Abstract
Colorectal cancer (CRC) is a lethal and common malignancy worldwide. Non-coding (nc)RNAs have been shown to modulate tumor progression in several types of cancer. The present study aimed to investigate the role of hsa_circ_0000212 in CRC, as a sponge of microRNA (miR)-491. The expression levels of miR-491 and forkhead box P4 (FOXP4) were analyzed using data from The Cancer Genome Atlas. The association between miR-491 and FOXP4 and the clinicopathological characteristics were also analyzed. A novel circular (circ)RNA, hsa_circ_0000212, was found to sponge miR-491 based on bioinformatics analysis. The potential binding site between miR-491 and FOXP4 or circ-0000212 was validated using luciferase and RNA immunoprecipitation assays. The expression levels and distribution of circ-0000212 was also determined. Cell Counting Kit-8 and colony formation assays were performed to determine the role of miR-491 or circ-0000212 on the proliferation of the CRC cells. Decreased miR-491 or increased FOXP4 expression levels were associated with the pathological stage in patients with CRC. In addition, miR-491 inhibited cell proliferation by targeting FOXP4. circ-0000212 was increased in CRC tissues and was predominantly localized in the cytoplasm. Furthermore, circ-0000212 augmented viability of the CRC cells by sponging miR-491 and modulating FOXP4. In conclusion, circ-0000212 may serve as a novel tumor-promoter and drug target in CRC.
Keywords: circular RNA, microRNA-491, forkhead box P4, proliferation, colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most lethal malignancies worldwide. It is the third leading cause of cancer-associated death and the third most commonly diagnosed cancer in both men and women (1). Despite improvements in treatment options, such as surgery and adjuvant therapeutic modalities in recent years, CRC still has a low survival rate. Thus, there is an urgent requirement to identify the important signaling pathways involved, to aid in the development of novel effective treatment strategies for management of CRC.
Non-coding (nc)RNAs have been shown to be involved in various types of disease, including cancer, and an increasing number of studies are identifying the associations between ncRNAs and cancer (2–4). MicroRNAs (miRNAs/miRs) are a class of small regulatory ncRNAs and are ~22 bp in length. They are evolutionarily conserved across species and can negatively regulate the expression levels of their targets by binding with the 3′-untranslated region (UTR) of mRNAs (5). Increasing evidence has shown that miRNAs serve crucial roles in oncogenesis, progression and prognosis of CRC (6–8).
Circular (circ)RNAs are a novel group of ncRNAs, which have covalently closed continuous loops without a polyadenylated tail or 5′ to 3′ polarity (9). circRNAs originate from exons, introns or intergenic regions and are resistant to RNase R (10). It has been found that circRNAs are conserved across multiple species and display tissue-specific and developmental-stage specific expression levels (11); however, their functional significance remains unknown. Previously, research has focused on investigating the function of circRNAs. Studies have shown that circRNAs exert functions in neurological diseases, cardiovascular disorders, prion diseases and cancer (12,13). circRNAs are principally localized in the cytoplasm, where they exert their key roles in human diseases (14). Accumulating data have suggested that circRNAs can function as miRNA sponges and interact with RNA-binding proteins to widely regulate gene expression levels (10). circAGFG1 promoted stemness and metastasis in CRC by regulating YY1/CTNNB1 and sponging miRNAs (15). circHIPK3 also inhibited cancer proliferation, acting as a miR-124 sponge in multiple types of cancer (16). These studies demonstrate that circRNAs are novel targets for drug development and clinical management in cancer.
In the present study, the role of circ-0000212, a circRNA derived from the SFMBT2 gene, in CRC was investigated. circ-0000212 expression was significantly higher in CRC and could promote tumor progression by acting as a competing endogenous RNA of miR-491 by modulating forkhead box P4 (FOXP4) expression levels. Therefore, circ-0000212 may act as a suppressive factor in tumors and thus serve as a novel target to treat CRC.
Materials and methods
Patient characteristics
Clinical data and miRNA expression data were downloaded from The Cancer Genome Atlas (TCGA) database using the TCGAbiolinks package (17). A total of 500 samples were downloaded for RNA analysis, including 41 normal tissues and 459 cancer tissues, and 465 samples were downloaded for miRNA analysis, including 8 normal tissues and 457 cancer tissues. TCGA data are publicly available and all written informed consent was acquired as stated by TCGA. A total of 20 pairs of CRC and adjacent normal tissues were harvested from patients with CRC, who received surgical treatment at The Second Affiliated Hospital of Harbin Medical University (Harbin, China). The median age of the patients was 64 years old, (age range, 48–81 years) and among the 20 cases, seven were female patients and 13 were male patients. All tissues were snap-frozen, then stored at −80°C until use. The study adhered to the principles of the Declaration of Helsinki. Written informed consent was provided by all patients and the present study was approved by the Institutional Review Board of The Second Affiliated Hospital of Harbin Medical University (Heilongjiang, China).
Cell culture
The normal colon-derived epithelial FHC cell line, 293T cells, and the CRC cell lines, SW620, SW480, LoVo, HT29 and HCT116 were purchased from the American Type Culture Collection. The FHC cells were cultured in DMEM: F12 Medium, and 293T cells and the CRC cell lines were cultured in DMEM (all from Gibco; Thermo Fisher Scientific, Inc.). All media were supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5% CO2.
Reverse transcription-quantitative PCR (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract RNA from the tissues and cells and RT was performed using a RT kit according to the manufacturer's protocol (Thermo Fisher Scientific, Inc.). qPCR was performed with the SYBR Green mix kit (Takara Bio, Inc.). The thermocycling conditions were as follows: 94°C for 2 min, followed by 40 cycles at 94°C for 15 sec and 60°C for 1 min. NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Inc.) were used to isolate the cytoplasmic and nuclear components, respectively. The following primers were used: circ-0000212 forward, 5′-GTTTGGGAGGCTTCTTGGTAAT-3′ and reverse, 5′-AAAGCCAGTTTCCTCCAAGC-3′; SFMBT2 forward, 5′-TCTGCGCTACTGCGGTTAC-3′ and reverse 5′-ACCAGTCAAGTCACGTATGAGAA-3′; U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; miR-491 stem loop, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCTCATG-3′, forward, 5′-ACACTCCAGCTGGGAGTGGGGAACCCTTC-3′ and reverse, 5′-TGGTGTCGTGGAGTCG-3′; FOXP4 forward, 5′-ATCGGCAGCTGACGCTAAATGAGA-3′ and reverse, 5′-AAACACTTGTGCAGGCTGAGGTTG-3′; actin forward, 5′-GGCGGCAACACCATGTACCCT-3′ and reverse, 5′-AGGGGCCGGACTCGTCATACT-3′. U6 and actin were used as the loading controls.
Plasmids, synthesized nucleotide sequences and transfection
An overexpression plasmid (pcDNA3.1) containing the FOXP4 transcript, small interfering (si)RNA targeting circ-0000212, miR-491 mimics and miR-491 inhibitors were purchased from Shanghai GenePharma Co., Ltd. The following siRNA sequence targeting circ-0000212 was used: 5′-GGGAGGCTTCTTGGTAATTTT-3′. The negative control used for overexpression of FOXP4 was empty pcDNA3.1 plasmid. The siRNA negative control sequence was as follows: 5′-TTUUGAACCAAGAAGCCUCCC-3′. The miR-491 mimic sequence was 5′-AGUGGGGAACCCUUCCAUGAGG-3′ and negative control used for miR-491 mimics was 5′-UUCUCCGAACGUGUCACGUTT-3′. The miR-491 inhibitor sequence was 2′-methoxy modified: 5′-mCm CmU mCm AmU mGm GmA mAm GmG mGm UmU mCm CmC mCm AmC mU-3′, and the negative control for the miR-491 inhibitor was 5′-CAGUACUUUUGUGUAGUACAA-3′. The miR-491 inhibitor was 2′-methoxy modified because this modification could more effectively inhibit the function of miRNA (18). The cells were seeded 24 h prior to transfection, at 50–60% confluence, then transfected using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The cells were transfected with 50 nM siRNA, 2.5 µg plasmid, 30 nM miRNA mimics or 30 nM miRNA inhibitor, according to the manufacturer's protocol. Subsequent experiments were performed 48 h post transfection.
Bioinformatics analysis
TargetScan online database (Release 7.2, http://www.targetscan.org/vert_72/) was used to identify potential mRNA targets of miRNA-491. CircBase (http://www.circbase.org/) and starBase (http://starbase.sysu.edu.cn/index.php) were used to identify potential circRNAs that could bind with miR-491. The sequence of miR-491 is 5′-ggaguaccuuccCAAGGGGUGa-3′ and the binding site of hsa-circ-0000212 is 5′-ggagcaagugcuGCUCCCCACa-3′ (upper-case letters refer to binding site).
Cell Counting Kit-8 (CCK-8) proliferation assay
A total of 2×103 cells were seeded in each well of a 96-well plate containing 100 µl culture medium. The cells were cultured for 24, 48 and 72 h, and 10 µl CCK-8 solution (Abcam) was then added to each well and cultured for 2 h in the incubator. The absorbance was measured at 460 nm using a microplate reader.
Colony formation assay
A total of 500 cells were seeded in each well of a 6-well plate and incubated at 37°C for 7 days. To maintain the effect of the siRNA, miRNA mimic or inhibitor, the cells were re-transfected on day 3. The colonies were fixed with 4% paraformaldehyde at room temperature for 15 min, then stained with 0.01% crystal violet solution at room temperature for 30 min. The number of cell colonies was then analyzed. A colony was defined to consist of ≥50 cells and colonies were detected under a light microscope (magnification, ×4).
Western blot analysis
The cells were harvested and the total protein was extracted using RIPA lysis buffer with protease-phosphatase inhibitor (EMD Millipore). A Pierce BCA Protein assay kit (Thermo Fisher Scientific, Inc.) was used to quantify protein expression. Proteins (30 µg) were loaded on a 15% SDS-gel, resolved using SDS-PAGE then transferred to PVDF membranes. Membranes were blocked using 3% non-fat milk at room temperature for 2 h. The primary antibodies used were anti-FOXP4 (1:1,000; cat. no. b17726) and anti-β-actin (1:2,500; cat. no. ab8227) (both from Abcam), and membranes were incubated with the antibodies overnight at 4°C. Subsequently, a HRP-conjugated secondary antibody, goat anti-rabbit IgG H&L (1:5,000; cat. no. b6721; Abcam), was incubated with the membrane at room temperature for 1 h. The membranes were visualized using an ECL kit (Bio-Rad Laboratories, Inc.) according to the manufacturer's protocol.
Luciferase reporter assay
The 293T cells were seeded in 96-well plates and co-transfected with either pRL-CMV Renilla luciferase reporter, firefly luciferase reporter, or miR-491 mimic. The luciferase activities of Renilla and firefly reporter vectors were quantified using a dual-luciferase reporter assay according to the manufacturer's protocol (Promega Corporation) after 48 h of incubation. The 3′ untranslated regions (UTRs) were taken from FOXP4, and the binding site of circ-0000212 with miR-491 was taken from circ-0000212. The sequences containing the miR-491-binding sites or point mutations in the binding sites were inserted between SpeI and HindIII sites in the firefly luciferase reporter vectors, in order to generate wild-type and mutant luciferase reporter vectors, respectively. These sequences were all obtained from Shanghai GenePharma Co., Ltd. The pRL-CMV Renilla luciferase reporter was purchased from Promega Corporation. Lipofectamine 2000 was used to perform transfection according to the manufacturer's protocol. Briefly, the 293T cells were seeded 24 h prior to transfection at 50–60% confluence. The cells were transfected once they reached ~70% confluence with 50 ng plasmids, and 20 nM miRNA mimics or miRNA inhibitor, according to the manufacturer's protocol. Luciferase activity was measured 48 h post-transfection and Renilla luciferase activity was used as a control.
RNA immunoprecipitation (RIP) assay
A RIP assay was performed using a Magna RIP kit (EMD Millipore) with samples from SW620 cells, according to the manufacturer's protocol. The anti-AGO2 (cat. no. b156870) and IgG antibodies (cat. no. b133470) were purchased from Abcam.
Actinomycin D and RNase R treatment
Actinomycin D (2 mg/ml; Sigma-Aldrich; Merck KGaA) was added to inhibit transcription. The RNA from SW620 cells (2 µg) was treated at 37°C for 30 min with 3 U/µg RNaseR (LGC Biosearch Technologies). After treatment with Actinomycin D and RNase R, the RNA expression levels of SFMBT2 and circ-0000212 were determined using RT-qPCR.
Statistical analysis
All the experiments were repeated at least three times. Statistical analysis was performed using SPSS version 20.0 (IBM Corp.). A Student's t-test (paired or unpaired depending on the data) or Fisher's exact test was used to compare differences between two groups. Differences between multiple groups were compared using a one-way ANOVA with a post hoc Tukey's test. Kaplan-Meier curves with a log-rank test were used to assess survival. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-491 is downregulated in CRC tissues and associated with certain clinicopathological characteristics of CRC
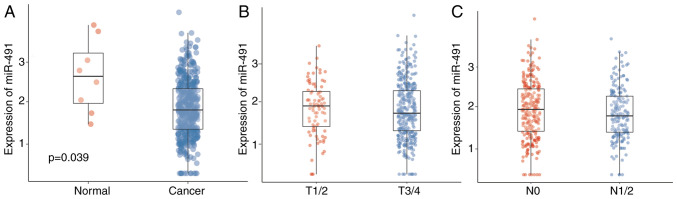
Data from TCGA was first analyzed to confirm the expression levels of miR-491 and the potential role of miR-491 in CRC. Table I shows the baseline characteristics of the patients from TCGA. miR-491 was upregulated in normal tissues compared with that in the cancer tissues (Fig. 1A), indicating an inhibitory effect on CRC. The expression levels of miR-491 in the cases with different T stages was also analyzed; there were no significant differences between the different T stages; however, late T stage had a lower expression level of miR-491 (Fig. 1B). Similarly, there was only a trend that patients with advanced N stage had lower miR-491 expression levels (Fig. 1C). Patients were further divided into two groups based on the miR-491 expression level, and it showed that lower miR-491 expression level was associated with advanced tumor and N staging (Table II).
Table I.
Baseline clinicopathological characteristics of the cases obtained from TCGA.
| Characteristics | N (%) |
|---|---|
| Age, years | |
| <60 | 124 (27.0) |
| ≥60 | 332 (72.2) |
| Sex | |
| Female | 216 (47.2) |
| Male | 242 (52.8) |
| Stage | |
| I | 76 (17.0) |
| II | 178 (39.7) |
| III | 129 (28.8) |
| IV | 65 (14.5) |
| T stage | |
| T1 | 11 (2.4) |
| T2 | 78 (17.0) |
| T3 | 312 (68.1) |
| T4 | 56 (12.2) |
| Tis | 1 (0.2) |
| N stage | |
| N0 | 269 (58.7) |
| N1/2 | 189 (41.2) |
| M Stage | |
| M0 | 336 (83.8) |
| M1 | 65 (16.2) |
| Radiation therapy | |
| No | 379 (97.7) |
| Yes | 9 (2.3) |
| Residual tumor | |
| R0 | 330 (85.7) |
| R1 | 4 (1.0) |
| R2 | 26 (6.8) |
| RX | 25 (6.5) |
| Histological type | |
| Adenocarcinoma | 391 (86.3) |
| Mucinous adenocarcinoma | 62 (13.7) |
| Ethnicity | |
| American Indiana or Alaskan native | 1 (0.4) |
| Asian | 11 (3.9) |
| Black or African American | 59 (20.8) |
| White | 213 (75.0) |
As described in TCGA. TCGA, The Cancer Genome Atlas.
Figure 1.
miR-491 is downregulated in CRC tissues. (A) miR-491 expression was significantly decreased in 457 cancer tissues compared with 8 normal tissues. (B) Expression of miR-491 in the early (T1/2) and late T stages (T3/4). (C) Expression of miR-491 in the early (N) and late N stages (N1/2). miR, microRNA; CRC, colorectal cancer.
Table II.
Association between miR-491 or FOXP4 expression with the clinicopathological characteristics.
| miR-491, n | FOXP4, n | |||||
|---|---|---|---|---|---|---|
| Characteristics | High | Low | P-value | High | Low | P-value |
| Age | 0.3684 | 0.2504 | ||||
| <60 | 56 | 66 | 73 | 51 | ||
| ≥60 | 163 | 155 | 172 | 157 | ||
| Sex | 0.297 | 0.889 | ||||
| Female | 111 | 100 | 115 | 100 | ||
| Male | 109 | 122 | 131 | 109 | ||
| Stage | 0.0455a | <0.001c | ||||
| I | 46 | 27 | 39 | 36 | ||
| II | 83 | 86 | 75 | 103 | ||
| III | 53 | 72 | 82 | 46 | ||
| IV | 34 | 31 | 44 | 20 | ||
| T stage | 0.0506 | 1 | ||||
| T1/2 | 51 | 34 | 48 | 40 | ||
| T3/4 | 169 | 187 | 198 | 168 | ||
| N stage | 0.026a | <0.001c | ||||
| N0 | 140 | 117 | 125 | 143 | ||
| N1/2 | 80 | 105 | 121 | 66 | ||
| M Stage | 0.768 | 0.016a | ||||
| M0 | 158 | 162 | 172 | 162 | ||
| M1 | 34 | 31 | 44 | 20 | ||
| Radiation therapy | 0.949 | 1 | ||||
| No | 179 | 188 | 202 | 175 | ||
| Yes | 5 | 4 | 5 | 4 | ||
| Residual tumor | 0.009b | 0.7435 | ||||
| R0 | 146 | 168 | 170 | 159 | ||
| R1 | 2 | 2 | 2 | 2 | ||
| R2 | 21 | 5 | 15 | 9 | ||
| RX | 11 | 14 | 12 | 13 | ||
| Histological type | 0.397 | 0.1008 | ||||
| Adenocarcinoma | 184 | 192 | 216 | 172 | ||
| Mucinous adenocarcinoma | 34 | 27 | 27 | 35 | ||
| Ethnicity | 0.014a | 0.6216 | ||||
| American Indiand or Alaskan native | 1 | 0 | 1 | 0 | ||
| Asian | 3 | 8 | 5 | 6 | ||
| Black or African American | 35 | 24 | 32 | 27 | ||
| White | 82 | 131 | 125 | 88 | ||
P<0.05
P<0.01
P<0.001
as described in TCGA. Data were obtained from TCGA. miR-491, microRNA-491; FOXP4, forkhead box P4; TCGA, The Cancer Genome Atlas.
miR-491 inhibits CRC cell proliferation
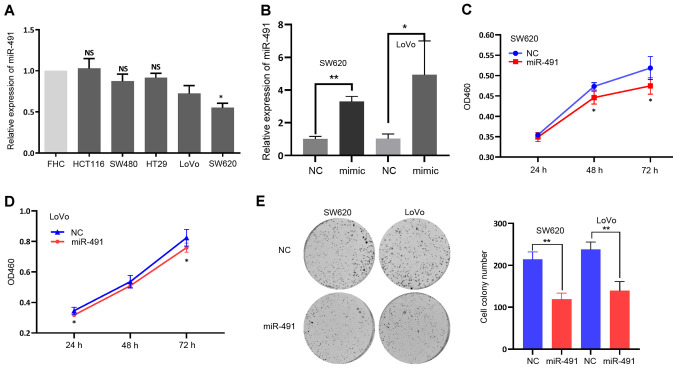
Based on the aforementioned findings, it was hypothesized that miR-491 inhibited cell malignancy. To validate this hypothesis, miR-491 expression levels were first detected in different CRC cell lines, as well as in a normal colon epithelial cell line. As shown in Fig. 2A, the CRC cell lines, particularly SW620 cells, had a relatively low expression level of miR-491 compared with that in the FHC cell line, indicating a potential tumor suppressor role. miR-491 was then transiently overexpressed in the SW620 and LoVo cell lines using miR-491 mimic (Fig. 2B), and the CCK-8 and colony formation assays were performed. miR-491 significantly inhibited the proliferation and viability of both the SW620 and LoVo cells (Fig. 2C-E).
Figure 2.
miR-491 inhibits CRC cell proliferation. (A) miR-491 expression in the different colorectal cancer cell lines (HCT116, SW480, HT29, LoVo and SW620) and normal colon cell line (FHC). *P<0.05 vs. FHC. (B) Expression of miR-491 was increased after transfection of miR-491 mimic both in LoVo and SW620 cells. miR-491 inhibited (C) SW620 and (D) LoVo cell proliferation, based on the Cell Counting Kit-8 assays. (E) miR-491 suppressed SW620 and LoVo cell colony formation ability. Right panel, representative figures; left panel, quantitative analysis. *P<0.05, **P<0.01 vs. NC. Experiments were repeated three times. miR, microRNA; CCK-8, Cell Counting Kit-8; NC, negative control; OD, optical density; NS, not significant.
miR-491 functionally targets FOXP4
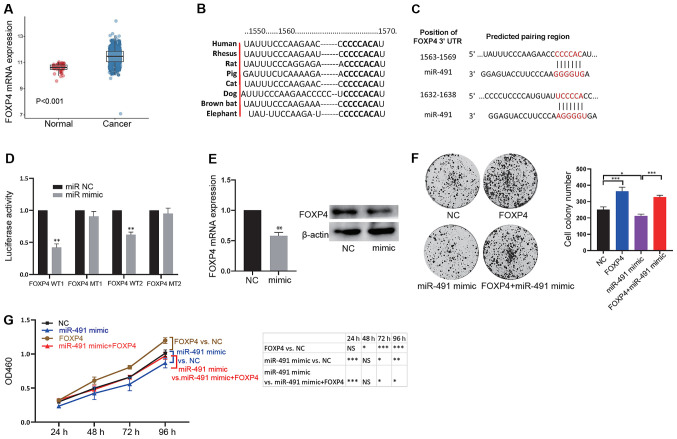
miRNAs exert their functions by inhibiting downstream targets. From the data analysis of patients with CRC from TCGA, it was found that while miR-491 was significantly reduced in cancer tissues, the expression levels of numerous mRNAs were significantly increased. Thus, the potential downstream targets of miRNA regulation may be within the upregulated mRNAs. FOXP4 was found to be upregulated in CRC tissues (adenocarcinoma tissues) compared with that in normal tissues (Fig. 3A). Higher expression levels of FOXP4 were also associated with late tumor, N and M stages (Table II). Thus, the association between miR-491 and FOXP4 expression levels with survival were analyzed, and no association was found (Fig. S1). Next, bioinformatics analysis was used to screen potential targets of miR-491, as miRNAs could exert their functions by inhibiting downstream targets. The results showed that FOXP4 could bind with miR-491 and the binding sites were conserved across different species (Fig. 3B; Table SI). There were 2 binding sites in the 3′-UTR of FOXP4 (Fig. 3C), indicating an interaction between miR-491 and FOXP4. To further confirm binding between miR-491 and FOXP4, a luciferase reporter assay was performed. The results showed that miR-491 mimic could significantly inhibit the luciferase activity of the vector containing the wild-type binding site compared with that in the vector containing the mutant binding site (Fig. 3D). In addition, miR-491 reduced the mRNA and protein expression levels of FOXP4 (Fig. 3E). It was then examined whether FOXP4 was a functional target of miR-491. First, FOXP4 and miR-491 were overexpressed, and the results showed that FOXP4 could promote cell colony formation ability, whereas overexpression of miR-491 reduced colony formation. Notably, FOXP4 reversed the inhibitory effects on cell colony formation ability of miR-491 (Figs. 3F and S2A). Furthermore, similar results were observed in the CCK-8 assay (Fig. 3G). These results confirmed that miR-491 could functionally target FOXP4 in colon cancer.
Figure 3.
miR-491 functionally targets FOXP4. (A) FOXP4 expression was significantly increased in cancer tissues compared with normal tissues. (B) The binding site between FOXP4 and miR-491 is conserved across different species. (C) Potential binding sites between the 3′-UTR in FOXP4 with miR-491. (D) miR-491 mimic decreased luciferase activity of the vector containing WT binding sites in 293T cells. *P<0.05 vs. miR NC. (E) miR-491 decreased FOXP4 mRNA (left) and protein (right) expression levels. **P<0.01 vs. NC. (F) SW620 cells were divided into four groups: NC, overexpression of FOXP4 (FOXP4), overexpression of miR-491 (miR-491 mimic), and concurrent overexpression of FOXP4 and miR-491 (FOXP4 + miR-491 mimic). Cell colony formation assays were performed in the different groups of cells: Right, representative figures; left, quantitative analysis. (G) SW620 Cells were divided into four groups: NC, overexpression of FOXP4 (FOXP4), overexpression of miR-491 (miR-491 mimic), and concurrent overexpression of FOXP4 and miR-491 (FOXP4 + miR-491 mimic). Cell Counting Kit-8 assays were performed in the different groups of transfected cells. *P<0.05, **P<0.01, ***P<0.001, as indicated. Experiments were repeated three times. miR-491, microRNA-491; FOXP4, forkhead box P4; UTR, untranslated region; NC, negative control; WT, wild-type; MT, mutant; OD, optical density.
Biological characteristics of circ-0000212
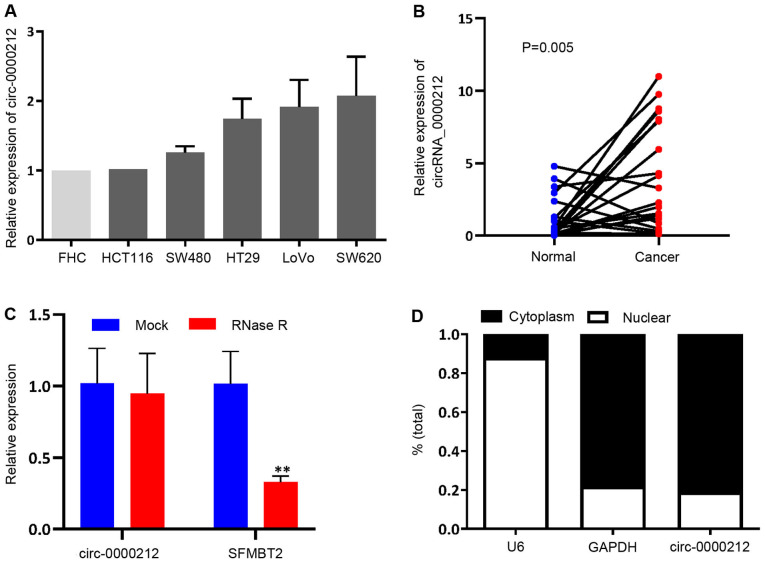
An increasing number of studies have demonstrated that circRNAs act as a novel class of miRNAs to regulate the target expression level and therefore, may be involved in oncogenesis and progression (19). To identify the circRNAs that could sponge miR-491 in CRC, circBase and starBase databases were used to identify potential circRNAs, that could bind to and sponge miR-491 (Table SII). As SFMBT2 is widely involved in cancer processes (20,21), from the results, hsa-circ-0000212 was selected, which is derived from the SFMBT2 gene (chromosome 10, 7405839-7423911). The expression levels of circ-0000212 were assessed in the CRC cell lines, and it was found that circ-0000212 was notably increased when compared with that in the normal FHC cell line, although the difference was not significant (Fig. 4A), opposite to the expression pattern of miR-491. Furthermore, circ-0000212 was also detected in 20 paired cancer and adjacent normal tissues, which revealed that circ-0000212 was expressed at a higher level compared with that in the normal adjacent tissues (Fig. 4B). According to Fig. 4C, compared with linear SFMBT2, circ-0000212 showed resistance to RNase R, indicating that circ-0000212 possessed a loop structure (Fig. 4C). Cytoplasmic and nuclear RNA analysis using RT-qPCR showed that circ-0000212 was primarily distributed in the cytoplasm (Fig. 4D).
Figure 4.
Biological characteristics of circ-0000212. (A) Expression of circ-0000212 in the different colorectal cancer cell lines and the normal colonic cell line FHC. (B) Relative expression of circ-0000212 in the 20 paired normal colon tissues and cancer tissues. (C) Expression of circ-0000212 or linear SFMBT2 mRNA after treatment of RNase R. **P<0.01 vs. Mock. (D) Localization of circ-0000212 in SW620 cells. Experiments were repeated three times. circ, circular RNA; NS, not significant.
circ-0000212 acts as a sponge for miR-491
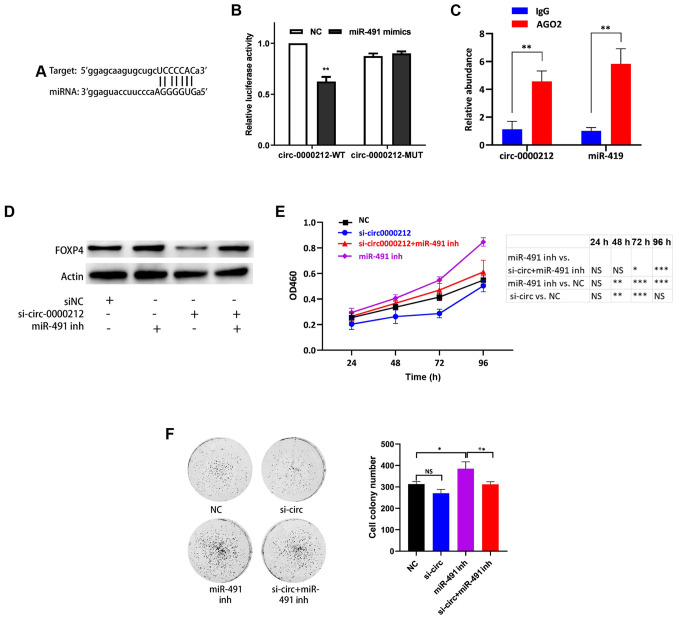
Considering that circRNAs can sponge miRNAs, and circ-0000212 was enriched in the cytoplasm, it was hypothesized that circ-0000212 could act as a sponge for, and bind to, miR-491. The potential binding site between circ-0000212 and miR-491 was identified (Fig. 5A), then the sequence was constructed and ligated downstream in the luciferase reporter plasmid. It was hypothesized that miR-491 could decrease the luciferase activity of circ-0000212. The luciferase reporter vector for circ-0000212 was co-transfected with miR-491 mimic and the results indicated that miR-491 markedly reduced the luciferase activity compared with that in the vector with the mutant binding sequence (Fig. 5B). Furthermore, a RIP assay was performed for AGO2, and the levels of endogenous circ-0000212 and miR-491 pulled-down from AGO2-expressing cells was determined using RT-qPCR analysis. The results demonstrated that circ-0000212 and miR-491 were highly enriched in the AGO2 pellet compared with that in the input control (Fig. 5C). As a sponge of miR-491, circ-0000212 could regulate the targets of miR-491. The cells were than transfected with either si-circ-0000212 and miR-491 inhibitor or si-circ-0000212 and miR-491 inhibitor at the same time. Notably, si-circ-0000212 decreased the expression levels of FOXP4, a target of miR-491, while inhibition of miR-491 increased the expression levels of FOXP4 (Figs. 5D, S2B and C). Furthermore, si-circ-0000212 reversed the effects of miR-491 on the regulation of FOXP4 (Figs. 5D, S2B and C). Finally, the ability of circ-0000212 to modulate cell proliferation in CRC was investigated. si-circ-0000212 significantly reduced the proliferative ability of the CRC cells (Figs. 5E, S2B and C), and consistently, it could also reverse the ability of the miR-491 inhibitor to increase cell proliferation (Figs. 5E and F, S2B and C). Taken together, circ-0000212 could act as sponge of miR-491.
Figure 5.
circ-0000212 acts as a sponge for miR-491. (A) Binding site between circ-0000212 and miR-491. (B) In the 293T cells, transfection of the miR-491 mimic decreased luciferase activity of the vector containing the wild type binding site of circ-0000212. **P<0.01 vs. NC. (C) AGO2 RNA immunoprecipitation assay to determine the quantity of circ-0000212 and miR-491. **P<0.01 vs. IgG. (D) Western blot analysis of the expression levels of FOXP4 after transfection with si-circ-0000212 + miR-491 inh in SW620 cells. (E) Cell Counting Kit-8 assay of SW620 cells after transfection with si-circ-0000212 + miR-491 inh. *P<0.05, **P<0.01, ***P<0.001. (F) Colony formation assay of SW620 cells after transfection with si-circ-0000212 + miR-491 inh. *P<0.05, **P<0.01, as indicated. Experiments were repeated three times. miR-491, microRNA-491; miR-491 inh, miR-491 inhibitor; si, small interfering; circ, circular RNA; OD, optical density; NC, negative control; FOXP4, forkhead box P4.
Discussion
Previous studies have shown that FOXP4 functions as a tumor promoter in various types of cancer (22,23), and is upregulated in cancer tissues (23). In the present study, the expression levels of FOXP4 were increased in a large CRC cohort based on data obtained from TCGA. Dysregulated miR-491 expression has been identified in various types of tumors. In breast cancer, miR-491 was downregulated in cancer tissues and functioned as a tumor suppressor by targeting TPX2 (24). In glioma, miR-491 overexpression inhibited cell proliferation via the Wnt3a/β-catenin pathway as well as invasion-mediated epithelial-mesenchymal transition (25). miR-491 also suppressed cell proliferation, motility and invasion in gastric cancer by targeting HMGA2 (26), or SNAIL and FGFR4 (27). Furthermore, miR-491 inhibited cell proliferation, motility and progression in prostate cancer (28) and nasopharyngeal carcinoma (29). Even though miR-491 has been investigated in CRC (30), there is limited research in this area. In the present study, there was a difference in the sample sizes between the normal and cancer tissues (8 vs. 457); however, the data were normally distributed, and the two groups were of equal variances. Therefore, the results are reliable. To the best of our knowledge, for the first time, these results showed that the miR-491 mimic suppressed cell proliferation by targeting FOXP4. Based on the association between miR-491 and FOXP4 in CRC tissues, the association between miR-491 and FOXP4 expression levels with the clinicopathological characteristics in patients with CRC were investigated. Both high miR-491 and low FOXP4 expression levels were associated with pathological and N stages, highlighting its potential as a diagnostic marker for patients with CRC. The aforementioned results show that miR-491 may act as a tumor suppressor in cancer. However, the results suggested that there was no association between miR-491 and reduced invasion of the CRC cells, as it was not associated with M stage, which has been shown in other types of cancer (24). This might be due to heterogeneity of different types of cancer and cell lines; therefore, further investigation is required to determine the role of miR-491 in different types of cancer.
An increasing number of studies has demonstrated that circRNAs are more than junk or by-products of errors during the splicing process. circRNAs can functionally regulate transcriptional activity and also play roles as miRNA sponges and therefore, modulate oncogenesis and cancer progression (31–34). In addition, circRNAs may also function as protein scaffolds, sequester proteins and function co-operatively to achieve measurable effects (31).
Genome-wide research has discovered a large number of new circRNAs with different functions in cancer. circRNAs can act as tumor promoters. circCCDC66 and circHIAT1 increased tumor proliferation and metastasis in various types of cancer (35,36). Conversely, other circRNAs have also been shown to inhibit cancer progression. circREPS2 and circ_0000353 suppresses gastric progression (37) and metastasis of non-small cell lung cancer (38), respectively, via modulation of multiple signaling pathways.
In the present study, circ-0000212 was selected. It is a circRNA derived from SFMBT2, which was shown to sponge miR-491. SFMBT2 has been shown to be dysregulated in several types of cancer and is involved in cell proliferation. It has been shown that SFMBT2 promoted cell viability through the epigenetic modulation of HOXB13 gene expression in prostate cancer cells (39). Furthermore, SFMBT2 positively regulated chondrocyte proliferation via SOX9 (40). Notably, it was discovered that circ-0000212 was upregulated in CRC tissues compared with that in adjacent normal tissues. In addition, compared with linear SFMBT2, circ-0000212 displayed stable expression in CRC cells and was preferentially distributed in the cytoplasm. Functional experiments found that circ-0000212 increased cell proliferation by sponging miR-491 and modulated FOXP4 expression levels. These data suggest that circ-0000212 may function as a tumor promoter in CRC via regulation of a miR-491/FOXP4 signaling axis. Notably, the CRC cell lines used in the present study had different expression levels of miR-491 and circ-0000212. The HCT116, SW480, HT29, LoVo and SW620 cells are classified as stages IV, II, III, III, and III, respectively. There was no significant association between the expression levels and the cell lines characteristics. Thus, other factors, such as gene mutation and chromosomal stability may affect the expression levels of miR-491 and circ-0000212.
circRNAs are stable and present different immunogenicity when synthesized in vitro, due to their specific loop structure, and therefore could be used as promising potential biomarkers for cancer diagnosis and prognosis (31,36). Previously, research has focused on the application of circRNA technologies to probe and operate intracellular processes (41), manipulate miRNA expression levels (42), repress innate immune responses (43), augment immune functions (44) and serve as biomarkers (45).
In conclusion, circ-0000212 may sponge miR-491 and therefore modulate FOXP4 expression in CRC cells. The present study lays the foundation for future studies, and circ-0000212 may serve as a novel target for the development of treatments to manage CRC.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 8170110698), Heilongjiang Province Natural Science Foundation (grant no. QC2017095), Heilongjiang Postdoctoral Fund (grant no. LBH-Z18270) and China Postdoctoral Science Foundation (grant no. 2019M651319).
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 8170110698), Heilongjiang Province Natural Science Foundation (grant no. QC2017095), Heilongjiang Postdoctoral Fund (grant no. LBH-Z18270) and China Postdoctoral Science Foundation (grant no. 2019M651319).
Availability of data and materials
TCGA dataset is available from https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga. The other datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QZ conceived the study. GW provided administrative support and analyzed data. YT, performed the experiments, collected the study materials and patient data. HW performed the experiments. WZ analyzed and interpretated the data. All authors confirm the authenticity of all the raw data. All authors wrote the manuscript, and read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of The Second Affiliated Hospital of Harbin Medical University (approval no. KY2017-031). All patients consent to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127:761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lekka E, Hall J. Noncoding RNAs in disease. FEBS Lett. 2018;592:2884–2900. doi: 10.1002/1873-3468.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzi L, Avila Cobos F, Decock A, Everaert C, Helsmoortel H, Lefever S, Verboom K, Volders PJ, Speleman F, Vandesompele J, Mestdagh P. Long noncoding RNA expression profiling in cancer: Challenges and opportunities. Genes Chromosomes Cancer. 2019;58:191–199. doi: 10.1002/gcc.22709. [DOI] [PubMed] [Google Scholar]
- 5.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corté H, Manceau G, Blons H, Laurent-Puig P. MicroRNA and colorectal cancer. Dig Liver Dis. 2012;44:195–200. doi: 10.1016/j.dld.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Belaguli N, Berger DH. MicroRNA and colorectal cancer. World J Surg. 2009;33:638–646. doi: 10.1007/s00268-008-9865-5. [DOI] [PubMed] [Google Scholar]
- 8.Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer-a brief overview. Adv Biol Regul. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Wilusz JE, Sharp PA. Molecular biology. A circuitous route to noncoding RNA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu S, Zhong Y, Shang R, Zhang X, Song W, Kjems J, Li H. The emerging landscape of circular RNA in life processes. RNA Biol. 2017;14:992–999. doi: 10.1080/15476286.2016.1220473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou DN, Ye CS, Deng YF. CircRNAs: Potency of protein translation and feasibility of novel biomarkers and therapeutic targets for head and neck cancers. Am J Transl Res. 2020;12:1535–1552. [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Zhang X, Yan M, Li H. Emerging role of circular RNAs in cancer. Front Oncol. 2020;10:663. doi: 10.3389/fonc.2020.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong Z, Huang M, Lv M, He Y, Duan C, Zhang L, Chen J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Barrett SP, Salzman J. Circular RNAs: Analysis, expression and potential functions. Development. 2016;143:1838–1847. doi: 10.1242/dev.128074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Dong X, Yan B, Yu W, Shan L. CircAGFG1 drives metastasis and stemness in colorectal cancer by modulating YY1/CTNNB1. Cell Death Dis. 2020;11:542. doi: 10.1038/s41419-020-2707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM, Castiglioni I, et al. TCGAbiolinks: An R/bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44:e71. doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zellweger T, Miyake H, Cooper S, Chi K, Conklin BS, Monia BP, Gleave ME. Antitumor activity of antisense clusterin oligonucleotides is improved in vitro and in vivo by incorporation of 2′-O-(2-methoxy)ethyl chemistry. J Pharmacol Exp Ther. 2001;298:934–940. [PubMed] [Google Scholar]
- 19.Zhao ZJ, Shen J. Circular RNA participates in the carcinogenesis and the malignant behavior of cancer. RNA Biol. 2017;14:514–521. doi: 10.1080/15476286.2015.1122162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwak J, Jeong H, Lee K, Shin JY, Sim T, Na J, Kim J, Ju BG. SFMBT2-mediated infiltration of preadipocytes and TAMs in prostate cancer. Cancers (Basel) 2020;12:2718. doi: 10.3390/cancers12092718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwak J, Shin JY, Lee K, Hong SK, Oh S, Goh SH, Kim WS, Ju BG. SFMBT2 (Scm-like with four mbt domains 2) negatively regulates cell migration and invasion in prostate cancer cells. Oncotarget. 2016;7:48250–48264. doi: 10.18632/oncotarget.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, Zhang J. Upregulation of FOXP4 in breast cancer promotes migration and invasion through facilitating EMT. Cancer Manag Res. 2019;11:2783–2793. doi: 10.2147/CMAR.S191641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Zhang G. Upregulation of FoxP4 in HCC promotes migration and invasion through regulation of EMT. Oncol Lett. 2019;17:3944–3951. doi: 10.3892/ol.2019.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan GZ, Li M, Tan X, Shi ML, Mou K. miR-491 suppresses migration and invasion via directly targeting TPX2 in breast cancer. Eur Rev Med Pharmacol Sci. 2019;23:9996–10004. doi: 10.26355/eurrev_201911_19566. [DOI] [PubMed] [Google Scholar]
- 25.Meng Y, Shang FR, Zhu YL. miR-491 functions as a tumor suppressor through Wnt3a/β-catenin signaling in the development of glioma. Eur Rev Med Pharmacol Sci. 2019;23:10899–10907. doi: 10.26355/eurrev_201912_19793. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Lü Y, Jiang Q, Yang Y, Dang C, Sun R. miR-491 inhibits BGC-823 cell migration via targeting HMGA2. Int J Biol Markers. 2019;34:364–372. doi: 10.1177/1724600819874488. [DOI] [PubMed] [Google Scholar]
- 27.Yu T, Wang LN, Li W, Zuo QF, Li MM, Zou QM, Xiao B. Downregulation of miR-491-5p promotes gastric cancer metastasis by regulating SNAIL and FGFR4. Cancer Sci. 2018;109:1393–1403. doi: 10.1111/cas.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Hou R, Lu Q, Zhang Y, Chen L, Zheng Y, Hu B. miR-491-5p negatively regulates cell proliferation and motility by targeting PDGFRA in prostate cancer. Am J Cancer Res. 2017;7:2545–2553. [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Q, Xu X, Jiang H, Xu L, Li Q. Knockdown of long non-coding RNA XIST suppresses nasopharyngeal carcinoma progression by activating miR-491-5p. J Cell Biochem. 2018;119:3936–3944. doi: 10.1002/jcb.26535. [DOI] [PubMed] [Google Scholar]
- 30.Hanisch C, Sharbati J, Kutz-Lohroff B, Huber O, Einspanier R, Sharbati S. TFF3-dependent resistance of human colorectal adenocarcinoma cells HT-29/B6 to apoptosis is mediated by miR-491-5p regulation of lncRNA PRINS. Cell Death Discov. 2017;3:16106. doi: 10.1038/cddiscovery.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 32.Zhou ZB, Huang GX, Fu Q, Han B, Lu JJ, Chen AM, Zhu L. circRNA.33186 contributes to the pathogenesis of osteoarthritis by sponging miR-127-5p. Mol Ther. 2019;27:531–541. doi: 10.1016/j.ymthe.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Yu Y, Huang Z, Kong Y, Hu X, Xiao W, Quan J, Fan X. CircRNA-5692 inhibits the progression of hepatocellular carcinoma by sponging miR-328-5p to enhance DAB2IP expression. Cell Death Dis. 2019;10:900. doi: 10.1038/s41419-019-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, Tsai SJ. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77:2339–2350. doi: 10.1158/0008-5472.CAN-16-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, Sun Y, Tao W, Fei X, Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12. doi: 10.1016/j.canlet.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Dai X, Liu J, Cheng A, Qin C, Wang Z. Circular RNA circREPS2 Acts as a sponge of miR-558 to suppress gastric cancer progression by regulating RUNX3/β-catenin signaling. Mol Ther Nucleic Acids. 2020;21:577–591. doi: 10.1016/j.omtn.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao WX, Tang YL, Wang WH, Bao MW. Up-regulation of circ_0000353 impedes the proliferation and metastasis of non-small cell lung cancer cells via adsorbing miR-411-5p and increasing forkhead box O1. Cancer Biomark. 2020;29:25–37. doi: 10.3233/CBM-190812. [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Na W, Maeng JH, Wu H, Ju BG. Regulation of DU145 prostate cancer cell growth by Scm-like with four mbt domains 2. J Biosci. 2013;38:105–112. doi: 10.1007/s12038-012-9283-6. [DOI] [PubMed] [Google Scholar]
- 40.Hussain S, Sun M, Guo Y, Mushtaq N, Zhao Y, Yuan Y, Hussain N, Osoro E, Suleiman A, Sadiq M, et al. SFMBT2 positively regulates SOX9 and chondrocyte proliferation. Int J Mol Med. 2018;42:3503–3512. doi: 10.3892/ijmm.2018.3894. [DOI] [PubMed] [Google Scholar]
- 41.Litke JL, Jaffrey SR. Highly efficient expression of circular RNA aptamers in cells using autocatalytic transcripts. Nat Biotechnol. 2019;37:667–675. doi: 10.1038/s41587-019-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jost I, Shalamova LA, Gerresheim GK, Niepmann M, Bindereif A, Rossbach O. Functional sequestration of microRNA-122 from hepatitis C virus by circular RNA sponges. RNA Biol. 2018;15:1032–1039. doi: 10.1080/15476286.2018.1435248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CX, Li X, Nan F, Jiang S, Gao X, Guo SK, Xue W, Cui Y, Dong K, Ding H, et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell. 2019;177:865–880.e21. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 44.Chen YG, Kim MV, Chen X, Batista PJ, Aoyama S, Wilusz JE, Iwasaki A, Chang HY. Sensing self and foreign circular RNAs by intron identity. Mol Cell. 2017;67:228–238.e5. doi: 10.1016/j.molcel.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT, Xiao X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61:221–230. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
TCGA dataset is available from https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga. The other datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.