Abstract
Postoperative cognitive dysfunction (POCD) is a common complication following cardiopulmonary bypass (CPB). U50488H, a κ-opioid receptor (KOR) agonist, can specifically activate KORs on hippocampal nerve cells, resulting in neuroprotective effects. The present study established a CPB rat model, observed the protective effect of U50488H on CPB-induced POCD and brain damage and explored the regulatory mechanism of the PI3K/AKT/nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase (HO)-1 pathway. Sprague-Dawley rats were divided into the following groups: Sham operation (Sham group), CPB (CPB group), KOR agonist (U50488H) + CPB (U50488H group), CPB + U50488H + HO-1 antagonist (ZnPP-IX; ZnPP group) and CPB + U50488H + PI3K antagonist (LY294002; LY294002 group), with 10 rats in each group. Neurological scores and the Morris water maze test were used to evaluate cognitive function; hematoxylin and eosin and terminal deoxynucleotidyl transferase dUTP nick end labeling assays were performed to observe hippocampal neuron damage in rats. Immunofluorescence was used to detect reactive oxygen species, glial fibrillary acidic protein and Nrf2 expression in the hippocampus. Enzyme-linked immunosorbent assays were used to detect inflammatory and oxidative stress factors. Western blotting was used to examine the expression of PI3K/AKT/Nrf2/HO-1-related proteins. It was demonstrated that U50488H significantly reduced the neural function score of rats with POCD induced by CPB, relieved cognitive dysfunction, reduced hippocampal neuron damage, inhibited the rate of apoptosis, repaired oxidative stress injury and protected against brain damage caused by CPB. In addition, U50488H could promote Nrf2 entry into the nucleus and upregulate HO-1 and thioredoxin 1 (Trx1) expression. In CPB rats treated with PI3K inhibitors, less Nrf2 was detected in the nucleus and HO-1 and Trx-1 expression levels were reduced in the nucleus. Therefore, U50488H, a KOR agonist, can activate Nrf2/HO-1 via the PI3K/AKT pathway to improve cognitive function and reduce brain damage in CPB rats.
Keywords: κ-opioid receptor agonist, cardiopulmonary bypass, postoperative cognitive dysfunction, phosphatidylinositol 3-kinase/AKT signaling pathway, nuclear factor erythroid 2-related factor 2/heme oxygenase 1
Introduction
Following cardiopulmonary bypass (CPB), the incidence rates of short-term cognitive abnormalities, memory and learning ability decline and visual-motor response declines by 60–80% (1). The incidence of postoperative cognitive dysfunction (POCD) is ~50–70% at 1 week following open-heart surgery (2). This incidence declines to 30–50% after 2 months; however, in 40% of patients cognitive function is not restored to the preoperative level after 5 years (3,4). Therefore, it is necessary to prevent and treat POCD induced by CPB during cardiac surgery, which remains a challenge and difficulty for clinicians.
The mechanism of POCD following CPB is complicated. Persistent perfusion with blood flowing through a CPB machine with a simulated human respiratory cycle, temperature decrease, damage to important molecules in the blood, ischemia and reperfusion and release of cytokines (IL-6 and TNF-α) and reactive oxygen species (ROS) in large amounts caused by endotoxemia can directly trigger systemic inflammatory response syndrome during CPB (5,6) and oxidative stress (7), leading to vital organ dysfunction and even permanent damage. Among these factors, oxidative stress serves an important role in the occurrence of POCD (8). Additionally, changes in the central cholinergic system and levels of Tau protein, calcium ions and γ-aminobutyric acid in the hippocampus following CPB can promote the occurrence of POCD (9).
In recent years, the protective effects mediated by endogenous heme oxygenase 1 (HO-1) on cognitive functions have been investigated (10). The antioxidant enzyme HO-1 serves an important role in oxidative stress (11). HO-1 is overexpressed in a variety of situations, including organ transplantation, acute kidney injury, hypertension and atherosclerosis (12–16). HO-1 has anti-inflammatory, anti-proliferative, anti-oxidative and anti-apoptotic activities and remains a promising target for the treatment of oxidative stress-related diseases (17). Nrf2, an anti-oxidative transcription factor, binds to the antioxidant responsive element (ARE) to initiate the expression of multiple antioxidant and anti-inflammatory proteins as well as downstream detoxification enzymes, which are key factors in regulating the transcription and expression of HO-1 (18). Nrf2 can be activated by protein kinases including PI3K/AKT (19). Activation of the AKT/mTOR pathway reduces the occurrence of POCD in rats (20). A previous study demonstrated that following PI3k/AKT pathway activation by phytoestrogens, the downstream protein GSK-3β is inactivated, which subsequently upregulates HO-1 expression, thereby inhibiting the neurotoxic effects of amyloid β (Aβ)25-35 (21). Puerarin has been confirmed to activate Nrf2 to upregulate HO-1 expression and protect primary cultured hippocampal neurons in rats from Aβ25-35-induced damage following GSK-3β inactivation (22).
Opioids are currently the most widely used analgesic drugs in clinical practice. Three types of classic opioid receptors exist including µ-opioid receptors, δ-opioid receptors and κ-opioid receptors (KORs). KORs are abundantly expressed in the prefrontal cortex and other brain regions and can regulate mood and cognitive functions. KORs have been demonstrated to alleviate brain damage and improve functional recovery in animal models with both systemic and regional cerebral ischemia (23). The KOR agonist U50488H was administered in the hippocampus during nerve injury induced by ischemia and the results demonstrate significant decline in cognitive impairment (24). However, its specific regulatory mechanism remains to be elucidated. Charron et al (24) only reported that KOR agonists were beneficial to the activation of hippocampal cholinergic neurons, which thus counteracted the memory impairment caused by scopolamine. However, whether KOR agonists induce transcription and expression of HO-1 by regulating the PI3K/AKT/Nrf2 pathway in the hippocampus of rats, inhibit oxidative stress following CPB and improve cognitive functions following CPB are rarely examined. In the present study, bloodless priming rat models of CPB were used and the KOR agonist U50488H was administered to the rats to investigate the protective effects of KORs on cognitive functions following CPB. The HO-1 antagonist ZnPP-IX and the PI3K antagonist LY294002 were then separately administered to observe the role of the PI3K/AKT/Nrf2/HO-1 signaling pathway in the protective effects on cognitive functions induced by KOR agonists following CPB. Hence, the present study aimed to investigate possible protective mechanisms and to provide an experimental basis for the clinical application of KOR agonists.
Materials and methods
Experimental animals and grouping
Specific pathogen free Sprague-Dawley rats (n=50; male; 400–480 g) were purchased from Liaoning Changsheng Biological Co., Ltd. [SCXK (Liao): 2017–0001]. The present study was approved by the China Medical University Laboratory Animal Welfare and Ethics Committee (IACUC no. 2018048R). All experimental procedures were performed in strict accordance with the guidelines for management and protection of laboratory animals. The animals were divided into the following groups: Sham operation (Sham group), CPB (CPB group), KOR agonist (U50488H) + CPB (U50488H group), CPB + U50488H + HO-1 antagonist (ZnPP-IX; ZnPP group) and CPB + U50488H + PI3K antagonist (LY294002; LY294002 group) based on a random number table, with 10 rats in each group. All rats were cultivated in individual ventilated cages at 24±2°C and 40–70% humidity in a 12-h light/dark cycle. Standard pelleted chow and drinking water were available ad libitum.
Establishment of bloodless priming CPB models with a beating heart
CPB rat models were established according to the procedure reported by Sun et al (25). The rats were fasted for 6 h before the operation but were allowed to drink freely. Rats were anesthetized with 30 mg/kg 2% sodium pentobarbital (Sigma-Aldrich; Merck KGaA) and underwent tracheal intubation (16G trocar) using a light transmission method. Intraoperative maintenance was conducted by intermittent administration of sodium pentobarbital and 1% rocuronium (Zhejiang Xianju Pharmaceutical Co., Ltd.). Vital signs including the heart rate, pulse oximetry and body temperature were monitored. Catheter insertion (24G) was performed by puncturing the left femoral artery and the pressure was measured. The needle (22G) was inserted in the right femoral vein as a channel for fluid supplementation. Another needle (22G) was indwelled in the caudal artery for CPB perfusion. Catheter insertion (18G with a porous tip) was performed via the right neck vein and the catheter was placed at the level of the right atrium to be used as a drainage end. Priming solution was prepared using 3 ml of succinylated gelatin, 1 ml of lactated Ringer's solution, 1 ml of 20% mannitol, 250 IU/kg heparin and 0.5 ml of 5% sodium bicarbonate and 10 mg/kg furosemide. Systemic heparinization (400 IU/kg) was injected into the left femoral vein once the activated clotting time reached 480 sec. CPB was then started and mechanical ventilation was halted. During CPB, the mean arterial pressure was maintained above 60 mmHg. Based on the blood gas report, drug application and respiratory parameters were adjusted, the pH value was maintained at 7.35–7.45, PaCO2 was 35–45 mmHg, hemoglobin was >70 g/l and the hematocrit was maintained >25%. Subsequently, 10 min before the completion of CPB, the drainage and bypass rates were gradually slowed. Mechanical ventilation was recovered following completion of CPB. Catheters in various blood vessels were removed in sequence and the anal temperature was maintained at 36.5–37.5°C. Fluid was supplemented appropriately, or vasoactive drugs were applied if needed.
For rats in the Sham group, catheter insertion was performed under anesthesia, but the CPB model was not established. For CPB group rats, CPB models were established following catheter insertion under anesthesia and bypass was maintained for 1 h. Rats in the U50488H group were intravenously injected with U50488H (1.5 mg/kg, cat. no. 0495/25; Tocris Bioscience) before CPB surgery. Rats in the ZnPP and LY294002 groups were first intravenously injected with ZnPP-IX (5 mg/kg; Sigma-Aldrich; Merck KGaA) or LY294002 (0.3 mg/kg; Sigma-Aldrich; Merck KGaA) and 30 min later intravenously injected with U50488H (1.5 mg/kg) before CPB surgery.
Neurological deficit score
According to the neurological scoring method of Longa et al (26), rats were scored for neurological deficits following the Morris water maze test. The criteria were as follows: No symptoms of neurological deficits (0 points); signs of flexion of the left upper limb after lifting the tail, not fully extended (1 point); showing rotation to the left and moving in circles (2 points); crawling to the left (3 points); involuntary movement and exhibiting disturbance of consciousness (4 points).
Morris water maze test
To observe the changes in cognitive abnormalities and memory and learning capability of CPB rats under U50488U treatment, the Morris water maze test (Shanghai Xinsoft Information Technology Co., Ltd.)was performed for rats in all groups on the third day following CPB (27). The water temperature was maintained at 22.0±10°C. A platform with an area of ~38 cm2 was located 2–3 cm below the horizontal plane. Black non-toxic stain was added to the water so that the rat could not see the platform. The pool was evenly divided into four quadrants (left top, right top, left bottom, right bottom) and the platform was placed in the center of the left bottom quadrant.
Acquisition test (hidden platform training): Prior to the operation, the rats were trained to find the platform twice each day and once each night for 5 days. The rats were randomly placed in the water in any of the four quadrants. The latency period of finding the hidden platform was calculated from the time the rat entered the water to the time that it climbed onto the platform. Each training period was limited to 1 min. If the rat could not find the hidden platform within 1 min, the latency was recorded as 1 min. Then, the rat was placed on the platform to rest for 1 min in order to help the rat locate the position of the platform. The movement paths of the rats were imaged and recorded by the system. In addition, the distance, duration, resting time, number of times entering the water and rates were analyzed. This experiment was used to assess the short-term memory and learning ability of the animals.
Memory retention test (spatial exploration): The platform was removed on day 6 and the rats were placed into the water from the right top quadrant. The time spent swimming in each quadrant, number of times crossing over the platform and swimming distance within 1 min were recorded and analyzed by the system automatically. The residence time, swimming distance and number of times crossing over the original platform in the left bottom quadrant (the quadrant where the platform was originally placed) were recorded to evaluate memory storage as well as retrieval and replication abilities of the rats.
Hematoxylin and eosin (H&E) staining and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
On the 7th day after the establishment of the rat CPB model, the rats received an intraperitoneal injection of sodium pentobarbital (200 mg/kg body weight) for sacrifice. The heartbeat of the rats was assessed for ≥5 min to confirm mortality. The hippocampal tissue was collected and post-fixed in perfusion fixative for 2 h at room temperature, immersed in 20% sucrose solution, then immersed in wax and embedded. The following day, the hippocampus was coronally sectioned at 5-µm-thick sections for H&E staining and TUNEL. A hematoxylin-eosin staining kit was purchased from Boster Biological Technology (Wuhan, Hubei, China) for H&E staining. A TUNEL assay kit (In situ Cell Death Detection kit-POD; Sigma cat. no. 11684817910) was used for TUNEL assay. The protocol was according to the manufacturer's protocol. The H&E results and TUNEL-positive cells on each slice were then observed using light microscopy (Olympus Corporation). Three typical 200 × fields of view were randomly selected from each section for quantification.
Immunofluorescence (IF)
After the wax-embedded hippocampal tissue sections were deparaffinized and then rehydrated. For antigen retrieval, the 5-µm paraffin sections were autoclaved (121°C, 20 min) in a solution of 0.1 M citric acid and 0.1 M sodium citrate. The sections were blocked in the blocking solution and incubated at 37°C for 30 min. The serum was decanted and glial fibrillary acidic protein (GFAP) antibodies (1:500; cat. no. ab7260; Abcam) and Mito-Tracker Red CMXRos (Shanghai Biyuntian Biotechnology Co., Ltd.) were added and the sections were incubated overnight at 4°C. After washed with PBS, the sections were incubated in Alexa Fluor 488-conjugated secondary antibodies (1:400) at 37°C for 30 min, followed by 4′,6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei. Sections were imaged using a Zeiss Axio Observer fluorescence microscope. Three typical 200 × fields of view were randomly selected from each section for quantification.
ELISA
The S100 β (cat. no. SEA567Ra) and neuron specific enolase (NSE; cat. no. SEA537Ra) expression levels in the rat hippocampus, the serum expression levels of the inflammatory factors IL-1β (cat. no. SEA563Ra) and IL-6 (cat. no. SEA079Ra) and the expression levels of the oxidative stress indicators superoxide dismutase (SOD; cat. no. SES134Ra), malondialdehyde (MDA, CEA597Ge) and myeloperoxidase (MPO, cat. no. SEA601Ra) were detected by ELISA kits. These ELISA kits were purchased from Wuhan USCN Business Co., Ltd. The protocol was performed according to the manufacturer's instructions.
Western blot analysis
The hippocampal tissues were weighed, ground, homogenized and centrifuged at 10,000 × g for 15 min at 4°C. pre-cooled RIPA (cat. no. 89900; Thermo Fisher Scientific, Inc.) lysate was added and was lysed on ice for 30 min. The supernatant was extracted to detect the protein levels through BCA method (cat. no. 23225; Thermo Fisher Scientific, Inc.). The proteins (30 µg/well) were loaded and separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The proteins were transferred to 12% polyvinylidene fluoride (PVDF) membrane for 1 h. The PVDF membrane was washed with PBS-T (PBS plus 0.1% Tween-20). Then, 5% skimmed milk blocking solution was added and the membrane was placed in a shaker at 4°C overnight. Then, the membrane was washed and primary antibodies to Bcl2 (1:1,000: cat. no. ab59348; Abcam), Bax (1:1,000; cat. no. ab32503; Abcam), PI3K (1:1,000; cat. no. ab191606; Abcam), AKT (1:1,000; cat. no. ab179463; Abcam), phosphorylated (p)-AKT (1:1,000; cat. no. ab131443; Abcam), HO-1 (1:1,000; cat. no. ab13248; Abcam), Nrf2 (1:1,000; cat. no. ab92946; Abcam), thioredoxin 1 (Trx1; 1:1,000; cat. no. ab185544; Abcam), H3 (1:1,000; cat. no. ab1791; Abcam) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1,000; cat. no. ab9485; Abcam) were added and incubated for 1 h. The membrane was washed three times consecutively. Then, the secondary antibody, goat anti-rabbit IgG H&L (HRP; 1:10,000; cat. no. ab6721; Abcam) antibody, was added and the membrane was placed on a shaker for 1 h. The membrane was rinsed and developed by luminescence (ECL; Amersham; Cytiva). A gel imaging system (Gel Doc™ XR; Bio-Rad Laboratories, Inc.) was used for capturing images. Absorbance values were analyzed using ImageJ (v1.8.0; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS 19.0 statistical software (IBM Corp.). Experimental data are expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) was performed followed with Tukey's post-hoc test for comparison tests. A χ2 test was used to compare ratios. A Kruskal-Wallis test with post-hoc Dunn's tests was performed in Figs. 1A and D, 5A and S1D. Each experiment was repeated at least three times. P<0.05 was considered to indicate a statistically significant difference.
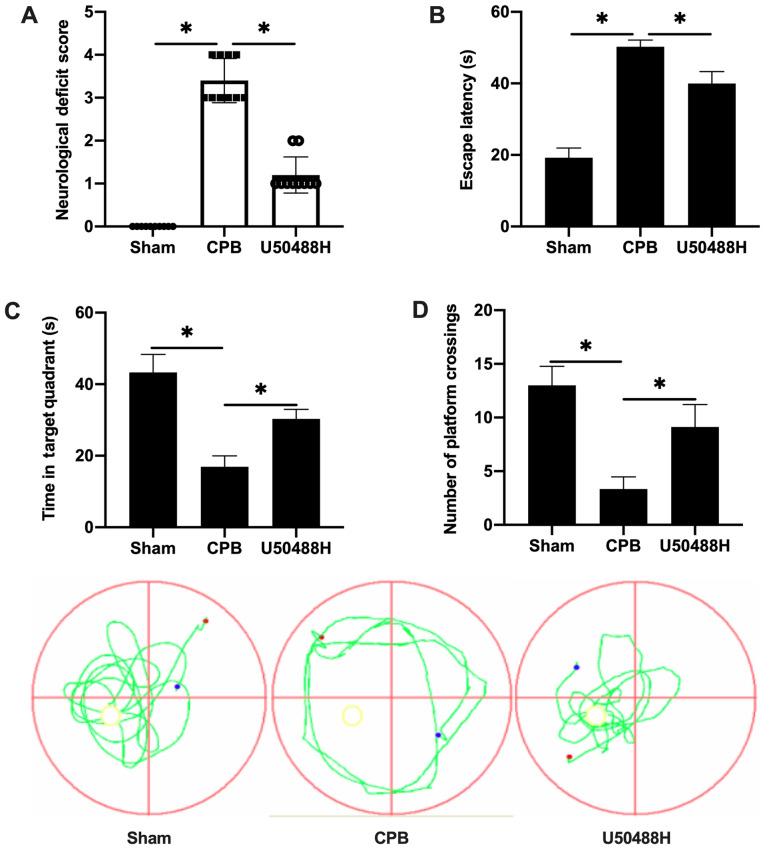
Figure 1.
KOR agonist improves cognitive function following CPB in rats. (A) Neurological function score. (B) Time of escape latency. (C) Time in target quadrant. (D) Number of platform crossings. n=10; *P<0.05. KOR, κ-opioid receptor; CPB, cardiopulmonary bypass.
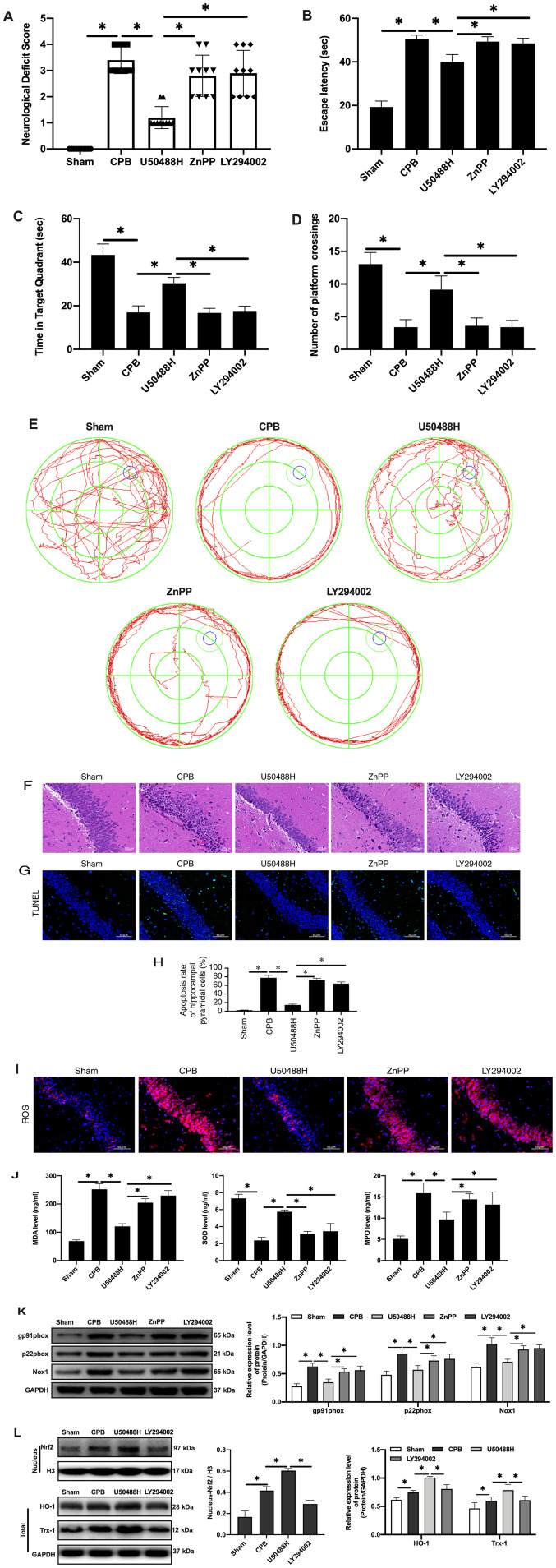
Figure 5.
KOR agonist activates Nrf2/HO-1 via PI3K/AKT pathway to improve cognitive function in CPB rats. (A) Neurological deficit score. (B) Time of escape latency. (C) Time in target quadrant. (D) Number of platform crossings. (E) Water maze track. (F) Hematoxylin and eosin staining scale bar, 100 µm). (G) TUNEL staining (scale bar, 50 µm; n=10). (H) Apoptosis rate. (I) Expression of ROS detected by immunofluorescence (scale bar, 50 µm; n=10). (J) Oxidative stress factors detect by ELISA. (K) Expression of gp91phox, p22phox and Nox1 proteins detected by western blotting. (L) Expression of Nrf2/HO-1 signaling pathway-related proteins detected by western blotting (n=10; *P<0.05). KOR, κ-opioid receptor; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1; CPB, cardiopulmonary bypass; ROS, reactive oxygen species; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1; Nox1, NADPH Oxidase 1; Trx1, thioredoxin 1.
Results
KOR agonist improves cognitive function following CPB in rats
To observe the cognitive abnormalities and memory and learning capability changes following CPB surgery, a rat CPB model was successfully established and the Longa method used to score neurological deficits. Treatment with U50488H improved the performance of CPB rats and significantly decreased the neurological deficit score (Fig. 1A). The Morris water maze navigation experiment results demonstrated that the escape latency gradually decreased. The escape latency in the CPB group was significantly longer than that in the Sham group and U50488H could shorten the escape latency of CPB rats (Fig. 1B). A spatial exploration experiment was performed to assess the spatial memory ability of rats. For rats in the CPB group, the retention time in the target quadrant was significantly less compared with that in the Sham group, however, rats in the U50488H group exhibited longer target quadrant retention times compared with those in the CPB group (Fig. 1C). In addition, the number of crossing platform in U50488H group was significantly more compared with that in CPB group (Fig. 1D). These results demonstrated that the KOR agonist significantly improved the cognitive function of CPB rats.
KOR agonist improves brain injury in CPB rats
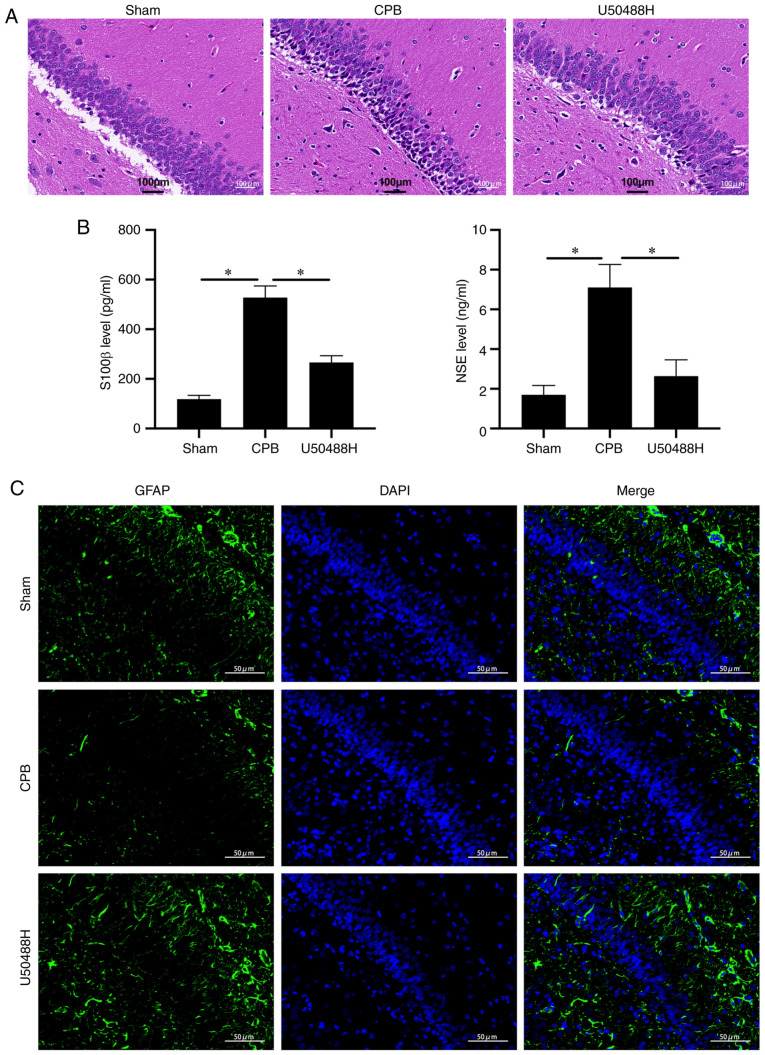
To observe the effect of U50448H on hippocampal neuronal injury, H&E staining was performed and it demonstrated that, in the CPB group, pyramidal cells in the hippocampal CA1 area were significantly reduced, the cytoplasm was severely decreased, nuclear volume was increased, nuclear vacuoles were enlarged and the intercellular space was widened. Neuronal cell damage was alleviated following U50488H treatment (Fig. 2A). When brain tissue is damaged, S100 β and NSE proteins are continuously released into the plasma, glial cells are damaged to varying degrees and blood-brain barrier permeability increases (28). The plasma levels of S100 β and NSE were significantly increased in CPB model rats and could be significantly reduced by U50488 treatment (Fig. 2B). In addition, under IF detection, U50488H was found to increase GFAP content, suggesting protection of hippocampal pyramidal cells (Fig. 2C). These results suggested that the KOR agonist significantly improved hippocampal neuron damage in rats subjected to CPB.
Figure 2.
KOR agonist improves brain injury in CPB rats. (A) Hematoxylin and eosin staining (scale bar, 100 µm). (B) Brain damage markers were detected by ELISA. (C) The GFAP expression of pyramidal cells in the hippocampus was detected by immunofluorescence (scale bar, 50 µm). n=10; *P<0.05. KOR, κ-opioid receptor; CPB, cardiopulmonary bypass; GFAP, glial fibrillary acidic protein; NSE, neuron specific enolase.
KOR agonist activates PI3K/AKT-related proteins to inhibit neuronal apoptosis in CPB rats
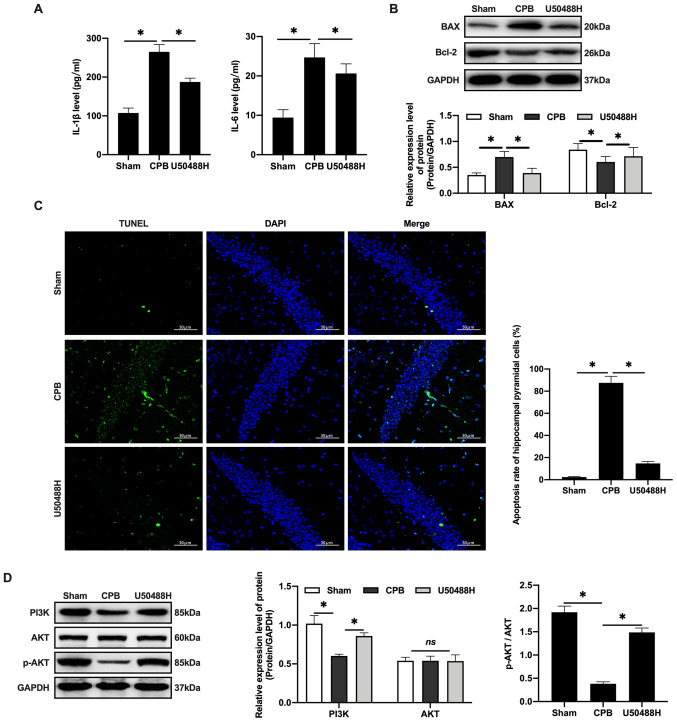
CPB surgery can promote the release of inflammatory factors, increase IL-1β and IL-6 plasma levels (Fig. 3A) and promote Bax and inhibit Bcl-2 in hippocampal neurons (Fig. 3B). U50488H treatment was demonstrated to decrease the apoptosis rate in rats (Fig. 3C). Previous studies have demonstrated that activating the PI3K/AKT signaling pathway can reduce the apoptosis rate of neurons caused by ischemia and hypoxia and inhibit the expression of Bax, thereby exerting brain protective effects (29–32). These results confirmed that U50488H can activate PI3K to phosphorylate of AKT and the activated AKT could regulate downstream proteins (Fig. 3D) and inhibit apoptosis, thus protecting cerebral tissue.
Figure 3.
KOR agonist activates PI3K/AKT-related proteins to inhibit neuronal apoptosis in CPB rats. (A) Inflammatory factors detected by ELISA. (B) Western blot were used to detect apoptosis factors of Bax and Bcl-2. (C) TUNEL staining (scale bar, 50 µm). (D) Expression levels of PI3K/AKT-related proteins were detected by western blotting. n=10; *P<0.05. KOR, κ-opioid receptor; CPB, cardiopulmonary bypass; p-, phosphorylated.
KOR agonist activates Nrf2/HO-1 to inhibit oxidative stress injury in CPB rats
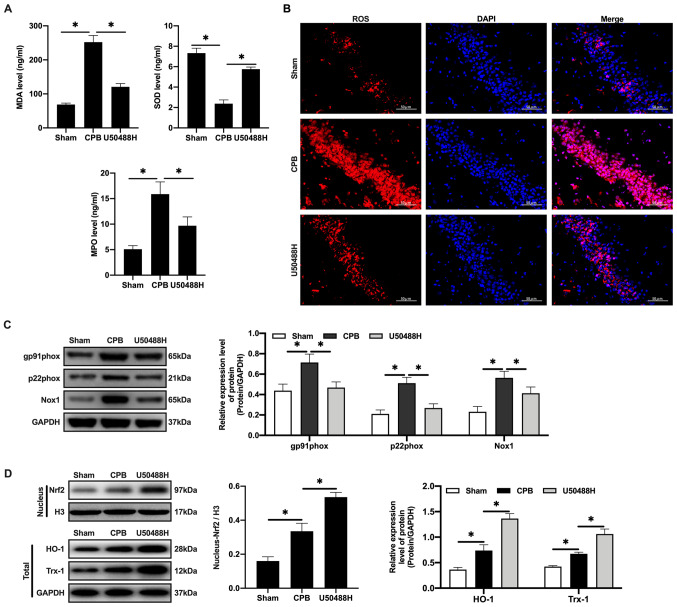
Oxidative stress is the main cause of neuronal apoptosis (33). Following CPB, it is difficult for cerebral cells to produce ATP through aerobic respiration (34,35). Therefore, the MDA and MPO levels increase and the SOD content decreases in plasma (Fig. 4A). Under U50448H treatment the MDA and MPO levels decreased, the SOD content increased and ROS expression in cerebral tissues was significantly reduced compared with the CPB group (Fig. 4B). In addition, the expression of the NADPH oxidase subunit components gp91phox, p22phox and NADPH Oxidase 1 was upregulated in the CBP group (Fig. 4C) and the expression of these components was also reversed by U50448H treatment (Fig. 4C). Nrf2/HO-1 is the main regulatory pathway of oxidative stress. CPB surgery could induce oxidative stress in the whole rat body and activate and increase Nrf2 content in the nucleus (Fig. 4D). KOR agonists can promote entrance of Nrf2 into the nucleus, which then combines with the ARE to initiate transcription of proteins downstream of ARE, including HO-1 and Trx-1, thus inhibiting injury (Fig. 4D).
Figure 4.
KOR agonist activates Nrf2/HO-1 to inhibit oxidative stress injury in CPB rats. (A) Oxidative stress factors detected by ELISA. (B) The expression of ROS detected by immunofluorescence (scale bar, 50 µm). (C) The expression of Nrf2/HO-1 signaling pathway-related proteins detected by western blotting. (D) Expression levels of gp91phox, p22phox and Nox1 were detected by western blotting. n=10; *P<0.05. KOR, κ-opioid receptor; CPB, cardiopulmonary bypass; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1; MDA, malondialdehyde; SOD, superoxide dismutase; MPO, myeloperoxidase; ROS, reactive oxygen species; Nox1, NADPH Oxidase 1; Trx1, thioredoxin 1.
KOR agonist activated Nrf2/HO-1 via the PI3K/AKT pathway to improve cognitive function in CPB rats
Nrf2 has been reported to be regulated by the PI3K/AKT signaling pathway (36). To explore the regulatory mechanism of PI3K/AKT/Nrf2/HO-1 in CPB, rats were treated with PI3K and HO-1 inhibitors to observe the protective effects of the KOR agonist on brain injury in CPB rats. The results demonstrated that compared with the U50488H group, rats treated with PI3K or HO-1 inhibitors demonstrated significantly increased neurological deficit scores (Fig. 5A), extended escape latency in the navigation experiment in the Morris water maze assay (Fig. 5B-E), fewer pyramidal cells and increased damage in the hippocampal CA1 area (Fig. 5F), an increased apoptosis rate of neuronal cells (Fig. 5G-H), promotion of oxidative stress injury (Fig. 5I-J) and increased oxidative stress injury related protein expression (Fig. 5K). Notably, when PI3K inhibitors were added, entrance of Nrf2 into the nucleus was inhibited and HO-1 and Trx-1 expression levels were reduced (Fig. 5L). Therefore, KOR agonists can improve cognitive function and reduce brain damage in CPB rats through activation of Nrf2/HO-1 and regulation of the PI3K/AKT pathway.
Discussion
POCD is a common complication following cardiac surgery and may lead to an extended stay in an intensive care unit or hospital, increased perioperative complications and mortality and a decrease in the ability of the patient to lead an independent life (2). At 1 week following cardiac surgery, the incidence of POCD is as high as 50–70 and ~40% of patients still have cognitive dysfunction at five years following surgery (37,38). CPB surgery can reduce postoperative neurocognitive ability because of hypoperfusion or low mean arterial pressure, hemodynamic instability, cerebral thrombosis, systemic inflammatory responses, anemia, hyperglycemia and extracorporeal circulation trauma (39). In the present study, a CPB rat model was successfully established and treated with a KOR agonist and then the cognitive function, inflammatory response, oxidative stress injury and apoptosis were observed. The results demonstrated that a KOR agonist could improve cognitive function and reduce brain damage in CPB rats, which is related to the activation of the PI3K/AKT signaling pathway, and activate Nrf2/HO-1, thus inhibiting oxidative stress injury.
KORs are distributed in the central nervous system tissue and mRNAs of KORs can be detected in the hippocampal dentate gyrus, hypothalamus, certain thalamic nuclei, cerebral cortex and spinal cord (40). KORs have been demonstrated to regulate emotional and cognitive functions, reduce brain tissue damage and improve functional recovery in animal models of cerebral ischemia (5). Previous studies have also demonstrated that KOR expression is reduced in the brain of Alzheimer's disease patients (41,42). Activation of KORs in the brain of Alzheimer's rats can improve cognitive impairment and protect neuronal cells by inhibiting the formation of Aβ and its neurotoxicity (43). Charron et al (24) demonstrated that U50488H, a KOR agonist, can specifically activate hippocampal cholinergic neurons by activating KORs on hippocampal nerve cells, which can significantly reduce hippocampal nerve damage caused by ischemia. In the present study, U50488H (1.5 mg/kg) was injected into the lateral ventricle before CPB surgery. It was found that U50488H could reduce the neurological deficit score of rats, shorten the escape latency, increase the number of crossings of the original platform and extend the swimming distance and time in the target quadrant in the Morris water maze and reduce hippocampal injury. These results suggested that U50488H can improve cognitive function and reduce brain injury in CPB rats.
The cerebral tissue damage induced by CPB is caused by the difficulty for brain cells to produce ATP through aerobic respiration (34,35). Glial cells activated following CPB can secrete a variety of chemokines and inflammatory mediators, leading to a large increase in the level of cell adhesion molecules and disrupting the permeability of vascular endothelial cells, causing cell dysfunction and eventually leading to apoptosis (24). Following the activation of KOR receptors in stroke rats, injury inhibits glutamic acid release and NO production at the presynaptic membrane (43). Activation of KOR receptors also reduces neurotoxicity, improves the survival rate of damaged neurons, reduces neuronal apoptosis and the incidence of cerebral infarction and can reverse the memory impairment caused by CPB (44). Using the CPB rat model, the present study confirmed that oxidative stress served an important role in brain injury following CPB. CPB stimulated the oxidative stress response of neuronal cells, which generated high concentrations of ROS, affecting nerve cells, adversely affecting metabolism and increasing the rate of neuronal cell apoptosis. U50488H treatment could inhibit oxidative stress damage, reduce the ROS content and increase the SOD concentration, thereby effectively removing excess oxygen free radicals to avoid oxidative stress.
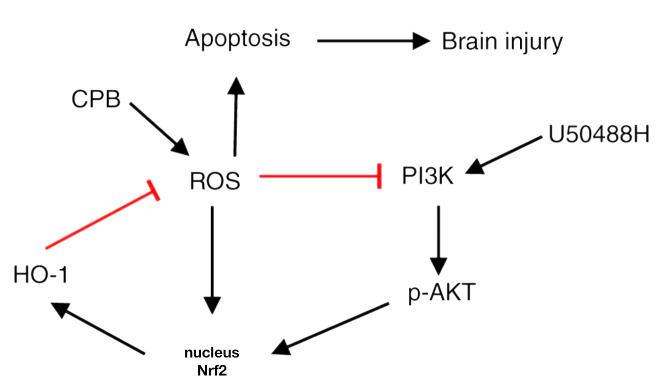
Harmful external environmental changes including hypoxia can induce the expression of HO-1, which can protect cells from a variety of harmful extracellular stimuli, including hypoxia (24). Additionally, HO-1 is regulated by the transcription factor Nrf2 (36). Under normal physiological conditions, Nrf2 in combination with the cytoplasmic linker protein Kelch-like ECH-associated protein 1 localizes to the cytoplasm and is maintained at a low level (6). When oxidative stress occurs, Nrf2 is not degraded by the ubiquitin proteasome and is thus maintained at a high level (5). The PI3K/AKT/Nrf2/HO-1 pathway is an important pathway in protection against epilepsy and seizure-induced brain injury under Dynorphin treatment through activation of κ-opioid receptor (36). The present study demonstrated that U50488H could activate Nrf2, promote its nuclear translocation, increase HO-1 expression and then protect cells from oxidative stress damage. However, when the HO-1 inhibitor ZnPP-IX was administered, the protective effect of U50488H on CPB rats was blocked, suggesting that HO-1 mediates U50488H activation of KORs in the hippocampus of CPB rats, which may be an important mechanism of neural protection. To further explore the regulatory mechanism of U50488H, the expression of the upstream protein Nrf2 was detected. As a result of Nrf2 expression, the PI3K/AKT signaling pathway was promoted and activated AKT participated in the anti-apoptosis and oxidative stress processes under U50488H treatment. In addition, PI3K inhibitor administration could inhibit Nrf2 and HO-1 expression. Therefore, the present study demonstrated that KOR agonists could activate Nrf2/HO-1 through the PI3K/AKT pathway to improve cognitive function and reduce brain damage in CPB rats (Fig. 6).
Figure 6.
Flow chart of Kappa opioid receptor agonist action for attenuates brain injury induced by CPB. KOR agonists can reduce brain damage of CPB rat, which is related to the activation of PI3K/AKT signaling pathway, to further activate Nrf2/HO-1, thus inhibiting oxidative stress injury. KOR, κ-opioid receptor; CPB, cardiopulmonary bypass; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1; p-, phosphorylated.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by the Key Scientific Research Project of Liaoning Province (grant no. 2020JH2/10300051).
Funding
This study was supported by the Key Scientific Research Project of Liaoning Province (grant no. 2020JH2/10300051).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JF, LL and PQ performed the animal experiment, prepared the CPB rat model and performed Neurological deficit score and Morris water maze tests. LL and PQ also performed the H&E staining, TUNEL assay and immunofluorescence. YD and YS contributed to acquisition of funding support. JF designed the study. YD and YS conceived and designed the study, acquired data, interpreted the results and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the China Medical University Laboratory Animal Welfare and Ethics Committee (IACUC no. 2018048R).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Martin J, Cheng DC. Evidence-Based Practice in Perioperative Cardiac Anesthesia and Surgery. Springer; 2020. Neurologic complications after cardiac surgery: Stroke, Delirium, Postoperative Cognitive Dysfunction, and Peripheral Neuropathy; pp. 619–636. [Google Scholar]
- 2.Keith JR, Puente AE, Malcolmson KL, Tartt S, Coleman AE, Marks HF., Jr Assessing postoperative cognitive change after cardiopulmonary bypass surgery. Neuropsychology. 2002;16:411–421. doi: 10.1037/0894-4105.16.3.411. [DOI] [PubMed] [Google Scholar]
- 3.Kumpaitiene B, Svagzdiene M, Sirvinskas E, Adomaitiene V, Petkus V, Zakelis R, Krakauskaite S, Chomskis R, Ragauskas A, Benetis R. Cerebrovascular autoregulation impairments during cardiac surgery with cardiopulmonary bypass are related to postoperative cognitive deterioration: Prospective observational study. Minerva Anestesiol. 2019;85:594–603. doi: 10.23736/S0375-9393.18.12358-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen K, Sun Y, Dong W, Zhang T, Zhou N, Yu W, Diao Y, Guo S, Tian Y. Activated A7nachr improves postoperative cognitive dysfunction and intestinal injury induced by cardiopulmonary bypass in rats: Inhibition of the proinflammatory response through the Th17 immune response. Cell Physiol Biochem. 2018;46:1175–1188. doi: 10.1159/000489068. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takaya K, Suzuki T, Motohashi H, Onodera K, Satomi S, Kensler TW, Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med. 2012;53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HH, Chen YT, Huang YW, Tsai HJ, Kuo CC. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic Biol Med. 2012;52:1054–1066. doi: 10.1016/j.freeradbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Netto MB, de Oliveira Junior AN, Goldim M, Mathias K, Fileti ME, da Rosa N, Laurentino AO, de Farias BX, Costa AB, Rezin GT, et al. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun. 2018;73:661–669. doi: 10.1016/j.bbi.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS One. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong-Qiang H, Mang-Qiao S, Fen X, Shan-Shan L, Hui-Juan C, Wu-Gang H, Wen-Jun Y, Zheng-Wu P. Sirt1 mediates improvement of isoflurane-induced memory impairment following hyperbaric oxygen preconditioning in middle-aged mice. Physiol Behav. 2018;195:1–8. doi: 10.1016/j.physbeh.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 12.Yu M, Wang J, Fang Q, Liu P, Chen S, Zhe N, Lin X, Zhang Y, Zhao J, Zhou Z. High expression of heme oxygenase-1 in target organs may attenuate acute graft-versus-host disease through regulation of immune balance of TH17/Treg. Transpl Immunol. 2016;37:10–17. doi: 10.1016/j.trim.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Wu B, Song HL, Yang Y, Yin ML, Zhang BY, Cao Y, Dong C, Shen ZY. Improvement of liver transplantation outcome by heme oxygenase-1-transduced bone marrow mesenchymal stem cells in rats. Stem Cells Int. 2016;2016:9235073. doi: 10.1155/2016/9235073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billings FT IV, Yu C, Byrne JG, Petracek MR, Pretorius M. Heme oxygenase-1 and acute kidney injury following cardiac surgery. Cardiorenal Med. 2014;4:12–21. doi: 10.1159/000357871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal. 2016;25:165–183. doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang M, Xue J, Sharma V, Habtezion A. Protective role of hemeoxygenase-1 in gastrointestinal diseases. Cell Mol Life Sci. 2015;72:1161–1173. doi: 10.1007/s00018-014-1790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu L, Chen W, Tian F, Yuan C, Wang H, Yue H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed Pharmacother. 2018;106:1484–1489. doi: 10.1016/j.biopha.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 18.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang J, Wang H, Zhou J, Dai W, Zhu Y, Zhou Y, Wang X, Zhou M. Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Des Devel Ther. 2018;12:2497–2508. doi: 10.2147/DDDT.S163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Cao J, Liu N, Ma L, Zhou X, Zhang H, Wang Y. Protective effects of edaravone in adult rats with surgery and lipopolysaccharide administration-induced cognitive function impairment. PLoS One. 2016;11:e0153708. doi: 10.1371/journal.pone.0153708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng X, Wang M, Sun G, Ye J, Zhou Y, Dong X, Wang T, Lu S, Sun X. Attenuation of Aβ25-35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol Appl Pharmacol. 2014;279:63–75. doi: 10.1016/j.taap.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Zou Y, Hong B, Fan L, Zhou L, Liu Y, Wu Q, Zhang X, Dong M. Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: Involvement of the GSK-3β/Nrf2 signaling pathway. Free Radic Res. 2013;47:55–63. doi: 10.3109/10715762.2012.742518. [DOI] [PubMed] [Google Scholar]
- 23.Chunhua C, Chunhua X, Megumi S, Renyu L. Kappa opioid receptor agonist and brain ischemia. Transl Perioper Pain Med. 2014;1:27–34. [PMC free article] [PubMed] [Google Scholar]
- 24.Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta 1-opioid agonists in a rat model of global ischemia. Physiol Behav. 2008;93:502–511. doi: 10.1016/j.physbeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Song D, Wang M, Chen K, Zhang T. α7 nicotinic acetylcholine receptor agonist attenuates the cerebral injury in a rat model of cardiopulmonary bypass by activating the Akt/GSK3β pathway. Mol Med Rep. 2017;16:7979–7986. doi: 10.3892/mmr.2017.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Mackensen GB, Sato Y, Nellgård B, Pineda J, Newman MF, Warner DS, Grocott HP. Cardiopulmonary bypass induces neurologic and neurocognitive dysfunction in the rat. Anesthesiology. 2001;95:1485–1491. doi: 10.1097/00000542-200112000-00031. [DOI] [PubMed] [Google Scholar]
- 28.Johnsson P, Lundqvist C, Lindgren A, Ferencz I, Alling C, Ståhl E. Cerebral complications after cardiac surgery assessed by S-100 and NSE levels in blood. J Cardiothorac Vasc Anesth. 1995;9:694–699. doi: 10.1016/S1053-0770(05)80231-9. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Li G, Geng F, Zhang Z, Li J, Yang M, Dong L, Gao F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis. 2014;19:946–957. doi: 10.1007/s10495-014-0977-0. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Zhang J, Zhu X, Wang P, Wang X, Li D. Progesterone reduces inflammation and apoptosis in neonatal rats with hypoxic ischemic brain damage through the PI3K/Akt pathway. Int J Clin Exp Med. 2015;8:8197. [PMC free article] [PubMed] [Google Scholar]
- 31.Pachori AS, Smith A, McDonald P, Zhang L, Dzau VJ, Melo LG. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J Mol Cell Cardiol. 2007;43:580–592. doi: 10.1016/j.yjmcc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Qu Y, Tang J, Chen D, Fu X, Mao M, Mu D. PI3K/Akt signaling pathway is required for neuroprotection of thalidomide on hypoxic-ischemic cortical neurons in vitro. Brain Res. 2010;1357:157–165. doi: 10.1016/j.brainres.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Trushina E, McMurray C. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Schurr A. Lactate, glucose and energy metabolism in the ischemic brain (Review) Int J Mol Med. 2002;10:131–136. [PubMed] [Google Scholar]
- 35.Arrica M, Bissonnette B. Therapeutic hypothermia. Semin Cardiothorac Vasc Anesth. 2007;11:6–15. doi: 10.1177/1089253206297409. [DOI] [PubMed] [Google Scholar]
- 36.Dai H, Wang P, Mao H, Mao X, Tan S, Chen Z. Dynorphin activation of kappa opioid receptor protects against epilepsy and seizure-induced brain injury via PI3K/Akt/Nrf2/HO-1 pathway. Cell Cycle. 2019;18:226–237. doi: 10.1080/15384101.2018.1562286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy DO, Haskell CF. Cerebral blood flow and behavioural effects of caffeine in habitual and non-habitual consumers of caffeine: A near infrared spectroscopy study. Biol Psychol. 2011;86:298–306. doi: 10.1016/j.biopsycho.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Neema PK, Dharan BS, Singha SK, Sethuraman M, Rathod RC. Entropy score, patent ductus arteriosus (PDA), and cardiopulmonary bypass (CPB): Ligation of PDA on CPB can compromise cerebral blood flow. Ann Card Anaesth. 2011;14:203–205. doi: 10.4103/0971-9784.84017. [DOI] [PubMed] [Google Scholar]
- 39.Gottesman RP, Grega MA, McKhann GM, Selnes OA. Brain disorders in critical illness: Mechanisms, diagnosis, and treatment. Camb Univ Press; 2011. Neurological complications of cardiac surgery: Stroke, encephalopathy, and cognitive decline; pp. 410–418. [Google Scholar]
- 40.Simonin F, Gavériaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattéi MG, Charron G, Bloch B, Kieffer B. kappa-opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci USA. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silvia RC, Slizgi GR, Ludens JH, Tang AH. Protection from ischemia-induced cerebral edema in the rat by U-50488H, a kappa opioid receptor agonist. Brain Res. 1987;403:52–57. doi: 10.1016/0006-8993(87)90121-1. [DOI] [PubMed] [Google Scholar]
- 42.Cai Z, Ratka A. Opioid system and Alzheimer's disease. Neuromolecular Med. 2012;14:91–111. doi: 10.1007/s12017-012-8180-3. [DOI] [PubMed] [Google Scholar]
- 43.Rácz B, Halasy K. Kappa opioid receptor is expressed by somatostatin- and neuropeptide Y-containing interneurons in the rat hippocampus. Brain Res. 2002;931:50–55. doi: 10.1016/S0006-8993(02)02259-X. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Sun Y, Jin Q, Song D, Diao Y. Kappa opioid receptor agonists improve postoperative cognitive dysfunction in rats via the JAK2/STAT3 signaling pathway. Int J Mol Med. 2019;44:1866–1876. doi: 10.3892/ijmm.2019.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.