Abstract
The relationship of ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) proteases with inflammatory processes was anticipated since their discovery. Although knowledge of these extracellular proteases in different contexts continues to grow, many questions remain unanswered. In this review, we summarize the most important studies of ADAMTSs and their substrates in inflammation and in the immune system of non-oncological disorders. In addition, we update the findings on cancer and highlight their emerging role in the tumor immune microenvironment. Although the overall functions of extracellular molecules are known to be modulated by proteolysis, specific activities attributed to intact proteins and cleaved fragments in the context of inflammation are still subject to debate. A better understanding of ADAMTS activities will help to elucidate their contribution to the immune phenotype and to open up new therapeutic and diagnostic possibilities.
Keywords: ADAMTS proteases, Extracellular matrix, Inflammation, Tumor immune microenvironment
Highlights
-
•
ADAMTS proteases have been widely associated with inflammatory and immune-related conditions in non-oncological disorders.
-
•
Some well-known ADAMTS substrates have also been studied in immune-related diseases regardless of their proteolysis status.
-
•
From all ADAMTS proteases, only ADAMTS1 and ADAMTS4 have been directly connected with the tumor immune microenvironment.
-
•
Knowing their substrates is key to understanding immunomodulation by ADAMTSs and designing new strategies against cancer.
Introduction
The tumor microenvironment (TME) and its immune compartments have emerged as critical players in tumor progression and metastasis. The confluence of major technological advances over recent decades has provided a fuller understanding of the components of the extracellular matrix (ECM) in the TME and their contribution to normal development, homeostasis and tumor growth [[1], [2], [3]]. Given its complex highly dynamic multifunctional composition, the ECM has been difficult to explore. The regulation and organization of the ECM depend on cellular entities present in specific niches, which secrete key molecules such as ECM-modifying enzymes. These include extracellular proteases, which have been the subject of much interest due to their ability to cleave and degrade ECM molecules which facilitates cell migration and invasion. Matrix metalloproteinases (MMPs), in particular, have been studied in depth in the overall context of tumor biology and tissue injury and have a considerable impact on inflammation processes [[3], [4], [5]]. Following flawed studies on the use of MMP inhibitors [6], subsequent analyses of other extracellular proteases such as a disintegrin and metalloproteinase (ADAMs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) received less interest from translational researchers and the pharmaceutical industry. However, advances in basic and experimental research have demonstrated the importance of these proteases, which require the development of more specifically targeted therapeutic tools.
Since the discovery of the first ADAMTS in a cachexia model in 1997 [7], these proteases have been found to be associated with a variety of physiological and pathological processes such as cell proliferation, migration, angiogenesis and inflammation, and to be directly related to certain human disorders [[8], [9], [10], [11], [12]]. Genomic studies have revealed a total of 19 secreted proteins in humans [9,11], whose structures contain a highly conserved N-terminal region composed of a prodomain, catalytic and disintegrin domains and a central thrombospondin type I repeat (TSR), as well as cysteine-rich and spacer regions. The variable C-terminal region consists of a varying number of TSRs and additional motifs such as the PLAC (protease and lacunin) module, as well as the gon-1-like and mucin-like motifs, with specific binding and interacting properties [9,11]. In addition to the catalytic activity of ADAMTS proteases in ECM components [13,14], proteolytic-independent functions have also been reported [10,11,13].
From an oncological perspective, ADAMTS proteases are involved in tumor progression, particularly in the tumor vasculature [10,[15], [16], [17], [18], [19], [20]]. In addition, attempts have recently been made to elucidate the role of several ADAMTSs and their substrates in tumoral immune responses (see below) [18,[21], [22], [23]]. However, it is first necessary to better understand their contribution to immune regulation and inflammation processes in other diseases.
Beyond cancer: ADAMTSs, their substrates and the immune system
Given the broad impact of the human immune system, studies of ADAMTSs and their substrates often allude to immune-related factors. In many cases, despite their collateral nature, we have assessed references in order to gain new insights into the involvement of ADAMTSs in tumors. During inflammatory events, leukocytes and other immune cells extravasate the vasculature and encounter the ECM, regarded as a physical barrier and as a reservoir of factors and signals that facilitate the activation and specialization of these complex multi-functional cells [4,5,24]. In line with the growing effort to understand how proteolytic enzymes generate molecules with immunomodulatory potential, in this section, we describe ADAMTS proteases and their substrates in the context of different diseases and conditions in which immune regulation plays an important role.
Beyond cancer: ADAMTSs and the immune system
ADAMTS proteases are directly involved in inflammatory responses under different non-oncological conditions in multiple ways (Table 1). Before discussing their involvement in different disorders, it is necessary to highlight some findings on ADAMTSs in immune cells during homeostasis and their connection with inflammatory responses with no pathological features. The original discovery of Adamts1 in mice already anticipated its inflammation features, since its induction occurred after systemic inflammation caused by lipopolysaccharide (LPS) [7]. Later research by Oveland et al. also described a similar LPS effect on rats which induced Adamts1 production in the spleen [25]. Interestingly, a study of Adamts1-knockout (Ats1-KO) mice under healthy conditions revealed a splenomegaly accompanied by an increase in pro-inflammatory T cells in the spleen and in bone marrow (BM) myeloid cells, suggesting that Adamts1 plays an immunomodulatory role [21]. In a comparable Adamts12-deficient mouse model, which produced different outcomes, Moncada-Pazos et al. found that Adamts12, which affects neutrophil apoptosis, is required for normal inflammatory responses to occur [26]. These deficient mice show delayed recovery from illnesses such as colitis, sepsis and pancreatitis, while monocytes and neutrophils in affected tissues increased and lymphocytes decreased. Another study has shown that ADAMTS12 plays a protective role during asthma and allergic inflammation [27]. These ambiguous findings reveal a complex scenario in which the crosstalk between ADAMTSs and ECM components require comprehensive research.
Table 1.
ADAMTS and diseases or conditions that proceed with an inflammatory component.
| ADAMTS | Disease/condition | Pro-/Anti-inflammatory | Mouse modela | Cell typeb | Substratec |
|---|---|---|---|---|---|
| ADAMTS1 | Aortic aneurysm and dissections [40,41,43,44] | Pro/Anti | Inducible whole-body knockout [43], Constitutive whole-body heterozygous [44] | Versican [40,41] | |
| Atherosclerosis [31,35,36] | Pro/Anti | THP-1, macrophages [30,31] | Versican [35] | ||
| LPS-induced inflammation [7,25] | Pro | ||||
| Regulation of immune populations [21] | n.d. | Constitutive whole-body knockout [21] | Versican [21] | ||
| ADAMTS2 | Dermatitis and skin dysfunction [[51], [52], [53]] | Anti | Constitutive whole-body knockout [52] | Monocytes, macrophages [53] | |
| ADAMTS4 | Aortic aneurysm and dissections [40,42] | Pro | Constitutive whole-body knockout [42] | Versican [40,42] | |
| Atherosclerosis [[30], [31], [32],34] | Pro | Constitutive whole-body knockout [34] | THP-1, macrophages [30,31] | Versican [32,34] | |
| Ischemic stroke and CNS disorders [45] | Anti | ||||
| Osteoarthritis [54] | Pro | Aggrecan [54] | |||
| ADAMTS5 | Atherosclerosis [30,31] | n.d. | THP-1, macrophages [30,31] | ||
| Influenza virus infection [60] | Pro | Constitutive whole-body knockout [60] | Versican [60] | ||
| Osteoarthritis [[54], [55], [56], [57]] | Pro | Aggrecan [57] | |||
| ADAMTS7 | Atherosclerosis [30,33,37,38] | Pro | Constitutive whole-body knockout [38] | THP-1, Macrophages [30,33] | |
| ADAMTS8 | Atherosclerosis [30] | Pro | THP-1 [30] | ||
| ADAMTS9 | Atherosclerosis [30] | Pro | THP-1 [30] | ||
| ADAMTS12 | Asthma or allergy [27] | Anti | Constitutive whole-body knockout [27] | ||
| Inflammatory response [26] | Anti | Constitutive whole-body knockout [26] | |||
| Osteoarthritis and rheumatoid arthritis [58,59] | Pro | COMP [58] | |||
| ADAMTS13 | Autoimmune encephalomyelitis [62] | Anti | |||
| Multiple sclerosis [61] | Anti | ||||
| Thrombotic thrombocytopenic purpura [47,50] | Anti | Von Willebrand Factor [47] | |||
| Traumatic microvascular injury [48] | n.d. | Constitutive whole-body knockout [49] | Von Willebrand Factor [48] | ||
| ADAMTS14 | Skin dysfunction [52] | Anti | Constitutive whole-body knockout [52] |
n.d.: not determined.
Mouse models used to study the referred ADAMTS protease in a specific disease or condition.
Immune cell type expressing the referred ADAMTS protease.
Substrate contributing to the mentioned pro/anti-inflammatory role.
Below, we describe a series of diseases in whose inflammatory processes and immune regulation ADAMTSs have been reported to be involved.
Cardiovascular diseases
Since earlier studies of the human ADAMTS1 [28], the anti-angiogenic TSR motif, present in all ADAMTSs, has stimulated considerable research in the field of vascular biology [10]. As with the widely studied connection between vasculature and inflammatory-related responses [29], many researchers have reported the involvement of ADAMTS proteases in cardiovascular pathologies and related disorders.
ADAMTS1, 4, 5, 7 and 8 have been widely reported to be found in macrophage-rich human atherosclerotic plaques [[30], [31], [32], [33]]. In vitro studies have shown that the differentiation of THP-1 monocytes into macrophages induces ADAMTS1, 4, 5, and 8 expression, while ADAMTS9 is downregulated [30,31]. From a functional perspective, treatment of differentiated macrophages with key atherosclerotic cytokines reveals the anti-inflammatory and plaque-stabilizing activities of ADAMTS1 as compared to the pro-inflammatory and destabilizing properties of ADAMTS4, 7, 8 and 9 [30,31]. Using ApoE−/−Adamts4−/− double knockout mice, Kumar et al. have confirmed the increase in plaque vulnerability caused by ADAMTS4 [34]. Other studies have also shown that ADAMTS1 promotes atherogenesis and accelerates plaque progression [35,36], in contradiction with the above mentioned reference. Genome-wide association studies have highlighted the important role of the ADAMTS7 gene locus in coronary artery disease [37]. In addition, Adamts7-deficient mice presented a reduction in atherosclerosis [38] and co-localization with macrophages in advanced atherosclerosis and vulnerable human atherosclerotic plaques [33]. The contribution of ADAMTS proteases to atherosclerosis has been linked to the aggregating proteoglycan versican in the ECM of blood vessels. In studies of mice, this proteoglycan appears to be less proteolyzed in the absence of ADAMTS4, which correlates with lower macrophage infiltration levels and more stable plaques [34]. Patients with high levels of ADAMTS4 were also found to have lower levels of versican and more vulnerable atherosclerotic plaques [32].
Advances have also been made in aortic aneurysm and dissection (AAD), another major vascular disorder, following research into ADAMTS proteases. AAD is characterized by inflammatory cell infiltration and the rupture of the aortic wall, particularly at the level of the thorax and abdomen [39]. Patients with thoracic AAD present elevated levels of ADAMTS1 and ADAMTS4 [40,41], whose pro-inflammatory role has been confirmed in experimental AAD mouse models [42,43]. The genetic depletion of these ADAMTSs reduces macrophage migration both in vitro [40] and in vivo [42,43]. With some variations, an important study of Marfan syndrome, characterized by thoracic AAD, has shown reduced levels of ADAMTS1 in the aorta of these patients, in conjunction with an increase in nitric oxide synthase 2 (NOS2), a recognized marker of pro-inflammatory macrophages. This study also demonstrates that Adamts1 deficiency in mice boosts the aneurysm accompanied by NOS2 overexpression [44]. The contradictory promoter [43] or protective [44] role of ADAMTS1 in thoracic AAD shown in these models could be related to the timing of ADAMTS1 deficiency at the postnatal or embryonic stage, respectively, but also to the AAD-inducing agent used in each case. Finally, as with atherosclerosis, AAD is associated with the degradation of versican, whose proteolysis increases in patients due to the presence of high levels of versicanases [40,42].
Recent research into ischemic strokes has also revealed the important role of ADAMTSs with regard to their inflammatory components. It is worth noting that, while high levels of ADAMTS4 have been reported in the brain of stroke patients, several experimental approaches have highlighted its anti-inflammatory activity [45]. While treatment of LPS-activated astrocytes with human recombinant ADAMTS4 decreases nitric oxide (NO) production and pro-inflammatory cytokine secretion, the silencing of ADAMTS4 reverses these processes. Additional modeling of cerebral artery stroke occlusion in mice has shown that ADAMTS4 reduces macrophage infiltration and promotes an anti-inflammatory landscape in central nervous system (CNS) injuries [45]. Nevertheless, these experimental procedures were unable to provide a full understanding of the mode of action of the protease.
Many studies show that there is a close connection between vascular thrombotic disorders and the distinctive proteolytic effect of ADAMTS13 on von Willebrand factor (VWF), a major coagulation glycoprotein [46,47]. It is important to note that plasma from patients with traumatic brain injury (TBI) presents reduced ADAMTS13 activity, which is probably mediated by the release of cytokines under acute systemic inflammation, resulting in high levels of VWF [48]. The anti-inflammatory activity of this protease has been corroborated using Adamts13-deficient mice, which exhibit increased leukocyte adhesion in inflamed veins, as well as enhanced neutrophil extravasation, both critical features of inflammation and thrombosis in various pathologies [49]. ADAMTS13 could also make a significant therapeutic contribution to helping patients with autoimmune thrombotic thrombocytopenic purpura (TTP), in which splenic memory B cells produce anti-ADAMTS13 autoantibodies that trigger severe enzyme deficiency [47,50].
Spontaneous atopic dermatitis
A key advance was made with the study of the pro-collagenase activity of some ADAMTS proteases in skin disorders, which found that ADAMTS2-inactivating mutations result in the inherited connective tissue condition Ehlers–Danlos syndrome (EDS) type VIIC [51], characterized by extreme skin fragility. Colige et al., who confirmed this finding in Adamts2-deficient mice, also observed spontaneous epidermal lesions in 2-month-old Adamts2/Adamts14 double knockout mice, with extensive immune cell infiltration reported around the lesions [52]. These deficient mice had a more active and proliferating population of pro-inflammatory CD4+ T lymphocytes in the blood and spleen, suggesting that these proteases play a regulatory role in the immune system, although the authors note that mesenchymal cells are also significantly involved in this process [52]. From a therapeutic perspective, the use of glucocorticoids (GC) for wound repair stimulates peripheral blood mononuclear cells and induces ADAMTS2 in monocytes and macrophages [53]. These procedures confirm the beneficial role played by ADAMTS2 and could be applied to other pathologies.
Arthritis
ADAMTS proteases have also been found to have an impact on osteoarthritis (OA) and rheumatoid arthritis (RA), which are both characterized by cartilage erosion and damaged joints with systemic inflammation [54,55]. The term aggrecanases is widely used to describe ADAMTS proteases which have specific catalytic effects on aggrecan, the cartilage-specific proteoglycan core protein responsible for these pathologies. With regard to inflammation, the release of specific ADAMTS-cleaved aggrecan fragments appears to activate toll-like receptor 2 (TLR2) in in vitro OA models [56]. The accumulation of aggrecan resistant to aggrecanase activity also provided protection against cartilage erosion and inflammation in an OA mouse model [57]. Proteases, principally ADAMTS4 and ADAMTS5, have been the subject of intense study in order to develop novel therapeutic strategies based on specific protease inhibitors and blocking monoclonal antibodies. However, these strategies produce adverse cardiovascular side effects due not only to the presence of aggrecan which regulates the elasticity and stiffness of large vessels, but also to the existence of other proteolytic targets [55]. In addition, ADAMTS12 has been linked to both OA and RA particularly through its impact on the cartilage oligomeric matrix protein (COMP) [58,59].
Other immune-related diseases
It is important to note that ADAMTS5 acts as a regulator of immune responses to viral infections [60], a subject of particular relevance given the ongoing COVID-19 pandemic. McMahon et al., in their study of Adamts5-deficient mice infected with the influenza virus, show that the absence of ADAMTS5 delays virus clearance and hampers T cell migration and activation in the lung and spleen [60]. The authors also point out that the proteoglycan versican mediates deficient immune responses in the absence of ADAMTS5. This mechanism, as well as therapies to control virus-induced threats, requires further research.
Finally, studies on multiple sclerosis (MS), an inflammatory demyelinating disease caused by autoreactive T cell infiltration in the CNS, reveal reduced levels of ADAMTS13 in plasma of patients [61]. Lu et al. also found reduced ADAMTS13 activity in a related experimental autoimmune encephalomyelitis (EAE) mouse model [62]. Treatment with recombinant human ADAMTS13 led to a decrease in T cell, neutrophil and monocyte infiltration in the spinal cord, while peripheral inflammatory responses remained unaffected. These studies suggest that ADAMTS13 could be used in patients with MS and related diseases, although further research is needed to better understand the secondary effects of this treatment.
Beyond cancer: ADAMTS substrates and the immune system
In previous sections, we reviewed the direct and indirect involvement of certain ADAMTS substrates in different diseases. Below, we summarize studies in which major substrates are directly associated with the immune system, regardless of their capacity of be cleaved or degraded.
Versican
The large hyaluronan-binding proteoglycan versican, which plays a central role in immune system evasion and modulation, has been widely studied during development and adult life [63]. Apart from its proteolysis by ADAMTSs, considerable controversy surrounds the pro-inflammatory and immunosuppressive properties of versican. The accumulation of versican in the ECM has been described in the early stages of inflammation in a large number of human disorders such as atherosclerosis [34], aneurysms [55], lung infection [64,65], wound healing [66] and carcinogenesis [67]. Negatively charged chondroitin sulfate (CS) chains of versican release pro-inflammatory cytokines from macrophages and splenocytes, which regulate antigen presentation and T-cell activation [63]. The binding of versican to TLR2 on dendritic cells (DCs) also increases the release of interleukin 6 (IL6) and IL10, leading to cytotoxic T lymphocyte dysfunction [67].
Versican has been studied in great depth in tissue repair processes. On the one hand, an increase in versican deposition in mice leads to more rapid wound repair, a process mediated by higher recruitment of macrophages and CD4+ cells in the injured area [66], which has also been observed in lung inflammation [65] and systemic sclerosis patients [63]. Other studies have shown that the deletion of specific macrophage-derived versican in mice with induced lung inflammation increases inflammatory cell recruitment accompanied by reduced immunosuppressive cytokine expression [64]. Given these contradictory data, it has been suggested that the modulatory role of versican in immune responses could depend on the cellular source, although the involvement of proteolytic events, such as those induced by ADAMTSs, has not been addressed in these studies.
Syndecan 4
Various studies have highlighted the crucial role played by the transmembrane heparan sulfate proteoglycan syndecan 4 in immune reactions to allergens and in inflammation events in RA. Polte et al. have reported that syndecan 4 is needed to ensure DCs motility to drain lymph nodes and to promote the presence of activated antigen-specific T cells in an asthma model [68]. In a RA mouse model, Endo et al. have also shown that syndecan 4 is required to modulate B-cell migration, which affects the formation of germinal centers in lymph nodes and autoantibody production [69], while inflammation and RA incidence were attenuated [69]. As with versican, it is important to note that LPS-treated macrophages induce syndecan 4 expression [64], while experiments with Sdc4−/− mice demonstrate its role in limiting pulmonary inflammation [70]. Finally, syndecan 4 has been shown to enhance the efficacy of a Food and Drug Administration (FDA)-approved platelet-derived growth factor-BB (PDGF-BB) treatment for non-healing ulcers in diabetic patients. This heparan sulfate proteoglycan plays an immunomodulatory role in M2 macrophage polarization, thereby inhibiting excess inflammation which impedes proper wound closure [71].
Collagens
As mentioned above, ADAMTS2, 3 and 14 are responsible for converting procollagens into collagens (I, II and III) [11]. Although an overall balance between collagen synthesis and turnover is necessary to maintain tissue homeostasis, less is known about the direct role of collagens in inflammation. Major research carried out on tissue injury and fibrosis has recognized macrophages and fibroblasts as the main providers of collagen for tissue remodeling [[72], [73], [74]]. In addition, key collagen-dependent characteristics of the ECM such as density and stiffness are associated with modulating immune cell behavior. Recent studies have shown that high collagen density is associated with M2 macrophage polarization [75,76] and lower levels of cytotoxic T cell activation, especially with regard to collagen I [72,77,78].
Nidogens
The multi-domain glycoproteins, nidogens 1 and 2, regulate the assembly and adhesion of the basement membrane between cells and the ECM [79]. As with the proteolytic degradation of both these nidogens, current knowledge of their effects on the immune system is limited. Nevertheless, an earlier study did find that an increase in neutrophil adhesion and chemotaxis was mediated by the interaction of the Arg-Gly-Asp (RGD) domain of nidogen 1 [80]. Another interaction between soluble nidogen 1 and neutrophils, mediated by NKp44 receptors in this case, inhibits the cytotoxic activity of these immune cells [81]. In addition, Balzano et al. report that nidogen 1 ensures that pro-B cells, mostly localized in an IL7-enriched BM niche, are maintained in a dedifferentiated condition [82].
Thrombospondin 1
The glycoprotein thrombospondin 1 (THBS1), whose anti-angiogenic properties are modulated by ADAMTS1 cleavage [83], is involved in tissue repair and immune responses given its capacity to activate TGFβ, although the underlying mechanisms implicated are not fully understood [84,85]. The genetic depletion of Thbs1 in mice highlights its anti-inflammatory role which is mediated by TGFβ in models of pulmonary hypertension [86], diabetic nephropathy and fibrotic disease [85]. The functions of THBS1 are mediated by interactions with several receptors; for example, the binding of THBS1 to CD36 in macrophages and endothelial cells modulates inflammation by activating macrophages through TLR4 [84]. In a cutaneous inflammation model, THBS1 activity is mediated by CD47 expression in polymorphonuclear cells, which inhibits effector T cells, induces regulatory T cells and enhances T cell apoptosis [87]. These immunosuppressive effects are also boosted by an increase in the tolerance of DCs to antigens. While the inhibition of Thbs1 or Cd47 in DCs protects mice against bacterial meningitis [84], the interaction of Thbs1 with CD47 is also associated with improved immunological responses, triggering T cell activation and proliferation [88]. Finally, increased macrophage mobilization and M2 polarization observed in a systemic sclerosis model also suggest that THBS1 plays an immunosuppressive role, which was corroborated in patients with brachio-cervical inflammatory myopathy (BCIM) [89].
Von Willebrand factor
As mentioned previously, ADAMTS13-mediated VWF proteolysis has a major impact on inflammation and thrombotic processes in MS. The effects of VWF include leukocyte rolling and adhesion, as well as extravasation at infection and injury sites, a hallmark of inflammation. The direct and indirect means by which VWF modulates inflammation include other cell entities such as endothelium and platelets [90]. Different experimental approaches, as well as the use of anti-VWF antibodies and human VWF infusion, have confirmed that the absence of VWF reduces leukocyte rolling and compromises the specific recruitment of monocytes in atherosclerosis and other inflammatory cues [91]. VWF commonly affects vascular permeability, while the complexity of its pro-inflammatory mechanisms is reflected in the involvement of integrins, cell-adhesion molecules and other interacting partners.
ADAMTS activity in tumors: an update
After delving into the literature regarding ADAMTS proteases and their known substrates in non-oncological immune-related processes, in this section, we focus on the links between ADAMTSs and cancer. Fortunately, there are comprehensive reviews of this subject [8,10,11,92,93], in addition to elaborate studies of ECM remodeling and cancer that are worth citing [1,3,94].
As highlighted in the above-mentioned reviews, gene expression studies and experimental approaches have described the pro- and anti-tumorigenic properties of ADAMTS. However, over the last five years, a new series of gene expression and correlation-based studies have revealed the increase and decrease of ADAMTS proteases in different tumor types, aspects of the immune microenvironment and novel mechanistic insights. This new research has placed particular emphasis on the pro-tumorigenic and metastatic effects of ADAMTS1 in breast [95], ovarian [96], and renal [97] carcinomas, and on the induction of stemness and endothelial-like features in the early stages of uveal melanoma [19], which is in line with previous studies of tumor plasticity [98]. Recent analyses of Adamts1-deficient mice in a syngeneic tumor model have also revealed that Adamts1 plays a major role in tumor stroma and the immune system [18,21]. ADAMTS4 [99,100], ADAMTS5 [101] and ADAMTS12 [102] have been found upregulated in gastrointestinal cancers such as colorectal cancer (CRC), which correlates with tumor progression and poor clinical outcomes. However, the ADAMTS9 gene, which acts as a tumor suppressor, appears to be considerably silenced in CRC tumors [103]. Likewise, in functional assays of gastric cancer, the ADAMTS5 gene acts as a tumor suppressor [104], while ADAMTS18 has an anti-tumorigenic impact on lung [105] and breast [106] cancers in a tumor-specific methylation pattern.
Furthermore, specific ADAMTS substrates such as versican have a determining influence on tumor progression. The contribution of this proteoglycan, in its intact and cleaved forms, has been highlighted in multiple scenarios. For example, Ricciardelli et al. have reported the Adamts1-dependent accumulation of cleaved versican in stromal components of breast tumors, suggesting that cleaved versican promotes cancer cell motility and progression, which, in turn, affects tumor grade and metastatic burden [107]. Using a different mouse model with several tumor cell lines, other authors have reported that total versican promotes tumor growth and angiogenesis, although a distinction is made between the stromal localization of intact versican and that of ADAMTS-generated fragments, which are mainly found in the tumor vascular endothelium [108]. Other studies have suggested that versican could be therapeutically targeted in uterine leiomyoma, given the increased levels of cleaved versican found in this type of tumor, which correlates with the upregulation of ADAMTS4 and ADAMTS15 [109]. However, ADAMTS15 has been reported to be involved in protease-dependent tumor suppression in prostate cancer, with both intact and cleaved forms of versican playing a balanced role [110]. These findings, together with the known involvement of versican in the immune system, highlight the importance of this research.
The dual role of ADAMTS1 during tumorigenesis is also associated with ECM substrates such as semaphorin 3C, which promotes a migratory phenotype in breast cancer cells [111], and basement membrane components nidogens 1 and 2, whose proteolysis is linked to anti-tumorigenic ADAMTS1 activity [16]. Studies of the transmembrane syndecan 4, a substrate of ADAMTS1 and 4 [112], also revealed a complex scenario with multiple fronts. Edwards et al. report that syndecan 4 mediates the effects on cell migration and angiogenesis of ADAMTS1 [113] and ADAMTS15 [114]. The presence of co-factors also modulate these processes in different cellular models, such as fibulin 1 in the case of ADAMTS1 [113,115], or fibulin 2 for ADAMTS12, whose interaction has a tumor-suppressive effect on breast cancer cells [116]. Significantly, this second interaction protects the ECM glycoprotein fibulin 2 against proteolysis by ADAMTS5 [117]. This study suggests that the cleavage of fibulin 2 by ADAMTS5 promotes the invasive breast cancer cell phenotype. In general, these reports highlight slight differences between the pro- and anti-tumorigenic properties of the proteases mentioned above, depending on their specific TME.
The proteolytic activities of ADAMTS4 and ADAMTS5 have also been the subject of much analysis in glioma studies. The high levels of ADAMTS4 and ADAMTS5 in glioblastoma samples [118,119] clearly correlate with ADAMTS-cleaved brain-enriched hyaluronan binding (BEHAB)/brevican which induces glioma cell aggressiveness and invasiveness [[120], [121], [122], [123]]. Both ADAMTS4 and ADAMTS5 also cleave the small leucine-rich proteoglycan (SLRP) biglycan [124], whose elevated expression levels in tumor vessels may contribute to the high motility of endothelial tumor cells [125]. Asano et al. have reported that the inhibitory effect of ADAMTS4 in the early stages of tumor development does not match biglycan expression in tumor vessels, which suggests the presence of other substrates [126].
ADAMTS activities in the tumor immune microenvironment and future perspectives
To date, few studies have described the direct involvement of ADAMTSs in tumor immune response. In this section, we assess the involvement of these proteases in the tumor immune microenvironment and their immunomodulatory role during the infiltration and polarization of specific immune cell populations (Fig. 1).
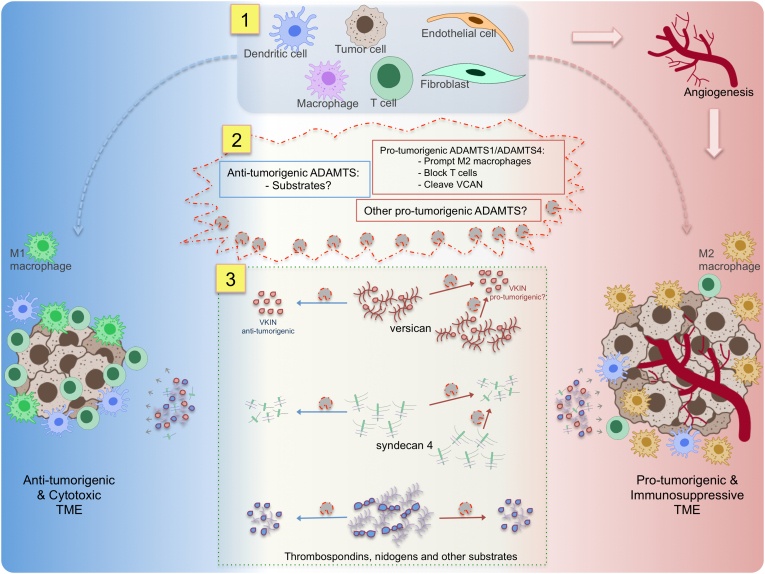
Fig. 1.
Effects of ADAMTSs on the tumor immune microenvironment. In the complex TME, a fine balance exists between immune cytotoxic anti-tumorigenic and immunosuppressive pro-tumorigenic phenotypes (left and right sides in the figure, respectively). In addition to various immune cell populations, the TME includes cellular entities (top, section 1), such as fibroblasts, endothelial and tumor cells that constantly contribute to the complex extracellular environment through the secretion of ECM proteases (middle, section 2) and ECM components, including ADAMTS substrates (bottom, section 3). Among proteases, ADAMTS1 and ADAMTS4 have been identified as promoting immunosuppression, prompting M2 polarization and shaping other immune cell populations. These proteases cleave versican, an immunomodulatory substrate. Overall, ADAMTSs affect the regulatory capacities of ECM molecules including versican, syndecan 4 and other substrates. The function of these ECM proteins, in intact or cleaved forms, which remains controversial, depends on their location and partnership profiles. Some ADAMTS proteases are also involved in angiogenesis and other vital mechanisms which may contribute to overall tumor progression. As these functions are still under discussion, a full understanding of ADAMTS activity would open up new therapeutic and diagnostic possibilities.
The close relationship between tumorigenesis and chronic inflammation has been the subject of intense research for many years. In a worst case scenario, the tumor ECM contributes to immunosuppressive activity over time which sustains immune escape mechanisms in cancers, leading to a worsening of the prognosis and reduced survival rates. In terms of inflammation, tumors have been roughly classified into hot tumors, which have a better overall prognosis and higher recruitment of CD8+ cytotoxic T cells, B cells and macrophages, and into cold tumors, with poor T cell recruitment and weak innate immune responses, leading to immune failure [127]. One of the main goals of therapeutic strategies for combating cancer [128] is to convert these cold tumors into hot tumors. To accomplish this goal, a full understanding of the proteolytic activity involved in the ECM is critical in order to manage the immunomodulatory capacity of complex TMEs [5,24].
To our knowledge, this crosstalk with immune cells has only been described in relation to ADAMTS1 and ADAMTS4, which have already been discussed in non-oncological settings and whose roles in macrophage differentiation and migration bear many similarities. It is worth noting that these proteases appear to mediate the regulation of macrophages and other cell populations, such as T cells and DCs, in tumor settings.
While the mouse homolog was originally found to be an inflammatory-related molecule [7], a closer study of ADAMTS1 functions in tumor immune infiltrates was not carried out until more than a decade later using Adamts1-knockout mice. Ricciardelli et al. report that the presence of ADAMTS1, which is necessary for tumor progression and metastasis in a spontaneous murine breast cancer model [107], correlates with reduced infiltration of CD45+ leukocytes and increased levels of cleaved versican. Although specific cell populations such as CD3+ T cells and F4/80+ macrophages remained stable, tumors in Adamts1-deficient mice switched to a Th1 phenotype, suggesting that cytotoxic host responses block tumor growth [107]. Using a syngeneic B16F1 melanoma model in Adamts1 knockout mice, a more recent study revealed a different immune output but with comparable consequences for overall tumor growth [21]. This study also showed a reduction in tumor volume in the absence of host Adamts1, as well as considerable changes in immune infiltrates. Major cell populations, such as CD3+ lymphocytes and CD11b+ macrophages, were found to increase in tumors in Adamts1-deficient mice, which had experienced decreased tumor growth. In addition, M2 macrophage marker Cd163 was also downregulated in a less immunosuppressive tumor microenvironment in the absence of stromal Adamts1. Unlike the above-mentioned breast tumor model, with lower levels of cleaved versican in Adamts1-deficient tumors [107], this more recent study showed reduced deposition of both intact and cleaved versican in Adamts1 knockout mouse tumors [21]. This overall reduction in versican reveals a distinct scenario, suggesting the involvement of other proteases. It is also worth noting that distant organs such as the spleen and bone marrow in Adamts1-knockout mice exhibited an increased presence of CD3+ and myeloid cells [21], changes which reflect the multifaceted and systemic immunomodulatory role of ADAMTS1.
The involvement of ADAMTS4 in modulating immune tumor infiltrates is associated with its pro-tumorigenic properties and higher expression in CRC patients with a poorer prognosis [99,100]. In addition, the close correlation between ADAMTS4 gene expression and macrophage markers CD68 and CD163 highlights the immunosuppressive role of this protease [100]. While the inhibition of ADAMTS4 attenuates xenograft tumor growth, its overexpression induces larger tumors together with increased infiltration of macrophages.
The phylogenetically close proteases, ADAMTS1 and ADAMTS4 [9], proteolyze similar substrates, such as certain proteoglycans which play a modulatory role in immunity and cancer-associated inflammation [14,127]. These proteoglycans include versican, which has both pro-tumorigenic [108] and anti-tumorigenic properties [129]. As mentioned above, intact and ADAMTS-proteolyzed forms of versican are widely associated with immune regulation. Kim et al. have shown that versican, a key molecule secreted in lung cancers, is responsible for activating macrophages through TLR2 which then promote metastasis [130]. Increased levels of versican, which is also present in F4/80+ macrophages in MDA-MB-231-derived tumors [108], correlate with an accumulation of pro-tumorigenic tumor-associated macrophages (TAMs) in different models [108,131,132]. The immunomodulatory capacity of versican also affects T lymphocytes in cervical cancer patients [133], with increased levels of versican correlating with a reduction in intraepithelial T cell infiltration, particularly of CD8+ cells.
Given these complex effects, studies of versican proteolysis have clarified many aspects of its immunomodulatory role [63,134]. In myeloma, while intact versican has tolerogenic properties through TLR2 binding, versican proteolysis generates N-terminal versikine fragments that attract CD8+ T cells [23,135]. Treatment with versikine from BM-derived TAMs in myeloma patients induces inflammatory cytokines such as IL1β or IL6. Research shows that versican is mainly produced by myeloid cells, while ADAMTS1 is expressed in stromal cells, suggesting that cell-to-cell crosstalk regulates versikine production and the tolerogenic properties of intact versican [23]. In CRC, the presence of versikine appears to be essential for T cell trafficking and antitumor responses, which enhance CD8+ cell accumulation and promote conventional DC differentiation in BM, while intact versican blocks these processes [22]. Current findings regarding the pro-tumorigenic properties of ADAMTS1 and ADAMTS4 appear to contradict those relating to the intact and cleaved forms of versican, suggesting that a more comprehensive analysis in different settings is required.
Like versican, heparan sulfates have been reported to affect DC maturation and CD8+ T cell responses [136], with the involvement of transmembrane syndecans also being reported [127]. Studies of Lewis lung carcinoma (LLC) syngeneic tumors in syndecan 4-deficient mice show higher levels of mature DCs and natural killer cells with robust antitumor activity, accompanied by enhanced CD8+ T cell responses [137,138]. As none of these studies have examined the effect of proteolytic activity on syndecans, further research could provide a better understanding of this finely tuned regulatory process.
As mentioned above, the immunomodulatory properties of collagens have been studied without reference to the effect of ADAMTSs on pro-collagen molecules. In the early 1980s, using co-cultures on collagen type I-coated plates, Henry et al. observed crosstalk between macrophages and carcinoma cells. While tumor cells induce collagen degradation by releasing collagenase, these cells are also affected by factors secreted by macrophages [139]. In addition, the ways in which immune cells are modulated by collagen in cancer patients continue to be the subject of considerable research. For example, collagen promotes the differentiation of monocytes into macrophages, with an enhanced M2 phenotype, and DCs [72]. High density collagen correlates with increased infiltration of TAMs in breast cancer samples, although additional research is required to design new therapies [140]. High-density collagen matrices also reduce T cell proliferation, leading to an increase in the ratio of CD4+ to CD8+ T cells, in which cytotoxic markers are downregulated and tumoricidal activity is blocked [78]. However, the specific role of pro-collagenase ADAMTSs in these complex regulatory mechanisms, previously described in non-oncological disorders, remains unexplored given the difficulty of analyzing their involvement in earlier collagen maturation events. Nevertheless, a study of the anti-inflammatory capacities of pro-collagenases in high-density collagen tumors associated with worse prognoses should lead to the discovery of important regulatory mechanisms.
This updated review of the literature highlights the vital importance of studying the regulation of proteolysis by different ADAMTSs together with other components of the tumor immune microenvironment. Great strides have also been made in the study of appropriate treatments [141] and in the promising use of immunotherapies and checkpoint inhibitors [142]. For example, we have described how the impact of collagen density [78] and proteolytic activity on versican [63,135] modulates the status and function of major immune cell populations such as T cells and macrophages [77]. Analysis of the tumor ECM in patients would be an effective way of assessing prognoses and ensuring the success of cancer therapies, making the evaluation of the tumor immune microenvironment an emerging tool in personalized medicine [2,141].
With regard to proteases-related pathways, scenarios such as the close connection between ADAMTSs and substrates including syndecans, thrombospondins and nidogens, remain unexplored. Despite studies of their involvement in immune and inflammatory responses, key functional features of proteases and substrates are little known mainly due to the lack of functional information regarding their cleaved fragments [143]. Advanced technologies should enable post-translational changes to be identified.
New therapies, including the use of specific peptides and proteolytic fragments to mimic or block the immunomodulatory capacities of ADAMTS substrates, are being developed. However, determining how the TME is affected and targeted by different therapeutic regimes presents a formidable challenge to researchers. Analysis of the immune tumor landscape, both before and after treatment, should provide valuable information in the context of these complex scenarios [144] in order to target the TME and neutralize resistance to anticancer drugs.
Conclusion
The hallmarks of cancer are directly influenced by the biochemical and biomechanical properties of the tumor ECM, whose inflammatory phenotype is also affected. During these processes, the heterogeneous nature of the TME either improves or worsens the prognosis and also contributes to overcoming the effects of anti-tumorigenic treatments. Significant studies have demonstrated the dynamism of the ECM, which exerts both pro- and anti-tumorigenic actions, including its modification by enzymes such as ADAMTS and other proteases. Still, these elements need to be explored in greater depth in order to ensure more effective therapeutic outcomes, including the identification of the specific cell populations producing them. While many questions remain unanswered with regard to how ADAMTS proteases modulate the ECM, this review of the current literature on inflammatory and immune-related settings brings together important information in order to shed light on the tumor immune microenvironment and to open up new fronts in the treatment of cancer.
Abbreviations
- AAD
aortic aneurysm and dissection
- ADAMs
a disintegrin and metalloproteinase
- ADAMTSs
a disintegrin and metalloproteinase with thrombospondin motifs
- BCIM
brachio-cervical inflammatory myopathy
- BM
bone marrow
- CNS
central nervous system
- COMP
cartilage oligomeric matrix protein
- CRC
colorectal cancer
- CS
chondroitin sulfate
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- ECM
extracellular matrix
- EDS
Ehlers-Danlos syndrome
- GC
glucocorticoids
- LPS
lipopolysaccharide
- MMPs
matrix metalloproteinases
- MS
multiple sclerosis
- NO
nitric oxide
- NOS2
nitric oxide synthase 2
- OA
osteoarthritis
- PDGF-BB
platelet derived growth factor-BB
- RA
rheumatoid arthritis
- SLRP
small leucine-rich proteoglycan
- TAM
tumor-associated macrophage
- TBI
traumatic brain injury
- THBS1
thrombospondin 1
- TLR2
toll-like receptor 2
- TSR
thrombospondin type I repeat
- TTP
thrombocytopenia purpura
- TME
tumor microenvironment
- VWF
Von Willebrand factor
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This research was funded by Ministerio de Economía y Competitividad and Instituto de Salud Carlos III from Spain, co-financed by FEDER (PI16/00345 to JCRM), by Ministerio de Ciencia, Innovación y Universidades from Spain (PID2019-104416RB-I00), and by Consejería de Salud, Junta de Andalucía (OH-0028-2018, PE-0225-2018, both to JCRM).
References
- 1.Pickup M.W., Mouw J.K., Weaver V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Reports. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., Vonderheide R.H., Pittet M.J., Jain R.K., Zou W., Howcroft T.K., Woodhouse E.C., Weinberg R.A., Krummel M.F. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karamanos N.K., Theocharis A.D., Neill T., Iozzo R.V. Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases. Matrix Biology. 2019;75–76:1–11. doi: 10.1016/j.matbio.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorokin L. The impact of the extracellular matrix on inflammation. Nature Reviews. Immunology. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 5.Vaday G.G., Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. Journal of Leukocyte Biology. 2000;67:149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- 6.Coussens L.M., Fingleton B., Matrisian L.M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 7.Kuno K., Kanada N., Nakashima E., Fujiki F., Ichimura F., Matsushima K., Nakashirma E., Fujiki F., Ichimura F., Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. The Journal of Biological Chemistry. 1997;272:556–562. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- 8.Cal S., López-Otín C. ADAMTS proteases and cancer. Matrix Biology. 2015;44–46:77–85. doi: 10.1016/j.matbio.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Apte S.S. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. The International Journal of Biochemistry & Cell Biology. 2004;36:981–985. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Manzaneque J.C., Fernández-Rodríguez R., Rodríguez-Baena F.J., Iruela-Arispe M.L. ADAMTS proteases in vascular biology. Matrix Biology. 2015;44–46:38–45. doi: 10.1016/j.matbio.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter S., Clark I.M., Kevorkian L., Edwards D.R. The ADAMTS metalloproteinases. Biochemical Journal. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mead T.J., Apte S.S. ADAMTS proteins in human disorders. Matrix Biology. 2018;71–72:225–239. doi: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubail J., Apte S.S. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biology. 2015;44–46:24–37. doi: 10.1016/j.matbio.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Theocharis A.D., Karamanos N.K. Proteoglycans remodeling in cancer: underlying molecular mechanisms. Matrix Biology. 2019;75–76:220–259. doi: 10.1016/j.matbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Lo P.H.Y., Lung H.L., Cheung A.K.L., Apte S.S., Chan K.W., Kwong F.M., Ko J.M.Y., Cheng Y., Law S., Srivastava G., Zabarovsky E.R., Tsao S.W., Tang J.C.O., Stanbridge E.J., Lung M.L. Extracellular protease ADAMTS9 suppresses esophageal and nasopharyngeal carcinoma tumor formation by inhibiting angiogenesis. Cancer Research. 2010;70:5567–5576. doi: 10.1158/0008-5472.CAN-09-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino-Echarri E., Fernández-Rodríguez R., Rodríguez-Baena F.J., Barrientos-Durán A., Torres-Collado A.X., del Carmen Plaza-Calonge M., Amador-Cubero S., Cortés J., Reynolds L.E., Hodivala-Dilke K.M., Rodríguez-Manzaneque J.C. Contribution of ADAMTS1 as a tumor suppressor gene in human breast carcinoma. Linking its tumor inhibitory properties to its proteolytic activity on nidogen-1 and nidogen-2. International Journal of Cancer. 2013;133:2315–2324. doi: 10.1002/ijc.28271. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S., Sharghi-Namini S., Rao N., Ge R. ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. The American Journal of Pathology. 2012;181:1056–1068. doi: 10.1016/j.ajpath.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Rodríguez R., Rodríguez-Baena F.J., Martino-Echarri E., Peris-Torres C., del Carmen Plaza-Calonge M., Rodríguez-Manzaneque J.C. Stroma-derived but not tumor ADAMTS1 is a main driver of tumor growth and metastasis. Oncotarget. 2016;7:34507–34519. doi: 10.18632/oncotarget.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peris-Torres C., del C. Plaza-Calonge M., López-Domínguez R., Domínguez-García S., Barrientos-Durán A., Carmona-Sáez P., Rodríguez-Manzaneque J.C. Extracellular protease ADAMTS1 is required at early stages of human uveal melanoma development by inducing stemness and endothelial-like features on tumor cells. Cancers (Basel) 2020;12:801. doi: 10.3390/cancers12040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Hour M., Moncada-Pazos A., Blacher S., Masset A., Cal S., Berndt S., Detilleux J., Host L., Obaya A.J., Maillard C., Foidart J.M., Ectors F., Noel A., Lopez-Otin C. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene. 2010;29:3025–3032. doi: 10.1038/onc.2010.49. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Baena F.J., Redondo-García S., Peris-Torres C., Martino-Echarri E., Fernández-Rodríguez R., del C. Plaza-Calonge M., Anderson P., Rodríguez-Manzaneque J.C. ADAMTS1 protease is required for a balanced immune cell repertoire and tumour inflammatory response. Scientific Reports. 2018;8:13103. doi: 10.1038/s41598-018-31288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hope C., Emmerich P.B., Papadas A., Pagenkopf A., Matkowskyj K.A., Van De Hey D.R., Payne S.N., Clipson L., Callander N.S., Hematti P., Miyamoto S., Johnson M.G., Deming D.A., Asimakopoulos F. Versican-derived Matrikines regulate Batf3–dendritic cell differentiation and promote T cell infiltration in colorectal Cancer. Journal of Immunology. 2017;199:1933–1941. doi: 10.4049/jimmunol.1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hope C., Foulcer S., Jagodinsky J., Chen S.X., Jensen J.L., Patel S., Leith C., Maroulakou I., Callander N., Miyamoto S., Hematti P., Apte S.S., Asimakopoulos F. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood. 2016;128:680–685. doi: 10.1182/blood-2016-03-705780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badylak S.F. Extracellular matrix and the immune system: friends or foes. Nature Reviews. Urology. 2019;16:389–390. doi: 10.1038/s41585-019-0196-0. [DOI] [PubMed] [Google Scholar]
- 25.Oveland E., Karlsen T.V., Haslene-Hox H., Semaeva E., Janaczyk B., Tenstad O., Wiig H. Proteomic evaluation of inflammatory proteins in rat spleen interstitial fluid and lymph during LPS-induced systemic inflammation reveals increased levels of ADAMST1. Journal of Proteome Research. 2012;11:5338–5349. doi: 10.1021/pr3005666. [DOI] [PubMed] [Google Scholar]
- 26.Moncada-Pazos A., Obaya A.J., Llamazares M., Heljasvaara R., Suárez M.F., Colado E., Noël A., Cal S., López-Otín C. ADAMTS-12 metalloprotease is necessary for normal inflammatory response. The Journal of Biological Chemistry. 2012;287:39554–39563. doi: 10.1074/jbc.M112.408625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulissen G., El Hour M., Rocks N., Guéders M.M., Bureau F., Foidart J.-M., Lopez-Otin C., Noel A., Cataldo D.D. Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12. Journal of Immunology. 2012;189:4135–4143. doi: 10.4049/jimmunol.1103739. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez F., Hastings G., Ortega M.-A.A., Lane T.F.T.F., Oikemus S., Lombardo M., Iruela-Arispe M.L.L., Vázquez F., Hastings G., Ortega M.-A.A., Lane T.F.T.F., Oikemus S., Lombardo M., Iruela-Arispe M.L.L. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. The Journal of Biological Chemistry. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- 29.Van Hinsbergh V.W.M. Endothelium - role in regulation of coagulation and inflammation. Seminars in Immunopathology. 2012;34:93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wågsäter D., Björk H., Zhu C., Björkegren J., Valen G., Hamsten A., Eriksson P. ADAMTS-4 and -8 are inflammatory regulated enzymes expressed in macrophage-rich areas of human atherosclerotic plaques. Atherosclerosis. 2008;196:514–522. doi: 10.1016/j.atherosclerosis.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Ashlin T.G., Kwan A.P.L., Ramji D.P. Regulation of ADAMTS-1, -4 and -5 expression in human macrophages: differential regulation by key cytokines implicated in atherosclerosis and novel synergism between TL1A and IL-17. Cytokine. 2013;64:234–242. doi: 10.1016/j.cyto.2013.06.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong H., Du T., Premaratne S., Zhao C.X., Tian Q., Li Y., Yan S., Zhang W.W. Relationship between ADAMTS4 and carotid atherosclerotic plaque vulnerability in humans. Journal of Vascular Surgery. 2018;67:1120–1126. doi: 10.1016/j.jvs.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 33.Bengtsson E., Hultman K., Dunér P., Asciutto G., Almgren P., Orho-Melander M., Melander O., Nilsson J., Hultgårdh-Nilsson A., Gonçalves I. ADAMTS-7 is associated with a high-risk plaque phenotype in human atherosclerosis. Scientific Reports. 2017;7:3753. doi: 10.1038/s41598-017-03573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S., Chen M., Li Y., Wong F.H.S., Thiam C.W., Hossain M.Z., Poh K.K., Hirohata S., Ogawa H., Angeli V., Ge R. Loss of ADAMTS4 reduces high fat diet-induced atherosclerosis and enhances plaque stability in ApoE(−/−) mice. Scientific Reports. 2016;6:31130. doi: 10.1038/srep31130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jönsson-Rylander A.-C., Nilsson T., Fritsche-Danielson R., Hammarström A., Behrendt M., Andersson J.-O., Lindgren K., Andersson A.-K., Wallbrandt P., Rosengren B., Brodin P., Thelin A., Westin A., Hurt-Camejo E., Lee-Søgaard C.-H. Role of ADAMTS-1 in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:180–185. doi: 10.1161/01.ATV.0000150045.27127.37. [DOI] [PubMed] [Google Scholar]
- 36.Ong M.-H.-L., Wong H.-K., Tengku-Muhammad T.-S., Choo Q.-C., Chew C.-H. Pro-atherogenic proteoglycanase ADAMTS-1 is down-regulated by lauric acid through PI3K and JNK signaling pathways in THP-1 derived macrophages. Molecular Biology Reports. 2019;46:2631–2641. doi: 10.1007/s11033-019-04661-6. [DOI] [PubMed] [Google Scholar]
- 37.Reilly M.P., Li M., He J., Ferguson J.F., Stylianou I.M., Mehta N.N., Burnett M.S., Devaney J.M., Knouff C.W., Thompson J.R., Horne B.D., Stewart A.F.R., Assimes T.L., Wild P.S., Allayee H., Nitschke P.L., Patel R.S., Martinelli N., Girelli D., Quyyumi A.A., Anderson J.L., Erdmann J., Hall A.S., Schunkert H., Quertermous T., Blankenberg S., Hazen S.L., Roberts R., Kathiresan S., Samani N.J., Epstein S.E., Rader D.J. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: Two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer R.C.C., Tohyama J., Cui J., Cheng L., Yang J., Zhang X., Ou K., Paschos G.K.K., Zheng X.L.L., Parmacek M.S.S., Rader D.J.J., Reilly M.P.P. Knockout of Adamts7 , a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation. 2015;131:1202–1213. doi: 10.1161/CIRCULATIONAHA.114.012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quintana R.A., Taylor W.R. Introduction to the compendium on aortic aneurysms. Circulation Research. 2019;124:470–471. doi: 10.1161/CIRCRESAHA.119.314765. [DOI] [PubMed] [Google Scholar]
- 40.Ren P., Zhang L., Xu G., Palmero L.C., Albini P.T., Coselli J.S., Shen Y.H., LeMaire S.A. ADAMTS-1 and ADAMTS-4 levels are elevated in thoracic aortic aneurysms and dissections. The Annals of Thoracic Surgery. 2013;95:570–577. doi: 10.1016/j.athoracsur.2012.10.084.ADAMTS-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y., Wu W., Yu C., Zhong F., Li G., Kong W., Zheng J. A disintegrin and metalloproteinase with thrombospondin motif 1 (ADAMTS1) expression increases in acute aortic dissection. Science China. Life Sciences. 2016;59:59–67. doi: 10.1007/s11427-015-4959-4. [DOI] [PubMed] [Google Scholar]
- 42.Ren P., Hughes M., Krishnamoorthy S., Zou S., Zhang L., Wu D., Zhang C., Curci J.A., Coselli J.S., Milewicz D.M., LeMaire S.A., Shen Y.H. Critical role of ADAMTS-4 in the development of sporadic aortic aneurysm and dissection in mice. Scientific Reports. 2017;7:12351. doi: 10.1038/s41598-017-12248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S., Liu Y., Zhao G., He L., Fu Y., Yu C., Wang Z., Zhao T., Cao F., Gao Y., Kong W., Zheng J. Postnatal deficiency of ADAMTS1 ameliorates thoracic aortic aneurysm and dissection in mice. Experimental Physiology. 2018;103:1717–1731. doi: 10.1113/EP087018. [DOI] [PubMed] [Google Scholar]
- 44.Oller J., Méndez-Barbero N., Ruiz E.J., Villahoz S., Renard M., Canelas L.I., Briones A.M., Alberca R., Lozano-Vidal N., Hurlé M.A., Milewicz D., Evangelista A., Salaices M., Nistal J.F., Jiménez-Borreguero L.J., De Backer J., Campanero M.R., Redondo J.M. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nature Medicine. 2017;23:200–212. doi: 10.1038/nm.4266. [DOI] [PubMed] [Google Scholar]
- 45.Lemarchant S., Dunghana H., Pomeshchik Y., Leinonen H., Kolosowska N., Korhonen P., Kanninen K.M., García-Berrocoso T., Montaner J., Malm T., Koistinaho J. Anti-inflammatory effects of ADAMTS-4 in a mouse model of ischemic stroke. Glia. 2016;64:1492–1507. doi: 10.1002/glia.23017. [DOI] [PubMed] [Google Scholar]
- 46.Fujikawa K., Suzuki H., McMullen B., Chung D. Purification of human von Willebrand factor–cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001;98:1662–1666. doi: 10.1182/blood.V98.6.1662. [DOI] [PubMed] [Google Scholar]
- 47.Zheng X., Chung D., Takayama T.K., Majerus E.M., Sadler J.E., Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic Purpura. The Journal of Biological Chemistry. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 48.Kumar M.A., Cao W., Pham H.P., Raju D., Nawalinski K., Maloney-Wilensky E., Schuster J., Zheng X.L. Relative deficiency of plasma A disintegrin and metalloprotease with thrombospondin type 1 repeats 13 activity and elevation of human neutrophil peptides in patients with traumatic brain injury. Journal of Neurotrauma. 2019;36:222–229. doi: 10.1089/neu.2018.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chauhan A.K., Kisucka J., Brill A., Walsh M.T., Scheiflinger F., Wagner D.D. ADAMTS13: a new link between thrombosis and inflammation. The Journal of Experimental Medicine. 2008;205:2065–2074. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas M.R., de Groot R., Scully M.A., Crawley J.T.B. Pathogenicity of anti-ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. EBioMedicine. 2015;2:942–952. doi: 10.1016/j.ebiom.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colige A., Nuytinck L., Hausser I., van Essen A.J., Thiry M., Herens C., Adès L.C., Malfait F., De Paepe A., Franck P., Wolff G., Oosterwijk J.C., Sillevis Smitt J.H., Lapière C.M., Nusgens B.V. Novel types of mutation responsible for the dermatosparactic type of Ehlers–Danlos syndrome (type VIIC) and common polymorphisms in the ADAMTS2 gene. The Journal of Investigative Dermatology. 2004;123:656–663. doi: 10.1111/j.0022-202X.2004.23406.x. [DOI] [PubMed] [Google Scholar]
- 52.Dupont L., Ehx G., Chantry M., Monseur C., Leduc C., Janssen L., Cataldo D., Thiry M., Jerome C., Thomassin J.-M., Nusgens B., Dubail J., Baron F., Colige A. Spontaneous atopic dermatitis due to immune dysregulation in mice lacking Adamts2 and 14. Matrix Biology. 2018;70:140–157. doi: 10.1016/j.matbio.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Hofer T.P.J., Frankenberger M., Mages J., Lang R., Hoffmann R., Colige A., Ziegler-Heitbrock L. Tissue-specific induction of ADAMTS2 in monocytes and macrophages by glucocorticoids. Journal of Molecular Medicine. 2008;86:323–332. doi: 10.1007/s00109-007-0284-0. [DOI] [PubMed] [Google Scholar]
- 54.Verma P., Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. Journal of Cellular Biochemistry. 2011;112:3507–3514. doi: 10.1002/jcb.23298. [DOI] [PubMed] [Google Scholar]
- 55.Santamaria S. ADAMTS-5: a difficult teenager turning 20. International Journal of Experimental Pathology. 2020;101:4–20. doi: 10.1111/iep.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma N., Drobinski P., Kayed A., Chen Z., Kjelgaard-Petersen C.F., Gantzel T., Karsdal M.A., Michaelis M., Ladel C., Bay-Jensen A.C., Lindemann S., Thudium C.S. Inflammation and joint destruction may be linked to the generation of cartilage metabolites of ADAMTS-5 through activation of toll-like receptors. Osteoarthritis and Cartilage. 2020;28:658–668. doi: 10.1016/j.joca.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Little C.B., Meeker C.T., Golub S.B., Lawlor K.E., Farmer P.J., Smith S.M., Fosang A.J. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. The Journal of Clinical Investigation. 2007;117:1627–1636. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C.J., Kong W., Xu K., Luan Y., Ilalov K., Sehgal B., Yu S., Howell R.D., Di Cesare P.E. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. The Journal of Biological Chemistry. 2006;281:15800–15808. doi: 10.1074/jbc.M513433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nah S.S., Lee S., Joo J., Kim H.K., Sohn D.R., Kwon J.T., Woo K.M., Hong S.J., Kim H.J. Association of ADAMTS12 polymorphisms with rheumatoid arthritis. Molecular Medicine Reports. 2012;6:227–231. doi: 10.3892/mmr.2012.867. [DOI] [PubMed] [Google Scholar]
- 60.McMahon M., Ye S., Izzard L., Dlugolenski D., Tripp R.A., Bean A.G.D., McCulloch D.R., Stambas J. ADAMTS5 is a critical regulator of virus-specific T cell immunity. PLoS Biology. 2016;14 doi: 10.1371/journal.pbio.1002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziliotto N., Bernardi F., Jakimovski D., Baroni M., Marchetti G., Bergsland N., Ramasamy D.P., Weinstock-Guttman B., Schweser F., Zamboni P., Ramanathan M., Zivadinov R. Hemostasis biomarkers in multiple sclerosis. European Journal of Neurology. 2018;25:1169–1176. doi: 10.1111/ene.13681. [DOI] [PubMed] [Google Scholar]
- 62.Lu K., Liu L., Xu X., Zhao F., Deng J., Tang X., Wang X., Zhao B.Q., Zhang X., Zhao Y. ADAMTS13 ameliorates inflammatory responses in experimental autoimmune encephalomyelitis. Journal of Neuroinflammation. 2020;17:67. doi: 10.1186/s12974-020-1713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wight T.N., Kang I., Evanko S.P., Harten I.A., Chang M.Y., Pearce O.M.T., Allen C.E., Frevert C.W. Versican—a critical extracellular matrix regulator of immunity and inflammation. Frontiers in Immunology. 2020;11:1–12. doi: 10.3389/fimmu.2020.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang M.Y., Kang I., Gale M., Jr., Manicone A.M., Kinsella M.G., Braun K.R., Wigmosta T., Parks W.C., Altemeier W.A., Wight T.N., Frevert C.W., Gale M., Manicone A.M., Kinsella M.G., Braun K.R., Wigmosta T., Parks W.C., Altemeier W.A., Wight T.N., Frevert C.W. Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am. J. Physiol. Cell. Mol. Physiol. 2017;313:L1069–L1086. doi: 10.1152/ajplung.00353.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang I., Harten I.A., Chang M.Y., Braun K.R., Sheih A., Nivison M.P., Johnson P.Y., Workman G., Kaber G., Evanko S.P., Chan C.K., Merrilees M.J., Ziegler S.F., Kinsella M.G., Frevert C.W., Wight T.N. Versican deficiency significantly reduces lung inflammatory response induced by polyinosine-polycytidylic acidstimulation. The Journal of Biological Chemistry. 2017;292:51–63. doi: 10.1074/jbc.M116.753186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Islam S., Chuensirikulchai K., Khummuang S., Keratibumrungpong T., Kongtawelert P., Kasinrerk W., Hatano S., Nagamachi A., Honda H., Watanabe H. Accumulation of versican facilitates wound healing: implication of its initial ADAMTS-cleavage site. Matrix Biology. 2020;87:77–93. doi: 10.1016/j.matbio.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Keire P.A., Kang I., Wight T.N. 2017. Versican: Role in Cancer Tumorigenesis; pp. 51–74. [DOI] [Google Scholar]
- 68.Polte T., Petzold S., Bertrand J., Schütze N., Hinz D., Simon J.C., Lehmann I., Echtermeyer F., Pap T., Averbeck M. Critical role for syndecan-4 in dendritic cell migration during development of allergic airway inflammation. Nature Communications. 2015;6:7554. doi: 10.1038/ncomms8554. [DOI] [PubMed] [Google Scholar]
- 69.Endo T., Ito K., Morimoto J., Kanayama M., Ota D., Ikesue M., Kon S., Takahashi D., Onodera T., Iwasaki N., Uede T. Syndecan 4 regulation of the development of autoimmune arthritis in mice by modulating B cell migration and germinal center formation. 2015;67:2512–2522. doi: 10.1002/art.39193. [DOI] [PubMed] [Google Scholar]
- 70.Tanino Y., Chang M.Y., Wang X., Gill S.E., Skerrett S., McGuire J.K., Sato S., Nikaido T., Kojima T., Munakata M., Mongovin S., Parks W.C., Martin T.R., Wight T.N., Frevert C.W. Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. American Journal of Respiratory Cell and Molecular Biology. 2012;47:196–202. doi: 10.1165/rcmb.2011-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Das S., Majid M., Baker A.B. Syndecan-4 enhances PDGF-BB activity in diabetic wound healing. Acta Biomaterialia. 2016;42:56–65. doi: 10.1016/j.actbio.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Jürgensen H.J., van Putten S., Nørregaard K.S., Bugge T.H., Engelholm L.H., Behrendt N., Madsen D.H. Cellular uptake of collagens and implications for immune cell regulation in disease. Cellular and Molecular Life Sciences. 2020;77:3161–3176. doi: 10.1007/s00018-020-03481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jürgensen H.J., Silva L.M., Krigslund O., van Putten S., Madsen D.H., Behrendt N., Engelholm L.H., Bugge T.H. CCL2/MCP-1 signaling drives extracellular matrix turnover by diverse macrophage subsets. Matrix Biol. Plus. 2019;1:100003. doi: 10.1016/j.mbplus.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simões F.C., Cahill T.J., Kenyon A., Gavriouchkina D., Vieira J.M., Sun X., Pezzolla D., Ravaud C., Masmanian E., Weinberger M., Mayes S., Lemieux M.E., Barnette D.N., Gunadasa-Rohling M., Williams R.M., Greaves D.R., Trinh L.A., Fraser S.E., Dallas S.L., Choudhury R.P., Sauka-Spengler T., Riley P.R. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nature Communications. 2020;11:600. doi: 10.1038/s41467-019-14263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sapudom J., Mohamed W.K.E., Garcia-Sabaté A., Alatoom A., Karaman S., Mahtani N., Teo J.C. Collagen fibril density modulates macrophage activation and cellular functions during tissue repair. Bioengineering. 2020;7:33. doi: 10.3390/bioengineering7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsen A.M.H., Kuczek D.E., Kalvisa A., Siersbæk M.S., Thorseth M.-L., Johansen A.Z., Carretta M., Grøntved L., Vang O., Madsen D.H. Collagen density modulates the immunosuppressive functions of macrophages. Journal of Immunology. 2020;205:1461–1472. doi: 10.4049/jimmunol.1900789. [DOI] [PubMed] [Google Scholar]
- 77.Jiang H., Hegde S., DeNardo D.G. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunology, Immunotherapy. 2017;66:1037–1048. doi: 10.1007/s00262-017-2003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuczek D.E., Larsen A.M.H., Thorseth M.-L., Carretta M., Kalvisa A., Siersbæk M.S., Simões A.M.C., Roslind A., Engelholm L.H., Noessner E., Donia M., Svane I.M., Straten P. thor, Grøntved L., Madsen D.H. Collagen density regulates the activity of tumor-infiltrating T cells. Journal for Immunotherapy of Cancer. 2019;7:68. doi: 10.1186/s40425-019-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho M.S.P., Böse K., Mokkapati S., Nischt R., Smyth N. Nidogens-extracellular matrix linker molecules. Microscopy Research and Technique. 2008;71:387–395. doi: 10.1002/jemt.20567. [DOI] [PubMed] [Google Scholar]
- 80.Senior R.M., Gresham H.D., Griffin G.L., Brown E.J., Chung A.E. Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-asp (RGD) domain and the leukocyte response integrin. The Journal of Clinical Investigation. 1992;90:2251–2257. doi: 10.1172/JCI116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaggero S., Bruschi M., Petretto A., Parodi M., Del Zotto G., Lavarello C., Prato C., Santucci L., Barbuto A., Bottino C., Candiano G., Moretta A., Vitale M., Moretta L., Cantoni C. Nidogen-1 is a novel extracellular ligand for the NKp44 activating receptor. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2018.1470730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balzano M., De Grandis M., Manh T.P. Vu, Chasson L., Bardin F., Farina A., Sergé A., Bidaut G., Charbord P., Hérault L., Bailly A.L., Cartier-Michaud A., Boned A., Dalod M., Duprez E., Genever P., Coles M., Bajenoff M., Xerri L., Aurrand-Lions M., Schiff C., Mancini S.J.C. Nidogen-1 contributes to the interaction network involved in pro-B cell retention in the peri-sinusoidal hematopoietic stem cell niche. Cell Reports. 2019;26:3257–3271. doi: 10.1016/j.celrep.2019.02.065. e8. [DOI] [PubMed] [Google Scholar]
- 83.Lee N.V., Sato M., Annis D.S., Loo J.A., Wu L., Mosher D.F., Iruela-Arispe M.L. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. The EMBO Journal. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gutierrez L.S., Lopez-Dee Z., Pidcock K. Thrombospondin-1: multiple paths to inflammation. Mediators of Inflammation. 2011;2011 doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy-Ullrich J.E. Thrombospondin 1 and its diverse roles as a regulator of extracellular matrix in fibrotic disease. The Journal of Histochemistry and Cytochemistry. 2019;67:683–699. doi: 10.1369/0022155419851103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar R., Mickael C., Kassa B., Sanders L., Hernandez-Saavedra D., Koyanagi D.E., Kumar S., Pugliese S.C., Thomas S., McClendon J., Maloney J.P., Janssen W.J., Stenmark K.R., Tuder R.M., Graham B.B. Interstitial macrophage-derived thrombospondin-1 contributes to hypoxia-induced pulmonary hypertension. Cardiovascular Research. 2020;116:2021–2030. doi: 10.1093/cvr/cvz304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamy L., Foussat A., Brown E.J., Bornstein P., Ticchioni M., Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. Journal of Immunology. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 88.Hayat S.M.G., Bianconi V., Pirro M., Jaafari M.R., Hatamipour M., Sahebkar A. CD47: role in the immune system and application to cancer therapy. Cellular Oncology. 2020;43:19–30. doi: 10.1007/s13402-019-00469-5. [DOI] [PubMed] [Google Scholar]
- 89.Suárez-Calvet X., Alonso-Pérez J., Castellví I., Carrasco-Rozas A., Fernández-Simón E., Zamora C., Martínez-Martínez L., Alonso-Jiménez A., Rojas-García R., Turón J., Querol L., de Luna N., Milena-Millan A., Corominas H., Castillo D., Cortés-Vicente E., Illa I., Gallardo E., Díaz-Manera J. Thrombospondin-1 mediates muscle damage in brachio-cervical inflammatory myopathy and systemic sclerosis. Neurol. - Neuroimmunol. Neuroinflammation. 2020;7 doi: 10.1212/NXI.0000000000000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawecki C., Lenting P.J., Denis C.V. von Willebrand factor and inflammation. Journal of Thrombosis and Haemostasis. 2017;15:1285–1294. doi: 10.1111/jth.13696. [DOI] [PubMed] [Google Scholar]
- 91.Denis C.V., Andre P., Saffaripour S., Wagner D.D. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proceedings of the National Academy of Sciences. 2001;98:4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Binder M.J., McCoombe S., Williams E.D., McCulloch D.R., Ward A.C. The extracellular matrix in cancer progression: role of hyalectan proteoglycans and ADAMTS enzymes. Cancer Letters. 2017;385:55–64. doi: 10.1016/j.canlet.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Sun Y., Huang J., Yang Z. The roles of ADAMTS in angiogenesis and cancer. Tumor Biology. 2015;36:4039–4051. doi: 10.1007/s13277-015-3461-8. [DOI] [PubMed] [Google Scholar]
- 94.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 95.Tan I.A., Frewin K., Ricciardelli C., Russell D.L. ADAMTS1 promotes adhesion to extracellular matrix proteins and predicts prognosis in early stage breast cancer patients. Cellular Physiology and Biochemistry. 2019;52:1553–1568. doi: 10.33594/000000108. [DOI] [PubMed] [Google Scholar]
- 96.Lima M.A., dos Santos L., Turri J.A., Nonogaki S., Buim M., Lima J.F., de Jesus Viana Pinheiro J., de Toledo Osório C.A. Bueno, Soares F.A., Freitas V.M. Prognostic value of ADAMTS proteases and their substrates in epithelial ovarian cancer. Pathobiology. 2016;83:316–326. doi: 10.1159/000446244. [DOI] [PubMed] [Google Scholar]
- 97.Wen Y.C., Lin Y.W., Chu C.Y., Yang Y.C., Yang S.F., Liu Y.F., Hsiao M., Lee W.J., Chien M.H. Melatonin-triggered post-transcriptional and post-translational modifications of ADAMTS1 coordinately retard tumorigenesis and metastasis of renal cell carcinoma. Journal of Pineal Research. 2020;69 doi: 10.1111/jpi.12668. [DOI] [PubMed] [Google Scholar]
- 98.Casal C., Torres-Collado A.X., Plaza-Calonge M.C.D.C., Martino-Echarri E., y Cajal S. Ramon, Rojo F., Griffioen A.W., Rodriguez-Manzaneque J.C., Cajal S.R.Y., Rojo F., Griffioen A.W., Rodríguez-Manzaneque J.C., Cajal S.R.Y., Rojo F., Griffioen A.W., Rodriguez-Manzaneque J.C. ADAMTS1 contributes to the acquisition of an endothelial-like phenotype in plastic tumor cells. Cancer Research. 2010;70:4676–4686. doi: 10.1158/0008-5472.CAN-09-4197. [DOI] [PubMed] [Google Scholar]
- 99.Shang X.-Q., Liu K.-L., Li Q., Lao Y.-Q., Li N.-S., Wu J. ADAMTS4 is upregulated in colorectal cancer and could be a useful prognostic indicator of colorectal cancer. Revista da Associação Médica Brasileira. 2020;66:42–47. doi: 10.1590/1806-9282.66.1.42. [DOI] [PubMed] [Google Scholar]
- 100.Chen J., Luo Y., Zhou Y., Qin S., Qiu Y., Cui R., Yu M., Qin J., Zhong M. Promotion of tumor growth by ADAMTS4 in colorectal cancer: focused on macrophages. Cellular Physiology and Biochemistry. 2018;46:1693–1703. doi: 10.1159/000489245. [DOI] [PubMed] [Google Scholar]
- 101.Haraguchi N., Ohara N., Koseki J., Takahashi H., Nishimura J., Hata T., Mizushima T., Yamamoto H., Ishii H., Doki Y., Mori M. High expression of ADAMTS5 is a potent marker for lymphatic invasion and lymph node metastasis in colorectal cancer. Mol. Clin. Oncol. 2017;6:130–134. doi: 10.3892/mco.2016.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C., Luo X., Huang B., Wang X., Deng Y., Zhong Z. ADAMTS12 acts as a cancer promoter in colorectal cancer via activating the Wnt/β-catenin signaling pathway in vitro. Ann. Transl. Med. 2020;8:301. doi: 10.21037/atm.2020.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen L., Tang J., Feng Y., Li S., Xiang Q., He X., Ren G., Peng W., Xiang T. ADAMTS9 is silenced by epigenetic disruption in colorectal cancer and inhibits cell growth and metastasis by regulating Akt/p53 signaling. Cellular Physiology and Biochemistry. 2017;44:1370–1380. doi: 10.1159/000485534. [DOI] [PubMed] [Google Scholar]
- 104.Huang J., Sun Y., Chen H., Liao Y., Li S., Chen C., Yang Z. ADAMTS5 acts as a tumor suppressor by inhibiting migration, invasion and angiogenesis in human gastric cancer. Gastric Cancer. 2019;22:287–301. doi: 10.1007/s10120-018-0866-2. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Xu H., Mu J., Guo S., Ye L., Li D., Peng W., He X., Xiang T. Inactivation of ADAMTS18 by aberrant promoter hypermethylation contribute to lung cancer progression. Journal of Cellular Physiology. 2019;234:6965–6975. doi: 10.1002/jcp.27439. [DOI] [PubMed] [Google Scholar]
- 106.Xu H., Xiao Q., Fan Y., Xiang T., Li C., Li C., Li S., Hui T., Zhang L., Li H., Li L., Ren G. Epigenetic silencing of ADAMTS18 promotes cell migration and invasion of breast cancer through AKT and NF-κB signaling. Cancer Medicine. 2017;6:1399–1408. doi: 10.1002/cam4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ricciardelli C., Frewin K.M., de A Tan I., Williams E.D., Opeskin K., Pritchard M.A., Ingman W.V., Russell D.L. The ADAMTS1 protease gene is required for mammary tumor growth and metastasis. The American Journal of Pathology. 2011;179:3075–3085. doi: 10.1016/j.ajpath.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Asano K., Nelson C.M., Nandadasa S., Aramaki-Hattori N., Lindner D.J., Alban T., Inagaki J., Ohtsuki T., Oohashi T., Apte S.S., Hirohata S. Stromal Versican regulates tumor growth by promoting angiogenesis. Scientific Reports. 2017;7:17225. doi: 10.1038/s41598-017-17613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gueye N.-A., Mead T.J., Koch C.D., Biscotti C.V., Falcone T., Apte S.S. Versican proteolysis by ADAMTS proteases and its influence on sex steroid receptor expression in uterine leiomyoma. The Journal of Clinical Endocrinology and Metabolism. 2017;102:1631–1641. doi: 10.1210/jc.2016-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Binder M.J., McCoombe S., Williams E.D., McCulloch D.R., Ward A.C. ADAMTS-15 has a tumor suppressor role in prostate cancer. Biomolecules. 2020;10:682. doi: 10.3390/biom10050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Esselens C., Malapeira J., Colome N., Casal C., Rodriguez-Manzaneque J.C., Canals F., Arribas J. The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. The Journal of Biological Chemistry. 2010;285:2463–2473. doi: 10.1074/jbc.M109.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodríguez-Manzaneque J.C., Carpizo D., del C. Plaza-Calonge M., Torres-Collado A.X., Thai S.N.-M., Simons M., Horowitz A., Iruela-Arispe M.L. Cleavage of syndecan-4 by ADAMTS1 provokes defects in adhesion. The International Journal of Biochemistry & Cell Biology. 2009;41:800–810. doi: 10.1016/j.biocel.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lambert J., Makin K., Akbareian S., Johnson R., Alghamdi A.A.A., Robinson S.D., Edwards D.R. ADAMTS-1 and syndecan-4 intersect in the regulation of cell migration and angiogenesis. Journal of Cell Science. 2020;133 doi: 10.1242/jcs.235762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kelwick R., Wagstaff L., Decock J., Roghi C., Cooley L.S., Robinson S.D., Arnold H., Gavrilović J., Jaworski D.M., Yamamoto K., Nagase H., Seubert B., Krüger A., Edwards D.R. Metalloproteinase-dependent and -independent processes contribute to inhibition of breast cancer cell migration, angiogenesis and liver metastasis by a disintegrin and metalloproteinase with thrombospondin motifs-15. International Journal of Cancer. 2015;136:E14–E26. doi: 10.1002/ijc.29129. [DOI] [PubMed] [Google Scholar]
- 115.Mohamedi Y., Fontanil T., Cobo T., Vega J.A., Cobo J.L., García-Suárez O., Cobo J., Cal S., Obaya Á.J. The molecular interaction of ADAMTS-1 and fibulin-1 and its potential contribution to breast cancer biology. J. Cancer Metastasis Treat. 2019;5:37. doi: 10.20517/2394-4722.2018.81. [DOI] [Google Scholar]
- 116.Fontanil T., Rúa S., Llamazares M., Moncada-Pazos A., Quirós P.M., García-Suárez O., Vega J.A., Sasaki T., Mohamedi Y., Esteban M.M., Obaya A.J., Cal S. Interaction between the ADAMTS-12 metalloprotease and fibulin-2 induces tumor-suppressive effects in breast cancer cells. Oncotarget. 2014;5:1253–1264. doi: 10.18632/oncotarget.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fontanil T., Álvarez-Teijeiro S., Villaronga M.Á., Mohamedi Y., Solares L., Moncada-Pazos A., Vega J.A., García-Suárez O., Pérez-Basterrechea M., García-Pedrero J.M., Obaya A.J., Cal S. Cleavage of Fibulin-2 by the aggrecanases ADAMTS-4 and ADAMTS-5 contributes to the tumorigenic potential of breast cancer cells. Oncotarget. 2017;8:13716–13729. doi: 10.18632/oncotarget.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matthews R.T., Gary S.C., Zerillo C., Pratta M., Solomon K., Arner E.C., Hockfield S. Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. The Journal of Biological Chemistry. 2000;275:22695–22703. doi: 10.1074/jbc.M909764199. [DOI] [PubMed] [Google Scholar]
- 119.Nakada M., Miyamori H., Kita D., Takahashi T., Yamashita J., Sato H., Miura R., Yamaguchi Y., Okada Y. Human glioblastomas overexpress ADAMTS-5 that degrades brevican. Acta Neuropathologica. 2005;110:239–246. doi: 10.1007/s00401-005-1032-6. [DOI] [PubMed] [Google Scholar]
- 120.Nutt C.L., Zerillo C.A., Kelly G.M., Hockfield S. Brain enriched hyaluronan binding (BEHAB)/brevican increases aggressiveness of CNS-1 gliomas in Lewis rats. Cancer Research. 2001;61:7056–7059. [PubMed] [Google Scholar]
- 121.Zhang H., Kelly G., Zerillo C., Jaworski D.M., Hockfield S. Expression of a cleaved brain-specific extracellular matrix protein mediates glioma cell invasion in vivo. The Journal of Neuroscience. 1998;18:2370–2376. doi: 10.1523/JNEUROSCI.18-07-02370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu B., Kong L.L., Matthews R.T., Viapiano M.S. The proteoglycan brevican binds to fibronectin after proteolytic cleavage and promotes glioma cell motility. The Journal of Biological Chemistry. 2008;283:24848–24859. doi: 10.1074/jbc.M801433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Viapiano M.S., Hockfield S., Matthews R.T. BEHAB/brevican requires ADAMTS-mediated proteolytic cleavage to promote glioma invasion. Journal of Neuro-Oncology. 2008;88:261–272. doi: 10.1007/s11060-008-9575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Melching L.I., Fisher W.D., Lee E.R., Mort J.S., Roughley P.J. The cleavage of biglycan by aggrecanases. Osteoarthritis and Cartilage. 2006;14:1147–1154. doi: 10.1016/j.joca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 125.Yamamoto K., Ohga N., Hida Y., Maishi N., Kawamoto T., Kitayama K., Akiyama K., Osawa T., Kondoh M., Matsuda K., Onodera Y., Fujie M., Kaga K., Hirano S., Shinohara N., Shindoh M., Hida K. Biglycan is a specific marker and an autocrine angiogenic factor of tumour endothelial cells. British Journal of Cancer. 2012;106:1214–1223. doi: 10.1038/bjc.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Asano K., Edamatsu M., Hatipoglu O.F., Inagaki J., Ono M., Ohtsuki T., Oohashi T., Hirohata S. Host-produced ADAMTS4 inhibits early-stage tumor growth. Acta Medica Okayama. 2018;72:257–266. doi: 10.18926/AMO/56071. [DOI] [PubMed] [Google Scholar]
- 127.Tzanakakis G., Neagu M., Tsatsakis A., Nikitovic D. Proteoglycans and immunobiology of cancer-therapeutic implications. Frontiers in Immunology. 2019;10:1–8. doi: 10.3389/fimmu.2019.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duan Q., Zhang H., Zheng J., Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends in Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 129.Fanhchaksai K., Okada F., Nagai N., Pothacharoen P., Kongtawelert P., Hatano S., Makino S., Nakamura T., Watanabe H. Host stromal versican is essential for cancer-associated fibroblast function to inhibit cancer growth. International Journal of Cancer. 2016;138:630–641. doi: 10.1002/ijc.29804. [DOI] [PubMed] [Google Scholar]
- 130.Kim S., Takahashi H., Lin W.W., Descargues P., Grivennikov S., Kim Y., Luo J.L., Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Z., Li Z., Wang Y., Cao D., Wang X., Jiang M., Li M., Yan X., Li Y., Liu Y., Luo F. Versican silencing improves the antitumor efficacy of endostatin by alleviating its induced inflammatory and immunosuppressive changes in the tumor microenvironment. Oncology Reports. 2015;33:2981–2991. doi: 10.3892/or.2015.3903. [DOI] [PubMed] [Google Scholar]
- 132.Pappas A.G., Magkouta S., Pateras I.S., Skianis I., Moschos C., Vazakidou M.E., Psarra K., Gorgoulis V.G., Kalomenidis I. Versican modulates tumor-associated macrophage properties to stimulate mesothelioma growth. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2018.1537427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gorter A., Zijlmans H.J., van Gent H., Trimbos J.B., Fleuren G.J., Jordanova E.S. Versican expression is associated with tumor-infiltrating CD8-positive T cells and infiltration depth in cervical cancer. Modern Pathology. 2010;23:1605–1615. doi: 10.1038/modpathol.2010.154. [DOI] [PubMed] [Google Scholar]
- 134.Schmitt M. Versican vs versikine: tolerance vs attack. Blood. 2016;128:612–613. doi: 10.1182/blood-2016-06-721092. [DOI] [PubMed] [Google Scholar]
- 135.Dhakal B., Pagenkopf A., Mushtaq M.U., Cunningham A.M., Flietner E., Morrow Z., Papadas A., Hope C., Leith C., Hematti P., Hari P., Callander N.S., Asimakopoulos F. Versican proteolysis predicts immune effector infiltration and post-transplant survival in myeloma. Leukemia & Lymphoma. 2019;60:2558–2562. doi: 10.1080/10428194.2019.1585836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yin X., Johns S.C., Kim D., Mikulski Z., Salanga C.L., Handel T.M., Macal M., Zúñiga E.I., Fuster M.M. Lymphatic specific disruption in the fine structure of heparan sulfate inhibits dendritic cell traffic and functional T cell responses in the lymph node. Journal of Immunology. 2014;192:2133–2142. doi: 10.4049/jimmunol.1301286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.El Ghazal R., Yin X., Johns S.C., Swanson L., Macal M., Ghosh P., Zuniga E.I., Fuster M.M. Glycan sulfation modulates dendritic cell biology and tumor growth. Neoplasia. 2016;18:294–306. doi: 10.1016/j.neo.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta P., Johns S.C., Kim S.Y., El Ghazal R., Zuniga E.I., Fuster M.M. Functional cellular anti-tumor mechanisms are augmented by genetic proteoglycan targeting. Neoplasia (United States) 2020;22:86–97. doi: 10.1016/j.neo.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Henry N., Van Lamsweerde A.L., Vaes G. Collagen degradation by metastatic variants of Lewis lung carcinoma: cooperation between tumor cells and macrophages. Cancer Research. 1983;43:5321–5327. [PubMed] [Google Scholar]
- 140.Huo C.W., Chew G., Hill P., Huang D., Ingman W., Hodson L., Brown K.A., Magenau A., Allam A.H., McGhee E., Timpson P., Henderson M.A., Thompson E.W., Britt K. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Research. 2015;17:79. doi: 10.1186/s13058-015-0592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mushtaq M.U., Papadas A., Pagenkopf A., Flietner E., Morrow Z., Chaudhary S.G., Asimakopoulos F. Tumor matrix remodeling and novel immunotherapies: the promise of matrix-derived immune biomarkers. Journal for Immunotherapy of Cancer. 2018;6:65. doi: 10.1186/s40425-018-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Diskin B., Adam S., Cassini M.F., Sanchez G., Liria M., Aykut B., Buttar C., Li E., Sundberg B., Salas R.D., Chen R., Wang J., Kim M., Farooq M.S., Nguy S., Fedele C., Tang K.H., Chen T., Wang W., Hundeyin M., Rossi J.A.K., Kurz E., Haq M.I.U., Karlen J., Kruger E., Sekendiz Z., Wu D., Shadaloey S.A.A., Baptiste G., Werba G., Selvaraj S., Loomis C., Wong K.-K., Leinwand J., Miller G. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nature Immunology. 2020;21:442–454. doi: 10.1038/s41590-020-0620-x. [DOI] [PubMed] [Google Scholar]
- 143.Ricard-Blum S., Vallet S.D. Fragments generated upon extracellular matrix remodeling: biological regulators and potential drugs. Matrix Biology. 2019;75–76:170–189. doi: 10.1016/j.matbio.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 144.Klemm F., Joyce J.A. Microenvironmental regulation of therapeutic response in cancer. Trends in Cell Biology. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]