Abstract
Background
More than 20% of hospitalized patients with COVID-19 demonstrate ARDS requiring ICU admission. The long-term respiratory sequelae in such patients remain unclear.
Research Question
What are the major long-term pulmonary sequelae in critical patients who survive COVID-19?
Study Design and Methods
Consecutive patients with COVID-19 requiring ICU admission were recruited and evaluated 3 months after hospitalization discharge. The follow-up comprised symptom and quality of life, anxiety and depression questionnaires, pulmonary function tests, exercise test (6-min walking test [6MWT]), and chest CT imaging.
Results
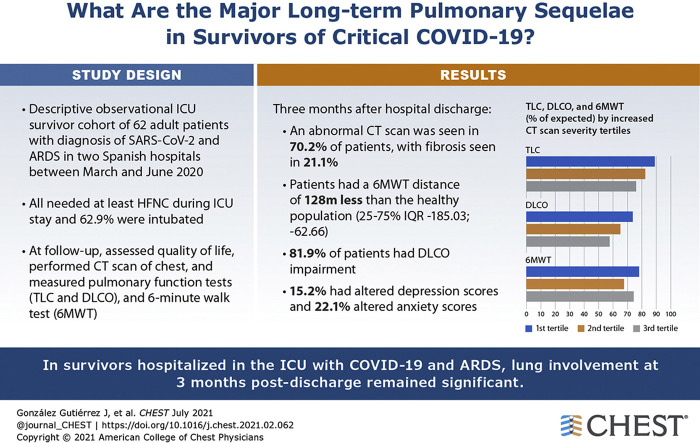
One hundred twenty-five patients admitted to the ICU with ARDS secondary to COVID-19 were recruited between March and June 2020. At the 3-month follow-up, 62 patients were available for pulmonary evaluation. The most frequent symptoms were dyspnea (46.7%) and cough (34.4%). Eighty-two percent of patients showed a lung diffusing capacity of less than 80%. The median distance in the 6MWT was 400 m (interquartile range, 362-440 m). CT scans showed abnormal results in 70.2% of patients, demonstrating reticular lesions in 49.1% and fibrotic patterns in 21.1%. Patients with more severe alterations on chest CT scan showed worse pulmonary function and presented more degrees of desaturation in the 6MWT. Factors associated with the severity of lung damage on chest CT scan were age and length of invasive mechanical ventilation during the ICU stay.
Interpretation
Three months after hospital discharge, pulmonary structural abnormalities and functional impairment are highly prevalent in patients with ARDS secondary to COVID-19 who required an ICU stay. Pulmonary evaluation should be considered for all critical COVID-19 survivors 3 months after discharge.
Key Words: COVID-19, CT abnormalities, ICU, lung function, SARS, SARS-CoV-2, sequelae
Graphical abstract
FOR EDITORIAL COMMENT, SEE PAGE 15
In December 2019, SARS-CoV-21 was identified as the cause of COVID-19. Through person-to-person transmission,2 it spread rapidly across China3 and many other countries,4 causing a global pandemic and a public health emergency of international concern.5 By October 3, 2020, there were 34,680,199 confirmed cases, including 1,029,525 deaths globally.
SARS-CoV-2 infection has a wide range of clinical presentations, with the majority of patients having mild disease with a favorable prognosis.6 However, for a significant proportion of hospitalized patients (20%-67%), SARS-CoV-2 may cause severe illness with rapid disease progression resulting in ARDS.7 , 8 This results in a high rate of ICU admission (26%-32%) and death (4.3%-15%).8 , 9
Patients with this type of critical illness could show major long-term sequelae, prompting the characterization of post-ICU syndrome. This syndrome is defined as “new or worsening impairment in physical, cognitive or mental status arising after critical illness and persisting beyond discharge from the acute care setting.”10 After ARDS, regardless of its origin, patients frequently show several functional impairments across biopsychosocial domains.11
Similarly, lung damage, impaired lung function, and psychological impairment are common and can last for months or even years in patients who have recovered from other types of coronavirus pneumonia, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).12 , 13 In follow-up studies of these patients lasting 0.3 to 2 years,14 , 15 impaired lung diffusing capacity for carbon monoxide (Dlco), defective total lung capacity (TLC), and poor 6-min walking test (6MWT) outcomes were the most common lung function abnormalities. Moreover, approximately one-third of SARS and MERS survivors may have psychological dysfunction, such as depression and anxiety, beyond 6 months.12
Regarding SARS-CoV-2, recent research has demonstrated that nearly half of discharged patients show residual abnormalities on chest CT scan.16 , 17 Moreover, these studies showed that in early convalescence (1 month after discharge), approximately three-quarters of patients with COVID-19 demonstrated pulmonary function impairment, represented most frequently again by declines in Dlco.16 A recent study of patients with noncritical disease18 demonstrated that a considerable proportion of COVID-19 survivors showed radiologic (70%) and pulmonary function (25%) abnormalities 3 months after discharge. Although short-term radiologic and pulmonary function outcomes have been reported16 , 19 in patients with noncritical disease, little is known about the outcomes of patients with critical COVID-19 3 months after discharge. Furthermore, ARDS resulting from COVID-19 shows a unique phenotype20 , 21 that requires different management strategies for ARDS in the acute phase22 , 23 and a more exhaustive and close short-term follow-up,24 including respiratory, mental health, and quality of life assessment.
Herein, we report the first descriptive observational cohort of recruited patients with COVID-19 who underwent an ICU stay. Participants were followed up 3 after months hospital discharge and underwent an evaluation of symptoms (involving the Short-Form Health Survey [SF-12] and Hospital Anxiety and Depression Scale [HADS]) and characterization of pulmonary function, including lung volume (TLC and residual volume), Dlco assessments, and 6MWT assessment. Moreover, we performed chest CT scan.
Methods
Ethical Statement
The study was approved by the Medical Ethics Committee of Hospital Universitary Arnau de Vilanova (Identifier: CEIC/2273). Informed consent was acquired for most patients by using emergency consent mechanisms in accordance with the ethics approval guidelines for the study.
Study Design and Population
This was a descriptive observational study performed to include all patients who experienced a critical care admission resulting from COVID-19 in Hospital Universitari Arnau de Vilanova and Hospital Universitari Santa Maria in Lleida, Spain, between March and June 2020. The study is a subset of the ongoing multicenter study Centro de Investigación Biomédica En Red Enfermedades Respiratorias Unidad de Cuidados Intensivos COVID (ClinicalTrials.gov Identifier: NCT04457505). The main objective was to determine the risk of and prognostic factors for critical illness in patients with COVID-19, as well as the impact of COVID-19 on respiratory and cardiovascular function within the first year of follow-up.
All patients showed positive results for SARS-CoV-2, were older than 18 years, met the Berlin definition of ARDS,25 and had undergone an ICU stay. Patients were unable to follow-up if they were transferred to another hospital during ICU hospitalization or later, if they were receiving palliative care, or if they had a severe mental disability that made it impossible to carry out pulmonary function tests after discharge.
Clinical Data Collection
Baseline and ICU Stay
Patient sociodemographic and comorbidity data were obtained. Clinical, vital, ventilatory, and laboratory parameters were recorded at ICU admission. The latter included general blood tests with acute markers of inflammation, such as D-dimer, ferritin, C-reactive protein, procalcitonin, lactate dehydrogenase, and fibrinogen. In addition to the baseline records, we collected data such as length of stay and the need for and duration of invasive and noninvasive mechanical ventilation, including high-flow nasal canula and prone positioning, during the ICU stay.
Three-Month Follow-up
General and respiratory symptoms, including anosmia, ageusia, fever, dry and wet cough, wheeze, dyspnea measured by the modified Medical Research Council, asthenia, and muscular fatigue, were assessed in the consultation. To complete the clinical evaluation, answers to the SF-12 and the HADS questionnaire were self-reported by all patients. The SF-12 is a well-known health-related quality-of-life questionnaire consisting of 12 questions that measure eight health domains to assess physical and mental health. These eight multi-item variables include general health, physical functioning, role physical, body pain, vitality, social functioning, role emotional, and mental health. This questionnaire has been validated in healthy populations and in patients with several chronic diseases and conditions.26 , 27 The SF-12 was scored according to the normative standards established by Ware et al28 such that persons with a normal health-related quality of life would have an average SF-12 score of 50, with an SD of 10. This scoring system can be used to assess the degree of well-being and functional status of people older than 14 years, identifying positive and negative physical health and mental health states, through the analysis of eight dimensions. Scores of < 50 indicate a poor health-related quality of life, whereas scores of > 50 indicate a good health-related quality of life. Its use to evaluate the functional status after hospitalization in patients who survived ARDS has been validated.29 The HADS is a 14-item self-report screening scale that was developed originally to indicate the possible presence of anxiety and depression states in medical nonpsychiatric outpatient clinic settings.30 The HADS assesses symptoms over the preceding week and consists of a seven-item anxiety subscale and a seven-item depression subscale. Each item on the questionnaire is scored as 0 to 3, with a maximum score of 21. A general cutoff of 8 of 21 is used to identify a possible case of anxiety or depression.31
Pulmonary Function Tests
Airway function (spirometry, lung volume, and diffusing capacity) was measured in all participants using a flow spirometer (MasterScreen; Jaeger) according to the guidelines of the American Thoracic Society.32 Pulmonary parameters included TLC, FVC, residual volume, FEV1, FEV1 to FVC ratio, and Dlco. The results were expressed as a percentage of the predicted value according to the European Community Lung Health Survey.33 Additionally, spirometric postbronchodilation measurements were determined 15 min after inhalation of 400 μg of salbutamol. The 6MWT was performed according to the current American Thoracic Society guidelines.34
Chest CT Scan Examinations
Patients were scanned using a 16- and 64-slice multidetector CT scanner (Brilliance 16 and 64; Philips Healthcare) with the following scan parameters: 16 × 1.5-mm slice collimation, 0.75-s gantry rotation time, 120-kV tube voltage, and 3-mm section thickness with a 1.5-mm reconstruction interval. Images were acquired with patients in the supine position in the craniocaudal direction at end-inspiration. The resulting images were visualized with an image archiving and communication system with standard lung (level, –450 Hounsfield units [HU]; width, 1,600 HU) and mediastinal (level, 40 HU; width, 400 HU) windows. Chest CT imaging was not performed or was not available for five patients.
Image Analysis and Quantification
All CT images were reviewed by a pulmonologist (J. G.) with experience in imaging who was blinded to the clinical data. CT images were evaluated as described previously17 for the presence of the following characteristics: (1) density: ground-glass opacities, mixed ground-glass opacities, or consolidation; (2) internal structures: air bronchogram, interlobular septal thickening, cavitation, pulmonary nodules; (3) number of lobes affected by ground-glass or consolidative opacities; (4) fibrotic or reticular lesions; (5) pleural effusion; (6) thoracic lymphadenopathy; and (7) underlying lung disease (TB, emphysema, or interstitial lung disease). Fibrotic pattern was defined according to the Fleischner Society glossary of terms for thoracic imaging: reticulation, architectural distortion, traction bronchiectasis, and honey combing.35 Because of the nature and the atypical presentation of COVID-19, most frequently we saw these components separately, and the clinical image did not fit into a classic interstitial lung disease pattern. The coexistence of ground-glass opacities with a predominantly upper and sometimes bilateral, but usually asymmetrical (often unilateral), presentation without immediate subpleural sparing and the coexistence in some cases of pulmonary nodules does suggest a typical pattern of usual interstitial pneumonia or nonspecific interstitial pneumonia. For that reason, we used the term “fibrotic pattern.”
To quantify the severity of lung affectation, the total severity score (TSS) was assessed. Each of the five lung lobes was determined for the percentage of lobar involvement. After this, the severity of each lobe was classified as none (0%), minimal (1%-25%), mild (26%-50%), moderate (51%-75%), or severe (76%-100%), with a corresponding score of 0, 1, 2, 3, or 4, respectively. The TSS is calculated by summing the five lobe scores (range, 0-20).36
Statistical Analysis
Descriptive statistics of the mean (SD) or median (interquartile range [IQR]) were estimated for quantitative variables with a normal or nonnormal distribution, respectively. The normality of the distribution was analyzed using the Shapiro-Wilk test. The absolute and relative frequencies were used for qualitative variables. To assess the pulmonary inflammation severity, the CT scan score was categorized by tertiles. Lung function parameters were compared according to pulmonary inflammation severity using the appropriate tests (analysis of variance or a nonparametric Kruskal-Wallis test for quantitative variables and Fisher exact test for qualitative variables). The P value for trend was computed from the Spearman rank correlation coefficient when the variable was continuous and from the χ 2 test for trend if the variable was categorical.
Furthermore, we evaluated the associations among demographic data, clinical data, and ICU stay in patients with pulmonary inflammation measured by CT scan score at the 3-month follow-up. Selection of variables was carried out using a relaxed least absolute shrinkage and selection operator (LASSO) model.37 , 38 Five-fold cross-validation was carried out to determine the lambda parameter of the LASSO model.39 Lambda was selected as the value that minimized the mean square error. A Spearman correlation test between the independent risk factors from the LASSO analysis and the rest of the variables was performed. To perform the LASSO analysis, missing values were replaced by the means of the nonmissing values. The same analysis was performed for the presence of lung lesions (reticular or fibrotic/no lesions) and type of lesion (reticular/fibrotic) among patients with lesions. R statistical software version 4.0.1 (R Foundation for Statistical Computing) was used for all the analyses.
Results
Characteristics of the Study Population and ICU Stay
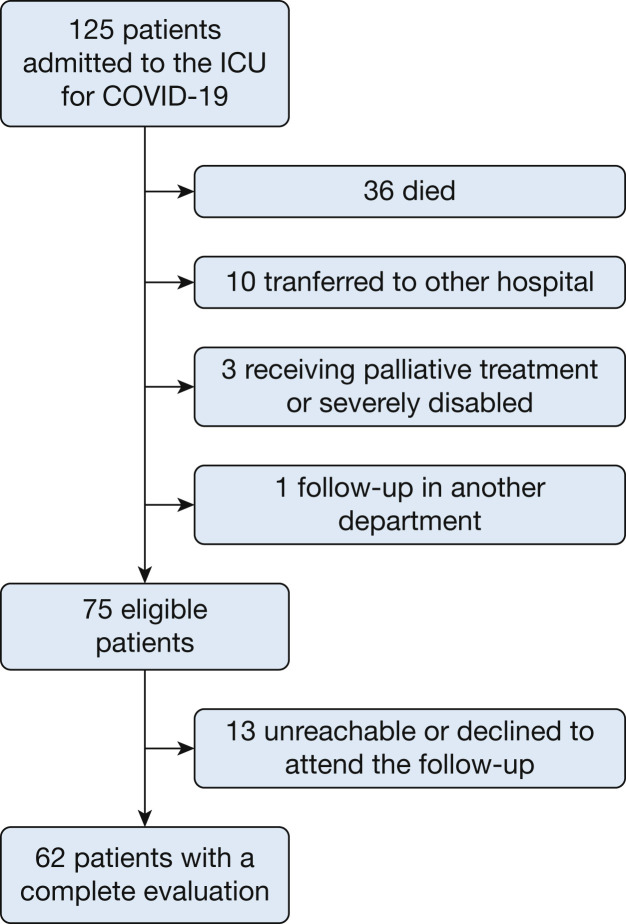
Figure 1 shows the flowchart of the study. One hundred twenty-five critically ill patients with ARDS resulting from COVID-19 were admitted during the study period. Thirty-six died during the ICU stay (28.8%), and 10 were transferred to other hospitals (only two patients were transferred to undergo extracorporeal membrane oxygenation). After hospital discharge, three patients were receiving palliative treatment or were severely disabled and one was undergoing follow-up in another center. Of the 75 eligible patients who completed the 3-month follow-up, 13 were unreachable or decided not to participate in follow-up. The latter did not differ in sociodemographic or clinical characteristics compared with the final cohort. A total of 62 patients completed the evaluation.
Figure 1.
Flowchart showing patients with critical COVID-19 included in the study.
The characteristics of the study population are displayed in Table 1 . Briefly, they were predominantly middle-aged, overweight, and male. Most of them were former smokers. The prevalence of pre-existing hypertension, diabetes mellitus, chronic heart disease, asthma, and COPD was 37.1%, 14.5%, 9.7%, 4.8%, and 4.8%, respectively. The median ICU stay was 14.5 days (IQR, 7.0-25.8 days), and the median overall hospitalization was 26 days (IQR, 15-38.5 days). Thirty-nine survivors (62.9%) required invasive mechanical ventilation (IMV) for a median duration of 17.4 days (SD, 8.5 days). In seven patients, both IMV and noninvasive mechanical ventilation modes were used. During the ICU stay, prone positioning was needed in 35 of the patients (56.5%). All patients needed a high-flow nasal canula during the ICU stay. Patients mostly were treated with hydroxychloroquine (98.4%), corticosteroids (58.3%), tocilizumab (25.8%), and interferon-β (17.7%). Ninety percent of patients received methylprednisolone and 10% hydrocortisone with a median maximum dose of 500 mg (IQR, 300 mg). Lopinavir plus ritonavir was used in only two patients (3.23%). All patients received antibiotic treatment, and only one patient received antifungal treatment. Only two patients were readmitted after the hospital discharge, one because respiratory problems and the other for other causes.
Table 1.
Demographic and Biological Characteristics of Patients Who Survived Critical COVID-19 Included in the Follow-up
| Characteristic | Data (N = 62) |
|---|---|
| Age, y | 60 (48-65) |
| Sex | |
| Male | 46 (74.2) |
| Female | 16 (25.8) |
| BMI, kg/m2 | 28.2 (25.4-32.6) |
| Smoking history | |
| Current | 3 (5.0) |
| Former | 31 (51.7) |
| Nonsmoker | 26 (43.3) |
| Comorbidities | |
| Hypertension | 23 (37.1) |
| Diabetes mellitus | 9 (14.5) |
| Chronic heart disease | 6 (9.7) |
| Asthma | 3 (4.8) |
| COPD | 3 (4.8) |
| ICU stay | |
| Length, d | 14.5 (7.0-25.8) |
| Mechanical ventilation | |
| Invasive | 39 (62.9) |
| Length, d | 17.4 ± 8.5 |
| Noninvasive | 30 (49.2) |
| Length, d | 2 (1-4) |
| Prone positioning | 35 (56.5) |
| Length, h | 43.9 ± 30.7 |
| Hydroxychloroquine | 61 (98.4) |
| Interferon-β | 11 (17.7) |
| Tocilizumab | 16 (25.8) |
| Methylprednisolone | 35 (56.4) |
| Maximum daily dose, mg | 500 (120-500) |
| Antibiotics | 62 (100) |
| APACHE | 13.5 ± 4.3 |
| Worst Pao2 to Fio2 ratio | 126.0 (90.1-173.0) |
| Worst Spo2 to Fio2 ratio | 172 (124-215) |
| Laboratory data on ICU admission | |
| CRP, mg/dL | 182 (102-243) |
| Hemoglobin, g/L | 13.2 ± 1.84 |
| Platelet count, × 109/L | 224 (172-305) |
| White blood count, × 109/L | 9.18. (5.99-10.40) |
| Lymphocyte count, × 109/L | 0.80 (0.57-1.14) |
| Urea nitrogen, mM/L | 29 (24-48) |
| Creatinine, mg/dL | 0.8 (0.65-0.95) |
| LDH, U/L | 836 ± 341 |
| Ferritin, mg/dL | 602 (464-2112) |
| D-dimer, mg/L | 430 (285-756) |
Data are presented as No. (%), mean ± SD, or median (interquartile range). No. of missing: smoking history, n = 2; BMI, n = 2; Pao2 to Fio2 ratio, n = 11; CRP, n = 2; LDH, n = 44; ferritin, n = 45; D-dimer, n = 14; and worst Pao2 to Fio2 ratio, n = 11. APACHE = Acute Physiology And Chronic Health Evaluation; CRP = C-reactive protein; LDH = lactate dehydrogenase.
Symptoms and SF-12 and HADS Questionnaires at the 3-Month Follow-up
At the 3-month follow-up, the most common symptoms were dyspnea (46.7%), followed by muscular fatigue (29.5%) and wet and dry cough (18.0% and 16.4%, respectively) (Table 2 ). Only one patient experienced fever or anosmia after discharge, and none experienced wheeze, anosmia, or abdominal pain. Five patients were receiving supplemental oxygen after hospital discharge. Only one patient showed incidental pulmonary thromboembolism on chest CT scan after discharge.
Table 2.
Symptoms and Quality-of-Life, Anxiety, and Depression Questionnaire Results at the 3-Month Follow-up
| Variable | Data |
|---|---|
| Symptoms | |
| Asymptomatic | 16 (27) |
| Dry cough | 10 (16.4) |
| Wet cough | 11 (18.0) |
| Dyspnea | |
| 0 | 32 (53.3) |
| 1 | 19 (31.7) |
| 2 | 8 (13.3) |
| 4 | 1 (1.67) |
| Muscular fatigue | 18 (29.5) |
| Questionnaires | |
| SF-12 | |
| Physical score | 45.9 (36.1-54.4) |
| Mental score | 55.8 (40.6-58.0) |
| HADS | |
| Depression score | 1.0 (0.5-4.5) |
| Normal (0-7) | 50 (84.7) |
| Borderline abnormal (8-10) | 6 (10.2) |
| Abnormal (11-21) | 3 (5.0) |
| Anxiety score | 3 (1-6) |
| Normal (0-7) | 46 (78.0) |
| Borderline abnormal (8-10) | 7 (11.9) |
| Abnormal (11-21) | 6 (10.2) |
Data are presented as No. (%) or median (interquartile range). No. of missing: SF-12, n = 7; HADS, n = 3; asymptomatic, n = 3; dry cough, n = 1; wet cough, n = 1; and dyspnea, n = 2. HADS = Hospital Anxiety and Depression Scale; SF-12 = Short-Form Health Survey.
The SF-12 showed median scores of 45.9 (IQR, 36.1-54.4) and 55.8 (IQR, 40.6-58) in the physical and mental domains, respectively. Degree of dyspnea showed a strong correlation only with the physical component of the SF-12 (e-Fig 1). A total of 15.2% and 22.1% of patients showed altered depression and anxiety scores, respectively, on the HADS questionnaire (Table 2).
Lung Function, 6MWT Results, and Chest CT Scan Findings at the 3-Month Follow-up
The pulmonary function and exercise test results are detailed in Table 3 . Only one patient showed lung airway obstruction. Fifty survivors (82%) showed an abnormal Dlco (< 80% predicted). Moreover, 23 survivors (37.1%) showed altered TLC. The median distance in the exercise test was 400 m (IQR, 362-440 m), with a mean oxygen saturation of 96%. Severe decrease of oxygen saturation (< 88%) was shown in only one patient. We calculated the difference in the distance walked on the 6MWT between our population and reference values from a healthy population using validated reference equations40 adjusted by sex, age, weight, and height. The results show a significant reduction in the distance in the study patients compared with the healthy population (median difference, –128.43 [IQR, –185.03 to –62.66]; P < .001) (e-Table 1).
Table 3.
Pulmonary Function, 6MWT Results, and Chest CT Scan Findings in All Patients at the 3-Month Follow-up
| Variable | Data |
|---|---|
| Pulmonary function (n = 62) | |
| FVC, % | 81.5 ± 16.7 |
| FEV1, % | 88.9 ± 19.1 |
| FEV1 to FVC ratio | 81.4 ± 4.8 |
| TLC, % | 83.8 ± 16.4 |
| ≥ 80% | 39 (62.9) |
| ≤ 50%-80% | 22 (35.5) |
| < 50% | 1 (1.61) |
| RV, % | 89.4 ± 37.9 |
| Dlco, mL/min/mm Hg | 67.8 ± 12.5 |
| ≥ 80% | 11 (18.0) |
| ≤ 60%-80% | 34 (55.7) |
| < 60% | 16 (26.2) |
| 6MWT (n = 60) | |
| Distance, m | 400 (362-440) |
| Oxygen saturation, % | |
| Average | 96 (94.5-97) |
| Initial | 97 (96-97) |
| Final | 96 (94.8-96) |
| Minimal | 94 (93-96) |
| Chest CT scan findings (n = 57) | |
| Density | |
| Ground-glass | 34 (59.6) |
| Mixed ground-glass | 9 (15.8) |
| Consolidation | 9 (15.8) |
| Internal structures | |
| Interlobular septal thickening | 46 (80.7) |
| Bronchiectasis | 41 (71.9) |
| Atelectasis | 14 (24.6) |
| Solid nodule | 22 (38.6) |
| Nonsolid nodule | 2 (3.5) |
| No. of lobes affected by ground-glass or consolidative opacities | 2.7 ± 2.0 |
| Lesions | |
| Reticular | 28 (49.1) |
| Fibrotic | 12 (21.1) |
| None | 17 (29.8) |
| TSS score | 4.8 ± 3.9 |
Data are presented as No. (%), mean ± SD, or median (interquartile range). 6MWT = 6-min walking test; Dlco = diffusing capacity for carbon monoxide; RV = residual volume; TLC = total lung capacity; TSS = total severity score.
Overall, those who underwent an ICU stay continued to demonstrate a wide array of abnormalities on CT scans at the 3-month follow-up (Table 3). Ground-glass opacities and consolidations were found in 59.6% and 15.8% of patients, respectively. Interlobular septal thickening and bronchiectasis were the most frequent abnormalities seen in chest CT imaging (80.7% and 71.9%, respectively). Only 10 patients had emphysema confirmed as a pre-existing comorbidity. The most frequent was the centrilobular subtype, located in the upper lobes with mild severity.
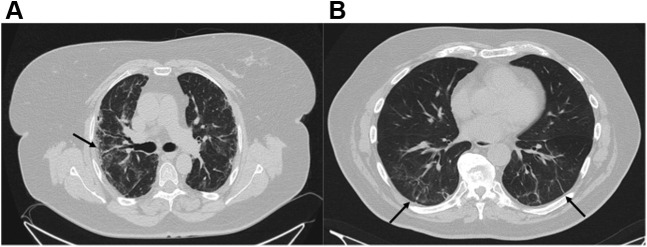
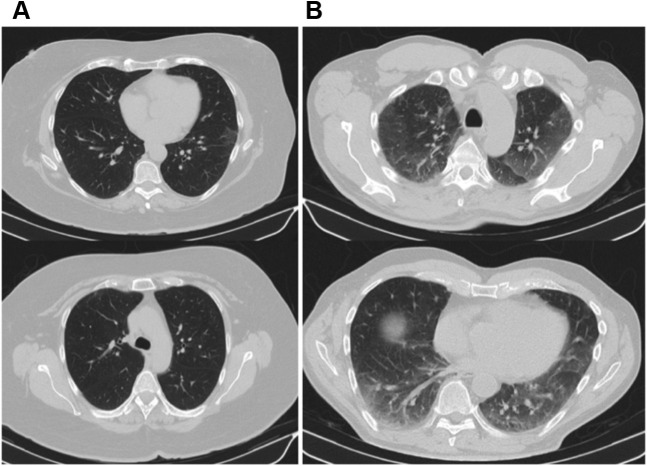
Importantly, 40 patients showed the presence of reticular (n = 28 [49.1%]) or fibrotic (n = 12 [21.1%]) lesions (Fig 2 ). Furthermore, the mean number of lobes affected by ground-glass or consolidative opacities per patient was 2.7 (SD, 2.0), and 34 patients showed at least one affected lobe. The mean TSS, which quantifies the severity of pulmonary inflammation, was 4.8 (SD, 3.9). Representative CT scans from those discharged from the ICU with low and high TSS scores 3 months after hospital discharge are shown in Figure 3 .
Figure 2.
A, B, CT scans comparing a patient with a fibrotic pattern (A) and a patients with a reticular pattern (B). A, CT scan showing subpleural predominant affectation, visible parenchymal bands, architectural distortion with irregular interface, and traction bronchiectasis (black arrow). B, CT scan showing only subpleural reticular bands (black arrow) accompanied by interlobular septal thickening.
Figure 3.
A, B, CT scans comparing a patient with a total severity score (TSS) score of 2 (A) and a more severe patient with a TSS score of 14 (B).
Correlation of Pulmonary Function and Exercise Testing Characteristics According to CT Scan Alterations
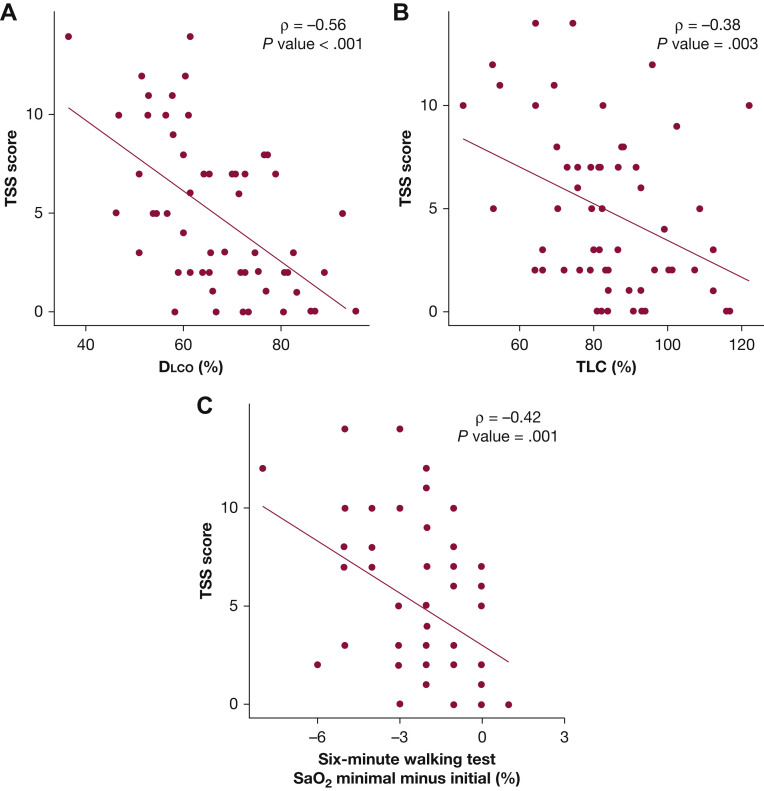
As expected, chest CT scan results severity measured by TSS score were associated intimately with respiratory abnormalities in those who underwent an ICU stay at 3 months after hospital discharge (e-Table 2). Survivors with CT scan scores in the higher tertiles showed an impaired Dlco, with a mean Dlco of 65.6 (95% CI, 60.1-71.0) in the second tertile and 57.8 (95% CI, 51.7-63.8) in the third tertile (P ≤ .001 for trend). Consequently, the CT scan score was correlated intimately with diffusing capacity (ρ = –0.56) (Fig 4 ). For the exercise test, although the mean 6MWT results were similar across CT scan score tertiles (P = .124), patients with severe abnormalities on CT scan showed a decreased average oxygen saturation, as well as decreased final and minimal oxygen saturation levels (P = .028 and P = .011, respectively). A robust and inverse correlation between the CT scan score and the percentage of change in oxygen during the walking test also was observed (ρ = –0.42) (Fig 4).
Figure 4.
A-C, Scatterplots showing correlation between lung function and the rate of decrease in SaO2 and TSS score. P values was computed from the Spearman rank correlation coefficient. A, Scatterplot showing correlation between diffusing capacity for carbon monoxide and TSS score. B, Scatterplot showing correlation between total lung capacity and TSS score. C, Scatterplot showing correlation between rate of decrease in SaO2 in six-minute walking test and TSS score. Dlco = diffusing capacity for carbon monoxide; SaO2 = oxygen saturation; TLC = total lung capacity; TSS = total severity score.
A predictor selection procedure (LASSO model) was performed using variables from the medical history and ICU stay to explore further the association of clinical profiles of those discharged from the ICU and the outcomes reported for CT scan anomalies: presence of lesions and type of lesions. The results from this analysis suggested an association between CT scan abnormalities and two predictors: age and the length of IMV during the ICU stay. The CT scan score was higher in those patients with longer duration of IMV, and age at admission was robust and correlated positively with the CT scan score (ρ = 0.40) (e-Fig 2). However, the requirement of IMV was the only variable selected for predicting the risk of lung lesions (e-Fig 3). Additionally, we explored which variables were important for the determination of the type of injury among patients who harbored any pulmonary lesion. The LASSO model showed that the duration of IMV was the only factor that allowed us to discriminate between the types of lesions (e-Fig 3). No other significant differences or correlations (ICU or hospital stay) were found.
Discussion
Since the beginning of the SARS-CoV-2 outbreak, various studies have been performed to describe pulmonary sequelae in patients with COVID-19 after hospital discharge. To our knowledge, this is the first well-characterized descriptive, observational study of COVID-19 survivors who underwent an ICU stay. The most striking finding is the high proportion of patients with Dlco impairment (81.9%) and lung injury (70.2%) 3 months after discharge. The magnitude of lung damage found in this cohort has no precedent,41 even in previous coronavirus outbreaks (SARS and MERS).13 , 14 The quality of life of COVID-19 survivors showed mean scores that are substantially lower than those of healthy people (e-Fig 4),26 those with other chronic diseases,12 and healthy Spanish people.42 This finding usually is observed in interstitial lung diseases and is important because it correlates with pulmonary function and physical activity.43 Additionally, the levels of anxiety and depression scores were higher than the reference values.
A recent meta-analysis12 of clinical outcomes after hospitalization or ICU admission in patients with SARS and MERS showed a pooled prevalence of impaired Dlco at 6 months of 24.35% (95% CI, 11.05%-45.46%). Two studies44 , 45 included some patients with SARS who required ICU treatment, showing a prevalence of impaired Dlco ranging from 13.5% to 24% at the 3- and 12-month follow-ups. Regarding SARS-CoV-2, a recent study19 performed at the time of hospital discharge and excluding patients with critical disease found Dlco and TLC anomalies in 47.2% and 25%, respectively, being more frequent in patients with severe pneumonia (84%; P = .001). Recent research in early convalescence (1 month after discharge) demonstrated that approximately three-quarters of patients with COVID-19 experience pulmonary function impairment, which is represented by a decline in Dlco in more than half of patients.16
A similar phenomenon is notable with exercise capacity. Previous SARS studies including patients requiring ICU treatment showed a mean 6MWT distance at the 3-month follow-up ranging from 454 m46 to 464 m,44 with a pooled distance estimate of 461.18 m (95% CI, 449.66-472.71 m).12 Those distances are significantly longer than the mean distance observed in the present patients (400 m). In SARS-CoV-2, the 6MWT distance was significantly shorter in patients with severe disease (517 m) than in patients with nonsevere disease (573.52 m), indicating poor exercise tolerance.16
The same phenomenon can be noted in chest CT scan abnormalities found during follow-up examinations of patients who survive COVID-19. In a recent SARS-CoV-2 series, the rate of radiologic abnormalities remained high 3 months after discharge (74.55%), although it was lower than that at the time of admission (84%).47 , 48 A study also performed at the 3-month follow-up showed that fibrosis was present in 23.6% of patients and the mean TSS was 8 in the group with abnormal CT scan findings.18 This finding is in line with our results, although the rate of reticular and fibrotic lesions was higher in the present cohort (49.1% and 47.1%, respectively). This rate was even higher than that of residual radiographic survivors with other viral pneumonias, including SARS, H1N1, and H7N9.49 , 50 As expected and as described previously,16 , 18 severity, as measured by TSS on chest CT imaging, correlated with pulmonary function. However, we also demonstrated this correlation with the decrease in oxygen saturation during the 6MWT.
Our data suggest different clinical and pulmonary effects for COVID-19 in comparison with other forms of viral respiratory illnesses, regardless of important ICU variables (need and length of tracheal intubation). Recent studies51 showed that patients with COVID-19 are hospitalized more often and have longer hospitalizations with a higher probability of ARDS developing than patients with other acute respiratory illnesses. This hypothesis could be explained by the particular pathologic findings that occur in patients with severe COVID-19 compared with those with other viral pneumonias.52 In addition to the typical diffuse alveolar damage, compared with lungs from patients with H1N1, lungs from patients with critical COVID-19 disease showed severe endothelial injury associated with the presence of intracellular virus, disrupted cell membranes, and a higher prevalence of thrombosis and microangiopathy.52 Moreover, patients with severe COVID-19 show a unique inflammatory and proteomic profile as well as a different immune response compared with patients with noncritical COVID-19,21 leading to organ-specific cellular death and damage.
Our findings have several clinical implications. Because more severe differential lung involvement is seen in critically ill patients with COVID-19, close monitoring after discharge is deserved. Therapies such as pulmonary rehabilitation and physical conditioning should be the cornerstone of follow-up. Empirical treatment with systemic glucocorticosteroids should be considered in selected patients. The long-term pulmonary sequelae are unknown, but these data encourage close follow-up of these patients.
This study has some limitations. First, the study examined a small cohort from a single city, and a larger sample size from different hospitals would be ideal for this type of study. However, generalization of our results is facilitated by the cohort being well characterized and prospective. Moreover, because of the methodologic characteristics of the study, we did not calculate a previous sample size. Second, even if the 13 patients lost to follow-up were considered, a minimal influence on the results would have ensued because no sociodemographic and clinical differences are available to compare with the final cohort. Third, it is uncertain whether lung lesions were present before the study. Fourth, the reversibility of the parenchymal involvement is uncertain because of short-term follow-up. Further long-term analysis should clarify this issue.
Interpretation
In conclusion, survivors of critical COVID-19 show a higher proportion of Dlco impairment and chest CT scan abnormalities at the 3-month follow-up. A complete evaluation including chest CT imaging and pulmonary function and exercise tests 3 months after discharge should be considered for these survivors of critical COVID-19.
Take-home Points.
Study Question: What are the major long-term pulmonary sequelae in patients who survive critical COVID-19?
Results: At the 3-month follow-up, 82% of patients who survives critical COVID-19 showed a lung diffusing capacity of less than 80% and abnormal chest CT scan results in 70.2%, with reticular lesions in 49.1% and fibrotic pattern in 21.1%.
Interpretation: Pulmonary structural abnormalities and functional impairment are highly prevalent in patients who survive critical COVID-19 at 3 months after hospital discharge. A complete evaluation including chest CT imaging and pulmonary function and exercise tests at this time should be considered for these patients.
Acknowledgments
Author contributions: F. B. is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. J. G., G. T., D. G.-G., A. T., and F. B. were responsible for conception, design, interpretation, and drafting of the manuscript for important intellectual content. Statistical analysis was performed by I. D. B. Patient recruitment and evaluation were carried out by P. C., S. S., A. Monge, A. M.-M., C. G.-P., L. P., A. C., and M. Z. The rest of CIBERESUCICOVID group (R. F., A. C., L. F., A. Motos, J. R., R. M., D. G.-G., O. P., J. F. B.-M., G. L., and J. C.) contributed to correcting and improving the manuscript.
Financial/nonfinancial disclosures: None declared.
∗CIBERESUCICOVID Project (COV20/00110, ISCIII) collaborators: Rosario Amaya Villar,MD, PhD (Department of Intensive Care Medicine, Hospital Universitario Virgen de Rocío); José M. Añón, MD, PhD (Department of Intensive Care Medicine. Hospital Universitario La Paz. IdiPAZ. Madrid. Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Madrid, Spain); Carme Barberà, MD (Hospital Santa Maria; IRBLleida, Lleida, Spain); José Barberán, MD, PhD (Hospital Universitario HM Montepríncipe, Universidad San Pablo-CEU, Madrid, Spain); Aaron Blandino Ortiz, MD (Department of Intensive Care Medicine, Hospital Universitario Ramón y Cajal, Madrid); Elena Bustamante-Munguira, MD, PhD (Department of Intensive Care Medicine, Hospital Clínico Universitario Valladolid, Valladolid, Spain); Jesús Caballero, MD (Department of Intensive Care Medicine, Hospital Universitari Arnau de Vilanova; IRBLleida, Lleida, Spain); Cristina Carbajales, MD (Hospital Álvaro Cunqueiro, Vigo, Spain); Nieves Carbonell, MD, PhD (Department of Intensive Care Medicine, Hospital Clínico y Universitario de Valencia, Valencia, Spain); Mercedes Catalán-González, MD (Department of Intensive Care Medicine, Hospital Universitario 12 de Octubre, Madrid, Spain); Cristóbal Galbán, MD (Department of Medicine, CHUS, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela, Spain); Victor Daniel Gumucio, MD (Intensive Care, Hospital Universitari de Bellvitge, Barcelona, Spain); Maria del Carmen de la Torre, MD (Hospital de Mataró de Barcelona, Spain); Emili Díaz, MD (Department of Medicine, Universitat Autònoma de Barcelona (UAB) and Critical Care Department, Corporació Sanitària Parc Taulí, Sabadell, Barcelona, Spain); Ángel Estella, MD (Departamento Medicina Facultad Medicina Universidad de Cádiz. Hospital Universitario de Jerez, Jerez de la Frontera, Cádiz. Spain); Elena Gallego, MD (Department of Intensive Care Medicine, Hospital San Pedro de Alcántara, Cáceres, Spain); José Luis García Garmendia, MD, PhD (Department of Intensive Care Medicine, Hospital San Juan de Dios del Aljarafe, Sevilla, Spain); José Garnacho-Montero, MD, PhD (Intensive Care Clinical Unit, Hospital Universitario Virgen Macarena, Seville, Spain); José M. Gómez, MD (Hospital General Universitario Gregorio Marañón, Madrid, Spain); Arturo Huerta, MD, PhD (Pulmonary and Critical Care Division; Emergency Department Clínica Sagrada Família, Barcelona, Spain); Ruth Noemí Jorge García, MD (Intensive Care Department, Hospital Nuestra Señora de Gracia, Zaragoza, Spain); Ana Loza-Vázquez; MD (Department of Intensive Care Medicine, Hospital Universitario Virgen de Valme, Sevilla, Spain); Judith Marin-Corral MD, PhD (Critical Care Department, Hospital del Mar-IMIM, Barcelona, Spain); Amalia Martínez de la Gándara, MD (Department of Intensive Medicine, Hospital Universitario Infanta Leonor, Madrid, Spain); Ignacio Martínez Varela, MD (Critical Care Department, Hospital Universitario Lucus Augusti, Lugo, Spain); Juan Lopez Messa, MD (Complejo Asistencial Universitario de Palencia, Palencia, Spain); Guillermo M. Albaiceta, MD (Departamento de Biología Funcional, Instituto Universitario de Oncología del Principado de Asturias, Universidad de Oviedo and Instituto de Investigación Sanitaria del Principado de Asturias, Hospital Central de Asturias, Oviedo, Spain); Mariana Andrea Novo, MD (Department of Intensive Care Medicine, Hospital Universitari Son Espases, Palma de Mallorca, Illes Balears, Spain); Yhivian Peñasco, MD (Department of Intensive Care Medicine, Hospital Universitario Marqués de Valdecilla, Santander, Spain); Juan Carlos Pozo-Laderas, MD (Department of Intensive Care Medicine, Hospital Universitario Reina Sofia, Instituto Maimonides IMIBIC, Córdoba, Spain); Pilar Ricart Martí, MD, PhD (Department of Intensive Care Medicine, Hospital Universitari Germans Trias, Badalona, Spain); Ferran Roche-Campo, MD, PhD (Hospital Verge de la Cinta, Tortosa, Tarragona); Angel Sánchez-Miralles, MD (Hospital de Sant Joan d'Alacant, Alacant, Spain); Susana Sancho Chinesta, MD (Department of Intensive Care Medicine. Hospital Universitario y Politécnico La Fe. Valencia); Lorenzo Socias, MD, PhD (Intensive Care Unit, Hospital Son Llàtzer, Palma de Mallorca, Illes Balears, Spain); Jordi Solé-Violan, MD (Critical Care Department, Hospital Dr. Negrín Gran Canaria, Las Palmas, Gran Canaria, Spain); Fernando Suares Sipmann, MD (Intensive Care Unit, Hospital Universitario La Princesa, Madrid, Spain); Luis Tamayo Lomas, MD (Critical Care Department, Hospital Universitario Río Hortega de Valladolid, Valladolid, Spain); José Trenado, MD (Department of Intensive Care Medicine, Hospital Universitario Mútua de Terrassa, Terrassa, Barcelona, Spain).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported in part by the Instituto de Salud Carlos III [Grant CIBERESUCICOVID, COV20/00110] and was cofunded by European Regional Development Funds, “Una manera de hacer Europa.” D. d. G.-C. has received financial support from the Instituto de Salud Carlos III [Grant Miguel Servet 2020: CP20/00041], co-funded by the European Social Fund “Investing in Your Future.” L. P. acknowledges receiving financial support from the Ministry of Science, Innovation and Universities for the Training of University Lecturers (FPU19 / 03526).
Contributor Information
CIBERESUCICOVID Project (COV20/00110, ISCIII):
Rosario Amaya Villar, José M. Añón, Carme Barberà, José Barberán, Aaron Blandino Ortiz, Elena Bustamante-Munguira, Jesús Caballero, Cristina Carbajales, Nieves Carbonell, Mercedes Catalán-González, Cristóbal Galbán, Victor Daniel Gumucio, Maria del Carmen de la Torre, Emili Díaz, Ángel Estella, Elena Gallego, José Luis García Garmendia, José Garnacho-Montero, José M. Gómez, Arturo Huerta, Ruth Noemí Jorge García, Ana Loza-Vázquez, Judith Marin-Corral, Amalia Martínez de la Gándara, Ignacio Martínez Varela, Juan Lopez Messa, Guillermo M. Albaiceta, Mariana Andrea Novo, Yhivian Peñasco, Juan Carlos Pozo-Laderas, Pilar Ricart Martí, Ferran Roche-Campo, Angel Sánchez-Miralles, Susana Sancho Chinesta, Lorenzo Socias, Jordi Solé-Violan, Fernando Suares Sipmann, Luis Tamayo Lomas, and José Trenado
Supplementary Data
References
- 1.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;22;395(10224):565-574. [DOI] [PMC free article] [PubMed]
- 2.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toit A Du. Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18(3):123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohrabi C., Alsafi Z., O’Neill N., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls [Internet] StatPearls Publishing; 2021. Features, evaluation, and treatment of coronavirus (COVID-19) [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawal G., Yadav S., Kumar R. Post-intensive care syndrome: an overview. J Transl Intern Med. 2017;5(2):90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herridge M.S., Moss M., Hough C.L., et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed H., Patel K., Greenwood D.C., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 13.Chan K.S., Zheng J.P., Mok Y.W., et al. SARS: prognosis, outcome and sequelae. Respirology. 2003;8(suppl 1):S36–S40. doi: 10.1046/j.1440-1843.2003.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui D.S., Joynt G.M., Wong K.T., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngai J.C., Ko F.W., Ng S.S., To K.W., Tong M., Hui D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y., Tan C., Wu J., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y.M., Shang Y.M., Song W.B., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mo X., Jian W., Su Z., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6):2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattinoni L., Coppola S., Cressoni M., et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10) doi: 10.1164/rccm.202003-0817LE. 1299-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filbin MR, Mehta A, Schneider AM, et al. Plasma proteomics reveals tissue-specific cell death and mediators of cell-cell interactions in severe COVID-19 patients. bioRxiv [Preprint]. 2020 Nov 3:2020.11.02.365536.
- 22.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 23.Bain W., Yang H., Shah F.A., et al. COVID-19 versus non-COVID ARDS: comparison of demographics, physiologic parameters, inflammatory biomarkers and clinical outcomes [published online ahead of print February 5, 2021] Ann Am Thorac Soc. 2021 doi: 10.1513/AnnalsATS.202008-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gassel RJJ, Bels J.L.M., Raafs A., et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med. 2021;203(3):371–374. doi: 10.1164/rccm.202010-3823LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranieri V.M., Rubenfeld G.D., Thompson B.T., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 26.Mols F., Pelle A.J., Kupper N. Normative data of the SF-12 health survey with validation using postmyocardial infarction patients in the Dutch population. Qual Life Res. 2009;18(4):403–414. doi: 10.1007/s11136-009-9455-5. [DOI] [PubMed] [Google Scholar]
- 27.Cheak-Zamora N.C., Wyrwich K.W., McBride T.D. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual Life Res. 2009;18(6):727–735. doi: 10.1007/s11136-009-9483-1. [DOI] [PubMed] [Google Scholar]
- 28.Ware J.E., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Biehl M., Kashyap R., Ahmed A.H., et al. Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: a population-based study. Crit Care. 2015;19:356. doi: 10.1186/s13054-015-1062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 32.Celli B.R., MacNee W., Agusti A., et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 33.Roca J., Burgos F., Sunyer J., et al. References values for forced spirometry. Eur Respir J. 1998;11(6):1354–1362. doi: 10.1183/09031936.98.11061354. [DOI] [PubMed] [Google Scholar]
- 34.Crapo R.O., Casaburi R., Coates A.L., et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 35.Lynch D.A., Sverzellati N., Travis W.D., et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 36.Ooi G.C., Khong P.L., Müller N.L., et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 37.Leisman D.E., Harhay M.O., Lederer D.J., et al. Development and reporting of prediction models. Crit Care Med. 2020;48(5):623–633. doi: 10.1097/CCM.0000000000004246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hastie T., Tibshirani R., Tibshirani R.J. Extended comparisons of best subset selection, forward stepwise selection, and the lasso. Crit Care Med. 2020;48(5):623–633. [Google Scholar]
- 39.Zhang Y., Yang Y. Cross-validation for selecting a model selection procedure. J Econom. 2015;187.1(2015):95–112. [Google Scholar]
- 40.Enrichi P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 41.Ramani C, Davis EM, Kim JS, Provencio JJ, Enfield KB, Kadl A. Post-ICU COVID-19 outcomes: a case series. Chest. 2021;159(1):215-218. [DOI] [PMC free article] [PubMed]
- 42.Vilagut G., Valderas J.M., Ferrer M., et al. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: componentes físico y mental. Med Clin (Barc) 2008;130(19):726–735. doi: 10.1157/13121076. [DOI] [PubMed] [Google Scholar]
- 43.Bahmer T., Kirsten A.M., Waschki B., et al. Clinical correlates of reduced physical activity in idiopathic pulmonary fibrosis. Respiration. 2016;91(6):497–502. doi: 10.1159/000446607. [DOI] [PubMed] [Google Scholar]
- 44.Hui D.S., Wong K.T., Ko F.W., et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herridge M.S., Cheung A.M., Tansey C.M., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 46.Li T.S., Gomersall C.D., Joynt G.M., et al. Long-term outcome of acute respiratory distress syndrome caused by severe acute respiratory syndrome (SARS): an observational study. Crit Care Resusc. 2006;8(4):302–308. [PubMed] [Google Scholar]
- 47.Xiong Y., Sun D., Liu Y., et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. 2020;55(6):332–339. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han X., Cao Y., Jiang N., et al. Novel coronavirus disease 2019 (COVID-19) pneumonia progression course in 17 discharged patients: comparison of clinical and thin-section computed tomography features during recovery. Clin Infect Dis. 2020;71(15):723–731. doi: 10.1093/cid/ciaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng C.K., Chan J.W.M., Kwan T.L., et al. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59(10):889–891. doi: 10.1136/thx.2004.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q., Zhang Z., Shi Y., Jiang Y. Emerging H7N9 influenza a (novel reassortant avian-origin) pneumonia: radiologic findings. Radiology. 2013;268(3):882–889. doi: 10.1148/radiol.13130988. [DOI] [PubMed] [Google Scholar]
- 51.Shah S.J., Barish P.N., Prasad P.A., et al. Clinical features, diagnostics, and outcomes of patients presenting with acute respiratory illness: a retrospective cohort study of patients with and without COVID-19. EClinicalMedicine. 2020;27:100518. doi: 10.1016/j.eclinm.2020.100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.