatostatin receptor expression (A; arrow indicates meningioma). Corresponding T1-weighted, gadolinium-enhanced magnetic resonance images (B, coronal; C, axial). Axial T1-weighted, gadolinium-enhanced magnetic resonance images from August 2018 prior to initiation of lutetium Lu 177-dotatate treatment (D) and April 2019 after 4 cycles of lutetium Lu 177-dotatatate (E).
The FDA approval of 177Lu-dotatate for SSTR-positive gastroenteropancreatic neuroendocrine tumors in January 2018 led to consideration of off-label treatment with 177Lu-dotatate for this patient. Consistent with the methods of the NETTER (Neuroendocrine Tumors Therapy) trial,10 this patient was treated with a 4-cycle regimen of 200 mCi intravenously, administered every 8 weeks. The rationale behind this approach was that this dosing schedule was known to be safe and effective in neuroendocrine tumors and there was no prior evidence to suspect that a different dosing regimen would be superior in meningioma. Treatment with
177Lu-dotatate was initiated in August 2018, and serial MRI and volumetric analyses were conducted throughout the treatment to monitor for tumor progression.
Outcomes and Follow-up
The treatment was well tolerated with no notable adverse events. Specifically, there was no evidence of substantial hematologic or renal toxicity and no evidence of cerebral radiation necrosis. Two months after initiation of treatment, the patient experienced 2 breakthrough partial seizures, which were controlled with an increase in levetiracetam dose. Neurologic examination findings remained unremarkable other than a mild gait abnormality that preceded initiation of 177Lu-dotatate therapy.
Magnetic resonance imaging confirmed that the tumor volume had slightly increased to 98 cm3 after 1 cycle of treatment with subsequent decrease to 91 cm3 after cycle 2. The tumor volume remained essentially stable thereafter for the duration of therapy, with a volume of 93 cm3 after completion of cycle 4, 8 months after treatment onset (Figure E). The degree of perilesional T2-weighted hyperintensities and mass effect of the tumor were also essentially stable during therapy.
Two months after completion of 177Lu-dotatate therapy, the patient presented to the clinic with increased seizure frequency, fatigue, and right-sided weakness. Magnetic resonance imaging revealed interval tumor enlargement (tumor volume, 116 cm3) with an increase in vasogenic edema. Meningioma-directed therapy was not considered in the immediate term because of the need for reconstructive surgery for a wound defect. Dexamethasone and levetiracetam doses were adjusted for symptomatic relief.
Five months after the completion of treatment, the patient presented to the emergency department with altered mental status. Magnetic resonance imaging revealed a substantial increase in tumor volume (146 cm3) compared with the previously stable volume of 93 cm3. Ultimately, the decision was made to proceed with symptomatic management only, and the patient entered hospice care, dying shortly thereafter.
Discussion
Meningiomas represent one of the largest subgroups of intracranial neoplasms, accounting for about 34% of all central nervous system tumors. They are usually benign, slow-growing tumors. The WHO subclassifies meningioma as grade I, grade II (atypical), and grade III (anaplastic), with progressively more aggressive behavior and poorer prognosis.2,11 Surgical procedures remain the standard initial treatment for most meningiomas.12 However, it is not unusual for WHO grade II and III meningiomas to recur, thus leading to multiple surgical resections and repeated radiation therapy.13
Treatment options for relapsing and treatment-refractory anaplastic meningiomas are limited. Recent studies have found that tumor growth in meningioma is related to hormonal factors.14 Meningiomas have been found to express estrogen and progesterone receptors, androgens, and nonsteroid hormones, including somatostatin. In vitro and in vivo studies have found that meningioma, regardless of histology and classification, may express SSTRs, and immunohistochemical studies document the presence of 5 SSTR subtypes (SSTR1 through SSTR5) in tumor tissue, with predominance of SSTR2 and SSTR5.3 The presence of SSTRs within meningiomas can be noninvasively documented by 111In-octreotide single-photon emission computed tomography, which historically has been the standard imaging modality for this purpose. More recently, gallium Ga 68-dotatate positron emission tomography has become the test of choice because it offers higher resolution and shorter imaging time and should more closely approximate the distribution of 177Lu-dotatate therapy. The use of SSTR analogues for meningioma treatment is recognized in European studies, with reported long-term stable disease and long overall survival in rapidly progressive multiple recurrent anaplastic meningiomas.6,10
177Lu-dotatate is a radioconjugate consisting of the tyrosine-containing SSTR analogue Tyr3-octreotate (TATE) conjugated with the bifunctional, macrocyclic chelating agent tetra-azacyclododecanetetra-acetic acid (DOTA) and radiolabeled with the beta-emitting radioisotope 177Lu. 177Lu-dotatate binds to SSRTs, with high affinity for SSTR2. On binding and internalization, this radioconjugate specifically delivers a cytotoxic dose of beta radiation to these SSTR-positive cells, with a very short beta particle length limiting radiation exposure of surrounding brain tissue.
In January 2018, 177Lu-dotatate therapy was FDA approved in the United States for adult patients with SSTR-positive gastroenteropancreatic neuroendocrine tumor, including those of the foregut, midgut, and hindgut. This FDA approval was mainly based on the results of the phase III NETTER trial.10 Eligible patients were adults who had inoperable midgut neuroendocrine tumors that had metastasized or were locally advanced and those who had disease progression on computed tomography or MRI. The patients were treated with 7.4 GBq (200 mCi) of 177Lu-dotatate infused intravenously every 8 weeks for 4 cycles. Treated patients had notable improvements in response rate, progression-free survival, and mortality.
A transient increase in tumor volume was observed after the first cycle, which may have represented delayed treatment effect or pseudoprogression secondary to vasogenic edema. Subsequently, the tumor volume decreased by 7% and remained stable with minimal change in volume throughout the remainder of treatment. Over the 4 cycles (8 months) of treatment, the tumor volume increased only 16% during 177Lu-dotatate therapy in comparison to the rapid increase of 169% during the 8 months prior to 177Lu-dotatate initiation. However, the tumor resumed its previous rapid growth after therapy cessation.
There was no evidence of any major biochemical and hematologic toxicities during therapy or posttreatment. The short path length of 177Lu-dotatate in comparison to other agents like yttrium Y 90-dotatoc may have allowed for better results with lower risk of cerebral radiation necrosis given previous external beam radiation and gamma knife radiosurgery.15
A phase II clinical trial has been activated at Mayo Clinic to determine the progression-free survival associated with 177Lu-dotatate in patients with recurrent meningioma.16
Conclusion
177Lu-dotatate is a theranostic radioisotope for targeted radionuclide therapy of SSTR-positive gastroenteropancreatic neuroendocrine tumors that may also be a reasonable consideration in SSTR-expressing meningiomas. In this case, treatment was well tolerated and demonstrated biological effect by temporarily halting growth in a previous rapidly growing WHO grade III meningioma refractory to numerous previous therapies. Although benefit was transient in this patient, this observation nonetheless builds confidence that 177Lu-dotatate may be a safe and effective strategy for the treatment of SSTR-positive recurrent meningiomas. Until additional data are available from larger efficacy studies and cost-benefit analyses, this approach should be considered only as a salvage therapy option once other standard therapies have been exhausted.
Acknowledgments
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Grant Support: This work was supported in part by grant K12CA090628 from the National Cancer Institute, United States (S.H.K).
Potential Competing Interests: The authors report no competing interests.
References
- 1.Koch M.J., Agarwalla P.K., Royce T.J., et al. Brachytherapy as an adjuvant for recurrent atypical and malignant meningiomas. Neurosurgery. 2019;85(5):E910–E916. doi: 10.1093/neuros/nyz115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom Q.T., Cioffi G., Gittleman H., et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncology. 2019;21(suppl_5):v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva C.B., Ongaratti B.R., Trott G., et al. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int J Clin Exp Pathol. 2015;8(10):13185–13192. [PMC free article] [PubMed] [Google Scholar]
- 4.Barresi V., Alafaci C., Salpietro F., Tuccari G. Sstr2A immunohistochemical expression in human meningiomas: is there a correlation with the histological grade, proliferation or microvessel density? Oncol Rep. 2008;20(3):485–492. [PubMed] [Google Scholar]
- 5.Chamberlain M.C., Glantz M.J., Fadul C.E. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue [published correction appears in Neurology. 2008;70(4):325]. Neurology. 2007;69(10):969–973. doi: 10.1212/01.wnl.0000271382.62776.b7. [DOI] [PubMed] [Google Scholar]
- 6.Seystahl K., Stoecklein V., Schüller U., et al. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016;18(11):1538–1547. doi: 10.1093/neuonc/now060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graillon T., Peyre M., Kalamarides M., et al. OS6.6 CEVOREM trial: Combination of EVerolimus and Octreotide in REsistant Meningiomas; presentation and preliminary results [abstract] Neuro Oncol. 2016;18(suppl 4):iv15. [Google Scholar]
- 8.Shih K.C., Chowdhary S., Rosenblatt P., et al. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol. 2016;129(2):281–288. doi: 10.1007/s11060-016-2172-3. [DOI] [PubMed] [Google Scholar]
- 9.Nayak L., Iwamoto F.M., Rudnick J.D., et al. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109(1):187–193. doi: 10.1007/s11060-012-0886-4. [DOI] [PubMed] [Google Scholar]
- 10.Strosberg J., El-Haddad G., Wolin E., et al. NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis D.N., Ohgaki H., Wiestler O.D., et al. The 2007 WHO classification of tumours of the central nervous system [published correction appears in Acta Neuropathol. 2007 Nov;114(5):547] Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaikh N., Dixit K., Raizer J. Recent advances in managing/understanding meningioma. F1000Res. 2018;7:F1000. doi: 10.12688/f1000research.13674.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaley T., Barani I., Chamberlain M., et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. doi: 10.1093/neuonc/not330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blitshteyn S., Crook J.E., Jaeckle K.A. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26(2):279–282. doi: 10.1200/JCO.2007.14.2133. [DOI] [PubMed] [Google Scholar]
- 15.Kunikowska J., Królicki L., Hubalewska-Dydejczyk A., Mikołajczak R., Sowa-Staszczak A., Pawlak D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: which is a better therapy option? Eur J Nucl Med Mol Imaging. 2011;38(10):1788–1797. doi: 10.1007/s00259-011-1833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutathera for the treatment of inoperable, progressive meningioma after external beam radiation therapy. ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT04082520.ClinicalTrials.gov Identifier: NCT04082520 Accessed September 1, 2020.